Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has already affected millions worldwide. The emergence of multiple SARS-CoV-2 variants may pose a significant threat to our efforts in controlling the pandemic. The impact of SARS-CoV-2 variants on the efficacy of available vaccines, therapeutics, and diagnostics is currently being investigated. SARS-CoV-2 has been implicated to be originated from animals due to cross-species jumping and raises zoonotic concerns due to the potential for reintroduction into the human populations via interspecies transmission between humans and animals. Natural SARS-CoV-2 infections have been reported in domestic animals (dog, cat, and ferret), captive animals (tiger, lion, snow leopard, puma, otter, and gorilla), and wild and farmed minks. Vaccination of domestic animals can prevent the possible introduction of SARS-CoV-2 into the feral population and subsequent transmission to wildlife. Although the need to vaccinate susceptible animal species, such as cats, minks, and great apes, might seem irrational from a public health standpoint, the successful elimination of SARS-CoV-2 will only be possible by controlling the transmission in all susceptible animal species. This is necessary to prevent the re-emergence of SARS-CoV-2 in the future.
Keywords: COVID-19, SARS-CoV-2, Vaccination, Wildlife, Re-emergence, Travel restriction
1. Introduction
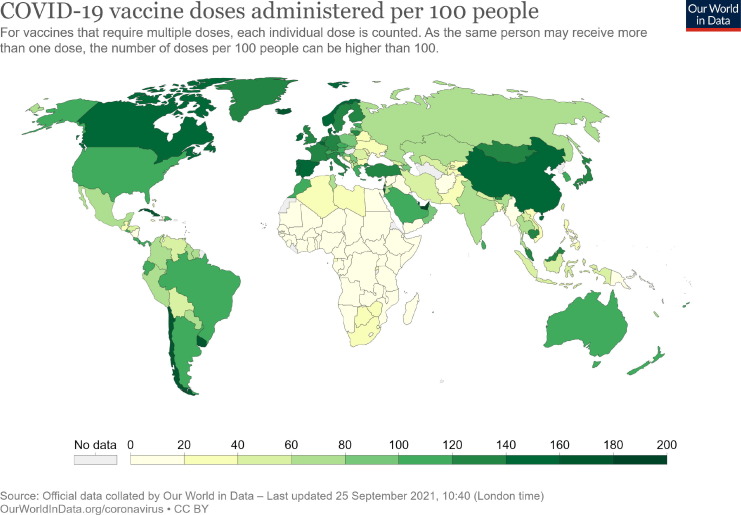
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has already affected more than 230 million1 individuals, causing over 4.7 million1 deaths worldwide as of September 24, 2021. International travel via air transport played an important role in the initial spread of COVID-19 across borders [1]. Although the initial preventive measures, such as lockdowns, travel restrictions, and the establishment of active surveillance frameworks at airports and seaports, were able to control the rapid rise in COVID-19 cases, these measures were not considered to be a permanent solution [1]. The successful rollout of several vaccine candidates worldwide following phase 3 clinical trials has given great hope for controlling this pandemic. However, we are far from achieving global vaccination coverage due to the lack of equitable access to vaccines, especially in low-income countries [2]. Even under ideal conditions, it will take years to achieve a significant level of global immunity through vaccination. The number of COVID-19 vaccine doses administered per 100 people in different countries is represented in Fig. 1 . Countries like the United Arab Emirates, China, the United Kingdom, Germany, and the United States of America are at the top of this list.2
Fig. 1.
Total number of vaccination doses administered per 100 people in the total population. The total number of vaccination doses administered per 100 people vary significantly across the world: United Arab Emirates (197.93), Israel (168.84), China (151.95), India (60.83), USA (115.53), Canada (146.31), and United Kingdom (136.86). The values do not represent the number of people vaccinated since several available COVID-19 vaccines require multiple doses. Source: Official data collected by “Our World in Data” - last updated on September 24, 2021. Available at: https://ourworldindata.org/covid-vaccinations.
The emergence of multiple SARS-CoV-2 variants, including the mink-associated variant, Delta (B.1.617.2), and Delta Plus (AY.1 or B.1.617.2.1) variants, may pose a significant threat to our efforts in controlling the pandemic [3], [4], [5], [6]. Preliminary findings indicate that the mink-associated variant (Y453F mutation) has the potential to escape detection by four of the commercial neutralizing monoclonal antibodies, partially due to weak recognition of the spike glycoproteins [7]. In addition, these mink-associated SARS-CoV-2 variants accounted for 40% of the total COVID-19 cases in humans initially in the Netherlands. However, they were found to be less lethal and infective compared to those identified in humans [8]. The SARS-CoV-2 Delta variant caused a rapid surge of cases during the second COVID-19 pandemic wave in India during April-June 2021 [9]. The Delta variant has also been detected from several countries across the globe, initiating a third wave [10]. The recent reports of the emergence of Delta Plus variants further raise concerns in terms of transmissibility and disease severity. Furthermore, the Delta Plus variant had a high prevalence of mutations in Spike (G142D, A222V, and T95I) compared to the Delta variant. Some of these mutations in the Spike (K417N, V70F, and W258L) were exclusive for the Delta Plus variant [6]. The impact of SARS-CoV-2 variants on the efficacy of available vaccines, therapeutics, and diagnostics is currently being investigated. The emergence of novel variants of concern (VOC) may warrant the need to update COVID-19 vaccines in the future as few mutations could render variants to be less optimally targeted by the presently available vaccines. However, some of these vaccines have been found to protect from the emerging variants but with variable efficacies [5], [11], [12].
Animals might have played a crucial role in the COVID-19 pandemic. In addition, SARS-CoV-2 has been implicated to be originated from animals, animal spillover events, cross-species jumping, zooanthroponotic transmission, and raises zoonotic concerns due to the potential for reintroduction into the human populations via interspecies transmission between humans and animals [4], [13], [14], [15]. Therefore, it is necessary to perform in-depth investigations to explore the interrelationships between animals and humans for disease transmission, spread, and adopt appropriate prevention and control strategies following interdisciplinary and holistic approaches [13], [14], [15], [16], [17], [18]. In addition, the key binding sites on the human ACE2 receptor are most conserved in the primates (especially Cercopithecoidae and Hominidae families) [19]. Furthermore, the ACE2 sites are highly conserved in some species belonging to Carnivora, Lagomorpha, Proboscidea, Artiodactyla, Chiroptera, Rodentia, Sirenia, and Perissodactyla orders indicating a potential for spillover SARS-CoV-2 infection from humans [19].
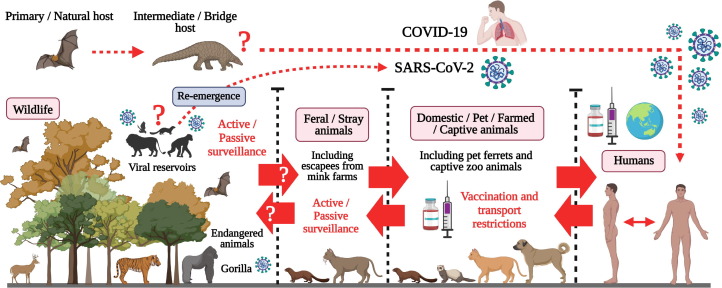
According to the World Organisation for Animal Health (OIE) database, natural SARS-CoV-2 infections have been reported in domestic animals (dog, cat, and ferret), captive animals (tiger, lion, snow leopard, puma, otter, and gorilla), and wild and farmed minks (Supplementary Table 1) [20], [21]. The increasing reports of SARS-CoV-2 infection in domestic and captive wild animals can be linked to the human-to-animal transmission of the virus [18], [21], [22], [23], [24]. However, infected domestic animals could transmit the virus to susceptible wild animal species, thereby establishing a reservoir of SARS-CoV-2 (Fig. 2 ) [18], [25]. Circulation of SARS-CoV-2 in wild animal populations could contribute to the occasional re-emergence of COVID-19 in humans at the human-animal interface.
Fig. 2.
Illustrates the potential route for establishing a viral reservoir host of SARS-CoV-2 in wildlife and the strategies to counter it. There is a need to establish active and passive surveillance systems to identify interspecies transmission of SARS-CoV-2 among wild animal species and feral animals. This will ensure the early identification and control of possible re-emergence of SARS-CoV-2 at the human-animal interface. In addition, the implementation of mandatory SARS-CoV-2 vaccination and transport restriction will further help prevent infection in susceptible animal species (domestic/pet/captive animals). Created with BioRender.com.
Although cats and dogs do not play a major role in the maintenance and/or transmission of SARS-CoV-2 to humans, experimental studies have confirmed the potential of cat-to-cat viral transmission [26], [27]. Therefore, vaccination of domestic cats can prevent the possible introduction of SARS-CoV-2 into the feral cat population and subsequent transmission to wildlife. The LinearDNA™ COVID-19 vaccine candidate is currently being developed through a joint effort between Applied DNA Sciences (United States) and EvviVax (Italy) for use in cats. The vaccine received regulatory approval from the United States Department of Agriculture (USDA) to advance to clinical trials for evaluating the safety and immunogenicity of the vaccine in domestic felines [28]. The preliminary analysis indicates that the vaccine candidate is well tolerated and effective in adult domestic felines. In addition, a single dose of vaccine induced high titers of SARS-CoV-2 neutralizing antibodies in cats [29]. Thus, the successful immunization of felines with this vaccine should help prevent feline SARS-CoV-2 infections and prevent onward transmission to their human owners, although cat-to-human transmission is unlikely to occur.
The captive orangutans and bonobos at the San Diego Zoo in the United States of America became the first non-human primates to receive an experimental COVID-19 vaccine developed specifically for animals by the veterinary pharmaceutical company Zoetis [20]. This move was triggered by the confirmation of SARS-CoV-2 infection in a troop of eight gorillas. Although this experimental vaccine was specifically developed for dogs and cats, minks were also included at the later stage because of their higher susceptibility and transmission potential for SARS-CoV-2. Its efficacy in dogs and cats has been demonstrated in preliminary studies [30]. The vaccine serology study was conducted in five animals from each group (placebo and vaccine formulated with two different adjuvants). The vaccinated animals mounted a robust antibody response against the SARS-CoV-2 Spike antigen [31]. Zoetis is currently discussing with the USDA for obtaining conditional approval for using the vaccine in animals [30]. They are also donating more than 11,000 doses of SARS-CoV-2 vaccines to immunize the captive mammals of several zoos, conservatories, and sanctuaries. The captive animals like hyenas, chimpanzees, and mountain lions have already received the first dose of the experimental vaccine developed by Zoetis [32]. In addition, several COVID-19 vaccine candidates already being used in humans underwent in vivo studies in animal models, such as rhesus macaques, ferrets, and hamsters [26]. The translation of the findings from these in vivo studies to other animal species will be easy, as the fundamentals of the vaccine platforms will remain the same, and mainly the antigen dose will need to be changed. Russia has recently registered the world's first SARS-CoV-2 vaccine for animals, Carnivac-Cov (inactivated vaccine). The vaccine has undergone clinical trials in dogs, cats, foxes, and minks, following which it was given regulatory approval for mass production [33]. Based on the findings of clinical trials, vaccination with Carnivac-Cov induced immunity that lasted for at least six months after the vaccination [34]. Such a vaccine will protect the susceptible animals and help prevent the emergence of novel SARS-CoV-2 variants as a result of viral mutations [35].
The concept of “vaccination passport” is now becoming a hot topic of debate [36]. A vaccination passport provides proof of immunization and enables an individual to travel freely. This is entirely different from an “immunity passport,” which documents past infection [37]. The vaccination passport would be a more practical substitute for an immunity passport, as vaccination is a better correlate of protection. Several countries have already implemented vaccination passports to exempt travelers from testing and quarantine requirements [36]. A similar strategy should be implemented to transport susceptible animal species across international borders, especially domestic and captive felines and endangered species. In addition to the COVID-19 vaccine rollout in humans, developing specific SARS-CoV-2 vaccines for animals along with strengthening of one health approaches should be considered as a vital part of pandemic recovery and future preparedness plans for countering pandemics of human-animal interface involvement [4], [18], [25], [35].
Although the need to vaccinate susceptible animal species, such as cats, minks, and great apes, might seem irrational from a public health standpoint, the successful elimination of SARS-CoV-2 will only be possible by controlling the transmission in all susceptible animal species. This is necessary to prevent the re-emergence of SARS-CoV-2 in the future. Furthermore, the current circumstances warrant the need to implement a mandatory vaccine passport to transport domestic and captive/wild animals across international borders. Such a move will further strengthen our efforts to prevent the introduction of SARS-CoV-2 into regional domestic/wild animal populations and its subsequent re-emergence. In addition to implementing stringent transport restrictions across international borders, active or passive surveillance frameworks must be established for domestic, captive, and wild animals while adopting the one health approach at a wider level to limit the circulation of SARS-CoV-2 in domestic and wild animal species. This will also prevent the possible emergence of novel variants such as the SARS-CoV-2 mink variant at the human-animal interface, facilitating the successful prevention and control of this pandemic.
Funding
The authors received no funding in relation to this article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.10.053.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Sharun K., Tiwari R., Natesan S., Yatoo M.I., Malik Y.S., Dhama K. International travel during the COVID-19 pandemic: implications and risks associated with 'travel bubbles'. J Travel Med. 2020;27(8):taaa184. doi: 10.1093/jtm/taaa184. PMID: 33009813; PMCID: PMC7665670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharun K., Dhama K. COVID-19 vaccine diplomacy and equitable access to vaccines amid ongoing pandemic. Arch Med Res. 2021;52(7):761–763. doi: 10.1016/j.arcmed.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauring A.S., Hodcroft E.B. Genetic variants of SARS-CoV-2-what do they mean? JAMA. 2021;325(6):529–531. doi: 10.1001/jama.2020.27124. PMID: 33404586. [DOI] [PubMed] [Google Scholar]
- 4.Sharun K., Tiwari R., Natesan S., Dhama K. SARS-CoV-2 infection in farmed minks, associated zoonotic concerns, and importance of the One Health approach during the ongoing COVID-19 pandemic. Vet Q. 2021;41(1):50–60. doi: 10.1080/01652176.2020.1867776. PMID: 33349165; PMCID: PMC7833041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raman R., Patel K.J., Ranjan K. COVID-19: unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies. Biomolecules. 2021;11(7):993. doi: 10.3390/biom11070993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kannan S.R., Spratt A.N., Cohen A.R., Naqvi S.H., Chand H.S., Quinn T.P., et al. Evolutionary analysis of the Delta and Delta Plus variants of the SARS-CoV-2 viruses. J Autoimmun. 2021;124:102715. doi: 10.1016/j.jaut.2021.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi T., Yaegashi N., Konishi I. Effect of RBD mutation (Y453F) in spike glycoprotein of SARS-CoV-2 on neutralizing antibody affinity. bioRxiv. 2020 doi: 10.1101/2020.11.27.401893. [DOI] [PubMed] [Google Scholar]
- 8.Konishi T. SARS-CoV-2 mutations among minks show reduced lethality and infectivity to humans. PLoS One. 2021;16(5):e0247626. doi: 10.1371/journal.pone.0247626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tareq A.M., Emran T.B., Dhama K., Dhawan M., Tallei T.E. Impact of SARS-CoV-2 delta variant (B16172) in surging second wave of COVID-19 and efficacy of vaccines in tackling the ongoing pandemic. Hum Vaccin Immunother. 2021:1–2. doi: 10.1080/21645515.2021.1963601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallapaty S. Delta threatens rural regions that dodged earlier COVID waves. Nature. 2021;596(7872):325–326. doi: 10.1038/d41586-021-02146-w. PMID: 34354278. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi T., Malik J.A., Mulla A.H., Al Hagbani T., Almansour K., Ubaid M.A., Alghamdi S., Anwar S. An overview of SARS-COV-2 epidemiology, mutant variants, vaccines, and management strategies. J Infect Public Health. 2021 doi: 10.1016/j.jiph.2021.08.014. S1876-0341(21)00232-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharun K., Tiwari R., Dhama K., Emran T.B., Rabaan A.A., Al Mutair A. Emerging SARS-CoV-2 variants: impact on vaccine efficacy and neutralizing antibodies. Hum Vaccin Immunother. 2021:1–4. doi: 10.1080/21645515.2021.1923350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhama K., Patel S.K., Sharun K., Pathak M., Tiwari R., Yatoo M.I., et al. SARS-CoV-2 jumping the species barrier: Zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel Med Infect Dis. 2020;37:101830. doi: 10.1016/j.tmaid.2020.101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari R., Dhama K., Sharun K., Iqbal Yatoo M., Malik Y.S., Singh R., et al. COVID-19: animals, veterinary and zoonotic links. Vet Q. 2020;40(1):169–182. doi: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee A., Mossman K., Baker M.L. Zooanthroponotic potential of SARS-CoV-2 and implications of reintroduction into human populations. Cell Host Microbe. 2021;29(2):160–164. doi: 10.1016/j.chom.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerrini A. Animals, vaccines, and COVID-19. Endeavour. 2021;45(3):100779. doi: 10.1016/j.endeavour.2021.100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korath A.D.J., Janda J., Untersmayr E., Sokolowska M., Feleszko W., Agache I., et al. One Health: EAACI Position Paper on coronaviruses at the human-animal interface, with a specific focus on comparative and zoonotic aspects of SARS-Cov-2. Allergy. 2021 doi: 10.1111/all.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharun K., Dhama K., Pawde A.M., Gortázar C., Tiwari R., Bonilla-Aldana D.K., et al. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Vet Q. 2021;41(1):181–201. doi: 10.1080/01652176.2021.1921311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Y., Aris P., Farookhi H., Xia X. Predicting mammalian species at risk of being infected by SARS-CoV-2 from an ACE2 perspective. Sci Rep. 2021;11(1):1702. doi: 10.1038/s41598-020-80573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daly N. (Updated March 3, 2021). First great apes at U.S. zoo receive COVID-19 vaccine made for animals. Available at: https://www.nationalgeographic.com/animals/article/first-great-apes-at-us-zoo-receive-coronavirus-vaccine-made-for-animals [accessed on: March 7, 2021].
- 21.OIE, (Updated February 23, 2021). Events in animals. Available at: https://www.oie.int/en/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/events-in-animals/ [accessed on: March 7, 2021].
- 22.Oude Munnink B.B., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371(6525):172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamer S.A., Ghai R.R., Zecca I.B., Auckland L.D., Roundy C.M., Davila E., et al. SARS-CoV-2 B.1.1.7 variant of concern detected in a pet dog and cat after exposure to a person with COVID-19, USA. Transbound Emerg Dis. 2021 doi: 10.1111/tbed.14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra Anamika, Kumar Naveen, Bhatia Sandeep, Aasdev Ashutosh, Kanniappan Sridhar, Sekhar Abelraj Thaya, et al. SARS-CoV-2 Delta Variant among Asiatic Lions, India. Emerg Infect Dis. 2021;27(10):2723–2725. doi: 10.3201/eid2710.211500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delahay R.J., de la Fuente J., Smith G.C., Sharun K., Snary E.L., Flores Girón L., et al. Assessing the risks of SARS-CoV-2 in wildlife. One Health Outlook. 2021;3(1) doi: 10.1186/s42522-021-00039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharun Khan, Tiwari Ruchi, Patel Shailesh Kumar, Karthik Kumaragurubaran, Iqbal Yatoo Mohd, Malik Yashpal S., et al. Coronavirus disease 2019 (COVID-19) in domestic animals and wildlife: advances and prospects in the development of animal models for vaccine and therapeutic research. Hum Vaccin Immunother. 2020;16(12):3043–3054. doi: 10.1080/21645515.2020.1807802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharun K., Saied A.A., Tiwari R., Dhama K. SARS-CoV-2 infection in domestic and feral cats: current evidence and implications. Vet Q. 2021:1–7. doi: 10.1080/01652176.2021.1962576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brook S. (Updated November 30, 2020). Applied DNA, EvviVax, and GVS Receive Regulatory Approval to Conduct Veterinary Clinical Trial for Linear COVID-19 Vaccine Candidate. Available at: https://www.businesswire.com/news/home/20201130005340/en/Applied-DNA-EvviVax-and-GVS-Receive-Regulatory-Approval-to-Conduct-Veterinary-Clinical-Trial-for-Linear-COVID-19-Vaccine-Candidate [accessed on: March 7, 2021].
- 29.Applied DNA Sciences. Applied DNA and Evvivax Announce Positive Preliminary Results of Phase I Clinical Trial for LinearDNA™ COVID-19 Vaccine Candidate in Felines. Available at: https://adnas.com/feline-clinical-trial-preliminary-results/ [accessed on 30 August 2021].
- 30.Zoetis, (Updated January 29, 2021). Zoetis' Emerging Infectious Disease Capabilities Support COVID-19 Solutions for Great Apes and Minks. Available at: https://www.zoetis.com/news-and-media/feature-stories/posts/zoetis-emerging-infectious-disease-capabilities-support-covid-19-solutions-for-great-apes-and-minks.aspx [accessed on: March 7, 2021].
- 31.Wappel S, Hainer N, Horst HV, Hutchinson K, King V, Dunham S, et al. Efficacy of a SARS-CoV-2 recombinant vaccine via serologic response in cats and dogs. Available at: https://www.zoetis.com/_locale-assets/pdf/innovation/sars-cov-2-vaccine-world-one-health-congress-poster070521.pdf [accessed on 30 August 2021].
- 32.Daly N. (Updated August 20, 2021). Bears, baboons, tigers are getting COVID vaccines at zoos across the U.S. Available at: https://www.nationalgeographic.com/animals/article/bears-baboons-tigers-are-getting-covid-vaccines-at-zoos-across-the-us [accessed on: September 25, 2021].
- 33.Tass, (Updated March 31, 2021). Russia registers world's first anti-coronavirus vaccine for animals. Available at: https://tass.com/world/1272331 [accessed on: April 5, 2021].
- 34.WION Web Team Russia Produces First Batch of COVID-19 Vaccine for Animals. In: WION. [accessed on 14 May 2021]; 2021 Available online: https://www.wionews.com/world/russia-produces-first-batch-of-covid-19-vaccine-for-animals-381651.
- 35.Chavda V.P., Feehan J., Apostolopoulos V. A veterinary vaccine for SARS-CoV-2: the first COVID-19 vaccine for animals. Vaccines (Basel) 2021;9(6):631. doi: 10.3390/vaccines9060631. PMID: 34200587; PMCID: PMC8228738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharun K., Tiwari R., Dhama K., Rabaan A.A., Alhumaid S. COVID-19 vaccination passport: prospects, scientific feasibility, and ethical concerns. Hum Vaccin Immunother. 2021:1–4. doi: 10.1080/21645515.2021.1953350. Epub ahead of print. PMID: 34292128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L.H., Freedman D.O., Visser L.G. COVID-19 immunity passport to ease travel restrictions? J Travel Med. 2020;27(5):taaa085. doi: 10.1093/jtm/taaa085. PMID: 32463093; PMCID: PMC7313786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.