Abstract
Objectives:
To define the clinical characteristics of hospitalized children with moderate traumatic brain injury (modTBI) and identify factors associated with deterioration to severe TBI.
Design:
Retrospective Cohort Study
Setting:
Tertiary Children’s Hospital with Level 1 Trauma Center designation
Patients:
Inpatient children less than 18 years of age with an international classification of diseases code for TBI and an admission Glasgow Coma Scale (GCS) score of 9–13.
Measurements and Results:
We queried the National Trauma Data Bank for our institutional data and identified 177 patients with modTBI from 2010–2017. These patients were then linked to the electronic health record (EHR) to obtain baseline and injury characteristics, laboratory data, serial GCS scores, CT findings, and neurocritical care interventions. Clinical deterioration was defined as ≥2 recorded values of GCS ≤8 during the first 48 hours of hospitalization. Thirty-seven patients experienced deterioration. Children who deteriorated were more likely to require intubation (73 vs 26%), have generalized edema, subdural hematoma or contusion on CT scan (30 vs 8%, 57 vs 37%, 35 vs 16% respectively), receive hypertonic saline (38 vs 7%), undergo intracranial pressure monitoring (24 vs 0%), were more likely to be transferred to inpatient rehabilitation following hospital discharge (32% vs 5%), and incur greater costs of care ($25,568 vs $10,724) (all p<0.01). There was no mortality in this cohort. Multivariable regression demonstrated that a higher injury severity score, a higher initial INR, and a lower admission GCS score were associated with deterioration to severe TBI in the first 48 hours (p<0.05 for all).
Conclusions:
A substantial subset (21%) of children presenting with modTBI at a Level 1 pediatric trauma center experienced deterioration in the first 48 hours, requiring additional resource utilization associated with increased cost of care. Deterioration was independently associated with an increased INR, higher ISS and a lower admission GCS.
Keywords: traumatic brain injury, moderate, pediatric, coagulopathy, injury severity score
Introduction
Pediatric traumatic brain injury (TBI) is a leading cause of death and disability in the pediatric population. It is estimated that TBI results in more than 600,000 emergency visits, 50,000 hospitalizations, and 7000 deaths; costs for TBI exceed $1 billion every year in the United States alone [1–3]. In addition to acute care costs, children with severe injuries often require lifelong care and rehabilitation, leading to significant financial burden [2, 4]
Moderate TBI (modTBI) represents subset of TBI with heterogenous outcomes ranging from complete return to baseline to severe impairment and death [5]. Rather than evaluating it as a distinct entity, most studies to date have grouped pediatric modTBI together with mild or severe TBI in terms of patient characteristics, clinical course and outcomes. As a result, insight regarding the epidemiology and clinical characteristics of this population is limited [6–9]. To date, only one study has examined epidemiology and outcome of modTBI in children using the data collected by the National Trauma Databank (NTDB) [9]. This study by reported that 59% of patients were admitted to an intensive care unit (ICU), 3% received intracranial pressure (ICP) monitoring and 0.7% died; notably, this mortality figure is markedly lower than that seen in adults with modTBI [5]. Furthermore, older age, low admission GCS score, high injury severity score (ISS) and the presence of polytrauma were associated with unfavorable short-term outcomes. This study indicated that there are certain subgroups of patients in the modTBI cohort who are vulnerable to deterioration and worse outcomes. Early identification of children at risk could enable triage of these children to centers with pediatric intensive care expertise for optimal management during hospitalization.
The majority of the studies investigating the risk factors for deterioration in modTBI are limited either by a small sample size or by the use of an administrative database [5]. We identified pediatric modTBI patients admitted to our hospital using the NTDB and then linked this information with our institutional EHR to obtain baseline and injury characteristics, laboratory data, serial GCS scores, CT findings, and neurocritical care interventions to identify factors associated with neurological deterioration.
Methods
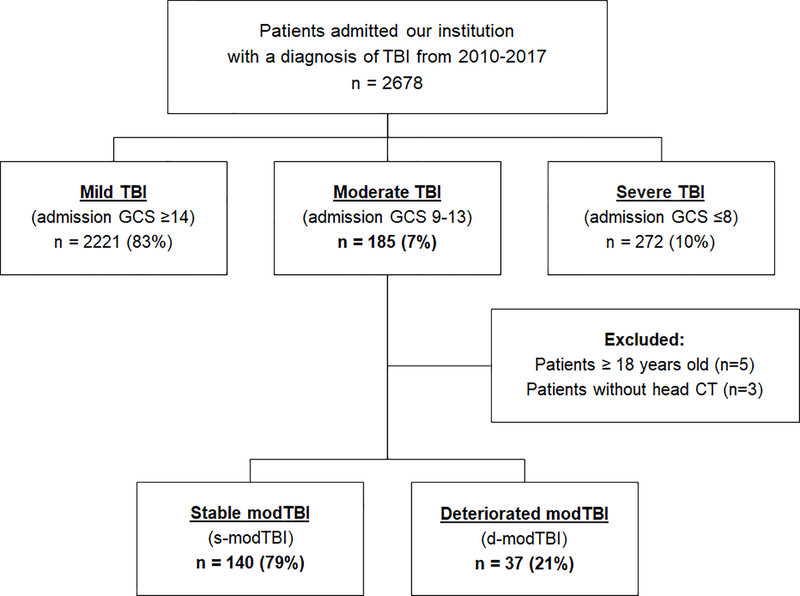
We conducted a retrospective cohort study querying the NTDB for our Level 1 Trauma Center’s data related to a head Abbreviated Injury Scale (AIS) code consistent with TBI from 2010–2017. Patients identified in the NTDB were linked to the EHR. ModTBI was defined as an admission GCS score of 9–13 as reported by the EHR. Patients without head CT imaging were excluded due to potential misclassification of spinal cord-injury related motor deficits. Patients were divided into two groups: 1) stable modTBI (s-modTBI), who remained clinically stable through their stay and 2) deteriorated modTBI (d-modTBI), whose clinical condition worsened to severe TBI. To avoid over-classification of temporary changes, clinical deterioration was defined as ≥2 recorded values of GCS ≤8 during the first 48 hours of hospitalization. Additionally, as sedation can lead to inaccurate measurement of GCS scores, University of Michigan Sedation Scale (UMSS) scores were extracted from the EHR and patients with a sedation score of >2 at the time when GCS was <9 were included in the s-modTBI group as these were not considered as true neurological deterioration (Figure 1) [10]. This study was approved by the institutional review board of the University of Pittsburgh (STUDY20050220).
Figure 1:
Flowchart of children with moderate traumatic brain injury (modTBI) characterized by a Glasgow Coma Scale (GCS) score of 9–13 on admission in electronic medical records.
Patient Characteristics
Data obtained from the NTDB included age, sex, race, cause of injury, AIS for each body region and ISS. Cause of injury was divided into 6 categories: falls, motor vehicle collisions (MVC), abusive head trauma (AHT), bicycle injuries, struck by/against, and other. Polytrauma was defined as an AIS score of >2 in body regions other than the head.
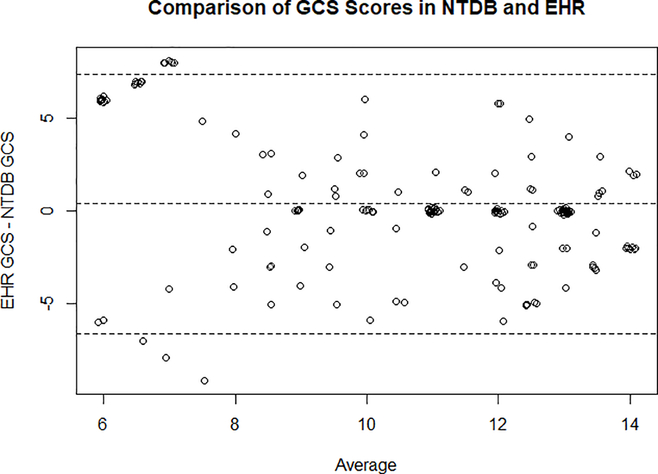
All data regarding clinical characteristics including hospital length of stay (LOS), pediatric ICU (PICU) admission, PICU LOS, and neurocritical care interventions were obtained from the EHR. CT findings on admission were extracted from radiology reports by chart review to identify radiographic diagnoses of traumatic subarachnoid hemorrhage (tSAH), epidural hematoma (EDH), subdural hematoma (SDH), skull fracture, contusion, midline shift and generalized brain edema. Rotterdam scores were obtained from the first available non-contrast head CT obtained during admission and were calculated according to methods described by Maas et.al [11]. Neurosurgical interventions included hematoma evacuation, burr hole placement, craniotomy, skull fracture elevation and decompressive craniectomy. Geographically-adjusted costs of care were obtained for each encounter from the Pediatric Health Information System database. To analyze differences between GCS values obtained from EHR and NTDB, we extracted the first GCS recorded for all patients from both databases separately and compared these values in a Bland-Altman Plot (Figure 2).
Figure 2:
Comparison of GCS scores from different database sources of children presenting with an ED GCS of 9–13 in either the NTDB or the EHR. The vertical axis shows the difference between GCS scores from NTDB and EHR. The horizontal axis represents the average of these two measures.
Data are presented as mean ± standard deviation (SD) or median interquartile range (IQR). For univariate analysis, continuous data were compared with a two-sided t-test and the Mann-Whitney test, and proportions were compared with Fisher’s exact or Chi-squared tests. Associations between independent variables were assessed with a stepwise logistic regression model that accounts for significant interactions between variables. Variables included in the analysis were: age, gender, cause of injury, ISS, admission GCS, first hemoglobin, INR and glucose. Presence of polytrauma and CT findings were excluded from the analysis due to collinearity between both variables and ISS. Missing values for laboratory parameters were assumed normal and imputed with medians. INR values were standardized to have a mean of 0 and a standard deviation of 1 to demonstrate clinically meaningful change for final logistic regression analysis. All analysis was conducted using R version 3.5.1 (The R Foundation for Statistical Computing, Institute for Statistics and Mathematics, Vienna, Austria). A p-value < 0.05 was considered statistically significant.
Results
A total of 2,678 patients were treated in our institution for TBI from 2010–2017. Based on initial GCS scores, 2,221 (83%) had mild, 185 (7%) had moderate, and 272 (10%) had severe TBI. In the modTBI cohort, we excluded 5 patients older than 18 years, 3 patients with no brain imaging after injury, and 4 patients with low GCS scores from sedation. The final number of patients with modTBI for this analysis was 177, and of this group, 37 (21%) experienced clinical deterioration. Median time to deterioration was 5.6 hours and 75% of patients deteriorated within 8 hours of presentation.
Patient demographics and admission characteristics are shown on Table 1. Characteristics were similar for both s-modTBI and d-modTBI groups. MVC and AHT were more common and falls were less common in d-modTBI than s-modTBI patients, although these differences did not reach statistical significance (30 vs. 26%, 30 vs 13%, 36 vs 19%, respectively, p= 0.12). The median GCS on admission was 11. Admission GCS was lower in d-modTBI vs. s-modTBI (10 vs 12, p<0.01). d-modTBI patients had higher median ISS (22 vs 13), higher AIS-Head (4 vs 3), and a higher percentage of polytrauma (37 vs. 15%, all p<0.01).
Table 1:
Characteristics of Children with Moderate Traumatic Brain Injury
| Admission Characteristics | All (n=177) | Stable (n=140) | Deteriorated (n=37) | p-value |
|---|---|---|---|---|
| Median Age (IQR) | 5 y (9 m, 11 y) | 5 y (9 m, 11 y) | 6 y (15 m – 10 y) | 0.83 |
| Male (%) | 122 (69) | 95 (68) | 27 (73) | 0.63 |
| Cause of Injury (%) | 0.12 | |||
| Fall | 58 (33) | 51 (36) | 7 (19) | |
| MVC | 47 (27) | 36 (26) | 11 (30) | |
| AHT | 29 (16) | 18 (13) | 11 (30) | |
| Bike | 16 (9) | 13 (9) | 3 (8) | |
| Other | 27 (15) | 22 (16) | 6 (16) | |
| Median Head AIS | 3 (2, 4) | 3 (2, 4) | 4 (3, 4) | < 0.01 |
| Median ISS (IQR) | 16 (9, 23) | 13 (9, 19) | 22 (16, 27) | < 0.01 |
| Polytrauma (%) | 35 (20) | 20 (15) | 15 (37) | <0.01 |
| Median GCS score on Admission (IQR) | 11 (10, 12) | 12 (10, 13) | 10 (9, 10) | < 0.01 |
| Initial CT Scan Findings (%) | ||||
| Epidural Hematoma | 28 (16) | 23 (16) | 5 (14) | 0.8 |
| Subdural Hematoma | 73 (41) | 52 (37) | 21 (57) | 0.040 |
| Subarachnoid Hemorrhage | 30 (17) | 23 (16) | 7 (19) | 0.80 |
| Cranial Fractures | ||||
| Basilar Fracture | 30 (17) | 24 (17) | 6 (16) | 1 |
| Vault Fracture | 58 (33) | 51 (36) | 7 (19) | 0.049 |
| Contusion | 37 (21) | 24 (17) | 13 (35) | 0.022 |
| Midline Shift | 16 (9) | 9 (6) | 7 (19) | 0.046 |
| Generalized Edema | 26 (15) | 11 (8) | 15 (30) | < 0.01 |
| CT Rotterdam Scores | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0.59 |
(MVC=motor vehicle accident, AHT=abusive head trauma, AIS=Abbreviated Injury Scale, ISS=Injury Severity Score, GCS=Glasgow Coma Scale, y=years, m=months)
The initial head CT showed at least one intracranial abnormality in 75% of patients with modTBI (Table 1). d-modTBI patients were more likely to have SDH (57 vs 37%,), midline shift (19 vs 6%), generalized edema (39 vs 7%) and contusion (37 vs 16%) on initial neuroimaging compared to s-modTBI (all p<0.05). The median Rotterdam Scores in both groups were 0 (IQR 0–1). Repeat CT scans of patients in the d-modTBI group showed worsening of SDH or EDH in 14 (38%) of patients. On univariate analysis, deterioration was also associated with a lower initial serum hemoglobin (mean ± SD: 12.3 ± 2.0 vs 12.28 ± 2.1 g/dL), higher initial glucose (mean ± SD: 158 ± 62 vs 134 ± 50) and a higher INR (mean ± SD: 1.3 ± 0.2 vs 1.1 ± 0.2) (all p<0.05) (Table 2).
Table 2:
Laboratory Parameters of Children with Moderate Traumatic Brain Injury at the Time of Presentation to the Emergency Department
| Initial Laboratory Parameters | All (n=177) | Stable (n=140) | Deteriorated (n=37) | p-value |
|---|---|---|---|---|
| Initial Hgb, mean ± SD | 12.06 ± 2.11 | 12.3 ± 2.03 | 11.2 ± 2.20 | 0.01 |
| Initial INR, mean ± SD | 1.16 ± 0.19 | 1.12 ± 0.17 | 1.27 ± 0.23 | < 0.01 |
| Initial Glucose, mean ± SD | 139 ± 53.69 | 134 ± 50.2 | 158 ± 62.1 | 0.03 |
| Initial Sodium, mean ± SD | 139 ± 2.86 | 139 ± 2.75 | 139 ± 3.07 | 0.82 |
Multivariable analysis using logistic regression modeling showed that a higher ISS, a higher INR and a lower admission GCS were associated with deterioration to severe TBI in the first 48 hours (Supplemental Digital Content 1). Each unit increase in ISS score was associated with a 1.07 (95% CI 1.02–1.14) greater odds of deterioration, whereas each unit increase in GCS was associated with a 0.51 (95% CI 0.36–0.71) lower odds of deterioration. Standardized INR values revealed that each standard deviation increase in INR level was associated with a 1.62 (95% CI 1.07–2.71) greater odds of deterioration.
A summary of critical care interventions is shown in Table 3. The median UMSS score of patients in the d-modTBI group at the time of deterioration was 1. The median UMSS score did not change within one hour of deterioration. 80% of patients with modTBI were admitted to the PICU with a median PICU LOS of 33 hours. PICU admission rate (100% vs. 74%), PICU LOS (6 vs. 1 days) and hospital LOS (9 vs. 3 days) were significantly higher in those with d-modTBI (all p<0.01). Children with d-modTBI were more likely to require intubation (73 vs. 26%), receive hypertonic saline (38 vs. 7%), and undergo ICP monitoring and (24 vs. 0%)(all p<0.01). ICP monitors were placed exclusively in 9 d-modTBI patients following neurologic deterioration; no s-modTBI patients required ICP monitoring. Of 14 patients who received hypertonic saline, 12 of them received the treatment after deterioration. Neurosurgical interventions included 13 EDH evacuations, 4 SDH evacuations, and 1 decompressive craniectomy and duraplasty; there was no difference in need for surgical intervention between d-mod and s-mod TBI patients.
Table 3:
Admission Course of Children with Moderate Traumatic Brain Injury
| Neurocritical Care Usage Parameters | All Patients (n=177) | Stable (n=140) | Deteriorated (n=37) | p-value |
|---|---|---|---|---|
| Median Hospital LOS days | 4 (2, 7) | 3 (2, 5) | 9 (7, 14) | < 0.01 |
| ICU Admission (%) | 141 (80) | 104 (74) | 37 (100) | < 0.01 |
| Median ICU LOS hours (IQR) | 33 (11–68) | 23 (0–47) | 133 (67–184) | < 0.01 |
| Neurosurgical Intervention | 18 (10) | 12 (8) | 6 (16) | 0.12 |
| Hypertonic Saline | 24 (14) | 10 (7) | 14 (38) | < 0.01 |
| Mechanical Ventilation | 68 (38) | 33 (26) | 27 (73) | < 0.01 |
| ICP Monitor | 9 (5) | 0 (0) | 9 (24) | < 0.01 |
| Median Cost (IQR) | 12,610 (7,170, 20,537) | 10,724 (6,509, 16,729) | 25,568 (19,385, 43,028) | < 0.01 |
(LOS=Length of Stay, ICU=Intensive Care Unit, ICP=Intracranial Pressure)
The median cost of hospitalization was significantly higher in d-modTBI vs s-modTBI ($25,568 vs $10,724, p<0.01). We observed no mortality in the modTBI cohort. Patients who deteriorated during their stay were more likely to be transferred to a rehabilitation facility following discharge (32% vs 5%, p<0.01;Supplemental Digital Content 2).
Discussion
ModTBI represents a spectrum of disease, with reported outcomes ranging from complete recovery to severe disability and death. Major organizations including ATLS and CDC define modTBI as an initial GCS score of 9–12 [12, 13]. NTDB however, defines mild TBI as a GCS of 14–15 and severe TBI as <9. Additionally, studies have shown that outcomes of patients with an initial GCS of 13 were more similar to modTBI than the mild TBI population[14, 15], leading to inclusion patients with a GCS of 13 in moderate TBI analysis[5, 16]. We included patients with GCS 13 given this patient population is at risk for worse outcomes. We aimed to define the characteristics of the modTBI population and identify subgroups of patients at risk for deterioration and associated critical care and hospital resource utilization, and worse short-term outcomes. In our cohort, 23% of patients initially presenting as modTBI with a GCS of 9–13 experienced clinical deterioration (d-modTBI). As anticipated, these children were admitted to the PICU more frequently, had longer LOS, incurred higher costs of care, and were more likely to receive additional neurocritical care interventions. Our logistic regression model demonstrated that higher ISS, higher INR and lower admission GCS scores were associated with deterioration to severe TBI.
The NTDB is the largest aggregation of standardized trauma registry data from the U.S. ever assembled and has yielded vital insights regarding the epidemiology and management of trauma. However, germane to TBI, EHR data can offer a higher level of detail (i.e. laboratory values, imaging findings and serial GCS measurements), with less missing data than administrative data. This study capitalizes on the comprehensive reporting of trauma to the NTDB, combined with the higher level of detail offered by the EHR.
The association of a lower GCS score with worse outcomes is consistent with previous findings in both children and adults with modTBI[5, 16]. Although the GCS score is prone to misinterpretation due to confounding factors including sedation and intubation, it has been the most extensively used method to define TBI severity. Previous studies have shown that even within the modTBI cohorts, patients had worse outcomes if they presented with a GCS of 9–10 compared to a GCS of 11–13.[5, 17]. Similarly, our study showed that the risk of deterioration was higher with a lower GCS score on admission within the moderate category.
The ISS score is a measure of trauma severity. It is derived from AIS, an anatomical classification system that codes eight different body regions with a score of 1–6; 1 being minor and 6 being nonsurvivable injuries[18]. A high ISS might be caused by a high head AIS in an isolated TBI case, or it may indicate the presence of other injuries in different body regions. Previous studies in pediatric modTBI showed that a high ISS and polytrauma were associated with a poor outcome[5, 16]. Interestingly, logistic regression models using polytrauma instead of ISS scores did not show any significantly increased risk of deterioration in our cohort. Thus, it may be that the association between deterioration and high ISS in our cohort is mediated by head injury.
Coagulopathy is an established prognostic indicator in children with trauma and particularly, TBI. [19–22]. It has been demonstrated that the probability of having an abnormal coagulation test increases as TBI severity increases [23]. A study in combat support hospitals showed that 27% of children with trauma had presented with early coagulopathy (INR >1.5). Coagulopathy was associated with a higher ISS and both were independently associated with mortality [24]. Similar findings on coagulopathy have also been demonstrated in children with different mechanisms of injury, including AHT [25, 26]. A study Leeper et. al showed that while elevated INR strongly predicted mortality, product transfusion did not influence the INR trend or the outcome; indicating that INR serves as a marker for systemic dysregulation rather than a treatment target [19].
These findings together imply that there are subgroups of severity in the modTBI cohort itself at higher risk for deterioration. From the perspective of initial diagnostic information, our data suggest clinical parameters that could direct care—namely, that pediatric modTBI patients with an initial GCS of 9–10, higher ISS, elevated INR, and/or with either diffuse swelling or contusion on CT predict the greatest risk. Such patients may benefit for consideration of interventions such as a more intensive nurse staffing ratio in the initial 48 h, or heightened vigilance in carrying out serial monitoring of GCS or neurological examinations, repeat imaging, the need for ICP monitoring, and/or therapy directed at brain edema. This concept is supported by recent initiatives, largely in adults, to more fully phenotype patients with TBI—and that the traditional classification of mild, moderate, and severe may be inadequate [27] [28]. Our findings also highlight the fact that current severe TBI guidelines fail to provide recommendations for pediatric modTBI patients [29], and even the consensus and evidence-based algorithm for bedside care does not address this important gap [30]. Retrospectively, we showed that only 22% of d-mod TBI patients had ICP monitors placed and 34% received hypertonic saline in the d-modTBI group; 0% of the s-mod TBI and 7% of the s-modTBI group received it as well underwent these interventions, respectively. The inconsistency between critical care interventions in the deterioration group can also be explained by the lack of evidence based guidelines for the deteriorated modTBI patients; therefore the decision to transition them to a severe TBI protocol was made on a case-by-case basis.
There are several limitations to this study. First, this is a retrospective study and long-term data on cognitive outcome was not available. In addition, we had no mortality in this cohort of 177 cases, suggesting a high level of care. That finding, however, limited our ability to link deterioration with either long term outcome or mortality. Nevertheless, our primary study goal was focused on factors associated with deterioration. Our study utilizes GCS as an indicator of brain injury, though GCS can be influenced by neurologic changes that are not a result of the primary brain trauma. Additional confounding factors regarding deterioration include variations in management that could impact progression of brain swelling, such as differences in fluid management/balance in the cohort, or other therapies [31].
This study shows that a subset of children presenting with modTBI at our level 1 pediatric trauma center experienced significant deterioration in the first 48 hours, requiring additional resource utilization associated with significant cost of care. Deterioration was independently associated with an increased INR, higher ISS and a lower admission GCS. Additional work is necessary to verify characteristics heralding potential neurologic deterioration in this unique and heterogeneous population.
Supplementary Material
Report in context.
Numerous studies have investigated the epidemiology and clinical characteristics of mild and severe TBI and identified independent risk factors for poor outcomes. However, there is paucity of data on risk factors for poor outcome after modTBI, particularly in children.
Administrative databases provide large sample sizes and have yielded vital insights regarding the epidemiology of trauma; however, they are limited by voluntary participation, missing data, lack of important parameters, which may be mitigated by use of data extracted from EHR.
To our knowledge, this study is the first study that identifies clinical characteristics associated with deterioration in children with modTBI using EHR data in conjunction with administrative databases.
At the bedside.
A substantial subset (23%) of patients initially presenting as modTBI with a GCS of 9–13 experienced clinical deterioration and required additional critical care resource utilization.
A GCS of 9–10, higher ISS and elevated INR were independently associated with clinical deterioration in pediatric modTBI patients.
Acknowledgements.
This work is supported, in part, by grants from the National Institutes of Health (NS061817, NS076511), the scientific program of the UPMC Children’s Hospital of Pittsburgh and the Children’s Hospital of Pittsburgh Trust Young Investigator’s Award.
Copyright form disclosure: Drs. Soysal and Bayir received support for article research from the UPMC Children’s Hospital of Pittsburgh Foundation Trust Young Investigator Award. Dr. Tyler-Kabara’s institution received funding from RegenexBio, Tekeda, and the National Institutes of Health (NIH). Dr. Kochanek’s institution received funding from NIH and US Army; he has received funding from the Society of Critical Care Medicine (Editor-in-Chief); and he has served as an expert witness on several cases over the past 36 months. Dr. Bayir received support for article research from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References.
- 1.Dewan MC, Mummareddy N, Wellons JC 3rd et al. : Epidemiology of Global Pediatric Traumatic Brain Injury: Qualitative Review. World Neurosurg 2016, 91:497–509 e491. [DOI] [PubMed] [Google Scholar]
- 2.Schneier AJ, Shields BJ, Hostetler SG et al. : Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics 2006, 118(2):483–492. [DOI] [PubMed] [Google Scholar]
- 3.Langlois JA, Rutland-Brown W, Thomas KE: The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil 2005, 20(3):229–238. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe KM, Massagli TL, Martin KM et al. : Pediatric traumatic brain injury: acute and rehabilitation costs. Arch Phys Med Rehabil 1993, 74(7):681–686. [DOI] [PubMed] [Google Scholar]
- 5.Chandee T, Lyons VH, Vavilala MS et al. : Critical Care Resource Utilization and Outcomes of Children With Moderate Traumatic Brain Injury. Pediatr Crit Care Med 2017, 18(12):1166–1174. [DOI] [PubMed] [Google Scholar]
- 6.Liesemer K, Bratton SL, Zebrack CM et al. : Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: rates, risk factors, and clinical features. J Neurotrauma 2011, 28(5):755–762. [DOI] [PubMed] [Google Scholar]
- 7.Teasdale G, Jennett B: Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2(7872):81–84. [DOI] [PubMed] [Google Scholar]
- 8.Wilde EA, Chu Z, Bigler ED et al. : Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma 2006, 23(10):1412–1426. [DOI] [PubMed] [Google Scholar]
- 9.Wilde EA, Hunter JV, Newsome MR et al. : Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J Neurotrauma 2005, 22(3):333–344. [DOI] [PubMed] [Google Scholar]
- 10.Malviya S, Voepel-Lewis T, Tait AR et al. : Depth of sedation in children undergoing computed tomography: validity and reliability of the University of Michigan Sedation Scale (UMSS). Br J Anaesth 2002, 88(2):241–245. [DOI] [PubMed] [Google Scholar]
- 11.Maas AI, Hukkelhoven CW, Marshall LF et al. : Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2005, 57(6):1173–1182; discussion 1173–1182. [DOI] [PubMed] [Google Scholar]
- 12.Kortbeek JB, Al Turki SA, Ali J et al. : Advanced trauma life support, 8th edition, the evidence for change. J Trauma 2008, 64(6):1638–1650. [DOI] [PubMed] [Google Scholar]
- 13.Lumba-Brown A, Yeates KO, Sarmiento K et al. : Centers for Disease Control and Prevention Guideline on the Diagnosis and Management of Mild Traumatic Brain Injury Among Children. JAMA Pediatr 2018, 172(11):e182853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Servadei F, Teasdale G, Merry G et al. : Defining acute mild head injury in adults: a proposal based on prognostic factors, diagnosis, and management. J Neurotrauma 2001, 18(7):657–664. [DOI] [PubMed] [Google Scholar]
- 15.Mena JH, Sanchez AI, Rubiano AM et al. : Effect of the modified Glasgow Coma Scale score criteria for mild traumatic brain injury on mortality prediction: comparing classic and modified Glasgow Coma Scale score model scores of 13. J Trauma 2011, 71(5):1185–1192; discussion 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanitanon A, Lyons VH, Lele AV et al. : Clinical Epidemiology of Adults With Moderate Traumatic Brain Injury. Crit Care Med 2018, 46(5):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godoy DA, Rubiano A, Rabinstein AA et al. : Moderate Traumatic Brain Injury: The Grey Zone of Neurotrauma. Neurocrit Care 2016, 25(2):306–319. [DOI] [PubMed] [Google Scholar]
- 18.Baker SP, O’Neill B: The injury severity score: an update. J Trauma 1976, 16(11):882–885. [DOI] [PubMed] [Google Scholar]
- 19.Leeper CM, Kutcher M, Nasr I et al. : Acute traumatic coagulopathy in a critically injured pediatric population: Definition, trend over time, and outcomes. J Trauma Acute Care Surg 2016, 81(1):34–41. [DOI] [PubMed] [Google Scholar]
- 20.Talving P, Lustenberger T, Lam L et al. : Coagulopathy after isolated severe traumatic brain injury in children. J Trauma 2011, 71(5):1205–1210. [DOI] [PubMed] [Google Scholar]
- 21.Whittaker B, Christiaans SC, Altice JL et al. : Early coagulopathy is an independent predictor of mortality in children after severe trauma. Shock 2013, 39(5):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peiniger S, Nienaber U, Lefering R et al. : Balanced massive transfusion ratios in multiple injury patients with traumatic brain injury. Crit Care 2011, 15(1):R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miner ME, Kaufman HH, Graham SH et al. : Disseminated intravascular coagulation fibrinolytic syndrome following head injury in children: frequency and prognostic implications. J Pediatr 1982, 100(5):687–691. [DOI] [PubMed] [Google Scholar]
- 24.Patregnani JT, Borgman MA, Maegele M et al. : Coagulopathy and shock on admission is associated with mortality for children with traumatic injuries at combat support hospitals. Pediatr Crit Care Med 2012, 13(3):273–277. [DOI] [PubMed] [Google Scholar]
- 25.Hymel KP, Abshire TC, Luckey DW et al. : Coagulopathy in pediatric abusive head trauma. Pediatrics 1997, 99(3):371–375. [DOI] [PubMed] [Google Scholar]
- 26.Merck LH, Yeatts SD, Silbergleit R et al. : The Effect of Goal-Directed Therapy on Patient Morbidity and Mortality After Traumatic Brain Injury: Results From the Progesterone for the Treatment of Traumatic Brain Injury III Clinical Trial. Crit Care Med 2019, 47(5):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawryluk GW, Manley GT: Classification of traumatic brain injury: past, present, and future. Handb Clin Neurol 2015, 127:15–21. [DOI] [PubMed] [Google Scholar]
- 28.Nielson JL, Cooper SR, Yue JK et al. : Uncovering precision phenotype-biomarker associations in traumatic brain injury using topological data analysis. PLoS One 2017, 12(3):e0169490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochanek PM, Tasker RC, Bell MJ et al. : Management of Pediatric Severe Traumatic Brain Injury: 2019 Consensus and Guidelines-Based Algorithm for First and Second Tier Therapies. Pediatr Crit Care Med 2019, 20(3):269–279. [DOI] [PubMed] [Google Scholar]
- 30.Kochanek PM, Tasker RC, Carney N et al. : Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines, Executive Summary. Pediatr Crit Care Med 2019, 20(3):280–289. [DOI] [PubMed] [Google Scholar]
- 31.Ekseth K, Abildgaard L, Vegfors M et al. : The in vitro effects of crystalloids and colloids on coagulation. Anaesthesia 2002, 57(11):1102–1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.