Abstract
On December 20, 2019, the FDA granted accelerated approval to fam-trastuzumab deruxtecan-nxki (DS-8201a, T-DXd, tradename ENHERTU®) for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting. Approval was based on data from study DS8201-A-U201 (DESTINY-Breast01) with supportive safety data from study DS8201-A-J101. The primary efficacy endpoint in DESTINY-Breast01 was overall response rate (ORR) based on confirmed responses by blinded independent central review (ICR) using RECIST v1.1 in all participants who were assigned to receive the recommended dose of 5.4 mg/kg while secondary endpoints included duration of response (DoR). The confirmed ORR based on ICR in these 184 patients was 60.3% (95% CI: 52.9, 67.4) and the median DoR was 14.8 months (95% CI: 13.8, 16.9). Interstitial lung disease (ILD), including pneumonitis, was experienced in patients treated with T-DXd and can be severe, life-threatening or fatal. In addition, neutropenia and left ventricular dysfunction were included as Warnings and Precautions in labeling. Other important common adverse reactions were nausea, fatigue, vomiting, alopecia, constipation, decreased appetite, anemia, diarrhea, and thrombocytopenia. Overall, the totality of efficacy and safety data supported the accelerated approval of T-DXd for the intended indication.
Introduction
Breast cancer is the most commonly diagnosed cancer and second leading cause of death in women. (1). In the United States, more than 270,00 cases are diagnosed with at least 40,000 deaths annually (2). Cases are rare in men, with approximately 2,600 diagnosed per year (3). Among patients with metastatic breast cancer, 15–20% are human epidermal growth factor receptor 2 (HER2)-positive with a 5-year relative survival rate ranging from 37–44%, depending on hormone-receptor (HR) status (4). HER2-positive disease is associated with a more aggressive tumor and a younger patient population (5). The standard of care for first-line metastatic HER2-positive breast cancer in the US is the combination of trastuzumab, pertuzumab, and a taxane while ado-trastuzumab emtansine (T-DM1) is currently the preferred second-line option based on results from trials in these settings (6, 7). Beyond the second-line, treatment options are more limited and could include lapatinib and capecitabine, or trastuzumab combined with a chemotherapeutic agent; however, although patients initially respond to these therapies, they are less durable and ultimately relapse will occur (8,9). After the accelerated approval ofT-DXd, there have been two additional approvals in this space. The combination of neratinib and capecitabine was granted regular approval in February 2020 for adult patients with advanced or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens in the metastatic setting (10). Tucatinib in combination with trastuzumab and capecitabine was granted regular approval in April 2020 for adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting (11). Although treatment with anti-HER2 therapies has improved disease outcomes in unresectable or metastatic HER2-positive breast cancer, the disease remains incurable in the metastatic setting. Therefore, there remains an unmet need for further effective therapies for metastatic HER2-positive breast cancer, especially in later lines of therapy. This article summarizes the data and FDA’s review leading to the accelerated approval of T-DXd (12).
Chemistry, Manufacturing, and Control
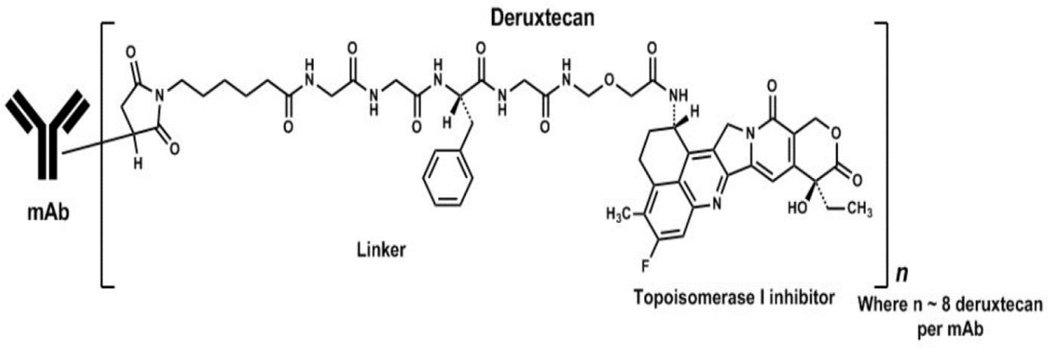
T-DXd is an antibody-drug conjugate (ADC) composed of a humanized anti-HER2 IgG1 monoclonal antibody (MAAL-9001) with the same amino acid sequence as trastuzumab, covalently linked to a topoisomerase inhibitor (MAAA-1181a, DXd) via a tetrapeptide based cleavable linker (Figure 1, ref. 13). The antibody is produced using recombinant DNA technology in Chinese hamster ovary cells, and the topoisomerase inhibitor and linker are produced by chemical synthesis. The target number of drug-linkers coupled to one antibody molecule is 8, resulting in a drug-to-antibody ratio (DAR) of approximately 8. The drug product is a sterile, white to yellowish white, preservative-free lyophilized powder in single-dose vials for reconstitution and further dilution.
Figure 1:
Structure of Fam-Trastuzumab Deruxtecan-nxki (15)
Nonclinical Pharmacology and Toxicology
In vitro, MAAL-9001 and T-DXdbound to human recombinant HER2 protein but not to recombinant EGFR, HER3, or HER4 proteins, and showed antibody-dependent cellular cytotoxic activity. DXd, an exatecan derivative, inhibited the activity of topoisomerase 1, in vitro, as shown by inhibition of supercoiled DNA relaxation in a concentration-dependent manner (11). T-DXd showed anti-tumor activity in HER2-positive or HER2-low-expressing breast cancer mouse tumor models (13).
Good laboratory practice (GLP)-compliant, repeat-dose general toxicology studies were conducted to evaluate the toxicity of T-DXd in rats and monkeys. The major observed target organs of toxicity following administration of T-DXd once every 3 weeks for up to 6 weeks to rats or 3 months to monkeys included the bone marrow, skin, lung, testis, kidney, and the gastrointestinal system. Except for the findings in the skin and kidneys in monkeys and testes in rats, all other toxicological findings were reversible by the end of the recovery period. The major findings induced by T-DXd except for those in the lung in monkeys , were observed in both species. Overall, the toxicity findings in animals administered T-DXd were consistent with clinical adverse reactions observed in clinical trials with T-DXd. DXd was clastogenic in both an in vivo rat bone marrow micronucleus assay and an in vitro Chinese hamster lung chromosome aberration assay but was not mutagenic in an in vitro bacterial reverse mutation assay. Based on postmarketing reports of oligohydramnios following use of trastuzumab during pregnancy and data showing that DXd is genotoxic, T-DXd can cause fetal harm when administered to a pregnant woman and a Warning and Precaution for embryo-fetal toxicity is included in the label (14,15).
Clinical Pharmacology
Based on the totality of data [Drug-drug interaction results, activity and safety], no dose adjustment was recommended in patients with moderate hepatic impairment, but close safety monitoring is recommended due to potentially increased exposure of DXd, the topoisomerase inhibitor. No data were available in patients with severe [total bilirubin >3 to 10 times upper limits of normal (ULN) and any aspartate aminotransferase (AST)] hepatic impairment. Additionally, no adjustments are recommended in patients with mild or moderate renal impairment.
Across all doses evaluated in clinical studies, only 0.6% (4/640) of evaluable patients developed anti-drug antibodies (ADA) against fam-trastuzumab deruxtecan-nxki following treatment. Therefore, no conclusions can be drawn concerning a potential effect of immunogenicity on efficacy or safety due to the limited number of patients who tested positive. The neutralizing activity of anti-T-DXd antibodies has also not been assessed.
Clinical Trial Design:
DESTINY-Breast01
This was a phase 2, multicenter, open-label, single arm, 2-part study designed to justify the recommended dose of T-DXd and investigate the safety and efficacy in trial participants with unresectable and/or metastatic HER2-positive BC previously treated with T-DM1. T-DXd was administered intravenously once every 3 weeks at various doses.
Part 1 of the study was randomized and consisted of 2 stages, a PK stage and a Dose-Finding stage. In the PK stage, trial participants were randomized in a 1:1:1 ratio to 3 doses (5.4, 6.4, and 7.4mg/kg), and in the dose-finding stage participants were randomized in a 1:1 ratio to 2 doses that were selected for further evaluation (5.4 and 6.4mg/kg). In Part 2 of the study, all patients received T-DXd at 5.4mg/kg every three weeks, which was determined to be the optimal dose from Part 1. Part 2 was divided into two cohorts consisting of Part 2a in participants with unresectable and/or metastatic HER2-positive BC previously treated with T-DM1 and Part 2b in participants who discontinued T-DM1 for reasons other than progressive disease. HER2 expression was confirmed from the most recent adequate tumor sample by central laboratory according to American Society of Clinical Oncology-College of American Pathologists (ASCO/CAP) 2013 guidelines.
Treatment continued until unacceptable toxicity or progressive disease. The primary efficacy endpoint was overall response rate (ORR) based on confirmed responses by blinded independent central review (ICR) using RECIST 1.1 in all participants who were assigned to receive the recommended dose of 5.4mg/kg. Secondary endpoints included duration of response (DoR). Tumor imaging was obtained every 6 weeks and CT/MRI of the brain was mandatory for patients with brain metastases at baseline. All responses had to be confirmed at the subsequent scan.
DS8201-A-J101
This was a Phase 1, 2-part dose escalation and dose expansion, non-randomized, open-label first-in-human study of T-DXd in various solid tumors that were either HER2-positive or with HER2-low expression. Part 1 (Dose Escalation) evaluated doses ranging from 0.8 mg/kg to 8.0 mg/kg and was intended to identify the maximum tolerated dose (MTD) or the recommended dose of T-DXd. Part 2 (Dose Expansion) was intended to further assess the safety and efficacy of T-DXd at the MTD/recommended doses of 5.4 mg/kg and 6.4 mg/kg. The dose expansion was conducted in 5 cohorts of subjects with various tumor types (breast cancer, gastric or GEJ adenocarcinoma, and others) and levels of HER2 expression.
The tumor HER2 status was determined locally either on archival tissue samples or on fresh tissue samples obtained after the last HER2-targeting treatment for metastatic disease, which differed from study DS8201-A-U201 in which HER2 status was confirmed centrally. Similar to DESTINY-Breast01, the primary efficacy endpoint was ORR based on confirmed responses by ICR and treatment continued until unacceptable toxicity or progressive disease.
Results:
Efficacy:
A total of 184 participants with HER2-positive breast cancer were enrolled at the 5.4mg/kg dose of T-DXd from Parts 1, 2a, and 2b in the DESTINY-Breast01 study, which formed the primary basis of the efficacy analysis. Patient demographics for both studies and the pooled population are presented in Table 1. All 184 patients in DESTINY-Breast01 that supported the target indication at a dose of 5.4mg/kg were female. The majority (76%) of these participants were <65 years of age, 55% White, and 55% ECOG 0. Twenty-nine percent of participants were enrolled at U.S. sites. Baseline disease characteristics for both studies and the pooled population for the targeted population are presented in Table 2. All patients in DESTINY-Breast-01 had received at least 2 lines of HER2-directed therapy in the metastatic setting. Ninety-two percent of participants had visceral disease, 29% had bone metastases, 13% had brain metastases, and 53% percent were hormone receptor positive. The sum of diameters of target lesions were < 5 cm in 42%, and ≥ 5 cm in 50% (not evaluable by central review in 8% of patients). The median number of prior cancer regimens in the locally advanced/metastatic setting was 5 (range: 2–17). All patients received prior trastuzumab, T-DM1, and 66% had prior pertuzumab.
Table 1:
Summary of Demographics for Studies DESTINY-Breast01 and DS8201-A-J101 and Pooled Population for the Target Indication (ITT population)(12)
| Pooled | Study | ||
|---|---|---|---|
| HER2-positive Breast Cancer 5.4mg/kg (N = 235) * | DESTINY-Breast01 HER2-positive Breast Cancer 5.4mg/kg (N = 184) | DS8201-A-J101 HER2-positive Breast Cancer 5.4mg/kg (N = 51) * | |
| Sex, n (%) | |||
| Male | 1 (0.4) | 0 | 1 (2) |
| Female | 234 (99.6) | 184 (100) | 50 (98) |
| Age (years) | |||
| Median | 56 | 55 | 58 |
| Range | 28–96 | 28–96 | 28–77 |
| Age Group | |||
| <65 years | 174 (74) | 140 (76) | 34 (67) |
| ≥65 years | 61 (26) | 44 (24) | 17 (33) |
| <75 years | 224 (95) | 175 (95) | 49 (96) |
| ≥75 years | 11 (5) | 9 (5) | 2 (4) |
| Race, n (%) | |||
| White | 120 (51) | 101 (55) | 19 (36) |
| Black or African American | 7 (3) | 4(2) | 3 (6) |
| Asian | 97 (42) | 72 (38) | 27 (54) |
| American Indian or Alaska Native | 2 (0.9) | 1 (0.5) | 1 (2) |
| Native Hawaiian or Pacific Islander | 1 (0.4) | 1 (0.5) | 0 |
| Other | 4 (1.7) | 3 (1.6) | 1 (2) |
| Missing | 4 (1.7) | 4 (2.2) | 0 |
| Region/Country of Enrollment, n (%) | |||
| North America | 83 (35) | 53 (29) | 30 (59) |
| United States | 83 (35) | 53 (29) | 30 (59) |
| Asia | 84 (36) | 63 (34) | 21 (41) |
| Japan | 51 (22) | 30 (16) | 21 (41) |
| Korea | 33 (14) | 33 (18) | 0 |
| Europe | 68 (29) | 68 (37) | 0 |
| Belgium | 7 (3.0) | 7 (3.8) | 0 |
| France | 19 (8) | 19 (10) | 0 |
| Italy | 9 (3.8) | 9 (4.9) | 0 |
| Spain | 21 (9) | 21 (11) | 0 |
| United Kingdom | 12 (5) | 12 (7) | 0 |
| ECOG | |||
| 0 | 135 (57) | 102 (55) | 33 (65) |
| 1 | 98 (42) | 81 (44) | 17 (33) |
| 2 | 1 (0.4) | 1 (0.5) | 0 |
| Missing | 1 (0.4) | 0 | 1 (2) |
One patient (10117015) was not dosed in DS8201-A-J101
Table 2:
Baseline Disease Characteristics for Studies DESTINY-Breast01 and DS8201-A-J101 and Pooled Population for the Targeted Indication (ITT population)(12)
| Pooled | Study | ||
|---|---|---|---|
| HER2-positive Breast Cancer 5.4mg/kg (N = 235) * | DESTINY-Breast01 HER2-positive Breast Cancer 5.4mg/kg (N = 184) | DS8201-A-J101 HER2-positive Breast Cancer 5.4mg/kg (N = 51) * | |
| ER Status, n (%) | |||
| Positive | 124 (53) | 93 (51) | 31 (61) |
| Negative | 108 (46) | 88 (48) | 20 (39) |
| Unknown or Unavailable | 3 (1.3) | 3 (1.6) | 0 |
| Hormone Receptor Status, n (%) | |||
| Positive | 129 (55) | 97 (53) | 32 (63) |
| Negative | 102 (43) | 83 (45) | 19 (37) |
| Unknown or Unavailable | 4 (1.7) | 4 (2.2) | 0 |
| HER2 Expression (IHC), n (%) | |||
| 0 | 0 | 0 | 0 |
| 1+ | 2 (0.9) | 2 (1.1) | 0 |
| 2+ | 39 (17) | 28 (15) | 11 (22) |
| 3+ | 194 (82.6) | 154 (84) | 40 (78) |
| Prior Pertuzumab, n (%) | |||
| Yes | 164 (70) | 121 (66) | 43 (84) |
| No | 71 (30) | 63 (34) | 8 (16) |
| Renal Impairment at Baseline, n (%) | |||
| Normal | 113 (48) | 90 (49) | 23 (45) |
| Mild | 91 (39) | 69 (38) | 22 (43) |
| Moderate | 29 (12) | 25 (14) | 4 (8) |
| Severe | 1 (0.4) | 0 | 1 (2) |
| Missing | 1 (0.4) | 0 | 1 (2) |
| Hepatic Impairment at Baseline, n (%) | |||
| Normal | 132 (56) | 105 (57) | 27 (53) |
| Mild | 99 (42) | 76 (41) | 23 (45) |
| Moderate | 1 (0.4) | 1 (0.5) | 0 |
| Missing | 3 (1.3) | 2 (1.1) | 1 (2) |
| Sum of Diameters of Target Lesions Based on ICR at Baseline, n (%) | |||
| <5cm | 97 (41) | 78 (42) | 19 (37) |
| ≥5cm | 118 (50) | 92 (50) | 26 (51) |
| Missing | 20 (9) | 14 (8) | 6 (12) |
| Visceral Disease at Baseline, n (%) | |||
| Yes | 220 (94) | 169 (92) | 51 (100) |
| No | 15 (6) | 15 (8) | 0 |
| Brain Metastasis, n (%) | |||
| Yes | 30 (13) | 24 (13) | 6 (12) |
| No | 205 (87) | 160 (87) | 45 (88) |
| Bone Metastasis, n (%) | |||
| Yes | 73 (31) | 53 (29) | 20 (39) |
| No | 162 (69) | 131 (71) | 31 (61) |
One patient (10117015) was not dosed in DS8201-A-J101
The confirmed ORR based on ICR in these 184 patients was 60.3% (95% CI: 52.9, 67.4). At data cut-off (DCO) of March 21, 2019, the median DoR for confirmed responses for the primary 5.4 mg/kg dose cohort was not estimable. An efficacy update for DoR occurred based on a DCO of August 1, 2019. The DCO for the efficacy update was 10.3 months after the last patient with HER2-positive breast cancer assigned to the 5.4 mg/kg dose group. The median duration of treatment was 9.97 months. The median DoR of the 111 responders was 14.8 months (95% CI: 13.8, 16.9), as shown in Table 3.
Table 3:
| Efficacy Parameter | DESTINY-Breast01 HER2-positive Breast Cancer 5.4mg/kg (N = 184) |
|---|---|
| Confirmed Objective Response Rate (95% CI) | 60.3% (52.9, 67.4) |
| Complete Response | 4.3% |
| Partial Response | 56% |
| Duration of Response* Median, months (95% CI)** | 14.8 (13.8, 16.9) |
ORR 95% CI calculated using Clopper-Pearson method
DOR is based on median duration of follow-up of 11.1 months.
Median DOR based on Kaplan-Meier estimate; 95% CI calculated using Brookmeyer-Crowley method
Safety:
The safety evaluation for T-DXd was based on a pooled total of 234 patients with HER2-positive breast cancer in studies DESTINY-Breast01 (184 participants) and DS8201-A-J101 (50 participants) that were treated with at least 1 dose of T-DXd at 5.4mg/kg. The median duration of treatment was 7 months (range: 0.7 to 31). Although 51 participants with HER2-positive breast cancer were enrolled in study DS8201-J101, one was not dosed; therefore, 50 patients were included in the safety analysis from this study.
Severe, life-threatening, or fatal interstitial lung disease (ILD), including pneumonitis, have been noted in patients treated with T-DXd. Of the 234 participants with HER2-positive breast cancer who received 5.4mg/kg, ILD occurred in 9.4% that was adjudicated by the independent ILD committee as related to the drug. Fatal outcomes due to ILD and/or pneumonitis occurred in 6 (2.6%) patients treated with T-DXd. All fatal cases of ILD were adjudicated as drug related. Median time to first onset was 4.1 months (range: 1.2 to 8.3).
Neutropenia and left ventricular dysfunction were included as Warnings and Precautions in the USPI. In the 234 patients with unresectable or metastatic HER2-positive breast cancer who received 5.4 mg/kg T-DXd, two cases (0.9%) of asymptomatic left ventricular ejection fraction (LVEF) decrease were reported as of the first clinical cut-off dates. Treatment with T-DXd has not been studied in patients with a history of clinically significant cardiac disease or LVEF less than 50% prior to initiation of treatment. However, as LVEF is a known class effect of HER2-directed therapies, it was justified to be included as a warning. Other important common adverse reactions (≥ 20%) were nausea, fatigue, vomiting, alopecia, constipation, decreased appetite, anemia, diarrhea, leukopenia, cough, and thrombocytopenia.
Regulatory Insights
T-DXd demonstrated adequate efficacy and safety in study DESTINY-Breast01 with supportive safety evidence from study DS8201-A-J101 to support an accelerated approval for the treatment of patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting (16–18). Prior to the accelerated approval, Fast Track Designation was granted by the FDA in November 2016 and Breakthrough Therapy Designation in August 2017 based on promising preclinical and early clinical data (18). At the time of filing, Priority Review was also granted to this application.
At the time of accelerated approval for T-DXd, treatment options for HER2-positive patients after two lines of therapy in the metastatic setting were limited, and this is an area of unmet need that continues to require further effective therapies. Prior lines of therapy from patient data were retrospectively reviewed to not include dose reductions or interruptions as a new line of therapy, and to only consider a regimen as a line of therapy if a new agent was added or removed. Based on this assessment, patients received a median of 5 therapies with a range from 2 to 17 lines as included in the label. At the time of accelerated approval of T-DXd, response rates for available therapies in third-line metastatic HER2-positive breast cancer ranged from approximately 14% for trastuzumab alone, to 24% for lapatinib plus capecitabine (9, 19). Therefore, T-DXd demonstrated a clear improvement of ORR and DOR over available therapies with an ORR of 60.3% (95% CI: 52.9, 67.4) and a DOR of 14.8 months (95% CI: 13.8, 16.9) in a heavily pretreated population. Phase 3, randomized trials with T-DXd are ongoing for HER2-positive breast cancer and these results may be used to confirm clinical benefit and support a regular approval in the future (Table 4).
Table 4:
Studies with T-DXd Currently Accruing/Ongoing in Tumors that are HER2-Positive, HER2-Low or Those with HER2 Activating Mutations (22)
| Studies | Disease Area | Phase | Study Details |
|---|---|---|---|
| HER2-positive | |||
| DS8201-A-U301 (DESTINY-Breast02) | Breast Cancer | 3 | Randomized, 2-arm, open-label study to compare the safety and efficacy of T-DXd versus the investigator’s choice of trastuzumab/capecitabine or lapatinib/capecitabine in subjects with HER2-positive, unresectable, and/or metastatic breast cancer previously treated with T-DM1. |
| DS8201-A-U302 (DESTINY-Breast03) | Breast Cancer | 3 | Randomized, 2-arm, open-label study to compare the safety and efficacy of T-DXd versus T-DM1 in subjects with HER2-positive, unresectable, and/or metastatic breast cancer previously treated with trastuzumab and taxane. |
| DS8201-A-U305 (DESTINY-Breast05) | Breast cancer | 3 | Randomized, open-label, active-controlled study of T-DXd versus T-DM1 in patients with high-risk HER2 positive primary breast cancer who have residual invasive disease in breast or axillary lymph nodes following neo-adjuvant therapy. High risk defined based on inoperable cancer at disease presentation (clinical stages T4, N0–3, M0 or T1–3, N2–3, M0) or operable at presentation (clinical stages T1–3, N0–1, M0) with positive pathological node status (ypN1–3) after neo-adjuvant therapy. |
| DS8201-A-U205 (DESTINY-Gastric02) | Gastric or GEJ adenocarcinoma | 2 | Single-arm, open-label, single-arm study of T-DXd in HER2-positive, unresectable or metastatic gastric or GEJ adenocarcinoma subjects who progressed on or after a trastuzumab-containing regimen. |
| D967LC00001 (DESTINY-Gastric03) | Gastric or GEJ adenocarcinoma | 1B/2 | Open-label study to evaluate the safety, tolerability, PK, immunogenicity, and preliminary antitumor activity in participants with HER2 overexpressing (IHC 3+ or IHC 2+/ISH+) locally advanced or metastatic, unresectable gastric cancer |
| DS8201-A-J203 (DESTINY-CRC01) | Colorectal cancer (CRC) | 2 | Single-arm, multicenter, open-label, 3-cohort study to investigate the safety and efficacy of DS-8201a in HER2-expressing advanced CRC subjects |
| DS8201-A-U105 | Breast or urothelial cancer | 1B | Single-arm, open-label, 2-part, multiple-dose study of T-DXd in combination with nivolumab in subjects with HER2-expressing advanced breast and urothelial cancer. Also enrolling HER2-low breast and urothelial cancer |
| DS8201-A-U106 | Breast or non-small cell lung cancer (NSCLC) | 1B | Single-arm, open-label, 2-part, multiple-dose study of T-DXd in combination with pembrolizumab, an anti-PD-1 antibody, in subjects with locally advanced/metastatic breast or NSCLC. Also enrolling HER2-low breast cancer and NSCLC, and HER2-mutant NSCLC |
| D967VC00001 (DESTINY-PanTumor02) | Solid tumors (urothelial bladder cancer, biliary tract cancer, cervical cancer, endometrial cancer, ovarian cancer, pancreatic cancer, and rare tumors) | 2 | Single-arm, open-label, multi-cohort, study to evaluate the efficacy and safety of T-DXd for the treatment of selected HER2-expressing tumors. Also enrolling HER2-low tumors |
| HER2-low disease | |||
| DS8201-A-U303 (DESTINY-Breast04) | Breast Cancer | 3 | Randomized, 2-arm, open-label, active-controlled study to compare the safety and efficacy of T-DXd versus the physician’s choice of capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel, in subjects with HER2-low, unresectable, and/or metastatic breast cancer. |
| D9670C00001 (DESTINY-Breast06) | Breast Cancer | 3 | Randomized, open-label study of T-DXd versus investigator’s choice chemotherapy (capecitabine, paclitaxel, or nab-paclitaxel) in HER2-low, hormone receptor positive breast cancer patients whose disease has progressed on endocrine therapy in the metastatic setting. |
| HER2-mutations | |||
| DS8201-A-U204 (DESTINY-Lung01) | Non-small cell lung cancer (NSCLC) | 2 | Single-arm, open-label, 2-cohort, Phase 2 study to investigate the safety and efficacy of T-DXd in subjects with HER2-overexpressing or HER2- mutated NSCLC. |
While the safety of T-DXd was acceptable for the intended population, ILD was recognized as a adverse reaction that required appropriate communication to the presecriber and patient and was thus included as a boxed warning in the U.S. prescribing information (USPI). The specific biologic mechanisms that may contribute to the development of ILD in those being treated with T-DXd is currently under investigation and may be clarified with results of ongoing studies. Management recommendations include corticiosteroid treatment with potential discontinuation of T-DXd depending on grade of the reaction. Those with a history of pneumonitis or interstitial disease or other chronic lung conditions may be more suseptitble to drug induced ILD however this data is limited as most patients with these underlying conditions were excluded from these trials for safety concerns.
Among HER2-negative disease, HER2-low expression (either immunohistochemistry [IHC] 1+ or IHC 2+ and in situ hybridization [ISH] negative) is estimated to compromise 40–50% including both luminal-type HR-positive and TNBC cancers (20). HER2-low disease is treated similarly to the HER2-negative population including use of endocrine therapies (for hormone receptor positive cancers), chemotherapy and targeted therapies as there are no specific approved treatments for this population. Therefore, there is an opportunity for drug development in the HER2-low space and various therapies are currently being investigated (21). Similarly, there are no specific therapies approved for HER2 activating mutations as not all tumors with these mutations are also considered HER2-positive. T-DXd is also being investigated in patients with HER2-low expressing tumors and those with HER2 activating mutations with enrollment into study DS8201-A-J101 (20). Table 4 summarizes the trials currently accruing with T-DXd (22). Some of these trials are evaluating the safety and efficacy of T-DXd across HER2-positive, HER2-low tumors as well as those with HER2 activating mutations regardless of tissue histology.
In DESTINY-Breast01, HER2 expression was determined based on archival tissue tested at a central laboratory prior to enrollment. All subjects enrolled in this study were required to have documented HER2-expressing tumors based on a validated testing method prior to study entry and were screened for HER2 amplification for previous HER2-directed therapies. Therefore, no companion diagnostic device was submitted for concurrent evaluation with this drug. As all participants in both studies received at least two prior therapies in the advanced or metastatic setting, the indication was restricted to the use in the third-line or later. However, in this refractory population prior HER2-based regimens were not specified to allow flexibility in the use of T-DXd as the landscape of therapies in the HER2-positive space continues to evolve.
The indication for T-DXd is inclusive of all adults with HER2-positive breast cancer to ensure flexibility in the use of this therapy across various patient populations. Historically, men have been excluded from breast cancer trials due to the low incidence of the disease in males and their management in clinical practice is extrapolated from data in women. The FDA has encouraged the inclusion of male patients in breast cancer trials and has released a guidance on this topic, which in part details that even when male enrollment is limited it may be possible to extrapolate findings in cases when no difference between males and females is anticipated based on the mechanism of action of the drug (23). Although only one male participant with breast cancer was enrolled in study DS8201-A-J101, it is not anticipated that T-DXd would have differing efficacy to exclude males from the indication.
The review of this NME application utilized the Assessment Aid (AAid), a multidisciplinary review template that is a voluntary submission from the applicant with the goal to focus and streamline the FDA’s written review on critical thinking and analysis (24). This in part enabled the accelerated approval of T-DXd on December 20, 2019, approximately 4 months ahead of the FDA goal date.
Conclusions
T-DXd represents a new treatment option for patients with heavily pretreated HER2-positive advanced or metastatic breast cancer. Results from DESTINY-Breast01 supported by data from study DS8201-A-J101 demonstrate a favorable benefit-risk profile (Table 5). This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Table 5:
FDA Benefit-Risk Assessment of T-DXd (12)
| Dimension | Evidence and Uncertainties | Conclusion and Reasons |
|---|---|---|
| Analysis of Condition | • Breast cancer is the most common cancer in women, with more than 260,000 new cases and 40,000 deaths annually. Approximately 20% of patients with breast cancer have HER2-positive tumors. • Breast cancer in male patients is rare and presents at a higher stage • Advanced or metastatic breast cancer is incurable. |
Advanced or metastatic HER2-positive breast cancer is a serious and life-threatening condition with ongoing unmet medical need in both female and male patients. |
| Current Treatment Options | • Metastatic HER2-positive breast cancer is not curable with treatment goals palliative in nature to delay disease progression, prolong survival, and reduced cancer-related symptoms. • The combination of trastuzumab, pertuzumab, and taxane is an FDA approved treatment for patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease. • T-DM1 is FDA approved for the treatment of patients with HER2- positive, metastatic breast cancer who previously received trastuzumab and a taxane. • Other options beyond 2 lines of HER2-based therapies can include lapatinib and capecitabine, or trastuzumab combined with either lapatinib, capecitabine, neratinib plus capecitabine*, tucatinib in combination with trastuzumab and capecitabine**, or another chemotherapeutic agent. |
Additional treatment options are needed forfemale and male patients with HER2-positive advanced or metastatic breast cancer whose disease has progressed on available HER2-directed therapies. |
| Benefit | • The efficacy of T-DXd was evaluated in study DS8201-A-U201, a multicenter, single-arm, trial that enrolled 184 female patients with HER2-positive, unresectable and/or metastatic breast cancer who had received two or more prior anti-HER2 therapies. • ORR was 60.3% (95% CI: 52.9, 67.4), with a 4.3% complete response rate and a 56% partial response rate. Median response duration was 14.8 months (95% CI: 13.8, 16.9). |
Study DS8201-A-U201 resulted in substantial and durable ORR that represents an improvement compared to that of available therapies and is reasonably likely to predict clinical benefit. |
| Risk and Risk Management | • The most common adverse reactions were nausea, fatigue, vomiting, alopecia, constipation, decreased appetite, anemia, neutropenia, diarrhea, leukopenia, cough, and thrombocytopenia. • Serious adverse reactions occurred in 20% of patients receiving T-DXd. • Fatalities due to adverse reactions occurred in 4.3% of patients including interstitial lung disease (2.6%). • Interstitial lung disease (ILD) is an important safety signal identified during the clinical development program for T-DXd. Fatal outcomes due to ILD occurred in 2.6% of patients. • Labeling includes a Boxed Warning to advise health professionals of the risk of interstitial lung disease (ILD), and embryo-fetal toxicity. |
The safe use of T-DXd can be managed through appropriate labeling, including boxed warnings for interstitial lung disease, and embryo-fetal toxicity. No REMS is indicated. |
Neratinib in combination with capecitabine was granted regular approval by the FDA in February 2020 after the accelerated approval of T-DXd.
Tucatinib in combination with trastuzumab and capecitabine was granted regular approval by the FDA in April 2020 after the accelerated approval of T-DXd.
Footnotes
Note: This is a U.S. Government work. There are no restrictions on its use.
Disclosure of Potential Conflicts of Interest: The authors report no financial interests or relationships with the commercial sponsors of any products discussed in this report.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Surveillance, Epidemiology and End Results Program (SEER). Female Breast Cancer. [cited 2020 Jul 2]. Available from: https://seer.cancer.gov/statfacts/html/breast.html [Google Scholar]
- 3.American Cancer Society. Key Statistics for Breast Cancer in Men. [cited 2020 Jul 2]. Available from: https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
- 4.National Cancer Institute. Surveillance, Epidemiology and End Results Program (SEER). Female Breast Cancer Subtypes. [cited 2020 Jun 2]. Available from: https://seer.cancer.gov/statfacts/html/breast-subtypes.html [Google Scholar]
- 5.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LAG, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014; 106(5): dju055 doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. The New England journal of medicine 2015; 372(8):724–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. New Engl J Med. 2012; 367(19):1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giordano SH, Temin S, Chandarlapaty S, Crews JR, Esteva FJ, Kirshner JJ, et al. Systemic Therapy for Patients With Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36(26):2736–40 [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network (NCCN) Guidelines. Breast Cancer [about 4 screens]. [cited 2020 Jul 2]. Available from https://www.nccn.org/professionals/physician_gls/default.aspx.
- 10.U.S. Food and Drug Administration: FDA approves neratinib for metastatic HER2-positive breast cancer [about 3 screens]. [cited 2020 Aug 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neratinib-metastatic-her2-positive-breast-cancer
- 11.U.S. Food and Drug Administration: FDA approves tucatinib for patients with HER2-positive metastatic breast cancer [about 3 screens]. [cited 2020 Aug 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tucatinib-patients-her2-positive-metastatic-breast-cancer
- 12.U.S. Food and Drug Administration: Drug Approval Package: ENHERTU. Multi-Discipline Review [about 2 screens].[cited 2020 Oct 17]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761139Orig1s000TOC.cfm
- 13.Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 2016; 22(20): 5097–5108. [DOI] [PubMed] [Google Scholar]
- 14.Pant S, Landon MB, Blumenfeld M, Farrar W, Shapiro CL. Treatment of breast cancer with trastuzumab during pregnancy. J Clin Oncol 2008; 26(9): 1567–1569. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Food and Drug Administration: Drugs@FDA: FDA-Approved Drugs. Labels for BLA 761139 Fam-trastuzumab deruxtecan-nxki [about 4 screens]. [cited 2020 Oct 17]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process
- 16.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. The New England journal of medicine 2020;382(7):610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration FDA News Release: FDA approves new treatment option for patients with HER2-positive breast cancer who have progressed on available therapies [about 3 screens]. [cited 2020 Jul 2]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-option-patients-her2-positive-breast-cancer-who-have-progressed-available
- 18.U.S. Food and Drug Administration: FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive breast cancer [about 2 screens]. [cited 2020 Jul 2]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2-positive-breast-cancer
- 19.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. The New England journal of medicine 2006;355(26):2733–43 [DOI] [PubMed] [Google Scholar]
- 20.Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: Results from a phase 1b study. J Clin Oncol 2020; 38(17): 1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-low breast cancer: Pathological and Clinical Landscape. J clin Oncol 2020; 38(17): 1951–1962. [DOI] [PubMed] [Google Scholar]
- 22.Clinicaltrials.gov. All studies with trastuzumab deruxtecan. [about 4 screens].[cited 2020 Oct 22]. Available from: https://clinicaltrials.gov/ct2/results?cond=&term=trastuzumab+deruxtecan&cntry=&state=&city=&dist=
- 23.U.S. Food and Drug Administration: Male Breast Cancer: Developing Drugs for Treatment [about 2 screens]. [cited 2020 Jun 5]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/male-breast-cancer-developing-drugs-treatment
- 24.U.S. Food and Drug Administration: Assessment Aid [about 3 screens]. [cited 2020 Jul 5]. Available from: https://www.fda.gov/about-fda/oncology-center-excellence/assessment-aid