Abstract
Objective:
Childhood abuse represents one of the most potent risk factors for developing psychopathology, especially in females. Evidence suggests that exposure to early-life adversity may be related to advanced maturation of emotion processing neural circuits. However, it remains unknown whether abuse is related to early circuit maturation and whether maturation patterns depend on the presence of psychopathology.
Methods:
A multi-site sample of 246 females (ages 8-18 years) completed clinical assessment, maltreatment histories, and high-resolution T1 structural magnetic resonance imaging (MRI). Girls were stratified by abuse history and internalizing disorder diagnosis: typically-developing (no abuse/no diagnosis), resilient (abuse/no diagnosis), and susceptible (abuse/current diagnosis). Machine learning models of normative brain development were aggregated in a stacked generalization framework, trained to predict chronological age using gray matter volume in whole-brain, emotion, and language circuit parcellations. Brain age gap estimates (BrainAGEs; predicted age minus true chronological age) were calculated as indices of relative circuit maturation.
Results:
Childhood abuse was related to reduced BrainAGE (delayed maturation) specific to emotion circuits. Delayed emotion circuit BrainAGE was further related to increased hyperarousal symptoms. Childhood physical neglect was associated with increased whole-brain BrainAGE (advanced maturation). Neural contributors to emotion circuit BrainAGE differed in girls with and without an internalizing diagnosis, especially in lateral prefrontal, parietal, insular cortices, and hippocampus.
Conclusions:
Abuse exposure in girls is associated with a delayed structural maturation pattern specific to emotion circuitry, a potentially adaptive mechanism enhancing threat generalization. Physical neglect, on the other hand, is associated with a broader brain-wide pattern of advanced structural maturation. The differential influence of fronto-parietal cortices and hippocampus on emotion circuit maturity in resilient girls may represent neurodevelopmental markers of reduced psychiatric risk following abuse.
INTRODUCTION
Exposure to potentially traumatic events during childhood is pervasive, with two thirds of children experiencing violence (physical abuse, sexual abuse, or witnessing community or domestic violence) by age 16(1). Early-life exposure to violence can markedly alter neurobiological, psychological, and social development (e.g. (2)). These changes increase the risk for developing both first-onset and comorbid internalizing psychopathology(3), which has especially high overlap in symptom expression in adolescent females(4). However, the neurodevelopmental mechanisms conferring resilience and susceptibility to disorder following abuse remain unclear. Recent models suggest that early-life adversity may alter maturation patterns in emotion processing circuits, but it remains unknown how neurodevelopmental maturity may influence the relationship between threat-related (versus deprivation-related) stress and risk for internalizing psychopathology. Identification of neural maturational markers of resilience and susceptibility could have important implications for clinical monitoring and treatment for youth victims of abuse.
Evolutionary trade-offs between an individual’s survival and development (e.g. investment in growth) and reproductive success (e.g. investment in sexual maturity) likely underlie individual differences in child development after early-life adversity. Life History Theory and the Differential Susceptibility Model suggest that these trade-offs are likely constrained by the susceptibility of various developmental milestones to early experiences. The Stress Acceleration Hypothesis extends these frameworks by integrating early-life adversity and child neurodevelopment(5). According to the hypothesis, early-life adversity may promote early development of emotion circuits, particularly those underlying threat-safety processing, to meet potentially-dangerous environmental demands. Indeed, stress-related changes in the recruitment of emotion circuits often show patterns suggesting advanced development. For example, early development of amygdala – medial prefrontal cortex (mPFC) functional connectivity has been observed in youth exposed to maternal deprivation stress(6), residing in disadvantaged socioeconomic neighborhoods(7), and longitudinally associated with the severity of early-life adversity generally(8). However, a more thorough review of the related literature indicates mixed results overall, equally suggesting both delayed maturation and no maturational differences(9). Importantly, the stress acceleration model of adversity and the development of emotion-related circuits may be dependent on the characteristics of adversity experienced; this has been suggested by recent work incorporating the Dimensional Model of Adversity and Psychopathology(10), where advanced biological aging measured via telomere shortening, epigenetic age, and pubertal development were specific to threat-related (e.g. abuse) versus deprivation-related (e.g. neglect) adversity(11).
The relationship between emotion circuit maturation after abuse and resilience or susceptibility to subsequent psychopathology is also unclear. Current evidence suggests that fast developmental strategies during childhood increases risk for psychopathology in adulthood(12). Additionally, abused youth are at increased risk for internalizing psychopathology earlier, with greater severity, and with more comorbidities(13,14). The Latent Vulnerability Model(15) suggests that resilience and susceptibility to psychopathology after early-life adversity depends on the degree of neurodevelopmental flexibility in systems underlying salience detection, threat appraisal, and emotion regulation in adapting to future adversity. Earlier-developing circuits underlying salience detection and threat appraisal (e.g. amygdala, insula, mPFC) are likely recalibrated toward increased recruitment to threat-related stimuli, spurring development toward a more mature threat processing phenotype. We suspected that vulnerability to psychopathology may then fundamentally depend on how later-developing circuits, especially in lateral (l)PFC, are recalibrated in response. In contrast to earlier-developing structures, lPFC does not reach developmental plateau until early adulthood, remaining highly plastic throughout adolescence by maintaining increased levels of synaptogenesis and experience-dependent pruning, myelination, and apoptosis(16). Therefore, such systems likely show greater variability in their developmental trajectories after early life adversity, leading to greater variability in executive control processes underlying resilience or susceptibility to psychopathology. We posit that adaptive flexibility in the maturation of lPFC is a key predictor for which youth develop internalizing psychopathology after early-life adversity, though this is likely highly dependent on the timing at which the adversity occurred.
In the current study, we asked whether abused girls show advanced structural maturation in emotion-related circuits, compared to whole-brain and language-related circuitry. Language-related circuitry was used as a control circuit to evaluate emotion circuit specificity. We asked whether the degree of structural maturity depended on the absence (resilience) or presence (susceptibility) of internalizing disorders and whether these effects were specific to abuse (threat) versus neglect (deprivation). The focus on internalizing disorders broadly was due to high rates of comorbidity between anxiety, depression, and post-traumatic stress disorders, which if segregated, would have considerably limited samples sizes and statistical power for psychopathology-related comparisons. Finally, we asked whether particular brain regions contributed to overall circuit maturation differently in internalizing resilient versus susceptible girls. Using machine learning in a stacked generalization framework, we implemented a normative development model trained to predict chronological age from regional gray matter volume estimates in typically-developing girls. We then used the normative model to calculate a brain age gap estimate (BrainAGE; predicted age minus chronological age), an index of relative circuit maturation. The BrainAGE has been shown to represent a biologically-meaningful index that is reliable(17,18), heritable(18), and associated with developmental neurophenotypes underlying illness(19,20). This index is specific to an individual’s brain at the time the data was collected and should not be interpreted as reflecting a developmental trajectory that brain will follow into the future. We hypothesized that abused girls, both resilient and susceptible to internalizing disorders, would show greater emotion circuit BrainAGE (indicating advanced maturation) relative to typically-developing girls. We also hypothesized that abuse-related gray matter volume in early-developing regions underlying salience detection and threat appraisal (e.g. amygdala, insula) would contribute to more positive (advanced) BrainAGE. Finally, we hypothesized that late-developing regions underlying attentional processes and executive control (e.g. lPFC) would best differentiate circuit maturation in resilient versus susceptible abused girls.
METHODS
Participant Recruitment and Assessment
Two hundred and forty-six adolescent females (chronological age range of 8-18 years) were pooled from research studies at three sites: Madison, Wisconsin; Little Rock, Arkansas; Seattle, Washington. We investigated violence exposure-related differences specific to abuse (physical, sexual, and emotional). Details regarding sample sizes, MRI parameters, and demographic information for each site are given in Table 1 and Supplemental Table 1. Written informed consent and/or verbal assent were obtained from all participants and study procedures were approved by institutional review boards. A detailed account of clinical assessments, including diagnostic battery, anxiety, depression, and PTSD symptom severity, and maltreatment history, can be found in Supplemental Material. Importantly, girls were segregated into three groups based on binary abuse exposure (unexposed vs. exposed) and internalizing diagnosis (presence vs. absence): typically-developing (unexposed, no diagnoses), Resilient (exposed, no diagnoses), and Susceptible (exposed, at least one diagnosis). It is important to note, we use the terms resilient and susceptible to refer to the current absence or presence of internalizing psychopathology after abuse respectively. Because many youth in this sample had yet to reach chronological age corresponding to the average age of onset for some internalizing disorders, in this context, resiliency does not imply that these youth may not be susceptible to disorder in the future.
Table 1.
Demographic, maltreatment, and clinical variables across the three study sites. Group comparison F, t, and p statistics are indicated with the effect direction/s for significant differences (p < 0.05) . Sus = Susceptible, Res = Resilient; CTQ = Childhood Trauma Questionnaire; PTSD = post-traumatic stress disorder; MFQ = Mood and Feelings Questionnaire; SCARED = Screen for Child Anxiety Related Emotional Disorders; PTSD-RI = PTSD Reaction Index; NS = non-significant * Indicates a variable that was not consistently available for all subjects (missing not-at-random). Statistics are provided for data that were available.
| Group | Group Comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Typically-Developing | Resilient | Susceptible | F | t | Direction | p | ||||
| n | 99 | 50 | 85 | - | ||||||
| Mean | SD | Mean | SD | Mean | SD | |||||
| Age | 13.78 | 2.64 | 14.15 | 2.25 | 14.55 | 2.32 | 2.59 | NS | - | 0.076 |
| IQ | 111.68 | 16.73 | 106.02 | 17.05 | 99.14 | 15.64 | 32.70 | −3.31 | Typically-Developing > Res | < 0.001 |
| −7.32 | Res > Sus | < 0.001 | ||||||||
| Tanner Stage* | 3.67 | 1.20 | 3.49 | 1.41 | 3.35 | 1.39 | 1.22 | NS | - | 0.296 |
| CTQ Abuse | 15.94 | 1.09 | 25.92 | 8.37 | 33.88 | 12.37 | 141.10 | 8.28 | Typically-Developing < Res | < 0.001 |
| 15.79 | Res < Sus | < 0.001 | ||||||||
| CTQ Physical Neglect | 6.76 | 3.08 | 8.76 | 3.57 | 9.69 | 4.44 | 13.82 | 2.74 | Typically-Developing < Res | < 0.001 |
| 5.00 | Typically-Developing < Sus | < 0.001 | ||||||||
| MFQ* | - | - | 4.60 | 4.39 | 17.01 | 10.53 | 171.20 | 12.97 | Res < Sus | < 0.001 |
| SCARED* | - | - | 19.83 | 11.50 | 32. 84 | 16.54 | 36.43 | 67.80 | Res < Sus | < 0.001 |
| PTSD-RI* | - | - | 20.58 | 15.42 | 43.53 | 17.62 | 89.72 | 3.07 | Res < Sus | < 0.001 |
| Count | Percentage | |||||||||
| Anxiety Disorder | - | - | 38 | 44.7% | - | |||||
| Depressive Disorder | - | - | 59 | 69.4% | - | |||||
| PTSD | - | - | 56 | 65.9% | - | |||||
Image Acquisition and Individual Preprocessing
A detailed account of image acquisition and individual preprocessing of magnetic resonance imaging (MRI) data can be found in Supplemental Material and in Supplemental Table 1. Briefly, mean voxel-wise cortical and subcortical gray matter volume was extracted from processed T1-weighted MRI scans using the Brainnetome Atlas(21). Neurosynth(22) was used to identify regions-of-interest belonging to emotion-related and language-related circuits, which are listed in Supplemental Table 2 and diagrammed in Supplemental Figure 1. Finally, each region-of-interest was scaled to total intracranial volume and harmonized across MR scanners.
Normative Models of Gray Matter Volume Development
Model Building and Training
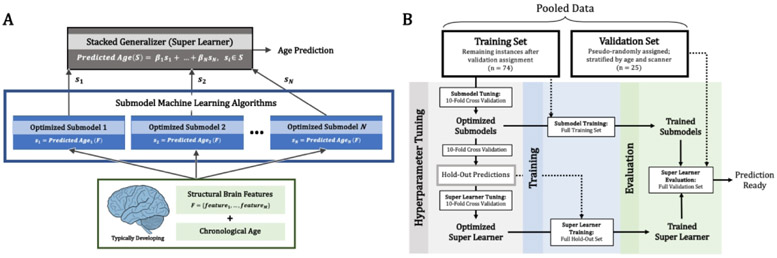
Ensemble machine learning using a stacked generalization(23) approach was used to build normative models of gray matter volume development with respect to whole-brain, emotion, and language circuit features. More specifically, a “super learner”, optimizing the aggregation of multiple learning algorithms minimizing cross-validation risk, was implemented for each neural feature set. Stacked generalization is a form of ensemble machine learning whereby individual, “lower-level” learning algorithms are aggregated to increase predictive power by utilizing the strengths of each base model (referred to as submodels). A “super learner”, therefore, is a final prediction aggregation model with the objective of finding the optimal combination of submodel predictions. A more detailed overview of the super learning algorithm (Figure 1A) and the model building protocol (Figure 1B) are provided in Supplemental Material.
Figure 1.
The super learner algorithm and normative model building protocol. (A) The super learner was implemented with an l2-penalized linear combination of predictions from N unique machine learning algorithms (submodels; including general linear ridge, multilayer perceptron, random forest, support vector machine, and gradient boosting machine regression). Submodel held-out predictions in each round of cross-validation were used to tune the super learner parameters. The full training set was used to train each submodel and the super learner coefficients (β1 … βN). Submodel predictions, S = (s1 … sN), were input to the super learner to make final age predictions and calculate BrainAGEs. (B) Schematic of the model building protocol for predicting chronological age and BrainAGE from gray matter volume features. Data were pooled and participants were pseudo-randomly assigned to the training or validation set (stratified by MR scanner and age). First, hyperparameters for each submodel algorithm were tuned using 10-fold cross-validation. The optimized submodels make predictions using 10-fold cross-validation and the hold-out set is used to tune the super learner hyperparameters. Finally, the optimized submodels are trained on the full training and the optimized super learner is trained on the full hold-out set. The trained super learner is then evaluated using the set-aside validation set.
Model Evaluation
The super learner and comprising submodels were evaluated with a validation set of typically-developing girls absent during training. Importantly, girls in the validation set were pseudorandomly assigned so as to remain representative of the training set (stratified by age and scanner). Mean absolute error was used to evaluate model performance. To ensure above-chance performance, a null distribution was created with 1000 bootstrap samples of the label vector (chronological age) and age was predicted with each bootstrap. Median differences between the true performance and null performance distributions were calculated. All models underwent a final evaluation step comparing predicted and chronological ages using Pearson correlation (r) and BrainAGEs were calculated for all abused girls. Here, a negative BrainAGE relative to typically-developing girls indicates the extent of delayed maturation and a positive BrainAGE indicates the extent of advanced maturation.
BrainAGE Group-Level Analyses
Linear mixed-effects models in R (lme4 package), were used to determine abuse- and diagnosis-related differences in BrainAGE from whole-brain, emotion, and language circuit features. BrainAGEs for abused girls were standardized to the typically-developing set and, for each neural feature set, an abuse by diagnosis interaction and main effects were included. As is common with the BrainAGE (24), there was an age bias in the super learner; therefore, chronological age was included as a covariate. Importantly, this bias correction was only included when analyzing BrainAGE at the group-level, and therefore doesn’t not change the interpretability of the BrainAGE as a maturational index. Additionally, IQ (gray matter volume is strongly associated with IQ in older children and adolescents(25)), scanner, and physical neglect experiences were included as covariates to safeguard against scanner effects and test specificity to abuse. Finally, an additional Bonferroni correction was applied across the three sets of circuit features (α = 0.017; p < 0.05/3). Post-hoc analyses were conducted if significant relationships were identified, investigating the relationship between BrainAGE differences and internalizing symptom severity (PTSD, depression, and anxiety symptoms) and pubertal stage. Additional details regarding all models tested, binarized versus continuous analyses, and covariates can be found in Supplemental Material.
Feature Influence Analysis
Calculating Regional Influence on BrainAGE
We hypothesized that regional gray matter volume phenotypes related to abuse may differentially influence BrainAGE estimates in Resilient versus Susceptible girls. In order to determine how influential abuse-related gray matter volume was to BrainAGE, a perturbation sensitivity approach was used (Supplemental Figure 3). Importantly, “perturbation” in this context did not refer to an anxiety phenotype, but instead referred to a change in model performance after that model had been altered in some way. The goals of perturbation sensitivity were two-fold: first, to determine if an altered distribution of BrainAGEs was significantly different than the unaltered distribution of BrainAGEs (significant feature influence), and second, to determine if perturbation with an abuse-related phenotype caused a shift in BrainAGE distribution relative to the distribution observed with typically-developing perturbation (beyond chance expectations). In pursuit of these goals, perturbation across more than two of the groups at once was ruled out as a possible approach. The rationale for this analysis is as follows: perturbing a typically-developing gray matter volume phenotype with an abuse-related gray matter volume phenotype should strongly impact the normative model’s age prediction (and related BrainAGE) if that region’s abuse phenotype is informative to either significantly increased or decreased BrainAGE. Similarly, if an abuse-related phenotype is not informative for the model’s age prediction, there will be no significant change in the distribution of BrainAGEs. Feature influence was calculated as the median change in BrainAGE distribution after perturbation (perturbed median BrainAGE - true median BrainAGE). More details regarding abuse-related gray matter volume sampling and statistical tests comparing BrainAGE distributions can be found in Supplemental Material.
RESULTS
Participant Demographics
Aggregated demographic, clinical, and maltreatment variables across study sites are found in Table 1 and are split by study site in Supplemental Table 1. The pooled cohort consisted of 246 adolescent females between the ages of 8 and 18 (mean age 14.15 ± 2.47). In total, 99 girls had no abuse exposure nor diagnosed internalizing psychopathology (typically-developing), 50 girls had abuse exposure and no diagnosis (Resilient), and 85 girls had abuse exposure and at least one diagnosis (Susceptible). Within the Susceptible girls, 38 (44.7%) had an anxiety disorder, 59 (69.4%) had a depressive disorder, and 56 (65.9%) had PTSD; 62 (72.9%) had at least 2 of these disorders.
Normative Model Performance and Bias
The super learner and its submodels performed significantly better than chance expectations (all p’s << 0.001). Model performances on the validation set of typically-developing girls for each super learner and comprising submodels are reported in Supplemental Table 4. Models generalized well to the validation set (means for whole-brain: mean absolute error = 2.463 years, r = 0.507, p = 0.048; means for emotion: mean absolute error = 1.851 years, r = 0.594, p = 0.003; means for language: mean absolute error = 2.326 years, r = 0.566, p = 0.009). For each feature set, the super learner performed better than all comprising submodels (whole-brain: mean absolute error = 1.666 years, r = 0.677, p < 0.001; emotion: mean absolute error = 1.602 years, r = 0.663, p < 0.001; language: mean absolute error = 1.569 years, r = 0.655, p < 0.001). There was an age bias in each super learner, over and under estimating BrainAGEs in younger and older girls respectively (Supplemental Figure 4; emotion circuitry: t 756 = −11.963, p << 0.001)
Group-Level BrainAGE Relationships
Group Differences in BrainAGE
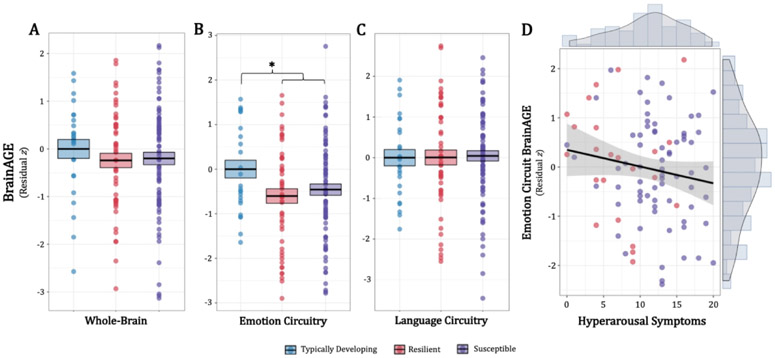
In the whole-brain and language circuitry analyses, no significant abuse- or diagnosis-related differences were identified (Figure 2A, 2C). In the emotion circuitry analysis, an abuse effect was identified, where girls exposed to abuse showed significantly reduced BrainAGE (Figure 2B; F1,150 = 15.680, t150 = −2.366, p = 0.014), an average of 0.70 years younger than typically-developing girls of the same chronological age. There was a significant effect of physical neglect severity for whole-brain BrainAGE, where physical neglect experiences were positively associated with BrainAGE across all girls (β = 0.064, F1,150 = 7.933; t150 = 2.817, p = 0.006). Importantly, there were no significant nor trending scanner effects (Supplemental Figure 5).
Figure 2.
Abuse- and internalizing diagnosis-related associations with BrainAGE for whole-brain, emotion circuit, and language circuit feature sets. BrainAGEs are represented as residualized z-scores relative to typically-developing girls, controlling for label (chronological age), MR scanner, IQ, and physical neglect. (A) For the whole-brain analysis, there were no significant differences in BrainAGE across groups. (B) For the emotion circuit analysis, an abuse main effect shows that Resilient and Susceptible girls show significantly reduced average BrainAGE relative to typically-developing girls. (C) For the language circuit analysis, there were no significant differences in BrainAGE across groups. (D) Symptom-level association with emotion circuit BrainAGEs across abused girls. Emotion circuitry BrainAGEs were negatively associated with hyperarousal symptoms (PTSD-RI subscore D). BrainAGE = brain age gap estimate; PTSD-RI = Post-Traumatic Stress Disorder Reaction Index.BrainAGE = brain age gap estimate; *p < 0.017 (after experiment-wide Bonferroni correction).
Symptom and Puberty Relationships with BrainAGE
In the symptom analyses with emotion circuit BrainAGEs, there was a significant effect of PTSD hyperarousal symptoms (PTSD-RI subscore D). Here, across all abused girls, hyperarousal symptom severity was negatively associated with BrainAGE (Figure 2D; β = −0.091, t 96 = −2.050, p = 0.043). This relationship was only identified when controlling for all previous group-level covariates, abuse severity, and the remaining PTSD-RI subscores (unadjusted β = −0.053, t98= −1.327, p = 0.187). Finally, there were no significant relationships between BrainAGE and total PTSD (t98= 1.142, p = 0.256), anxiety (t65= 0.354, p = 0.724), and depression (t110= 0.340, p = 0.734) symptoms, or pubertal milestones (t65= 1.114, p = 0.270).
Regional Influence on Abuse-Related BrainAGE Distribution
Regions Influencing BrainAGE: All Abused Girls
A summary of results from the feature influence analysis can be found in Table 2, separated by spatially overlapping (all abused girls) or unique (Resilient vs. Susceptible) contributors to BrainAGE. Resilient and Susceptible girls showed few overlapping influential regions. Generally, thalamic gray matter volume from abused girls contributed to a positive (advanced) shift in BrainAGE: gray matter volume in the left and right mediodorsal thalamus (medial prefrontal thalamus: adj. R2 = 0.087, FDR-p < 0.001; lateral prefrontal thalamus: adj. R2 = 0.053, FDR-p = 0.040), and left lateral pulvinar nucleus (caudal temporal thalamus: adj. R2 = 0.049, FDR-p = 0.005) all contributed to a positive shift in BrainAGE. Additionally, gray matter volume in the right caudal anterior cingulate cortex (ACC; adj. R2 = 0.076, FDR-p < 0.039) contributed to a positive shift in BrainAGE for both Resilient and Susceptible girls. No regions contributed to a negative (delayed) shift in BrainAGE.
Table 2.
Perturbation sensitivity (feature influence) analysis in emotion circuitry; regions are separated by overlapping vs. unique effects between abuse groups. Within these separations, regions are sorted by descending adjusted R 2. R 2 and FDR-corrected p -values are for the abuse-perturbed BrainAGE vs. typically-developing-perturbed (chance) BrainAGE comparison. All probability values for the abuse-perturbed BrainAGE vs. true BrainAGE comparison were less than 0.001 and are absent to avoid redundancy.
| Effect | Direction | Region | L/R | BA | Power et al. Network |
Atlas Label | Adj. R 2 | FDR p |
|---|---|---|---|---|---|---|---|---|
| Abuse Exposure | Greater BrainAGE | Medial Prefrontal Thalamus | L | - | Subcortical | Tha_8_1 | 0.087 | < 0.001 |
| Caudal Anterior Cingulate Cortex | R | 24 | Cingulo-Opercular | CG_7_5 | 0.076 | 0.039 | ||
| Lateral Prefrontal Thalamus | R | - | Subcortical | Tha_8_8 | 0.053 | 0.040 | ||
| Caudal Temporal Thalamus | L | - | Subcortical | Tha_8_7 | 0.049 | < 0.001 | ||
| Unique to Resilient | Greater BrainAGE | Rostral Inferior Parietal Lobule | L | 40 | Fronto-Parietal | IPL_6_6 | 0.098 | < 0.001 |
| Premotor Thalamus | L | - | Subcortical | Tha_8_2 | 0.092 | < 0.001 | ||
| Dorsal Inferior Parietal Lobule | R | 39 | Fronto-Parietal | IPL_6_2 | 0.087 | < 0.001 | ||
| Dorsolateral Prefrontal Cortex | R | 8 | Fronto-Parietal | MFG_7_5 | 0.087 | 0.006 | ||
| Primary Auditory Cortex | L | 41 | Auditory | STG_6_3 | 0.042 | 0.043 | ||
| Ventral Caudate Nucleus | L | - | Subcortical | BG_6_1 | 0.039 | 0.014 | ||
| Reduced BrainAGE | Posterior Parahippocampal Gyrus | R | 27 | Default-Mode | PhG_6_3 | 0.098 | 0.034 | |
| Dorsal Inferior Parietal Lobule | L | 39,40 | Dorsal Attention | IPL_6_3 | 0.095 | < 0.001 | ||
| Dorsal Agranular Insula | R | 13 | Cingulo-Opercular | INS_6_3 | 0.083 | 0.050 | ||
| Lateral Orbitofrontal Cortex | R | 12/47 | Cingulo-Opercular | OrG_6_6 | 0.080 | 0.034 | ||
| Lateral Opercular Prefrontal Cortex | R | 44 | Cingulo-Opercular | IFG_6_6 | 0.079 | < 0.001 | ||
| Unique to Susceptible | Greater BrainAGE | Occipital Thalamus | L | - | Subcortical | Tha_8_6 | 0.093 | < 0.001 |
| Rostral Temporal Thalamus | L | - | Subcortical | Tha_8_4 | 0.088 | < 0.001 | ||
| Dorsal Dysgranular Insula | L | 13 | Cingulo-Opercular | INS_6_6 | 0.081 | < 0.001 | ||
| Opercular Prefrontal Cortex | R | 44 | Cingulo-Opercular | IFG_6_5 | 0.051 | 0.028 | ||
| Reduced BrainAGE | Caudal Hippocampus | L | - | Default-Mode | Hipp_2_2 | 0.088 | < 0.001 | |
| Caudal Hippocampus | R | - | Default-Mode | Hipp_2_2 | 0.087 | < 0.001 | ||
| Rostral Temporal Thalamus | R | - | Subcortical | Tha_8_4 | 0.067 | < 0.001 |
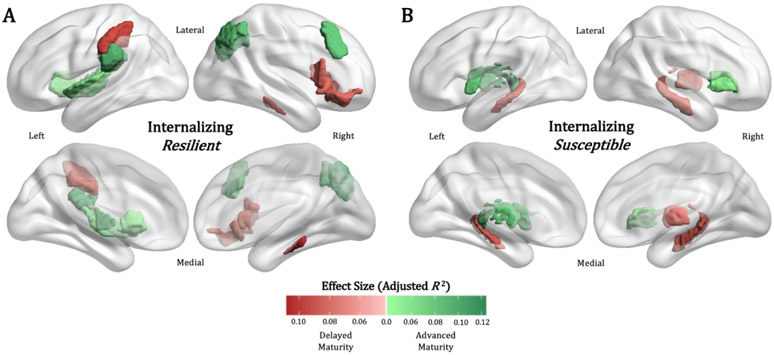
Regions Influencing BrainAGE: Resilient Girls
Resilient girls showed regional gray matter volume phenotypes that uniquely influenced BrainAGE relative to Susceptible girls (Table 2, Figure 3A). Regions contributing to a positive shift BrainAGE included the left ventral lateral thalamic nucleus (premotor thalamus: adj. R 2 = 0.092, FDR-p < 0.001), the left rostral inferior parietal lobule (BA40; adj. R 2 = 0.098, FDR-p < 0.001), right dorsal inferior parietal lobule (BA 39; adj. R 2 = 0.087, FDR-p < 0.001), and right dorsolateral prefrontal cortex (dlPFC, BA 8; adj. R 2 = 0.087, FDR-p = 0.006). Regions that contributed to a negative shift in BrainAGE included the right posterior parahippocampal gyrus (BA 27; adj. R 2 = 0.098, FDR-p < 0.034), right dorsal insular cortex (BA 13; adj. R 2 = 0.083, FDR-p = 0.050), right lateral orbitofrontal cortex (BA 12/47; adj. R 2 = 0.080, FDR-p = 0.034), and right lateral opercular prefrontal cortex (BA 44; adj. R 2 = 0.079, FDR-p < 0.001).
Figure 3.
Spatially unique feature influence results for (A) Resilient and (B) Susceptible girls from the perturbation sensitivity analysis. Region-of-interest color corresponds to the mean effect size (adj. R 2) of the BrainAGE distribution shift when the region is perturbed with an abuse-related gray matter volume phenotype. Darker green indicates greater positive shift and darker red indicates greater negative shift. PS = noise perturbation sensitivity; BrainAGE = brain age gap estimate
Regions Influencing BrainAGE: Susceptible Girls
Susceptible girls showed regional gray matter volume phenotypes that uniquely influenced BrainAGE relative to Resilient girls (Table 2, Figure 3B). Regions that contributed to a positive shift in BrainAGE included the left lateral geniculate nucleus (occipital thalamus: adj. R 2 = 0.093, FDR-p < 0.001), medial pulvinar nucleus (rostral temporal thalamus: adj. R 2 = 0.088, FDR-p < 0.001), left dorsal insular cortex (adj. R 2 = 0.081, FDR-p < 0.001), and right opercular PFC (BA 44; adj. R 2 = 0.051, FDR-p = 0.028). Regions that contributed to a negative shift in BrainAGE included bilateral caudal hippocampus (left: adj. R 2 = 0.088, FDR-p < 0.001; right: adj. R 2 = 0.087, FDR-p < 0.001).
DISCUSSION
Abused girls, regardless of diagnostic status, showed delayed maturity in emotion circuitry (counter to our original hypothesis), which was further associated with increased hyperarousal symptoms. Advanced whole-brain BrainAGE was associated with increased physical neglect severity, suggesting differential effects of threat versus deprivation stress on patterns of circuit-specific and whole-brain neurodevelopment. Further, we found unique regional contributors to emotion circuitry maturation in Resilient and Susceptible girls, most prominently in frontoparietal, hippocampal, and insular gray matter. Altogether, our findings provide new insights into brain maturational patterns related to threat- and deprivation-related adversity, internalizing psychopathology, and point to potential systems-level mechanisms differentiating resilient and susceptible developmental trajectories.
Abuse exposure was associated with delayed emotion circuit maturity relative to typically-developing girls, which was further related to increased hyperarousal symptoms (but only when controlling for group-level covariates and other PTSD-related symptoms). Although speculative, this suggests that delayed structural maturity in emotion circuits may underlie sensitive salience and threat detection systems in the brain, potentially leading to reduced threat-safety discrimination and states of generalized hypervigilance. Although enhanced threat bias in neurobiological reactivity is likely adaptive in abusive environments, this may lead to reliably misinterpreting safety cues as dangerous. Indeed, reduced threat-safety discrimination has been previously associated with younger developmental stage (children < adolescents < adults)(26,27), and with increased recruitment of threat processing circuits with maltreatment exposure and increased risk for psychopathology(28,29). For this reason, girls exposed to abuse may habitually recruit threat-related circuitry even in canonically safe contexts: this kind of threat generalization, typically only observed in younger children, likely delays increases in synaptic pruning, circuit myelination, and other related processes that result in age-appropriate reductions in gray matter volume.
Physical neglect experiences, on the other hand, were positively associated with whole-brain maturity across typically-developing and abused girls. Here, increased physical neglect corresponded to advanced whole-brain maturation. In contrast to the delayed emotion circuit maturation associated with abuse, this suggests that neglect and deprivation-related adversity may have global rather than circuit-specific effects on brain maturation. Indeed, the absence of expected age-typical cognitive and social inputs to the brain, as is often the case with physical neglect, likely affects association cortex broadly, representing a global acceleration of neuronal and synapse elimination mechanisms typical of low-complexity environments(10,30). These mechanisms would translate to decreased gray matter volume in a deprivation context for equivalent chronological age, an advanced maturation phenotype. This may also explain the seeming discrepancy between previously reported findings of advanced emotion circuit development with adversity and the reported findings here, as the majority of these previous reports documented adversity more specific to deprivation (maternal separation(6), disadvantaged socioeconomic neighborhoods(7)) or a combination of threat and deprivation (abuse with physical, emotional neglect (8)). Thus, we suspect that the current formulation of the Stress Acceleration Hypothesis, as it pertains to brain maturation, may more accurately account for broader whole-brain patterns specific to deprivation-related adversity. Additionally, because co-occurrence of deprivation- and threat-related adversity is common, global advanced maturation patterns specific to deprivation may mask threat-specific delayed maturation patterns only observed in emotion circuits when not examined separately, causing them to go unnoticed in earlier studies.
We observed unique gray matter volume phenotypes from abused girls contributing to significant shifts in BrainAGE distribution. Resilient, but not Susceptible, abused girls showed dlPFC and lateral IPL gray matter volume contributing to a positive (advanced) shift in emotion circuit maturation, supporting our original hypotheses. We have previously reported internalizing-susceptible youth show developmentally delayed gray matter volume reduction in dlPFC(31) and cortical expansion of dlPFC differentiated which youth showed remission versus persistence of PTSD symptoms at one-year follow-up(32). Although abnormal dlPFC structure has not been reliably associated with childhood abuse exposure(9), changes in dlPFC structure and function have been broadly associated with reduced internalizing symptoms(33,34) and recovery from trauma(35). For example, an inability to recruit the dlPFC during differentiation of threat from non-threat mediates the relationship between anxiety disorders and generalized fear(36). Additionally, repetitive transcranial magnetic stimulation of dlPFC, altering functional connectivity with the amygdala(37) and nucleus accumbens(38), has been shown to reduce both anxiety and depression symptoms. Therefore, fronto-parietal structures contributing to advanced emotion circuit maturation in Resilient girls may translate to a compensatory trajectory promoting more mature attentional and executive control processes, approaching adult patterns of function and decreasing psychiatric risk.
We also find that bilateral hippocampus gray matter volume contributed to negative (delayed) shifts in emotion circuit maturation in Susceptible, but not Resilient, abused girls. We have previously reported age-related hippocampus gray matter abnormalities in traumatized youth with PTSD(39), one of the most commonly-reported neural correlates of early-life stress in youth and adults. Here, both childhood maltreatment and trauma exposure generally are associated with decreased hippocampal volume(40-42), likely driven by a combination of decreased neurogenesis/neural progenitor cells(43), atrophy of dendrites and reduced postsynaptic dendritic spines(44), and reduced pool of stem cells into adulthood(45). In alignment with our findings, decreased hippocampal volume appears more pronounced in maltreatment victims with PTSD and other internalizing disorders relative to those without(42). Together, these results suggest that neurodevelopment underlying resilience to internalizing after abuse is critically dependent on regional patterns of maturation, where regions with a late (e.g. dlPFC) versus early (e.g. hippocampus) developmental plateau may have extended windows of change susceptibility in which to reorganize, a key factor in the development of pathology-inducing circuit phenotypes. Importantly, the factors determining which children will undergo resilient circuit reorganization and which will not is unknown and warrants future study.
The majority of differences in BrainAGE influential regions between Resilient and Susceptible girls were found in nodes of the cingulo-opercular network, including insular cortex, opercular PFC, and lateral orbitofrontal cortex (OFC). Gray matter volume from Susceptible and Resilient girls contributed to more positive (advanced) and more negative (delayed) shifts in emotion circuit maturation respectively. The cingulo-opercular network is a key substrate of threat processing circuitry and is recruited by salient or unexpected stimuli to reorient attention circuitry toward relevant cues and inform subsequent responses(46,47). Abnormal development of the OFC, cross-sectionally and longitudinally, has been reported in studies of abused youth(31,39). As the brain’s “engine of alertness”, abnormalities likely underlie symptoms of hyperarousal and hypervigilance commonly observed after abuse(48,49). In fact, meta-analyses across all DSM-IV axis I disorders suggest that gray matter volume in the insular cortex is the best differentiator of individuals with diagnosable psychopathology broadly versus those without(50,51). We suspect that differences in the maturation of the cingulo-opercular network underlie important differences between Resilient and Susceptible girls after abuse, presumably through biasing attentional processes toward threat detection and the promotion of emotional reactivity.
Our study is not without limitations. First, as is the case for many pediatric neuroimaging studies, the sample size of typically-developing and abused girls is modest. The small sample size for non-abused girls with internalizing diagnoses precluded us from an omnibus analysis interrogating maturation patterns specific to internalizing psychopathology. Second, given that many measures collected had substantial missing data (depression, anxiety symptoms, pubertal milestones), and these data were missing not-at-random, associated null findings should be interpreted with caution. For example, in a full sample we would expect that earlier pubertal milestones for the same chronological age would be associated with more positive BrainAGE, and accordingly, that abuse exposure would be associated with delayed pubertal milestones. This would represent an important step in evaluating the biological validity of the BrainAGE and its ability to test hypotheses regarding maturation. Finally, the current sample only contains female youth. While relevant given the higher prevalence of internalizing disorders in girls, future studies including boys could begin to disentangle which reported effects are sex-specific. Future research would be strengthened by using both structural and functional MRI data for abused youth simultaneously, supporting the functional implications of structural maturity differences. Longitudinal studies are required to confirm maturational differences associated with abuse, as well as whether pre-adversity gray matter volume accounts for developmental delays and whether clinical interventions can bring emotion circuit maturity back into a healthy range.
Despite these limitations, the current study has many strengths. First is our use of ensemble machine learning and feature influence analyses. To our knowledge, this is the first report interrogating multivariate, circuit-specific BrainAGEs, where constraining the neural feature set to specific domains of function allows a more precise indexing of maturation. This allows for the detection of altered circuit maturation which may normally go undetected in whole-brain analyses. These methods also allowed us to explore how abuse-related gray matter volume phenotypes contributed to changes in BrainAGE, an important step in understanding the neurodevelopmental relationships learned by the normative model. Second, our study focused on disentangling the effects of threat- versus deprivation-related adversity on circuit maturation, which are all too often aggregated into a single “adversity” cohort. Finally, this is one of only a handful of studies using the BrainAGE to interrogate important questions in development, and more specifically, related to early-life adversity and psychopathology in youth. Normative neurodevelopment models and the BrainAGE maturity index have the potential to allow researchers to test treatment strategies targeting specific circuits and help clinicians monitor the neurodevelopmental trajectories of their patients, with the aim of helping guide them back into healthy ranges.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to sincerely thank Rachael Meline, Shelby Weaver, and many others from the Herringa, McLaughlin, and Cisler labs for their work in the recruitment and data collection for this study. We’d also like to thank Drs. Andrew Sheldon and Richard Davidson for their help in early iterations of these analyses and interpretation of our results. Finally, we owe our sincerest gratitude to the youth and families who have given their time for this study.
Funding for this study was provided by the National Institutes of Health and National Institutes of Mental Health (R01 MH103291, R01 MH106482, and R56 MH119194 to KAM; R01 MH115910 to RJH, R01 MH117141 to RJH and XZ; R21 MH097784 and R21 MH106869 to JC; K08 MH100267 to RJH; 5T32 MH018931 to JDR), American Academy of Child and Adolescent Psychiatry Junior Investigator Award (to RJH), Arkansas Science and Technology Authority and the State of Arkansas (to JC), NARSAD Young Investigator Grant (to RJH and JC), University of Wisconsin Institute for Clinical and Translational Research Translational Pilot Grant Award (NIH/NCATS UL1TR000427, to RJH), National Science Foundation Graduate Research Fellowship Award (DGE 1747503 to TJK), University of Wisconsin Institute of Clinical and Translational TL1 Training Award (TL1TR000429, to SAH), and the University of Wisconsin School of Medicine and Public Health.
Footnotes
CONFLICT AND FUNDING DISCLOSURES
The author’s report no actual or perceived financial conflicts of interest in the preparation, data collection, analysis, interpretation, or writing of this manuscript.
REFERENCES
- 1.Finkelhor D, Turner HA, Shattuck A, Hamby SL. Prevalence of Childhood Exposure to Violence, Crime, and Abuse: Results From the National Survey of Children’s Exposure to Violence. JAMA Pediatr. 2015. August;169(8):746–54. [DOI] [PubMed] [Google Scholar]
- 2.Herrenkohl TI, Sousa C, Tajima EA, Herrenkohl RC, Moylan CA. Intersection of Child Abuse and Children’s Exposure to Domestic Violence. Trauma Violence Abuse. 2008. April 1;9(2):84–99. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, et al. Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry. 2013. August;52(8):815–830.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merikangas KR, Nakamura EF, Kessler RC. Epidemiology of mental disorders in children and adolescents. Dialogues Clin Neurosci. 2009. March;11(1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaghan BL, Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci. 2016. February 1;7:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013. September 24;110(39):15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramphal B, DeSerisy M, Pagliaccio D, Raffanello E, Rauh V, Tau G, et al. Associations between Amygdala-Prefrontal Functional Connectivity and Age Depend on Neighborhood Socioeconomic Status. Cereb Cortex Commun [Internet]. [cited 2020 Jul 30]; Available from: https://academic.oup.com/cercorcomms/article/doi/10.1093/texcom/tgaa033/5875522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JG, Ho TC, Humphreys KL, King LS, Foland-Ross LC, Colich NL, et al. Early Life Stress, Frontoamygdala Connectivity, and Biological Aging in Adolescence: A Longitudinal Investigation. Cereb Cortex [Internet]. [cited 2020 Mar 30]; Available from: https://academic.oup.com/cercor/advance-article/doi/10.1093/cercor/bhaa057/5811847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin KA, Weissman D, Bitrán D. Childhood Adversity and Neural Development: A Systematic Review. Annu Rev Dev Psychol. 2019;1(1):277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014. November;47:578–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colich NL, Rosen ML, Williams ES, McLaughlin KA. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychol Bull. 2020. August 3; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurst JE, Kavanagh PS. Life history strategies and psychopathology: The faster the life strategies, the more symptoms of psychopathology. Evol Hum Behav. 2017;38(1):1–8. [Google Scholar]
- 13.Hovens JGFM, Wiersma JE, Giltay EJ, van Oppen P, Spinhoven P, Penninx BWJH, et al. Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatr Scand. 2010. July;122(1):66–74. [DOI] [PubMed] [Google Scholar]
- 14.Hovens JGFM, Giltay EJ, Wiersma JE, Spinhoven P, Penninx BWJH, Zitman FG. Impact of childhood life events and trauma on the course of depressive and anxiety disorders. Acta Psychiatr Scand. 2012. September;126(3):198–207. [DOI] [PubMed] [Google Scholar]
- 15.McCrory EJ, Gerin MI, Viding E. Annual Research Review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry - the contribution of functional brain imaging. J Child Psychol Psychiatry. 2017. April;58(4):338–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gogtay N, Thompson PM. Mapping Gray Matter Development: Implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010. February;72(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franke K, Gaser C. Ten Years of BrainAGE as a Neuroimaging Biomarker of Brain Aging: What Insights Have We Gained? Front Neurol [Internet]. 2019. August 14 [cited 2019 Sep 3];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6702897/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole JH, Poudel RPK, Tsagkrasoulis D, Caan MWA, Steves C, Spector TD, et al. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage. 2017. December 1;163:115–24. [DOI] [PubMed] [Google Scholar]
- 19.Truelove-Hill M, Erus G, Bashyam V, Varol E, Sako C, Gur RC, et al. A multidimensional Neural Maturation Index reveals reproducible developmental patterns in children and adolescents. J Neurosci [Internet]. 2020. January 2 [cited 2020 Jan 7]; Available from: https://www.jneurosci.org/content/early/2020/01/02/JNEUROSCI.2092-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gur RE, Moore TM, Rosen AFG, Barzilay R, Roalf DR, Calkins ME, et al. Burden of Environmental Adversity Associated With Psychopathology, Maturation, and Brain Behavior Parameters in Youths. JAMA Psychiatry. 2019. May 29; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016. August 1;26(8):3508–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011. June 26;8(8):665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naimi AI, Balzer LB. Stacked Generalization: An Introduction to Super Learning. Eur J Epidemiol. 2018. May;33(5):459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beheshti I, Nugent S, Potvin O, Duchesne S. Bias-adjustment in neuroimaging-based brain age frameworks: a robust scheme. NeuroImage Clin. 2019. November 4;102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilke M, Sohn J-H, Byars AW, Holland SK. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. NeuroImage. 2003. September;20(1):202–15. [DOI] [PubMed] [Google Scholar]
- 26.Schiele MA, Reinhard J, Reif A, Domschke K, Romanos M, Deckert J, et al. Developmental aspects of fear: Comparing the acquisition and generalization of conditioned fear in children and adults. Dev Psychobiol. 2016. May 1;58(4):471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waters AM, Theresiana C, Neumann DL, Craske MG. Developmental differences in aversive conditioning, extinction, and reinstatement: A study with children, adolescents, and adults. J Exp Child Psychol. 2017. July;159:263–78. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, et al. Maltreatment Exposure, Brain Structure, and Fear Conditioning in Children and Adolescents. Neuropsychopharmacology. 2016. July;41(8):1956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lange I, Goossens L, Bakker J, Michielse S, van Winkel R, Lissek S, et al. Neurobehavioural mechanisms of threat generalization moderate the link between childhood maltreatment and psychopathology in emerging adulthood. J Psychiatry Neurosci JPN. 2019. May;44(3):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLaughlin KA, Sheridan MA, Nelson CA. Neglect as a Violation of Species-Expectant Experience: Neurodevelopmental Consequences. Biol Psychiatry. 2017. October 1;82(7):462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heyn SA, Keding TJ, Ross MC, Cisler JM, Mumford JA, Herringa RJ. Abnormal Prefrontal Development in Pediatric Posttraumatic Stress Disorder: A Longitudinal Structural and Functional Magnetic Resonance Imaging Study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019. February 1;4(2):171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heyn SA, Herringa RJ. Longitudinal cortical markers of persistence and remission of pediatric PTSD. NeuroImage Clin. 2019. January 1;24:102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, et al. Distinct Contributions of the Dorsolateral Prefrontal and Orbitofrontal Cortex during Emotion Regulation. PLOS ONE. 2012. November 7;7(11):e48107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren SL, Crocker LD, Spielberg JM, Engels AS, Banich MT, Sutton BP, et al. Cortical organization of inhibition-related functions and modulation by psychopathology. Front Hum Neurosci [Internet]. 2013. [cited 2019 Oct 16];7. Available from: https://www.frontiersin.org/articles/10.3389/fnhum.2013.00271/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyoo IK, Kim JE, Yoon SJ, Hwang J, Bae S, Kim DJ. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma: longitudinal brain imaging study among survivors of the South korean subway disaster. Arch Gen Psychiatry. 2011. July;68(7):701–13. [DOI] [PubMed] [Google Scholar]
- 36.Balderston NL, Hsiung A, Ernst M, Grillon C. Effect of Threat on Right dlPFC Activity during Behavioral Pattern Separation. J Neurosci Off J Soc Neurosci. 2017. 20;37(38):9160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eshel N, Keller CJ, Wu W, Jiang J, Mills-Finnerty C, Huemer J, et al. Global connectivity and local excitability changes underlie antidepressant effects of repetitive transcranial magnetic stimulation. Neuropsychopharmacology. 2020. May;45(6):1018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du L, Liu H, Du W, Chao F, Zhang L, Wang K, et al. Stimulated left DLPFC-nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl Psychiatry. 2018. March 9;7(11):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keding TJ, Herringa RJ. Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2015. February;40(3):537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010. October 1;34(7):1181–8. [DOI] [PubMed] [Google Scholar]
- 41.Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18(8):729–36. [DOI] [PubMed] [Google Scholar]
- 42.Calem M, Bromis K, McGuire P, Morgan C, Kempton MJ. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. NeuroImage Clin. 2017. January 1;14:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stress Kino T., glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders. Front Physiol [Internet]. 2015. [cited 2020 Jan 23];6. Available from: https://www.frontiersin.org/articles/10.3389/fphys.2015.00230/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall BS, Moda RN, Liston C. Glucocorticoid mechanisms of functional connectivity changes in stress-related neuropsychiatric disorders. Neurobiol Stress. 2014. November 18;1:174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youssef M, Atsak P, Cardenas J, Kosmidis S, Leonardo ED, Dranovsky A. Early life stress delays hippocampal development and diminishes the adult stem cell pool in mice. Sci Rep. 2019. March 11;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002. March;3(3):201–15. [DOI] [PubMed] [Google Scholar]
- 47.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008. May 8;58(3):306–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coste CP, Kleinschmidt A. Cingulo-opercular network activity maintains alertness. NeuroImage. 2016. March 1;128:264–72. [DOI] [PubMed] [Google Scholar]
- 49.Sadaghiani S, D’Esposito M. Functional Characterization of the Cingulo-Opercular Network in the Maintenance of Tonic Alertness. Cereb Cortex. 2015. September 1;25(9):2763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McTeague LM, Goodkind MS, Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res. 2016. December 1;83:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. Am J Psychiatry. 2017. March 21;174(7):676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.