Abstract
Identification of human miRNAs involved in coronavirus-host interplay is important due to the current COVID-19 pandemic. Therefore, this study aimed to measure the circulating plasma miR-155 expression level in COVID-19 patients and healthy controls to investigate its roles in the pathogenesis and severity of COVID-19 disease and to assess its usefulness as a clinical biomarker for the detection of COVID-19 disease and the severity of infection.
A total of 150 COVID-19 patients and 50 controls were enrolled into our study. Beside the routine laboratory work and chest computed tomography (CT) scans of COVID-19 patients, plasma miR-155 expression level was measured using reverse transcription quantitative real-time PCR (RT-qPCR) technique.
Our results demonstrated increased miR-155 expression level in COVID-19 patients compared to controls, in severe compared to moderate COVID-19 patients, and in non-survival compared to survival COVID-19 patients. miR-155 expression level also had significant correlation with clinicopathological characteristics of COVID-19 patients such as chest CT findings, CRP, ferritin, mortality, D-dimer, WBC count, and lymphocytes and neutrophils percentages. Also, our results showed that the area under the curve (AUC) for miR-155 was 0.986 with 90% sensitivity and 100% specificity when used as a biomarker for the detection of COVID-19 disease; while in detection of severity of COVID-19 disease, AUC for miR-155 was 0.75 with 76% sensitivity and specificity.
From these results we can conclude that miR-155 has a crucial role in the pathogenesis and severity of COVID-19; also, it could be a good diagnostic clinical biomarker for the detection of COVID-19 disease and the severity of infection.
Key words: Coronaviruses, COVID-19, SARS-CoV-2 infection, microRNAs (miRNAs), miR-155
Introduction
Coronavirus disease (COVID-19) is an outbreak of respiratory illnesses caused by a new coronavirus and declared as a pandemic by the World Health Organization (WHO) on 11 March 2020.1 In 1966, Tyrell and Bynoe first identified coronaviruses as positive single stranded, enveloped RNA viruses with a genomic size of 26–32 kb, which can infect both humans and mammals.2 The new Coronavirus (SARS-CoV-2) is the causative agent of the COVID-19 pandemic and was first identified in Wuhan, Hubei province, China.3 SARS-CoV-2 belongs to the β-subfamily of coronaviruses such as MERS-CoV and SARS-CoV. Among all other seven types of coronaviruses, β-subtype is the most fatal. SARS-CoV-2 contains four structural proteins (the spike, envelope, membrane, and nucleocapsid) and RNA viral genome. The structure of SARS-CoV-2 is considered as coreshell morphology with about 60–140 nm in diameter.4
SARS-CoV-2 infections may be asymptomatic or cause only mild symptoms in the majority of cases, but may deteriorate to interstitial pneumonia and acute respiratory distress syndrome (ARDS) in about 10–20% of cases, particularly in older and comorbid people.5 Globally, many people were infected by SARS-CoV-2 infection and the number of cases and deaths increased dramatically. On 28 June 2020, about 10 million people were infected, which increased to 20 million cases on 10 August, then 30 million cases by 17 September, and 40 million cases by 19 October 2020; as of 23 May 2021, there were 167,058,099 confirmed cases and 3,468,924 deaths.6 The mortality rate of COVID-19 is about 3.4–5.5%.
MicroRNAs (miRNAs), are highly conserved and short non-coding RNA molecules of about 18–25 nucleotides, present in animals, plants and even some viruses. Several studies have shown that many miRNAs play vital roles in gene expression regulation by targeting the mRNAs of protein-coding genes. Also, the dysregulation of miRNAs has been proved to be correlated with disease severity and therapeutic outcomes by different treatments.7
MiRNAs have rapidly emerged not only as critically contributing to various pulmonary diseases but also to normal development of the lung and maintenance of its homeostasis.8 Studies have proven that many miRNAs play crucial roles in fine-tuning important pathogenic pathways including the regulation of the effector function of T helper (Th)2 cells in allergic asthma, the regulation of host defense immune responses, and the repair and remodelling of the airways.9 Numerous reports have consistently demonstrated the role of miRNAs in viral infections, while others have focused on differential expressions of miRNAs in response to viral infection.10
miRNA-155 (miR-155) has been identified to have important roles in the regulation of immune responses and plays a critical role in tissue fibrosis in liver and lung.11 Also it is identified as the master regulator of inflammation.12 Therefore, this study aimed to measure the expression level of miR-155 in the plasma of positive COVID-19 Egyptian patients to investigate its roles in the pathogenesis and severity of COVID-19 disease and to assess its usefulness as a clinical biomarker for the detection of COVID-19 disease and the severity of infection.
Patients and methods
Ethics statement
This study was approved by the ethics committees of Faculty of Medicine, Port-Said University, Egypt [ERN MED (23/04/2020) S.no(5)MED]. Informed consents were obtained from all patients.
Human subjects
The study was conducted on 150 COVID-19 patients and 50 healthy controls. All patients were infected with SARS-CoV-2 and recruited from Port-Said hospital isolation department, Port-Said, Egypt. Sputum and throat swab specimens (for qPCR for SARS-Cov-2 RNA test) and blood samples (for laboratory tests and miR-155 expression level) were collected from all patients. Laboratory tests were conducted at admission, including a complete blood count, liver function tests (AST, ALT), kidney function tests (urea, creatinine), C-reactive protein (CRP), D-dimer and ferritin. Chest computed tomography (CT) scans were performed on all patients, and then these patients received supportive oxygen therapy, antiviral medication, and other supportive treatments according to Suspected COVID-19 Cases Management in Triage Hospitals by Ministry of Health and Population of Egypt. Severity of COVID-19 was graded according to Suspected COVID-19 Cases Management in Triage Hospitals by Ministry of Health and Population of Egypt. Our COVID-19 patients classified according to their clinical status into two main groups: moderate patients (patients with fever, respiratory manifestations, and radiological findings indicative of pneumonia), and severe patients [patients with respiratory distress, resting oxygen saturation ≤93%, respiratory failure requiring mechanical ventilation, or failure of other organs requiring intensive care unit (ICU) admission].
Determination of plasma expression level of miR-155 by reverse transcription quantitative real-time PCR (RT-qPCR)
miRNA extraction
Peripheral blood samples (4 mL) were collected in tubes containing EDTA and centrifuged at 10,000 rpm for 10 min at room temperature, then the supernatant was transferred to be stored immediately in RNase free tubes at –80oC until microRNA extraction. MicroRNA extraction was performed using miRNeasy Mini kit (cat # 217004; Qiagen, USA) according to manufacturer's instructions. The purity and the concentration of the purified miRNA was detected using spectrophotometer nano-drop (Quawell, Q-500; Scribner, USA) and stored at −80°C until further assessments.
Reverse transcription and cDNA preparation
Reverse transcription of miRNA was carried out using MiScript II reverse transcription kit (cat # 218160; Qiagen) according to the manufacturer's instructions and stored at −20°C until qPCR was performed.
Quantitative real-time PCR (qPCR)
Quantitative real-time PCR was performed using miScript primer assay for miR-155 (Hs_miR-155_2 miScript Primer Assay, MS00031486); the reaction was carried out using MiScript SYBR Green PCR kit (cat # 218073; Qiagen). Also, RNU6–2 (Hs_RNU6-2_11 miScript Primer Assay, MS00033740) was used as an endogenous control to normalise the expression levels of the investigated miRNAs. The primer sequences are listed in Table 1 .
Table 1.
Primer sequences for quantitative RT-PCR analysis
| Gene | Primer sequence |
|---|---|
| miR-155 | 5′- UUAAUGCUAAUCGUGAUAGGGGU-3′ |
| RNU6B | 5′-CUCGCUUCGGCAGCACAUAUACUAA-3′ |
The qPCR cycling conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95ºC for 15 s, 55ºC for 30 s, and 72ºC for 30 s in which fluorescence was acquired and detected by Stratagene Real-time PCR system (Max3005P QPCR system; Stratagene, Agilent Biotechnology, USA). The relative expression levels of the investigated miRNAs were evaluated using the 2−ΔΔCq method described by Livak and Schmittgen.13
Statistical analysis
Statistical analysis was performed using IBM SPSS software (version 23.0; IBM, USA), and data were presented as means ± SD. The comparison between COVID-19 patients and controls was made by chi-square test for categorical variables, post hoc test (LSD) for normal distributed variables. Pearson's correlation coefficient was used to determine significant correlations of miRNA-155 expression level and other clinical parameters. The receiver operating characteristic (ROC) curve was used to calculate the area under the curve (AUC) of circulating plasma miR-155 expression level in order to evaluate its sensitivity and specificity as a biomarker for the detection of COVID-19 disease and the severity of infection. The criterion for significance was p<0.05.
Results
Demographic and biochemical data of COVID-19 patients
The current study included 150 positive COVID-19 patients, 89 male and 61 female, with a mean age of 49.43±9.10 years. Twenty-six patients died during hospitalization. The clinical and biological data of healthy subjects and COVID-19 patients are summarised in Table 2 . As the study was designed to match the age and gender between cases and controls, no statistically significant difference was observed for these parameters (p>0.05).
Table 2.
Clinicopathological characteristics and CT findings of COVID-19 patients and controls
| Variable | COVID-19 patients |
Control (n=50) Mean±SD |
||
|---|---|---|---|---|
| Moderate (n=98) Mean±SD |
Severe (n=52) Mean±SD |
Total (n=150) Mean±SD |
||
| Age, years | 48.36±9.14 | 50.40±9.06 | 49.43±9.10 | 45.80±8.82 |
| Gender, n (%) | ||||
| Male | 56 (57.2%) | 33 (63.5%) | 89 (59.3%) | 32 (64%) |
| Female | 42 (42.8%) | 19 (36.5%) | 61 (40.7%) | 18 (36%) |
| Mortality, n (%) | 9 (9.2%) | 17 (32.7%) | 26 (17.3%) | – |
| Chest CT findings, n (%) | ||||
| Normal | 36 (37%) | 0 (0%) | 36 (24%) | – |
| Ground-glass opacity | 61 (62%) | 18 (35%) | 79 (53%) | |
| Ground-glass opacity with consolidation | 1 (1%) | 34 (65%) | 35 (23%) | |
| AST, U/L | 33.15±21.97 | 38.28±37.65a,c | 31.12±31.12 | 22.05±9.37 |
| ALT, U/L | 34.44±23.56a,c | 35.0±30.79a,c | 34.73±27.42a,c | 19.35±7.71 |
| Urea, mg/dL | 40.71±14.48a,c | 41.35±15.20a,c | 41.05±14.77a,c | 32.05±6.57 |
| Creatinine, mg/dL | 1.22±0.35a,c | 1.18±0.34a,c | 1.20±0.34a,c | 0.93±0.22 |
| CRP, mg/dL | 75.04±39.50b,c | 78.97±23.31b,c | 77.10±31.88b,c | 4.99±1.57 |
| WBC, 103/μL | 10.03±4.17a,c,b,e | 14.38±6.06b,c,d | 12.32±5.66b,c | 7.47±2.05 |
| Neutrophils, % | 73.76±7.19b,c,d | 79.26±5.57b,c,d | 76.65±6.92b,c | 65.00±5.06 |
| Lymphocytes, % | 19.89± 6.94b,c,d | 14.21±5.13b,c,d | 16.91±6.66b,c | 29.60±5.30 |
| D-dimer, mg/L | 0.43±0.35b,e | 2.64±3.34b,c,d | 1.59±2.66a,c | 0.06±0.01 |
| Ferritin, ng/mL | 213.86±135.24a,c,b,e | 494.11±260.96b,c,d | 361.0±252.47b,c | 104.35±50.85 |
aSignificant at p value <0.05.
bHighly significant at p value <0.001.
Significant difference versus control group.
Significant difference versus moderate group.
Significant difference versus severe group.
Data obtained from laboratory routine work of COVID-19 patients revealed a significant (p<0.05) increase in the serum ALT, urea, creatinine and D-dimer. A highly significant (p<0.001) increase in the serum CRP, ferritin, WBC count and neutrophils count was observed among the positive COVID-19 patients as compared to controls; lymphocytes count was highly significantly (p<0.001) decreased when compared to controls, as shown in Table 2.
Chest CT scan findings
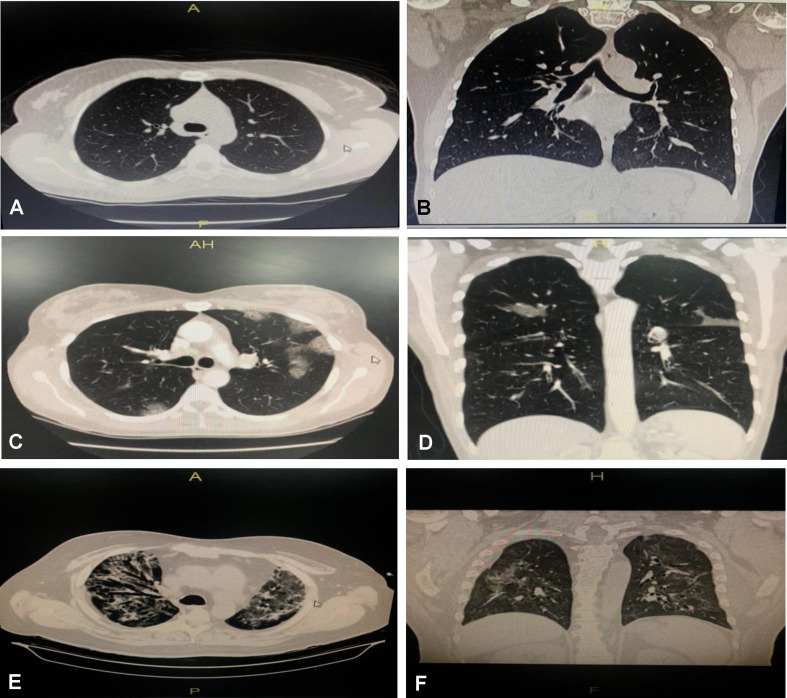
At admission time, chest CT scan of our COVID-19 patients showed that 36 (24%) had normal CT scans, 79 (53%) had ground-glass opacity only, and 35 (23%) had ground-glass opacity with consolidation, as shown in Table 2 and Fig. 1 .
Fig. 1.
Chest computed tomography (CT) findings of COVID-19 patients: axial and coronal CT chest window images. (A,B) Moderate COVID-19 patient showing normal findings. (C,D) Moderate COVID-19 patient showing peripheral ground glass opacities seen in upper and lower lung lobes, more on the left side, with a non-lobar distribution, which are associated with mild related interstitial septal thickening features specific for SARS-CoV-2 viral infection (CORAD 5), moderately affecting the lung. (E,F) Severe COVID-19 patient showing peripheral, and to a lesser extent central dense ground glass opacities, seen in both upper lung lobes in a non-lobar distribution, which are associated with moderate related interstitial septal thickening features specific for SARS-CoV-2 viral infection (CORAD 5), severely affecting the lung.
Expression level of plasma miR-155 in COVID-19 patients
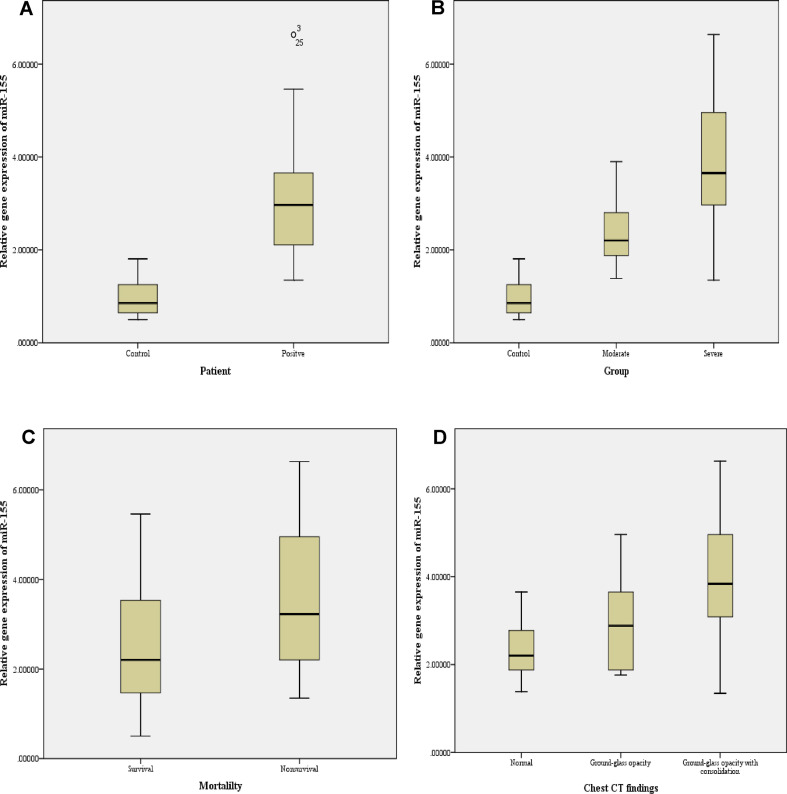
The plasma miR-155 had a differential expression pattern, as it was found to be highly significantly (p<0.001) increased in COVID-19 patients (3.11±1.26) when compared to the control group (0.98±0.40), as shown in Fig. 2 A. When COVID-19 patients were divided into moderate and severe patients, our results revealed that miR-155 expression level was highly significantly (p<0.001) increased in severe patients (3.68±1.37) when compared to moderate patients (2.48±0.71) or control group (0.98±0.40), as shown in Fig. 2B.
Fig. 2.
Differential expression level of plasma miR-155 in (A) COVID-19 patients and controls; (B) moderate and severe COVID-19 patients and controls; (C) survival and non-survival COVID-19 patients; and (D) COVID-19 patients with normal or ground-glass opacity with or without consolidation.
The expression level of miR-155 was also examined between survival and non-survival COVID-19 patients and our results showed that it was found to be significantly (p<0.05) increased in non-survival (3.49±1.52) when compared to survival (2.43±1.29) COVID-19 patients, as shown in Fig. 2C.
When the plasma miR-155 expression level was examined among different chest CT findings of COVID-19 patients, our results revealed it was significantly increased in patients with ground-glass opacity and consolidation (3.97±1.44) compared to patients with normal chest CT scans (2.45±0.71) or with ground-glass opacity only (2.82±0.92), as shown in Fig. 2D.
Correlation of miR-155 expression level with clinical variables in COVID-19 patients
Data recorded in Table 3 show the correlation matrix of miR-155 expression level with the different clinical parameters in this study. It was found that the plasma miR-155 expression level had highly significant positive correlations (p<0.001) with chest CT findings, CRP and ferritin; while it had significant positive correlations (p<0.05) with mortality, D-dimer, WBC count and neutrophils percentage. However, there was a significant negative correlation (p<0.05) between plasma miR-155 expression level and lymphocytes percentage.
Table 3.
Correlations of plasma miR-155 and different parameters among COVID-19 patients
| Variable | miR-155 |
|
|---|---|---|
| r | p value | |
| Mortality, n (%) | 0.322 | 0.001a |
| Chest CT findings | 0.504 | 0.000b |
| AST, U/L | 0.101 | 0.316 |
| ALT, U/L | 0.133 | 0.186 |
| Urea, mg/dL | 0.017 | 0.867 |
| Creatinine, mg/dL | 0.059 | 0.561 |
| CRP, mg/dL | 0.430 | 0.000b |
| WBC, 103/μL | 0.287 | 0.004a |
| Neutrophils, % | 0.243 | 0.015a |
| Lymphocytes, % | –0.259 | 0.009a |
| D-dimer, mg/L | 0.336 | 0.001a |
| Ferritin, ng/mL | 0.395 | 0.000b |
a Significant at p value <0.05.
b Highly significant at p value <0.001.
ROC curves analysis
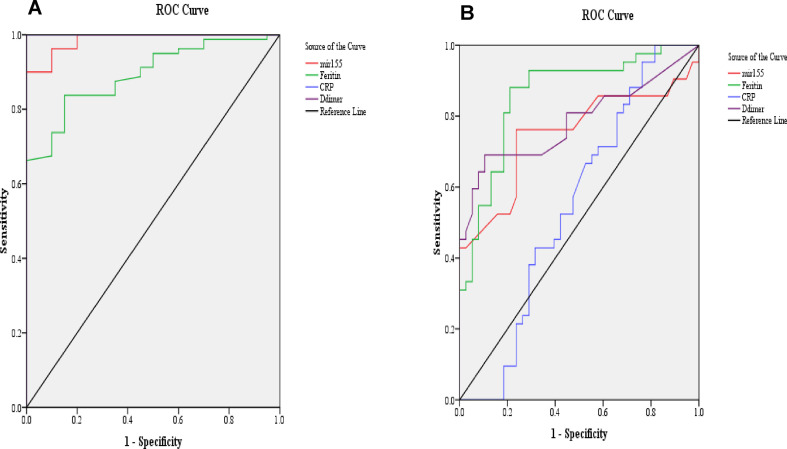
To evaluate the sensitivity and specificity of the circulating plasma miR-155 expression level as marker for the detection of COVID-19 infection and the severity of infection, ROC curve analysis was performed. Our results showed that AUC for miR-155 was 0.986 with 90% sensitivity and 100% specificity when used as a biomarker for the detection of COVID-19 disease, as shown in Table 4 A and Fig. 3 A; while in detection of severity of COVID-19 disease, AUC for miR-155 was 0.75 with 76% sensitivity and specificity, as shown in Table 4B and Fig. 3B.
Table 4.
Area under the curve (AUC), cut-off value, sensitivity and specificity of plasma miR-155 and other parameters in COVID-19 patients
| miR-155 | Ferritin | CRP | D-dimer | |
|---|---|---|---|---|
| (A) For the detection of COVID-19 disease | ||||
| AUC | 0.986 | 0.893 | 1 | 1 |
| Cut-off value | 1.81 | 134.8 | 9.1 | 0.09 |
| Asymptotic significance | 0.000a | 0.000a | 0.000a | 0.000a |
| Sensitivity | 90% | 84% | 100% | 100% |
| Specificity | 100% | 85% | 100% | 100% |
| (B) For the detection of COVID-19 disease severity | ||||
| AUC | 0.75 | 0.86 | 0.54 | 0.78 |
| Cut-off value | 2.88 | 286.5 | 71.8 | 0.65 |
| Asymptotic significance | 0.000a | 0.000a | 0.45 | 0.000a |
| Sensitivity | 76% | 81% | 57% | 69% |
| Specificity | 76% | 82% | 53% | 79% |
AUC, area under the curve.
aHighly significant at p value <0.001.
Fig. 3.
Receiver operating characteristic (ROC) curve of circulating plasma miR-155 and other parameters for detection of (A) COVID-19 disease and (B) severity of COVID-19 disease.
Discussion
In our previous work, we measured the levels of serum IP-10 and SAA in positive COVID-19 patients to explore their clinical values and significance in discrimination between moderate and severe COVID-19 infection and in predicting the severity and prognosis of COVID-19 disease.14 Here, we measured the circulating plasma miR-155 expression level in COVID-19 patients and controls to investigate its roles in the pathogenesis and severity of COVID-19 disease and to assess its usefulness as a clinical biomarker for the detection of COVID-19 disease and the severity of infection. Results of the present study revealed that the expression level of miR-155 was highly significantly increased in COVID-19 patients when compared to controls, and in severe COVID-19 patients when compared to moderate COVID-19 patients. Our findings indicate that miR-155 may play a role in the progression and severity of COVID-19 disease.
In humans, miR-155 is encoded by MIRHG155 (formerly known as BIC) gene, which is composed of three exons that span a 13 kb region within chromosome 21.12 It was found that miR-155 regulated many (about 140) target genes that encode for inflammatory-related proteins, immunomodulatory proteins and tumour-suppressor proteins. Therefore, the circulating miR-155 is associated with specific disorders such as inflammation, cardiovascular diseases and cancer.15 The target genes of miR-155 with most potential are SHIP1 (tumour suppressor) and SOCS1 (negative regulator to inhibit NF-kB and JAK2/STAT3 signalling pathways and therefore control inflammation).16
Pociask et al. 17 found that miR-155 knockout mice have decreased lung inflammation and recover faster from influenza infection. De Smet et al. 18 observed that the lung inflammation induced by cigarette smoke is reduced in miR-155 knockout mice and relieved by anti-miR-155 treatment. Adwanikar et al. 19 reported that the miR-155/NF-κB signalling cascade had a crucial role in the inflammatory response initiation. Koranteng et al. 20 found that the inhibition of miR-155/NF-κB was effective in relieving the inflammatory response. Meduri et al. 21 demonstrated that inflammatory cytokines (such as TNF-α, IL-6 and IL-1β) were associated with ARDS development, shock and multiple organ dysfunction syndromes. Liu et al. 22 hypothesised that the miR-155/NF-κB pathway might be an effective therapeutic target for the anti-inflammatory treatment of ARDS in neonatal pigs. From all these observations along with our results, we believe that miR-155 has a crucial role in the pathogenesis and severity of COVID-19. Our results demonstrate the increased miR-155 expression level in COVID-19 patients compared to controls, in severe compared to moderate COVID-19 patients, and in non-survival compared to survival COVID-19 patients; also, it has significant correlations with clinicopathological characteristics of COVID-19 patients such as chest CT findings, CRP, ferritin, mortality, D-dimer, WBC count and lymphocytes and neutrophils percentages.
The radiological evaluations and assessments such as chest CT imaging are crucial for detection and prognosis of SARS-CoV-2 infection, as chest radiography is not sensitive for detecting ground-glass opacity, and may show normal results in the initial stage of viral infection.23 , 24 Our results revealed that the circulating plasma miR-155 expression level was significantly increased in COVID-19 patients with ground-glass opacity and/or consolidation compared to patients with normal chest CT scans, which strengthens and emphasises our hypothesis that miR-155 has a crucial role in the pathogenesis and severity of COVID-19.
In agreement with our results, miR-155 is overexpressed in different pulmonary diseases such as asthma,25 cystic fibrosis,26 , 27 idiopathic pulmonary fibrosis,28 and acute lung injury.29
Several studies have found miRNA signatures representative of diseases in various diseased tissues, as well as in urine, serum, plasma, and other body fluids in numerous pathologies, making them an appropriate candidate for biomarkers.30, 31, 32 Therefore, the current study also aimed to elaborate and assess the potential role of miR-155 as a diagnostic biomarker for the detection of COVID-19 disease and the severity of infection. Our results showed that AUC for miR-155 was 0.986 with 90% sensitivity and 100% specificity when it was used as a biomarker for the detection of COVID-19 disease; while in detection of severity of COVID-19 disease, AUC for miR-155 was 0.75 with 76% sensitivity and specificity. This makes it a good diagnostic and prognostic biomarker for the detection of COVID-19 disease. Our results are consistent with Garg et al. 33 who found that miR-155 was able to differentiate between severely ill, mechanically-ventilated COVID-19 patients.
Finally, from these results we can conclude that the level of circulating miR-155 correlates with the presence and severity of COVID-19 disease. Hence, it could serve as a good diagnostic biomarker for COVID-19 disease.
Conflicts of interest and sources of funding
The authors state that there are no conflicts of interest to disclose. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Tariku M., Hajure M. Available evidence and ongoing hypothesis on corona virus (covid-19) and psychosis: is corona virus and psychosis related? A narrative review. Psychol Res Behav Manag. 2020;13:701–704. doi: 10.2147/PRBM.S264235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyrrell D.A., Bynoe M.L. Cultivation of viruses from a high proportion of patients with colds. Lancet. 1966;1:76–77. doi: 10.1016/s0140-6736(66)92364-6. [DOI] [PubMed] [Google Scholar]
- 3.Mirzaei R., Mahdavi F., Badrzadeh F., et al. The emerging role of microRNAs in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Int Immunopharmacol. 2021;90:107204. doi: 10.1016/j.intimp.2020.107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chauhan N., Jaggi M., Chauhan S.C., Yallapu M.M. COVID-19: fighting the invisible enemy with microRNAs. Expert Rev Anti Infect Ther. 2021;19:137–145. doi: 10.1080/14787210.2020.1812385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worldometer: COVID-19 Coronavirus Pandemic https://www.worldometers.info/coronavirus/ Cited 23 May 2021.
- 7.Li C., Hu X., Li L., Li J.H. Differential microRNA expression in the peripheral blood from human patients with COVID-19. J Clin Lab Anal. 2020;34 doi: 10.1002/jcla.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sessa R., Hata A. Role of microRNAs in lung development and pulmonary diseases. Pulm Circ. 2013;3:315–328. doi: 10.4103/2045-8932.114758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez M.J., Gomez J.L., Perez G.F., et al. Airway secretory microRNAome changes during rhinovirus infection in early childhood. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nersisyan S., Engibaryan N., Gorbonos A., Kirdey K., Makhonin A., Tonevitsky A. Potential role of cellular miRNAs in coronavirus-host interplay. PeerJ. 2020;8:e9994. doi: 10.7717/peerj.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang H., Mao J., Ye X., et al. SHIP-1, a target of miR-155, regulates endothelial cell responses in lung fibrosis. FASEB J. 2020;34:2011–2023. doi: 10.1096/fj.201902063R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahesh G., Biswas R. MicroRNA-155: a master regulator of inflammation. J Interferon Cytokine Res. 2019;39:321–330. doi: 10.1089/jir.2018.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Haroun R.A., Osman W.H., Eessa A.M. Interferon-γ-induced protein 10 (IP-10) and serum amyloid A (SAA) are excellent biomarkers for the prediction of COVID-19 progression and severity. Life Sci. 2021;269:119019. doi: 10.1016/j.lfs.2021.119019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan J., Xia L., Xu W., Lu N. Expression and function of miR-155 in diseases of the gastrointestinal tract. Int J Mol Sci. 2016;17:709. doi: 10.3390/ijms17050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang K., Yang J., Guo S., Zhao G., Wu H., Deng G. Peripheral circulating exosome-mediated delivery of miR-155 as a novel mechanism for acute lung inflammation. Mol Ther. 2019;27:1758–1771. doi: 10.1016/j.ymthe.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pociask D.A., Robinson K.M., Chen K., et al. Epigenetic and transcriptomic regulation of lung repair during recovery from influenza infection. Am J Pathol. 2017;187:851–863. doi: 10.1016/j.ajpath.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Smet E.G., Van Eeckhoutte H.P., Avila Cobos F., et al. The role of miR-155 in cigarette smoke-induced pulmonary inflammation and COPD. Mucosal Immunol. 2020;13:423–436. doi: 10.1038/s41385-019-0241-6. [DOI] [PubMed] [Google Scholar]
- 19.Adwanikar H., Karim F., Gereau R.W., 4th Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain. 2004;111:125–135. doi: 10.1016/j.pain.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Koranteng R.D., Swindle E.J., Davis B.J., et al. Differential regulation of mast cell cytokines by both dexamethasone and the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580. Clin Exp Immunol. 2004;137:81–87. doi: 10.1111/j.1365-2249.2004.02510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meduri G.U., Headley S., Kohler G., et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z.Q., Feng J., Shi L.L., Xu J., Zhang B.J., Chen L.J. Influences of miR-155/NF-κB signaling pathway on inflammatory factors in ARDS in neonatal pigs. Eur Rev Med Pharmacol Sci. 2019;23:7042–7048. doi: 10.26355/eurrev_201908_18746. [DOI] [PubMed] [Google Scholar]
- 23.Pan Y., Guan H. Imaging changes in patients with 2019-nCov. Eur Radiol. 2020;30:3612–3613. doi: 10.1007/s00330-020-06713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng M.Y., Lee E.Y.P., Yang J., et al. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol Cardiothorac Imag. 2020;2 doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y., Xiong S., Jiang P., et al. Glucocorticoids inhibit lipopolysaccharide-mediated inflammatory response by downregulating microRNA-155: a novel anti-inflammation mechanism. Free Radic Biol Med. 2012;52:1307–1317. doi: 10.1016/j.freeradbiomed.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Pottier N., Maurin T., Chevalier B., et al. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: implication in epithelial-mesenchymal interactions. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharyya S., Balakathiresan N.S., Dalgard C., et al. Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J Biol Chem. 2011;286:11604–11615. doi: 10.1074/jbc.M110.198390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oak S.R., Murray L., Herath A., et al. A micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaporidi K., Vergadi E., Kaniaris E., et al. Pulmonary microRNA profiling in a mouse model of ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L199–L207. doi: 10.1152/ajplung.00370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etheridge A., Lee I., Hood L., Galas D., Wang K. Extracellular microRNA: a new source of biomarkers. Mutat Res. 2011;717:85–90. doi: 10.1016/j.mrfmmm.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra P.J. MicroRNAs as promising biomarkers in cancer diagnostics. Biomark Res. 2014;2:19. doi: 10.1186/2050-7771-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Chen J., Sen S. MicroRNA as biomarkers and diagnostics. J Cell Physiol. 2016;231:25–30. doi: 10.1002/jcp.25056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg A., Seeliger B., Derda A.A., et al. Circulating cardiovascular microRNAs in critically ill COVID-19 patients. Eur J Heart Fail. 2021;23:468–475. doi: 10.1002/ejhf.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]