Abstract
Cardiac arrhythmias, endocarditis, or myocarditis was identified in 12 dogs, of which 11 were seroreactive to Bartonella vinsonii subspecies berkhoffii antigens. Historical abnormalities were highly variable but frequently included substantial weight loss, syncope, collapse, or sudden death. Fever was an infrequently detected abnormality. Cardiac disease was diagnosed following an illness of short duration in most dogs, but a protracted illness of at least 6 months' duration was reported for four dogs. Valvular endocarditis was diagnosed echocardiographically or histologically in eight dogs, two of which also had moderate to severe multifocal myocarditis. Four dogs lacking definitive evidence of endocarditis were included because of seroreactivity to B. vinsonii antigens and uncharacterized heart murmurs and/or arrhythmias. Alpha proteobacteria were not isolated from the blood by either conventional or lysis centrifugation blood culture techniques. Using PCR amplification and DNA sequencing of a portion of the 16S rRNA gene, B. vinsonii was identified in the blood or heart valves of three dogs. DNA sequence alignment of PCR amplicons derived from blood or tissue samples from seven dogs clustered among members of the alpha subdivision of the Proteobacteria and suggested the possibility of involvement of one or more alpha proteobacteria; however, because of the limited quantity of sequence, the genus could not be identified. Serologic or molecular evidence of coinfection with tick-transmitted pathogens, including Ehrlichia canis, Babesia canis, Babesia gibsonii, or spotted fever group rickettsiae, was obtained for seven dogs. We conclude that B. vinsonii subsp. berkhoffii and closely related species of alpha proteobacteria are an important, previously unrecognized cause of arrhythmias, endocarditis, myocarditis, syncope, and sudden death in dogs.
There is increasing evidence that Bartonella species and other closely related members of the alpha subdivision of the Proteobacteria are important cardiac pathogens in both dogs and people. During 1993, Bartonella quintana (35), Bartonella elizabethae (9), and Bartonella henselae (15) were identified for the first time as causal agents of endocarditis in human patients. In 1993, our laboratory isolated from a dog with endocarditis a novel Bartonella subspecies (6) that was subsequently designated Bartonella vinsonii subspecies berkhoffii (American Type Culture Collection type strain 51672) (22). In 1997, a new alpha-2 proteobacterium, provisionally designated Rasbo bacterium, was isolated from a chronically febrile patient with pericardial effusion and clinical evidence of myocardial disease (5). Because these organisms are highly fastidious, molecular diagnosis by PCR amplification and direct sequencing, as reported in recent studies of human endocarditis (references 13 and 19 and this study), may be necessary to confirm alpha-proteobacterial infection. Based on these recent observations, continued research efforts should be directed at clarifying the role of alpha proteobacteria in cardiovascular disease in dogs, man, and potentially other animal species.
Since its first association with endocarditis in 1993, Bartonella infection has become known as an important cause of culture-negative endocarditis in man (10, 19, 33, 36). Of microbiological and clinical importance, B. quintana or B. henselae was ultimately identified as the cause of endocarditis in nine human patients previously diagnosed with chlamydia endocarditis by seroreactivity to Chlamydia antigens. It is now known that Bartonella infection induces antibodies that cross-react with Chlamydia species (33). Although Bartonella quintana, which is transmitted by the human body louse, caused epidemics of trench fever during World War I, the clinical association of this fastidious organism with endocarditis was not reported until nearly a century later. More recently, it has been determined that B. quintana endocarditis can be associated with alcoholism, homelessness, and presumably body louse infestations (10, 33, 37). B. henselae endocarditis can be associated with cat contact, since cats throughout the world serve as a major reservoir for B. henselae and Bartonella clarridgeiae (21, 26, 33). Although a human pathogen, B. clarridgeiae has not yet been associated with endocarditis (25).
Evolving evidence indicates that B. vinsonii is a potentially important canine pathogen, and it has been implicated as a cause of endocarditis (6), granulomatous lymphadenitis, and granulomatous rhinitis (32). A seroepidemiological survey of sick dogs from North Carolina and Virginia identified tick exposure as a risk factor for the detection of B. vinsonii antibodies (30). Compared to a seroprevalence of 3.6% in the North Carolina State University Veterinary Teaching Hospital population, B. vinsonii antibodies were found in 36% of dogs that were seroreactive to Ehrlichia canis antigens, further supporting the potential of tick transmission of B. vinsonii subsp. berkhoffii. Examination of sera from dogs experimentally infected with Rickettsia rickettsii or E. canis did not identify cross-reactivity to B. vinsonii antigens (32). In a more recent prospective study of dogs from North Carolina naturally infected with E. canis, Ehrlichia chaffeensis, Ehrlichia equi, and/or Ehrlichia ewingii, serologic or molecular evidence of Bartonella infection was detected in 7 of 12 animals (7). Based on these observations, the extent to which coinfection with B. vinsonii influences the pathophysiologic consequences of E. canis infection in dogs deserves additional investigation.
The initial purpose of this study was to identify additional cases of endocarditis caused by infection with B. vinsonii subsp. berkhoffii in dogs. During the course of the investigation, definitive molecular evidence of B. vinsonii subsp. berkhoffii infection was obtained for three dogs. Unexpectedly, molecular evidence of infection with one or more alpha proteobacteria spp. was found in seven dogs. The remaining two dogs had serologic evidence of Bartonella infection, but DNA could not be amplified from blood or tissue samples.
MATERIALS AND METHODS
Dogs.
Since definitive diagnostic criteria for Bartonella species infection in dogs have not been established, inclusion in this study required electrocardiographic evidence of arrhythmias or conduction defects, echocardiographic evidence of endocarditis, or histopathologic evidence of endocarditis or myocarditis. In addition, one or more of the following conditions had to be met: a reciprocal indirect fluorescent antibody (IFA) antibody titer of ≥128 to B. vinsonii antigens, culture of B. vinsonii from blood, or PCR amplification of DNA from EDTA-treated blood samples or from tissues obtained at necropsy, using primers originally designed to detect Bartonella species (4). Eleven dogs were evaluated at North Carolina State University, and one dog was evaluated at the University of Florida Veterinary Teaching Hospital.
Clinical and pathologic findings.
The medical records of dogs meeting the above inclusion criteria were reviewed by two authors (E.B.B. and C.E.A.). Clinical, hematologic, serum biochemical, and urinalysis findings, available for all dogs, were summarized. When requested by the attending clinician, blood coagulation profiles, blood culture results, and all other ancillary diagnostic test results were reviewed. Cardiovascular findings, including the electrocardiogram and echocardiogram, were reviewed by a veterinary cardiologist (C.E.A.). Histopathologic analysis of biopsy samples or tissues obtained at necropsy was performed by a pathologist (T.T.B.).
Serology.
When serologic testing for tick-transmitted diseases had not been requested by the attending clinician and serum was stored in our research laboratory, frozen samples from these dogs were analyzed by indirect fluorescent antibody (IFA) testing for reactivity to B. vinsonii subsp. berkhoffii, E. canis, Babesia canis, and R. rickettsii antigens, using previously published procedures (6, 7).
DNA extraction and sequencing.
DNA was extracted from EDTA-treated blood or fixed tissues as specimens became available during the investigation period. DNA was extracted from 200 μl of stored, frozen (−70°C), EDTA-treated blood sample with phenol-chloroform after proteinase K digestion (27). Cultured B. vinsonii was used as the positive control. PCR amplification of Bartonella DNA was performed in a 100-μl reaction volume containing 1 μg of DNA template, 0.2 μM each primer (Bh16SF [AGAGTTTGATCCTGGCTCAG] and Bh16SR [CCGATAAATCTTTCTCCCTAA], and 1.25 U of Taq polymerase, using a previously described procedure (4). Amplification cycles included denaturation at 95°C for 30 s, annealing at 54°C for 1 min, and chain extension at 72°C for 45 s. This was repeated for 35 cycles and was followed by a final chain extension at 72°C for 5 min. When the specimen was obtained by necropsy or biopsy, PCR was performed to amplify a portion of the 16S rRNA gene from formalin-fixed, paraffin-embedded heart valve tissue, myocardium, or other tissues. In all instances, the DNA sequence of the amplicons was obtained through the North Carolina State University DNA Sequencing Facility.
RESULTS
Dogs.
The states of origin, signalments, approximate dates of onset, durations of illness, historical abnormalities, and cardiac abnormalities of animals in this study are summarized in Table 1. All dogs resided in North Carolina, South Carolina, or Florida. Dogs ranged in age from 6 months to 12 years (median age, 5.5 years), were predominantly male (9 of 12), and included only medium or large breeds. Historical abnormalities were highly variable but frequently included fever (n = 5), substantial weight loss (n = 5), syncope or collapse (n = 4), vomiting or diarrhea (n = 4), lameness (n = 2), ataxia (n = 3), hemorrhage (n = 2), and/or sudden death (n = 2). In nearly all instances, illness was severe, necessitating intensive care management and/or prolonged hospitalization or resulting in death.
TABLE 1.
Selected clinical findings in 12 dogs with cardiac abnormalities and evidence of alpha-proteobacterial infection
| Dog no. | City of origin | Signalmenta | Referral date (mo/yr) | Historical durationb | Historical abnormalitiesc | Cardiac abnormalitiesd |
|---|---|---|---|---|---|---|
| 1 | Greensboro, N.C. | 10-yr-old cocker spaniel (F/S) | 1/98 | 2 wks | Intermittent lameness, lethargy, anorexia, vomiting, collapse, submandibular/axillary lymphadenopathy | II/VI heart murmur; echo: aortic/mitral endocarditis |
| 2 | Raleigh, N.C. | 1-yr-old weimaraner (F/S) | 2/98 | 8 mo | Intermittent FUO post-OHE; lethargy, anorexia, poorly localized pain | Echo: decreased myocardial function; necropsy: aortic endocarditis |
| 3 | Raleigh, N.C. | 6-yr-old Newfoundland (M/C) | 2/98 | 2 days | Anorexia, lethargy, collapse | II/VI murmur, atrial fibrillation, decreased systolic function |
| 4 | Raleigh, N.C. | 12-yr-old coonhound (M) | 12/94 | 1 wk | Anorexia, vomiting, weight loss (29%), ataxia, collapse | Tachycardia, grade III/VI heart murmur, VPCs, ventricular tachycardia; echo: possible mitral endocarditis |
| 5 | Surfside Beach, S.C. | 5-yr-old German shepherd (M) | 1/96 | 1 wk | Acute collapse | 3° AV block; echo: aortic endocarditis, pacemaker |
| 6 | Henderson, N.C. | 5-yr-old rottweiler (M) | 5/96 | 6 mo | Weight loss (25%), shifting-leg lameness, epistaxis, coughing, penile bleeding | Intermittent VPCs; echo: aortic valve endocarditis |
| 7 | Madison, Fla. | 5-yr-old bull mastiff (M) | 6/96 | 1 wk | Acute fever (40.4°C), lethargy, weight loss (9%), sudden death | Aortic endocarditis, ventricular tachycardia |
| 8 | Rocky Mount, N.C. | 11-yr-old Labrador retriever (M/C) | 9/96 | 2 wks | Hematuria, lethargy, anorexia, abdominal pain, fever (40.8°C), weight loss | V/VI heart murmur, mitral endocarditis |
| 9 | Cary, N.C. | 10-yr-old German shepherd (M) | 8/88 | 1 day | Acute posterior paresis, collapse, fever (41°C) | Mild hypertrophic cardiomyopathy, ventricular tachycardia |
| 10 | Nashville, N.C. | 4-yr-old boxer (M) | 8/86 | 1 wk | Lethargy, exercise intolerance, sudden death | V/VI murmur; echo: aortic stenosis |
| 11 | Myrtle Beach, S.C. | 6-yr-old St. Bernard (F/S) | 9/95 | 6 mo | Progressive weight loss (40%), fever, diarrhea | Tachycardia, heart murmur; echo: aortic/mitral endocarditis |
| 12 | Bailey, N.C. | 6-mo-old boxer (M/C) | 12/96 | 7 mo | Acute vomiting, ataxia, head tilt | III/VI heart murmur, VPCs |
F/S, spayed female; M, male; M/C, castrated male.
Duration of illness prior to documentation of cardiac disease.
FUO, fever of unknown origin; OHE, ovariohysterectomy.
Echo, echocardiogram; VPCs, ventricular premature contractions; AV, atrioventricular.
Cardiovascular findings.
Clinical signs that were attributable to the cardiovascular system included heart murmurs (n = 6), syncope or collapse (n = 4), heart failure (n = 4), and exercise intolerance (n = 1). However, four dogs were presented for evaluation of abnormalities other than cardiac. At the time of initial presentation, eight dogs were in sinus rhythm, three had ventricular ectopy, and one had a complete atrioventricular block. Dog 3 subsequently developed atrial fibrillation. Endocarditis involved the aortic valve in four dogs, the mitral valve in two dogs, and both the aortic and mitral valves in two additional dogs. Based on echocardiographic and/or postmortem findings, two dogs had preexisting aortic stenosis.
Clinical and pathologic findings.
Hematologic parameters were highly variable, and frequently values were within laboratory reference ranges (Table 2). Hematologic abnormalities included anemia (hematocrit <36%; n = 6), thrombocytopenia (platelet count <200,000/μl; n = 5), and neutrophilia (segmented neutrophil count >11,500/μl; n = 10), rarely accompanied by a substantial left shift but occasionally accompanied by mild neutrophil toxicity, monocytosis (monocyte count >1,350/μl; n = 7), and eosinophilia (eosinophil count >750/μl; n = 1). Antierythrocyte antibodies were detected in dog 8, the only dog examined by Coombs' testing. Of the three dogs tested, antinuclear antibodies were detected only in dog 3 (reciprocal titer, 1,280). When present, hypoalbuminemia (serum albumin <2.8 g/dl; n = 9 of 12) and hyperglobulinemia (serum globulin >3.8 g/dl; n = 8 of 12) were of mild to moderate severity. With the exception of dog 4, in which there was chronic renal failure secondary to chronic renal fibrosis, hypercreatinemia (serum creatinine >1.8 mg/dl; n = 4) was usually associated with dehydration or decreased cardiac output and resolved if initial therapeutic interventions were successful. Four dogs were hyperglycemic (serum glucose >115 mg/dl), presumably a function of severe systemic stress or bacteremia-induced hyperglycemia. Hemoglobinuria, proteinuria, and bilirubinuria, as determined by urine dipstick quantitation (trace to 4+), were identified in 12 dogs.
TABLE 2.
Selected laboratory findings from 12 dogs with cardiac abnormalities and evidence of alpha-proteobacterial infection
| Dog no. | PCVa (%) | Platelet count/ μl (103) | No. of segmented neutrophils/μl | No. of band neutrophils/μl | Neutrophil toxicity | No. of monocytes/μl | No. of eosinophils/μl | Serum level of:
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Albumin (g/dl) | Globulin (g/dl) | Creatinine (mg/dl) | Glucose (mg/liter) | ||||||||
| 1 | 28 | 334 | 15,708 | 0 | —b | 1,496 | 561 | 2.8 | 4.0 | 1.6 | 118 |
| 2 | 48 | 212 | 22,640 | 1,981 | — | 1,981 | 0 | 2.4 | 3.4 | 1.0 | 99 |
| 3 | 20 | 124 | 18,450 | 246 | Mild | 3,198 | 0 | 2.4 | 4.2 | 1.0 | 76 |
| 4 | 46 | 479 | 11,704 | 304 | Mild | 1,200 | 0 | 3.8 | 4.9 | 4.7 | 162 |
| 5 | 39 | 87 | 27,473 | 331 | Mild | 2,317 | 178 | 2.5 | 4.2 | 2.4 | 166 |
| 6 | 31 | 280 | 8,300 | 0 | — | 400 | 550 | 2.7 | 4.4 | 1.1 | 86 |
| 7 | 25 | 35 | 29,460 | 1,320 | — | 1,320 | 170 | 1.9 | 3.9 | 1.4 | 63 |
| 8 | 14 | 297 | 34,997 | 4,430 | — | 2,215 | 1,092 | 1.8 | 6.2 | 1.1 | 106 |
| 9 | 39 | 83 | 15,416 | 0 | Mild | 492 | 0 | 2.4 | 3.2 | 2.9 | 68 |
| 10 | 43 | 318 | 8,928 | 0 | — | 1,736 | 744 | 2.2 | NAc | 1.3 | 96 |
| 11 | 36 | 99 | 15,456 | 0 | Mild | 1,656 | 0 | 2.8 | 3.0 | 2.3 | 93 |
| 12 | 60 | NDd | 13,272 | 158 | — | 632 | 158 | 4.0 | 4.3 | 1.2 | 120 |
PCV, packed cell volume.
—, toxicity not observed.
NA, not available.
ND, not determined (clumped).
Blood cultures and serology.
Conventional blood cultures from 10 dogs and lysis centrifugation blood cultures from 5 dogs failed to result in bacterial growth (Table 3).
TABLE 3.
Selected microbiological, serologic, and PCR-DNA sequencing results from 12 dogs with cardiac abnormalities and evidence of alpha-proteobacterial infection
| Dog no. | Blood culture resultsa
|
Reciprocal antibody titer
|
PCR sequencing results
|
|||||
|---|---|---|---|---|---|---|---|---|
| Conventional | Lysis centrifugation | B. vinsonii | E. canis | Babesia canis | R. rickettsii | Tissue | Organism | |
| 1 | − | NDb | 128 | 40 | 20 | <16 | Blood | B. vinsonii subsp. berkhoffii |
| 2 | − | ND | 128 | Negf | 20 | <16 | Blood, valve | B. vinsonii subsp. berkhoffii |
| 3 | ND | ND | 512 | Neg | 40 (160)c | <16 | Blood | B. vinsonii subsp. berkhoffii |
| 4 | − | − | 4,096 | 2,560 | 40 | 32 | Blood, valve | α-Proteobacterium |
| 5 | − | − | 8,192 | 80 | 40 | 64 | Blood | α-Proteobacterium |
| 6 | − | + | 8,192/512g | Neg | 20 | 128 | Blood | α-Proteobacterium |
| 7 | − | − | 8,192 | 320 | 40 | 64 | Blood, valve | α-Proteobacterium |
| 8 | + | ND | 128 | Neg | 20 | <16 | Valve | α-Proteobacterium |
| 9 | − | ND | 128 | Neg | 20d/2,560g | <16 | Prepucial masse | α-Proteobacterium |
| 10 | ND | ND | ND | ND | ND | ND | Valve, myocardium | α-Proteobacterium |
| 11 | + | ND | 8,192 | Neg | 40 | 128 | Blood | |
| 12 | − | − | 128 | 20 | 20 | <16 | Blood | |
−, negative, +, positive. In dogs 8 and 11, β-lactamase-positive Staphylococcus intermedius (three of three cutures) and Alcaligenes xylosoxidans (one of two cultures) were respectively isolated by conventional blood culture techniques, and an uncharacterized bacterium from which DNA was not amplified using primers described in Materials and Methods was isolated from dog 6 by lysis centrifugation blood culture.
ND, not done.
Titer in parenthesis represents seroreactivity to Babesia gibsonii antigens.
Babesia canis organisms observed on blood smear.
Four months prior to presentation for babesiosis and bartonellosis, a preputial mass was resected surgically. DNA was extracted from the mass, which was histologically compatible with a schwannoma but contained marked perivascular accumulations of lymphocytes and plasma cells.
Neg, negative.
Acute titer/convalescent titer.
Seroreactivity (reciprocal titers of ≥128) to B. vinsonii antigens, as determined by IFA testing, was documented in 11 of 12 dogs (Table 3). There was also serologic evidence of exposure to several other tick-transmitted pathogens. Seroreactivity to spotted fever group rickettsiae was documented for four dogs (reciprocal titers to R. rickettsii antigens, ≥64), seroreactivity to E. canis was evident for three dogs (reciprocal titers, ≥80), and seroreactivity to Babesia canis or Babesia gibsonii was found for dogs 3 and 10 (reciprocal titers, ≥80). Babesia canis organisms were observed on the blood smear of dog 10. Dog 3 had a reciprocal titer to Babesia gibsonii antigens of 160, and Babesia gibsonii DNA was subsequently amplified from a stored EDTA-treated blood sample. Retrospectively, based on DNA sequence similarity to Brucella canis (see below), stored frozen sera from 10 of 12 dogs (available for all except dogs 4 and 10) were tested by rapid slide agglutination for antibodies to Brucella canis antigens by Leland Carmichael, Cornell University. Brucella canis antibodies were not detected in any serum sample.
PCR and DNA sequencing.
DNA obtained from stored EDTA-treated blood samples or formalin-fixed, paraffin-embedded heart valve, myocardium, or other tissue was amplified by using 16S rRNA primers originally described as specific for the genus Bartonella (4). PCR performed at low stringency allowed amplification of a product of appropriate size (approximately 185 bp) from the blood of seven dogs, the heart valve or myocardium of five dogs, the kidney of one dog, and a preputial mass removed from dog 10 4 months prior to evaluation of fever and collapse (Table 3). Between 99 and 146 nucleotides of DNA sequence were derived from the 185-bp PCR products. A similarity search of GenBank sequence data indicated that three samples derived from dogs 1 to 3 were identical to B. vinsonii subsp. berkhoffii (Table 4). Amplicons derived from seven other dogs (cases 4 to 10) clustered among several alpha proteobacteria, including Rhizobium, Agrobacterium, Brucella, Methylobacterium, and the currently unnamed Rasbo agent (5). Given the lack of a bacterial isolate and the limited quantity of DNA available, it was not possible to definitively identify the bacteria in these seven dogs. However, when compared with the corresponding nucleotide sequences of blood culture isolates or of common contaminants (Staphylococcus aureus or Staphylococcus intermedius), other organisms associated with cardiac disease or bacteremia (Capnocytophaga canis or Chlamydia spp.), or bacteria, rickettsia, or bloodborne protozoa that some of these dogs were apparently exposed to or coinfected with (Serratia marcescens, E. canis, or Babesia canis), minimal sequence alignment was achieved. Bacterial DNA was not amplified from the available blood of dogs 11 and 12, despite the presence of Bartonella-reactive immunoglobulin G (IgG) and clinical signs compatible with bacteremia.
TABLE 4.
Comparative alignment of partial 16S rRNA gene sequences of B. henselae, B. vinsonii subsp. berkhoffii, and amplicons derived from dogs 1 to 3
| Organism | Dog | Sequencea
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 | 47 | 55 | 56 | 58 | 67 | 138 | 144 | 183 | 184 | I1b | I2 | I3 | I4 | I5 | I6 | I7 | I8 | I9 | I10 | I11 | I12 | 187 | 188 | 189 | 207 | ||
| B. henselae | A | G | G | C | G | A | A | A | G | T | —c | — | — | — | — | — | — | — | — | — | — | — | T | T | A | G | |
| B. vinsonii subsp. berkhoffii | A | G | N | C | N | A | C | N | G | T | C | A | C | A | G | G | G | A | G | A | C | C | T | T | T | G | |
| B. vinsonii subsp. berkhoffii | 1 | A | T | G | A | G | N | C | A | G | T | C | A | C | A | G | G | G | A | G | A | C | C | T | T | A | G |
| B. vinsonii subsp. berkhoffii | 2 | A | T | G | A | G | N | C | A | G | T | C | A | C | A | G | G | G | A | G | A | C | C | T | T | A | G |
| B. vinsonii subsp. berkhoffii | 3 | A | G | G | C | G | A | C | A | G | T | C | A | C | A | G | G | G | A | G | A | C | C | T | T | A | G |
The sequence of the noncoding RNA strand is shown. The numbers correspond to the Escherichia coli numbering scheme. Unless noted in the table, all intervening sequences between no. 45 and 207 for all isolates were identical.
Insertion sequence designated I1–I12.
—, no comparable sequence for this region.
Treatment.
Regimens differed considerably among individual dogs but included antibiotics in all cases (amoxicillin, enrofloxacin, cephalexin, doxycycline, and amikacin), diuretics for congestive heart failure for five dogs (furosemide), and various combinations of cardiovascular drugs (enalapril, digoxin, nitroglycerin, and diltiazem). Eight dogs were euthanized or died within the first month following presentation, two died within 2 to 7 months of presentation, 1 was lost to follow-up, and 1 remains alive. Dog 6, diagnosed with endocarditis during May 1996, has been treated continuously with enrofloxacin since that time. Sequential reciprocal B. vinsonii antibody titers were 8,192 (May 1996), 512 (November 1997), 512 (January 1998), and 256 (May 1998). The dog was still alive in November 1998 but had developed congestive heart failure.
Histopathology.
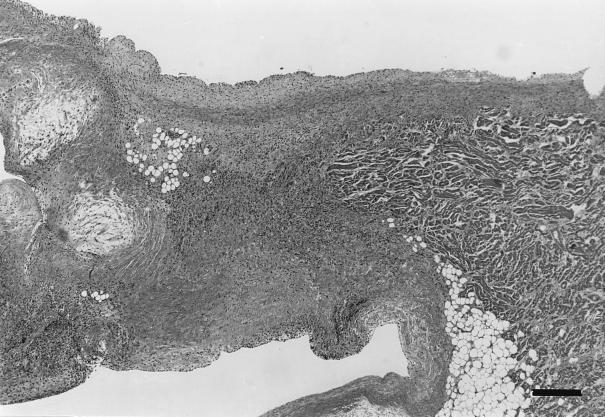
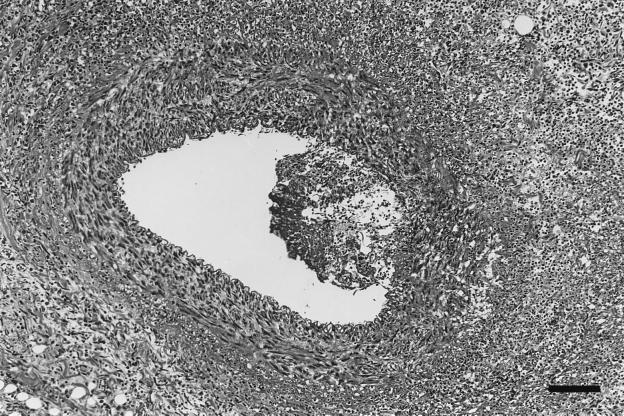
Four dogs were necropsied. Endocarditis was confirmed at necropsy in dog 2, infected with B. vinsonii subsp. berkhoffii, and in dogs 7 and 10, infected with an undetermined alpha proteobacterium. Dogs 2 and 7 also had myocarditis. Dog 2 had a 2-mm-diameter vegetative mass on a mitral valve leaflet (Fig. 1). There were multifocal areas of severe myocardial inflammation widely scattered in both the left and right sides of the heart. These inflammatory foci were often associated with thickened, severely inflamed coronary arteries that were often disrupted by areas of severe fibrinoid necrosis (Fig. 2). Inflammatory foci were characterized by myocardial fiber loss, neovascularization, and various numbers of neutrophils and macrophages. In a few areas, a purulent exudate was a prominent component of the inflammatory foci. The mitral valvular mass was a mixture of myxomatous tissue, neutrophils, and macrophages. Warthin Starry staining for bacteria was negative. In dog 4 (infected with an alpha proteobacterium), there were nodular myxomatous thickenings of the mitral valve leaflets, characteristic of valvular endocardiosis rather than endocarditis as suspected from the echocardiogram. Inflammatory cells were not observed in the valvular lesions, but occasional small myocardial inflammatory foci composed of various mixtures of lymphocytes, plasma cells, and macrophages were randomly scattered in the left ventricle. A 1.5-cm-diameter vegetative inflammatory mass was present on a cusp of the aortic valve of dog 7. This mass consisted of a mixture of fibrin, numerous neutrophils, macrophages, and cell debris. Myocardial inflammatory foci composed primarily of neutrophils, fibrin, macrophages, and cell debris were randomly scattered in the left ventricle, interventricular septum, and right atrium. Serratia marcescens was cultured from the aortic cusp mass, Warthin Starry staining was negative, and alignment of the DNA sequence of the PCR amplicon from the valve was consistent with an alpha proteobacterium, not Serratia marcescens. A 1.5-cm-diameter vegetative inflammatory mass was located on the cusps of the aortic valve of dog 10. The mass was composed of a mixture of well- to poorly differentiated mesenchymal tissue. Near the surface, the myxomatous tissue was mixed with cell debris and lymphocytes and was encrusted with amorphous eosinophilic debris containing numerous bacterial colonies and focal areas of mineralization. The bacterial colonies contained short, plump rods, consistent in size and shape with Bartonella spp., that stained positive with the Warthin Starry silver stain.
FIG. 1.
Left auricle and base of mitral valve of dog 2, infected with B. vinsonii, distended by inflammatory exudate. Inflammation extends into the adjacent auricular myocardium on the left. Hematoxylin and eosin stain; bar = 268 μm.
FIG. 2.
Coronary artery of dog 2, infected with B. vinsonii. Shown is the coronary artery with an adherent mass of inflammatory exudate bulging into the arterial lumen, with transmural inflammation of the arterial wall beneath the inflammatory exudate. Severe inflammation surrounding inflamed artery effaces the normal tissue architecture. Hematoxylin and eosin stain; bar = 107 μm.
DISCUSSION
In this study, the diagnosis of B. vinsonii endocarditis in 3 of 12 dogs was suggested by serology and confirmed by PCR and DNA sequencing. The DNA sequences for these three dogs (no. 1 to 3) were essentially identical (Table 4) and included the 12-bp insert that is consistent with B. vinsonii subsp. berkhoffii. Unexpectedly, when the partial 16S rRNA gene sequence derived from blood or other tissue samples from the other seven dogs was compared to GenBank sequences for other, closely related bacteria, including B. henselae, B. vinsonii, Brucella canis, and the alpha proteobacterium provisionally designated Rasbo, all sequences clustered together and were most closely related to Brucella canis and Rasbo bacterium (Fig. 3) (5). Although several Brucella spp. have been associated with endocarditis in human patients (3), Brucella canis has been infrequently associated with endocarditis in dogs (1). Since antibodies to Brucella antigens were not detected in 10 of 12 dogs tested retrospectively, it seems unlikely that the partial sequences are from Brucella spp. Since amplicons were not obtained from blood samples from the remaining two dogs, DNA sequencing could not be performed to determine the infecting bacterial species.
FIG. 3.
Comparative alignment of partial 16S rRNA gene sequences of Brucella canis, Rasbo bacterium, and amplicons derived from dogs 4 to 10. The sequence of the noncoding RNA strand is shown. The numbers correspond to the Escherichia coli numbering scheme. Unless noted in boldface type in the table, all intervening sequences between no. 45 and 207 for all isolates were identical. Dashes (a) indicate the lack of comparable sequence. aP, alpha proteobacterium.
Historically, the duration and severity of disease presentation varied considerably among these dogs. An acute onset of illness characterized by fever, lethargy, anorexia, weight loss, and collapse was identified in eight dogs. In contrast, cardiac disease was diagnosed in four dogs following a protracted illness of at least 6 months' duration. When the historical duration of illness was compared to the Bartonella-reactive antibody titer, there was a poor correlation. Of the five dogs with reciprocal antibody titers of 4,096 or higher, three had an acute onset of illness whereas two dogs were ill for at least 6 months prior to the diagnosis of endocarditis. These serologic observations appear to support a more chronic course of infection with acute cardiac decompensation. On an evolutionary basis, Bartonella species appear to be well adapted to facilitate intracellular persistence within most host species (21, 26, 27, 29). Although persistent infection of 16 months' duration with B. vinsonii has been documented in a healthy dog (26), similar data based on culturing the organism from the blood of sick dogs is lacking. Persistent infection with B. henselae or B. clarridgeiae spanning years in duration has been documented in both naturally and experimentally infected cats (21, 27). Although the duration of Bartonella infection in various domestic and wild animal species requires additional clarification, documentation of endocarditis presumably represents a manifestation of chronic Bartonella infection with eventual bacterial localization in the heart valve.
Comparatively low Bartonella-specific serum antibody titers were found in several dogs; dogs 2 and 12 had low antibody titers, despite a prolonged duration of illness. Similar to findings in experimentally infected cats (23), wild animal species such as deer (8), and a recently described human case of B. quintana bacteremia (11), this observation may relate to failure of selected individuals to develop a strong humoral immune response when chronically infected with Bartonella (cases 1 to 3) (34). Specifically, low or undetectable levels of Bartonella-specific antibodies have been observed in culture-positive animals or human patients, even when the homologous organism is used as the test antigen. A similar observation was made in the case of a Swedish patient infected with the Rasbo bacterium, who failed to develop a detectable IgG antibody response to the organism despite a chronic course of infection (5). Alternatively, failure to detect an IgG-specific immune response to these organisms may reflect differences in culture- or tissue culture-grown organisms compared to the antigenic properties of the organisms in vivo.
In contrast to the low Bartonella-reactive titers, four of the highest reciprocal antibody titers to B. vinsonii antigens (4,096 to 8,192) were in dogs with PCR evidence of infection with a species of proteobacteria other than Bartonella. Presumably, this organism(s) cross-reacts serologically with B. vinsonii antigens, or some of these dogs may have been coinfected with more than one alpha-proteobacteria species. Our results indicate that B. vinsonii-reactive serum does not cross-react with Brucella canis, E. canis, or R. rickettsii antigens (31). Until less technically demanding procedures are developed, detection of Bartonella-reactive antibodies, in conjunction with PCR amplification and sequencing of DNA from blood or tissue specimens, would seem the most beneficial approach for detecting B. vinsonii or alpha-Proteobacteria spp. in dogs with endocarditis or arrhythmias.
Based on the results of this study, endocarditis associated with Bartonella and other alpha proteobacteria occurs in large-breed dogs. There also appears to be a strong predisposition for these organisms to infect the aortic valve (70%), in contrast to a review of five studies of bacterial endocarditis, involving 187 dogs, in which the mitral valve was affected nearly three times as often as the aortic valve (67% versus 23%) (20). Preexisting valvular disease, such as subaortic stenosis, might explain the increased predilection for aortic valve involvement, particularly in boxers, a breed predisposed to congenital aortic stenosis. Although B. henselae was the third most frequent infectious agent identified in 146 children with fever of unknown origin (18), fever was not found in over half of the dogs in this study. Hematologic abnormalities such as neutrophilia, band neutrophils, and neutrophil toxic change are frequently not detected. Hemoglobinuria and proteinuria, potentially a reflection of glomerulonephritis or renal microinfarction due to bacteremia, were identified in most of the dogs in this series. Despite the severity of illness documented in most of these dogs, neither conventional blood culture nor lysis centrifugation blood culture was of value for the isolation of alpha proteobacteria from blood.
Concurrent isolation of other bacterial organisms from three dogs was an unexpected finding. These isolates may represent infection, isolation contaminants, catheter-acquired infections associated with intensive care management, or postmortem contamination. In dog 7, Serratia marcescens was isolated at necropsy from an aortic cusp mass and gram-negative bacteria were visualized in the tissues. However, this dog had a reciprocal B. vinsonii titer of 8,192, and alpha-proteobacterial DNA was amplified from EDTA-treated blood and valve tissue on two independent occasions. In this instance, S. marcescens may have been a postmortem contaminant, or S. marcescens bacteremia may have developed secondary to chronic alpha-proteobacterial infection. In human endocarditis patients, isolation of multiple bacterial organisms generally occurs in association with severe immunosuppression or intravenous drug use (3). Additional efforts to establish whether these organisms can contribute to immunosuppression in dogs appear justified. Clinical observations related to human infection with Bartonella bacilliformis in South America support an immunosuppressive role for the organism, potentially leading to death from concurrent bacterial infections (12). Concurrent viral infections, including those caused by Epstein-Barr virus or the human immunodeficiency virus, can markedly influence the severity and clinical course of B. henselae or B. quintana infection in humans (20, 22, 39). Similarly, cats coinfected with B. henselae and the feline immunodeficiency virus were more likely to exhibit lymphadenopathy and gingivitis than cats infected with only one of these organisms (38). Previously, we documented severe suppression of circulating CD8 lymphocytes in dogs experimentally infected with B. vinsonii (31). Although these and other, uncited observations support a potentially important role for the immune system in determining the clinical outcome of Bartonella infections, the immunopathogenesis associated with human or canine infection remains incompletely understood (29, 34).
The spectrum of histologic changes observed in the four dogs with endocardial lesions may provide some insight into possible pathogenic mechanisms associated with alpha-proteobacterial infections. Dog 4 had mitral valvular endocardiosis; however, the myocardium contained mild inflammatory foci, and DNA sequencing of amplicons from both the blood and the valvular region were identical and indicative of an undetermined alpha-Proteobacteria species. In dogs 2 (infected with B. vinsonii), 7, and 10 (both infected with alpha proteobacteria), the valvular lesions were inflammatory and more severe. Additionally, the myocardium of dogs 2 and 7 contained multifocal areas of inflammation. Alpha proteobacteria may preferentially colonize damaged tissue sites, such as degenerative heart valve leaflets. Once valvular colonization is established, the inflammatory response to the organism results in a progressive valvular inflammatory lesion that may serve as a source of inflammatory debris, spreading the organism to other areas of the heart or to more distal sites.
Based on review of our cases, the prognosis for alpha-proteobacterial endocarditis is generally poor, as is described for other causes of bacterial endocarditis in dogs (1). However, based on comparative medical data, therapeutic elimination of alpha-proteobacterial infection, particularly in dogs or human patients with endocarditis or myocarditis, may be difficult to attain. Although enrofloxacin was more efficacious than doxycycline for the treatment of B. henselae or B. clarridgeiae infection in experimentally or naturally infected cats, neither drug eliminated the infection in all animals, even when administered for a duration of 4 weeks (24). Similarly, despite prolonged treatment with numerous antibiotics, including doxycycline and imipenem, the Swedish patient infected with the Rasbo bacterium experienced at least three relapses during a 1-year period (5). In the present study, dog 6 is notable in that it has lived 3 years beyond the initial diagnosis of endocarditis while being maintained on continuous therapy with enrofloxacin by the referring veterinarian. Despite a seemingly favorable therapeutic response and a gradual decline in serum antibody titers, this dog has remained seroreactive to B. vinsonii antigens, alpha-proteobacteria DNA has been amplified from pretreatment and early posttreatment blood samples, and cardiac performance has deteriorated to the point of congestive heart failure. Since a recent prospective randomized double-blind placebo-controlled study indicated that azithromycin was of clinical benefit to patients with typical cat scratch disease, macrolide antibiotics may hold additional promise for the treatment of chronic Bartonella or alpha-proteobacterial infections (2).
Although myocarditis was documented histologically in only two dogs, the potential of alpha-Proteobacteria spp. contributing to myocardial involvement in these dogs was supported by clinical findings such as syncope, acute collapse, or sudden death and by the documentation of conduction abnormalities, ventricular arrhythmias, or decreased myocardial contractility. Myocarditis was identified in a 60-year-old male who died suddenly during a running competition (17). Similar to the dogs in this report, serologic and molecular evidence implicated infection with Bartonella or a closely related species of bacteria. Recently, focal myocardial inflammation, consisting predominantly of mononuclear cells, has been observed in cats experimentally infected with B. henselae (14, 27).
Complete atrioventricular block has been associated with Borrelia burgdorferi infection in dogs and human patients in regions with endemic Lyme Disease (28). Recently, it has been determined that Ixodes scapularis ticks in these regions can cotransmit multiple tickborne pathogens, including B. burgdorferi, Babesia microti, a granulocytic Ehrlichia species (presumably E. equi), and an as-yet-uncharacterized Bartonella species (16). In this study, complete atrioventricular block required pacemaker implantation in dog 5 (alpha-proteobacterium infected), which was seroreactive to B. vinsonii, E. canis, and R. rickettsii antigens. Collectively, these observations serve to illustrate the potential difficulty in establishing causation in dogs or people coinfected with multiple tick-transmitted pathogens. Since certain Borrelia, Ehrlichia, Babesia, and Bartonella species can induce chronic, insidious infection in dogs (7), the relative role of each of these organisms in the pathogenesis of a specific disease manifestation in a sick dog will be difficult to establish in nature.
Findings generated through this study indicate that B. vinsonii and other closely related alpha-Proteobacteria spp. can be detected in dogs with cardiac arrhythmias, endocarditis, or myocarditis. Importantly, infection with these organisms may contribute to syncope, collapse, conduction defects, arrhythmias, or sudden death. Since the initial intent of this study was to identify dogs with serologic evidence of Bartonella endocarditis, case selection was biased toward Bartonella-seroreactive dogs with clinical, echocardiographic, or necropsy evidence of endocarditis. Therefore, the role of alpha proteobacteria as a cause of cardiac arrhythmias or myocarditis might well be expanded through additional studies that target different patient populations.
ACKNOWLEDGMENTS
This work was supported by the state of North Carolina, by a grant from Intervet Inc., and through a donation from Heska Corporation.
We acknowledge the technical support of KwangOk Shin, Brandee Pappalardo, Barbara Hegarty, Susan Hancock, Robin Gager, and Julie Bradley.
REFERENCES
- 1.Atkins C E. Bacterial endocarditis. In: Allen D G, editor. Small animal medicine. J. B. Philadelphia, Pa: Lippincott Company; 1991. pp. 299–308. [Google Scholar]
- 2.Bass J W, Freitas B C, Freitas A D, Sisler C L, Chan D S, Vincent J M, Person D A, Claybaugh J R, Wittler R R, Weisse M E, Regnery R L, Slater L N. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat scratch disease. Pediatr Infect Dis J. 1998;17:447–452. doi: 10.1097/00006454-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Berbari E F, Cockerill F R, Steckelberg J M. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin Proc. 1997;72:532–542. doi: 10.4065/72.6.532. [DOI] [PubMed] [Google Scholar]
- 4.Bergmans A M C, Groothedde J W, Schellekens J F P, van Embden J D, Ossewaarde J M, Schouls L M. Etiology of cat scratch disease: comparison of polymerase chain reaction detection of Bartonella (formerly Rochalimaea) and Afipia felis DNA with serology and skin tests. J Infect Dis. 1995;171:916–923. doi: 10.1093/infdis/171.4.916. [DOI] [PubMed] [Google Scholar]
- 5.Blomqvist G, Wesslén L, Påhlson C, Hjelm E, Pettersson B, Nikkilä T, Allard U, Svensson O, Uhlén M, Morein B, Friman G. Phylogenetic placement and characterization of a new alpha-2 proteobacterium isolated from a patient with sepsis. J Clin Microbiol. 1997;35:1988–1995. doi: 10.1128/jcm.35.8.1988-1995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitschwerdt E B, Kordick D L, Malarkey D E, Keene B, Hadfield T L, Wilson K. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol. 1995;33:154–160. doi: 10.1128/jcm.33.1.154-160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitschwerdt E B, Hegarty B C, Hancock S I. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645–2651. doi: 10.1128/jcm.36.9.2645-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomel B B, Kasten R W, Chang C C, Yamamoto K, Heller R, Maruyama S, Ueno H, Simpson D, Swift P A, Jang S S, Piemont Y, Pedersen N C. In Proceedings of the International Conference on Emerging Infectious Diseases, Atlanta, Ga. 1998. Isolation of Bartonella spp. from California wildlife, abstr. P-21-10. [Google Scholar]
- 9.Daly J S, Worthington M G, Brenner D J, Moss C W, Hollis D G, Weyant R S, Steigerwalt A G, Weaver R E, Daneshvar M I, O'Connor S P. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drancourt M, Mainardi J L, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 11.Drancourt M, Maol V, Brunet P, Dussol B, Berland Y, Raoult D. Bartonella (Rochalimaea) quintana infection in a seronegative hemodialyzed patient. J Clin Microbiol. 1996;34:1158–1160. doi: 10.1128/jcm.34.5.1158-1160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Caceres U, Garcia F U. Bartonellosis: an immunodepressive disease and the life of Daniel Carrion. Am J Clin Pathol. 1991;95(Suppl. I):S58–S66. [PubMed] [Google Scholar]
- 13.Goldenberger D, Künzli A, Vogt P, Zbinden R, Altwegg M. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol. 1997;35:2733–2739. doi: 10.1128/jcm.35.11.2733-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guptill L, Slater L, Wu C, Lin T, Glickman L, Welch D, HogenEsch H. Experimental infection of young specific pathogen-free cats with Bartonella henselae. J Infect Dis. 1997;176:206–216. doi: 10.1086/514026. [DOI] [PubMed] [Google Scholar]
- 15.Hadfield T L, Warren R, Kass M, Brun E, Levy C. Endocarditis caused by Rochalimaea henselae. Hum Pathol. 1993;24:1140–1141. doi: 10.1016/0046-8177(93)90196-n. [DOI] [PubMed] [Google Scholar]
- 16.Hofmeister E K, Kolbert C P, Abdulkarim A S. Cosegregation of a novel Bartonella species with Borrelia burgdorferi and Babesia microti in Peromyscus leucopus. J Infect Dis. 1998;177:409–416. doi: 10.1086/514201. [DOI] [PubMed] [Google Scholar]
- 17.Holmberg M, Wesslen L, Hjelm E, Pahlson C, Lindquist O, Friman G, Regnery R. In Proceedings of the 13th Sesquiannual Meeting of the American Society for Rickettsiology, Champion, Pa. 1997. Bartonella spp. in a 60 year-old Swedish male with myocarditis who succumbed to sudden death, abstr. 1. [Google Scholar]
- 18.Jacobs R F, Schutze G E. Bartonella henselae as a cause of prolonged fever and fever of unknown origin in children. Clin Infect Dis. 1997;26:80–84. doi: 10.1086/516256. [DOI] [PubMed] [Google Scholar]
- 19.Jalava J, Kotilainen P, Nikkari S, Skurnik M, Vanttinen E, Lehtonen O, Eerola E, Toivanen P. Use of the polymerase chain reaction and DNA sequencing for detection of Bartonella quintana in the aortic valve of a patient with culture-negative infective endocarditis. Clin Infect Dis. 1995;21:891–896. doi: 10.1093/clinids/21.4.891. [DOI] [PubMed] [Google Scholar]
- 20.Koehler J E, Sanchez M A, Garrido C S, Whitfeld M J, Chen F M, Berger T G, Rodriguez-Barradas M C, LeBoit P E, Tappero J W. Molecular epidemiology of Bartonella infections in patients with bacillary angiomatosis-peliosis. N Engl J Med. 1997;337:1876–1883. doi: 10.1056/NEJM199712253372603. [DOI] [PubMed] [Google Scholar]
- 21.Kordick D L, Wilson K H, Sexton D J, Hadfield T L, Berkhoff H A, Breitschwerdt E B. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J Clin Microbiol. 1995;33:3245–3251. doi: 10.1128/jcm.33.12.3245-3251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kordick D L, Swaminathan B, Greene C E, Wilson K H, Whitney A M, O'Connor S, Hollis D G, Matar G M, Steigerwalt A G, Malcolm G B, Hayes P S, Hadfield T L, Breitschwerdt E B, Brenner D J. Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol. 1996;46:704–709. doi: 10.1099/00207713-46-3-704. [DOI] [PubMed] [Google Scholar]
- 23.Kordick D L, Breitschwerdt E B. Relapsing bacteremia following blood transmission of Bartonella henselae in cats. Am J Vet Res. 1997;58:492–497. [PubMed] [Google Scholar]
- 24.Kordick D L, Papich M G, Breitschwerdt E B. Efficacy of enrofloxacin or doxycycline for treatment of Bartonella henselae or Bartonella clarridgeiae infection in cats. Antimicrob Agents Chemother. 1997;41:2448–2455. doi: 10.1128/aac.41.11.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kordick D L, Hilyard E J, Hadfield T L, Wilson K H, Steigerwalt A G, Brenner D J, Breitschwerdt E B. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kordick D L, Breitschwerdt E B. Persistent infection of pets within a household with three Bartonella species. Emerg Infect Dis. 1998;4:325–328. doi: 10.3201/eid0402.980225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kordick D L, Brown T T, Shin K, Breitschwerdt E B. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats. J Clin Microbiol. 1999;37:1536–1547. doi: 10.1128/jcm.37.5.1536-1547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy S A, Duray P H. Complete heart block in a dog seropositive for Borrelia burgdorferi. J Vet Intern Med. 1988;2:138–144. doi: 10.1111/j.1939-1676.1988.tb02810.x. [DOI] [PubMed] [Google Scholar]
- 29.Minnick M F, Mitchell S J, McAllister S J. Cell entry and the pathogenesis of Bartonella infections. Trends Microbiol. 1996;4:343–347. doi: 10.1016/0966-842x(96)10055-x. [DOI] [PubMed] [Google Scholar]
- 30.Pappalardo B L, Correa M T, York C C, Peat C Y, Breitschwerdt E B. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am J Vet Res. 1997;58:467–471. [PubMed] [Google Scholar]
- 31.Pappalardo B L, Gebhard D H, Breitschwerdt E B. In Proceedings of the 13th Sesquiannual Meeting of the American Society for Rickettsiology, Champion, Pa. 1997. Cyclic CD8 lymphopenia associated with dogs experimentally infected with Bartonella vinsonii subspecies berkhoffii, abstr. 19. [Google Scholar]
- 32.Pappalardo, B. L., T. T. Brown, J. L. Gookin, C. L. Morrill, and E. B. Breitschwerdt. Bartonella induced granulomatous disease in two dogs. J. Vet. Intern. Med., in press. [DOI] [PubMed]
- 33.Raoult D, Fournier P E, Drancourt M, Marrie T J, Etienne J, Cosserat J, Cacoub P, Poinsignon Y, Leclercq P, Sefton A M. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646–652. doi: 10.7326/0003-4819-125-8-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Barradas M C, Bandres J C, Hammill R J, Trial J, Clarridge III J E, Baughn R E, Rossen R D. In vitro evaluation of the role of humoral immunity against Bartonella henselae. Infect Immun. 1995;63:2367–2370. doi: 10.1128/iai.63.6.2367-2370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spach D H, Callis K P, Paauw D S, Houze Y B, Schoenknecht F D, Welch D F, Rosen H, Brenner D J. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol. 1993;31:692–694. doi: 10.1128/jcm.31.3.692-694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spach D H, Kanter A S, Daniels N A, Nowowiejski D J, Larson A M, Schmidt R A, Swaminathan B, Brenner D J. Bartonella (Rochalimaea) species as a cause of apparent “culture-negative” endocarditis. Clin Infect Dis. 1995;20:1044–1047. doi: 10.1093/clinids/20.4.1044. [DOI] [PubMed] [Google Scholar]
- 37.Spach D H, Kanter A S, Dougherty M J, Larson A M, Coyle M B, Brenner D J, Swaminathan B, Matar G M, Welch D F, Root R K, Stamm W E. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med. 1995;332:424–428. doi: 10.1056/NEJM199502163320703. [DOI] [PubMed] [Google Scholar]
- 38.Ueno H, Hohdatsu T, Muramatsu Y, Koyama H, Morita C. Does coinfection of Bartonella henselae and FIV induce clinical disorders in cats? Microbiol Immunol. 1996;40:617–620. doi: 10.1111/j.1348-0421.1996.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 39.Zbinden R, Kurer S B, Altwegg M, Weber R. Generalized infection with Bartonella henselae following infection due to Epstein-Barr virus. Clin Infect Dis. 1996;23:1184–1185. doi: 10.1093/clinids/23.5.1184. [DOI] [PubMed] [Google Scholar]