Abstract
Background
Pandemics have significantly modified our societal behaviour over the millennia, and the COVID-19 pandemic is no exception.
Types of Articles Reviewed
In this article, the authors review the history of pandemics, the probable reasons for their emergence, and the COVID-19 pandemic due to the severe acute respiratory syndrome virus 2 (SARS-CoV-2) and its variants, as well as its possible impact on dentistry during the postpandemic period.
Results
There are multiple reasons why catastrophic pandemics occur due to new infectious organisms that cross the species barrier from animals to humans. These include, population explosion, mass migration, and prolonged survival of debilitated and susceptible cohorts on various immunosuppressants. Coupled with global warming and the resultant loss of habitats, such vicissitudes of humans and nature lead to microbes evolving and mutating at an exponential pace, paving the way for pandemics. The contemporary epidemics and pandemics beginning with the HIV pandemic have modulated dentistry beyond recognition, now with assiduous and robust infection control measures in place.
Conclusions and Practical Implications
Because COVID-19 may become an endemic disease, particularly due to emerging SARS-CoV-2 variants the dental community should adopt modified infection control measures, teledentistry, and point-of-care diagnostics, among other measures. It is likely, that clinical ecosystems in future would be rendered even safer by predicting how pathogens evolve and priming the human immune system for the next wave of microbial combatants through vaccines produced using deep mutational scanning in which artificial intelligence and machine learning can predict the next variants even before their arrival.
Key Words: Pandemics, past, present, future, impact, oral health care
Abbreviation Key: DHCW, Dental health care worker; POC, Point of care; SARS, Severe acute respiratory syndrome; SARS-Cov-2, Severe acute respiratory syndrome coronavirus 2

In a retrospective review published in The Journal of the American Dental Association about the severe acute respiratory syndrome (SARS) outbreak in 2003, the authors stated that “… the dental community cannot let down its guard, and must be constantly aware of impending infectious threats in various guises, as well as the recrudescence of disease, that may challenge the current infection control regimens.”1 This prediction has proven to be true with the COVID-19 pandemic, as the organism has mutated into a newer, more infectious, and deadlier variant of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), affecting the world. Moreover, it has evolved and mutated into additional variants, such as Delta and Lambda, which have exhibited higher infectivity, contagiousness, and immune resistance.2 In the context of the SARS-CoV-2 pandemic, we will provide a historical overview of pandemics, the reasons for their emergence, and the probable impact of the pandemic on the practice of dentistry now and in the future.
Historical Aspects
Epidemics and pandemics have affected, molded, and modified our societal behavior substantially over the millennia. When humans were hunters and gatherers living in small population groups within a limited locale, devoid of yet-undiscovered means of transportation, contagions were limited to focal population groups. This resulted in either the annihilation of the small group after succumbing to the virulent disease or the few survivors acquiring active and heritable immunity and overcoming the infectious agents, with the fittest surviving and strengthening the gene pool of their descendants to withstand additional onslaughts from similar infectious agents.3 However, there was a radical disjuncture in these population dynamics when, approximately 12,000 years ago, humans began domesticating animals, growing crops, storing food, and leading a relatively sedentary lifestyle in larger population groups, and they evolved into a communal agrarian society. Such human and animal cosocialization in proximity was the cue for zoonotic microbes to habituate to humans, leading to communicable diseases, such as tuberculosis and smallpox from cattle, influenza from pigs and ducks, and pertussis from pigs and dogs.4
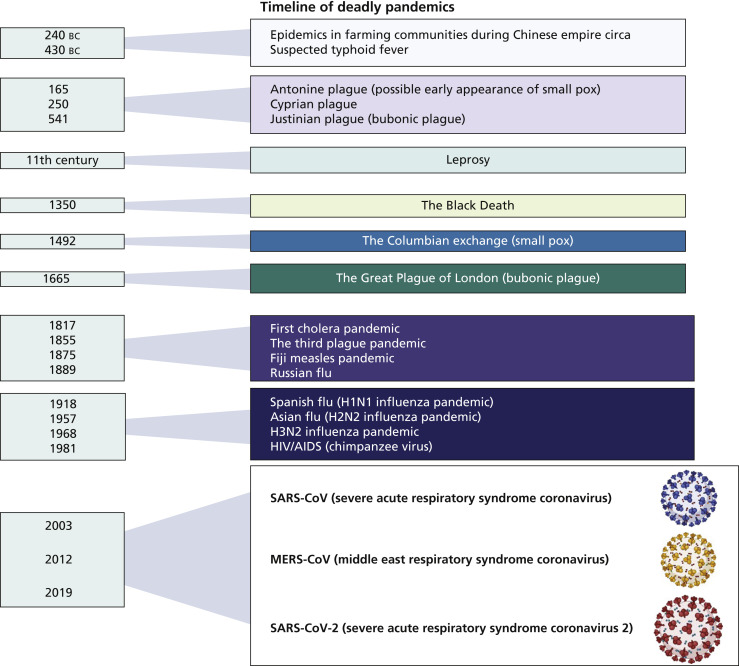
The oldest such recorded epidemics in farming communities go back to the beginning of the Chinese Empire circa 240 bc (Figure 1 ).4 Historians have estimated that more than 450 epidemics, including pandemics, have occurred in the Middle Kingdom over the last 2 millennia.4 The resemblances of the COVID-19 pandemic and the SARS epidemic of 2003, which originated from animals, to these oldest pandemics are stark, as they can be traced back to the animal to human infection transmission dynamics we now know of.5
Figure 1.
Timeline of deadly pandemics.
Reasons For The Emergence Of Epidemics And Pandemics
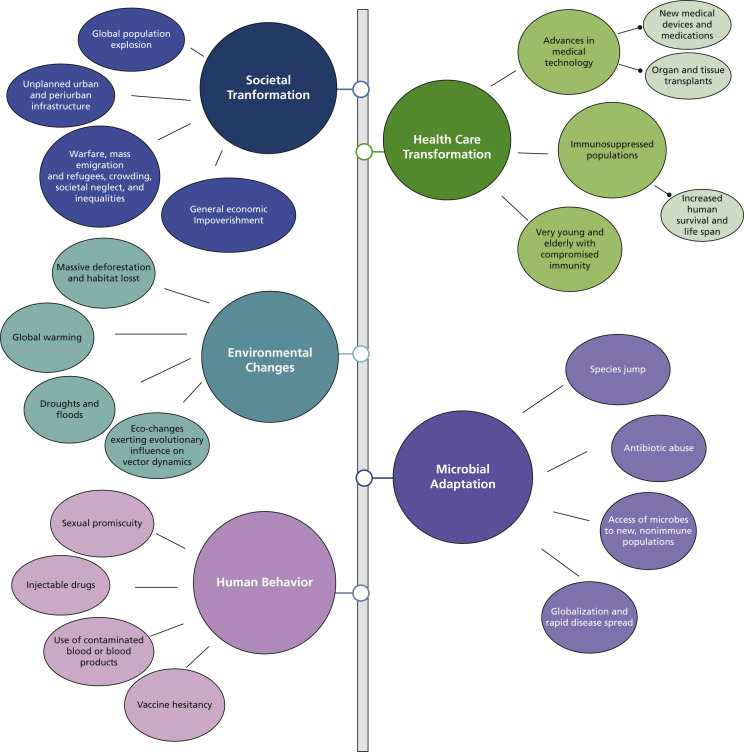
Although the societal and cultural transformations from hunters and gatherers to a more nomadic and communal existence might have contributed to the initial epidemics and pandemics, it is unclear why they continue to recur at a regular pace. In 1992, the National Institutes of Health convened a committee of the Institute of Medicine to answer this question.6 In their report, the committee identified the following key factors as most likely responsible for the emergence of pandemics (Figure 2 ).
Figure 2.
Reasons for the emergence of new infections leading to epidemics and pandemics.
Societal transformation
With improvements in living conditions, particularly health care, there has been an explosion of the global population, reaching 7.7 billion in 2019.7 Such a large mass of humanity competing for finite resources in a resource-dwindling world has led to malnutrition and poor sanitation and hygienic conditions. These, in turn, have led to waves of emigration, migration, and internecine wars and conflicts, with millions of fleeing refugees living in dire, crowded habitats with little or no medical care, all of which are conducive to disease spread.
Health care transformation
The advent of technological improvements, novel medical devices, and medications that suppress immunity have facilitated widespread organ and tissue transplants and a substantial cohort that are debilitated and chronically immunosuppressed. The latter, coupled with rampant antibiotic abuse and a growing elderly population with a relatively weaker immune systems (due to immunosenescence), provide fertile ground for emerging and multidrug-resistant infectious agents.6
Human behavior
Increasing affluence and ever-changing societal norms have transformed human behavior, leading to increased sexual promiscuity and rampant injectable drug abuse associated with contaminated blood and blood products. These behaviors have contributed immensely to the HIV pandemic and hepatitis B and C epidemics. In addition, although not stated in the Institute of Medicine’s report,6 religious, cultural, and political dogma and beliefs leading to vaccine hesitancy have resulted in the resurrection of viral infections, such as mumps in the West8 and COVID-19.
Environmental changes
Fluctuations in the global climate due to human-made disasters, such as massive deforestation, accelerated urbanization, and loss of biodiversity, have resulted in droughts, floods, and global warming. These environmental changes and habitat loss have led to the proliferation and spread of intermediate hosts, such as mosquito vectors spreading diseases including malaria and yellow fever, and displacement of animals harboring viruses that may find humans as fresh reservoirs of infection.
Microbial adaptation
Although in the foregoing we have described human-made outcomes, the microbes have evolved over the millennia, transforming into formidable adversaries by means of developing virulence attributes, such as the antigenic drifts and shifts seen in viruses (for example, the SARS-CoV-2 Delta variant), toxin production, and drug resistance of bacteria.
The Institute of Medicine’s findings,6 which are still relevant, were important in highlighting the critical importance of keeping a close watch on the reasons for the emergence of new epidemic- and pandemic-inducing infections. Until the advent of the COVID-19 pandemic, humankind apparently paid little heed to such ominous warnings of impending disasters.
From The Past To The Present
As we have mentioned, a pivotal reason for the beginning of a pandemic is the ability of animal viruses to cross the species barrier from animals to humans. Although mired in controversy, it is plausible that a bat virus (horseshoe bat [Rhinolophus species]) crossing the species barrier and infecting an intermediate host, pangolin (Pholidota order), may have initiated the COVID-19 pandemic, as pangolins are consumed as food in China.9
A common and ominous characteristic of coronaviruses is their ability to spread through the air in aerosolized particles.10 Some of these infections have affected health care workers disproportionately in the past, as has been the case of the SARS coronavirus infection,11 by virtue of the fact that aerosol-generating procedures are used commonly in medical and dental procedures. The COVID-19 pandemic, which has led to the deaths of medical personnel worldwide, is no exception. However, dental health care workers (DHCWs) appear to have been more fortunate, being spared to a great extent from the SARS-CoV-2 infection.12 The cause for the minimal reported transmission of coronavirus infections in dental settings is unknown, but may be due to a combination of the additional stringent infection control measures used in dentistry and the likelihood of patients with a severe acute infection not undergoing elective dental treatments during the pandemic.13 The dental profession has learned lessons from the HIV and SARS pandemics, and the resultant extensive regimentation of their infection control measures might have led to the low rate of infections during the COVID-19 pandemic. In the sections that follow, we described how the profession arrived there and the renewed challenges it faces in the wake of the COVID-19 pandemic.
Epidemics, pandemics, and infection control
It is hard to visualize practicing clinical dentistry with ungloved hands or devoid of a surgical mask. However, until the late 1960s, the dental profession in most countries paid only lip service to infection control and personal protective equipment and ignored such guidelines even if they existed in various jurisdictions.14 A wake-up call for the dental profession came in the late 1980s with reports of a number of oral surgeons’ and dentists’ transmitting hepatitis B infection to some of their patients in both the United States and United Kingdom and the concomitant adverse publicity in the media.15 , 16 HIV, a viral pandemic that is still widely prevalent, brought the lapses in infection control in dentistry into sharp focus when a patient in Florida, Kimberly Bergalis, claimed that she (and 6 others subsequently) acquired the HIV infection from her dentist, Dr. David Acer, when she attended his clinic for treatment. However, after assiduous and protracted inquiry, the possibility of HIV transmission from the dentist to the patient in the dental operatory was ruled out,17 but not before the reputational damage to the profession reverberated throughout the world.
This case highlighted the critical importance of infection control in dentistry and led to its reinforcement the world over. Furthermore, owing to the insidious nature of HIV, with a prolonged incubation period, there was a need to recognize and consider that all patients attending a clinic, irrespective of their health status, could be infectious and should be treated with blood and body fluid precautions. Since then, and considering extant diseases, such as HIV and hepatitis B, and multidrug–resistant tuberculosis posing a threat to health care workers, health authorities in the United States and United Kingdom issued guidelines on infection control in dental settings.18 The formal promulgation of infection control guidelines for medical and dental practices, entitled “Recommendations for Prevention of HIV Transmission in Health-Care Settings,” occurred in 1987.19
By the early 1980s, an enhanced advisory termed standard precautions was also promulgated to reduce the spread of blood-borne and other pathogens in hospitals and clinics. In conjunction with these, additional transmission-based precautions were introduced to reduce the risk of causing droplet, aerosol, and contact transmission of airborne and highly infectious diseases, such as tuberculosis and vancomycin-resistant Staphylococcus aureus infections.18 The latter guidelines are in force during the COVID-19 era, with additional modifications, owing to the high probability of aerosol transmission of SARS-CoV-2 and its high infectivity.20 The standard infection control measures, revised several times, also include a comprehensive set of recommendations for vaccines for DHCWs.11 , 21
These incessant and often recurrent epidemics and pandemics have changed the practice of dentistry, and the COVID-19 pandemic is no exception. In the following sections, we discussed some features of dentistry that might irreversibly change how we practice dentistry during the postpandemic period.
Postpandemic Dentistry
COVID-19 vaccines and vaccinations
The unparalleled rapid development of various COVID-19 vaccines within a record short period of 9 months is a testament to human ingenuity and technological prowess in the face of immense adversity.22 More than 8 major COVID-19 vaccines produced on various vaccine platforms23 have slowed the pandemic considerably where vaccine delivery is quick and efficacious and uptake is high. The wave of resurgent pandemic in the United States and many other countries in the West has been called the “pandemic of the unvaccinated,” implying the high efficacy of the approved COVID-19 vaccines. However, there are many pitfalls ahead on the road to success. These include vaccine shortages, compromised logistical networks of vaccine distribution, and the vaccination processes itself, particularly in developing countries. These are compounded by vaccine hesitancy among a substantial proportion of the populace in both developed and developing countries.24
Centers for Disease Control and Prevention’s standard infection control guidelines state that oral health care workers must be vaccinated against several occupationally acquired transmissible infections.21 Therefore, a vaccine against COVID-19 is likely to be a new entrant to the list of stipulated vaccines for DHCWs, which range from hepatitis B to seasonal influenza.20 However, introducing a COVID-19 vaccination mandate for DHCWs will likely generate several complex administrative, cultural, political, and logistical issues that will need to be addressed. These include managing expectations of DHCWs who refuse to get the vaccine owing to their beliefs and convictions, contraindicated comorbidities for vaccination, and necessity for booster vaccine doses and frequency of their administration. The data indicate that booster vaccinations are required to maintain immunity after the 2-dose vaccination regimens.25 Finally, the annual vaccination regimen for COVID-19 needs urgent consideration because of circulating new and old variants and the temporal waning of antibody levels.26 Many of these issues have not yet been resolved.
Point-of-care (POC) diagnostic tests
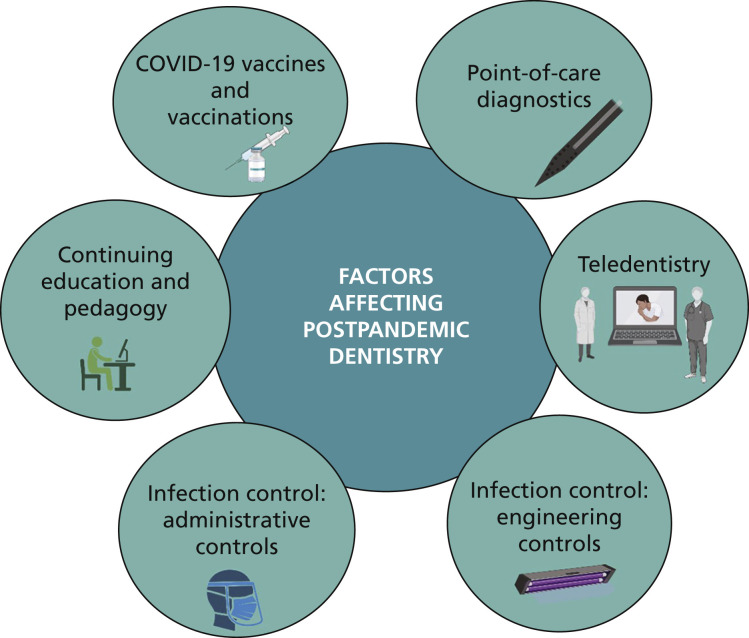
As COVID-19 is likely to be an endemic infection, it is crucial that DHCWs ensure that the patients they treat on a daily basis are free of viral reservoirs that can create new clusters of infection. One way to forestall such an outcome is to perform rapid POC diagnostic tests for SARS-CoV-2 antigens and antibodies for all patients immediately before treatment (Figure 3).
Figure 3.
Factors affecting postpandemic dentistry.
An array of new POC diagnostic tests are available for this purpose,27 and more than 450 such tests are in the developmental stages for expedited identification of patients with COVID-19 in clinical and other settings (for example, airports).28 These POC tests are not widely deployed in routine dentistry, possibly owing to their novelty and the initial outlay involved. However, it is highly likely that robust, reliable, sensitive, and specific POC tests will be mass produced and available in the near future as inexpensive kits to facilitate easy administration by any dental team member.27 However, DHCWs must be aware of the consequences of false-negative test results and importance of strict compliance with enhanced infection control protocols.
The battery of rapid POC tests for COVID-19 are essentially based on molecular technology, such as polymerase chain reaction, lateral flow, and loop-mediated isothermal amplification technology.27 However, newer next-generation technologies are being evaluated, including the use of holography and artificial intelligence–based methods, which can deliver results from a saliva sample within 30 seconds. Other testing methods in development are based on biorecognition and include electrochemical sensors, field-effect transistor–based biosensors, magnetic biosensors, immunosensors, enzyme-based sensors, and DNA biosensors. Biosensors have already been evaluated for detection of infectious diseases, such as influenza.29
Finally, in terms of the practicality of administering POC diagnostic tests as a component of a structured screening program in dentistry, there are a number of critical questions that need to be answered. For instance, should every patient be tested at every dental appointment? What should the frequency of testing be? What if a patient refuses to be tested or does not want to bear the cost of testing? Should the DHCW protocolize a patient's referral to a preidentified confirmatory testing facility? These questions need to be answered through dialogue, particularly with medical care providers, once the evolutionary demographics of COVID-19 are clearer.
Teledentistry
Minimizing physical contact with patients or presymptomatic people will be critical in curbing the spread of COVID-19 in a postpandemic world. Teledentistry, defined as “the remote facilitating of dental treatment, guidance, and education via the use of information technology instead of direct face-to-face contact with patients,”30 could be an essential arm of the patient care management protocol, particularly during the sporadic resurgence of the infection in regional pockets or in areas where the pandemic may linger for months.
Teledentistry comprises several components, such as teleconsultation, telediagnosis, teletriage, and telemonitoring, and each has essential functions relevant to dental practice. For example, in the context of the COVID-19 pandemic, teledentistry would be particularly useful for remote consultation with patients with acute COVID-19 infections and those in quarantine or for follow-up advice and assessment during the presymptomatic exposure period, postrecovery period, and posttreatment period. In addition, continuing care for preexisting oral diseases, such as cancer rehabilitation therapy and follow-up of suspect oral lesions, could also be performed using teledentistry. This is particularly important, as it is likely that routine oral health care of patients has been overlooked during the pandemic for several reasons, including inaccessibility of care facilities due to travel restrictions.
There are several challenges to adopting teledentistry as a care management tool, such as its novelty and the resulting reluctance among both dentists and patients to accept it.31 Such concerns need to be allayed to popularize its utility, which will undoubtedly come of age as a robust diagnostic and patient care management tool owing to the increasing use of cloud-based data services, artificial intelligence, and big data resolution through bioinformatics in the not too distant future.32
Pedagogy and continuing education
During the postpandemic period, universities and similar establishments need to consider how best to provide dental education in a safe, disease-free environment for the benefit of all stakeholders. A proper balance must be struck between offering students the necessary skill acquisition and training and maintaining essential infection control measures. This could take the form of administrative controls, such as virtual lectures and small group tutorials; fine-tuning the use of haptics-based, distant operative dentistry training protocols; optimizing student-to-patient proportions in teaching clinics; and appropriate fallow periods between patient treatment sessions, all of which should minimize disease transmission in the clinic environments.
The dental profession is well placed to join in public health initiatives and must be empowered to advocate for patient immunizations (including influenza, human papillomavirus, and childhood vaccination regimens). Organized dentistry and education delivery establishments must actively lobby for immunization. In addition, in view of pressing public health priorities, the profession must address ethics and occupational and patient safety concerns to mandate COVID-19 immune sufficiency certificates in dental clinics. All students and other personnel attending to patients may be required to produce COVID-19 immune sufficiency certificates (either electronic or hard copy) before attending the institution and at periodic intervals afterward, depending on the disease dynamics of the region.
Infection control: engineering and administrative controls
Aerosol-generating procedures are part and parcel of clinical dentistry. This makes it imperative that the dental operatory redesigns or retrofitting entail modern, state-of-the-art engineering controls to prevent aerosol-transmitted diseases. Such engineering control measures required to reduce bioaerosols include extraoral high-volume evacuation, negative pressure ventilation, enhanced efficacious air filtration, ultraviolet irradiation of the operatories during fallow periods, and transparent barriers in patient–administrator communication portals.33
Lessons Learned And Future Preparedness
The COVID-19 pandemic was a wake-up call for the entire world. The inadequate preparedness of humanity for such a devastating disease was evident early in the pandemic and with the somewhat dysfunctional vaccine programs, particularly in many developing regions of the world. Although the dental profession was a step ahead in dealing with COVID-19 owing to universally used rigorous infection control measures, the profession has to be forearmed not only for a recurring and possibly endemic disease of various SARS-CoV-2 viral variants, but also new infections emerging, which are predicted to arrive periodically in various guises. One exception during this period of crisis was the negligible role the dental profession played in serving the community in health care institutions, except in a few jurisdictions, such as Singapore.34 There might be several reasons for this, but going forward the profession needs to take the lead by means of broadening the dental curriculum through interprofessional education programs and extending the repertoire of dentistry beyond oral health care. Last but not least, policy makers and regulatory bodies need to take a broad and deep look at how the pandemic has affected the dental profession and review and modify the guidelines and recommendations across the whole spectrum of dental practice and training.33 , 35
Conclusion: Predicting The Future?
We have described several measures that may help the dental profession alleviate the burden of the epidemics and pandemics that will unpredictably emerge from complex, changing ecosystems, often far from scrutiny or regulatory control. However, there is a silver lining in this dark cloud, as it appears that technological advances in vaccine production and other predictive tools that forecast the next great pandemic might save humanity from such recurrent perils. For instance, forecasting how various pathogens evolve and then priming the human immune system for the next wave of combatants is one way to win this battle. One such approach, called “deep mutational scanning,” observes surface antigen drifts and shifts of the pathogens in silico using artificial intelligence and machine learning.36 This means that vaccines for an upcoming viral variant or even a new pathogen could be predicted far in advance, and the infection addressed before becoming a pandemic. It has been said that humanity has made it to the life raft, but deep mutational scanning and related technologies may help us remain on “dry land” and brace for and survive the oncoming pandemic “floods.”37
Biographies
Dr. Samaranayake is a professor emeritus and an immediate-past dean, Faculty of Dentistry, University of Hong Kong, Hong Kong.
Dr. Fakhruddin is a lecturer, Departments of Preventive and Restorative Dentistry, College of Dental Medicine, University of Sharjah, United Arab Emirates.
Footnotes
Disclosure. Drs. Samaranayake and Fakhruddin did not report any disclosures.
References
- 1.Samaranayake L.P., Peiris M. Severe acute respiratory syndrome and dentistry: a retrospective view. JADA. 2004;135(9):1292–1302. doi: 10.14219/jada.archive.2004.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura I, Kosugi Y, Wu J, et al. SARS-CoV-2 Lambda variant exhibits higher infectivity and immune resistance. Preprint. Published online July 28, 2021. bioRxiv 454085. https://doi.org/10.1101/2021.07.28.454085
- 3.Kenny C. Scribner; 2021. The Plague Cycle: The Unending War Between Humanity and Infectious Disease. [Google Scholar]
- 4.Høiby N. Pandemics: past, present, future—that is like choosing between cholera and plague. APMIS. 2021;129(7):352–371. doi: 10.1111/apm.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., Harrich D., Li Z., Hu D., Li D. The unique features of SARS-CoV-2 transmission: comparison with SARS-CoV, MERS-CoV and 2009 H1N1 pandemic influenza virus. Rev Med Virol. 2021;31(2) doi: 10.1002/rmv.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute of Medicine (US) Committee on Emerging Microbial Threats to Health . In: Emerging Infections: Microbial Threats to Health in the United States. Lederberg J., Shope R.E., Oaks S.C. Jr, editors. National Academies Press; 1992. [PubMed] [Google Scholar]
- 7.World Population Prospects 2019: Highlights United Nations. June 17, 2019. Accessed October 23, 2021. https://www.un.org/development/desa/publications/world-population-prospects-2019-highlights.html
- 8.Dubé E., Laberge C., Guay M., Bramadat P., Roy R., Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9(8):1763–1773. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyranoski D. Did pangolins spread the China coronavirus to people? Nature. Published online February 7, 2020. https://doi.org/10.1038/d41586-020-00364-2 [DOI] [PubMed]
- 10.Wang C.C., Prather K.A., Sznitman J., et al. Airborne transmission of respiratory viruses. Science. 2021;373(6558) doi: 10.1126/science.abd9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R.W., Leung K.W., Sun F.C., Samaranayake L.P. Severe acute respiratory syndrome (SARS) and the GDP, part II: implications for GDPs. Br Dent J. 2004;197(3):130–134. doi: 10.1038/sj.bdj.4811522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araujo M.W.B., Estrich C.G., Mikkelsen M., et al. COVID-2019 among dentists in the United States: a 6-month longitudinal report of accumulative prevalence and incidence. JADA. 2021;152(6):425–433. doi: 10.1016/j.adaj.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommerstein R., Fux C.A., Vuichard-Gysin D., et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9(1):100. doi: 10.1186/s13756-020-00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan B., Samaranayake L.P. The surgical mask unmasked: a review. Oral Surg Oral Med Oral Pathol. 1990;70(1):34–36. doi: 10.1016/0030-4220(90)90174-q. [DOI] [PubMed] [Google Scholar]
- 15.Goodman R.A., Ahtone J.L., Finton R.J. Hepatitis B transmission from dental personnel to patients: unfinished business. Ann Intern Med. 1982;96(1):119. doi: 10.7326/0003-4819-96-1-119_1. [DOI] [PubMed] [Google Scholar]
- 16.Lewis J.D., Enfield K.B., Sifri C.D. Hepatitis B in healthcare workers: transmission events and guidance for management. World J Hepatol. 2015;7(3):488–497. doi: 10.4254/wjh.v7.i3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown D. The 1990 Florida dental investigation: theory and fact. Ann Intern Med. 1996;124(2):255–256. doi: 10.7326/0003-4819-124-2-199601150-00010. [DOI] [PubMed] [Google Scholar]
- 18.Siegel J.D., Rhinehart E., Jackson M., Chiarello L., Health Care Infection Control Practices Advisory Committee 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 suppl 2):S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control Recommendations for prevention of HIV transmission in health-care settings. MMWR. 1987;36(suppl 2S):1S–18S. [PubMed] [Google Scholar]
- 20.CDC updates COVID-19 infection prevention and control guidance Centers for Disease Control and Prevention. Updated July 13, 2021. Accessed October 23, 2021. https://www.cdc.gov/oralhealth/infectioncontrol/statement-COVID.html
- 21.Kohn W.G., Collins A.S., Cleveland J.L., Harte J.A., Eklund K.J., Malvitz D.M. Guidelines for infection control in dental health-care settings: 2003. MMWR Recomm Rep. 2003;52(RR-17):1–61. [PubMed] [Google Scholar]
- 22.Ekström A.M., Berggren C., Tomson G., Gostin L.O., Friberg P., Ottersen O.P. The battle for COVID-19 vaccines highlights the need for a new global governance mechanism. Nat Med. 2021;27(5):739–740. doi: 10.1038/s41591-021-01288-8. [DOI] [PubMed] [Google Scholar]
- 23.Zimmer C., Corum J., Wee S.-L. Coronavirus vaccine tracker. The New York Times. Accessed October 23, 2021. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
- 24.Dabla-Norris E., Khan H., Lima F., Sollaci A. Who doesn’t want to be vaccinated? Determinants of vaccine hesitancy during COVID-19. IMF Working Paper. International Monetary Fund. Published May 6, 2021. Accessed October 7, 2021. https://www.imf.org/en/Publications/WP/Issues/2021/05/06/Who-Doesnt-Want-to-be-Vaccinated-Determinants-of-Vaccine-Hesitancy-During-COVID-19-50244
- 25.Keehner J., Horton L.E., Binkin N.J., et al. Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. N Engl J Med. 2021;385:1330–1332. doi: 10.1056/NEJMc2112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samaranayake LP, Seneviratne CJ, Fakhruddin KS. Coronavirus disease 2019 (COVID-19) vaccines: a concise review. Oral Dis. Published online May 15, 2021. https://doi.org/10.1111/odi.13916 [DOI] [PMC free article] [PubMed]
- 27.Samaranayake L., Kinariwala N. Point-of-care (POC) diagnostics for coronavirus disease 2019 (COVID-19) and their potential impact on dentistry. Dent Update. 2021;48(7):585–590. [Google Scholar]
- 28.Emergency use authorizations for medical devices US Food and Drug Administration. Accessed October 23, 2021. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations-medical-devices
- 29.Wu K., Klein T., Krishna V.D., Su D., Perez A.M., Wang J.-P. Portable GMR handheld platform for the detection of influenza A virus. ACS Sens. 2017;2(11):1594–1601. doi: 10.1021/acssensors.7b00432. [DOI] [PubMed] [Google Scholar]
- 30.Ghai S. Teledentistry during COVID-19 pandemic. Diabetes Metab Syndr. 2020;14(5):933–935. doi: 10.1016/j.dsx.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estai M., Kanagasingam Y., Xiao D., et al. End-user acceptance of a cloud-based teledentistry system and Android phone app for remote screening for oral diseases. J Telemed Telecare. 2017;23(1):44–52. doi: 10.1177/1357633X15621847. [DOI] [PubMed] [Google Scholar]
- 32.Schwendicke F., Samek W., Krois J. Artificial intelligence in dentistry: chances and challenges. J Dent Res. 2020;99(7):769–774. doi: 10.1177/0022034520915714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recommendations for the Re-Opening of Dental Services: A Rapid Review of International Sources. COVID-19 Dental Services Evidence Review (CoDER) Working Group. Accessed October 23, 2021. https://oralhealth.cochrane.org/sites/oralhealth.cochrane.org/files/public/uploads/covid19_dental_review_16_may_2020_update.pdf
- 34.Seneviratne C.J., Lau M.W.J., Goh B.T. The role of dentists in COVID-19 is beyond dentistry: voluntary medical engagements and future preparedness. Front Med (Lausanne) 2020;7:566. doi: 10.3389/fmed.2020.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kathree B.A., Khan S.B., Ahmed R., Maart R., Layloo N., Asia-Michaels W. COVID-19 and its impact in the dental setting: a scoping review. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0244352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fowler D.M., Fields S. Deep mutational scanning: a new style of protein science. Nat Methods. 2014;11(8):801–807. doi: 10.1038/nmeth.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipsitch M, Grad Y. Navigating the Covid-19 pandemic: we’re just clambering into a life raft—dry land is far away. STAT. April 1, 2020. Accessed October 25, 2021. https://www.statnews.com/2020/04/01/navigating-covid-19-pandemic/