Abstract
Objective:
To describe blood component usage in transfused children with congenital heart disease (CHD) undergoing cardiopulmonary bypass (CPB) surgery across peri-operative settings and diagnostic categories.
Design:
Datasets from US hospitals participating in the National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) were analyzed.
Setting:
Inpatient admissions from three US hospitals from 2013–2016.
Patients:
Transfused children with CHD undergoing single (SV), biventricular (BV) surgery, extracorporeal membrane oxygenation (ECMO).
Interventions:
None
Measurements and Main Results:
882 transfused patients were included. Most of the 185 children with SV surgery received multiple blood products: 81% red blood cells (RBCs), 79% platelets, 86% plasma, and 56% cryoprecipitate. In the 678 patients undergoing BV surgery, 85% were transfused plasma, 75% platelets, 74% RBCs and 48% cryoprecipitate All 19 patients on ECMO were transfused RBCs, plasma, and cryoprecipitate, and 18 were transfused platelets. Intra-operatively, patients commonly received all 3 components, while post-operative transfusions were predominantly single blood components. Pre-transfusion hemoglobin (Hb) values were normal/low-normal for age for all phases of care for SV surgery (median Hb 13.2 – 13.5 g/dL). Pre-transfusion Hb values for BV surgeries were higher intra-operatively as compared to other timing (12.2 g/dL vs. 11.2 pre-op and post-op, p<0.0001). Plasma transfusions for all patients were associated with a near normal INR: SV surgeries median INR was 1.3 post-op vs. 1.8 intraoperative and BV surgeries median INR was 1.1 intraoperative vs 1.7 post-op. Intraoperative platelet transfusions with BV surgeries had higher median platelet count compared to post-operative pre-transfusion platelet count (244 ×109/L intra-op vs 69×109/L post-op).
Conclusion:
Children with CHD undergoing CPB surgery are transfused many blood components both intra- and post-operatively. Multiple blood components are transfused intra-operatively at seemingly normal/low-normal pre-transfusion values. Pediatric evidence guiding blood component transfusion in this population at high risk of bleeding and with limited physiologic reserve is needed to advance safe and effective blood conservation practices.
Keywords: blood transfusion, cardiopulmonary bypass, critical illness, pediatrics, Plasma, Platelet transfusion
Introduction
Children with cardiac defects undergoing surgical repair or surgical palliation requiring cardiopulmonary bypass (CPB) are a highly transfused pediatric cohort.1–10 Factors contributing to blood component transfusion include complex surgeries at high risk of bleeding, hemodilution from CPB prime in small sized infants, and baseline cyanosis in the single ventricle population with poor tolerance for anemia.1,11
Though blood transfusion in adults after cardiac surgery is associated with increased mortality and morbidity, the heterogeneity of types of pediatric cardiac surgeries, and age-related hemostatic differences limits the ability to extrapolate adult evidence to pediatrics.12–14 The limited pediatric studies vary in many aspects including the cardiac population studied (neonate1, 3,7, single1,3,7,15 or two ventricle repair1, 3,7,16,17), blood components reported (red blood cell (RBC)1, 3,6,7,16, 17, 18,19, plasma1,6,7, platelets1,6,7, cryoprecipitate6,7) and phase of surgical care (operative1,6,7,16, 17 or post-operative3,15, 17 transfusion). Outcomes associated with blood component transfusion have also varied, with reporting of no increase in complications19, to less pulmonary complications, shorter mechanical ventilation duration, less acute kidney injury, and less infections or shorter hospital stay for those transfused at more restrictive RBC thresholds1,7,10,19. Other studies have shown worse outcome with restrictive transfusion practices, including a report of worsened psychomotor development one year after discharge.16 Cryoprecipitate and platelet transfusions have been associated with decreased 30-day mortality, but increased pulmonary complications.7
Much is unknown regarding the utilization of blood products across hospital settings in children undergoing cardiac surgery requiring CPB. A greater understanding of the epidemiology of blood product utilization in this complex patient population would facilitate targeted algorithm development and patient blood management efforts.
The National Heart, Lung, and Blood Institute (NHLBI) sponsored REDS-III (Recipient Epidemiology and Donor Evaluation Study) Program includes comprehensive clinical and transfusion data.20 The pediatric data within REDS-III has recently been reported, showing the highest transfusion rates in neonates and children undergoing CPB.8,9
In this study, we sought to describe the utilization of all blood components in a multicenter cohort of transfused children with congenital heart disease (CHD) undergoing surgery with CPB, both as a whole and analyzed across peri-operative settings and diagnostic categories.
Methods
Data Source
The datasets from the REDS-III Domestic Program were queried for this analysis. The REDS-III program, as well as the individual components of the datasets, have been previously described.20 In brief, four blood centers in the United States (Connecticut, Pennsylvania, Wisconsin and California) served as hubs for 12 participating hospitals, three of which included pediatric cardiac surgery patients. Patient data were collected from the electronic health record from January 1, 2013 to December 31, 2016. The study was approved by the Institutional Review Boards at each participating hospital (including Medical College of Wisconsin/Froedtert Hospital IRB #00011516; Yale University IRB #1201009518; Bridgeport Hospital IRB #031305), as well as the data coordinating center. The dataset was de-identified from any personal health information and the treating hospital. Encoded timing was derived to maintain data temporal relationships.
Study Definitions
We included all inpatient admissions for neonates, infants and children age ≤18 years with CHD requiring CPB (as coded by ICD 9 39.6 and ICD 10 PCS code 5A1221Z), who received a transfusion during the admission. Complete data were not able to be obtained for patients without a transfusion and these patients were excluded. A patient encounter was defined as an inpatient admission with a unique start date/time and discharge date, with patients having multiple encounters. Hospital diagnoses were recorded by ICD-9/10 coding and primary and supporting diagnoses were included. Cardiac surgical diagnoses were grouped into three mutually exclusive categories: single ventricular repair/palliation (SV), biventricular repair (BV), and extracorporeal membrane oxygenation (ECMO). All diagnoses and their corresponding codes are listed in Supplemental Table 1. A patient who had both SV and BV diagnoses was categorized as ‘single ventricle’. Patients receiving ECMO during their inpatient hospitalization were categorized as ‘ECMO’ and the patient and transfusions were not included in either SV or BV categories. Children born in the hospital during the admission that included cardiac surgery were included and categorized as ‘birth encounters’.
A transfusion event was defined as the issuance of a blood product from the transfusion service. Products returned to the blood bank without transfusing were not considered a transfusion event. Data captured on the issued product included issue time, issue location (intensive care unit [ICU], or operating room [OR]), and a barcode (Codabar or ISBT 128) from which product type was extracted. RBC, plasma, platelet, cryoprecipitate, and whole blood transfusions were included. The volume of transfusion was not captured in the database. Blood products used to prime the CPB circuit were included and could not be distinguished from transfusions given to the patient in the OR. The transfusion laboratory values reported were hemoglobin levels (Hb) (g/dL), platelet counts (×109 cells/L) and international normalized ratios (INR) obtained closest to and within 24 hours prior to the transfusion.
Statistical Analysis
Demographic and clinical characteristics were described as counts and percentages or median and interquartile range (IQR), as appropriate. The percent of encounters during which a blood product was transfused is presented by patient demographics, selected diagnoses and blood component transfused. To account for potential correlation in repeated encounters among patients, we used generalized estimating equations to calculate p-values. Two-sided p-values below 0.05 were considered significant. All models were adjusted for site. Box plots were constructed showing the distribution of pre-transfusion laboratory values by SV or BV diagnosis and transfusion location. Analyses were conducted with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and the Venn diagrams were produced with R version 4.1.0 (R-project, Vienna, Austria).
Results
Patient Characteristics (Table 1,2,Supplemental Table 2)
Table 1.
Specific Blood Product Transfusion in Single Ventricle Cardiac Surgeries
| Encounters | Any RBC N (%) |
p-value | Any Plasma N (%) |
p-value | Any Platelet N (%) |
p-value | Any Cryo N (%) |
p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 185 | 149 (80.5) | 159 (85.9) | 146 (78.9) | 103 (55.7) | ||||
| Sex | 0.22 | 0.088 | 0.10 | 0.82 | |||||
| Female | 86 | 65 (75.6) | 70 (81.4) | 63 (73.3) | 46 (53.5) | ||||
| Male | 99 | 84 (84.8) | 89 (89.9) | 83 (83.8) | 57 (57.6) | ||||
| Age (years) | 0.0006 | 0.24 | 0.07 | 0.02 | |||||
| Birth encountera | 30 | 29 (96.7) | 27 (90.0) | 27 (90.0) | 24 (80.0) | ||||
| <1 | 61 | 58 (95.1) | 48 (78.7) | 46 (75.4) | 37 (60.7) | ||||
| 1 to <6 | 74 | 49 (66.2) | 68 (91.9) | 55 (74.3) | 31 (41.9) | ||||
| 6 to <13 | 16 | 10 (62.5) | 13 (81.3) | 15 (93.8) | 9 (56.3) | ||||
| 13 to <18 | 4 | 3 (75.0) | 3 (75.0) | 3 (75.0) | 2 (50.0) | ||||
| Race | 0.31b | 0.69 | 0.98 | 0.89 | |||||
| White | 65 | 53 (81.5) | 55 (84.6) | 51 (78.5) | 38 (58.5) | ||||
| Black | 30 | 23 (76.7) | 24 (80.0) | 23 (76.7) | 14 (46.7) | ||||
| Asian | 10 | 10 (100.0) | 9 (90.0) | 8 (80.0) | 7 (70.0) | ||||
| Not specified/Unknown | 80 | 63 (78.8) | 71 (88.8) | 64 (80.0) | 44 (55.0) | ||||
| Ethnicity | 0.25 | 0.43 | 0.63 | 0.58 | |||||
| Non-Hispanic | 108 | 91 (84.3) | 91 (84.3) | 84 (77.8) | 62 (57.4) | ||||
| Hispanic | 77 | 58 (75.3) | 68 (88.3) | 62 (80.5) | 41 (53.2) |
NOTE: 9 patients on ECMO excluded from table
RBC-red blood cell transfusion; Platelet-platelet transfusion; Plasma-plasma transfusion; Cryo-cryoprecipitate transfusion;
Birth encounter- admitted to hospital on day of birth, exclusive of patient age category <1 year.
Not adjusted for potential correlation in repeated encounters due to small cell sizes
Table 2.
Specific Blood Product Transfusion in Biventricular Cardiac Surgeries
| Encounters | Any RBC N (%) |
p-value | Any Plasma N (%) |
p-value | Any Platelet N (%) |
p-value | Any Cryo N (%) |
p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 678 | 503 (74.2) | 577 (85.1) | 509 (75.1) | 324 (47.8) | ||||
| Sex | 0.39 | 0.67b | 0.44 | 0.33b | |||||
| Female | 298 | 227 (76.2) | 254 (85.2) | 222 (74.5) | 148 (49.7) | ||||
| Male | 380 | 276 (72.6) | 323 (85.0) | 287 (75.5) | 176 (46.3) | ||||
| Age (years) | <0.0001b | 0.067b | 0.007 | <0.0001b | |||||
| Birth encountera | 55 | 55 (100.0) | 52 (94.6) | 43 (78.2) | 35 (63.6) | ||||
| <1 | 231 | 197 (85.3) | 199 (86.2) | 183 (79.2) | 139 (60.2) | ||||
| 1 to <6 | 246 | 167 (67.9) | 209 (85.0) | 162 (65.9) | 107 (43.5) | ||||
| 6 to <13 | 90 | 56 (62.2) | 71 (78.9) | 71 (78.9) | 24 (26.7) | ||||
| 13 to <18 | 56 | 28 (50.0) | 46 (82.1) | 50 (89.3) | 19 (33.9) | ||||
| Race | 0.54 | 0.82b | 0.8892 | 0.39b | |||||
| White | 281 | 198 (70.5) | 229 (81.5) | 205 (73.0) | 119 (42.3) | ||||
| Black | 66 | 47 (71.2) | 58 (87.9) | 47 (71.2) | 29 (43.9) | ||||
| Asian | 64 | 52 (81.3) | 58 (90.6) | 48 (75.0) | 33 (51.6) | ||||
| Not specified/Unknown | 267 | 206 (77.2) | 232 (86.9) | 209 (78.3) | 143 (53.6) | ||||
| Ethnicity | 0.60 | 0.70b | 0.6919 | 0.69b | |||||
| Non-Hispanic | 468 | 348 (74.4) | 396 (84.6) | 348 (74.4) | 220 (47.0) | ||||
| Hispanic | 208 | 154 (74.0) | 180 (86.5) | 160 (76.9) | 103 (49.5) |
NOTE: 10 patients on ECMO excluded from this table and 2 patients with unknown ethnicity excluded from this table
RBC-red blood cell transfusion; Platelet-platelet transfusion; Plasma-plasma transfusion; Cryo-cryoprecipitate transfusion;
Birth encounter- admitted to hospital on day of birth, exclusive of patient age category <1 year.
Not adjusted for potential correlation in repeated encounters due to small cell sizes
There were 882 patient encounters from three sites that met inclusion criteria. Fifty-five percent were male with a median (IQR) age of 1 (0–4) years. Eleven percent of patient encounters were birth admissions. The demographics and the number and type of products transfused are described for SV surgeries in Table 1, BV in Table 2, and those supported by ECMO in Supplemental Table 2. Infants (birth encounter and the 0–1-year groups) had increased frequency of RBC transfusion (88%) as compared to 50% in 13–18 year group (p<.0001).
No patients undergoing single ventricular repair or ECMO received whole blood. Fourteen patients undergoing biventricular repair received whole blood (13 intra-operatively and 1 with unknown setting), and of those patients, only one received an RBC transfusion as well.
Transfusion Characteristics (Figure 1 and Supplemental Figure 1)
Figure 1.
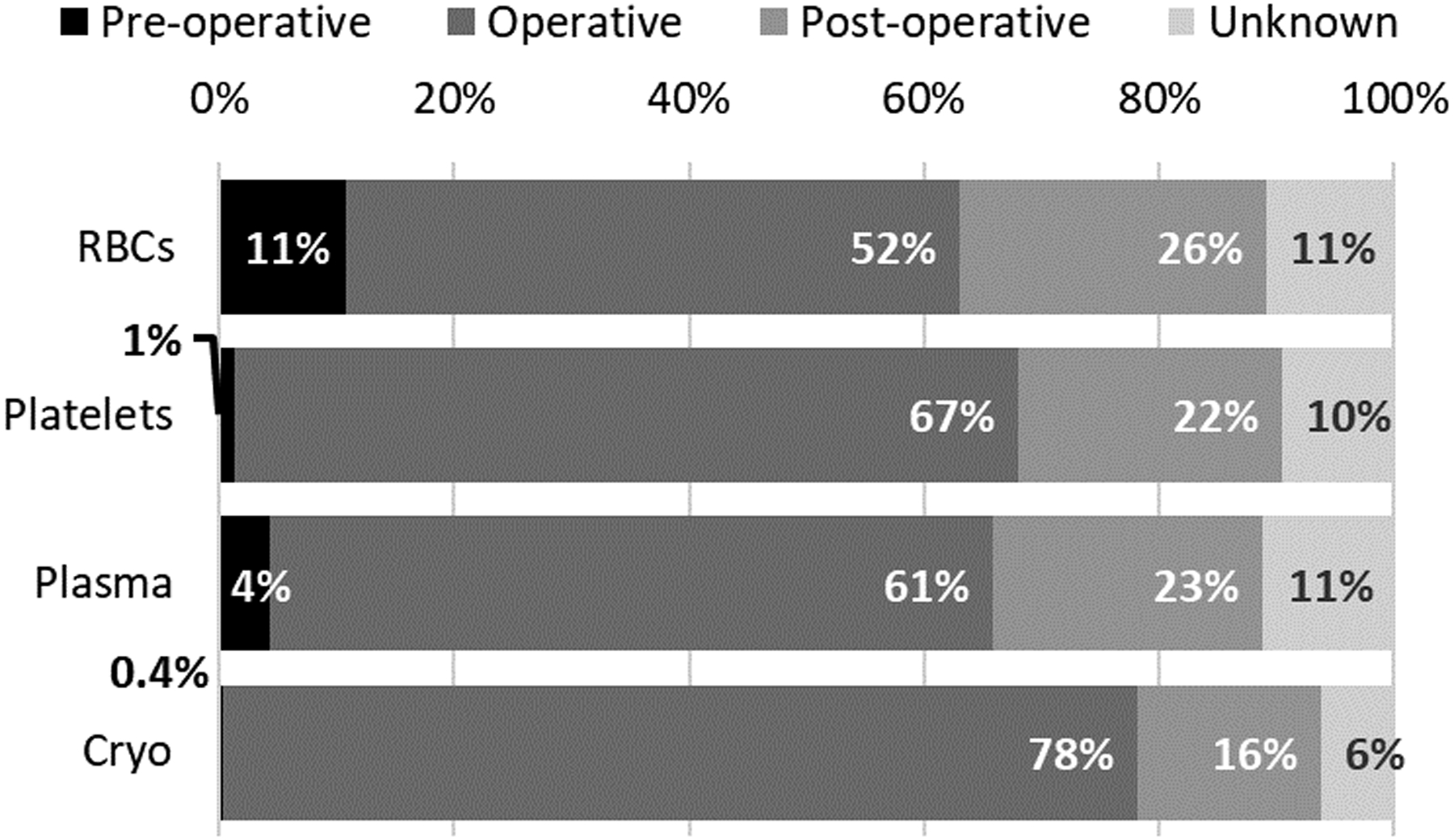
Specific Blood Product Transfusion by Setting (pre-operative, operative, or post-operative). Percent distribution of blood product transfusion (Red Blood Cell (RBC), Platelets, Plasma or Cryoprecipitate (Cryo)) in each operative setting.
Figure 1 describes the settings in which the transfusions were administered. Few platelet (1%, n=6) and plasma (4%, n=27) transfusions were administered in the pre-operative period. The majority of transfusions were given intra-operatively: 52% of RBC, 61% of plasma, 67% of platelet, and 78% of cryoprecipitate. Post-operative transfusions were most commonly RBC, followed by plasma, platelet, and cryoprecipitate.
The Venn diagrams in Supplemental Figure 1 illustrate the combination of blood components transfused across the operative settings by SV and BV status. Intra-operatively, patients most commonly received all 3 components: 51% for SV and 49% for BV. Twenty-four (65%) of pre-operative transfusions to children with SV and 65 (71%) of pre-operative transfusions to children with BV were transfused solely RBCs. Post-operative transfusions were mostly single blood components (59% for SV and 70% for BV).
Pre-Transfusion Laboratory Values (Table 3, Supplemental Figures 2, 3 and 4)
Table 3.
Pre-transfusion laboratory values, by cardiac surgery category and setting
| HEMOGLOBIN | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery Type | Timing | n | mean | min | P_1 | P_10 | P_20 | P_25 | P_50 | P_75 | P_90 | P_99 | max |
| Bi-Ventricular Diagnosis | pre-operative | 107 | 11.38 | 6.5 | 7.1 | 8.7 | 9.6 | 9.8 | 11.2 | 13.0 | 14.4 | 15.5 | 18.1 |
| operative | 281 | 12.41 | 7.8 | 8.2 | 10.1 | 10.8 | 11.2 | 12.2 | 13.6 | 15.0 | 17.5 | 19.1 | |
| post-operative | 389 | 11.18 | 5.7 | 6.8 | 8.8 | 9.5 | 9.9 | 11.2 | 12.4 | 13.6 | 16.0 | 17.2 | |
| unknown | 153 | 11.36 | 6.1 | 6.3 | 8.4 | 9.4 | 9.7 | 11.5 | 12.8 | 14.4 | 17.3 | 19.6 | |
| Single Ventricle Diagnosis | pre-operative | 69 | 13.16 | 9.5 | 9.5 | 11.0 | 11.6 | 12.0 | 13.2 | 13.9 | 15.8 | 18.1 | 18.1 |
| operative | 266 | 13.62 | 9.1 | 9.6 | 11.4 | 12.1 | 12.4 | 13.5 | 14.8 | 16.2 | 18.0 | 18.4 | |
| post-operative | 284 | 13.30 | 8.8 | 9.1 | 11.4 | 12.2 | 12.5 | 13.3 | 14.2 | 15.2 | 17.9 | 18.5 | |
| unknown | 39 | 13.45 | 10.2 | 10.2 | 10.8 | 12.2 | 12.5 | 13.6 | 14.7 | 15.3 | 16.6 | 16.6 | |
| INR | |||||||||||||
| Surgery Type | Timing | n | mean | min | P_1 | P_10 | P_20 | P_25 | P_50 | P_75 | P_90 | P_99 | max |
| Biventricular Diagnosis | pre-operative | 25 | 1.82 | 1.0 | 1.0 | 1.0 | 1.2 | 1.4 | 1.7 | 2.3 | 2.7 | 3.4 | 3.4 |
| operative | 233 | 1.21 | 0.7 | 0.8 | 1.0 | 1.0 | 1.0 | 1.1 | 1.3 | 1.6 | 2.6 | 5.3 | |
| post-operative | 199 | 1.82 | 0.8 | 0.8 | 1.3 | 1.4 | 1.5 | 1.6 | 1.9 | 2.4 | 6.1 | 7.2 | |
| unknown | 53 | 1.65 | 0.9 | 0.9 | 1.0 | 1.2 | 1.2 | 1.4 | 1.6 | 1.9 | 11.0 | 11.0 | |
| Single Ventricle Diagnosis | pre-operative | 14 | 1.65 | 1.1 | 1.1 | 1.2 | 1.3 | 1.3 | 1.6 | 1.8 | 2.3 | 2.6 | 2.6 |
| operative | 140 | 1.71 | 0.9 | 0.9 | 1.0 | 1.1 | 1.2 | 1.5 | 1.8 | 2.5 | 6.3 | 7.5 | |
| post-operative | 110 | 1.90 | 0.8 | 0.9 | 1.4 | 1.5 | 1.5 | 1.8 | 2.1 | 2.6 | 4.2 | 4.3 | |
| unknown | 14 | 1.73 | 1.1 | 1.1 | 1.2 | 1.3 | 1.3 | 1.6 | 1.8 | 2.7 | 3.2 | 3.2 | |
| TOTAL PLATELET COUNT | |||||||||||||
| Surgery Type | Timing | n | mean | min | P_1 | P_10 | P_20 | P_25 | P_50 | P_75 | P_90 | P_99 | max |
| Biventricular Diagnosis | pre-operative | 4 | 90.50 | 9.0 | 9.0 | 9.0 | 9.0 | 21.5 | 50.0 | 159.5 | 253.0 | 253.0 | 253.0 |
| operative | 213 | 258.83 | 19.0 | 24.0 | 103.0 | 169.0 | 189.0 | 244.0 | 324.0 | 411.0 | 565.0 | 671.0 | |
| post-operative | 155 | 84.98 | 10.0 | 16.0 | 32.0 | 41.5 | 48.0 | 70.0 | 92.0 | 144.0 | 398.0 | 414.0 | |
| unknown | 48 | 158.15 | 18.0 | 18.0 | 25.0 | 47.0 | 54.5 | 108.0 | 168.5 | 394.0 | 670.0 | 670.0 | |
| Single Ventricle Diagnosis | pre-operative | 9 | 72.78 | 4.0 | 4.0 | 4.0 | 17.0 | 23.0 | 38.0 | 79.0 | 309.0 | 309.0 | 309.0 |
| operative | 133 | 129.20 | 5.0 | 6.0 | 14.0 | 28.0 | 33.0 | 72.0 | 226.0 | 318.0 | 497.0 | 515.0 | |
| post-operative | 83 | 67.82 | 5.0 | 5.0 | 17.0 | 28.0 | 32.0 | 54.0 | 79.0 | 125.0 | 359.0 | 359.0 | |
| unknown | 9 | 148.56 | 47.0 | 47.0 | 47.0 | 52.0 | 75.0 | 138.0 | 229.0 | 287.0 | 287.0 | 287.0 | |
Pre-transfusion laboratory values of Hemoglobin, INR, and total Platelet Count are compared by Cardiac surgical categories of Bi-ventricular or Single Ventricle Diagnosis across operative settings (pre-operative, operative, or post-operative). Mean, minimum and Maximum values, and percentage distribution are shown. (P_1 =1st percentile, P_50=50th percentile (median), P_99=99th percentile)
Median (IQR) laboratory values obtained closest to and within 24 hours of transfusion are presented for specific blood component transfusions by operative setting and surgical category in Table 3 and Supplemental Figure 2. Supplemental Figures 3 and 4 show the pre-transfusion thresholds by age, surgical category, and operative setting. BV patients transfused RBC were transfused with lower Hb values pre-operatively (p=0.0006) and post-operatively (p<0.0001) compared to intraoperatively (median Hb (g/dL) 11.2 pre-op vs. 12.2 intra-op vs. 11.2 post-op) (Table 3). These differences were more pronounced when analyzed by age group, with older children having greater differences between pre-, intra-, and post-operative hemoglobin values prior to transfusion (Supplemental Figures 3 and 4). For all age groups the difference in pre-transfusion hemoglobin values were significantly higher when comparing operative to post-operative values, p<0.05. Patients with SV were transfused RBC at higher Hb values in all 3 phases of care (median Hb (g/dL) 13.2 pre-op vs 13.5 intra-op vs. 13.3 post-op) compared to patients with BV (p<.0001). Pre-transfusion Hb levels were lower post-operatively in patients ≥6 years of age with BV, with a median pre-transfusion Hg level of 9.1 g/dL, compared to patients <6 years (11.4 g/dL), p<.0001.
Recognizing that the upper limit of normal for INR varies by age, plasma transfusions in all settings and ages were associated with only mild coagulopathy, reflected by a median INR <1.8. Intraoperative plasma transfusions for patients with biventricular surgeries were associated with a lower INR (median 1.7 pre-op (p=0.0006 vs intra-op) vs INR 1.1 intra-op vs 1.6 post-op (p<0.0001 vs intra-op). Patients with SV were transfused plasma with an associated median INR of 1.6 pre-op vs 1.5 intra-op vs. 1.8 post-op (p=NS).
Pre-transfusion platelet levels varied widely, even within an operative setting or surgical setting as shown in Table 3. The median pre-transfusion platelet count was highest for intraoperative BV platelet transfusions (median (× 109/L) 50 pre-op (p=0.004 vs intra-op) vs 244 intra-op vs 70 post-op (p<0.0001 vs. intra-op). Age-based differences were observed with SV and BV (Supplemental Figures 3 and 4), with infants having little variability in pre-transfusion laboratory values based on setting and older children having significant variability. Intraoperative platelet transfusions to SV patients were associated with the highest platelet counts (median (× 109 cells/L) 38 pre-op (p=0.04 vs intra-op) vs 72 intra-op vs 54 post-op (p=0.02 vs. intra-op).
Discussion
In this multicenter cohort of transfused children with CHD undergoing surgery with CPB, the transfusion of blood components is common across the operative settings. Infancy was associated with more RBC and cryoprecipitate transfusions for both SV and BV repairs compared to older ages. There was no association by age or single ventricle status for plasma or platelet transfusions. In this transfused cohort, birth encounters of any diagnosis and infants with SV were almost universally transfused RBC. Intra-operative and ECMO status were associated with transfusion of all blood components while post-operative transfusion was more targeted, with most patients receiving a single blood component. Blood conservation strategies targeting intra-operative transfusion including CBP equipment, priming composition and techniques, cell-salvage and hemostasis may be appropriate given the frequent and diffuse transfusion practices shown in this study.
RBC transfusion occurred most frequently and at the highest pre-transfusion Hb values in infants and SV repair, likely reflecting the surgical complexity, hemodilution from CPB prime in small sized infants, increased bleeding risk and/or baseline cyanosis with poor tolerance for anemia in this population. As these infants, including those undergoing stage 1 palliation, were excluded from many of the pediatric transfusion studies, there is very little evidence to describe transfusion practices in this vulnerable population. One clinical trial of 162 infants showed no differences in measures of oxygen delivery and clinical outcomes between restrictive (Hb of 7.0 g/dL in SV and 9.5 g/dL in BV) and liberal thresholds (Hb of 9.5 g/dL in SV and 12.0 g/dL in BV).21 Based on an extensive systematic review, the Transfusion Anemia eXpert Initiative (TAXI), published after the REDSIII study, recently recommended not to transfuse if Hb > 7.0 g/dL in stable infants undergoing BV. For infants undergoing SV palliation, they recommend avoiding transfusing based on Hb alone if > 9.0 g/dL.22 They recommend further studies investigating anemia, RBC transfusion, oxygen delivery and utilization and outcomes. The ideal Hb associated with optimal outcomes is unknown, and it is not known if this Hb threshold would be constant across operative settings.
Outside of birth and infant transfusion encounters where RBC transfusion was the most frequent, plasma was the most frequent blood component transfused. A recent single center study demonstrated that nearly one-quarter of children received a hemostatic transfusion following CPB.4 We found the median pre-transfusion INR values ranged 1.1 to 1.8 depending on the operative setting and diagnosis. In a previous pediatric study, plasma transfusion for bleeding from coagulopathy resulted in improved INR only in those children with a pre-transfusion INR >2.5.23 There is no evidence to support empiric prophylactic plasma transfusion for patients perceived to be at risk of bleeding.24 Further research to investigate the barriers to limiting plasma transfusion to massive or critical bleeding are needed in this specific pediatric cardiac population including practices to empirically replace chest tube output with plasma.
Pre-transfusion platelet values were highest for transfusions given in the intraoperative setting. Lower pre-transfusion values were found pre- and post-operatively in the 38–70 ×109/L range for both SV and BV. There are no pediatric guidelines, nor evidence, for post-cardiac surgical platelet transfusion thresholds to minimize post-operative bleeding. The AABB suggests platelet transfusion for adult patients having CPB who exhibit perioperative bleeding with thrombocytopenia and/or with evidence of platelet dysfunction.25 The AABB recommendations for children are limited to consideration of platelet transfusion to any bleeding child with platelet dysfunction.26 Qualitative platelet defects are common in patients on ECMO, and studies have shown possible benefit of including viscoelastic testing and evaluation of platelet function to help guide the decision to transfuse platelets in this patient population.27, 28 As the pre-transfusion laboratory values were selected based on the platelet count closest to the transfusion within 24 hours, it cannot be determined if that threshold platelet count reflects the platelet count at the time of the intra-operative bleeding. Additional significant clinical information, such as active bleeding, will be important to explain the difference in pre-transfusion platelet counts between pre-, intra-, and post-operative settings.
Limitations of this study are common to any secondary analysis. The indication for transfusions, site transfusion guidelines, patient physiologic state (active bleeding or hemodynamic compromise) and other patient factors such as co-morbidities and complexity of the cardiac surgery including the STAT (The Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery) score are unknown. Patient data on non-transfused patients was not available, so comparison with transfused patients was unable to be obtained. The use of blood and blood products for CPB pump priming could not be distinguished from direct patient transfusion in the intraoperative setting. Intraoperative collection and reinfusion of cell-saver blood or other patient blood management methods was not available. We did not assess the use of fibrinogen concentrate in this study, which may be relevant to this surgical population.29 Pre-transfusion laboratory values may reflect a different setting than the transfusion; for example, the most recent laboratory value of platelets prior to an intraoperative transfusion may have been pre-operative. These values cannot be interpreted as a transfusion threshold as the clinical factors at the time of transfusion, such as active bleeding or hemodynamic stability, couldn’t be assessed. Transfusion volume, and therefore the dose, was unknown and response to transfusions couldn’t be reported. However, this study captured blood component utilization across multiple ages, diagnoses, and geographical and operative settings, reflecting contemporary transfusion practice for this population.
Prior pediatric studies describing blood component usage in this population are scarce and often limited to a single blood component or peri-operative setting. This study describes the transfusion practices of all blood components in this critically ill cardiac surgical population across the peri-operative settings. Given our findings, the development of transfusion algorithms in all settings, but in particular, intraoperative strategies for patient blood management are essential. Targeting a single blood component for study may limit the effectiveness of blood conservation efforts, as concomitant transfusions of both RBC and hemostatic blood components are common.
Conclusion
Our study confirms that children with CHD undergoing surgery requiring CBP are a group that is transfused many blood components both intra- and post-operatively. Infants are most likely to be transfused RBC and cryoprecipitate, while plasma is the most frequent blood component transfused outside of infancy. Multiple blood components are transfused intraoperatively at the most liberal pre-transfusion laboratory values. Post-operative transfusions are targeted, with transfusion of single or multiple blood components at pre-transfusion levels higher than general pediatric transfusion guidelines. Pediatric evidence guiding blood component transfusion in this population at high risk of bleeding and with limited physiologic reserve is needed to advance safe and effective blood conservation practices.
Supplementary Material
Research in Context:
Children undergoing cardiac surgery with cardiopulmonary bypass are at high risk of requiring transfusions, both in the operating room and following their procedure.
Though TAXI guidelines have suggested hemoglobin thresholds for RBC transfusion post-operatively, few recommendations exist to guide the transfusion of all blood components during and after cardiac surgery.
This secondary analysis of the NHLBI Recipient Epidemiology and Donor Database provides a report of the current utilization of blood components across hospital settings.
At the Bedside:
Infants more commonly receive RBC and cryoprecipitate transfusions whereas platelet and plasma transfusions are not associated with age in this cohort.
Development of transfusion algorithms for children undergoing cardiac surgeries in all settings, in particular intraoperative strategies for patient blood management, are essential.
Patient blood management strategies should address all blood components, as concomitant transfusions of both RBC and hemostatic blood components are common.
Acknowledgements
The NHLBI Recipient Epidemiology Donor Evaluation Study - IV - Pediatric (REDS-IV-P) domestic program is the responsibility of the following persons:
Hubs
A.E. Mast and J.L. Gottschall, Versiti Wisconsin, Milwaukee, WI
E.A. Hod, Columbia University Medical Center, New York, NY and B.S. Sachais, New York Blood Center, New York, NY
B.S. Custer, Vitalant Research Institute, San Francisco, CA and E.P. Vichinsky, Benioff Children’s Hospital Oakland, Oakland, CA
J.E. Hendrickson, Yale University School of Medicine, New Haven, CT and B.R. Spencer, American Red Cross, Dedham, MA
Data coordinating center
S.M. Mathew and D.R. Harris, Westat, Rockville, MD
N.L. Luban, Children’s National Medical Center, Washington, D.C.
Central laboratory
M.P. Busch and P.J. Norris, Vitalant Research Institute, San Francisco, CA
Publications Committee Chairman
P.M. Ness, Johns Hopkins University, Baltimore, MD
Steering Committee Chairpersons
S.H. Kleinman, University of British Columbia, Victoria, BC, Canada
C.D. Josephson, Emory University, Atlanta, GA
National Institute of Child Health and Human Development (NICHD)
R. Tamburro
National Heart, Lung, and Blood Institute, National Institutes of Health
S.A. Glynn and K. Malkin
Funding/Support:
The authors were supported by research contracts from the National Heart, Lung, and Blood Institute (NHLBI Contracts HHSN 75N92019D00032, HHSN 75N92019D00034, 75N92019D00035, HHSN 75N92019D00036, and HHSN 75N92019D00037). Additional funding was provided by the National Institute of Child Health and Human Development (NICHD).
Copyright Form Disclosure:
Drs. Hanson, Birch, and Gottschall’s institutions received funding from the National Heart, Lung, and Blood Institute (NHLBI). Dr. Hanson received support for article research from the NHLBI and the National Institute of Child Health and Human Development. Dr. Birch’s institution received funding from the Centers for Disease Control and Prevention and the Environmental Protection Agency; she disclosed work for hire for Westat. Drs. Birch, Goel, Patel, Sola-Visner, Sachais, Hauser, Gottschall, Josephson, Hendrickson, Karafin, and Nellis received support for article research from the National Institutes of Health (NIH). Dr. Patel received funding from the NHLBI REDS-IV-P Program and Mednax. Dr. SolaVisner’s institution received funding form Sysmex America, Inc. Dr. Gottschall disclosed government work. Dr. Hendrickson’s institution received funding from the NIH. Dr. Karafin received funding from the NIH from a R23 and R01. Drs. Karam and Luban have disclosed that they do not have any potential conflicts of interest.
Footnotes
Tweet: Children with #CHD surgery in #PedsICU receive many #transfusion products at liberal thresholds. Research to guide #PBM algorithms needed.
References
- 1.Székely A, Cserép Z, Sápi E et al. : Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg 2009; 87(1):187–97 [DOI] [PubMed] [Google Scholar]
- 2.Keung CY, Smith KR, Savoia HF, and Davidson AJ: An audit of transfusion of red blood cell units in pediatric anesthesia. Paediatr Anaesth 2009;19(4):320–8 [DOI] [PubMed] [Google Scholar]
- 3.Hanson SJ, Owen EB, McDonald MJ et al. Standardized Implementation of Evidence-based Guidelines to Decrease Blood Transfusions in Pediatric Intensive Care Units. Pediatr Qual Saf 2019;4(3):e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Closson R, Mauer E, Stock A, et al. : The Use of Hemostatic Blood Products in Children Following Cardiopulmonary Bypass and Associated Outcomes. Critical care explorations, 2019; 2(8), e0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nellis ME, Karam O, Mauer E, et al. : Platelet Transfusion Practices in Critically Ill Children. Crit Care Med. 2018;46(8):1309–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato H, Chasovskyi K, Gandhi SK. Are Blood Products Routinely Required in Pediatric Heart Surgery? Pediatric Cardiology. 2020. June;41(5):932–938 [DOI] [PubMed] [Google Scholar]
- 7.Iyengar A, Scipione CN, Sheth P, et al. : Association of Complications with Blood Transfusions in Pediatric Cardiac Surgery Patients. Ann Thorac Surg 2013;96:910–6 [DOI] [PubMed] [Google Scholar]
- 8.Nellis M, Goel R, Hanson S, et al. : Pediatric Transfusion Practice in the United States. Abstract Presentations from the AABB Virtual Annual Meeting, October 3–5, 2020. Available at: https://aabb.confex.com/aabb/2020/meetingapp.cgi/Paper/7401 Accessed January 18, 2021 [Google Scholar]
- 9.Patel R, Sola-Visner M2, Nellis ME et al. : Variation in Neonatal Transfusion Practice in the United States. Abstract Presentations from the AABB Virtual Annual Meeting, October 3–5, 2020. Available at: https://aabb.confex.com/aabb/2020/meetingapp.cgi/Paper/7117 Accessed January 18, 2021 [Google Scholar]
- 10.Mille FK, Badheka A, Yu P et al. : Red Blood Cell Transfusion After Stage I Palliation Is Associated with Worse Clinical Outcomes, J Am Heart Assoc. 2020;9:e015304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faraoni D, Willems A, Romlin BS et al. : Development of a specific algorithm to guide haemostatic therapy in children undergoing cardiac surgery-A single-centre retrospective study. European Journal of Anaesthesiology (EJA): 2015; 32: 320–329 [DOI] [PubMed] [Google Scholar]
- 12.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007. November 27;116(22):2544–52 [DOI] [PubMed] [Google Scholar]
- 13.Hajjar LA, Vincent J, Galas FRBG, et al. Transfusion Requirements After Cardiac Surgery: The TRACS Randomized Controlled Trial. JAMA. 2010;304(14):1559–1567 [DOI] [PubMed] [Google Scholar]
- 14.Siemens K; Sangaran DP, Hunt BJ et al. : .;Strategies for Prevention and Management of Bleeding Following Pediatric Cardiac Surgery on Cardiopulmonary Bypass: A Scoping Review Ped Crit Care Med 2018; 19(1): 40–47 [DOI] [PubMed] [Google Scholar]
- 15.Cholette J, Rubenstein J, Alfieris G, et al. Children with single-ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red-cell transfusion strategy. Pediatr Crit Care Med. 2011; 12:39–45 [DOI] [PubMed] [Google Scholar]
- 16.Jonas RA, Wypij D, Roth SJ, et al. :The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants, J Thorac Card Surg 2003; 126 (6):1765–1774 [DOI] [PubMed] [Google Scholar]
- 17.de Gast-Bakker DH, de Wilde RB, Hazekamp MG, et al. : Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients: a randomized controlled trial. Intensive Care Med. 2013. November;39(11):2011–9 [DOI] [PubMed] [Google Scholar]
- 18.Salvin JW, Scheurer MA, Laussen PC et al. : Blood Transfusion After Pediatric Cardiac Surgery Is Associated With Prolonged Hospital Stay, Ann Thorac Surg. 2011; 91(1): 204–210 [DOI] [PubMed] [Google Scholar]
- 19.Willems A, Harrington K, Lacroix J. et al. TRIPICU investigators; Canadian Critical Care Trials Group; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Crit Care Med. 2010; 38: 649–656 [DOI] [PubMed] [Google Scholar]
- 20.Kleinman S, Busch MP, Murphy EL, et al. The National Heart, Lung and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion. 2014;54(3 Pt 2):942–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cholette JM, Swartz MF, Rubenstein J, et al. Outcomes Using a Conservative Versus Liberal Red Blood Cell Transfusion Strategy in Infants Requiring Cardiac Operation. Ann Thorac Surg. 2017. January;103(1):206–214 [DOI] [PubMed] [Google Scholar]
- 22.Cholette JM, Willems A, Valentine SL, et al. ; Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI); Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Recommendations on RBC Transfusion in Infants and Children With Acquired and Congenital Heart Disease From the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018. September;19(9S Suppl 1):S137–S148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karam O, Demaret P, Shefler A, et al. Indications and effects of Plasma transfusions in critically ill children. Am J Respir Crit Care Med. (2015) 191:1395–402. doi: 10.1164/rccm.201503-0450OC [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Stanworth S, Hopewell S, et al. Is fresh-frozen Plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion. (2012) 52:1673–86; quiz. doi: 10.1111/j.1537-2995.2011.03515.x [DOI] [PubMed] [Google Scholar]
- 25.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. (2015) 162:205–13. doi: 10.7326/M14-1589 [DOI] [PubMed] [Google Scholar]
- 26.Josephson C. ME. Neonatal and pediatric transfusion practice. In: Fung M, editor. AABB Technical Manual. 18th ed. Bethesda, MD: AABB; (2005). p. 571–97 [Google Scholar]
- 27.Dieu A, Van Regemorter V, Detaille T, et al. Combined Use of Rotational Thromboelastometry (Rotem) and Platelet Impedance Aggregometry (Multiplate Analyzer) in Cyanotic and Acyanotic Infants and Children Undergoing Cardiac Surgery With Cardiopulmonary Bypass: Subgroup Analysis of a Randomized Clinical Trial. J Cardiothorac Vasc Anesth. 2020. October 1:S1053-0770(20)31051-X. [DOI] [PubMed] [Google Scholar]
- 28.Auci E, Vetrugno L, Riccardi I, et al. Multiple Electrode Aggregometry After Cardiopulmonary Bypass to Assess Platelet (Dys)-Function and Transfusion Threshold: A Concordance Study. J Cardiothorac Vasc Anesth. 2020. June 24:S1053-0770(20)30602-9. [DOI] [PubMed] [Google Scholar]
- 29.Siemens K, Hunt BJ, Harris J, et al. Individualized, Intraoperative Dosing of Fibrinogen Concentrate for the Prevention of Bleeding in Neonatal and Infant Cardiac Surgery Using Cardiopulmonary Bypass (FIBCON) A Phase 1b/2a Randomized Controlled Trial. Circulation: Cardiovascular Interventions. 2020. Ahead of Print 10.1161/CIRCINTERVENTIONS.120.009465 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


