Abstract
Objectives
The coronavirus disease 2019 (COVID-19) pandemic has led to significant morbidity and mortality. Although COVID-19 vaccination is available, therapeutic options are still needed. The goal of the present manuscript is to report on a treatment strategy used in a naturopathic medical practice for mild and moderate COVID-19.
Design
A retrospective chart review was conducted of 30 consecutive patients diagnosed with mild and moderate COVID-19 who were provided multi-nutrient, herbal, and probiotic treatment in a rural, out-patient, naturopathic primary care setting.
Main outcomes measures
The primary outcome was treatment safety; secondary outcomes included changes in symptoms, progression to severe COVID-19, incidence of long COVID, and recovery time.
Results
No side effects or adverse events were reported from treatment and all patients experienced resolution of symptoms presumed to be associated with COVID-19 infection. One patient who had been ill for 28 days prior to presentation was hospitalized. Five patients had an illness duration of more than one month. Time to treatment was correlated with duration of illness post-treatment (r = 0.63, p < 0.001) and more symptoms at presentation was correlated with a longer duration of illness (r = 0.52, p < 0.01).
Conclusions
In this retrospective chart review, a multi-nutrient, herbal, and probiotic therapeutic approach for mild and moderate COVID-19 appeared to be well-tolerated. Delay in seeking treatment after symptom onset, as well as more symptoms at presentation, were correlated with a longer duration of illness. This treatment strategy may have clinical benefit, warranting prospective clinical trials with confirmed COVID-19 cases.
Keywords: COVID-19, SARS-CoV-2, Herbal medicine, Phytotherapy, Vitamins, Minerals, Nutrition therapy, Orthomolecular therapy, Intravenous infusions, Immune system, Complementary therapies, Naturopathic medicine, Integrative medicine, Case reports
1. Introduction
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to a pandemic resulting in nearly 4 million deaths globally to date and has resulted in chronic sequelae (‘long COVID’ or ‘post-COVID syndrome’) in 10–90% of those infected.1., 2., 3., 4., 5, 6 Multiple COVID-19 vaccines are now available internationally, however, several dilemmas remaining include: (1) vaccine availability and completion of the recommended vaccine schedule, (2) vaccine effectiveness in the immunocompromised, (3) vaccine effectiveness against new SARS-CoV-2 variants, (4) vaccine breakthrough infections, and (5) vaccine hesitancy.7, 8, 9 Those with weakened immune function, estimated at greater than 6% of the population, are less likely to respond to vaccination.10, 11, 12., 13., 14, 15 Therefore, there is still a need for therapeutic agents for COVID-19 infection.
Risk factors identified for developing severe COVID-19 infection include older age and comorbidities, including conditions associated with chronic systemic inflammation, such as obesity and cardiovascular disease.2 Prominent risk factors for long COVID include initial acute COVID-19 severity, presence of more than five early symptoms, and female sex.2., 3. Aside from COVID-19 vaccine development, there have been significant efforts dedicated to investigating drug treatment and some efforts dedicated to investigating natural therapeutics.16., 17., 18 In this retrospective chart review, we report on the clinical outcomes from a multi-nutrient, herbal, and probiotic treatment strategy designed to provide immune support and regulation, and therefore limit inflammation, in 30 consecutive patients diagnosed with COVID-19 in a rural, out-patient, naturopathic primary care setting. This report was completed for clinical quality improvement to evaluate current clinical practices supporting patients with COVID-19.
2. Methods
This retrospective chart review was prepared according to recent standardized guidelines.19, 20, 21., 22. The study population included the first thirty consecutive patients of any age diagnosed with mild and moderate COVID-19 who were provided multi-nutrient, herbal, and probiotic treatment in a rural, outpatient, naturopathic primary care setting between March 2020 and February 2021. There were no exclusion criteria to prevent selection bias. Illness severity was determined based on the National Institutes of Health (NIH) treatment guidelines.23 Treatment was delivered by two licensed naturopathic physicians. Deidentified data was collected using a data abstraction instrument hosted on the Research Electronic Data Capture (REDCap) platform.24, 25 The tool developed was a hybrid version of a prior case report data collection tool (CARE Capture) and a registry form designed to collect deidentified data on patients with COVID-19 treated with traditional, complementary, and integrative medicine.26, 27 Collected information addressed the primary outcome of treatment safety and secondary outcomes including changes in symptoms, disease progression, incidence of long COVID, and recovery time. Microsoft Excel Version 2107 was used for analysis. Categorical data was summarized using frequency and percentages. Continuous variables were presented as means. Pearson correlations were calculated to evaluate if duration of illness was correlated with other variables including: presence of comorbid conditions, presence of five or more symptoms, and time to treatment. Because of the small number of patient cases, variable treatments, and heterogeneous duration of treatment, multivariable statistical modeling was not conducted. Therefore, these findings should be interpreted as descriptive and exploratory.
3. Results
3.1. Patient information
Patients were predominantly female, Caucasian, and non-Hispanic with a mean age of 46. About one-third were from the Russian Orthodox community and 80% were longstanding patients ( Table 1). The most common presenting symptoms were fatigue, myalgia, dry cough, fever, chills, and dyspnea; 70% of patients had five or more symptoms at presentation (Table 1). Most patients had a symptom duration of 5 days or less before seeking treatment. Ninety-three percent had mild severity and 7% had moderate severity at presentation.23 One patient had been hospitalized for a myocardial infarction, community acquired pneumonia (CAP), and concurrent COVID-19 infection before establishing care.
Table 1.
Patient demographic characteristics and clinical features.
| Characteristic | Frequency | Percentage (%) |
|---|---|---|
| Age (mean ± SD) < 65 years old ≥ 65 years old |
45.47 ± 22.13 21 9 |
70 30 |
| Sex Male Female |
12 18 |
40 60 |
| Race and Ethnicity Caucasian Hispanic |
30 3 |
100 10 |
| BMI (mean ± SD) (n = 24) | 30.01 ± 11.55 | |
| Pregnant | 1 | 3 |
| Comorbidities | 19 | 63 |
| Vitamin D Status (mean ± SD) Lab normal (>30 ng/mL) Insufficient (20–30 ng/mL) Deficient (<20 ng/mL) Unknown |
49.94 ± 12.23 18 1 1 10 |
60 3 3 33 |
| Most common symptoms at presentation Fatigue Myalgia Dry cough Fever (>38 °C) Chills Dyspnea Loss of taste or smell Sore throat |
27 24 19 18 18 16 9 8 |
90 80 63 60 60 53 30 27 |
| Total number of symptoms at presentation < 5 symptoms ≥ 5 symptoms |
9 21 |
30 70 |
Sixty-three percent of the 30 patients had comorbid conditions, the most common included hypertension (30%), cardiovascular disease (17%), obesity (10%), and COPD with asthma (7%) (Table 1). One patient had a blood clotting disorder, one an autoimmune condition, and another was four months pregnant. Mean BMI was 30 (n = 24). Two patients had a history of tobacco use. Vitamin D status (serum 25-hydroxycholecalciferol or 25-(OH)D) was sufficient (>30 ng/mL) for 60% of the patients, insufficient or deficient (<30 ng/mL) for 6%, and unknown for 33%. Mean 25(OH)D was 50 ng/mL (Table 1).
Due to the unprecedented nature of the COVID-19 global pandemic, modifications to routine practices were necessary. All patients provided consent to be seen via telehealth and/or in the clinic parking lot to reduce risk for COVID-19 transmission. Two patients were seen in person initially due to the absence of symptoms suggestive of COVID-19 infection.
Medications and supplements patients were taking at presentation are summarized in Table 2. The most common herbal supplement patients were taking at the time of COVID-19 diagnosis was the root of Curcuma longa or turmeric. Patients were taking dietary supplements for a wide array of health concerns, including cardiovascular and digestive complaints.
Table 2.
Baseline medications and dietary supplements.
| Category | Frequency | Percentage (%) |
|---|---|---|
| Taking medication | 17 | 57 |
| Taking dietary supplement | 28 | 93 |
| Medication | Frequency | Percentage (%) |
| Angiotensin-converting enzyme inhibitor (ACEI) antihypertensive | 4 | 13 |
| Non-ACEI antihypertensive | 4 | 13 |
| Acetaminophen | 4 | 13 |
| Anti-asthma | 4 | 13 |
| Other cardiovascular | 3 | 10 |
| Antibiotic | 3 | 10 |
| Non-chloroquine antiviral | 1 | 3 |
| Lipid-lowering | 1 | 3 |
| Topical corticosteroid | 1 | 3 |
| Non-steroidal anti-inflammatory | 1 | 3 |
| Central nervous system stimulant | 1 | 3 |
| Selective serotonin reuptake inhibitor | 1 | 3 |
| Antigout | 1 | 3 |
| Hormone replacement | 1 | 3 |
| Antihistamine | 1 | 3 |
| Antiglaucoma | 1 | 3 |
| Dietary Supplement | Frequency | Percentage (%) |
| Vitamin/mineral | 26 | 87 |
| Vitamin D | 21 | 70 |
| Omega 3 fatty acids | 19 | 63 |
| Herbal | 17 | 57 |
| Curcuma longa root | 8 | 27 |
| Probiotic | 11 | 37 |
| Amino acids | 10 | 33 |
| Digestive enzymes | 6 | 20 |
3.2. Clinical findings and diagnostic assessment
There were limitations to physical examination due to the nature of virtual and parking lot visits. Approximately half of the patients had elevated blood pressure (n = 14) and seven had dyspnea and tachypnea (>20 respirations per minute, but <30 respirations per minute). No patients had tachycardia (>100 beats per minute) at the initial encounter. Due to the limited availability of testing kits in the United States (and internationally) at the time care was delivered and the virtual nature of some of the visits, not all patients were tested for COVID-19. Fifty-three percent (16 patients) tested positive for SARS-CoV-2 via single-test nasopharyngeal swab PCR, 43% (13 patients) were presumed positive due to symptom presentation and cohabitation with a confirmed positive case, and 3% (1 patient) was presumed positive due to symptom presentation alone. Access to testing kits improved by mid-June 2020.
3.3. Therapeutic intervention
There were no U.S. Food and Drug Administration (FDA) approved treatments for COVID-19 for outpatient settings at the time treatment was initiated for these 30 patients.28 Therefore, therapeutic agents known to (1) be involved in innate and adaptive immunity, (2) be supportive in respiratory infections, and (3) modulate inflammation (due to risk of hyperinflammation and coagulopathy from COVID-19) were identified and included in our treatment protocol ( Table 3). Treatment recommendations included dietary recommendations, oral dietary supplementation including vitamins, minerals, probiotics, and an herbal tincture. Intravenous (IV) nutrient therapy was reserved for patients with moderate COVID-19 infection and/or comorbid conditions, including cardiovascular, metabolic, and pulmonary conditions. Treatment options were personalized to patients as needed (i.e., in the case of pregnancy, other medication use, and comorbid conditions). In November 2020, the FDA granted emergency use authorization for monoclonal antibody treatment for ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease.28 No patients fit this criterion when this therapy became available.
Table 3.
Mild and moderate COVID-19 treatment protocol.
|
Diet | ||
|---|---|---|
| Food Item and Preparation | Dosing Regimen | Rationale and References |
| Bone Broth (homemade or store-bought; made from beef, elk, buffalo, and/or chicken bones) | ½−1 cup 2 times daily until 3–5 days after symptom resolution | Bone broth contains immune-system supporting amino acids (i.e., arginine), minerals (i.e., selenium, magnesium)29., 30, and glutathione (GSH) precursors (GSH is involved in activation of T-lymphocytes and polymorphonuclear leukocytes)31; has anti-inflammatory activity31., 32., 33. |
| Beetroot and Greens (steamed, roasted, or juiced) | ¾−1 cup 2–3 times daily until 3–5 days after symptom resolution | Beetroot increases NO, which plays a role in the innate immune response in the airways and has bronchoprotective effects†34; is protective against oxidative stress and inflammation with potential to decrease vascular inflammation†35, 36, 37., 38, 39 |
|
Oral Supplementation | ||
|---|---|---|
| Dietary Supplement | Dosing Regimen | Rationale and References |
Vitamin C 1000 Plus (Professional Formulas):
|
2000 mg PO 3–5 times daily to bowel tolerance, continue for 3–5 days after symptom resolution | Vitamin C has antiviral effects40, 41; regulates proliferation and function of T- and B-cells and natural killer (NK) cells40, 41, 42.; can prevent and repair oxidative damage30, 40, 41; improves endothelial dysfunction41; reduces acute respiratory infection (ARI) symptoms, severity, and duration†43., 44., 45; decreased mortality rate in patients with SARS-CoV-2 pneunomia†46 |
D3 10,000 + K (Metagenics):
|
10,000 IU daily for 3–5 days if vitamin D levels are WNL; continue for 3–5 days after symptom resolution if vitamin D status is unknown and measure levels; 10,000 IU daily for 3 months if vitamin D is deficient and recheck levels |
Vitamin D is involved in chemotaxis, phagocytosis, and B- and T-cell function30, 47; may reduce viral cellular infection via angiotensin-converting enzyme 2 and lower viral replication30, 48; deficiency associated with increased risk of ARI, acute respiratory distress syndrome (ARDS), and SARS-CoV-2 mortality†30, 47, 48; may curb inflammation and lower need for oxygen therapy and intensive care support in patients with COVID-19†49, 50, 51 Vitamin K2 prevents hypercalcemia that can result from vitamin D supplementation52 |
Zinc 30 (Pure Encapsulations)
|
30–60 mg with food 1–2 times daily (60 mg max daily dose), continue for 3–5 days after symptom resolution | Zinc is essential for immune cell growth and differentiation30; involved in phagocytosis, NK activity, and T-cell response to infection30, 42., 53.; modulates cytokine release30; has potential to decrease virulence and incidence, severity, and duration of upper respiratory infection (URI)†30,44,53,54; cofactor for over 200 enzymes involved in antioxidant defense30; deficiency is associated with an increased risk of infection and mortality due to pneumonia and COVID-19 and higher rates of complications in patients with COVID-19†30, 55., 56 |
Vitamin A Mulsion (Professional Formulas):
|
12,000 IU daily for 3–5 days | Vitamin A regulates neutrophil, macrophage, NK cell, T- and B-cell activity30, 57; influences differentiation of dendritic cell precursors30, 58; required for mucin secretion and supports mucosal integrity30, 57; deficiency can lead to histopathological changes of pulmonary epithelium and lung parenchyma and increase the risk for respiratory disease and lung failure30, 58; may decrease morbidity and mortality from infectious disease57; inhibits proliferation of several types of viruses58, 59, 60.; may help with olfactory loss†61; modulates Th1-Th2 cell balance and may promote Treg response (and inhibit progression to Th17)57, 60. |
| Ther-biotic Complete Powder (Klaire Labs) (100 billion CFU in a base of inulin derived from chicory root and a proprietary polysaccharide complex): 24 billion CFU:
|
25–100 billion CFUs daily until 3–5 days after symptom resolution | Probiotics enhance innate and adaptive immunity (including Th1 and Treg function), prevent colonization by pathogens, reduce incidence and severity of infections and increase clearance (including respiratory infections)†30, 62, 63, 64; healthy gut microbiota is associated with lower systemic inflammation and prevention of an excessive immune response30, 62; commensal microbes (e.g., Lactobacillus plantarum) and their metabolites (e.g., bioactive peptides) may block viral entry into cells (e.g., SARS-CoV-2 via ACE receptor sites) and viral replication30, 62, 65, 66; some patients with SARS-COV-2 exhibit intestinal microbial dysbiosis characterized by low numbers of commensal microbes (e.g., Bifidobacterium spp. and Lactobacillus spp.)62, 66; probiotic treatment may prevent gastrointestinal dysbiosis and reduce risk of secondary infections30, 64, 66 |
Herbal tincture equal parts (all extracts in 30% grain alcohol and purified water):
|
½ teaspoon PO 5 times daily for 3–5 days, then ½ teaspoon PO three times daily for 7–10 days |
Elecampane may support a Th1 response67; contains an antimicrobial compound (alantolactone) which enhances phagocytosis, decreases reactive oxygen species (ROS) and superoxide production, inhibits pro-inflammatory cytokines (e.g., tumor necrosis factor alpha or TNF-α, IL-1β, IL-6, IL-8), suppresses nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB) activation, stimulates the release of anti-inflammatory mediators (e.g., IL-10, transforming growth factor beta or TGF-β), and upregulates nuclear factor erythroid 2-related factor 2 (Nrf2) activity68., 69., 70., 71.; may reduce inflammation in respiratory tract via controlling chemotaxis of neutrophils69; may attenuate lung injury70 Osha has anti-inflammatory effects72; may support the viability of peripheral blood lymphocytes via an increase in reduced glutathione levels and activity of superoxide dismutase and catalase73 Echinacea is a virucidal agent74, 75, 76, 77; shown to decrease severity and duration of ARI when taken at symptom onset†78,79; decreases pro-inflammatory cytokines involved in cytokine storm and ARDS†78 Oregon grape has antioxidant and immunomodulatory activity80, 81, 82, 83 |
|
IV Nutrient Therapy* | |||
|---|---|---|---|
| Ingredient | Dosing Regimen | Rationale and References | |
| IV Bag 1 | Magnesium chloride (magnesium sulfate used when MgCl was not available) | 1500–2000 mg diluted in 250 mL Lactated Ringer and infused over 45 min (IV bag 1) | Magnesium activates and enhances functionality of Vitamin D52., 84, 85; can inhibit calcium influx in immunocompetent cells which limits NF-ĸB activation, IL-6, and CRP and therefore reduces systemic inflammation85; coadministration with Vitamins D3 and B12 in hospitalized patients with COVID-19 was associated with a significant reduction in the requirement for oxygen therapy and intensive care support†86; several aspects of COVID-19 can mimic metabolic events shown to occur during latent subclinical magnesium deficiency85 |
| Ascorbic acid/ Vitamin C | 2500–12,500 mg diluted in 250 mL Lactated Ringer and infused over 45 min (IV bag 1) | (Also see PO above); IV Vitamin C is shown to block components of cytokine storms40; shortens intensive care unit (ICU) length of stay and duration of mechanical ventilation in ICU patients†40; decreases inflammatory markers and fraction of inspired oxygen (FiO2) requirements, normalizes lymphocyte and CD4+ T cell counts in patients with COVID-1987, 88 | |
| Methylcobalamin/Vitamin B12 | 2000 mcg delivered via push through the port after completion of IV bag 1 and before starting IV bag 2 of IV nutrients | Vitamin B12 is involved in leukocyte production and may enhance macrophage function and number of cytotoxic T-cells against viral infections†57, 89, 90; has anti-inflammatory effects via regulation of NF-ĸB57; may inhibit non-structural protein (nsp) 12 protein (involved in SARS-CoV-2 replication)91 | |
| IV Bag 2 | Zinc sulfate | 5–25 mg diluted in 50 mL 0.9% saline and infused over 15 min (IV bag 2) | (Also see PO above); IV Zinc protects various organs, including the heart, kidneys, and liver, against damage caused by hypoxia92 |
| Pyridoxine/ Vitamin B6 | 100–200 mg diluted in 50 mL 0.9% saline and infused over 15 min (IV bag 2) | Vitamin B6 is involved in antibody, T-cell, and interleukin production and lymphocyte maturation57, 93.; enhances the interaction between cytokines and chemokines93; reduces ROS production93; attenuates platelet aggregation and clot formation57; pyridoxal phosphate (PLP) levels inversely correlated with systemic inflammation57; reduces IL-1β production by suppressing NLR family pyrin domain containing 3 (NLRP3) inflammasome93 | |
Clinical evidence
For patients with moderate or severe symptom presentation and/or comorbid conditions
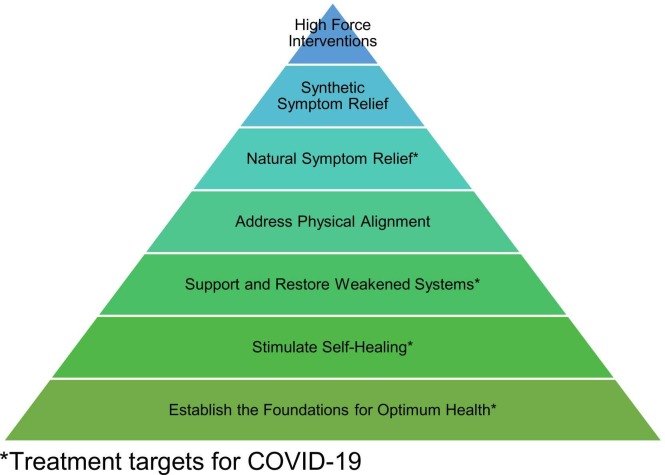
Selected therapies targeted the following tiers of the naturopathic therapeutic order: addressing pathology and providing natural symptom relief while establishing the foundations for optimum health, stimulating self-healing, and supporting and restoring weakened systems ( Fig. 1).94
Fig. 1.
The therapeutic order of naturopathic medicine and treatment strategies for COVID-19.94
For concurrent oral dietary supplementation, if patients were already taking the product or a combination product containing the constituent (e.g., vitamin D3), they were instructed to continue and adjust dosing as needed to follow the dosing listed in Table 3. One patient did not take the probiotic supplement as she had poor tolerance (i.e., probiotic supplementation historically led to side effects of abdominal cramping and bloating in this patient). The patient who was pregnant was not recommended high dose vitamin A dietary supplementation due to risk of teratogenicity.95
Patients were recommended a commercial herbal product which included root of Echinacea purpurea, rhizome and rootlet of Hydrastis canadensis, root of Inula helenium, whole flowering plant of Spilanthes acmella, herb of Eriodictyon californicum, root of Armoracia rusticana, rhizome of Zingiber officinale, fruit of Sambucus nigra, flowering tops of Achillea millefolium, and root of Baptisia spp in 54–64% organic cane alcohol, distilled water, and vegetable glycerin (Rapid Immune Boost by Herb Pharm). If this product was unavailable, patients were offered an herbal formula with similar constituents (Table 3). Neither herbal formula was prescribed to the pregnant patient due to safety concerns.96, 97. As an alternative, the pregnant patient elected to consume the fruit of Sambucus nigra or elderberry, prepared as a syrup, dosed at 1903 mg three times daily. Fourteen patients were already taking herbs with antiviral properties at home and opted to take these instead. These herbs were part of encapsulated products, teas, syrups, and tinctures; the most common was a syrup of the fruit of Sambucus nigra (n = 6).
Before IV nutrient therapy was administered, patients were screened for glucose-6-phosphate dehydrogenase (G6PD) deficiency, which would have excluded them from receiving IV vitamin C. They were also screened for kidney disease and congestive heart failure. No patients had G6PD deficiency. One patient had chronic kidney disease (GFR was 56 mL/min/1.73 m2) and one patient was on warfarin therapy; both were dosed at the low end of the dose range for vitamin C (Table 3) and administered IV fluids over a longer time period (75–85 min vs. 60 min or less). The pregnant patient, who had a moderate symptom presentation, received IV nutrient therapy with the exception of zinc as she already had sufficient levels of zinc in her prenatal vitamin. Following guidelines from the United States Pharmacopeia (USP), no more than three ingredients were provided in each IV bag because no compounding hood (laminar airflow workbench) was available for use at the clinic (Table 3). The solution osmolarity ranged from 308 to 460 mOsm/L (isotonic to slightly hypertonic).
Prior to IV infusion, patients received instructions to eat, be well-hydrated, and urinate at home before coming to the clinic. After receiving patient consent, IV nutrients were delivered in the parking lot while the patient remained in the vehicle. Those who were IV “naive” were started at the low end of the dose range listed in Table 3. If they responded well to treatment, doses were titrated up with subsequent treatments. Patients received two to six IV treatments (up to four times per week) depending on how quickly symptoms resolved.
Two patients were prescribed oral antibiotics (azithromycin 500 mg for the first day and 250 mg for days two to five). The first patient was prescribed this regimen for a bacterial superinfection, after allergic rhinosinusitis, a few days prior to COVID-19 infection. The second patient prescribed azithromycin had signs and symptoms consistent with community acquired pneumonia (CAP) and COVID-19 infection. SARS-CoV-2 testing was not available at the time of presentation for this patient.
Prior to establishing care, the patient taking warfarin had been unable to receive a prothrombin time (PT)/international normalized ratio (INR) by his primary care provider (PCP); lab error was suspected. No value was reported again with repeat testing. His PCP had prescribed him an antibiotic and antitussive medication which the patient was irregularly taking. This patient had been sick with COVID-19 for 28 days prior to presentation. He received IV nutrient therapy, however, he progressed to more severe infection and was referred to the emergency department (ED) four days later.23 At the ED, his SpO2 was 85%, his plasma prothrombin time was 67.9 s (reference range (RR) 11–13 s), INR 6.3 (RR 2.0–3.0 on warfarin therapy), and serum ferritin 5443 ng/mL (RR 24–336 ng/mL for males). He was hospitalized for seven days, received supplemental oxygen, and his warfarin dose was adjusted. He reinitiated treatment post-hospitalization. Another patient had previously been hospitalized for a myocardial infarction, CAP, and concurrent COVID-19 infection prior to establishing care; she remained outpatient during this episode of care.
3.4. Follow-up and outcomes
The length of follow-up from the initial visit at the time this manuscript was written was greater than one year for ten patients, nine months to less than one year for three patients, and six months to less than nine months for 17 patients. Therapy duration is listed in Table 3 and therapies utilized by patients are outlined in Table 4. One-half of patients experienced complete symptom resolution within ten days of initiating treatment (n = 15) ( Table 5). Two patients ended treatment prematurely, but resumed treatments for the recommended course when symptoms recurred. All patients eventually reported complete resolution of symptoms. There was no repeat SARS-CoV-2 testing. When questioned at follow-up visits, no patients reported side effects, including presentation of new or different symptoms, or adverse events from treatment. The pregnant patient had a full-term pregnancy and delivered a healthy infant without complications. This was her eighth pregnancy.
Table 4.
Therapies utilized by patients from treatment protocol.
| Therapy | Frequency | Percentage |
|---|---|---|
| Diet (bone broth and beets) | 11 | 37 |
| Dietary Supplement: | ||
| Vitamin C | 30 | 100 |
| Vitamin D3 | 29 | 97 |
| Zinc | 27 | 90 |
| Vitamin A | 14 | 47 |
| Probiotics | 26 | 87 |
| Antiviral Herbal Product | 30 | 100 |
| Herbal Tincture (from clinic) | 5 | 17 |
| Rapid Immune Boost | 10 | 33 |
| Other herbal product | 15 | 50 |
| IV Nutrient Therapy | ||
| Magnesium | 10 | 33 |
| Vitamin C | 10 | 33 |
| Zinc | 9 | 30 |
| B6 | 10 | 33 |
| B12 | 10 | 33 |
Table 5.
Duration of illness.
| Time Period | Time to Treatment No. of Patients (%) |
Symptom Duration Post-Treatment No. of Patients (%) |
Total Symptom Duration No. of Patients (%) |
|---|---|---|---|
| 0–3 days | 21 (70) | 0 | 0 |
| 4–6 days | 2 (7) | 7 (23) | 5 (17) |
| 7–10 days | 4 (13) | 8 (27) | 5 (17) |
| 11–14 days | 0 | 5 (17) | 7 (23) |
| >2 weeks to <3 weeks | 1 (3) | 3 (10) | 4 (13) |
| 3–4 weeks | 2 (7) | 3 (10) | 4 (13) |
| >1 month and <3 months | 0 | 2 (7) | 3 (10) |
| 3–6 months | 0 | 1 (3) | 1 (3) |
| >6 months and <1 year | 0 | 1 (3) | 1 (3) |
|
Time to Treatment (mean ± SD) |
Symptom Duration Post-Treatment (mean ± SD) |
Total Symptom Duration (mean ± SD) |
|
| No. of Days | 4.1 ± 7.56 | 25.9 ± 52.47 | 30 ± 57.52 |
The patient on anticoagulant therapy was the only patient to be hospitalized and receive supplemental oxygen. Although, one patient had been hospitalized prior to establishing care. The patient on anticoagulant therapy had the longest duration of illness of 307 days before he had complete symptom resolution. No patients required intubation during this episode of care.
The five patients who had a recovery longer than one-month post-treatment included three patients who delayed treatment for more than two weeks after symptom onset (Table 5). Of these three patients who delayed treatment, two patients (including one patient who had COVID-19 pneumonia) had a lingering cough which eventually resolved. The third was the patient on anticoagulant therapy who was hospitalized; he experienced ongoing dyspnea on exertion and chest tightness. Cardiology work-up was unremarkable aside from recommending statin therapy for mildly elevated LDL. This patient’s symptoms resolved with repeat IV nutrient therapy. The remaining two patients experienced ongoing fatigue. One of these patients had been hospitalized for a myocardial infarction, CAP, and COVID-19 prior to this episode of care. When feeling better, she prematurely ended recommended treatment and when experiencing lingering fatigue, she completed treatment and the fatigue eventually resolved. The other patient with fatigue was pregnant and when diagnosed with iron deficiency anemia and treated, her fatigue resolved.
There was a strong positive correlation between time to treatment and duration of illness post-treatment (r = 0.63, p < 0.001), suggesting there may have been a treatment effect. This correlation no longer remained significant when removing from analysis the patients who delayed treatment for more than two weeks. The presence of five or more symptoms at presentation was correlated with a longer duration of illness (r = 0.52, p < 0.01). Duration of illness did not correlate with presence of comorbid conditions (r = 0.05, p = 0.81).
4. Discussion
To our knowledge, this is the first retrospective chart review to report on a natural treatment strategy for mild to moderate COVID-19. Notable contributions of this retrospective, observational study of 30 consecutive patients with COVID-19 include presentation of a safe and well-tolerated treatment strategy for COVID-19 administered in an ambulatory setting. Based on patient risk factors (e.g., comorbid conditions), concomitant medication and dietary supplement use, and patient preference, our treatment protocol could be personalized to different patients. Patients with more complex disease (i.e., more symptoms at presentation), older patients, and patients with multiple comorbid conditions appeared to respond well to treatment. The ultimate resolution of symptoms in those who had a longer disease course with repeat treatment may suggest the ability to resolve "entrenched" illness. From the perspective of clinical quality improvement, the findings of this review support current practices, however, current practices could be further optimized by learning about the specific contributions of each component of the treatment protocol to the outcomes observed, which would take multiple clinical trials with large sample sizes. It is possible there was a synergistic effect between treatments.
Evidence suggests the coordinated response between innate and adaptive immunity, and a timely immune response, play a critical role in determining the clinical course of SARS-CoV-2 infection.2., 98, 99, 100 Nutritional deficiencies can impair immune function and increase the risk for a more severe and chronic course of disease.30, 47, 48, 49, 50, 51, 101. Malnutrition has been noted in 26–45% of patients with COVID-19.5 A study of 50 hospitalized patients with SARS-CoV-2 revealed 76% were vitamin D deficient and 42% selenium deficient.102 Older age and comorbid conditions including cardiovascular disease and metabolic syndrome are risk factors for complications of COVID-19.2., 103 Interestingly, nutritional deficiencies have been reported in older individuals (>70 years of age) including vitamins C and E, and in patients with metabolic syndrome, with significantly lower plasma levels of A, C, E, and D when compared to healthy individuals.60 An ecological study demonstrated that intake levels of Vitamins D, C, B12, and iron are inversely associated with SARS-COV-2 incidence and mortality, particularly in populations genetically predisposed to lower micronutrient status.104 Therefore, our goal was to provide multi-nutrient treatment early in the course of disease.
In comparison to other recently published studies and protocols recommending natural approaches, the protocol used herein was similar in many ways including use of dietary factors and supplements (e.g., zinc and vitamin D), concurrent use of oral and IV vitamin C, and botanical herbs with immunomodulatory effects.100, 105., 106, 107, 108, 109., 110, 111, 112., 113, 114, 115, 116 Some ways our protocol deviated from what has been published include administration of IV nutrient therapy in an outpatient setting, exclusion of pharmaceutical medications unless indicated for concurrent infection (e.g., CAP), and use of probiotics. IV nutrient therapy in other studies has largely been reserved for patients who are severely ill, in the ICU, and on FiO2; in contrast, IV nutrient therapy was administered preemptively to those with moderate COVID-19 and risk factors for progression to severe disease.87 Due to the risk for hyperinflammation from SARS-Co-V-2 infection, we included probiotic and herbal agents with virucidal properties and agents that have been shown to demonstrate promotion of a more immediate immune response and control inflammation. A proposed mechanism for a hyperinflammatory response is the ability of SARS-CoV-2 to inhibit and delay the type 1 interferon (IFN) responses in infected cells, which leads to the triggering of a more “exuberant immune response” as the immune system essentially tries to catch up. This impaired early type 1 IFN response is likely a predictor of acute COVID-19 severity; therefore, this was a therapeutic target.2
Most therapies for COVID-19 have been symptomatic supportive care.112., 117 Strengths of our treatment approach to COVID-19 include addressing risk factors and etiologies associated with progression to a more severe disease state and chronic sequelae. IV nutrient therapy was offered in an out-patient setting to patients with risk factors and moderate presentation instead of waiting for progression to more severe disease. In theory, if one nutrient were to be deficient, this could serve as a “linchpin” and lead to an ineffective or dysregulated immune response. Our therapeutic approach was comprehensive and employed multiple nutrients, herbs, and probiotics. No side effects or adverse events from treatment were reported. After receiving patient consent, patients were managed via telemedicine and in the clinic parking lot to prevent transmission in the clinic.
A limitation to this study includes reporting on a small sample of patients and thus observations may not be representative of the general population. Patients also had varying durations of illness at presentation. Treatment for patients with severe COVID-19 infection was not within the scope of this review, as this was a primary care out-patient setting. Not all patients were able to be tested due to limited test kit availability in the United States until mid-June 2020. Therefore, some patients were presumed positive based on symptom presentation and cohabitation with a confirmed positive case. The commercial combination herbal product also had limited availability. Due to the comprehensive treatment approach and with no comparison or control groups, we cannot determine which treatment(s) had the greatest bearing on outcomes. Many of the patients were also already on pharmaceutical and dietary supplements prior to this episode of care. Patient characteristics (i.e., those who seek naturopathic medical care, take dietary supplements, etc.) may have also accounted for responses observed. No conclusions can be made about causation based on this chart review. Because patients were managed via a telemedicine capacity and in the clinic parking lot, physical exams and monitoring laboratory biomarkers were limited.
Based on the results of these cases, we hypothesize if acute COVID-19 infection is treated at first onset with nutrients known to be essential to immune function and probiotics and herbs with immunomodulatory and antiviral activity, this approach could lead to a more rapid clearance of COVID-19, prevent progression to severe disease, as well as the development of chronic sequelae. Future directions include investigating a more comprehensive treatment strategy for confirmed COVID-19 cases in the early symptomatic stages. Because we do not have sophisticated means to readily risk stratify all patients (e.g., including testing for nutrient deficiencies), investigating treatments options that have “safety margins superior to those of reference drugs and enough levels of evidence” should be a priority.79
5. Conclusions
This retrospective chart review describes complete resolution of mild and moderate symptoms presumed to be associated with COVID-19 infection in 30 patients treated with multi-nutrient, probiotic, and herbal therapies. This comprehensive treatment approach appeared to be safe. Delay in seeking treatment after symptom onset, as well as more symptoms at presentation, were correlated with a longer duration of illness. Prospective clinical trials with confirmed COVID-19 cases are warranted.
Patient Perspectives
“[Treatment] really helped with the shortness of breath. While… sick, there were times that I don’t remember much. I had shortness of breath, loss of taste and smell, fatigue, and sinus headaches. The fatigue lasted for weeks. Eventually I regained all of my sense of taste and smell… [and] had no lingering symptoms except fatigue, which eventually dissipated.”
"[I had] no side effects to the treatment, I felt great with the treatment. When I got sick, I recovered fine.”
“[I] loved getting IV treatment when feeling run down."
Funding
This research utilized REDCap which is supported by grant UL1 TR000445 from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH), United States. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Melissa S. Barber: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, and edited of the manuscript. Richard Barrett: Conceptualization, Methodology, Project administration, and drafted and editing the manuscript. Ryan D. Bradley: Formal analysis, Methodology, and editing of the manuscript. Erin Walker: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, and editing of the manuscript. All authors critically viewed and approved the final version of the manuscript.
Declaration of Competing Interest
Authors M.S.B., R.B., R.D.B, and E.W. have no competing interests to declare.
Acknowledgments
Authors would like to thank Dr. Douglas Hanes for his contribution to the statistical analysis and would like to thank patients and their families for their participation and interest in reporting this data.
References
- 1.COVID-19 dashboard. Center for Systems Science and Engineering, Johns Hopkins University; Published 2021. Accessed 5 July 2021. 〈https://coronavirus.jhu.edu/map.html〉.
- 2.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27(1):28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 3.Yong S.J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 2021;0(0):1–18. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayoubkhani D. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 1 April 2021. Off Natl Stat. Published online 2021:1–16.
- 5.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(April):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenhalgh T., Knight M., Court C.A., Buxton M., Husain L. Management of post-acute covid-19 in primary care. Br Med J. 2020;370(m3026):3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 7.Chen J., Lu H. New challenges to fighting COVID-19: virus variants, potential vaccines, and development of antivirals. Biosci Trends. 2021;15(2):126–128. doi: 10.5582/bst.2021.01092. [DOI] [PubMed] [Google Scholar]
- 8.Lin C., Tu P., Beitsch L. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines. 2020;9(1):1–32. doi: 10.3390/VACCINES9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coustasse A., Kimble C., Maxik K. COVID-19 and vaccine hesitancy: a challenge the United States must overcome. J Ambul Care Manag. 2021;44(1):71–75. doi: 10.1097/JAC.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 10.Achiron A., Mandel M., Dreyer-Alster S., et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14 doi: 10.1177/17562864211012835. 17562864211012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gresham L.M., Marzario B., Dutz J., Kirchhof M.G. An evidence-based guide to SARS-CoV-2 vaccination of patients on immunotherapies in dermatology. J Am Acad Dermatol. 2021;84(6):1652–1666. doi: 10.1016/j.jaad.2021.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shroff RT, Chalasani P., Wei R., et al. Immune responses to COVID-19 mRNA vaccines in patients with solid tumors on active, immunosuppressive cancer therapy. medRxiv Prepr Serv Heal Sci. Published online May 14, 2021. doi: 10.1101/2021.05.13.21257129. [DOI]
- 13.Agha M., Blake M., Chilleo C., Wells A., Haidar G.. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv Prepr Serv Heal Sci. Published online April 7, 2021:2021.04.06.21254949. doi: 10.1101/2021.04.06.21254949. [DOI]
- 14.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose sars-cov-2 mrna vaccine series in solid organ transplant recipients. J Am Med Assoc. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel M., Chen J., Kim S., et al. Analysis of MarketScan data for immunosuppressive conditions and hospitalizations for acute respiratory illness, United States. Emerg Infect Dis. 2020;26(8):1720–1730. doi: 10.3201/eid2608.191493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NIAID Strategic Plan for COVID-19 Research - 2021 Update. Accessed 7 July 2021. 〈https://www.niaid.nih.gov/sites/default/files/NIAID-COVID-19-Strategic-Plan-2021.pdf〉.
- 17.BIO COVID-19 Therapeutic Development Tracker. Accessed July 7, 2021. 〈https://www.bio.org/policy/human-health/vaccines-biodefense/coronavirus/pipeline-tracker〉.
- 18.Schiller L. COVID-19: developing drugs and biological products for treatment or prevention; guidance for industry; availability. Fed Regist. 2020;85(97):29949–29951. doi: 10.1016/0196-335x(80)90058-8. [DOI] [Google Scholar]
- 19.Gagnier J.J., Kienle G., Altman D.G., Moher D., Sox H., Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-201554. bcr2013201554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riley D.S., Barber M.S., Kienle G.S., et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–235. doi: 10.1016/j.jclinepi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Vassar M., Matthew H. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi: 10.3352/jeehp.2013.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gearing R.E., Mian I.A., Baber J., Ickowicz A. A methodology for conducting retrospective chart review research in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2006;15(3):126–134. [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Accessed 8 August 2021. 〈https://www.covid19treatmentguidelines.nih.gov/〉. [PubMed]
- 24.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95:103208. doi: 10.1016/J.JBI.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barber M.S., Bradley R.D., Riley D.S., Tippens K.M. Testing a web-based data collection tool for clinical case reporting: NCNM-CR. Integr Med A Clin J. 2016;15(1):42. [Google Scholar]
- 27.Weeks J. Call to action: announcing the Traditional, Complementary and Integrative Health and Medicine COVID-19 Support Registry. J Alter Complement Med. 2020;26(4):256–258. doi: 10.1089/acm.2020.29083.jjw. [DOI] [PubMed] [Google Scholar]
- 28.Bhimraj A., Morgan R., Shumaker A., et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Infectious Diseases Society of America 2021. Accessed 2 August 2021. 〈https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-12〉. [DOI] [PMC free article] [PubMed]
- 29.Sheppard Davenport J., Fitzgerald K., Hodges R.. Bone Broth Report.; 2019. doi: 10.1891/9781617052385.0096. [DOI]
- 30.Iddir M., Brito A., Dingeo G., et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6):1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu G., Fang Y., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J Nutr. 2003;134:489–492. doi: 10.1093/jn/134.3.489. 〈http://www.ncbi.nlm.nih.gov/pubmed/14988435〉 [DOI] [PubMed] [Google Scholar]
- 32.Frasca G., Cardile V., Puglia C., Bonina C., Bonina F. Gelatin tannate reduces the proinflammatory effects of lipopolysaccharide in human intestinal epithelial cells. Clin Exp Gastroenterol. 2012;5(1):61–67. doi: 10.2147/CEG.S28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rennard B.O., Ertl R.F., Gossman G.L., Robbins R.A., Rennard S.I. Chicken soup inhibits neutrophil chemotaxis in vitro. Chest. 2000;118(4):1150–1157. doi: 10.1378/chest.118.4.1150. [DOI] [PubMed] [Google Scholar]
- 34.Kroll J.L., Werchan C.A., Rosenfield D., Ritz T. Acute ingestion of beetroot juice increases exhaled nitric oxide in healthy individuals. PLoS One. 2018;13(1):1–11. doi: 10.1371/journal.pone.0191030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Files D.C., Heinrich T., Shields K.L., et al. A randomized pilot study of nitrate supplementation with beetroot juice in acute respiratory failure. Nitric Oxide - Biol Chem. 2020;94(October 2019):63–68. doi: 10.1016/j.niox.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Raubenheimer K., Hickey D., Leveritt M., et al. Acute effects of nitrate-rich beetroot juice on blood pressure, hemostasis and vascular inflammation markers in healthy older adults: a randomized, placebo-controlled crossover study. Nutrients. 2017;9(11):1270. doi: 10.3390/nu9111270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ninfali P., Angelino D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia. 2013;89(1):188–199. doi: 10.1016/j.fitote.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Zielińska-Przyjemska M., Olejnik A., Kostrzewa A., Śuczak M., Jagodziński P.P., Baer-Dubowska W. The beetroot component betanin modulates ROS production, DNA damage and apoptosis in human polymorphonuclear neutrophils. Phyther Res. 2012;26(6):845–852. doi: 10.1002/ptr.3649. [DOI] [PubMed] [Google Scholar]
- 39.Cho J., Bing S.J., Kim A., et al. Beetroot (Beta vulgaris) rescues mice from γ-ray irradiation by accelerating hematopoiesis and curtailing immunosuppression. Pharm Biol. 2017;55(1):306–316. doi: 10.1080/13880209.2016.1237976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F., Zhu Y., Zhang J., Li Y., Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ. 2020;10(7):e039519. doi: 10.1136/bmjopen-2020-039519. 039519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colunga Biancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and Vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front Immunol. 2020;11:1–11. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wintergerst E.S., Maggini S., Hornig D.H. Immune-enhancing role of Vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 43.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1–25. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fashner J., Ericson K., Werner S. Treatment of the common cold in children and adults. Am Fam Physician. 2012;86(2):153–159. 〈http://www.aafp.org/afp/2012/0715/p153.html〉 [PubMed] [Google Scholar]
- 45.Schloss J., Lauche R., Harnett J., et al. Advances in integrative medicine efficacy and safety of vitamin C in the management of acute respiratory infection and disease: a rapid review. Adv Integr Med. 2020;7(4):187–191. doi: 10.1016/j.aimed.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnan S., Patel K., Desai R., et al. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. J Clin Anesth. 2020;67:110005. doi: 10.1016/j.jclinane.2020.110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradley R., Schloss J., Brown D., Celis D., Finnell J. The effects of vitamin D on acute viral respiratory infections: a rapid review. Adv Integr Med. 2020;7(4):192–202. doi: 10.1016/j.aimed.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant W.B., Lahore H., Mcdonnell S.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan C.W., Ho L.P., Kalimuddin S., et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutrition. 2020;79–80:111017. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., Alcalá Díaz J.F., López Miranda J., Bouillon R.Q.G.J. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020 doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohaegbulam K.C., Swalih M., Patel P., Smith M.A., Perrin R. Vitamin D supplementation in COVID-19 patients: a clinical case series. Am J Ther. 2020;27(5):e485–e490. doi: 10.1097/MJT.0000000000001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goddek S. Vitamin D3 and K2 and their potential contribution to reducing the COVID-19 mortality rate. Int J Infect Dis. 2020;99:286–290. doi: 10.1016/j.ijid.2020.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh M., Das R.R. Zinc for the common cold. Cochrane Database Syst Rev. 2015;2015(4) doi: 10.1002/14651858.CD001364.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allan G., Arroll B. Prevention and treatment of the common cold: making sense of the evidence. Can Med Assoc J. 2014;186(3):190–199. doi: 10.1503/cmaj.121442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carlucci P.M., Ahuja T., Petrilli C., Rajagopalan H., Jones S., Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020;69:1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jothimani D., Kailasam E., Danielraj S., Nallathambi B. COVID-19: poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.BourBour F., Mirzaei Dahka S., Gholamalizadeh M., et al. Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Arch Physiol Biochem. 2020:1–10. doi: 10.1080/13813455.2020.1791188. [DOI] [PubMed] [Google Scholar]
- 58.Allegra A., Tonacci A., Pioggia G., Musolino C., Gangemi S. Vitamin deficiency as risk factor for SARS-CoV-2 infection: correlation with susceptibility and prognosis. Eur Rev Med Pharmacol Sci. 2020;24:9721–9738. doi: 10.26355/eurrev_202009_23064. [DOI] [PubMed] [Google Scholar]
- 59.Li R., Wu K., Li Y., et al. Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19. Aging. 2020;12(15):15784–15796. doi: 10.18632/aging.103888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiorino S., Gallo C., Zippi M., Sabbatani S., Manfredi R., Moretti R. Cytokine storm in aged people with CoV‑2: possible role of vitamins as therapy or preventive strategy. Aging Clin Exp Res. 2020;32(10):2115–2131. doi: 10.1007/s40520-020-01669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hummel T., Whitcroft K.L., Rueter G., Haehner A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur Arch Otorhinolaryngol. 2017;274(7):2819–2825. doi: 10.1007/s00405-017-4576-x. [DOI] [PubMed] [Google Scholar]
- 62.Olaimat A.N., Aolymat I., Al-holy M., et al. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci Food. 2020;4:17. doi: 10.1038/s41538-020-00078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stefan K.L., Kim M.V., Iwasaki A., Kasper D.L. Commensal microbiota modulation of natural resistance to virus infection. Cell. 2020;183(5):1312–1324. doi: 10.1016/j.cell.2020.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shinde T., Hansbro P., Singh Sohal S., Dingle P., Eri R., Standley R. Microbiota modulating nutritional approaches to countering the effects of viral respiratory infections including SARS-CoV-2 through promoting metabolic and immune fitness with probiotics and plant bioactives. Microorganisms. 2020;8(921):1–21. doi: 10.3390/microorganisms8060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anwar F., Altayb H.N., Al-Abbasi F.A., Al-Malki A.L., Kamal M.A. Antiviral effects of probiotic metabolites on COVID-19. J Biomol Struct Dyn. 2020;39(11):4175–4184. doi: 10.1080/07391102.2020.1775123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tiwari S.K., Dicks L., Popov I.V., et al. Probiotics at war against viruses: what is missing from the picture? Front Microbiol. 2020;11:1877. doi: 10.3389/fmicb.2020.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danilets M.G., Bel’skiĭ I., Gur’ev A.M., et al. Effect of plant polysaccharides on Th1-dependent immune response: screening investigation. Eksp i Klin Farmakol. 2010;73(6):19–22. [PubMed] [Google Scholar]
- 68.Gierlikowska B., Gierlikowski W., Demkow U. Alantolactone enhances the phagocytic properties of human macrophages and modulates their proinflammatory functions. Front Pharmacol. 2020;11:1–13. doi: 10.3389/fphar.2020.01339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gierlikowska B., Gierlikowski W., Bekier K., Skalicka-Woźniak K., Czerwińska M.E., Kiss A.K. Inula helenium and Grindelia squarrosa as a source of compounds with anti-inflammatory activity in human neutrophils and cultured human respiratory epithelium. J Ethnopharmacol. 2020;249(September 2019) doi: 10.1016/j.jep.2019.112311. [DOI] [PubMed] [Google Scholar]
- 70.Yuan C. bo, Tian L., Yang B., Zhou H. yan. Isoalantolactone protects LPS-induced acute lung injury through Nrf2 activation. Microb Pathog. 2018;123:213–218. doi: 10.1016/j.micpath.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 71.Wang M., Wang K., Gao X., Zhao K., Chen H., Xu M. Anti-inflammatory effects of isoalantolactone on LPS-stimulated BV2 microglia cells through activating GSK-3β-Nrf2 signaling pathway. Int Immunopharmacol. 2018;65(October):323–327. doi: 10.1016/j.intimp.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Mazzio E.A., Li N., Bauer D., et al. Natural product HTP screening for antibacterial (E.coli 0157:H7) and anti-inflammatory agents in (LPS from E. coli O111:B4) activated macrophages and microglial cells; focus on sepsis. BMC Complement Alter Med. 2016;16(1):467. doi: 10.1186/s12906-016-1429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen K., Sparks J., Omoruyi F.O. Investigation of the cytotoxicity, antioxidative and immune-modulatory effects of Ligusticum porteri (Osha) root extract on human peripheral blood lymphocytes. J Integr Med. 2016;14(6):465–472. doi: 10.1016/S2095-4964(16)60280-7. [DOI] [PubMed] [Google Scholar]
- 74.Signer J., Jonsdottir H.R., Albrich W.C., et al. In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol J. 2020;17(1):172. doi: 10.1186/s12985-020-01439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nugraha R.V., Ridwansyah H., Ghozali M., Khairani A.F., Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evid-Based Complement Alter Med. 2020:2560645. doi: 10.1155/2020/2560645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hensel A., Bauer R., Heinrich M., et al. Challenges at the time of COVID-19: Opportunities and innovations in antivirals from nature. Planta Med. 2020;86(10):659–664. doi: 10.1055/a-1177-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khalifa S.A.M., Yosri N., El-mallah M.F., Ghonaim R. Screening for natural and derived bio-active compounds in preclinical and clinical studies: one of the frontlines of fighting the coronaviruses pandemic. Phytomedicine. 2020;85 doi: 10.1016/j.phymed.2020.153311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aucoin M., Cooley K., Saunders P.R., Carè J. The effect of Echinacea spp. on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: a rapid review. Adv Integr. 2020;7(4):203–217. doi: 10.1016/j.aimed.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silveira D., Prieto-Garcia J.M., Boylan F., et al. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front Pharm. 2020;11(September):581840. doi: 10.3389/fphar.2020.581840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kostalova D., Bukovsky M., Koscova H., Kardosova A. Anticomplement activity of Mahonia aquifolium bisbenzylisoquinoline alkaloids and berberine extract. Ces Slov Farm. 2001;50(6):286–289. 〈http://www.ncbi.nlm.nih.gov/pubmed/11797199〉 [PubMed] [Google Scholar]
- 81.Andreicuț A.D., Fischer-Fodor E., Pârvu A.E., et al. Antitumoral and immunomodulatory effect of Mahonia aquifolium extracts. Oxid Med Cell Longev. 2019;2019:6439021. doi: 10.1155/2019/6439021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cernakova M., Košťálová D. Antimicrobial activity of berberine - a constituent of Mahonia aquifolium. Folia Microbiol. 2002;47(4):375–378. doi: 10.1007/BF02818693. [DOI] [PubMed] [Google Scholar]
- 83.Slobodníková L., Košt’álová D., Labudová D., Kotulová D., Kettmann V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phyther Res. 2004;18(8):674–676. doi: 10.1002/ptr.1517. [DOI] [PubMed] [Google Scholar]
- 84.Cooper I.D., Crofts C.A.P., DiNicolantonio J.J., et al. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: rationale for clinical management. Open Hear. 2020;7(2) doi: 10.1136/openhrt-2020-001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wallace T. Combating COVID-19 and building immune resilience: a potential role for magnesium nutrition? J Am Coll Nutr. 2020;39(8):685–693. doi: 10.1080/07315724.2020.1785971. [DOI] [PubMed] [Google Scholar]
- 86.Tan C.W., Ho L.P., Kalimuddin S., et al. Cohort study to evaluate effect of vitamin D, magnesium, and vitamin B12 in combination on severe outcome progression in older patients with coronavirus (COVID-19) Nutrition. 2020;79–80:111017. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hiedra R., Lo K.B., Elbashabsheh M., et al. The use of IV vitamin C for patients with COVID-19: a case series. Expert Rev Anti Infect Ther. 2020;18(12):1259–1261. doi: 10.1080/14787210.2020.1794819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao B., Ling Y., Li J., et al. Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: a retrospective case series study. Ann Palliat Med. 2021;10(2):1599–1609. doi: 10.21037/apm-20-1387. [DOI] [PubMed] [Google Scholar]
- 89.Tamura J., Kubota K., Murakami H., et al. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol. 1999;116(1):28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaplan S., Basford R.E. Effect of vitamin B12 and folic acid deficiencies on neutraphil function. Blood. 1976;47(5):801–806. [PubMed] [Google Scholar]
- 91.Narayanan N., Nair D.T. Vitamin B12 may inhibit RNA-dependent-RNA polymerase activity of nsp12 from the SARS-CoV-2 virus. IUBMB Life. 2020;72(10):2112–2120. doi: 10.1002/iub.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perera M., El Khoury J., Chinni V., et al. Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV-2 (COVID-19) positive critically ill patients: trial protocol. BMJ Open. 2020;10(12) doi: 10.1136/bmjopen-2020-040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumrungsee T., Zhang P., Chartkul M., Yanaka N., Kato N. Potential role of vitamin B6 in ameliorating the severity of COVID-19 and its complications. Front Nutr. 2020;7(October):1–5. doi: 10.3389/fnut.2020.562051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeff J.L., Snider P., Myers S.P., DeGrandpre Z. A Hierarchy of Healing: The Therapeutic Order. Fourth ed. Elsevier; 2013. [Google Scholar]
- 95.Rothman K., Moore L., Singer M., Nguyen U., Mannino S., Milunsky A. Teratogenicity of high vitamin A intake. N Engl J Med. 1995;333(21):1369–1373. doi: 10.1056/NEJM199511233332101. [DOI] [PubMed] [Google Scholar]
- 96.Bernstein N., Akram M., Yaniv-Bachrach Z., Daniyal M. Is it safe to consume traditional medicinal plants during pregnancy? Phyther Res. 2021;35:1908–1924. doi: 10.1002/ptr.6935. [DOI] [PubMed] [Google Scholar]
- 97.Brinker F. Herb Contraindications and Drug Interactions. Third Edit. (Stodart N., ed.). Eclectic Medical Publications; 2001.
- 98.Carsetti R., Zaffina S., Piano Mortari E., et al. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front Immunol. 2020;11:610300. doi: 10.3389/fimmu.2020.610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stephenson E., Reynolds G., Botting R.A., et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med. 2021;27:904–916. doi: 10.1038/s41591-021-01329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yanuck S., Pizzorno J., Messier H., Fitzgerald K. Evidence supporting a phased immuno-physiological approach to COVID-19 from prevention through recovery. Integr Med A Clin J. 2020;19:8–35. [PMC free article] [PubMed] [Google Scholar]
- 101.Wu D., Lewis E.D., Pae M., Meydani S.N. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. 2019;9(January):1–19. doi: 10.3389/fimmu.2018.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Im J.H., Je Y.S., Baek J., Chung M.H., Kwon H.Y., Lee J.S. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/nejmcp2009249. [DOI] [PubMed] [Google Scholar]
- 104.Galmés S., Serra F., Palou A. Current state of evidence: influence of nutritional and nutrigenetic factors on immunity in the COVID-19 pandemic framework. Nutrients. 2020;12(9):2738. doi: 10.3390/nu12092738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marik P.E., Kory P., Varon J., Iglesias J., Meduri G.U. MATH+ protocol for the treatment of SARS-CoV-2 infection: the scientific rationale. Expert Rev Anti Infect Ther. 2021;19(2):129–135. doi: 10.1080/14787210.2020.1808462. [DOI] [PubMed] [Google Scholar]
- 106.Zhang K., Tian M., Zeng Y., et al. The combined therapy of a traditional Chinese medicine formula and Western medicine for a critically ill case infected with COVID-19. Complement Ther Med. 2020;52 doi: 10.1016/j.ctim.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14(1):64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 108.Wang J.B., Wang Z.X., Jing J., et al. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin J Integr Med. 2020;26(9):648–655. doi: 10.1007/s11655-020-3426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marik P.. EVMS Critical Care COVID-19 Management Protocol; 2020. 〈https://www.evms.edu/covid-19/〉.
- 110.Alschuler L., Weil A., Horwitz R., et al. Integrative considerations during the COVID-19 pandemic. EXPLORE. 2020;16(6):354–356. doi: 10.1016/j.explore.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alschuler L., Chiasson A.M., Horwitz R., et al. Integrative medicine considerations for convalescence from mild-to- moderate COVID-19 disease. EXPLORE. 2020:S1550–8307–1. doi: 10.1016/j.explore.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kory P., Meduri G.U., Iglesias J., Varon J., Marik P.E. Clinical and scientific rationale for the “MATH+” hospital treatment protocol for COVID-19. J Intensive Care Med. 2021;36(2):135–156. doi: 10.1177/0885066620973585. [DOI] [PubMed] [Google Scholar]
- 113.Girija P.L.T., Sivan N. Ayurvedic treatment of COVID-19/SARS-CoV-2: a case report. J Ayurveda Integr Med. 2021:1–5. doi: 10.1016/j.jaim.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mishra A., Bentur S.A., Thakral S., Garg R., Duggal B. The use of integrative therapy based on Yoga and Ayurveda in the treatment of a high‑risk case of COVID ‑ 19/SARS‑CoV‑2 with multiple comorbidities: a case report. J Med Case Rep. 2021;15(1):95. doi: 10.1186/s13256-020-02624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Joshi J., Puthiyedath R. Outcomes of Ayurvedic care in a COVID-19 patient with hypoxia - A case report. J Ayurveda Integr Med. 2020 doi: 10.1016/j.jaim.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El Sayed S.M., Aboonq M.S., Aljehani Y.T., et al. TaibUVID nutritional supplements help rapid cure of COVID-19 infection and rapid reversion to negative nasopharyngeal swab PCR: for better public prophylaxis and treatment of COVID-19 pandemic. Am J Blood Res. 2020;10(6):397–406. [PMC free article] [PubMed] [Google Scholar]
- 117.Liu F., Zhu Y., Zhang J., Li Y., Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. Br Med J. 2020;10(7):e039519. doi: 10.1136/bmjopen-2020-039519. [DOI] [PMC free article] [PubMed] [Google Scholar]