Abstract
Background:
Few data exist on long-term outcome in patients undergoing combined coronary CT angiography (CTA) and myocardial CT perfusion imaging (CTP) as well as invasive coronary angiography (ICA) and single photon emission tomography (SPECT).
Methods:
At 16 centers, 381 patients were followed for major adverse cardiac events (MACE) for the CORE320 study. All patients underwent coronary CTA, CTP, and SPECT before ICA within 60 days. Prognostic performance according binary results (normal/abnormal) was assessed by 5-year major cardiovascular events (MACE) free survival and area under the receiver-operating-characteristic curve (AUC).
Results:
Follow up beyond 2-years was available in 323 patients. MACE-free survival rate was greater among patients with normal combined CTA-CTP findings compared to ICA-SPECT: 85 vs. 80% (95% confidence interval [CI] for difference 0.1, 11.3) though event-free survival time was similar (4.54 vs. 4.37 years, 95% CI for difference: −0.03, 0.36). Abnormal results by combined CTA-CTP was associated with 3.83 years event-free survival vs. 3.66 years after abnormal combined ICA-SPECT (95% CI for difference: −0.05, 0.39). Predicting MACE by AUC also was similar: 65 vs. 65 (difference 0.1; 95% CI −4.6, 4.9). When MACE was restricted to cardiovascular death, myocardial infarction, or stroke, AUC for CTA-CTP was 71 vs. 60 by ICA-SPECT (difference 11.2; 95% CI −1.0, 19.7).
Conclusions:
Combined CTA-CTP evaluation yields at least equal 5-year prognostic information as combined ICA-SPECT assessment in patients presenting with suspected coronary artery disease. Noninvasive cardiac CT assessment may eliminate the need for diagnostic cardiac catheterization in many patients.
Clinical trial registration:
Keywords: Coronary heart disease, Coronary artery disease, Coronary atherosclerosis, Coronary imaging, CT angiography, Myocardial perfusion imaging
1. Introduction
More than 10,000,000 evaluations for coronary artery disease (CAD) are being performed each year in the US alone.1 To reduce the burden on our healthcare system, reliable and effective risk stratification of patients with suspected CAD is of utmost importance.2 Critical to the process of risk stratification is the identification of patients at high risk of adverse outcome who may benefit from coronary artery revascularization.2 Conversely, testing must not lead to invasive procedures that are unnecessary and costly. Evaluation for myocardial ischemia has been the traditional approach to patients with suspected CAD but non-invasive coronary angiography by CT (CTA) has recently been shown to be at least equally effective in this setting.3,4 Little is known about a combined approach of CTA and myocardial ischemia testing for the evaluation and risk stratification in patients with suspected CAD. The CORE320 international study reported high diagnostic accuracy of a combination of CTA with computed tomography myocardial perfusion imaging (CTP) to detect hemodynamically significant CAD, defined by an obstructive (≥50%) coronary artery stenosis on invasive coronary angiography (ICA) associated with a perfusion defect with single photon emission computed tomography (SPECT).5 Intermediate follow up after two years revealed similar risk assessment of CTA-CTP compared to ICA and SPECT but adverse events mostly consisted of revascularization procedures and there were few myocardial infarctions, strokes, or deaths, allowing limited conclusions on prognostic power.6 In the present study, we report the long-term follow up results of the CORE320 study. We hypothesized that CTP in combination with CTA predicts clinical events during 5 years of follow-up similarly as the combination of SPECT and ICA.
2. Methods
The Coronary Artery Evaluation using 320-row Multi-detector Computed Tomography Angiography and Myocardial Perfusion (CORE320) international study is a prospective, diagnostic study that enrolled participants at 16 centers in 8 countries between November 2009 and July 2011.5 The enrolled participants were between 45 and 85 years of age with suspected or known CAD and were referred for a clinical ICA. The detailed eligibility criteria, study design, main results and the 2-year follow-up results of the study were published in detail elsewhere.5–8 The study was approved by the institutional Review Boards at each enrolling center and all participants provided written informed consent for enrolment and for follow-up.
At baseline, all participants underwent coronary CTA, CTP, SPECT and ICA. Non-invasive imaging (CTA, CTP and SPECT) was completed within 60 days prior to ICA at CORE320 validated laboratories. Baseline assessment also included complete clinical history and physical examination. The images were interpreted in centralized core laboratories according to a standard, predefined protocol. Image acquisition and interpretation methods have been previously described.5,7–9
2.1. Endpoints
The primary endpoint was time-to-first major adverse cardiovascular event (MACE) within 5-years of follow-up. MACE included cardiac death, myocardial infarction, hospitalization for chest pain or congestive heart failure, late revascularization (beyond 30 days of index ICA), cardiac arrhythmia requiring hospitalization, death from non-cardiac causes, and cerebrovascular events. “Hard” events were defined as death, stroke, or non-fatal myocardial infarction. Hard cardiovascular events were defined as cardiac death, non-fatal myocardial infarction, or stroke.
2.2. Follow-up
All study participants were followed after ICA at 30 days, 6 months, 12 months, and annually thereafter for occurrence of any event. Preliminary data on health status and any potential adverse event or hospitalization were collected through office visits, telephone interviews or standardized questionnaires sent by mail. Additional data were then collected for subjects with a possible event by reviewing the medical records, and comprehensive patient interviews. Data in languages other than English was translated to English and all events were then reviewed by a dedicated adjudication committee consisting of representatives of study sites and members of the steering committee. Participants are considered lost to follow-up after three unsuccessful contact attempts.
2.3. Statistical analysis
Descriptive statistics were compared between MACE and non-MACE groups using the Wilcoxon rank-sum test or Pearson’s chi-squared test, as appropriate. Unadjusted comparisons of the distribution of 5-year survival used standard Kaplan-Meier curves and restricted mean survival times. Standard errors were estimated with the bootstrap method with 2000 replicates, resampling at the patient level to accommodate within-patient correlation due to the fact that each patient underwent all imaging tests. Restricted mean survival times are determined as the area under the Kaplan-Meier curve up to 5 years and can be interpreted as the expected time of event-free survival within 5 years after ICA. Area under the receiver operating characteristic curve (AUC) was used as a measure of diagnostic power, treating event occurrence within 5 years as a binary endpoint rather than time-to-event. In the AUC analyses, CTA, CTP, ICA, and SPECT were modelled as continuous variables, with the corresponding Leaman score also included in each model.10 Cox proportional hazard models were used to compute hazard ratios for adjusted models separately including disease status by CTA-CTP and by ICA-SPECT. Models were adjusted for age, sex, hypertension, diabetes, dyslipidemia, body max index (BMI), and history of myocardial infarction. Combined CTA-CTP and combined ICA-SPECT were defined as abnormal, i.e., presence of functionally significant CAD, if at least one vessel had a stenosis of 50% or greater by quantitative assessment and a corresponding myocardial perfusion defect was present in a corresponding territory by CTP (2) or SPECT (1).5,7,8 Conversely, results were considered normal if all coronary artery segments had less than 50% stenosis by CTA or ICA and CTP or SPECT did not have perfusion defects. For discrepant findings in the combination of tests, a consensus process was followed to determine if the study was normal or abnormal.9 In the Cox model, events which occurred within 3 months beyond the 5-year window were included; all later events captured in follow-up were excluded and subjects were censored as event-free at 5 years and 3 months. We tested whether the hazard ratio for CTA-CTP was non-inferior (within 5%) to that for ICA-SPECT using an adapted method from Chow et al.11 Only the first MACE event was considered in analyses (not repeated events).
3. Results
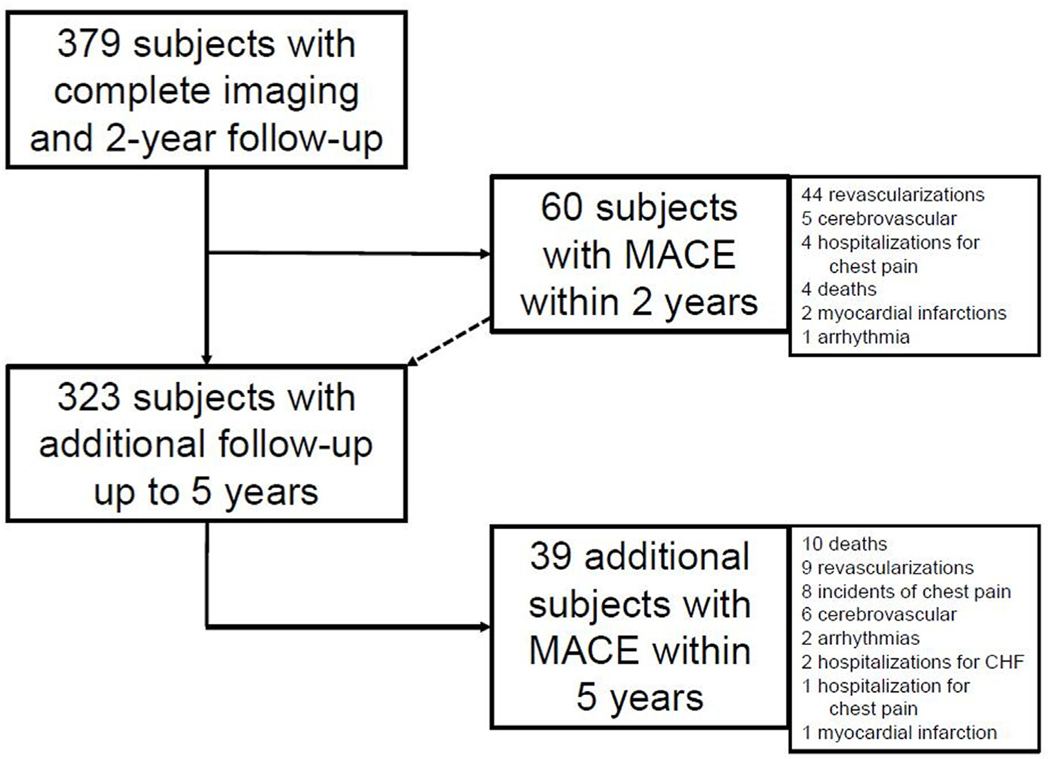
At baseline, 381 patients with clinical indication for ICA were enrolled in the CORE320 prospective multicenter study and underwent all imaging tests. Of those, complete imaging data are available in 379 patients with least 2 years of follow-up data (Fig. 1). Additional follow-up data through 5 years were available for 323 (85%) subjects. Eight participants withdrew consent after 2-year follow-up and 43 subjects were lost to follow-up after the second year—among them all 16 participants from Iwate Medical University, Japan, who could not have been reached after the Tsunami incident in 2011. Subjects without MACE who did not complete the additional 5-year follow-up were censored at the last follow-up time observed.
Fig. 1.
Flow of study patients and follow-up, An overview of patient flow and follow up is provided. Of 381 patients, 379 had complete imaging information and at least 2-year follow up. Of these, 56 patients were lost to follow up beyond 2 years while 323 had additional data collected.
3.1. Prediction of MACE for combined groups
During 1662 person-years of follow-up (median follow-up time of 4.97 years), 99 subjects experienced at least one MACE (6 cardiac deaths, 12 myocardial infarctions, 68 late revascularizations (>30 days after index ICA), 26 hospitalizations for chest pain, 4 hospitalizations for heart failure, 5 arrhythmias, 24 deaths from non-cardiac causes, and 16 strokes or cerebrovascular events). Baseline characteristics of the participants with and without MACE are summarized in Table 1. Subjects with MACE were predominantly white, older male participants, with higher rates of prior myocardial infarction and higher baseline coronary calcium scores (Table 1).
Table 1.
Baseline characteristics of patients with and without MACE.
| Characteristic | MACE (n = 99) | Non-MACE (n = 280) | P value |
|---|---|---|---|
|
| |||
| Age – year | 64 [58, 69] | 62 [55, 68] | 0.02 |
| Male sex | 76 (77%) | 176 (63%) | 0.01 |
| Ethnicity – number (%) | 0.03 | ||
| Hispanic | 5 (5%) | 25 (9%) | |
| Non-Hispanic | 83 (84%) | 243 (87%) | |
| Other | 11 (11%) | 12 (4%) | |
| Race | 0.005 | ||
| White | 66 (67%) | 147 (53%) | |
| Black | 6 (6%) | 33 (12%) | |
| Asian | 24 (24%) | 99 (35%) | |
| Other | 3 (3%) | 1 (<1%) | |
| Body mass index | 26 [24, 30] | 27 [24, 30] | 0.51 |
| Hypertension | 82 (83%) | 213 (77%) | 0.20 |
| Diabetes | 38 (38%) | 92 (33%) | 0.32 |
| Dyslipidemia | 66 (69%) | 186 (68%) | 0.84 |
| Previous myocardial infarction | 37 (37%) | 66 (24%) | 0.008 |
| Smoking | 0.50 | ||
| Current | 16 (17%) | 46 (17%) | |
| Past | 39 (41%) | 94 (35%) | |
| Never | 39 (41%) | 128 (48%) | |
| Family history of CAD | 49 (53%) | 112 (43%) | 0.09 |
| Prior percutaneous coronary intervention | 35 (35%) | 78 (28%) | 0.16 |
| History of unstable angina | 7 (7%) | 20 (7%) | 0.95 |
| Previous congestive heart failure | 14 (14%) | 34 (12%) | 0.61 |
| NYHA class I | 3 (21%) | 5 (15%) | |
| NYHA class II | 11 (79%) | 28 (82%) | |
| NYHA class III | 0 (0%) | 1 (3%) | |
| NYHA class IV | 0 (0%) | 0 (0%) | |
| Previous cerebrovascular accident | 5 (5%) | 6 (2%) | 0.14 |
| Previous transient ischemic attack | 0 (0%) | 10 (4%) | 0.06 |
| Cardiovascular Medications – n | |||
| (%) | |||
| ACEI/ARB | 47 (47%) | 121 (43%) | 0.46 |
| Beta-blocker | 60 (61%) | 143 (51%) | 0.10 |
| Salicylates | 68 (69%) | 174 (62%) | 0.24 |
| Nitrates | 21 (21%) | 47 (17%) | 0.32 |
| Other Anti-Hypertensive | 72 (73%) | 181 (65%) | 0.14 |
| Medication | |||
| Grace Risk Score | 101 [86, 120] | 94 [80, 110] | 0.008 |
| Diamond/Forrester Score | 0.18 | ||
| Low Risk | 3 (3%) | 5 (2%) | |
| Intermediate Risk | 59 (60%) | 195 (70%) | |
| High Risk | 37 (37%) | 80 (29%) | |
| Agatston Calcium Score | 326 [80, 734] | 115 [3, 454] | <0.0001 |
Baseline characteristics are presented for all patients with completed imaging at baseline (N = 379) according to the occurrence of MACE after 5-years follow up. Data in parentheses represent interquartile ranges or percentages, as applicable. MACE was defined as a composite of all-cause death, myocardial infarction, hospitalization for chest pain or congestive heart failure, stroke, late revascularization (beyond 30 days of index ICA) and arrhythmia.
Abbreviations: MACE: major adverse cardiovascular events; CAD: coronary heart disease; NYHA: New York Heart Association; ACEI: angiotensin-converting-enzyme inhibitor; ARB: angiotensin-receptor-blocker.
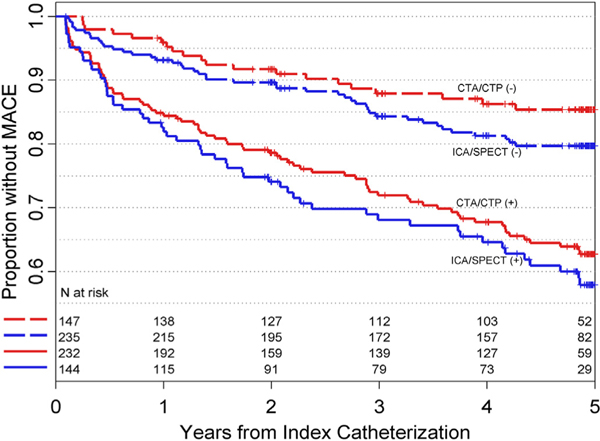
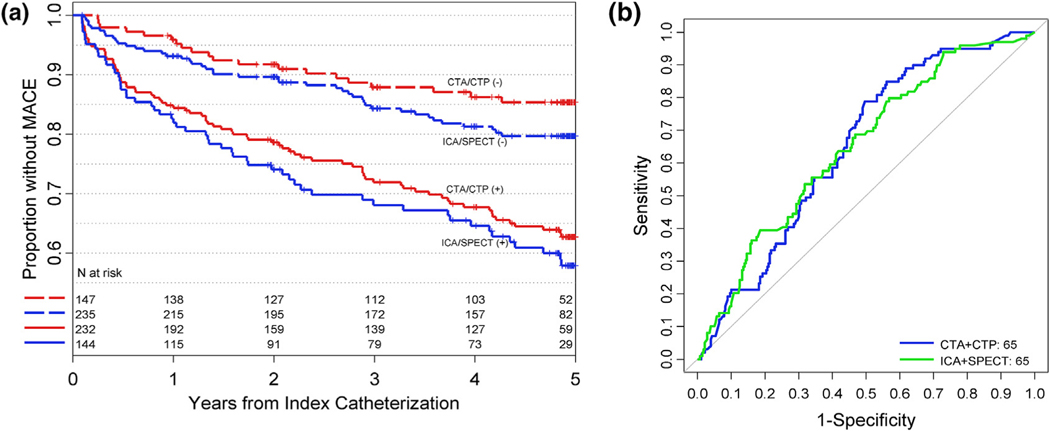
MACE-free survival was similar following normal CTA-CTP and normal ICA-SPECT (4.54 and 4.37 years, difference = 0.17; 95% CI: −0.03, 0.36) though survival rate was greater with normal CTA-CTP results compared to those with negative combined ICA-SPECT (85% versus 80%, difference = 5.7% (95% CI: 0.1, 11.3, Table 2a). MACE-free survival was significantly reduced (p < 0.001 for each) in patients with abnormal results both by CTA-CTP (3.83 years; 95% CI: 3.58, 4.06) and ICA-SPECT (3.66; 95% CI: 3.33, 3.98). Survival curves for both test combinations are shown in Fig. 2. CTA-CTP were similarly accurate as SPECT-ICA in predicting MACE (65; 95% CI: 59, 72) and 65 (95% CI: 59, 71, p > 0.05 for difference). Receiver-operating characteristic curves are presented in Fig. 3. Prediction of MACE was nominally better for CTA-CTP vs. ICA-SPECT when MACE was restricted to “hard” events, though without reaching statistical significance (Table 2a). Table 2b lists results for CTA and SPECT alone. Results were overall similar for CTA and SPECT with trends favoring CTA for prediction of hard cardiovascular events.
Table 2a.
Comparison of the diagnostic and prognostic performance by combined CTA-CTP and ICA-SPECT.
| CTA-CTP | ICA-SPECT | Difference | |
|---|---|---|---|
|
| |||
| Absence of hemodynamically significant coronary heart disease* | |||
| 5 Year Event-Free | |||
| Survival, % (95% CI) | |||
| MACE | 85 (79, 91) | 80 (74, 85) | 5.7 (0.1, 11.3) |
| Hard events | 94 (90, 98) | 92 (89, 96) | 1.7 (−1.8, 5.3) |
| Hard CV events | 98 (95, 100) | 95 (92, 98) | 2.7 (0.0, 5.6) |
| Restricted Mean Survival Time | |||
| MACE | 4.539 (4.323, 4.730) | 4.374 (4.189, 4.555) | 0.165 (−0.033, 0.363) |
| Hard events | 4.836 (4.705, 4.941) | 4.794 (4.676, 4.894) | 0.041 (−0.047, 0.143) |
| Hard CV events | 4.932 (4.839, 5.000) | 4.869 (4.769, 4.947) | 0.062 (−0.007, 0.152) |
| Presence of hemodynamically significant coronary heart disease | |||
| 5 Year Event-Free | |||
| Survival, % (95% CI) | |||
| MACE | 63 (56, 69) | 58 (49, 67) | 4.8 (−1.0, 11.1) |
| Hard events | 86 (81, 91) | 84 (76, 90) | 2.4 (−2.0, 6.6) |
| Hard CV events | 90 (85, 94) | 89 (83, 95) | 0.7 (−3.0, 4.4) |
| Restricted Mean Survival Time | |||
| MACE | 3.827 (3.581, 4.064) | 3.663 (3.325, 3.976) | 0.164 (−0.050, 0.387) |
| Hard events | 4.707 (4.574, 4.820) | 4.694 (4.523, 4.834) | 0.013 (−0.099, 0.116) |
| Hard CV events | 4.785 (4.664, 4.885) | 4.796 (4.655, 4.911) | −0.011 (−0.112, 0.073) |
| Diagnosis of 5 year events | |||
| AUC | |||
| MACE | 65 (59, 72) | 65 (59, 71) | 0.1 (−4.6, 4.9) |
| Hard events | 66 (58, 76) | 61 (55, 71) | 5.1 (−7.1, 12.9) |
| Hard CV events | 71 (62, 83) | 60 (54, 74) | 11.2 (−1.0, 19.7) |
Shown are the results for 5-year event-free survival among the 379 patients and prediction of MACE according to test results.
MACE was defined as a composite of all-cause death, myocardial infarction, hospitalization for chest pain or congestive heart failure, stroke, late revascularization (beyond 30 days of index ICA) and arrhythmia. Hard events were defined as myocardial infarction, all-cause death, or stroke. Hard cardiovascular events were defined as non-fatal myocardial infarction, cardiovascular death, or stroke.
Hemodynamically significant coronary heart disease was defined as a 50% or greater coronary stenosis with a corresponding myocardial perfusion defect.
Abbreviations: CTA: computed tomography angiography, CTP: computed tomography myocardial perfusion imaging, CV: cardiovascular; ICA: invasive coronary angiography, SPECT: single photon emission computed tomography, CI: confidence interval, MACE: major adverse cardiovascular events AUC: area under receiver operating characteristic curve.
Fig. 2.
Kaplan-Meier curves for 5-year event-free survival, Kaplan-Meier curves are shown for 5-year event-free survival in 323 patients according to the results (abnormal [+]; normal [−]) of combined CTA-CTP and ICA-SPECT assessment of hemodynamically significant coronary heart disease). Differences between the test combinations were not statistically significant. Abbreviations: MACE: major adverse cardiovascular event; CTA: computed tomography angiography; CTP: computed tomography perfusion; ICA: invasive coronary angiography; SPECT: single-photon-emission tomography.
Fig. 3.
Receiver operating characteristic curves for MACE events. Receiver operating characteristic (ROC) curves are shown for identifying patients who suffered MACE at 5-year follow up. Data were modelled as continuous variables with the Leaman score included in each model. Panel A shows the ROC curves for combined CTA-CTP and ICA-SPECT for predicting all MACE. Panel B shows the curves for combined CTA-CTP and ICA-SPECT for predicting “hard” events, defined as myocardial infarction, death, or stroke. Differences between the test combinations were not statistically significant. Abbreviations: MACE: major adverse cardiovascular event; ROC: receiver operating characteristic; CTA: computed tomography angiography; CTP: computed tomography perfusion; ICA: invasive coronary angiography; SPECT: single-photon-emission tomography.
Table 2b.
Comparison of the diagnostic and prognostic performance by CTA and SPECT.
| CTA | SPECT | Difference | |
|---|---|---|---|
|
| |||
| Absence of coronary heart disease* | |||
| 5 Year Event-Free | |||
| Survival, % (95% CI) | |||
| MACE | 86 (80, 92) | 80 (74, 86) | 5.9 (−1.1, 12.7) |
| Hard events | 93 (88, 98) | 94 (90, 97) | −0.9 (−5.7, 3.7) |
| Hard CV events | 96 (92, 99) | 95 (91, 98) | 1.2 (−2.6, 4.9) |
| Restricted Mean Survival | |||
| Time | |||
| MACE | 4.526 (4.295, 4.738) | 4.389 (4.178, 4.584) | 0.137 (−0.099, 0.371) |
| Hard events | 4.806 (4.653, 4.932) | 4.846 (4.730, 4.942) | −0.040 (−0.203, 0.110) |
| Hard CV events | 4.906 (4.793, 5.000) | 4.878 (4.767, 4.964) | 0.027 (−0.095, 0.142) |
| Presence of coronary heart disease | |||
| 5 Year Event-Free | |||
| Survival, % (95% CI) | |||
| MACE | ease 64 (57, 70) | 63 (56, 71) | 0.8 (−4.4, 6.4) |
| Hard events | 87 (82, 92) | 85 (79, 90) | 2.6 (−0.8, 6.2) |
| Hard CV events | 91 (87, 95) | 91 (86, 95) | 0.4 (−2.6, 3.3) |
| Restricted Mean Survival | |||
| Time | |||
| MACE | 3.884 (3.658, 4.098) | 3.830 (3.548, 4.097) | 0.054 (−0.126, 0.233) |
| Hard events | 4.731 (4.615, 4.835) | 4.670 (4.523, 4.808) | 0.062 (−0.051, 0.174) |
| Hard CV events | 4.809 (4.700, 4.897) | 4.807 (4.684, 4.908) | 0.002 (−0.086, 0.087) |
| Diagnosis of 5-year events | |||
| AUC | |||
| MACE | 64 (57, 70) | 61 (55, 67) | 2.8 (−4.1, 8.8) |
| Hard events | 62 (52, 72) | 62 (53, 72) | −0.0 (−7.1, 12.9) |
| Hard CV events | 65 (53, 76) | 59 (47, 71) | 5.5 (−5.3, 16.0) |
Shown are the results for 5-year event-free survival among the 379 patients and prediction of MACE according to normal/abnormal results by CTA or SPECT. MACE was defined as a composite of all-cause death, myocardial infarction, hospitalization for chest pain or congestive heart failure, stroke, late revascularization (beyond 30 days of index ICA) and arrhythmia. Hard events were defined as myocardial infarction, all-cause death, or stroke. Hard cardiovascular events were defined as non-fatal myocardial infarction, cardiovascular death, or stroke.
Coronary heart disease was defined as a 50% or greater coronary stenosis by CTA and by presence of a myocardial perfusion defect by SPECT.
Abbreviations: CTA: computed tomography angiography; CV: cardiovascular; SPECT: single photon emission computed tomography, CI: confidence interval, MACE: major adverse cardiovascular events AUC: area under receiver operating characteristic curve.
3.2. Predictors of MACE
In multivariable Cox regression analysis adjusted for clinical predictors, a history of previous myocardial infarction and abnormal imaging findings were found to be independent predictors of 5-year MACE (Table 3). When restricting the analysis to “hard” events, only a history of diabetes mellitus remained an independent predictor (Table 4a). Results for hard cardiovascular events are presented in Table 4b. A test comparing the hazard ratios for non-inferiority (within 5%) of CTA-CTP to ICA-SPECT had p < 0.0001, both adjusted for covariates and unadjusted, affirming that the two methods had similar predictive capability.
Table 3.
Cox proportional hazards model for associations with MACE.
| Characteristic | Model with CTA-CTP |
Model with ICA-SPECT |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
|
| ||||
| Age, years | 1.021 (0.996–1.046) | 0.11 | 1.025 (0.999–1.050) | 0.06 |
| Male sex | 1.279 (0.773–2.116) | 0.34 | 1.323 (0.802–2.181) | 0.27 |
| Hypertension | 1.039 (0.578–1.868) | 0.90 | 1.078 (0.601–1.933) | 0.80 |
| Diabetes | 1.308 (0.849–2.016) | 0.22 | 1.242 (0.805–1.916) | 0.33 |
| Dyslipidemia | 0.816 (0.517–1.286) | 0.38 | 0.894 (0.568–1.407) | 0.63 |
| BMI | 0.987 (0.939–1.037) | 0.60 | 0.986 (0.939–1.035) | 0.57 |
| Calcium score 1- 99 | 0.962 (0.395–2.342) | 0.93 | 1.098 (0.464–2.601) | 0.83 |
| Calcium score 100–399 | 1.083 (0.435–2.696) | 0.86 | 1.304 (0.552–3.078) | 0.55 |
| Calcium score ≥400 | 1.222 (0.483–3.091) | 0.67 | 1.453 (0.610–3.463) | 0.40 |
| Previous MI | 1.858 (1.175–2.939) | 0.008 | 1.773 (1.110–2.831) | 0.02 |
| Abnormal CTA- CTP | 2.047 (1.090–3.845) | 0.03 | n/a | n/a |
| Abnormal ICA- SPECT | n/a | n/a | 1.744 (1.087–2.798) | 0.02 |
Shown are results from Cox proportional hazard analysis for various predictors of MACE. MACE was defined as a composite of all-cause death, myocardial infarction, hospitalization for chest pain or congestive heart failure, stroke, late revascularization (beyond 30 days of index ICA) and arrhythmia. Disease classification by CTA-CTP and ICA-SPECT were included in separate models, adjusted for the same other predictors.
Abbreviations CTA: computed tomography angiography, CTP: computed tomography myocardial perfusion imaging, ICA: invasive coronary angiography, SPECT: single photon emission computed tomography, CI: confidence interval, MACE: major adverse cardiovascular events, AUC: area under receiver operating characteristic curve.
Table 4a.
Cox proportional hazards model for associations with 5-year hard events.
| Characteristic | Model with CTA-CTP |
Model with ICA-SPECT |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
|
| ||||
| Age, years | 1.018 (0.976–1.063) | 0.41 | 1.021 (0.979–1.066) | 0.33 |
| Male sex | 1.546 (0.650–3.679) | 0.32 | 1.572 (0.664–3.723) | 0.30 |
| Hypertension | 1.630 (0.579–4.584) | 0.35 | 1.625 (0.579–4.557) | 0.36 |
| Diabetes | 2.225 (1.131–4.377) | 0.02 | 2.155 (1.094–4.247) | 0.03 |
| Dyslipidemia | 0.421 (0.209–0.848) | 0.02 | 0.446 (0.223–0.892) | 0.02 |
| BMI | 0.970 (0.888–1.060) | 0.50 | 0.973 (0.891–1.062) | 0.54 |
| Calcium score 1- 99 | 0.794 (0.069–9.083) | 0.85 | 0.751 (0.065–8.607) | 0.82 |
| Calcium score 100–399 | 2.005 (0.398–10.105) | 0.40 | 2.184 (0.463–10.306) | 0.32 |
| Calcium score ≥400 | 2.116 (0.379–11.827) | 0.39 | 2.259 (0.443–11.515) | 0.33 |
| Previous MI | 1.127 (0.504–2.520) | 0.77 | 1.103 (0.493–2.467) | 0.81 |
| Abnormal CTA- CTP | 1.559 (0.606–4.014) | 0.36 | n/a | n/a |
| Abnormal ICA- SPECT | n/a | n/a | 1.559 (0.752–3.233) | 0.23 |
Shown are results from Cox proportional hazard analysis for various predictors of “hard” MACE, defined as a composite of all-cause death, myocardial infarction, or stroke. Disease classification by CTA-CTP and ICA-SPECT were included in separate models, adjusted for the same other predictors.
Abbreviations: CTA: computed tomography angiography, CTP: computed tomography myocardial perfusion imaging, ICA: invasive coronary angiography, SPECT: single photon emission computed tomography, CI: confidence interval, MACE: major adverse cardiovascular events AUC: area under receiver operating characteristic curve.
Table 4b.
Cox proportional hazards model for associations with 5-year hard CV events.
| Characteristic | Model with CTA-CTP |
Model with ICA-SPECT |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
|
| ||||
| Age, years | 1.013 (0.961–1.069) | 0.62 | 1.018 (0.966–1.073) | 0.50 |
| Male sex | 1.261 (0.445–3.572) | 0.66 | 1.572 (0.562–4.395) | 0.39 |
| Hypertension | 1.413 (0.426–4.695) | 0.57 | 1.489 (0.449–4.934) | 0.52 |
| Diabetes | 1.795 (0.752–4.287) | 0.19 | 1.773 (0.746–4.217) | 0.20 |
| Dyslipidemia | 0.332 (0.138–0.800) | 0.01 | 0.393 (0.162–0.951) | 0.04 |
| BMI | 0.974 (0.874–1.085) | 0.63 | 0.971 (0.872–1.082) | 0.59 |
| Previous MI | 1.059 (0.393–2.851) | 0.91 | 1.236 (0.452–3.378) | 0.68 |
| Statin use | 1.279 (0.529–3.089) | 0.59 | 1.281 (0.521–3.148) | 0.59 |
| Abnormal CTA- CTP | 4.425 (1.222–16.020) | 0.02 | n/a | n/a |
| Abnormal ICA- SPECT | n/a | n/a | 1.668 (0.682–4.078) | 0.26 |
Shown are results from Cox proportional hazard analysis for various predictors of “hard” MACE, defined as a composite of all-cause death, myocardial infarction, or stroke. Hard cardiovascular events were defined as non-fatal myocardial infarction, cardiovascular death, or stroke. Disease classification by CTA-CTP and ICA-SPECT were included in separate models, adjusted for the same other predictors.
Abbreviations: CTA: computed tomography angiography, CTP: computed tomography myocardial perfusion imaging, ICA: invasive coronary angiography, SPECT: single photon emission computed tomography, CI: confidence interval, MACE: major adverse cardiovascular events AUC: area under receiver operating characteristic curve.
Combined CTA-CTP (both normal) failed to identify 20 patients who experienced a MACE within 5 years of follow-up (6 revascularizations, 5 non-cardiac deaths, 3 cerebrovascular events, 3 incidents of chest pain, 2 hospitalizations for chest pain, 1 hospitalization for CHF), resulting in a negative predictive value of 86% (95% CI 80–91%). Of those, 9 had a stenosis by CTA but no associated perfusion defect, and 3 had a perfusion defect, despite no stenosis in a corresponding coronary artery by CTA; 8 of these patients had neither stenosis nor perfusion defect. Combined ICA-SPECT (both normal) did not identify 44 patients who had a subsequent MACE within 5 years (20 revascularizations, 1 MI, 1 cardiac death, 5 non-cardiac deaths, 6 cerebrovascular events, 6 incidents of chest pain, 3 hospitalizations for chest pain, 2 hospitalizations for CHF), resulting in a negative predictive value of 81% (95% CI 76–86%). Of those, 21 and 9 patients had positive results for either ICA or SPECT, respectively, but not for both; 14 patients did not have stenosis or perfusion defect in ICA or SPECT. The negative predictive values of the two tests were not significantly different (p = 0.08).
4. Discussion
In patients referred for invasive coronary angiography for evaluation of suspected CAD, combined CTA-CTP yields similar prognostic information as the traditional approach of ICA-SPECT. Given the noninvasive nature of CT as well as the ease and speed of acquisition, a combination of CTA-CTP may be an attractive alternative to nuclear stress testing and ICA for evaluating patients with suspected CAD.
In the US, approximately 1,000,000 cardiac catheterizations are being performed each year for the evaluation of CAD and only about 38–40% of patients actually have obstructive disease.12 While the adverse event rate associated with performing diagnostic cardiac catheterization is overall low, it is not trivial either. Data from the US National Catheterization Data Registry suggest an average rate of adverse events of 6%, with 0.2% suffering death, stroke, or myocardial infarction.13 Accordingly, several thousand patients suffer adverse events from diagnostic cardiac catheterization each year. Furthermore, a recent multi-center study showed that patients undergoing invasive coronary angiography are more likely to receive subsequent coronary intervention compared to patients randomly assigned to selective cardiac catheterization after abnormal CT angiography.14 While patient outcome was not different among the groups, healthcare costs were approximately 50% lower with the strategy of CTA guiding referral for cardiac catheterization.
CT coronary angiography has shown to be highly accurate in identifying patients with obstructive CAD by cardiac catheterization.15 Indeed, compared to independent reference standards of intravascular ultrasound and fractional flow reserve, diagnostic performance of CT coronary angiography was similar to that by ICA.16,17 Our results of at least equivalent risk stratification by non-invasive CT compared to ICA-SPECT represent a critical next step towards establishing cardiac CT as a valid alternative to our traditional approach of routine cardiac catheterization in patients with suspected CAD. While just missing criteria for statistical significance, absence of disease by CTA-CTP was associated with a larger percentage of patients surviving without events than with ICA-SPECT assessment. Results from the DISCHARGE multicenter trial, testing the hypothesis that outcome of low-intermediate risk patients with chest pain is superior when assigned to cardiac CT vs. cardiac catheterization may confirm this notion.18
In addition to determining the hemodynamic significance of coronary atherosclerosis, CT is also capable of providing total atheroma burden and details on atherosclerotic disease characteristics.19 While not yet validated for clinical application, these additional features may further increase the utility of cardiac CT in the near future. Among predictors of MACE, only a history of MI and abnormal imaging results are associated with significantly increased (adjusted) hazard but the high-risk study population must be considered for adequate interpretation. Obstructive CAD was found in more than 60% of patients in our cohort, limiting the predictive discrimination for MACE at follow up. Furthermore, collinearity is likely a factor among many predictors as they are associated with both disease burden and traditional risk factors. Adverse event risk is driven predominantly by the atherosclerotic disease burden and by risk factors for a prothrombotic state.20 Not surprisingly, diabetes mellitus (as a condition associated with inflammation and increased thrombosis risk) and high disease burden by imaging are linked with increased hazard ratios for events.21 Lack of statistical power likely explains few statistically significant associations when limiting analyses to hard events.
4.1. Limitations
We acknowledge several study limitations. Follow up beyond 2-years was completed in 85% of patients with missing data potentially inducing bias. Given the observational nature of our study, decisions about revascularization followed common practice patterns and therefore, were primarily based on results by cardiac catheterization and SPECT rather than CTA and CTP to which the decision makers were blinded. All CT scans were performed using 320-slice technology and results may not be applicable to other scanners. CT interpretation occurred in a central core laboratory by expert readers. Results by less experienced physicians may vary. Lastly, attenuation correction was available for SPECT imaging at only few sites which may have affected test specificity.
4.2. Conclusions
Combined CT coronary angiography and CT myocardial perfusion imaging using 320-slice CT performs similarly to a combination of ICA and SPECT imaging for predicting outcome of patients with suspected or known CAD. These results suggest that non-invasive cardiac CT imaging may represent a valid alternative to our traditional approach of using cardiac catheterization in this population.
Acknowledgments
Funding
This work was funded in part by intramural research support from the NHLBI, National Institutes of Health, USA. The CORE320 cross-sectional study with limited follow up was funded by Canon (formerly Toshiba) Medical Systems.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the american heart association. Circulation. 2019;139(10): e56–e528. 10.1161/CIR.0000000000000659 [doi]. [DOI] [PubMed] [Google Scholar]
- 2.Arbab-Zadeh A, Fuster V. From detecting the vulnerable plaque to managing the vulnerable patient. J Am Coll Cardiol. 2019;74(12):1582–1593. [DOI] [PubMed] [Google Scholar]
- 3.SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10): 924–933. [DOI] [PubMed] [Google Scholar]
- 4.Bittencourt MS, Hulten EA, Murthy VL, et al. Clinical outcomes after evaluation of stable chest pain by coronary computed tomographic angiography versus usual care: a meta-analysis. Circ Cardiovasc Imaging. 2016;9(4), e004419. [DOI] [PubMed] [Google Scholar]
- 5.Rochitte CE, George RT, Chen MY, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J. 2014; 35(17):1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen MY, Rochitte CE, Arbab-Zadeh A, et al. Prognostic value of combined CT angiography and myocardial perfusion imaging versus invasive coronary angiography and nuclear stress perfusion imaging in the prediction of major adverse cardiovascular events: the CORE320 multicenter study. Radiology. 2017;284(1): 55–65. 10.1148/radiol.2017161565 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vavere AL, Simon GG, George RT, et al. Diagnostic performance of combined noninvasive coronary angiography and myocardial perfusion imaging using 320 row detector computed tomography: design and implementation of the CORE320 multicenter, multinational diagnostic study. J Cardiovasc Comput Tomogr. 2011;5(6): 370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George RT, Arbab-Zadeh A, Cerci RJ, et al. Diagnostic performance of combined noninvasive coronary angiography and myocardial perfusion imaging using 320-MDCT: the CT angiography and perfusion methods of the CORE320 multicenter multinational diagnostic study. AJR Am J Roentgenol. 2011;197(4):829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerci RJ, Arbab-Zadeh A, George RT, et al. Aligning coronary anatomy and myocardial perfusion territories: an algorithm for the CORE320 multicenter study. Circ Cardiovasc Imaging. 2012;5(5):587–595. [DOI] [PubMed] [Google Scholar]
- 10.Leaman DM, Brower RW, Meester GT, Serruys P, van den Brand M. Coronary artery atherosclerosis: severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation. 1981;63(2):285–299. [DOI] [PubMed] [Google Scholar]
- 11.Chow S, Shao J, Wang H. Sample Size Calculations in Clinical Research. second ed. Boca Raton: Chapman and Hall/CRC; 2008. [Google Scholar]
- 12.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the american heart association. Circulation. 2020, CIR0000000000000757. 10.1161/CIR.0000000000000757 [doi]. [DOI] [PubMed] [Google Scholar]
- 13.Noto Tj J, Johnson LW, Krone R, et al. Cardiac catheterization 1990: a report of the registry of the society for cardiac angiography and interventions (SCA&I). Cathet Cardiovasc Diagn. 1991;24(2):75–83. [DOI] [PubMed] [Google Scholar]
- 14.Chang HJ, Lin FY, Gebow D, et al. Selective referral using CCTA versus direct referral for individuals referred to invasive coronary angiography for suspected CAD: a randomized, controlled, open-label trial. JACC Cardiovasc Imaging. 2018;12(7 Pt 2): 1303–1312. S1936–878X(18)30921–5 [pii]. [DOI] [PubMed] [Google Scholar]
- 15.Haase R, Schlattmann P, Gueret P, et al. Diagnosis of obstructive coronary artery disease using computed tomography angiography in patients with stable chest pain depending on clinical probability and in clinically important subgroups: meta-analysis of individual patient data. BMJ. 2019;365:l1945. 10.1136/bmj.l1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feuchtner G, Loureiro R, Bezerra H, et al. Quantification of coronary stenosis by dual source computed tomography in patients: a comparative study with intravascular ultrasound and invasive angiography. Eur J Radiol. 2012;81(1):83–88. [DOI] [PubMed] [Google Scholar]
- 17.Budoff MJ, Nakazato R, Mancini GB, et al. Head-to-head comparison of quantitative coronary angiography, and computed tomography angiography for the prediction of hemodyanmic significance in intermediate and severe lesions, using fractional flow reserve as reference standard. JACC Cardiovasc.Imaging. 2016;9(5):559–564. 10.1016/j.jcmg.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Napp AE, Haase R, Laule M, et al. Computed tomography versus invasive coronary angiography: design and methods of the pragmatic randomised multicentre DISCHARGE trial. Eur Radiol. 2017;27(7):2957–2968. 10.1007/s00330-016-4620-z [doi]. [DOI] [PubMed] [Google Scholar]
- 19.Sharma A, Arbab-Zadeh A. Assessment of coronary heart disease by CT angiography: current and evolving applications. J Nucl Cardiol. 2012;19(4):796–806. [DOI] [PubMed] [Google Scholar]
- 20.Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012;125(9):1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbab-Zadeh A, Fuster V. The risk continuum of atherosclerosis and its implications for defining CHD by coronary angiography. J Am Coll Cardiol. 2016;68(22): 2467–2478. [DOI] [PubMed] [Google Scholar]