Abstract
Purpose
The study aims to depict the radiological features of Cov-ROCM, depict the common routes of spread to orbits and intracranial compartment and look for an association of the risk factors with radiological severity of the disease.
Methods
96 patients who had COVID-19 infection in the past 3 months and were diagnosed with ROCM underwent CECT PNS examinations which were assessed by two experienced radiologists. They were divided into three groups based on the intraorbital and intracranial involvement and were correlated with various risk factors.
Results
The incidence of bony erosions which was the commonest finding (75%) was double in Cov-ROCM than the ROCM cases of pre COVID era (33–40%). The most common route of spread to orbit was through angioinvasion(52%) with intact orbital walls; and intracranial extension was via erosion of the cribriform plate(52%). Sphenoid sinus involvement is strongly associated with intracranial and intraorbital involvement.(p-value = .0004).
History of longer ICU stays and being on mechanical ventilation as a part of COVID management is associated with aggressive disease pattern(p-value = .002). Similarly, poor glycaemic control signified by raised HbA1c levels showed statistically significant correlation with severe Cov-ROCM(intraorbital/intracranial extension) (p-value = .040).
Conclusion
Amidst the COVID pandemic, it is pertinent to look at bony erosions in case of any sinusitis, especially bony maxillary walls and the turbinates. The intraorbital compartment must be viewed thoroughly even in the absence of bony erosions due to the angioinvasive nature of these fungi. Aggressive follow-up for patients with ICU stays for COVID and for glycaemic control would help reduce the morbidity.
Abbreviations: ROCM, Rhino-orbito-cerebral mucormycosis; COVID related ROCM, Cov-ROCM; CT, Computed tomography; NRBM, Non rebreather mask
Keywords: COVID, Rhino-orbito-cerebral mucormycosis, CT and clinico-radiological correlation
1. Introduction
The start of the second wave of the COVID epidemic in India saw a substantial rise in the cases of Rhino-Orbito-Cerebral mucormycosis (ROCM) which conventionally used to occur in patients of immunosuppressed individuals and Diabetic ketoacidosis.1 These cases of ROCM were seen within 3 months of COVID-19 infection with the mean interval between COVID infection and development of ROCM being 10 days.2
The risk factors predisposing to this newer entity, the COVID-associated ROCM (Cov-ROCM) were found to be diabetes mellitus irrespective of ketoacidotic state and administration of corticosteroid and broad-spectrum antibiotics as a part of the management of COVID infection.2., 3. Moreover, severe COVID infection itself being a risk factor was hypothesised.4
Most of the patients diagnosed with ROCM undergo surgical debridement of necrotic tissue to augment the effect of the antifungal amphotericin and prevent the spread of the disease to adjacent vital structures.2 Radiological imaging plays a vital role in exactly gauging the area of involvement and the regions that require debridement. Delayed diagnosis and treatment, can lead to rapid progression of the disease, with reported mortality rates from intra-orbital and intracranial complications of 50–80%.5 Contrast enhanced Computed Tomography (CT) scan of paranasal sinuses is usually the initial modality of imaging to depict the regions involved.6
Hence our primary objective is to depict the radiological features and routes of spread in Cov-ROCM to orbit and intracranial cavity. Another objective is to look for an association between the risk factors and radiologically severe Cov-ROCM.
2. Materials and methods
2.1. Study design and participants
This single centre cross-sectional study was approved by the Institutional Review Board, (IEC-III/EC/OA-126/2021). It included all patients between 1st March 2021 and 31st May 2021.
Medical records of all patients admitted with the following inclusion criteria: (a) patients with a history of COVID-19 confirmed by a positive SARS-CoV-2 RT-PCR in the past 3 months; (b) ROC mucormycosis diagnosed on CT; and (c) characteristic hyphae on KOH mount or histopathologically proven for filamentous fungi of the family Mucoraceae. Patients not willing to give informed consent, with age less than 18 years, or on immunosuppressant drugs for indications other than COVID-19 infection-related were excluded from the study.
Patients demographic data underlying comorbidities, all history pertaining to COVID-19 infection and HbA1c level were collected. Reports of the KOH mount or culture growth specific for Mucormycosis using necrosed tissue samples were recorded.
A plain and contrast-enhanced CT examination of the paranasal sinuses was performed. The area covered was from the vertex of the skull up to the hyoid bone. Brain and Orbit were covered to look for any intraorbital/intracranial extension, as the disease is angioinvasiveness and can affect sites distant from the affected paranasal sinuses. MDCT machine Philips Brilliance CT 64 channel-DS was used. All scans were reviewed by two Head and Neck Radiology specialists separately (one with experience of 20 years and another 4 years), who were blinded to previous images and clinical information. In cases of inter-reader discrepancies, a consensus was reached through discussion. Imaging findings of nasal cavity, paranasal sinuses, bones and routes of spread were corroborated with the FESS(Functional Endoscopic Sinus Surgery) findings. Also, in case of subtle/ indeterminate findings in the orbit and intracranial cavity, MRI brain/orbits were used to confirm them.
Each scan was evaluated for soft tissue involvement/irregular mucosal thickening of the nasal cavity and the paranasal sinuses. The presence of hyperdense contents (HU > 60) which are relatively specific for the presence of fungal elements after ruling out the possibility of haemorrhage was noted.
A focal area of discontinuity was labelled as bony erosions. Involvement of the subcutaneous tissue/fat stranding was looked for in specific areas, namely premaxillary and preseptal regions. Regional extensions/fat stranding were noted in the suprahyoid neck space especially the retroantral fat pad. Intraorbital extension of the soft tissue/fat stranding was evaluated in the extraconal and intraconal compartments with particular attention to extraocular muscles and involvement of the orbital apex. Abnormal enhancement of the optic nerve or fat stranding around the optic nerve was considered to be optic neuritis. Intracranial complications noted were pachymeningeal and leptomeningeal involvement, cavernous sinus thrombosis, internal carotid artery thrombosis, infarct, hematoma, or intraparenchymal abscess.
The exact routes of spread of the disease to the orbits and the intracranial cavity were recorded. In cases where there was no bony erosion or direct abnormal soft tissue extension into the intraorbital and intracranial compartments, the spread of the disease was classified as being via angioinvasion. In case of multiple routes of spread, the one with the bulk of soft tissue was considered. Aggressive Cov-ROCM in the study was labelled whenever there was involvement of the intraorbital or intracranial compartments.
Patients were divided into 3 groups based on the areas of involvement. Group A included patients with involvement limited to nasal cavity and paranasal sinuses, Group B consisted of patients with extension into intraorbital disease only and Group C involved patients with intracranial extension.
Data coding and entry were done in Microsoft Excel spread sheets. Descriptive and inferential statistical analysis was done by using SPSS version 21 (Statistical Package for Social Sciences) software. The raw data was compiled, classified, presented in a tabulated manner to bring out important details. Descriptive statistics like frequency, proportion, Mean, Standard deviation were used. One-way ANOVA, Kruskal Wallis Test, Independent t-test were used as inferential statistics. P less than 0.05 was considered statistically significant.
All patients who were eligible to participate in the study as per inclusion and exclusion criteria were included in study using the purposive sampling method.
3. Results
Out of the total 154 patients with ROC mucormycosis admitted to our hospital, 96 of them met the inclusion criteria (Fig. 1 ). Demographic and key comorbidities are shown in Table 1 . We observed that it was a male predominant disease with men being affected 3 times than women. Diabetes mellitus turned out to be the most common risk factor amongst these patients with mean HbA1c levels of 8.68%. Some form of steroid administration as a part of COVID-related management was noted in more than 80% of patients. The average CT severity score of the COVID infection fell into the moderate category (mean = 10.66) (Table 1).
Fig. 1.
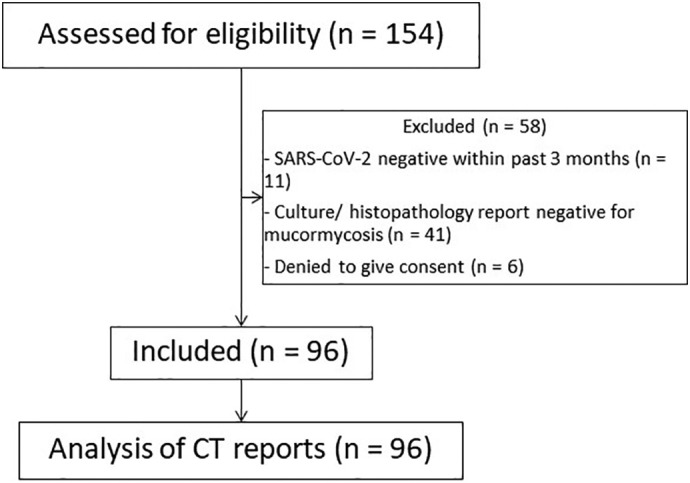
Flowchart for recruitment and analysis procedure of the study.
Table 1.
Demographics (M = Male, F=Female)
| Total patients | 96 |
|---|---|
| Age (yrs.) | 49.39 (21–76) |
| M:F | 73:23 |
| Diabetes mellitus (n) | 69 (M = 51,F = 18) |
| Hypertension (n) | 38 |
| Ischaemic heart disease | 6 |
| Chronic kidney disease | 2 |
| Obesity | 2 |
| Stroke | 1 |
| Steroid Intake | 79 |
| Mean HbA1c | 8.683 (5–16) |
| Mean CT severity | 10.66(2–25) |
3.1. Imaging spectrum
The most common sinuses to be involved were the maxillary and ethmoid sinuses. There was a statistically significant association between involvement of the sphenoid sinus and aggressive Cov-ROCM. (p-value = .0004/Chi-Square test) (Table 2 ). Bilateral sinusoidal involvement was seen in two-thirds of the cases. Air-fluid levels which suggest acute sinusitis were seen in only one-eighth of the patients. Post-contrast scans revealed mild enhancement in about 51% of the cases, while the rest of them had either heterogeneous enhancement or predominantly non-enhancing soft tissue (Table 3 ).
Table 2.
Sphenoid sinus association with aggressive Cov-ROCM
| Sphenoid sinus | Aggressive Cov-ROCM (Group B&C) | Group A |
|---|---|---|
| Involved | 53 | 22 |
| Uninvolved | 6 | 15 |
| p-value | 0.0004 | |
Table 3.
Radiological features and areas of involvement
| Imaging features | Features/sites | No. of patients (n) | % |
|---|---|---|---|
| Hyperdense contents | 30 | 31.2 | |
| Air fluid levels | 12 | 12.5 | |
| Bilateral Sinus involvement | 67 | 69.7 | |
| Enhancement pattern | Non | 26 | 27.0 |
| Mild | 49 | 51.0 | |
| Heterogenous | 21 | 21.8 | |
| Sinuses | Maxillary | 92 | 95.8 |
| Ethmoid | 88 | 91.6 | |
| Sphenoid | 75 | 78.2 | |
| Frontal | 61 | 63.6 | |
| Spaces | Retromaxillary Fat pad | 47 | 49 |
| PPF | 23 | 23.9 | |
| Masticator | 16 | 16.6 | |
| Bone | Maxillary sinus | 41 | 42.7 |
| Ethmoid sinus | 18 | 18.7 | |
| Sphenoid | 18 | 18.8 | |
| Superior turbinates | 2 | 2.1 | |
| Inferior/middle Turbinates | 36 | 37.5 | |
| Alveolar process | 13 | 13.5 | |
| Hard palate | 13 | 13.5 | |
| Nasal septum | 14 | 14.6 | |
| Inferior orbital wall | 7 | 7.3 | |
| Lamina | 24 | 25.0 | |
| Cribriform | 14 | 14.6 | |
| Uncinate | 16 | 16.6 | |
| Cellulitis | Preseptal | 11 | 11.4 |
| Premaxillary | 24 | 40.67 | |
| Orbit (n = 57) | Extraconal | 49 | 85.9 |
| EOM | 31 | 54.4 | |
| Intraconal | 21 | 36.8 | |
| Optic nerve | 22 | 38.5 | |
| Orbital Apex | 15 | 26.3 | |
| Intracranial (n = 21) | Cavernous sinus | 7 | 33.3 |
| ICA | 5 | 23.8 | |
| Abscess | 5 | 23.8 | |
| Infarct/Hematoma | 6 | 28.5 | |
| Pachymeningeal | 8 | 38.1 | |
| Trigeminal nerve | 5 | 23.8 | |
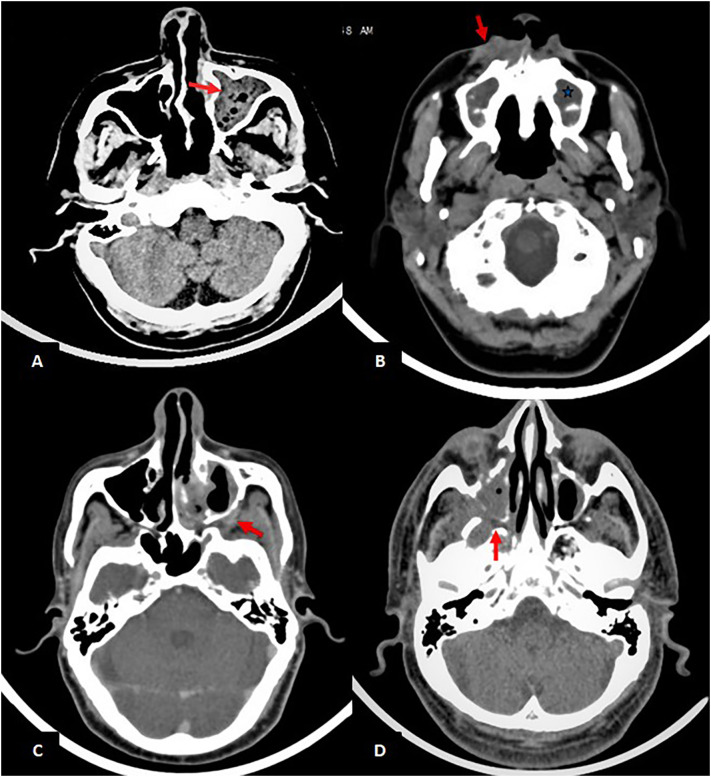
Bony erosions were found in 79% of the patients with maxillary sinus wall involvement being the commonest (Table 3). Amongst suprahyoid neck spaces, Retroantral fat pad was the most commonly involved (51%) while Pterygopalatine fossa (Fig. 2d) and Masticator space involvement was seen in 26 and 17 patients respectively.
Fig. 2.
Paranasal sinuses (Note-All the images are of different patients): (A) Axial section of unenhanced CT shows hyperdense content and air foci in left maxillary sinus (red arrow); (B) Axial section of unenhanced CT shows mucosal thickening in maxillary sinuses bilaterally (bluestar) and fat stranding in right premaxillary area (red arrow); (C) Axial section of contrast enhanced CT shows heterogeneously enhancing soft tissue density in left retroantral fat pad(red arrow)and surrounding fat stranding; (D) Axial section of unenhanced CT shows soft tissue density in the right maxillary sinus with extension into the right pterygopalatine fossa (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
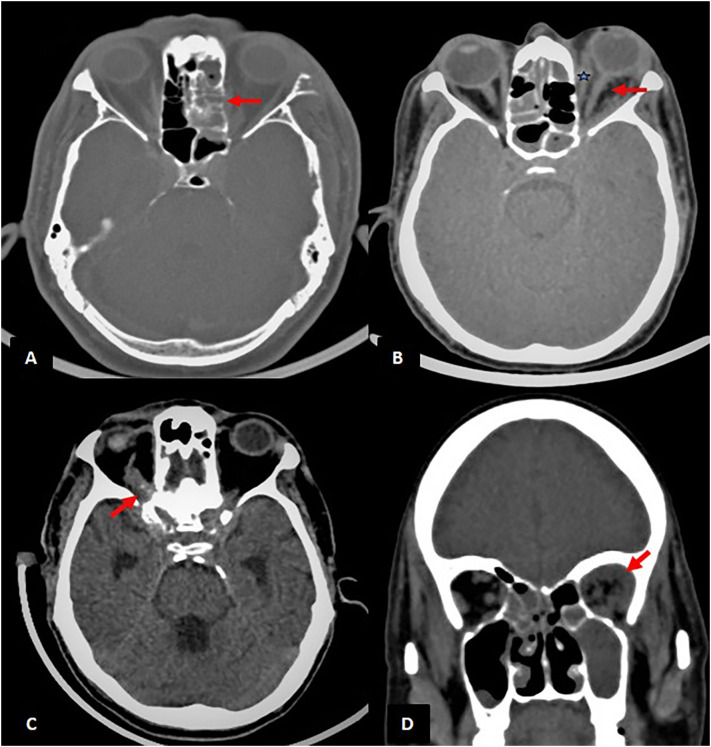
The orbital disease was seen in about 60% of the study population (n = 57) with intraconal involvement in 21 of them. Optic neuritis was seen in about 38.5% of the patients with orbital involvement (Fig. 3 a,b,c,d, Table 3).
Fig. 3.
Orbits (Note-All the images are of different patients): (A) Axial section of CT PNS at the level of orbits in bone window shows soft tissue in the left ethmoid sinus with erosion of the left lamina papyracea (red arrow).; (B) Axial section of unenhanced CT shows fat stranding surrounding the left optic nerve (red arrow) and bulky left medial rectus muscle (bluestar). There is associated proptosis of the left globe and tenting of the posterior coat; (C) Axial section of unenhanced CT shows thrombosed superior ophthalmic vein (red arrow) with cavernous sinus thrombosis; (D) Coronal section of unenhanced CT shows soft tissue density in the extraconal region of the left orbit (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
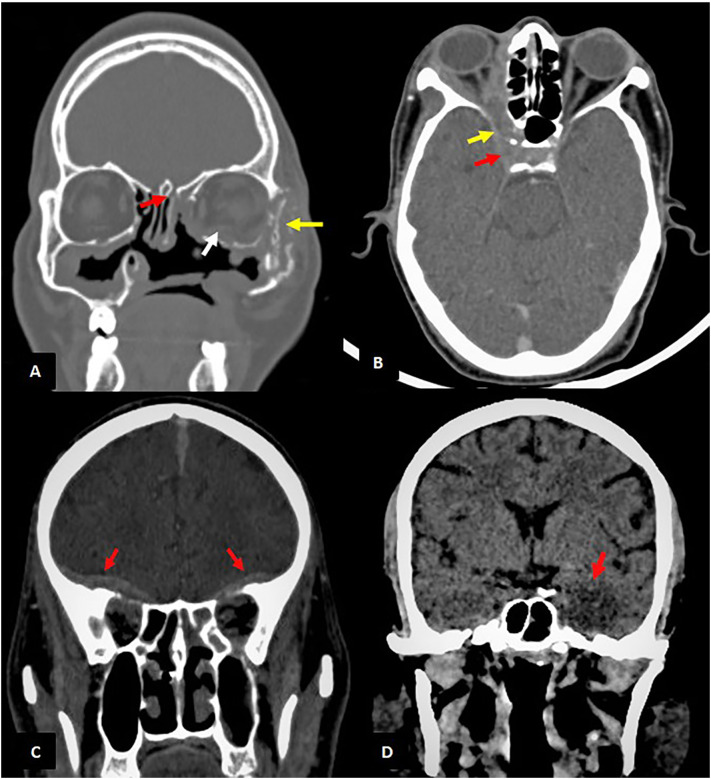
Common intracranial extensions were seen in the form of cavernous sinus thrombosis (n = 7) and pachymeningeal enhancement (n = 8) (Fig. 4c,d). Cerebral abscesses were seen in five patients (Table 3).
Fig. 4.
Intracranial involvement (Note-All the images are of different patients): (A) Coronal section of CT PNS of a patient with history of FESS in bone window shows erosion of left zygomatic arch (yellow arrow) and cribriform plate (red arrow). The extra ocular muscles in left orbit are bulky (white arrow) (B) Axial section of contrast enhanced CT shows peripherally enhancing soft tissue density involving right orbital apex (yellow arrow), extending into right cavernous sinus (red arrow) with resultant non-opacification suggestive of cavernous sinus thrombosis (C) Coronal sections of contrast enhanced CT PNS shows enhancing extra-axial dural thickening along basifrontal region bilaterally(red arrows) (D) Coronal sections of unenhanced CT shows an ill defined area of hypodensity with loss of grey-white matter junction in the left temporal lobe (red arrow) suggestive of infarct. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
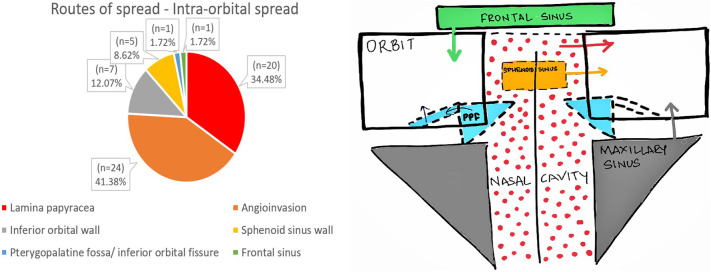
3.2. Pathways of spread
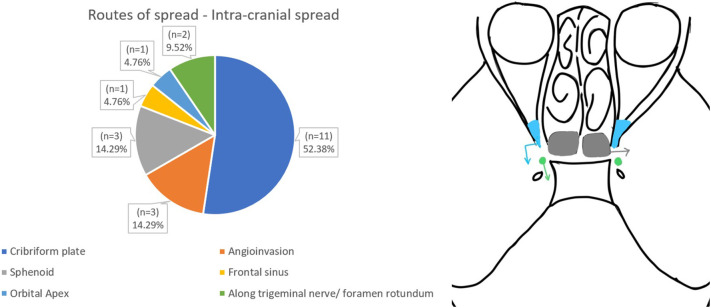
The routes of spread to the orbit and intracranial spread are shown in Fig. 5, Fig. 6 .
Fig. 5.
Routes of spread- Intra-orbital spread.
Fig. 6.
Routes of spread - Intra-cranial spread.
3.3. Clinico-radiological correlation
Poor glycaemic control (higher HbA1c levels) showed a statistically significant correlation with a more invasive form of ROC mucormycosis (p-value = .04/Kruskal-Willis test) (Table 4 ).
Table 4.
Clinico-radiological correlation.
| Sr no. | Characteristics | Group A (n = 37) | Group B (n = 38) | Group C (n = 21) | p- value |
|---|---|---|---|---|---|
| Quantitative data | |||||
| 1 | HbA1c (mmol) | 8.59 | 8.18 | 9.7 | 0.049 |
| 2 | CT Severity score (mean levels out of 25) | 10.35 | 10.5 | 11.48 | 0.6 |
| 3 | ICU Stay (average number of days) | 1.2 | 3.76 | 0.002 | |
| Qualitative data | |||||
| 4 | Steroids | 0.326 | |||
| None | 5 | 6 | 6 | ||
| Taken | 32 | 32 | 15 | ||
| 5 | Oxygen administration | 0.15 | |||
| a | None | 9 | 13 | 4 | |
| b | Nasal prongs | 7 | 6 | 3 | |
| c | face mask | 10 | 11 | 7 | |
| d | NRBM | 11 | 6 | 3 | |
| e | Mechanical ventilation | 0 | 2 | 4 | |
A statistically significant association between the average number of days of ICU stay and intracranial involvement (p = .002/t-test) was seen. An average of 1.2 days for Group A & B together compared to 3.7 days in Group C. Also, while 6 patients from Groups B & C were on mechanical ventilation, none had a similar history in group A (Table 4).
As seen in Table 4, there is no statistically significant correlation in the average CT severity score (p = .670/Chi-square test), or association of the number of patients with steroid intake (p-value = .320/Chi-square test) for each group. Similarly, no significant association was seen when comparing the modes of oxygen delivery (namely, nasal prongs, face mask, Non rebreather mask ventilation, and mechanical ventilation) across the 3 groups(p = .150/Chi-square test) (Table 4).
4. Discussion
The study revealed that bony erosions were seen in 75% of the patients of Cov-ROCM; with those of maxillary wall being the commonest. However, as per DelGaudio et al., Gupta K. et al. and Jacob Therekattu et al., the ROCM cases found in the pre-COVID era had bony erosions in only 33–40% of them.7., 8., 9. This is a distinct aggressive finding of COVID-associated ROCM as compared to those in the pre-COVID era. Moreover, they can be easily detected even on plain CT scans. Some of the characteristic erosions noted were in the nasal septum, hard palate, and alveolar process of the maxilla (which can present as loosening of teeth).
Since sphenoid sinus forms a part of the base of the skull and the orbital apex, its involvement predisposed to intra-orbital and intracranial extension.(p-value = .0004) Hyperdense intraluminal contents (Fig. 2a) in fungal sinusitis represent fungal hyphae and debris; which was seen in one-third of our patients.10 The enhancement pattern of the abnormal soft tissue varied from none to mild or heterogeneous enhancement; hence suggesting its unreliability in diagnosing the disease. Similar findings were also shown in cases of ROCM in the pre-COVID era by Jacob Therakathu et al.7
An early imaging finding in cases of ROCM is the involvement of the retroantral fat pad.11 Due to the angio-invasiveness of this group of fungi, it can occur without bony erosions by spreading through small vascular channels.12 This finding was seen in every other patient of our study group (n = 49). Similar results were also depicted in the study by Joshi et al.13 Additionally, angioinvasion was the commonest route of spread to orbit.
Superficial cellulitis is another early finding to diagnose ROCM, as the involvement of the superficially located subcutaneous fat is not common in non-fungal sinusitis. About 35% of the patients in the study showed features of superficial cellulitis, with the commonest being premaxillary fat stranding/soft tissue replacement (n = 27) (Fig. 2b).
The lethality of the ROCM is due to its invasiveness to involve critical surrounding areas, the orbit and the cranial cavity. The knowledge of pathways of spread to these areas would help the surgeon in targeting specific areas to prevent further spread or at least check its normalcy before completing the procedure. Moreover, a systematic approach with a checklist will greatly enhance the value of the preoperative imaging report for referring surgeons. CT proved useful in operated cases in order to know the exact status of bony landmarks before undergoing revision surgery. M Sen et al. showed that cavernous sinus thrombosis and cribriform plate erosion were the commonest routes of spread to the intracranial cavity, present in about 76% and 22% respectively.2 On the contrary, our study showed that cribriform plate erosion (37%) and cavernous sinus thrombosis (31%) were the commonest routes (Fig. 6a & b).
As per Prakash et al., the typical cases of ROCM have occurred in those with uncontrolled diabetes, diabetic ketoacidosis, or less commonly, in immunosuppressed hosts.14 Similar results were found by Sen et al., John et al. and Hoengil et al. which showed 78–94% diabetics amongst Cov-ROCM cases.2., 3., 15. In addition to the fact that our study population had 71% diabetics amongst them, we found that those with poor glycaemic control was associated with more invasive disease. (p-value = .040).In other words, severe DM did predispose to intraorbital or intracranial extension.
In the pre-COVID era, Yang et al. had shown that the SARS coronavirus had a propensity to cause Islet cell damage which would predispose individuals to develop DM or worsen glycaemic control in known cases.16 Additionally, the question of COVID itself being an independent risk factor has arisen. While we did not compare the ROCM cases with and without a history of COVID, we were able to show that radiological COVID severity depicted with the help of CT severity score was not associated with severity of Cov-ROCM. However, it is to be noted that since the average CT severity score of all the patients with Cov-ROCM in this study was in the moderate category, it can be concluded that COVID infection may predispose to ROCM. Also, Cov-ROCM was seen to be male dominant disease likely due to the fact that the diabetes was primarily male predominant (Table 1). COVID-19 data of our city showed that 54% of the COVID patients were male which does not support the male preponderance in Cov-ROCM.17
We found that higher ICU stay predisposed to Cov-ROCM with intracranial extension (Group C); as those with intracranial extension staying in the ICU as many as 3 times than those without (p-value = .046). Also shown is that amongst patients without a history of being on mechanical ventilation, none showed intraorbital/intracranial extension. Hence, an early aggressive approach in those patients with a history of ICU stay and being on mechanical ventilation would help control the spread of the disease and reduce the overall morbidity.
Since our study was a cross-sectional study, the course of the disease and its outcomes were not assessed. Our study includes CT examination of the patients, since it is the initial imaging modality of choice with easy availability and superiority in diagnosing bony erosions. MRI was not included in our study due to resource constraints and patient cooperation. The efficacy of CT vs MRI needs to be investigated to analyse the feasibility and practicality of one modality over the other.
In conclusion, Cov-ROCM can be tackled by using approaches of primary prevention, early diagnosis and targeted approach. Primary prevention in form of glycaemic control and close follow-up of patients with a history of mechanical ventilation. Early diagnosis can be enabled by the characteristic radiological findings in Cov - ROCM, these include bony erosion, hyperdense content within the sinuses and superficial cellulitis. These findings can raise the suspicion of Cov - ROCM on plain CT scans too. Moreover, due to the angioinvasiveness of the fungi, extension to the intraorbital compartment must be assessed irrespective of evidence of direct extension.
References
- 1.Pal R., Singh B., Bhadada S.K., Banerjee M., Bhogal R.S., Hage N., et al. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses. 2021 Dec;64(12):1452–1459. doi: 10.1111/myc.13338. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen M., Honavar S.G., Bansal R., Sengupta S., Rao R., Kim U., et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - collaborative OPAI-IJO study on mucormycosis in COVID-19 (COSMIC), report 1. Indian J Ophthalmol. 2021;69:1670–1692. doi: 10.4103/ijo.IJO_1565_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoenigl M., Danila S., Carvalho A., Rudramurthy S., Arastehfar A., Gangneux J.-P. 2021. The emergence of COVID-19 associated mucormycosis: analysis of cases from 18 countries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh A.K., Singh R., Joshi S.R., Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4) doi: 10.1016/j.dsx.2021.05.019. Jul-Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135:442–447. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alam A., Chander B., Sabhikhi G., Bhatia M. Sinonasal mucormycosis: diagnosis using computed tomography. Med J Armed Forces India. 2003;59:243–245. doi: 10.1016/S0377-1237(03)80018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therakathu J., Prabhu S., Irodi A., Sudhakar S.V., Yadav V.K., Rupa V. Imaging features of rhinocerebral mucormycosis: a study of 43 patients. Egypt J Radiol Nucl Med. 2018;49:447–452. [Google Scholar]
- 8.DelGaudio J.M., Swain R.E., Kingdom T.T., Muller S., Hudgins P.A. Computed tomographic findings in patients with invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 2003;129:236–240. doi: 10.1001/archotol.129.2.236. [DOI] [PubMed] [Google Scholar]
- 9.Gupta K., Saggar K. Analysis of computed tomography features of fungal sinusitis and their correlation with nasal endoscopy and histopathology findings. Ann Afr Med. 2014;13:119–123. doi: 10.4103/1596-3519.134398. [DOI] [PubMed] [Google Scholar]
- 10.Aribandi M., McCoy V.A., Bazan C. Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics. 2007;27:1283–1296. doi: 10.1148/rg.275065189. [DOI] [PubMed] [Google Scholar]
- 11.Silverman C.S., Mancuso A.A. Periantral soft-tissue infiltration and its relevance to the early detection of invasive fungal sinusitis: CT and MR findings. Am J Neuroradiol. 1998;19:321–325. [PMC free article] [PubMed] [Google Scholar]
- 12.Skiada A., Pavleas I., Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. 2020;6:265. doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi A.R., Muthe M.M., Patankar S.H., Athawale A., Achhapalia Y. CT and MRI findings of invasive Mucormycosis in the Setting of COVID-19: experience from a Single Center in India. Am J Roentgenol. 2021;217(6):1431–1432. doi: 10.2214/AJR.21.26205. AJR.21.26205. In press. [DOI] [PubMed] [Google Scholar]
- 14.Prakash H., Chakrabarti A. Global epidemiology of mucormycosis. 2019;5:E26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi Basel Switz. 2021;7:298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjOz9aP2J3zAhUIcCsKHQ9oDpAQFnoECCYQAQ&url=https%3A%2F%2Fstopcoronavirus.mcgm.gov.in%2Fassets%2Fdocs%2FDashboard.pdf&usg=AOvVaw2VIOaa816H8UucUOn2M3jx