Abstract
Anaplasma centrale (A. centrale) is an obligate red blood cell residing tick transmitted rickettsiae that has not been studied extensively for its prevalence in cattle along with its epidemiology. Aim of this investigation was to report the seasonal prevalence, phylogeny and epidemiological parameters associated with the prevalence of A. centrale in cattle breeds enrolled from District Layyah in Punjab, Pakistan. A total of 844 blood samples [Cross breed = 300, Holstein Friesian = 244, Sahiwal breed = 300)] were collected from apparently healthy cattle along with epidemiological data during 2017–18. PCR amplified 426 base pair fragment from 16S rRNA gene of A. centrale in 14.4% (122/844) of cattle. Amplified 16S rRNA partial gene sequence of A. centrale were confirmed by DNA sequencing and deposited to GenBank. Highest A. centrale prevalence was observed in spring (24%) followed by autumn (12.4%) summer (10%) and winter (7.1%) seasons. Sahiwal breed (18.3%) was most susceptible to A. centrale infection followed by cross (12.3%) and Holstein Friesian breed (12.3%). 69/844 (8.2%) of Giemsa stained cattle blood smears were also found positive for Anaplasma spp. Farms where animal use to drink pool water and farms where dogs and other dairy animals were living with cattle had higher A. centrale prevalence. Female cattle and dogs having tick burden were found associated with A. centrale infection. Hematological profile was severely disturbed in A. centrale positive cattle. It is recommended that A. centrale should be screened in cattle, in addition to A. marginale, for the effective control of tick born diseases in Pakistan.
Keywords: Cattle, PCR, 16S rRNA gene, Blood smear screening, Phylogenetic analysis
1. Introduction
Majority of Pakistani population, around 62%, is resident of rural areas and agriculture and livestock is their main source of earning (Rehman et al., 2017). That is why livestock sector is a major contributor in gross domestic product (GDP) of Pakistan. Contribution of this sector was 11.8% in GDP during financial year 2013/2014 (Pakistan Economic Survey 2013–14). During financial year 2014–15, a further 4.1% growth was recorded in livestock sector indicating its major contribution in national economy (Rehman et al., 2017). In Pakistan, the estimated population of dairy cattle is around 35–40 million heads yielding approximately 18,000–31,000 million tons of milk making Pakistan 4th leading milk producer worldwide after China, India and USA (Pakistan Economic Survey 2013–14).
Diseases in cattle are major constrain as they cause financial losses to livestock owners. Most common diseases in cattle are caused by ectoparasites and ticks are the most frequent vectors for pathogens (Srikant and Gaurav, 2014). Anaplasmosis is a common pathological condition in tropical and subtropical parts of the world and it has been reported in domestic as well as wild ruminants. Anaplasmosis is caused by obligate rickettsiae that belong to the genus Anaplasma (Rickettsiales: Anaplasmatacea) and infect red blood cells of their host. A. marginale is the usual causative agent of bovine anaplasmosis and A. centrale is not commonly known to cause this disease (Ashraf et al., 2013). A. centrale is usually transmitted by ticks (Ixodes spp., Dermacentor spp., Rhipicephalus spp. and Haemaphysailis spp.) (Jabbar et al., 2015, Battilani et al., 2017). A. centrale is generally considered to be less pathogenic for cattle when compared with the infection caused by A. marginale (Rar and Golovljova, 2011). A. centrale infection results in progressive anemia in host (Battilani et al., 2017). It has been documented that cattle infected with A. centrale develops an immunity against A. marginale (Kocan et al., 2010). Due to the high economic impact, in several countries of South America, Africa and in Australia, A. centrale is used as a live vaccine (Bock et al., 2003) and in these countries whenever A. centrale is detected in cattle, it considered as a result of vaccination (Khumalo et al., 2016). Pakistan is a developing country with limited health resources available for livestock and vaccination of cattle for A. centrale is not a usual practice here. Hence, there is no information available about the prevalence of this parasite in local cattle population. Also, very few studies have been cited in literature from worldwide regarding the molecular detection of A. centrale in cattle (Belkahia et al., 2015, Aktas et al., 2011). Keeping in view the above mentioned facts, present investigation was designed to report the seasonal prevalence of A. centrale in three breed of cattle from District Layyah by conventional (blood smear screening) as well as modern (Polymerase chain reaction) tools. We are also documenting the epidemiology of A. centrale and its effects on complete blood count of infected cattle, if any.
2. Materials and methods
2.1. Sample and data collection
Apparently healthy cattle [N = 844] were enrolled from various dairy farms in District Layyah of Punjab (Pakistan) during 2017 and 2018. Enrolled cattle included Holstein Friesian (N = 244), Sahiwal (N = 300) and cross breed (N = 300). Following the informed consent from livestock owners, animals were inspected by the veterinary doctor and blood sample was collected from Jugular vein (approximately 10 ml) in EDTA containing tubes on seasonal basis. May to July was summer, August till October was autumn, November to January was winter and February till April was considered as spring season. Blood samples were used for DNA extraction and to analyze complete blood count parameters. Epidemiological parameters (gender, tick load on cattle, water supply source at farm, other dairy animals associated with herd, dog associated with farm and tick load on dog) were recorded for each enrolled animal at the time of blood sampling. All the experimental procedures and lab protocols were reviewed and approved by the ethical review board of Institute of Pure and Applied Biology, Bahauddin Zakariya University Multan, Pakistan.
2.2. Blood smear screening
For each enrolled cattle, blood smear was prepared, air dried and fixed in ethanol on sampling site. Later on slides were stained with Geimsa (Takihi and Sandes 2013) and examined under the microscope with oil immersion lens for the presence of Anaplasma spp.
2.3. Tick collection and identification
Bodies of animals were thoroughly inspected for the presence of ticks at the time of blood sample collection and when ticks were observed they were carefully collected and preserved in 70% ethanol and later on identified following Madder et al. (2014).
2.4. DNA extraction from blood and ticks
An inorganic DNA extraction protocol was followed to extract DNA from cattle blood as described by Saeed et al. (2016). Following their identification, DNA from ticks was extracted following the protocol as described by Ammazzalorso et al. (2015).
2.5. PCR amplification
Primers, FF 5′CTGCTTTTAATACTGCAGGACTA 3′ and R 5′ATGCAGCACCTGTGTGAGGT 3′ and protocol of Kawahara et al. (2006) was used to amplify 426 base pairs of 16S rRNA gene of A. centrale in blood and tick samples. Anaplasma centrale positive samples that we had detected from large ruminants during previous investigations by Dr. Munir Aktas at University of Firat Turkey were used as positive control during PCR. For negative control, double distilled water was used instead of negative cattle DNA during each PCR.
2.6. DNA sequencing and phylogenetic analysis
Five PCR were selected randomly for DNA sequencing at First base Sequencing Service (Malaysia) in order to confirm the presence of A. centrale in cattle blood samples. BLAST analysis was performed on resultant sequences and they were submitted to NCBI GenBank.
Maximum Likelihood method was used to report the evolutionary history of A, centrale. Method was based on the Kimura 2-parameter model as reported by Kimura (1980). Phylogenetic analyses were done by MEGA7 (Kumar et al., 2016).
2.7. Complete blood count analysis
All collected cattle blood samples were analyzed for blood cell count and for parameters associated with blood cells by using hematology analyzer.
2.8. Statistical data analysis
Data were presented as Mean ± Standard error of mean And were analyzed by using Minitab (version 16, USA). Association between the presence of A. centrale and studied epidemiological parameters was assessed through Fisher’s exact test. Complete blood count parameters were compared between A. centrale positive and negative blood samples by using two sample t-test.
3. Results
3.1. Tick identification
Only small number of ticks (N = 38) were randomly collected from the enrolled cattle for identification during summer season. Rhipicephalus microplus and Haemaphysalis punctata were the ticks that were identified during present study.
3.2. Prevalence of Anaplasma spp. In blood smears
Microscopic examination of blood smears revealed that 69 (8.2%) cattle were found infected with Anaplasma spp. When data was analyzed on the basis of cattle breed, it was observed that highest Anaplasma spp. infection was observed in Sahiwal (12.3%), followed by cross breed (6.3%) and Holstein Friesian breed (5.3%) (data not shown here). When seasonal prevalence of Anaplasma spp. was analyzed blood smears prepared during spring were found to be most infected (10.5%) followed by blood smears prepared during autumn (7.3%), summer (5.2%) and winter (4.3%) seasons (data not shown here).
3.3. PCR based prevalence of A. Centrale
PCR detected A. centrale in 14.4% of enrolled cattle (122/844). Maximum A. centrale infection was observed in those cattle blood samples that were collected in spring (24%) and it was followed by blood samples collected in autumn (16.6%), summer (10%) and winter (7.6%) seasons (Table 1).
Table 1.
Over all and seasonal prevalence of Anaplasma centrale among cross, Holstein Friesian and Sahiwal breed of cattle blood samples collected from District Layyah during present study.
|
Season (Number of collected samples) |
A. centrale positive Cross Breed |
A. centrale positive Holstein Friesian Breed |
A. centrale positive Sahiwal Breed |
Over all A. centrale positive samples (% Prevalence) |
|---|---|---|---|---|
| Spring (N = 225) |
2 |
12 |
40 |
54 (24.0%) |
| Summer (N = 225) |
15 |
8 |
0 |
23 (10.2%) |
| Autumn (N = 169) |
16 |
0 |
12 |
28 (16.6%) |
| Winter (N = 225) |
4 |
10 |
3 |
17 (7.6%) |
|
A. centrale positive samples (% Prevalence) |
37/300 (12.3%) |
30/244 (12.3%) |
55/300 (18.3%) |
122/844 (over all prevalence 14.4%) |
Among the breeds, prevalence of A. centrale was highest in Sahiwal breed (18.3%) followed by cross breed (12.3%) and Holstein Friesian breed (12.3%) (Table.1).When seasonal prevalence of A. centrale was analyzed in three cattle breeds that were enrolled in present study, it was observed that highest A. centrale infection in Sahiwal breed was found in blood samples that were collected in spring (53.3%) and it was followed by blood samples collected in autumn (16.2%) and winter (4.2%). No parasite positive sample was found during summer season. For Cross breed, the highest infection rate for A. centrale was detected in blood samples that were collected in autumn (22.2%) and this was followed by 20.1% in summer, 5.3% in winter and 2.6% in spring season. For Holstein Friesian breed, maximum prevalence of A. centrale was detected in blood samples collected during spring (16.2%) followed by 13.1% in winter and 13.3% in summer season. None of the Holstein Friesian breed blood sampled in autumn was found infected with A. centrale (Table 1).
None of the collected tick was found infected by A. centrale during PCR based screening.
3.4. DNA sequencing and Phylogenetic analysis
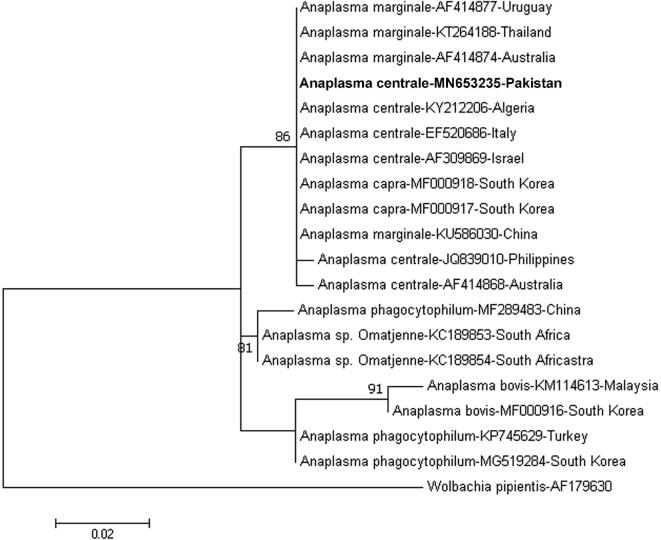
DNA sequences from 16S rRNA gene of A. centrale revealed high genetic similarity so only a single sequence was submitted to the GenBank (Accession number MN653235). Amplified DNA sequence of A. centrale was placed in stable monophyletic cluster along with 16S rRNA gene sequences of A. centrale deposited from Australia, Thailand, Uruguay, Algeria, Italy and Israel (Fig. 1).
Fig. 1.
Phylogenetic tree based on partial 16S rRNA gene sequence of Anaplasma centrale amplified from cattle in Pakistan and A. centrale sequences deposited from various parts of world in GenBank. The sequence of A. centrale submitted during present study is presented in bold.
3.5. Risk factor analysis
It was observed that prevalence of A. centrale in blood samples of Sahiwal breed was significantly higher in farms having dogs (P = 0.01) during spring season. During autumn in farms where cattle had tick load (P = 0.001), other animals were kept with cattle (P = 0.04), dogs were present at farms (P = 0.01) and dogs having tick load (P = 0.01) were found more infected with A. centrale. During winter, farms where animals used to drink water from pools (P = 0.003) had higher A. centrale prevalence (Table 2).
Table 2.
Association of studied epidemiological parameters of Sahiwal breed cattle collected from District Layyah with the prevalence of Anaplasma centrale. Prevalence (%) of Anaplasma centrale is given in parenthesis. P –values indicate the results of Fischer test calculated for each studied parameter
| Parameters | A centrale + ive samples collected during spring | P value | A centrale + ive samples collected during summer | P value | A centrale + ive samples collected during autumn | P value | A centrale + ive samples collected duringwinter | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Sex |
Male | 9/13 (69%) | 0.2 | # | # | 1/14 (7%) | 0.4 | 0/19 (0%) | 0.5 |
| Female | 31/62 (50%) | # | 11/61 (18%) | 3/56 (5%) | |||||
| Water resource | Pump | 38/73 (52%) | 0.4 | # | # | 9/57 (16%) | 1 | 0/63 (0%) | 0.003* |
| Pool | 2/2 (100%) | # | 3/18 (17%) | 3/12 (25%) | |||||
| Tick load | Yes | 32/63 (51%) | 0.3 | # | # | 8/70 (11%) | 0.001** | 3/70 (4%) | 1 |
| No | 8/12 (67%) | # | 4/5 (80%) | 0/5 (0%) | |||||
| Other dairy animals at farm | Yes | 36/68 (53%) | 1 | # | # | 11/61 (18%) | 0.4 | 3/48 (6%) | 0.5 |
| No | 4/7 (57%) | # | 1/14 (7%) | 0/27 (0%) | |||||
| Dogs at farm | Yes | 29/44 (66%) | 0.01* | # | # | 8/68 (12%) | 0.01* | 3/50 (6%) | 0.5 |
| No | 11/31 (35%) | # | 4/7 (57%) | 0/25 (0%) | |||||
| Tick load on dogs | Yes | 22/34 (65%) | 0.1 | # | # | 8/68 (12%) | 0.01* | 3/38 (8%) | 0.2 |
| No | 18/41(44%) | # | 4/7 (57%) | 0/37 (0%) | |||||
P > 0.05 = Non significant; P < 0.05 = Least significant (*); P < 0.01 = Significant (**); P < 0.001 = Highly significant (***)
# Statistical analysis was not possible
For cross breed cattle, during summer season, prevalence of A. centrale was higher in cattle with tick burden (P = 0.03). In autumn season, female cattle (P = 0.05) and farms supplied with water pool (P = 0.01) where other animals (P = 0.05) and dog (P = 0.04) with tick load (P = 0.05) were reared along with cattle were more infected with A. centrale (Data not shown here).
For Holstein Friesian breed, tick burden on cattle (P = 0.006) and dogs associated with farm (P = 0.0001) were corelated with prevalence of A. centrale during spring season. In summer season A. centrale infection was significantly higher among female cattle (P = 0.0001) and at farms where cattle and other dairy animals (P = 0.0001) as well as dogs (P = 0.0004) living together. Dogs with tick load (P = 0.0004) were also found associated with A. centrale infection during this season. While in winter season, farms where other animals were also reared with cattle (P = 0.02) were more infected with A. centrale (Data not shown here).
3.6. Complete blood count analysis
Sahiwal breed cattle that were found positive for A. centrale infection during autumn had reduced red blood cell (P = 0.025), heamoglobin (P = 0.011), hematocrit (P = 0.017) and mean cell hemoglobin (P = 0.011). In winter season, lymphocytes % (P = 0.002) and mean corpuscular hemoglobin concentration (P = 0.03) remained significantly lower in cattle positive for A. centrale. All other parameters remained unaffected (P > 0.05) (Table 3).
Table 3.
Comparison of complete blood count parameters between Anaplasma centrale positive and negative blood samples of Sahiwal breed cattle collected from District Layyah during 2017–2018. Data is expressed as mean ± standard error of mean (SEM). P-value indicates the result of two sample t –test calculated for each studied parameter when compared between parasite positive and negative blood samples collected during a particular season.
| Parameters |
Spring |
Autumn |
Winter |
|||
|---|---|---|---|---|---|---|
|
A. centrale (+ive) N = 40 |
A. centrale (- ive) N = 35 |
A. centrale (+ive) N = 12 |
A. centrale (- ive) N = 63 |
A. centrale (+ive) N = 3 |
A. centrale (- ive) N = 72 |
|
| White blood cell (x 103 μL−1) | 8.5 ± 0.35 | 8.6 ± 0.31 | 15.3 ± 6.5 | 9.7 ± 0.3 | 7.8 ± 0.5 | 8.1 ± 0.1 |
| Lymphocytes (%) | 37 ± 2.3 | 38 ± 2.7 | 27 ± 3.6 | 26 ± 1.6 | 31 ± 1.7** | 43 ± 1.4 |
| Monocytes (%) | 4.86 ± 0.33 | 4.84 ± 0.32 | 3.1 ± 0.1 | 3 ± 0.03 | 4.3 ± 0.6 | 3.4 ± 0.2 |
| Red blood cells (x 106μL−1) | 6.5 ± 0.7 | 5.8 ± 0.1 | 3.7 ± 0.1* | 4.2 ± 0.07 | 5.2 ± 1.1 | 4.4 ± 0.1 |
| Hemoglobin (gdL-1) | 9 ± 0.16 | 9.2 ± 0.17 | 9.1 ± 0.5* | 10.8 ± 0.2 | 9.2 ± 0.7 | 9 ± 0.09 |
| Mean cell volume (f L) | 44 ± 1.3 | 42 ± 0.7 | 81 ± 1.7 | 80 ± 0.9 | 79 ± 0.9 | 78 ± 0.6 |
| Hematocrit (%) | 26 ± 0.69 | 25 ± 0.66 | 28 ± 1.9* | 33 ± 0.8 | 40 ± 5 | 41 ± 0.6 |
| Mean cell hemoglobin (pg) | 14.9 ± 0.2 | 20.3 ± 3 | 25 ± 0.7* | 27 ± 0.4 | 30 ± 0.5 | 31 ± 0.6 |
| Mean corpuscular hemoglobin concentration (g/dl) | 33 ± 0.9 | 35 ± 1 | 33.1 ± 0.5 | 33.5 ± 0.2 | 31 ± 0.5* | 33 ± 0.3 |
| Platelets (x 103 μL−1) | 272 ± 21 | 249 ± 16 | 245 ± 20 | 245 ± 11 | 264 ± 52 | 287 ± 7 |
No parasite was detected in samples collected during summer season
P > 0.05 = Non significant; P < 0.01 = Significant (*); P < 0.001 = Significant (**)
For cross breed, mean corpuscular hemoglobin concentration (P < 0.001) in spring and platelet count in autumn (P = 0.05) was increased in cattle infected with A. centrale (Data not shown here). For Holstein Friesian breed, A. centrale positive animals had significantly lower white blood cells (P = 0.01) but had enhanced lymphocyte count (P = 0.03) during winter. All other parameters remained unaffected (P > 0.05) for the three cattle breeds (data not shown here).
4. Discussion
Anaplasmosis in cattle is caused by Anaplasma marginale and Anaplasma centrale and this disease is also known as yellow-bag or yellow-fever (Ashraf et al., 2013). A. marginale is commonly screened in cattle as part of tick borne disease control program but there are very few reports around the globe regarding the prevalence of A. centrale in cattle. Present study is a pioneer study from Pakistan regarding the prevalence of A. centrale in three cattle breeds from Punjab, Pakistan.
Tropical countries have elevated temperatures and favorable climatic conditions for tick growth and that is the reason that tick borne diseases are more prevalent in these countries (Sen et al., 2010). Rhipicephalus spp. and Haemaphysailis spp. can transmit A. centrale to a variety of vertebrate hosts (Leger et al., 2013). Our results also confirm these findings as we had also collected ticks belonging to Rhipicephalus spp. and Haemaphysalis spp. from the enrolled cattle during present study although we were unable to detect A. centrale in these ticks (data not shown here).
During present study, we used both modern (PCR) and conventional method (blood smear screening) for t A. centrale detection in cattle blood. The conventional blood smear microscopy is considered as less sensitive and less reliable techniques as it can only detect the parasite if they infect 106 erythrocytes per ml during acute infections. It has been reported that animals recovered from acute anaplasmosis sustain a persistent infection characterized by 102.5-107 infected erythrocytes. This level of rickettsiemia cannot be detected during microscopic examination (Selcuk et al., 2015). PCR is a sensitive and reliable tool for the detection of tick borne parasites in blood (Ashraf et al., 2013). Our results from present study are also in agreement with these established facts as PCR picked A. centrale (14.4%) in more blood samples as compared to samples that were found Anaplasma spp. during blood smears screening (8.2%).
Belkahia et al. (2015) had enrolled 232 cattle from three areas in Tunisia. They had detected Anaplasma spp. in enrolled cattle blood by real-time PCR and/or nested PCR. They found that 34.9% were infected with Anaplasma spp., 25.4%, were positive for A. marginale, 15.1% for A. centrale and 3.9% cattle were infected with A. bovis. Prevalence of A. centrale reported by Belkahia et al. (2015) is closer to the A. centrale prevalence reported in present study (14.4%).
Aktas et al. (2011) used reverse line blot (RLB) hybridization assay to screen 389 cattle blood samples collected from six provinces in Turkey. 9% of enrolled cattle were found infected with Anaplasma spp. Highest prevalence was observed by A. marginale (2.8%), followed by A. centrale (1.0%) and A. phagocytophilum (1.0%). Ceci et al. (2014) had reported 13.8% prevalence of A. centrale in cattle samples enrolled from Southern Italy. While Mamoudou et al. (2017) had reported 53.5% present prevalence of A. centrale through blood smear screening in cattle from Vina division in Cameroon. The different prevalence rates of A. centrale in cattle reported from various parts of the world are probably due to different environmental and geographical conditions, tick control programmes, farm management strategies and husbandry practices in these areas (Magona et al., 2011, Belkahia et al., 2015).
There is no data available in literature regarding the genetic diversity of A. centrale from any animal species in Pakistan. Phylogenetic analysis that was based on a 426 base pair fragment of 16S rRNA gene of A. centrale was performed to investigate the genetic background of A. centrale in cattle from Pakistan. Results indicated that our amplified sequence was similar to 16S rRNA gene sequences of A. centrale documented from Philippines, Australia, Thailand, Italy, Algeria and Israel indicating that all these sequences are closely related (Fig. 1).
We observed a seasonal variation in the prevalence of A. centrale during current investigation and spring was found to be the season with highest A. centrale prevalence in cattle blood (Table 1). Our results are in line with those of Rajpoot et al. (2005) who had reported the prevalence of Anaplasma spp. was highest in buffalo and cattle sample collected during the month of February, March and April (spring season) and they assumed that this time period had favorable conditions for tick’s development and infestation. Our result are contradictory to Khan et al. (2019) who had reported high prevalence of Anaplasma spp. in cattle samples collected during summer from Khyber Pakhtonkhwa province in Pakistan followed by samples collected during spring season.
We found that Sahiwal breed was most susceptible breed for A. centrale infection during present study (Table 1). There is no trend in Pakistan regarding vaccination of cattle with A. centrale. Hence, this proves that A. centrale is present in local cattle as a parasite and should be diagnosed and treated in addition to A. marginale. Prevalence of A. centrale was lower in Holstein Friesian breed, an exotic breed, and this due to the fact that the exotic cattle breed are vaccination with A. centrale that provide them immunity against this parasite (Hove et al., 2018). Similarly, cross breed cattle in Pakistan are produced by crossing exotic breeds with local giving them characteristics of both (Saeed et al., 2016) that is the probable reason that cross breed were found less infected with A. centrale than Sahiwal breed during present study. Our result is in line with result of Belkahia et al. (2015) who had showed Holstein cattle were significantly less infected by A. centrale (p < 0.001) as compared to the local breeds. However our results are contradictory to the findings of Tay et al. (2014) who had reported a significantly higher Anaplasma spp. infection rate in Holstein Friesian breed than in any other breeds under investigation. Our result is also contradictory to the findings of Rehman et al. (2015) who had reported that crossbred cattle were affected more with anaplasmosis than indigenous cattle.
Analysis of the epidemiological data revealed that female cross breed cattle samples collected during autumn season had higher A. centrale infection than male (P = 0.05). This is in accordance with Vetrivel et al. (2017) who had reported higher prevalence of anaplasmosis in female cattle. Alim et al. (2012) had documented that prevalence of Anaplasma spp. varies with the sex of host animal. Females have higher parasite prevalence as they have lower immunity due to pregnancy associated stress and disturbed hormonal levels (Kamani et al., 2010). Another reason for higher parasite prevalence in female is the fact that they are more exposed to contaminated needles that are used to inject hormones for letdown of milk (Atif et al., 2012a, Atif et al., 2012b). The dog associated with herd of cattle and tick load on dog were the parameters that had showed significant association with the A. centrale infection during present study confirming that ticks can be transported to cattle herd via dogs. Contrary to our observation, Ashraf et al. (2013) had found higher prevalence of Anaplasma spp. in buffalo herds that do not had dogs associated with them. During present study, we observed that prevalence of A. centrale was significantly higher in cattle having tick burden. Our results are consistent with Ashuma et al. (2013) who had also reported higher prevalence of Anaplasma species in tick infested cattle. We observed that farms that had traditional management practices (like no trend for vaccination and no proper water supply for animals) had higher prevalence of A. centrale. Swai et al., 2005, Atif et al., 2012b had also reported higher prevalence of Anaplasma spp. in traditionally managed farms.
Complete blood count analysis indicated that A. centrale infection disturb the red blood cell and associated parameters of cattle Gannguly et al. (2017) had also documented that Anaplasma spp. infection in cattle results to extremely low hemoglobin and packed cell volumes. Our result also coincide with those of Khan et al. (2016) who had reported anemia as the major clinical finding in Anaplasma spp. infected cattle along with decreased red blood cell associated hematological parameters. We observed that white blood cells and platelet count were significantly increased, while lymphocytes % was decreased in A. centrale positive cattle blood samples during present study. Our result are contradictory to the finding of Khan et al. (2016) who had recorded significant decrease in white blood cells in pre patent phase and a non-significant decrease during anaplasmosis in cows and buffaloes of District Charsadda in Pakistan.
In conclusion, we are reporting that Sahiwal breed was most susceptible to A. centrale infection A. centrale prevalence varied significantly with the sampling season. A. centrale infection also disturbs the complete blood count of cattle. This is a pioneer study regarding prevalence of A. centrale in cattle from Pakistan and this data will pave the way for the control of A. centrale in Pakistan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are grateful to the directorate of Research and External Linkages of Bahauddin Zakariya University Multan (Pakistan) for funding this project via grant number DR & EL/D-545. DNA sample of A. centrale was kindly donated by Prof. Kelly A. Brayton from Washington State University, USA was used as positive control in PCR.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Abdullah D. Alanazi, Email: aalanazi@su.edu.sa.
Furhan Iqbal, Email: furhan.iqbal@bzu.edu.pk.
References
- Aktas M., Altay K., Dumanli N. Molecular detection and identification of 520Anaplasma and Ehrlichia species in cattle from Turkey. Tick. Tick Born. Dis. 2011;2:52162–52165. doi: 10.1016/j.ttbdis.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Alim M.A., Das S., Roy K., Masuduzzaman M., Sikder S., Hassan M.M. Prevalence of hemoprotozoan diseases in cattle population of Chittagong Division. Bangladesh. Pak. Vet. J. 2012;32(2):221–222. [Google Scholar]
- Ammazzalorso A.D., Zolnik C.P., Daniels T.J., Kolokotronis S. To beat or not to beat a tick: comparison of DNA extraction methods for ticks (Ixodes scapularis) Peer J. 2015;3 doi: 10.7717/peerj.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf Q.U.A., Khan A.U., Khattak R.M., Ali M., Shaikh R.S., Ali M., Iqbal F. A report on high prevalence of Anaplasma spp. In buffaloes from two provices in Pakistan. Tick. Tick Born. Dis. 2013;4:395–398. doi: 10.1016/j.ttbdis.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Ashuma A., Sharma A., Singla L.D., Kaur P., Bal M.S., Batth B.K., Juyal P.D. Prevalence and haemato-biochemical profile of Anaplasma marginale infection in dairy animals of Punjab (India) Asia. Pac. J. Trop. Med. 2013;6:139–144. doi: 10.1016/S1995-7645(13)60010-3. [DOI] [PubMed] [Google Scholar]
- Atif F.A., Khan M.S., Iqbal H.J., Roheen T. Prevalence of tick-borne diseases in Punjab (Pakistan) and hematological profile of Anaplasma marginale infection in indigenous and crossbred cattle. Pak. J. Sci. 2012;64:11–15. [Google Scholar]
- Atif F.A., Khan M.S., Iqbal H.J., Arshad G.M., Ashraf E., Ullah S. Prevalence of Anaplasma marginale, Babesia bigemina and Theileria annulata infections among cattle in Sargodha District. Pakistan. Afr. J. Agricul. Res. 2012;7(22):302–3307. [Google Scholar]
- Battilani M., Arcangeli S.D., Balboni A., Dondi F. Genetic diversity and molecular epidemiology of Anaplasma. Infec. Genet. Evol. 2017;49:195–211. doi: 10.1016/j.meegid.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Belkahia H., Ben Said M., Alberti A., Abdi K., Issaoui Z., Hattab D., Messadi L. First molecular survey and novel genetic variants’ identification of Anaplasma marginale, A. centrale and A. bovis in cattle from Tunisia. Infect. Genet. Evol. 2015;34:361–371. doi: 10.1016/j.meegid.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Bock R.E., de Vos A.J., Kingston T.G., Carter P.D. Assessment of a low virulence Australian isolate of Anaplasma marginale for pathogenicity, immunogenicity and transmissibility by Boophilus microplus. Vet. Parasitol. 2003;118:121–131. doi: 10.1016/j.vetpar.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Ceci L., Iarussi F., Greco B., Lacinio R., Fornelli S., Carelli G. Retrospective study of hemoparasites in cattle in southern Italy by reverse line blot hybridization. J. Vet. Med. Sci. 2014;76:869–875. doi: 10.1292/jvms.13-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannguly A., Bilsa R.S., Singh H., Bhanot V., Kumar A., Kumar S., Maharana B.R., Ganguly I. Prevalence and haemato-biochemical changes of tick-borne haemoparasitic diseases in crossbred cattle of Haryana. India. J. Ani. Sci. 2017;87(5):552–557. [Google Scholar]
- Hove, P., Khumalo, Z.T.H., Mamohale, E., Chaisi, M.E., Oosthuizen, M. C., Brayton, K.A., Collins, N.E., 2018. Detection and Characterisation of Anaplasma marginale and A. centrale in SouthAfrica. Vet. Sci. 5, doi:10.3390/vetsci5010026. [DOI] [PMC free article] [PubMed]
- Jabbar A., Abbas T., Sandhu Z.U.D., Saddiqi H.A., Qamar M.F., Gasser R.B. Tick-borne diseases of bovines in Pakistan: major scope for future research and improved control. Parasit. Vect. 2015;8:283–289. doi: 10.1186/s13071-015-0894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamani J., Sannusi A., Eqwu O.K., Dogo G.I., Tanko T.J., Kemza S., Takarki A.E., Gbise D.S. Prevalence and significance of haemoparasitic infections of cattle in North-Central. Nigeria. Vet. World. 2010;3:445–448. [Google Scholar]
- Khan A., Saeed K., Nasreen S., Niaz S., Akhtar N. Prevalence of Anaplasmosis in Cows and Buffaloes of District Charsadda, Khyber Pakhtunkhwa. Pakistan. Glob. Vet. 2016;16(5):431–440. [Google Scholar]
- Khan N.U., Sarwar M.S., Ayaz S., Ali H., Ali A., Khan I., Khan M.A., Khan A.U., Hussain M., Ali M., Rashid G. Prevalence and risk factors analysis associated with anaplasmosis in symptomatic cattle under field conditions in southern Khyber Pakhtoonkhwa. Pakistan. Pure Appl. Biol. 2019;8(4):2119–2127. [Google Scholar]
- Kawahara M., Rikihisa Y., Lin Q., Isogai E., Tahara K., Itagaki A., Hiramitsu Y., Tajima T. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia spp. in wild deer and ticks on two major islands in Japan. Appl. Environ. Microbiol. 2006;72:1102–1109. doi: 10.1128/AEM.72.2.1102-1109.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khumalo Z.T., Catanese H.N., Liesching N., Hove P., Collins N.E., Chaisi M.E., Gebremedhin A.H., Oosthuizen M.C., Brayton K.A. Characterization of Anaplasma marginale subspecies centrale strains using msp1aS genotyping reveals a wildlife reservoir. J. Clin. Microbiol. 2016;54(10) doi: 10.1128/JCM.01029-16. https://journals.asm.org/doi/full/10.1128/JCM.01029-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. A simple method or estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kocan K.M., de la Fuente J., Bouin E.F., Coetzee J.F., Ewing S.A. The natural history of Anaplasma marginale. Vet. Parasitol. 2010;167:95107. doi: 10.1016/j.vetpar.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger data sets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger E., Vourc'h G., Vial L., Chevillon C., McCoy K.D. Changing distributions of ticks: causes and consequences. Exp. Appl. Acarol. 2013;59:219–244. doi: 10.1007/s10493-012-9615-0. [DOI] [PubMed] [Google Scholar]
- Magona J.W., Walubengo J., Olaho-Mukani W., Jonsson N.N., Welburn S.W., Eisler M.C. Spatial variation of tick abundance and seroconversion rates of indigenous cattle to Anaplasma marginale, Babesia bigemina and Therileria parva infections in Uganda. Exp. App. Acarol. 2011;55:203–213. doi: 10.1007/s10493-011-9456-2. [DOI] [PubMed] [Google Scholar]
- Madder, M., Horak, I., Stoltsz, H., (2014) Tick Identification. Pretoria: Facult. vet. Sci. Uni. Pretoria, 58.
- Mamoudou A., Nguetoum N.C., Sevidzem S.L., Manchang T.K., Ebene N.J., Zoli P.A. Bovine babesiosis and anaplasmosis in some cattle farms in the Vina Division. Adamaoua Pleateau. Int. J. Livestock Res. 2017;7(6):69–80. [Google Scholar]
- Pakistan Economic Survey 2013–14. Ministry of Finance, Islamabad: Government of Pakistan; 2014, 23–41.
- Rahman A., Sumon S., Khan M., Islam M. Current status of subclinical form of babesiosis and anaplasmosis in cattle at Rangpur district in Bangladesh. Prog. Agricul. 2015;26:51–59. [Google Scholar]
- Rar V., Golovljova I. Anaplasma, Ehrlichia and ‘‘Candidatus neoehrlichia’’ bacteria: pathogenicity, biodiversity and molecular genetic characteristics, a 735 review. Infect. Genet. Evol. 2011;11:1842–1861. doi: 10.1016/j.meegid.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Rajput Z.I., Song-hua H.U., Arijo A.G., Habib M., Khalid M. Comparative study of Anaplasma parasites in tick carrying buffaloes and cattle. J. Zhejiang Uni. Sci. 2005;6B(11):1057–1062. doi: 10.1631/jzus.2005.B1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman A., Jingdong L., Chandio A.A., Hussain I. Livestock production and population census in Pakistan: Determining their relationship with agricultural GDP using econometric analysis. Info. Process. Agricul. 2017;4(2):168–177. [Google Scholar]
- Saeed Z., Iqbal F., Hussain M., Shaikh R.S., Farooq U., Akbar A., Gulsher M., Ayaz M.M., Mahmood S.A., Ali M., Aktas M. Molecular Prevalence and Haematology of Tropical Theileriosis in in Cholistani Cattle from Nomadic Herds of the Cholistan Desert, Pakistan. Kafkas Uni. Vet. Fakultesi Dergisi J. 2016;22(2):281–286. [Google Scholar]
- Swai E.S., Karimuribo E.D., Ogden N.H., French N.P., Fitzpatrick J.L., Bryanto M.J. Seroprevalence estimation and risk factors for Anaplasma marginale on small holder dairy farmers in Tanzania. Trop. Anim. Health Prod. 2005;37:599–610. doi: 10.1007/s11250-005-4307-y. [DOI] [PubMed] [Google Scholar]
- Selcuk O., Alver O., Çatik S., Aydin L., Senlik B. Determination of Diagnostic Value of cELISA for the Diagnosis of Anaplasmosis in Clinically Suspected Ruminants. Kafkas Univ. Vet. Fak. Derg. 2015;21(5):691–695. [Google Scholar]
- Sen E., Uchishima Y., Okamoto Y., Fukui T., Kadosaka T., Kadosaka T., Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet. Parasitol. 2010;167:108–122. doi: 10.1016/j.vetpar.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Srikant G., Gaurav N. Problem of ticks and tick borne diseases in India with special emphasis on progress in tick control research: A review. J. Vect. Born. Dis. 2014;51:259–327. [PubMed] [Google Scholar]
- Takihi I.Y., Sandes A.F. Killers on the road: Klebsiella and Pseudomonas bacteremia detected on peripheral blood smear. Blood. 2013;122:1851. doi: 10.1182/blood-2013-04-496620. [DOI] [PubMed] [Google Scholar]
- Tay S.T., Koh F.X., Kho K.L., Ong B.L. Molecular survey and sequence analysis of Anaplasma spp. in cattle and ticks in a Malaysian farm. Trop. Biomed. 2014;31:769–776. [PubMed] [Google Scholar]
- Vetrivel D.A., Serma S.P.J., Shilpa J.S. A study on predisposing factors for the prevalence of anaplasmosis in dairy cattle. J. Entomol. Zool. 2017;5:1228–1232. [Google Scholar]