Abstract
In biological responses, fatty acids (FA) are absorbed and metabolized in the form of substrates for energy production. The molecular structures (number of double bonds and chain length) and composition of dietary FA impact digestion, absorption and metabolism, and the biological roles of FA. Recently, increasing evidence indicates that FA are essentially utilized as an energy source and are signaling molecules that exert physiological activity of gut microbiota and immune responses. In addition, FA could serve as natural ligands for orphan G protein-coupled receptors (GPCR), also called free fatty acid receptors (FFAR), which intertwine metabolic and immune systems via multiple mechanisms. The present review explores the recent findings on FA absorption and its impact on gut health, particularly addressing the mechanism by which dietary FA potentially influences intestinal microbiota and epithelial functions. Also, this work attempts to uncover research ideas for devising future strategies for manipulating the composition of dietary FA to regulate gut health and support a normal immune system for metabolic and immune disorders.
Keywords: Fatty acids, Gut, Absorption, Metabolism, G protein coupled receptor
1. Introduction
Fatty acids (FA) are critical reservoirs of energy for a large number of body tissues and exert several functions during the life cycle. FA are organics that show as long aliphatic hydrocarbon chains containing one carboxyl group at one end. Based on the length of hydrocarbon chain, FA can be divided into 3 classes: short chain fatty acids (SCFA), with aliphatic tails fewer than 6 carbons; middle chain fatty acids (MCFA), which have aliphatic tails of 6 – 12 carbons; and long-chain fatty acids (LCFA), having aliphatic tails with more than 12 carbons (Burdge and Calder, 2015). FA are important components to form lipids, such as triglycerides (TAG), phospholipids, cholesteryl esters, plant sterols and so on (Wang et al., 2013), which mainly contribute to energy metabolism, stability of the cell membrane and modulation in various cell processes (Glatz and Luiken, 2015).
FA and the associated ligands, including peroxisome proliferator-activated receptors (PPAR) potentially regulate the expression of associated genes contributing to the synthesis of fatty acids, oxidation, energy partitioning, glucose utilization, lipogenesis, insulin sensitivity, reverse cholesterol transport, thermoregulation, cholesterol synthesis, growth and differentiation, inflammatory responses (Grygiel-Gorniak, 2014; Hoppenbrouwers et al., 2019; Sekikawa et al., 2019), and low-density-lipoprotein-receptor expression. Our previous study found that gut immunity and microbiota dysbiosis are associated with altered fatty acids metabolism in LPS-challenged piglets (Xiao et al., 2021; Zong et al., 2019b). Accumulating evidence has shown the cardioprotective activity of monounsaturated fatty acids (MUFA) in dietary fat (Gillingham et al., 2011), especially with essential FA docosahexaenoic acid (Hammad et al., 2016). Further, conjugated linoleic acids have proved to be beneficial in preventing and managing several diseases, such as cancer, obesity, cardiovascular diseases, and diabetes (Fuke and Nornberg, 2017). Because of the inability to synthesize essential FA by bodies, these FA or their precursors must be ingested from dietary fat. Therefore, an understanding of FA absorption is essential.
Based on the current understanding, 3 groups of proteins (plasma membrane fatty acid-binding proteins [FABP] and free fatty acid receptors [FFAR]) potentially detect the type and presence of fatty acids whether in the cytosol, extracellular medium, or the nuclear matrix (Hara et al., 2013). Within the plasma membrane, FA activate G protein-coupled receptors (GPCR), also called FFAR. Independently, they are taken by FABP and channeled to specific metabolic pathways or subcellular structures in the cytosol. Eventually, nuclear receptors PPAR mediate the regulatory functions of fatty acids within the nucleus (Zolezzi and Inestrosa, 2013). The particular spatiotemporal expression pattern and multiple co-expression of isoform from each protein family in one cell depict a sensory and modulatory platform of cellular response to the presence of various FA, for instance, cell adaptation to functional or developmental needs. Thus, the signaling or regulatory functions of FA have widely been implicated in various pathological and physiological processes.
With this current understanding and interpretation of FA, the aim is to highlight recent discoveries related to FA absorption and the impact on health, particularly addressing the mechanisms by which dietary FA alters intestinal microbiota and epithelium function.
2. Absorption and metabolism of FA
2.1. Overview of dietary lipid digestion
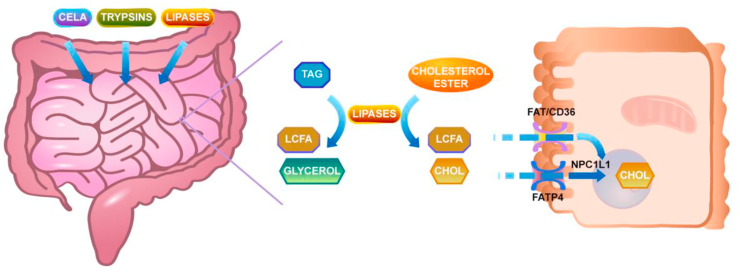
As shown in Fig. 1, dietary fat and other food ingredients form a bolus by chewing and stirring of the tongue. This bolus goes through the esophagus and hydrolyzes into diglycerides and free FA in the stomach by gastric lipase or lingual lipase (Hamosh and Scow, 1973). Following the gastric pre-digestion, the bolus enters the duodenum and the major digestion of TAG takes place here by hydrolysis with pancreatic lipase. Bile salts act as vehicles for TAG, diglycerides, cholesteryl esters, and other lipids to form micelles that can make fat contact with the water-phase as much as possible (Maldonado-Valderrama et al., 2011). Colipase helps pancreatic lipase to bind to the emulsion/water interface, which can highly facilitate the digestion of fat (van Tilbeurgh et al., 1999). Eventually, the digestion of fat results in monoglycerides (MAG) and free FA. The FA chain length influences the rate of dietary fat digestion. It will decrease with increasing FA chain length, i.e. the rate is in this order: short chain TAG > medium chain TAG > long chain TAG (Guo et al., 2017).
Fig. 1.
Intestinal fatty acids absorption. Dietary lipids are emulsified with bile salts and are hydrolyzed by different pancreatic lipases, resulting in the generation of free fatty acids and cholesterol. These products are taken up by the enterocytes involving various transporters and transported to the endoplasmic reticulum, where they are used for the synthesis of triacylglycerols. TAG = triglyceride; LCFA = long-chain fatty acids; CHOL = cholesterol.
2.2. The uptake of FA into enterocytes
The absorption of solutes by the gut depends on the amount of solutes that can get to the brush-border membrane (BBM), and the delivery of solute to the BBM depends upon the efficiency of luminal stirring (Levitt et al., 1990). There would be a permeability barrier between the digesta and brush border which is called the unstirred water layer (UWL) (Kono et al., 2016; Korjamo et al., 2009). The UWL exerts a significant function in the membrane transport process; transporting the rapidly penetrating solutes across membranes can be wholly or partly rate regulated by UWL (Thomson and Dietschy, 1984).
2.2.1. The uptake of SCFA in colonocytes
The absorbed SCFA, such as acetate, are sources of energy to the mammals, which can be rapidly absorbed, recycled and metabolized into the body to make the body functional (Latymer et al., 2010). Many researchers disagree about how SCFA get into the gut cells. Some hold the view that SCFA go through the cell membranes by simple diffusion, but others think it may need some proteins to transport SCFA. One study showed that formic, acetic and propionic acid permeate via the lipid bilayer membranes owing to nonionic diffusion as well as the permeability pattern, which is similar to other nonelectrolytes in lipid bilayers (Walter and Gutknecht, 1984). Researchers found that apical membrane vesicles obtained from rat distal colon uptake butyrate, which is a representative SCFA, by a bicarbonate gradient dependent, were pH-dependent, and carrier-mediated anion exchange process (Mascolo et al., 1991; Stein et al., 2000), which supports the latter opinion. The carrier is called monocarboxylate transporter (MCT), SLC5A8. A large volume of evidence has proven that the uptake of SCFA will be various in different situations. The uptake of SCFA monomers is limited by the BBM; however, the diffusion via the UWL is increasingly rate limiting with an increase in the chain length (Sallee and Dietschy, 1973; Thomson et al., 1993).
2.2.2. The uptake of MCFA and LCFA in enterocytes
The uptakes of MCFA and LCFA in enterocytes are similar, but some small differences exist. For LCFA and cholesterol, any passage across the UWL is rate-limiting, but not for MCFA. The rate liming step for MCFA is passaging across the brush-border membrane (Thomson et al., 1993).
SCFA and MCFA uptake by enterocytes do not require FA transporters, but LCFA require fatty acid transport proteins (Stahl et al., 2001), plasma membrane-embedded fatty acid translocase (FAT/CD36) (Drover et al., 2008), FABP (Gajda and Storch, 2015) and other proteins (Stremmel et al., 2017) for their cellular uptake (Schonfeld and Wojtczak, 2016). After entry into the enterocytes, FA and MAG are moved to the membrane of the endoplasmic reticulum (ER) by FABP; here, they resynthesize TAG to form the hydrophobic core of chylomicrons (CM) (Yen et al., 2015) in the cytoplasmic lipid droplets within the enterocytes (D'Aquila et al., 2016). TAG and other neutral lipids together with apolipoprotein (apo) B-48s and microsomal triglyceride transferases form pre-CM, which transport from ER to the Golgi complex by the pre-CM transport vesicles to form mature CM (Black, 2007). The mature CM exit the Golgi complex and fuse with the basolateral membrane, after which they are secreted into the lamina propria (Mansbach and Gorelick, 2007; Sabesin and Frase, 1977).
2.3. The metabolism of FA
Of note, FA are primarily synthesized in animals from carbohydrates in the adipose tissue, lactating mammary glands and liver (Hillgartner et al., 1995; Rinaldo and Matern, 2002). First, the most critical step in how carbohydrates are converted to FA, is the generation of carbohydrates to pyruvate through glycolysis (Olson, 1966). Then, pyruvate is decarboxylated, which forms acetyl-CoA within the mitochondria. Notably, the acetyl-CoA must be transported to the cytosol, the FA synthesis site. For cytosolic acetyl-CoA generation, the citrate released through acetyl-CoA condensation with oxaloacetic acid is eliminated from the citrate cycle. It is then taken to the cytosol via the inner mitochondrial membrane. At this point, it is cleaved to oxaloacetate and acetyl-CoA and catalyzed by ATP ci-tratelyase. Oxaloacetic acid is then taken back to the mitochondria in the form of malic acid (Ferre and Foufelle, 2007). Acetyl-CoA carboxylase mediates the carboxylation of cytosolic acetyl-CoA to malonyl-CoA; notably, this forms the initial committed step in FA synthesis. Malonyl-CoA participates in iterative reactions that prolong the FA chain by 2 carbons at a time. Consequently, nearly all natural FA exhibit an even number of carbon atoms. When the synthesis is complete, FA, in most cases, bind to glycerol (3 FA bind to 1 glycerol molecule) and form triglycerides, the main storage form of FA, thereby it produces the main energy source in animals. Also, synthesized FA are a crucial component of phospholipids, forming essential phospholipid bilayers in establishing the cell membranes.
Within the circulating plasma, FA are derived through lipolysis from reserved triglycerides (Zechner et al., 2005). Owing to the insoluble nature of FA in water, their transportation is aided by binding to plasma albumin. Consequently, the presence of albumin binding sites is limited by plasma FA levels. Besides, FA may be absorbed from circulating plasma by all mitochondria-containing cells, then are metabolized through β-oxidation and broken down to water and CO2 within the mitochondria. As a result, large quantities of energy are released in the form of ATP via the citric acid cycle and β-oxidation (Mcgarry and Foster, 1980). Thus, FA serve as an energy source in tissues. Cells in the central nervous system (CNS) have mitochondria; however, glucose is the primary source of energy in the mammalian brain. FA synthesis from carbohydrates takes place in CNS cells; this enhances the production and maintenance of the required phospho-lipids for organelles and cell membranes. At the same time, the transport mechanisms of FA from the plasma to the brain via the blood–brain barrier is dependent on the carbon length of FA (Tsuji, 2005; Vijay and Morris, 2014). For instance, FA obtained from dietary fat ingestion or from the triglycerides secreted in adipose tissue are distributed to cells, acting as a fuel source for systemic metabolism and muscle contraction.
3. Physiological functions of FA on gut health
Notably, FFA serve as essential energy sources and signaling molecules regulating distinct physiological functions and cellular processes, but that is dependent on the length of their carbon chain.
3.1. Impact of dietary FA on gut immune function
3.1.1. SCFA
Studies have revealed that SCFA are significantly crucial in improving intestinal health and limiting intestinal inflammation. The low potential of intestinal mucosa in the oxidation of butyrate has been described in ulcerative colitis pathogenesis (Scheppach et al., 1992). A study by Fang et al. (Fang et al., 2014) found that dietary supplementation of sodium butyrate (1 g/kg feed) remarkably lowered cases of diarrhea in weaned piglets from 15% to 11%. Further, there was enhanced immune function due to high serum IgG concentration and IgA+ cell count in the jejunum, which lowered the adverse impacts of weaning stress and maintained the intestinal mucosa integrity. There is a close correlation of the beneficial roles of butyrate with elevated proliferation and decreased apoptosis of enterocytes (Bartholome et al., 2004; Kien et al., 2007). Elsewhere, Hou et al. (Hou et al., 2014) demonstrated that 0.1% tributyrin dietary supplementation alleviated intestinal injury through inhibition of apoptosis, enhanced tight-junction establishment and activation of epidermal growth factor receptor signaling in a piglet colitis model induced via intrarectal acetic acid administration. With the intestinal porcine enterocytes J2 (IPEC-J2) cell model, we revealed that SCFA and their analogs induced porcine host defense peptide gene expression (Xiong et al., 2016). Collectively, dietary SCFA supplementation, in particular, butyrate, is key to intestinal health and attenuation of inflammation of the intestines.
3.1.2. MCFA and LCFA
MCFA and LCFA have been suggested to improve the gut morphology and epithelial barrier functions through distinct ways (Liu, 2015). Inflammation, in most cases, causes the repartitioning of the host's energy to other functions apart from digestion. Our study revealed that the absorption of LCFA is closely related to intestinal barrier function (Zong et al., 2019a). Also, MCFA is absorbed directly by the enterocytes to produce energy, thus supporting the intestine integrity (Jia et al., 2020). For instance, relative research has provided evidence that MCFA can induce the production of host defense peptides, such as β-defensins (Wang et al., 2018; Zhou et al., 2019). A diet with 0.5% capric acid fed to pigs could significantly protect against cyclophosphamide-induced intestinal inflammation, oxidative stress, and gut barrier function (Lee and Kang, 2017).
Reports have suggested MCFA or LCFA potentially improve gut health in inflammatory conditions. Sam et al. (2021) demonstrated that SCFA and MCFA have divergent immunomodulatory propensities. MCFA down-attenuated host pro-inflammatory IL-1β, IL-6, and TNFα response predominantly through the TLR2 pathway. Bertevello et al. (Bertevello et al., 2012) showed that partial substitution of n-6 fatty acids with MCT enhanced damage in experimental colitis of rats but improved colon cytokine response. Also, Papada et al. (Papada et al., 2014) revealed that a MCT-rich diet lowered the levels of intercellular adhesion molecule-1 (ICAM-1), IL-6, IL-8 and glutathione S-transferase (GST) activity, thus inducing anti-inflammatory functions in trinitrobenzenesulfonic acid (TNBS) induced colitis. Besides, in an Escherichia coli (ETEC) infected pig model, they found MCFA-protected sodium butyrate and heptanoate additive did not lead to reduce ETEC colonisation, but enterobacterial counts and goblet cell numbers in the ileum were increased and this followed higher serum TNF-α concentrations (Lopez-Colom et al., 2020). These observations reveal that adopting MCFA or MCT as supplements in attenuating intestinal inflammation may offer a promising strategy.
3.1.3. PUFA
The synthesis of essential fatty acids (EFA) in animals cannot take place endogenously; thus, they are availed exogenously from dietary reservoirs (Beare-Rogers et al., 2001). Two EFA families have been described: n-6 (ω-6) and n-3 (ω-3). Notably, linoleic acid (LA; C18:2n-6) and α-linolenic acid (ALA; C18:3n-3) represent the sourcing factors for n-6 and n-3 families, respectively (Swick, 2019). Numerous plant oils, such as soybean oils, sunflower, and corn, contain excellent n-6 fatty acid sourcing, primarily as LA. The diet-derived ALA/LA in pigs is processed into long-chain PUFA, for example, eicosapentaenoic acid (EPA; C20:5n-3), arachidonic acid (ARA; C20:4n-6), together with docosahexaenoic acid (DHA; C22:6n-3). However, there are limitations in the conversion efficiency owing to the low activity of desaturase (Jacobi et al., 2011; Xie and Innis, 2008).
Currently, researchers have shown immense interest in the mechanisms by which long-chain PUFA influence intestinal function. Through clinical trials and animal models, a wealth of literature reports have proven n-3 PUFA has positive functions over inflammatory bowel conditions (Scaioli et al., 2017). For instance, the long-chain n-3 PUFA, abundant within fish-oils, could reduce inflammation signaling transductions, especially the TLR4 pathway (Childs et al., 2019). Similarly, Liu et al. (Liu et al., 2012) revealed that dietary fish oil enhanced the integrity of the intestines through inhibiting TLR4 and NOD2 signaling pathways within breastfed developing porcines post-LPS confrontation. Interestingly, n-3 PUFA, mainly EPA, are able to alleviate LPS-induced Kupffer cells pyroptosis via abolishing NLRP3 inflammasome activation, thus inhibiting subsequent release of cytokines (Fan et al., 2021).
A few reports have demonstrated that n-6 PUFA, particularly, ARA in addition to its metabolites, enhances the recovery process for damaged intestinal mucosa (Lauridsen, 2020). Adrian et al. (Bartoszek et al., 2020) assessed the anti-inflammatory effect of walnut oil within dextran sulfate sodium (DSS)-induced colitis murine studies, and found that walnut oil treatment could increase the expression of tight junction proteins (TJ), decrease pro-inflammatory cytokines, and restore colonic wall permeability. Additional experiments using pigs revealed that dietary supplementation of prostanoids triggers the rapid recovery of barrier function, reduces inflammatory response, together with enhancing intestinal tight junction proteomic levels (Hu et al., 2020). In addition, Patterson et al. (Patterson et al., 2008) revealed that weaning piglets using 2% CLA-augmented sows led to lower intestinal inflammatory responses, together with elevated serum IgG/IgA levels, in comparison to control sows, following ETEC challenges. Additional health effects were found upon the provision of dietary intake of n-6/n-3 PUFA, Paleolithic-/Mediterranean-supplementations could be linked to reduced systemic inflammatory/oxidative stress responses within humans (Whalen et al., 2016). Thus, use of PUFA as a supplement is a practical means for improving the overall gut health, and should be considered as an immune-resolving approach.
3.2. Impact of dietary FA on intestinal microbiota
Diet composition is among the highly significant factors influencing the total diversity and population of the intestinal microbiota. Previous investigations highlighted influences by selected diet-derived FA sub-groups, including LCFA, MCFA, MUFA and PUFA, upon intestinal microbiomes (Castonguay-Paradis et al., 2020). A high-fat diet is known to impact on the composition of gut microbiota and its function; however, the majority of studies focus on the colonic or faecal microbiota (Zong et al., 2020). Furthermore, high-fat dietary regimes carrying palm oil were found to reduce microbiotic diversification (Mujico et al., 2013).
Information on diet-derived lipid influences over microbiotic composition is scanty. Mozes et al. ((Mozes et al., 2008) and other groups (Sefcikova et al., 2010), using 2-week-aged rat pups (over-fed high-fat-content milk), showed through fluorescent in situ hybridization (FISH), that such a diet regulated the number of Bacteroidetes (Bacteroides/Prevotella) while exacerbated Firmicutes (Lactobacillus/Enterococcus) populations within jejuni. Other than altering spatial distribution/microbial counts, high-fat dietary regimes severely impacted the microbiota constitution, with exacerbation of Firmicutes (appearance of Erysipelotrichia), Proteobacteria (Desulfovibrionales) and Verrucomicrobia, together with reductions in Bacteroidetes (Chang and Martinez-Guryn, 2019). Through a comparison of the dietary effects, antibiotic cocktails and broad-spectrum antibiotics, Poteres et al. (Poteres et al., 2020) revealed that antibiotics impacted the murine small intestine, faeces and caecum microbiota make-up, whereas dietary intake highly influenced caecum and jejunum microbiota, implying that dietary intake exerts specific and localized effect over gastro-intestinal microbiota. Thus, future exploration on how lipids influence the microbial content in human jejunum is needed to decipher the detailed relationship between microbiota, host, and diet.
Generally, it is concluded that the influence of dietary FA on the intestinal microbiota is dependent on SCFA (organic acids, e.g. formic, propionic, and lactic acids) and MCFA, which have been proved to exhibit antimicrobial properties. Moreover, dietary strategies with such lipids impede the survival of common pathogens such as Salmonella and E. coli within porcine digested matter post-breastfeeding; this could reduce possibility for gastrointestinal infections (Gunness et al., 2016; Yang et al., 2021). The effect of capric acids (C10:0) and caprylic (C8:0) on Gram-negative and Gram-positive bacterial organisms through non-animal investigations has been described (Zentek et al., 2011). Further, MCFA/SCFA tandem effects were explored based administering a mixed-feed containing MCFA (0.2% to 0.4%) and organic acids, and the antimicrobial activity over E. coli-confronted young porcines during breast-feeding lowered diarrheal cases (Lei et al., 2017). Also, marine n-3 potentially influences the diversity of the bacteria (colonic microbiota), creating a limited inflammatory condition within colon intestinal lining for such medical management (Calder, 2019). Notwithstanding, information on the fish oil/n-3 LCFA influences over composition of porcine bacteria remains scanty. Variation in diet-derived n-6-content: n-3-content in breastfed porcines does not affect the fecal microbial counts (Upadhaya et al., 2019); thus, there is an urgent need to explore whether the LCFA has significant effects on the composition of mucosal bacteria colonizing the upper intestinal portion, which is the lipid absorption site. Further, novel reports in mice demonstrated an existing interplay of intestinal-based microbial populations with the organism's energetic processing through LCFA ileal metabolytes (Miyamoto et al., 2019).
It is interesting to elucidate the mechanism by which enteric microbiota manipulates lipid absorption, digestibility, and processing. Antibiotic-based development enhancers shows a partial correlation with low bile salt hydrolase (BSH) activity (Lin, 2014); such gut microbiota-secreted enzymatic agent catalyzes splits within bound intestinal bile acids (Begley et al., 2006). Of note, such bound bile acids have higher efficiency as bio-detergents, compared to unbound bile acids, especially in the emulsification and solubilization of lipids. Thus, higher BSH activity negatively impacts the digestion and utilization of the host lipid. Orally administering low-dose antibiotics also influences intestinal-based microbial distribution, density and population strain (Lin, 2014). Similar to broilers, this additionally influenced degree of breakdown for fat-soluble vitamins/lipids (Engberg et al., 2000; Knarreborg et al., 2002). Eliminating antibiotic-based development enhancers from porcine rearing potentially influences microbiota make-up; this may consequently affect the breakdown, uptake, and processing for diet-derived fat.
Collectively, MCFA and SCFA modulate intestinal-antimicrobial roles, being instrumental in dietary regimes for reducing intestinal pathogenic risks. Additionally, manipulating gut-based microbial populations may alter FA breakdown/uptake; however, novel observations illustrate the LCFA metabolic functions of the intestinal microbiota.
4. Mechanism of action of FA: GPCR
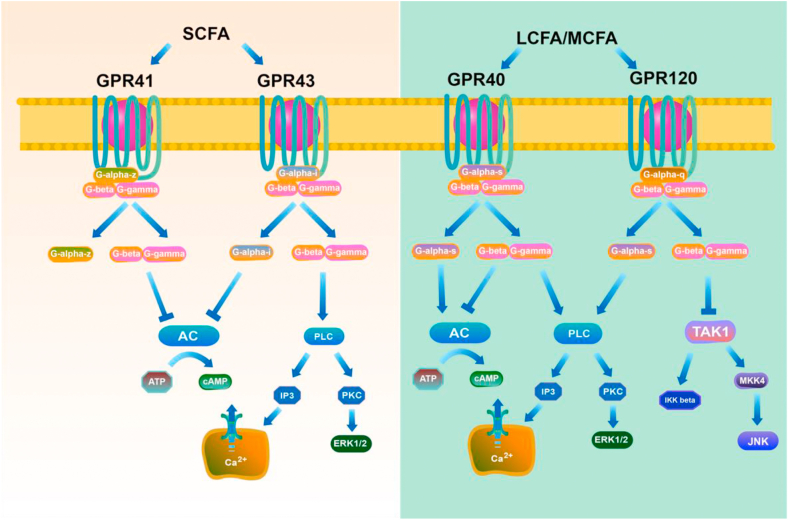
Studies have reported numerous orphan GPCR as FA receptors. Notably, the receptors GPR120 and GPR40 are activated by LCFA and MCFA, whereas GPR43 and GPR41 are activated by SCFA (Fig. 2) (Delzenne et al., 2011; den Besten et al., 2013; Donohoe et al., 2011; Liou et al., 2013). GPCR are seven transmembrane receptors that constitute an extensive protein family of receptors vital in the detection of extracellular molecules. They initiate the activation of intracellular signal transduction pathways, and eventually induce cellular responses (Lagerstrom and Schioth, 2008; Marinissen and Gutkind, 2001).
Fig. 2.
Signaling pathways and G protein-coupled receptors (GPCR) ligand specificity of fatty acids. LCFA and MCFA activate GPR40 and GPR120, whereas short chain fatty acids (SCFA) activate GPR41 and GPR43. An illustration of the signaling pathways downstream of each receptor is shown. LCFA = long-chain fatty acids; MCFA = middle chain fatty acids; AC = adenylate cyclase; DAG = diacylglycerol; IP3 = inositol trisphosphate; PKC = protein kinase C; PLC = phospholipase C.
4.1. GPR40
By 2003, GPR40 was eventually described as the receptor activated by LCFA and MCFA (Briscoe et al., 2003; Itoh et al., 2003). Unsaturated FA potentially activate GPR40 but not saturated FA; notably, DHA shows the highest potency as a GPR40 agonist among unsaturated FA (Grundmann et al., 2021). The activation of GPR40 by saturated FA is dependent on the carbon-chain length, while palmitate (C16) acts as a robust binding factor for saturated FA (Offermanns, 2014). GPR40 was initially found to be a Gq-proteomic-coupled receptor (Itoh et al., 2003; Shapiro et al., 2005), which activated phospholipase C, increasing the intracellular Ca2+ levels diacylglycerol-induced or IP3 protein kinase C (PKC) phosphorylation (Fig. 2). Extra-cellular signaling-modulated kinases (ERK1/2) activation was revealed to be part of down-stream GPR40-Gq protein signaling cascades (Itoh et al., 2003). Thus, GPR40 signaling may seem more complex than anticipated. Further exploration is necessary to elucidate the actual mechanism and satisfactory clinical demands.
4.2. GPR120
Unlike for GPR40, there are no confirmed reports on GPR120 coupling onto Gi/Gs proteomic players. Hirasawa et al. (Hirasawa et al., 2005) de-isolated GPR120 to be a secondary FFAR, revealing its strong affinity to LCFA. Also, they described how GLP-1 discharge could be enhanced through FFA, through GPR120 triggering within STC-1 cellular structures, with high plasma insulin/GLP-1 concentrations occurring within murines subjected to oral ALA treatment. GPR120/GPR40 each have 10% homology in AA sequencing (Hirasawa et al., 2005; Itoh et al., 2003); however, binding factor function (FA into GPR120) is similar to that of GPR40. GPR120 has a preference for C18 PUFA as internally-derived binding factors, following 50% effective concentration values generated, employing GPCR protein over-expressing cell lines (Ichimura et al., 2014). With downstream signaling, phospho-inositide 3-kinase (PI3K) and ERK1/2 activation have been reported after GPR120 activation within restricted investigational situations. Such a defined cascade, however, is elusive (Fig. 2) (Katsuma et al., 2005).
4.3. GPR43
Following reports on the structure–activity relationship, GPR43 prefers to be activated by acetate (C2) as well as other SCFA, including butyrate (C4) and propionate (C3) (Brown et al., 2003; Nilsson et al., 2003). GPR43 activation by SCFA through the Gi/o family impedes the generation of cAMP, also triggering ERK cascading system. Further, GPR43 triggering through SCFA, using Gq family members, elevates Ca2+ levels and induces MAPK cascade activation (Fig. 2) (Hudson et al., 2012; Hudson et al., 2013). Moreover, GPR43 signaling impeded the nuclear NF-κB translocating processes, thus down-regulating inflammation-related cytokines, including IL-6/IL-1β, within GPR43-transfected HeLa cellular populations (Fig. 2) (Lee et al., 2013). Of note, SCFA receptors GPR41/GPR43 bind onto pertussis toxin-sensitive Gi/o proteomic family; GPR43 binds onto pertussis toxin-insensitive Gq proteomic family (Bolognini et al., 2016; Hudson et al., 2011; Le Poul et al., 2003). Notwithstanding, the physiological parts played by such duet-combined signal transduction pathways via GPR43 are unclear.
4.4. GPR41
GPR41, which regulates energy homeostasis (Samuel et al., 2008b; Xiong et al., 2004), was deorphanized in 2003 and described as a SCFA receptor (Brown et al., 2003; Le Poul et al., 2003). GPR41 is activated by SCFA, including valerate (C5), butyrate (C4) and propionate (C3), all synthesized through microbial fermenting activity over colon-based diet-derived fiber-content (Brown et al., 2003; Le Poul et al., 2003). However, GPR41 exerts more robust reactions towards extended SCFAs, for example, valerate/caproate (C6) in comparison to GPR43 (Brown et al., 2003; Le Poul et al., 2003). GPR41 stimulation with SCFA promotes phosphorylation of ERK1/2 and impedes cAMP release. Additionally, these responses are managed using pertussis toxin, an implication of GPR41 coupling to Gi/o (Bolognini et al., 2019; Hudson et al., 2011) (Fig. 2). Reports show that GPR41 expression occurs in various body structures, including adipose tissue, immune cells, together with the peripheral nervous system, and orchestrates overall energetic balance through SCFA-driven signaling (Brown et al., 2003; Kimura et al., 2011; Le Poul et al., 2003; Samuel et al., 2008a). Of interest, one previous investigation utilizing bimolecular fluorescence complementation (BiFC), fluorescence resonance energy transfer (FRET), and proximity ligation assays demonstrated that the GPR43-GPR41 heteromer displays distinct signaling from its parent homomers (Ang et al., 2018).
Even though combining GPR43/GPR41 in any manner depicts differing signal transduction routes, defined functions for this are still unclear. Thus, further studies should probe additional pathophysiological functions for such receptors, elucidating if receptor heteromerizing activities are crucial, or otherwise, to develop such bespoke signal transduction roles.
5. Conclusion and perspectives
Based on the literature reports reviewed in the present study, fat-feeding covers multiple biologically important FAs with potential positive functions on gut health. The antibacterial fats (SCFA and MCFA) could provide rapid host energetic boosts. Contrarily, there is complexity in LCFA breakdown/uptake, though such FA function through inserting within membrane-based biologically-important functions tied to inflammation responses. Epithelial immunological and antimicrobial capacities become crucial for its barrier function. These findings demonstrate redundancy as a remarkable aspect of GPCR and FA for maintaining receptor triggering within specific levels. Although the role of GPCR over positive contributions by selected dietary regimes are still elusive, uncovering such mechanistics by which diet correlates with health may offer new therapeutic strategies. Finally, with the strategic use of FA gut development and function in the challenging phases, growth, function, and health of the gut could be optimized.
Author contributions
E. Xu: investigation, original draft preparation. Chao Chen, Jie Fu, Luoyi Zhu, and Junlan Shu: investigation, revision and reviewing. Minliang Jin: reviewing and editing. Yizhen Wang and Xin Zong: original draft preparation, writing, supervision, validation.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was funded by Zhejiang Provincial Key R&D Program of China (2021C02008), the National Natural Science Foundation of China (Grant Nos. 31630075, 32002185, and 31860648), Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ21C170002).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Yizhen Wang, Email: yzwang321@zju.edu.cn.
Xin Zong, Email: zongxin@zju.edu.cn.
References
- Ang Z., Xiong D., Wu M., Ding J.L. FFAR2-FFAR3 receptor heteromerization modulates short-chain fatty acid sensing. Faseb J. 2018;32:289–303. doi: 10.1096/fj.201700252RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholome A.L., Albin D.M., Baker D.H., Holst J.J., Tappenden K.A. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. Jpen-Parenter Enter. 2004;28:210–222. doi: 10.1177/0148607104028004210. [DOI] [PubMed] [Google Scholar]
- Bartoszek A., Makaro A., Bartoszek A., Kordek R., Fichna J., Salaga M. Walnut oil alleviates intestinal inflammation and restores intestinal barrier function in mice. Nutrients. 2020;12 doi: 10.3390/nu12051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare-Rogers J., Dieffenbacher A., Holm J.V. Lexicon of lipid nutrition. Pure Appl Chem. 2001;73:685–744. [Google Scholar]
- Begley M., Hill C., Gahan C.G.M. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006;72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertevello P.L., De Nardi L., Torrinhas R.S., Logullo A.F., Waitzberg D.L. Partial replacement of omega-6 fatty acids with medium-chain triglycerides, but not olive oil, improves colon cytokine response and damage in experimental colitis. Jpen-Parenter Enter. 2012;36:442–448. doi: 10.1177/0148607111421788. [DOI] [PubMed] [Google Scholar]
- Black D.D. Development and Physiological Regulation of Intestinal Lipid Absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293:G519–G524. doi: 10.1152/ajpgi.00189.2007. [DOI] [PubMed] [Google Scholar]
- Bolognini D., Barki N., Butcher A.J., Hudson B.D., Sergeev E., Molloy C., Moss C.E., Bradley S.J., Le Gouill C., Bouvier M., Tobin A.B., Milligan G. Chemogenetics defines receptor-mediated functions of short chain free fatty acids. Nat Chem Biol. 2019;15:489–498. doi: 10.1038/s41589-019-0270-1. [DOI] [PubMed] [Google Scholar]
- Bolognini D., Tobin A.B., Milligan G., Moss C.E. The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol. 2016;89:388–398. doi: 10.1124/mol.115.102301. [DOI] [PubMed] [Google Scholar]
- Briscoe C.P., Tadayyon M., Andrews J.L., Benson W.G., Chambers J.K., Eilert M.M., Ellis C., Elshourbagy N.A., Goetz A.S., Minnick D.T., Murdock P.R., Sauls H.R., Shabon U., Spinage L.D., Strum J.C., Szekeres P.G., Tan K.B., Way J.M., Ignar D.M., Wilson S., Muir A.I. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., Muir A.I., Wigglesworth M.J., Kinghorn I., Fraser N.J., Pike N.B., Strum J.C., Steplewski K.M., Murdock P.R., Holder J.C., Marshall F.H., Szekeres P.G., Wilson S., Ignar D.M., Foord S.M., Wise A., Dowell S.J. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Burdge G.C., Calder P.C. Introduction to fatty acids and lipids. World Rev Nutr Diet. 2015;112:1–16. doi: 10.1159/000365423. [DOI] [PubMed] [Google Scholar]
- Calder P.C. Is increasing microbiota diversity a novel anti-inflammatory action of marine n-3 fatty acids? J Nutr. 2019;149:1102–1104. doi: 10.1093/jn/nxz043. [DOI] [PubMed] [Google Scholar]
- Castonguay-Paradis S., Lacroix S., Rochefort G., Parent L., Perron J., Martin C., Lamarche B., Raymond F., Flamand N., Di Marzo V., Veilleux A. Dietary fatty acid intake and gut microbiota determine circulating endocannabinoidome signaling beyond the effect of body fat. Sci Rep-Uk. 2020;10 doi: 10.1038/s41598-020-72861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E.B., Martinez-Guryn K. Small intestinal microbiota: the neglected stepchild needed for fat digestion and absorption. Gut Microb. 2019;10:235–240. doi: 10.1080/19490976.2018.1502539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs C.E., Calder P.C., Miles E.A. Diet and immune function. Nutrients. 2019;11 doi: 10.3390/nu11081933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquila T., Hung Y.H., Carreiro A., Buhman K.K. Recent discoveries on absorption of dietary fat: presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim Biophys Acta. 2016;1861:730–747. doi: 10.1016/j.bbalip.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzenne N.M., Neyrinck A.M., Backhed F., Cani P.D. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D.R., Garge N., Zhang X.X., Sun W., O'Connell T.M., Bunger M.K., Bultman S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metabol. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drover V.A., Nguyen D.V., Bastie C.C., Darlington Y.F., Abumrad N.A., Pessin J.E., London E., Sahoo D., Phillips M.C. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem. 2008;283:13108–13115. doi: 10.1074/jbc.M708086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg R.M., Hedemann M.S., Leser T.D., Jensen B.B. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poultry Sci. 2000;79:1311–1319. doi: 10.1093/ps/79.9.1311. [DOI] [PubMed] [Google Scholar]
- Fan G.Q., Li Y.F., Chen J.L., Zong Y.B., Yang X.J. DHA/AA alleviates LPS-induced Kupffer cells pyroptosis via GPR120 interaction with NLRP3 to inhibit inflammasome complexes assembly. Cell Death Dis. 2021;12 doi: 10.1038/s41419-020-03347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C.L., Sun H., Wu J., Niu H.H., Feng J. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J Anim Physiol an N. 2014;98:680–685. doi: 10.1111/jpn.12122. [DOI] [PubMed] [Google Scholar]
- Ferre P., Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007;68:72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- Fuke G., Nornberg J.L. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit Rev Food Sci Nutr. 2017;57:1–7. doi: 10.1080/10408398.2012.716800. [DOI] [PubMed] [Google Scholar]
- Gajda A.M., Storch J. Enterocyte fatty acid-binding proteins (FABPs): different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot Essent Fatty Acids. 2015;93:9–16. doi: 10.1016/j.plefa.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham L.G., Harris-Janz S., Jones P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids. 2011;46:209–228. doi: 10.1007/s11745-010-3524-y. [DOI] [PubMed] [Google Scholar]
- Glatz J.F., Luiken J.J. Fatty acids in cell signaling: historical perspective and future outlook. Prostaglandins Leukot Essent Fatty Acids. 2015;92:57–62. doi: 10.1016/j.plefa.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Grundmann M., Bender E., Schamberger J., Eitner F. Pharmacology of free fatty acid receptors and their allosteric modulators. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22041763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications - a review. Nutr J. 2014;13 doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunness P., Williams B.A., Gerrits W.J.J., Bird A.R., Kravchuk O., Gidley M.J. Circulating triglycerides and bile acids are reduced by a soluble wheat arabinoxylan via modulation of bile concentration and lipid digestion rates in a pig model. Mol Nutr Food Res. 2016;60:642–651. doi: 10.1002/mnfr.201500686. [DOI] [PubMed] [Google Scholar]
- Guo Q., Ye A., Bellissimo N., Singh H., Rousseau D. Modulating fat digestion through food structure design. Prog Lipid Res. 2017;68:109–118. doi: 10.1016/j.plipres.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Hammad S., Pu S., Jones P.J. Current evidence supporting the link between dietary fatty acids and cardiovascular disease. Lipids. 2016;51:507–517. doi: 10.1007/s11745-015-4113-x. [DOI] [PubMed] [Google Scholar]
- Hamosh M., Scow R.O. Lingual lipase and its role in the digestion of dietary lipid. J Clin Invest. 1973;52:88–95. doi: 10.1172/JCI107177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Kimura I., Inoue D., Ichimura A., Hirasawa A. Free fatty acid receptors and their role in regulation of energy metabolism. Rev Physiol Biochem Pharmacol. 2013;164:77–116. doi: 10.1007/112_2013_13. [DOI] [PubMed] [Google Scholar]
- Hillgartner F., Salati L.M., Goodridge A.G. Physiological and molecular mechanisms involved in nutritional regulation of fatty-acid synthesis. Physiol Rev. 1995;75:47–76. doi: 10.1152/physrev.1995.75.1.47. [DOI] [PubMed] [Google Scholar]
- Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. J Pharmacol Sci. 2005;97:125p. doi: 10.1038/nm1168. 125pp. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers T., Hogervorst J.H.C., Garssen J., Wichers H.J., Willemsen L.E.M. Long chain polyunsaturated fatty acids (LCPUFAs) in the prevention of food allergy. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.Q., Wang L., Yi D., Ding B.Y., Chen X., Wang Q.J., Zhu H.L., Liu Y.L., Yin Y.L., Gong J., Wu G.Y. Dietary supplementation with tributyrin alleviates intestinal injury in piglets challenged with intrarectal administration of acetic acid. Br J Nutr. 2014;111:1748–1758. doi: 10.1017/S0007114514000038. [DOI] [PubMed] [Google Scholar]
- Hu R.Z., He Z.Y., Liu M., Tan J.J., Zhang H.F., Hou D.X., He J.H., Wu S.S. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J Anim Sci Biotechnol. 2020;11 doi: 10.1186/s40104-020-00492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B.D., Christiansen E., Tikhonova I.G., Grundmann M., Kostenis E., Adams D.R., Ulven T., Milligan G. Chemically engineering ligand selectivity at the free fatty acid receptor 2 based on pharmacological variation between species orthologs. Faseb J. 2012;26:4951–4965. doi: 10.1096/fj.12-213314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B.D., Due-Hansen M.E., Christiansen E., Hansen A.M., Mackenzie A.E., Murdoch H., Pandey S.K., Ward R.J., Marquez R., Tikhonova I.G., Ulven T., Milligan G. Defining the molecular basis for the first potent and selective orthosteric agonists of the FFA2 free fatty acid receptor. J Biol Chem. 2013;288:17296–17312. doi: 10.1074/jbc.M113.455337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B.D., Smith N.J., Milligan G. Experimental challenges to targeting poorly characterized GPCRs: uncovering the therapeutic potential for free fatty acid receptors. Adv Pharmacol. 2011;62:175–218. doi: 10.1016/B978-0-12-385952-5.00006-3. [DOI] [PubMed] [Google Scholar]
- Ichimura A., Hasegawa S., Kasubuchi M., Kimura I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H., Tanaka H., Maruyama M., Satoh R., Okubo S., Kizawa H., Komatsu H., Matsumura F., Noguchi Y., Shinobara T., Hinuma S., Fujisawa Y., Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Jacobi S.K., Lin X., Corl B.A., Hess H.A., Harrell R.J., Odle J. Dietary arachidonate differentially alters desaturase-elongase pathway flux and gene expression in liver and intestine of suckling pigs. J Nutr. 2011;141:548–553. doi: 10.3945/jn.110.127118. [DOI] [PubMed] [Google Scholar]
- Jia M.Y., Zhang Y.C., Gao Y.Q., Ma X. Effects of medium chain fatty acids on intestinal animals health of monogastric animals. Curr Protein Pept Sci. 2020;21:777–784. doi: 10.2174/1389203721666191231145901. [DOI] [PubMed] [Google Scholar]
- Katsuma S., Hatae N., Yano T., Ruike Y., Kimura M., Hirasawa A., Tsujimoto G. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J Biol Chem. 2005;280:19507–19515. doi: 10.1074/jbc.M412385200. [DOI] [PubMed] [Google Scholar]
- Kien C.L., Blauwiekel R., Bunn J.Y., Jetton T.L., Frankel W.L., Holst J.J. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J Nutr. 2007;137:916–922. doi: 10.1093/jn/137.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S., Kobayashi M., Hirasawa A., Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) P Natl Acad Sci USA. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knarreborg A., Simon M.A., Engberg R.M., Jensen B.B., Tannock G.W. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl Environ Microbiol. 2002;68:5918–5924. doi: 10.1128/AEM.68.12.5918-5924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Iwasaki A., Matsuoka K., Fujita T. Effect of mechanical agitation on cationic liposome transport across an unstirred water layer in caco-2 cells. Biol Pharm Bull. 2016;39:1293–1299. doi: 10.1248/bpb.b16-00050. [DOI] [PubMed] [Google Scholar]
- Korjamo T., Heikkinen A.T., Monkkonen J. Analysis of unstirred water layer in in vitro permeability experiments. J Pharmacol Sci. 2009;98:4469–4479. doi: 10.1002/jps.21762. [DOI] [PubMed] [Google Scholar]
- Lagerstrom M.C., Schioth H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- Latymer E.A., Keal H.D., Low A.G. Absorption and metabolism of [U-14C] acetic acid in growing pigs. Anim Prod. 2010;52:331–336. [Google Scholar]
- Lauridsen C. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J Anim Sci. 2020;98 doi: 10.1093/jas/skaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E., Loison C., Struyf S., Springael J.Y., Lannoy V., Decobecq M.E., Brezillon S., Dupriez V., Vassart G., Van Damme J., Parmentier M., Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- Lee S.I., Kang K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci Rep-Uk. 2017;7 doi: 10.1038/s41598-017-16561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.U., In H.J., Kwon M.S., Park B.O., Jo M., Kim M.O., Cho S., Lee S., Lee H.J., Kwak Y.S., Kim S. Beta-arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-kappa B. Biol Pharm Bull. 2013;36:1754–1759. doi: 10.1248/bpb.b13-00312. [DOI] [PubMed] [Google Scholar]
- Lei X.J., Park J.W., Baek D.H., Kim J.K., Kim I.H. Feeding the blend of organic acids and medium chain fatty acids reduces the diarrhea in piglets orally challenged with enterotoxigenic Escherichia coli K88. Anim Feed Sci Technol. 2017;224:46–51. [Google Scholar]
- Levitt M.D., Furne J.K., Strocchi A., Anderson B.W., Levitt D.G. Physiological measurements of luminal stirring in the dog and human small bowel. J Clin Invest. 1990;86:1540–1547. doi: 10.1172/JCI114873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. Antibiotic growth promoters enhance animal production by targeting intestinal bile salt hydrolase and its producers. Front Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou A.P., Paziuk M., Luevano J.M., Machineni S., Turnbaugh P.J., Kaplan L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.L. Fatty acids, inflammation and intestinal health in pigs. J Anim Sci Biotechnol. 2015;6 doi: 10.1186/s40104-015-0040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.L., Chen F., Odle J., Lin X., Jacobi S.K., Zhu H.L., Wu Z.F., Hou Y.Q. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J Nutr. 2012;142:2017–2024. doi: 10.3945/jn.112.164947. [DOI] [PubMed] [Google Scholar]
- Lopez-Colom P., Castillejos L., Rodriguez-Sorrento A., Puyalto M., Mallo J.J., Martin-Orue S.M. Impact of in-feed sodium butyrate or sodium heptanoate protected with medium-chain fatty acids on gut health in weaned piglets challenged with Escherichia coli F4(+) Arch Anim Nutr. 2020;74:271–295. doi: 10.1080/1745039X.2020.1726719. [DOI] [PubMed] [Google Scholar]
- Maldonado-Valderrama J., Wilde P., Macierzanka A., Mackie A. The role of bile salts in digestion. Adv Colloid Interface Sci. 2011;165:36–46. doi: 10.1016/j.cis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Mansbach C.M., 2nd, Gorelick F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastrointest Liver Physiol. 2007;293:G645–G650. doi: 10.1152/ajpgi.00299.2007. [DOI] [PubMed] [Google Scholar]
- Marinissen M.J., Gutkind J.S. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- Mascolo N., Rajendran V.M., Binder H.J. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 1991;101:331–338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Mcgarry J.D., Foster D.W. Regulation of hepatic fatty-acid oxidation and ketone-body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Miyamoto J., Igarashi M., Watanabe K., Karaki S., Mukouyama H., Kishino S., Li X., Ichimura A., Irie J., Sugimoto Y., Mizutani T., Sugawara T., Miki T., Ogawa J., Drucker D.J., Arita M., Itoh H., Kimura I. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat Commun. 2019;10 doi: 10.1038/s41467-019-11978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozes S., Bujnakova D., Sefcikova Z., Kmet V. Developmental changes of gut microflora and enzyme activity in rat pups exposed to fat-rich diet. Obesity. 2008;16:2610–2615. doi: 10.1038/oby.2008.435. [DOI] [PubMed] [Google Scholar]
- Mujico J.R., Baccan G.C., Gheorghe A., Diaz L.E., Marcos A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110:711–720. doi: 10.1017/S0007114512005612. [DOI] [PubMed] [Google Scholar]
- Nilsson N.E., Kotarsky K., Owman C., Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- Offermanns S. Free fatty acid (FFA) and hydroxy carboxylic acid (HCA) receptors. Annu Rev Pharmacol. 2014;54:407–434. doi: 10.1146/annurev-pharmtox-011613-135945. [DOI] [PubMed] [Google Scholar]
- Olson J.A. Lipid metabolism. Annu Rev Biochem. 1966;35:559. doi: 10.1146/annurev.bi.35.070166.003015. [DOI] [PubMed] [Google Scholar]
- Papada E., Kaliora A.C., Gioxari A., Papalois A., Forbes A. Anti-inflammatory effect of elemental diets with different fat composition in experimental colitis. Br J Nutr. 2014;111:1213–1220. doi: 10.1017/S0007114513003632. [DOI] [PubMed] [Google Scholar]
- Patterson R., Connor M.L., Krause D.O., Nyachoti C.M. Response of piglets weaned from sows fed diets supplemented with conjugated linoleic acid (CLA) to an Escherichia coli K88(+) oral challenge. Animal. 2008;2:1303–1311. doi: 10.1017/S1751731108002309. [DOI] [PubMed] [Google Scholar]
- Poteres E., Hubert N., Poludasu S., Brigando G., Moore J., Keeler K., Isabelli A., Ibay I.C.V., Alt L., Pytynia M., Ciancio M., Martinez-Guryn K. Selective regional alteration of the gut microbiota by diet and antibiotics. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo P., Matern D. Fatty acid oxidation disorders. Annu Rev Physiol. 2002;64:477–502. doi: 10.1146/annurev.physiol.64.082201.154705. [DOI] [PubMed] [Google Scholar]
- Sabesin S.M., Frase S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J Lipid Res. 1977;18:496–511. [PubMed] [Google Scholar]
- Sallee V.L., Dietschy J.M. Determinants of intestinal mucosal uptake of short- and medium-chain fatty acids and alcohols. J Lipid Res. 1973;14:475–484. [PubMed] [Google Scholar]
- Sam Q.H., Ling H., Yew W.S., Tan Z.H., Ravikumar S., Chang M.W., Chai L.Y.A. The divergent immunomodulatory effects of short chain fatty acids and medium chain fatty acids. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22126453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K., Hammer R.E., Williams S.C., Crowley J., Yanagisawa M., Gordon J.I. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. P Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K., Hammer R.E., Williams S.C., Crowley J., Yanagisawa M., Gordon J.I. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaioli E., Liverani E., Belluzzi A. The imbalance between n-6/n-3 polyunsaturated fatty acids and inflammatory bowel disease: a comprehensive review and future therapeutic perspectives. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18122619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheppach W., Sommer H., Kirchner T., Paganelli G.M., Bartram P., Christl S., Richter F., Dusel G., Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative-colitis. Gastroenterology. 1992;103:51–56. doi: 10.1016/0016-5085(92)91094-k. [DOI] [PubMed] [Google Scholar]
- Schonfeld P., Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57:943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefcikova Z., Kmet V., Bujnakova D., Racek L., Mozes S. Development of gut microflora in obese and lean rats. Folia Microbiol. 2010;55:373–375. doi: 10.1007/s12223-010-0061-2. [DOI] [PubMed] [Google Scholar]
- Sekikawa A., Mahajan H., Kadowaki S., Hisamatsu T., Miyagawa N., Fujiyoshi A., Kadota A., Maegawa H., Murata K., Miura K., Edmundowicz D., Ueshima H., Grp S.R. Association of blood levels of marine omega-3 fatty acids with coronary calcification and calcium density in Japanese men. Eur J Clin Nutr. 2019;73:783–792. doi: 10.1038/s41430-018-0242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H., Shachar S., Sekler I., Hershfinkel M., Walker M.D. Role of GPR40 in fatty acid action on the beta cell line INS-1E. Biochem Bioph Res Co. 2005;335:97–104. doi: 10.1016/j.bbrc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Stahl A., Gimeno R.E., Tartaglia L.A., Lodish H.F. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol Metabol. 2001;12:266–273. doi: 10.1016/s1043-2760(01)00427-1. [DOI] [PubMed] [Google Scholar]
- Stein J., Zores M., Schroder O. Short-chain fatty acid (SCFA) uptake into Caco-2 cells by a pH-dependent and carrier mediated transport mechanism. Eur J Nutr. 2000;39:121–125. doi: 10.1007/s003940070028. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Staffer S., Wannhoff A., Pathil A. The overall fatty acid absorption controlled by basolateral chylomicron excretion under regulation of p-JNK1. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:917–928. doi: 10.1016/j.bbalip.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Swick B. Recent advances in animal nutrition - Australia 2019 foreword. Anim Prod Sci. 2019;59:I. [I] [Google Scholar]
- Thomson A.B., Schoeller C., Keelan M., Smith L., Clandinin M.T. Lipid absorption: passing through the unstirred layers, brush-border membrane, and beyond. Can J Physiol Pharmacol. 1993;71:531–555. doi: 10.1139/y93-078. [DOI] [PubMed] [Google Scholar]
- Thomson A.B.R., Dietschy J.M. Springer; Berlin Heidelberg: 1984. The role of the unstirred water layer in intestinal permeation. Place: Published. [Google Scholar]
- Tsuji A. Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems. NeuroRx. 2005;2:54–62. doi: 10.1602/neurorx.2.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya S.D., Yun H.M., Lee K.Y., Kim I.H. Do varied dietary omega-6 to omega-3 ratios affect the performance, nutrient digestibility, immune status and faecal microbiota of weaner pigs? Anim Prod Sci. 2019;59:236–242. [Google Scholar]
- van Tilbeurgh H., Bezzine S., Cambillau C., Verger R., Carriere F. Colipase: structure and interaction with pancreatic lipase. Biochim Biophys Acta. 1999;1441:173–184. doi: 10.1016/s1388-1981(99)00149-3. [DOI] [PubMed] [Google Scholar]
- Vijay N., Morris M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharmaceut Des. 2014;20:1487–1498. doi: 10.2174/13816128113199990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A., Gutknecht J. Monocarboxylic acid permeation through lipid bilayer membranes. J Membr Biol. 1984;77:255–264. doi: 10.1007/BF01870573. [DOI] [PubMed] [Google Scholar]
- Wang J., Lu J.X., Xie X.W., Xiong J., Huang N.N., Wei H.K., Jiang S.W., Peng J. Blend of organic acids and medium chain fatty acids prevents the inflammatory response and intestinal barrier dysfunction in mice challenged with enterohemorrhagic Escherichia coli O157:H7. Int Immunopharm. 2018;58:64–71. doi: 10.1016/j.intimp.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Wang T.Y., Liu M., Portincasa P., Wang D.Q. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur J Clin Invest. 2013;43:1203–1223. doi: 10.1111/eci.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen K.A., McCullough M.L., Flanders W.D., Hartman T.J., Judd S., Bostick R.M. Paleolithic and mediterranean diet pattern scores are inversely associated with biomarkers of inflammation and oxidative balance in adults. J Nutr. 2016;146:1217–1226. doi: 10.3945/jn.115.224048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Cheng Y., Fu J., Lu Z., Wang F., Jin M., Zong X., Wang Y. Gut immunity and microbiota dysbiosis are associated with altered bile acid metabolism in LPS-challenged piglets. Oxid Med Cell Longev. 2021:6634821. doi: 10.1155/2021/6634821. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Innis S.M. Genetic variants of the FADS1 FADS2 gene cluster Are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr. 2008;138:2222–2228. doi: 10.3945/jn.108.096156. [DOI] [PubMed] [Google Scholar]
- Xiong H.T., Guo B.X., Gan Z.S., Song D.G., Lu Z.Q., Yi H.B., Wu Y.M., Wang Y.Z., Du H.H. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci Rep-Uk. 2016;6 doi: 10.1038/srep27070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y.M., Miyamoto N., Shibata K., Valasek M.A., Motoike T., Kedzierski R.M., Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. P Natl Acad Sci USA. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.T., Chen Y.J., Huang C.W., Wang Y.C., Mersmann H.J., Wang P.H., Ding S.T. Docosahexaenoic acid suppresses expression of adipogenic tetranectin through sterol regulatory element-binding protein and forkhead box O protein in pigs. Nutrients. 2021;13 doi: 10.3390/nu13072315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C.L., Nelson D.W., Yen M.I. Intestinal triacylglycerol synthesis in fat absorption and systemic energy metabolism. J Lipid Res. 2015;56:489–501. doi: 10.1194/jlr.R052902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R., Strauss J.G., Haemmerle G., Lass A., Zimmermann R. Lipolysis: pathway under construction. Curr Opin Lipidol. 2005;16:333–340. doi: 10.1097/01.mol.0000169354.20395.1c. [DOI] [PubMed] [Google Scholar]
- Zentek J., Buchheit-Renko S., Ferrara F., Vahjen W., Van Kessel A.G., Pieper R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim Health Res Rev. 2011;12:83–93. doi: 10.1017/S1466252311000089. [DOI] [PubMed] [Google Scholar]
- Zhou Z.X., Huang J., Hao H.H., Wei H.K., Zhou Y.F., Peng J. Applications of new functions for inducing host defense peptides and synergy sterilization of medium chain fatty acids in substituting in-feed antibiotics. J Funct Foods. 2019;52:348–359. [Google Scholar]
- Zolezzi J.M., Inestrosa N.C. Peroxisome proliferator-activated receptors and alzheimer's disease: hitting the blood-brain barrier. Mol Neurobiol. 2013;48:438–451. doi: 10.1007/s12035-013-8435-5. [DOI] [PubMed] [Google Scholar]
- Zong X., Cao X.X., Wang H., Xiao X., Wang Y.Z., Lu Z.Q. Cathelicidin-WA facilitated intestinal fatty acid absorption through enhancing PPAR-gamma dependent barrier function. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X., Fu J., Xu B.C., Wang Y.Z., Jin M.L. Interplay between gut microbiota and antimicrobial peptides. Anim Nutr. 2020;6:389–396. doi: 10.1016/j.aninu.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X., Zhao J., Wang H., Lu Z.Q., Wang F.Q., Du H.H., Wang Y.Z. Mettl3 deficiency sustains long-chain fatty acid absorption through suppressing traf6-dependent inflammation response. J Immunol. 2019;202:567–578. doi: 10.4049/jimmunol.1801151. [DOI] [PMC free article] [PubMed] [Google Scholar]