Abstract
Aortic arterial stiffness is a strong independent predictor of cardiovascular disease (CVD), however its dependence on mean arterial pressure (MAP) limits its clinical utility. The aortic-femoral arterial stiffness gradient (af-SG), a novel marker of CVD risk, may be a promising alternative, but its dependence on MAP is not known. The aim of this study was to determine the relationship between MAP and the af-SG in healthy older adults and those with established disease, including hypertension and diabetes. We evaluated the dependency of the af-SG on MAP in healthy older adults (n = 694, aged 74 ± 5 years), and adults with hypertension (n = 2040, aged 76 ± 5 years), and diabetes (n = 1405, aged 75 ± 5 years) as part of the community-based Atherosclerosis Risk in Communities (ARIC) Study. Carotid-femoral pulse-wave velocity (cfPWV), femoral-ankle PWV (faPWV) and blood pressure were measured using standardized protocols. The af-SG was calculated as faPWV divided by cfPWV. Multivariable regression analysis was performed to test the independent association of MAP with af-SG, with adjustments for known confounders including age, sex, body mass index, blood glucose and heart rate. The was no significant relationship between the af-SG and MAP in healthy (β = 0.002, p = .301), hypertension (β = −0.001, p = .298) or diabetes (β = −0.001, p = .063) population groups, with MAP explaining <0.1, <0.1 and 0.2% of the variance in the af-SG, respectively. These findings suggest that the af-SG may be regarded as a MAP independent index of arterial health and CVD risk in older adults.
Keywords: Arterial stiffness, pulse wave velocity, mean arterial pressure, pulse wave velocity ratio, cardiovascular disease
INTRODUCTION
Arterial stiffness measures are commonly used to investigate arterial health and assist in the evaluation of cardiovascular disease (CVD) risk [1,2]. Pulse wave velocity (PWV) is the referent standard measure of arterial stiffness, of which, carotid-femoral PWV, a measure of central aortic stiffness, is the most prominent, and a strong independent predictor of CVD [3,4]. However, an inherent limitation of arterial stiffness measures, including cfPWV, is that they are highly dependent on the operational mean arterial pressure (MAP) [5,6]. In turn, MAP is known to be affected by a range of physiological, mechanical, and psychological factors [7–9]. Whilst arterial stiffness measures can be adjusted for MAP, the curvilinear nature and individual distinctiveness of the pressure-diameter relationship are persistent limitations [10]. In particular, comparing arterial stiffness-related outcomes between individuals, tracking changes over time, and determining optimal treatment strategies can be challenging. This likely limits the widespread adoption of arterial stiffness measures in clinical practice [11]. A MAP independent measure of arterial stiffness may therefore be of significant clinical value. One promising biomarker, that has demonstrated MAP independence, is the central to peripheral arterial stiffness gradient [12].
The central to peripheral arterial stiffness gradient is typically characterized as the ratio of upper- or lower-extremity arterial stiffness to central arterial stiffness [13,14]. Expressing arterial health in this manner is suggested to provide a MAP independent index of vascular aging, given that both central and peripheral arterial stiffness are similarly impacted by MAP [10]. The most widely explored measure is the aortic-brachial stiffness gradient (ab-SG), defined as the ratio of carotid-radial PWV (crPWV) to cfPWV [13,15]. The ab-SG has been shown to predict incident CVD and all-cause mortality in dialysis patients [13], as well as healthy older adults [15]. But of relevance, whilst the ab-SG was shown to be MAP independent in populations with prevalent renal disease, hypertension and diabetes [12,16], it was not among healthy adults [16]. The presence of disease may therefore impact the MAP dependence of the ab-SG and, as such, its clinical value. Recently, our research group reported that the aortic-femoral arterial stiffness gradient (af-SG), defined as the ratio of femoral-ankle PWV (faPWV) to cfPWV, was strongly associated with prevalent CVD in older adults [14]. Specifically, a low af-SG, as that which might occur with age or in the presence of disease[17], was associated with coronary heart disease, heart failure and stroke, whilst a high cfPWV was not [14]. Inclusion of the lower-extremities, which make up a significant portion of the arterial tree, may permit the af-SG to provide a more comprehensive picture of hemodynamic integration than the ab-SG. But although the af-SG has demonstrated promising utility, the dependence of the af-SG on MAP, and whether or not this relationship is influenced by disease status, is not known. Should the af-SG demonstrate MAP independence in both healthy and diseased populations, it may be of significant clinical value.
The aim of this study was to determine the relationship between MAP and the af-SG in healthy subjects and those with established disease, specifically hypertension and diabetes patients. This aim was undertaken using a well characterized population of older men and women from the Atherosclerosis Risk in Communities (ARIC) Study cohort.
METHODS
This observational study is reported in accordance with STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines[18]. Participants provided written informed consent, and the study was approved by the Institutional Review Boards at all field centers, coordinating center, and central labs and reading centers.
STUDY POPULATION
The ARIC Study is a population-based, longitudinal study of 15,792 men and women aged 45–64 years enrolled between 1987 and 1989 from 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland). Details of the baseline visit have been previously described[19]. Prior to exclusions, the current analysis includes 6,538 participants who attended visit 5 between 2011 and 2013, 5,683 of whom had PWV measures completed.
We excluded participants with the following conditions due to concerns of PWV data quality: BMI ≥40 kg/m2, major arrhythmias (Minnesota codes 8-1-3, 8-3-1, and 8-3-2), Minnesota code 8-1-2 with evidence of biased PWV waveforms, aortic aneurysms, abdominal aorta ≥5 cm, history of aortic or peripheral revascularization or aortic graft, aortic stenosis, and moderate or greater aortic regurgitation. Additionally, we excluded participants whose race was other than white or African American (due to small sample size), with missing PWV or vascular risk factor data, as well as those with outlying PWV values, defined as PWV values 3 standard deviations above or below the mean.
We categorized the remaining 4,139 participants into the following groups: (i) Apparently healthy: participants who were free of hypertension, diabetes, prevalent CVD and were not using medications for those conditions; (ii) Participants with hypertension: a systolic BP (SBP) ≥140 mm Hg, diastolic BP (DBP) ≥90 mm Hg, or antihypertensive medication use; (iii) Participants with diabetes: fasting glucose ≥126 mg/dl, non-fasting glucose ≥200 mg/dl, antidiabetic medication use, or self-reported diagnosis of diabetes by a physician.
Participants were asked not to consume food or drink, and refrain from tobacco and vigorous physical activity after midnight prior to the clinic visit or for 8 hours prior to the visit. The visit 5 study examination included interviewer-administered questionnaires to obtain demographic data, medical history and lifestyle information, blood and urine collection, and assessment of vascular risk factors and cardiovascular phenotypes, including PWV.
EXPERIMENTAL MEASURES
BLOOD PRESSURE and PULSE WAVE VELOCITY
After participants were supine for 5–10 minutes, technicians measured blood pressure, cfPWV and faPWV following a standardized protocol, using the automated cardiovascular screening device VP-1000 Plus (Omron, Kyoto, Japan)[20]. The device simultaneously measured bilateral brachial blood pressures, and carotid, femoral and posterior tibial arterial pulse waves in the supine position. PWV was estimated as the distance between two arterial recording sites divided by transit time (TT): distance/TT (Figure 1). For cfPWV assessments, arterial waveforms were simultaneously acquired for 30 seconds by applanation tonometry sensors attached on the left common carotid artery (via neck collar) and left common femoral artery. The distance from the carotid to the femoral artery was directly measured with a segmometer (Rosscraft, Surrey, Canada) and calculated as the carotid to femoral distance minus the distance between the suprasternal notch to the carotid applanation site. For faPWV assessments, bilateral posterior-tibial arterial pressure waveforms were detected over 10 seconds by extremities cuffs connected to plethysmographic and oscillometric pressure sensors wrapped on both ankles. Distance for faPWV was automatically calculated by the VP-1000 Plus using height-based formulas, as previously described[21]. A minimum of two PWV measurements were taken per participant and the last two measurements were averaged. The average of left and right faPWV measures was included for analysis.
FIGURE 1.
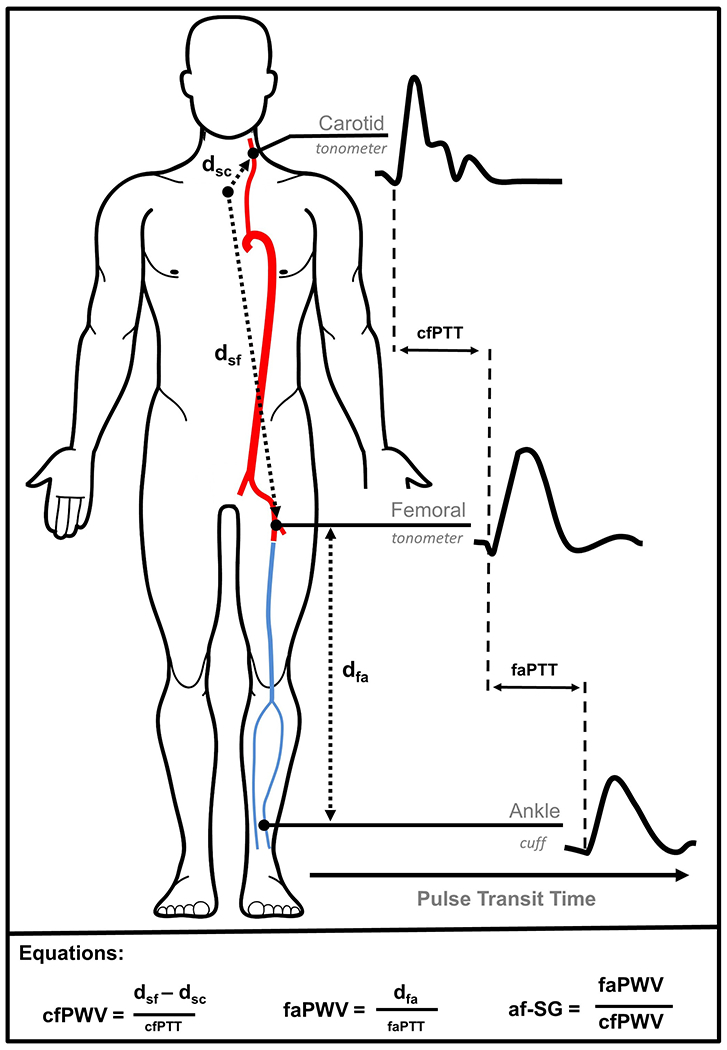
The aortic-femoral arterial stiffness gradient (af-SG) was calculated as femoral-ankle pulse wave velocity (faPWV) divided by carotid-femoral pulse wave velocity (cfPWV). Applanation tonometry was used to sequentially obtain waveforms at the left carotid and left femoral arteries, with cfPWV being estimated as the distance between the sternal notch and the femoral sampling site (dsf) minus the sternal notch to carotid sampling site (dsc), divided by the time delay (pulse transit time) between carotid and femoral waveforms (cfPTT). Simultaneously, bilateral posterior-tibial arterial pressure waveforms were detected using oscillometric cuffs at the ankles, with faPWV being estimated as the distance between femoral and ankle sampling sites determined using height-based formulas, divided by the time delay between femoral and ankle waveforms (faPTT).
The validity and reliability of the automatic device for measuring PWV have previously been described[20,22]. The device has been widely used in prospective observational studies and for independently predicting CVD and all-cause mortality[23,24], and is recommended by the American Heart Association as criterion device for the non-invasive validation studies [25]. Quality assurance for PWV included central training and recertification, quarterly equipment calibration, and ongoing quality control reviews by one of the authors (H.T.) on a stratified random sample of 40 records per month with feedback provided to technicians. Approximately 78% of records were considered optimal quality, 17% were good quality, 3% were acceptable, and none were poor or unacceptable.
Aortic-Femoral Arterial Stiffness gradient.
The af-SG was calculated by dividing the femoral-ankle PWV (faPWV) by carotid-femoral PWV (cfPWV). This method emphasizes the model arterial system, whereby in a healthy cardiovascular system arterial stiffness increases between central and distal arteries [26]. Although no clinical threshold has been identified, to give greater context, an af-SG greater than 1.0 (i.e. faPWV>cfPWV) can be considered physiologically normal, whereas an af-SG of 1.0 or less (i.e. faPWV < cfPWV) can be considered pathological[15].
DEMOGRAPHIC AND COVARIATE MEASUREMENTS
All covariate measures were collected as part of ARIC Visit 5.
Demographics.
Age was calculated from date of birth. Sex and race were self-reported. History of smoking was self-reported and analyzed as dichotomous (current versus noncurrent).
Anthropometrics.
Body weight was measured to the nearest 0.1 kg, and height was recorded to the nearest centimeter. Body mass index (BMI) was calculated body mass (kg) divided by height squared (m2).
Blood Markers.
Blood samples were obtained following a standardized venipuncture protocol and shipped weekly to ARIC central laboratories where assays for total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and fasting glucose concentration were performed. Total plasma cholesterol concentrations were determined enzymatically [27] using a Cobas-Bio analyzer with reagents purchased from Boehringer Mannheim Biochemicals, (Indianapolis, IN). Plasma low-density lipoprotein (LDL) cholesterol, concentration was calculated using the Friedewald equation, [28] and HDL concentrations were measured using the method of Warnick et al. [29].
Medications.
Participants were asked to bring to the clinical visit all prescription and nonprescription medications taken within the four preceding weeks. That information was transcribed and categorized using MediSPAN prescription codes and classified into medication categories. Participants also self-reported medication use. Medications used included β-blockers, α-blockers, calcium channel blockers, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers.
Prevalent Cardiovascular Diseases.
Prevalent CHD was defined by self-reported prior physician diagnosis of myocardial infarction or coronary revascularization, or prevalent myocardial infarction according to adjudicated ECG. Prevalent HF was classified by having at least one of the following: an adjudicated diagnosis of a HF event, International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) discharge code of 428.X in first position not overruled by a physician, any physician report of HF, self-reported HF or self-report of HF medication with pro-BNP greater than 125 pg/mL, or subsequent self-report of HF or HF medication (defined as medications participants reported taking for the treatment of HF). Prevalent stroke was defined by self-reported prior physician diagnosis of stroke or TIA and whether they had ever experienced the sudden onset of specific stroke symptoms (weakness, numbness, loss of vision, loss of understanding, inability to express). An ankle-brachial index (ABI) of less than or equal to 0.9 was used to indicate peripheral artery disease (PAD). An ABI was determined for each leg and calculated as the highest ankle systolic blood pressure divided by the highest of the right or left brachial systolic blood pressure [30].The lower value of right and left ABI was used for our analysis
STATISTICAL ANALYSIS
Statistical analyses were performed using R Statistical Software (R Foundation for Statistical Computing, Vienna, Austria). The α-level was set a-priori for all statistical procedures at α = 0.05. Cumulative frequency and Q-Q plots were used to compare the distributions of cfPWV, faPWV, and af-SG. Participant characteristics were estimated as means and standard deviation (SD), or frequencies and percent. Descriptive data across quartiles were compared using one-way analysis of variance (ANOVA) or Kruskal-Wallis for continuous outcomes, and Pearson’s chi-squared for categorical outcomes, with Bonferroni correction for multiple comparisons. Univariable and multivariable linear regression was used to assess the relationships between MAP and cfPWV, faPWV and af-SG. The impact of age, sex and race on the relationship between MAP and cfPWV, faPWV and af-SG was examined by introducing an interaction term between MAP and the variable under consideration, and assessed using the significance of the interaction term in univariable and multivariable models. Multivariable regression models were adjusted for known or potential confounders including age, sex, BMI, fasting blood glucose, heart rate, race and field center. For linear regression we report unstandardized and standardized β coefficient estimates and 95% confidence intervals (95% CI), and the R2 values for model fit. Partial R2 values for dependent variables were determined using semi-partial correlation analysis within the ppcor package in R[31]. Assumption of linearity, collinearity, homoscedasticity, and outliers were assessed for every model.
RESULTS
CHARACTERISTICS OF THE STUDY POPULATION
Of the 5,683 participants who attended visit 5 and underwent PWV measurements: 1500 were excluded using the following criteria: pre-existing condition (n=579), race other than white or African American (n=15), missing PWV data (n=529), PWV values 3 SDs above or below the mean (n=76), missing risk factor data (n=81), and missing covariates (n=220). Finally, 50 healthy (defined as above) participants were excluded due to prevalent CVD. Following exclusions, the sample included 4,139 cohort participants between the ages of 66 and 90 years, with 694, 2040, 1405 participants being categorized into apparently healthy, hypertension and diabetes groups, respectively.
Descriptive characteristics, stratified by patient group, are reported in Table 1. Healthy subjects were significantly younger, had lower BMI and systolic BP, and displayed a more favorable blood lipid profile than disease groups. cfPWV was significantly lower and the af-SG was significantly higher in the healthy when compared to disease groups, and both were significantly different between hypertension and diabetes groups. faPWV was significantly lower in the diabetes group than that of healthy and hypertension groups only.
TABLE 1.
Descriptive characteristics of ARIC visit 5 participants, stratified by healthy and disease groups.
| Healthy n = 694 |
Hypertension n = 2040 |
Diabetes n = 1405 |
P Value | ||||
|---|---|---|---|---|---|---|---|
|
Continuous Variables (Mean, SD)
| |||||||
| Age (years) | 74 | (4.6) | 75.5 | (5.04)b | 75.2 | (5.08)b | <0.001 |
| Body Mass Index (kg/m2) | 26.1 | (4.0) | 27.6 | (4.3) | 29.2 | (4.4) | <0.001a |
| Systolic blood pressure (mm Hg) | 122 | (11) | 133 | (18) | 130 | (18) | <0.001a |
| Diastolic blood pressure (mm Hg) | 65 | (8) | 67 | (10.9)b | 65 | (10.1)c | <0.001 |
| Heart rate (bpm) | 65 | (10) | 64 | (10.7) | 66 | (11.2)b,c | <0.001 |
| Fasting glucose (mg/dL) | 5.6 | (0.5) | 5.7 | (0.5) | 7.3 | (2.0) | <0.001a |
| LDL (mg/dL) | 3.1 | (0.8) | 2.8 | (0.8) | 2.4 | (0.9) | <0.001a |
| HDL (mg/dL) | 1.5 | (0.4) | 1.4 | (0.6) | 1.5 | (0.7) | <0.001a |
| Triglycerides (mg/dL) | 1.3 | (0.5) | 1.4 | (0.6)b | 1.4 | (0.6)b | <0.001 |
| Ankle-brachial index | 1.14 | (0.10) | 1.10 | (0.13)b | 1.09 | (0.15)b | <0.001 |
| Femoral-ankle PWV (m/s) | 11.0 | (1.6) | 10.9 | (1.7) | 10.6 | (1.69)b,c | <0.001 |
| Carotid-femoral PWV (m/s) | 10.5 | (2.4) | 11.5 | (3.0) | 12.3 | (3.2) | <0.001a |
| Aortic-femoral stiffness gradient | 1.10 | (0.3) | 1.01 | (0.3) | 0.93 | (0.3) | <0.001a |
|
| |||||||
|
Categorical Variables (No., %)
| |||||||
| Sex | b,c | ||||||
| Male | 261 | (38) | 771 | (38) | 635 | (45) | <0.001 |
| Female | 433 | (62) | 1269 | (62) | 770 | (55) | |
| Race | |||||||
| African American | 62 | (9) | 459 | (22) | 410 | (29) | <0.001a |
| White | 632 | (91) | 1581 | (78) | 995 | (71) | |
| Prevalent Cardiovascular Disease | |||||||
| Coronary heart disease | 0 | (0) | 294 | (14) | 269 | (19) | <0.001 a |
| Heart failure | 0 | (0) | 206 | (10) | 210 | (15) | <0.001a |
| Stroke | 0 | (0) | 46 | (2) | 60 | (4) | <0.001a |
| Ankle-brachial index <0.9 | 0 | (0) | 135 | (7) | 133 | (6) | <0.001a |
| Medication use | |||||||
| β-Blocker | 0 | (0) | 703 | (34)b | 464 | (33)b | <0.001 |
| α-Blocker | 0 | (0) | 71 | (3)b | 64 | (5)b | <0.001 |
| Diuretic | 0 | (0) | 916 | (45) | 695 | (49) | <0.001a |
| ACE Inhibitor | 0 | (0) | 517 | (25) | 430 | (31) | <0.001a |
| ANG II receptor blocker | 0 | (0) | 221 | (11) | 203 | (14) | <0.001a |
| Calcium channel blocker | 0 | (0) | 583 | (29)b | 448 | (32)b | <0.001a |
| Current smoker | 45 | (6) | 114 | (6) | 76 | (5) | 0.588 |
Abbreviations: PWV, pulse wave velocity; HDL, high-density lipoprotein cholesterol; LDL, Low-density lipoprotein cholesterol.
for the comparison between all groups;
vs. healthy;
vs. hypertension.
ASSOCIATIONS WITH MEAN ARTERIAL PRESSURE
Within the diabetes group only, there were significant sex by MAP interactions for af-SG and faPWV, and a race by MAP interaction for faPWV in univariable analyses (all P < 0.05), but none were significant following covariate adjustment in multivariable models. Univariable associations of the af-SG, cfPWV and faPWV with MAP by participant group are presented in Figure 2. The af-SG was not associated with MAP in healthy and hypertension groups, but was significantly associated with MAP in the diabetes group (P<0.05). MAP explained 0.1%, 0.1% and 0.4% of the variation in the af-SG within healthy, hypertension and diabetes groups, respectively. Both cfPWV (2.5 - 5.1%) and faPWV (6.6-9.3%) were significantly associated with MAP in all population groups. Multivariable associations of the af-SG, cfPWV and faPWV by group are presented in Table 2. Overall, multivariable adjustment had a small effect on MAP estimates; however, the significant association between af-SG and MAP in diabetes participants was no longer significant following covariate adjustment. In multivariable models MAP explained <0.1%, <0.1% and 0.2% of the variation in the af-SG within healthy, hypertension and diabetes groups, respectively.
FIGURE 2.
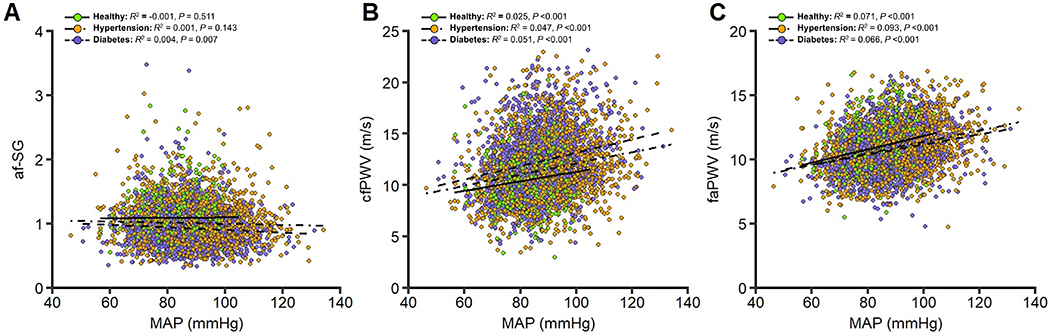
Relationship between mean arterial pressure and the aortic-femoral stiffness gradient, carotid-femoral pulse wave velocity, and femoral-ankle pulse-wave velocity in healthy (n=694), hypertension (n=2040), and diabetes (n=1405) population groups. Abbreviations: cfPWV, carotid-femoral pulse-wave velocity; faPWV, femoral-ankle pulse-wave velocity; af-SG, aortic-femoral arterial stiffness gradient; MAP, mean arterial pressure.
TABLE 2.
Multivariable linear regression models for the association between mean arterial pressure and the aortic-femoral stiffness gradient, carotid-femoral pulse wave velocity, and femoral-ankle pulse-wave velocity in healthy (n=694), hypertension (n=2040), and diabetes (n=1405) groups.
| HEALTHY |
HYPERTENSION |
DIABETES |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | Std. β | 95% CI | P | b R 2 | β | Std. β | 95% CI | P | b R 2 | β | Std. β | 95% CI | P | b R 2 | ||||
| cfPWV | a R 2 = | 0.211 | a R 2 = | 0.169 | a R 2 = | 0.143 | ||||||||||||
|
| ||||||||||||||||||
| MAP | 0.051 | 0.178 | 0.158 | 0.198 | <0.001 | 0.029 | 0.052 | 0.208 | 0.198 | 0.218 | <0.001 | 0.042 | 0.061 | 0.214 | 0.200 | 0.228 | <0.001 | 0.043 |
| Age | 0.163 | 0.314 | 0.279 | 0.349 | <0.001 | 0.095 | 0.150 | 0.255 | 0.231 | 0.279 | <0.001 | 0.062 | 0.143 | 0.230 | 0.200 | 0.261 | 0.000 | 0.051 |
| Sex | 0.759 | 0.154 | −0.200 | 0.509 | <0.001 | 0.020 | 0.423 | 0.069 | −0.182 | 0.320 | 0.001 | 0.004 | 0.490 | 0.077 | −0.236 | 0.391 | 0.002 | 0.006 |
| BMI | −0.062 | −0.104 | −0.146 | −0.062 | 0.004 | 0.009 | −0.083 | −0.121 | −0.150 | −0.093 | <0.001 | 0.013 | −0.031 | −0.044 | −0.080 | −0.008 | 0.090 | 0.002 |
| FBG | 0.407 | 0.085 | −0.248 | 0.417 | 0.016 | 0.007 | 0.128 | 0.024 | −0.200 | 0.248 | 0.264 | 0.001 | 0.125 | 0.079 | 0.001 | 0.157 | 0.002 | 0.006 |
| HR | 0.056 | 0.233 | 0.216 | 0.250 | <0.001 | 0.048 | 0.050 | 0.179 | 0.167 | 0.190 | <0.001 | 0.030 | 0.050 | 0.175 | 0.161 | 0.189 | 0.000 | 0.029 |
|
| ||||||||||||||||||
| faPWV | a R 2 = | 0.113 | a R 2 = | 0.190 | a R 2 = | 0.182 | ||||||||||||
|
| ||||||||||||||||||
| MAP | 0.061 | 0.313 | 0.299 | 0.327 | 0.000 | 0.089 | 0.046 | 0.316 | 0.310 | 0.322 | <0.001 | 0.096 | 0.043 | 0.282 | 0.274 | 0.289 | <0.001 | 0.074 |
| Age | 0.034 | 0.095 | 0.070 | 0.121 | 0.009 | 0.009 | 0.001 | 0.003 | −0.010 | 0.017 | 0.869 | 0.000 | −0.006 | −0.017 | −0.033 | 0.000 | 0.501 | 0.000 |
| Sex | −0.184 | −0.055 | −0.311 | 0.202 | 0.160 | 0.002 | −0.083 | −0.023 | −0.168 | 0.122 | 0.263 | 0.000 | 0.122 | 0.036 | −0.128 | 0.200 | 0.143 | 0.001 |
| BMI | −0.050 | −0.125 | −0.155 | −0.094 | 0.001 | 0.014 | −0.077 | −0.191 | −0.207 | −0.174 | <0.001 | 0.033 | −0.093 | −0.243 | −0.262 | −0.224 | 0.000 | 0.054 |
| FBG | −0.018 | −0.005 | −0.246 | 0.235 | 0.884 | 0.000 | 0.102 | 0.032 | −0.097 | 0.162 | 0.123 | 0.001 | 0.045 | 0.054 | 0.013 | 0.095 | 0.029 | 0.003 |
| HR | 0.015 | 0.091 | 0.079 | 0.104 | 0.016 | 0.007 | 0.024 | 0.145 | 0.138 | 0.151 | 0.000 | 0.020 | 0.022 | 0.144 | 0.137 | 0.151 | 0.000 | 0.003 |
|
| ||||||||||||||||||
| af-SG | a R 2 = | 0.075 | a R 2 = | 0.057 | a R 2 = | 0.054 | ||||||||||||
|
| ||||||||||||||||||
| MAP | 0.002 | 0.039 | 0.036 | 0.042 | 0.301 | 0.001 | −0.001 | −0.023 | −0.024 | −0.022 | 0.298 | 0.001 | −0.001 | −0.050 | −0.051 | −0.048 | 0.063 | 0.002 |
| Age | −0.014 | −0.193 | −0.199 | −0.188 | <0.001 | 0.036 | −0.012 | −0.186 | −0.189 | −0.183 | <0.001 | 0.033 | −0.010 | −0.164 | −0.167 | −0.161 | <0.001 | 0.026 |
| Sex | −0.099 | −0.142 | −0.196 | −0.087 | <0.001 | 0.017 | −0.043 | −0.066 | −0.095 | −0.038 | 0.003 | 0.004 | −0.028 | −0.046 | −0.078 | −0.014 | 0.084 | 0.002 |
| BMI | 0.000 | −0.005 | −0.011 | 0.001 | 0.897 | 0.000 | −0.001 | −0.012 | −0.015 | −0.009 | 0.591 | 0.000 | −0.006 | −0.089 | −0.092 | −0.085 | 0.001 | 0.007 |
| FBG | −0.047 | −0.069 | −0.120 | −0.018 | 0.070 | 0.004 | 0.004 | 0.006 | −0.019 | 0.032 | 0.775 | 0.000 | −0.006 | −0.042 | −0.050 | −0.034 | 0.112 | 0.002 |
| HR | −0.005 | −0.142 | −0.144 | −0.139 | <0.001 | 0.018 | −0.002 | −0.072 | −0.073 | −0.070 | 0.001 | 0.005 | −0.002 | −0.067 | −0.069 | −0.066 | 0.012 | 0.004 |
Abbreviations: cfPWV, carotid-femoral pulse-wave velocity; faPWV, femoral-ankle pulse-wave velocity; afPWV ratio, aortic-femoral pulse-wave velocity ratio; MAP, mean arterial pressure, BMI, body mass index; FBG, fasting blood glucose; HR, heart rate, β, beta coefficient; std. β, standardized beta coefficient;
R2, Model adjusted R squared coefficient;
R2, partial R squared coefficient.
Adjustments: age, sex, body mass index, fasting blood glucose, heart rate, race and field center.
SENSITIVITY AND ANCILLARY ANALYSIS
Analysis of af-SG determined using left and right faPWV measures separately revealed no notable differences to those determined using a mean of left and right faPWV measures. Independently, the exclusion of those participants with an ankle brachial index (ABI) ≤0.9 and all participants with prevalent CVD (hypertension and diabetes groups only) did not impact the key findings. Finally, compared to the primary findings, stratification of all subjects by quartiles of age (<71,71-74,75-79,>79 years) did not reveal any contrasting associations for the af-SG with MAP (Table S1).
DISCUSSION
The aim of this study was to determine the relationship between MAP and the af-SG in healthy older adults as well as those with established disease, specifically hypertension and diabetes. The principal finding was that, unlike cfPWV and faPWV, the af-SG was found not to be dependent on MAP in healthy, hypertension or diabetes population groups. This finding suggests that the af-SG may be regarded as a MAP independent index of arterial health and CVD risk in older adults.
LIMITATIONS AND STRENGTHS
The strengths and limitations of this study need to be addressed to best contextualize the findings and better facilitate comparisons to the existing literature. Firstly, the generalizability of our findings is limited to older populations and cannot be extended to younger, healthier cohorts. Further, the predominate inclusion of participants who had survived from baseline (1987-1989) and attended the Visit 5 examination (2011-2013), and were thus likely healthier compared to those who did not participate in the visit, may have generated a bias within the study population. Secondly, the use of height-based formulas to calculate faPWV were validated in a Japanese population and may not be applicable to other racial or ethnic groups. A major strength is that this is the first study to examine the association between the af-SG and MAP among different patient and healthy populations.
COMPARISON TO THE LITERATURE
A major finding of this study is that unlike cfPWV and faPWV, MAP was not associated with af-SG in a large population of community-dwelling older adults, regardless of health status. Following adjustment for known confounders in multivariable models, MAP explained <0.1, <0.1 and 0.2% of the variation in the af-SG in healthy, hypertension and diabetes groups, respectively. Whilst no other studies have investigated the MAP dependence of the af-SG, the present findings are consistent with the majority of previous literature investigating the MAP dependency of the upper-extremity derived ab-SG [12,16,32,33]. The ab-SG has demonstrated independence to MAP in dialysis and renal dysfunction patients [12,32], and comparable to the present study, diabetes and hypertension patient groups [16,33]. Of those reported, the variation in af-SG explained by MAP in the present study across populations (~0.2%) is lower than that reported for the ab-SG (0.4-9.6%)[12,16,32,33], supporting our inference of MAP independence. However, unlike the af-SG in the present study, the ab-SG was shown not to be MAP independent among healthy adults [16]. The divergent findings between the dependence of ab-SG and af-SG on MAP in healthy populations is likely due to several factors, including: i) the inherent difference in the structural characteristics of the peripheral vascular segments used to determine the respective arterial stiffness gradients, and, ii) the contrasting demographic characteristics of the populations in which MAP dependence was explored.
Arterial wall stiffness is dependent on both MAP and the intrinsic structure of the arterial wall. Distending tension is primarily borne by elastin-distensible fibres at low pressure, but an increase in MAP increases vessel diameter and transfers the distending load to the less extensible collagen fibres, leading to an augmentation in arterial stiffness [26]. Increased arterial stiffness shifts the pressure-diameter relationship upwards, meaning a higher pressure is required to induce a similar change in diameter[10]. Consequently, an arterial segment of higher arterial stiffness will inherently be impacted by MAP to a lesser degree. Relative to the upper-extremities, the lower-extremities incorporate a greater proportion of inelastic muscular conduit arteries. Additionally, lower-extremity arterial stiffness is typically higher than that of the upper extremities, in order to manage the greater hydrostatic load induced during orthostasis [34]. It is therefore plausible that the inclusion of a greater proportion, of intrinsically stiffer, arterial segments (femoro-tibial) may lessen the effect of MAP on the af-SG. However, no studies have directly compared the MAP dependence of ab-SG and af-SG measures.
The pressure-diameter relationship is also influenced by age and disease. With normal aging, the elastin-distensible fibres become fragmented and discontinuous, this, coupled with a reduction in elastin expression attenuating the elastin-collagen ratio, shifts the mechanical load to the stiffer collagen fibres [26,35]. The stiffness of elastin and collagen fibres is also increased via additional cross-linking by advanced glycation end-products (AGE)[35]. This degeneration is accelerated by the presence of disease, with calcification of the elastic lamellae and the cross-linking by AGEs occurring at an advanced rate with diabetes for example [36,37]. The progression of vascular dysfunction is partly offset by arterial dilation, with arterial cross-sectional area increasing with age [11]. However, these phenomena steepen the slope of the pressure-diameter relationship, lessening the effect of MAP on arterial stiffness measures. To illustrate, compared to healthy adults, hypertensive adults demonstrate an augmented aortic PWV [16,38], and an attenuated dependence of aortic stiffness on transmural pressure has been reported in hypertensive versus normotensive patients [39]. Therefore, as for the ab-SG [12,16,32,33], the consequences of vascular aging and disease likely contribute to the independence of the af-SG to MAP among older adults in the present study, particularly within hypertension and diabetes patient groups. Further, although it is difficult to discern the impact of age and disease, vascular aging also likely explains the contrasting findings between the pressure-dependence of ab-SG and af-SG measures in healthy adults. Whilst free from hypertension, diabetes and prevalent CVD, the healthy adults in the present study were significantly older (74 ± 5 years vs 51 ± 8 years) and, expectantly, had a ~30% greater cfPWV (10.5 ± 2.4 m/s vs 7.5 ± 1.8 m/s years), than the healthy adults in the study conducted by Armstrong et al. [16] to investigate the MAP dependence of the ab-SG. This may suggest that the observed independence of the af-SG to MAP in the present study could in-part be due to use of older adults who are further along the vascular aging pathway. However, our healthy adults demonstrated aortic stiffness measures which closely reflect age-specific reference values (10.4-11.7 m/s) for normotensive adults [40], and are therefore representative of healthy older adults in the general population. As such the af-SG may be regarded as a MAP independent index of arterial health in older adults.
IMPLICATIONS AND CONCLUSIONS
The assessment of aortic arterial stiffness, typically as cfPWV, to assist in the determination of CVD risk is now well established in epidemiological and clinical research settings[41]. However, notwithstanding the strong association of aortic stiffness with clinical outcomes [3,4], this persistent focus ignores the integrated role that peripheral muscular arteries play in the cardiovascular system. Although the peripheral vasculature is less impacted by age and disease compared to the central vasculature [17], there can be important pathophysiological changes within this region that may contribute to CVD risk [42]. In this respect, incorporation of peripheral arterial stiffness into risk prediction in the form of the ab-SG or af-SG has been shown to confer unique and prognostic information beyond cfPWV alone, with particular use in older-age and diseased populations [13–15], and may better explain the impact of pathophysiological changes in arterial stiffness on both myocardium and peripheral circulation [43]. For example, our research group demonstrated that the af-SG was associated with coronary heart disease, heart failure and stroke in community-dwelling older adults, whilst cfPWV was not [14]. The current study extends the scant arterial stiffness gradient literature by being the first to report that, unlike cfPWV, the af-SG is not dependent on MAP in older adults, regardless of health status. The independence of af-SG to MAP is a significant advantage, and overcomes a likely barrier to the widespread adoption of arterial stiffness measures into clinical practice. Collectively, these findings indicate that the af-SG may be of clinical utility as a simple non-invasive assessment of arterial health and identification of CVD risk. However, a number of gaps in the literature remain and need to be addressed in order to ascertain whether the af-SG is a clinically viable surrogate endpoint, including whether the af-SG predicts CVD events and mortality, and if it is sensitive to risk factor modification or pharmacological intervention [41]. Further, to confirm utility, future research should seek to identify if age or disease, in younger adults, impacts the dependency of the af-SG on MAP.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). The study was also supported by R01AG053938.
Footnotes
CONFLICTS OF INTEREST
There are no conflicts of interest to declare.
REFERENCES
- 1.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 2.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–448. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 5.Gavish B, Gavish L. Blood pressure variation in response to changing arm cuff height cannot be explained solely by the hydrostatic effect. J Hypertens 2011; 29:2099–2104. [DOI] [PubMed] [Google Scholar]
- 6.Lim J, Pearman ME, Park W, Alkatan M, Machin DR, Tanaka H. Impact of blood pressure perturbations on arterial stiffness. Am J Physiol Regul Integr Comp Physiol 2015; 309:R1540–1545. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, Davy KP, Seals DR. Cardiopulmonary baroreflex inhibition of sympathetic nerve activity is preserved with age in healthy humans. J Physiol 1999; 515 (Pt 1):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seals DR. Sympathetic activation during the cold pressor test: influence of stimulus area. Clinical physiology 1990; 10:123–129. [DOI] [PubMed] [Google Scholar]
- 9.Stoner L, Stone K, Zieff G, Hanson ED, Credeur D, Faulkner J, et al. The impact of upper-limb position on estimated central blood pressure waveforms. J Hum Hypertens 2019; 33:444–453. [DOI] [PubMed] [Google Scholar]
- 10.Fortier C, Desjardins MP, Agharazii M. Aortic-Brachial Pulse Wave Velocity Ratio: A Measure of Arterial Stiffness Gradient Not Affected by Mean Arterial Pressure. Pulse (Basel) 2018; 5:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spronck B, Heusinkveld MH, Vanmolkot FH, Roodt JO, Hermeling E, Delhaas T, et al. Pressure-dependence of arterial stiffness: potential clinical implications. J Hypertens 2015; 33:330–338. [DOI] [PubMed] [Google Scholar]
- 12.Fortier C, Sidibe A, Desjardins MP, Marquis K, De Serres SA, Mac-Way F, et al. Aortic-Brachial Pulse Wave Velocity Ratio: A Blood Pressure-Independent Index of Vascular Aging. Hypertension 2017; 69:96–101. [DOI] [PubMed] [Google Scholar]
- 13.Fortier C, Mac-Way F, Desmeules S, Marquis K, De Serres SA, Lebel M, et al. Aortic-brachial stiffness mismatch and mortality in dialysis population. Hypertension 2015; 65:378–384. [DOI] [PubMed] [Google Scholar]
- 14.Stone K, Fryer S, Meyer M, Kucharska-newton A, Faulkner J, Zieff G, et al. The Aortic-Femoral Arterial Stiffness Gradient: An Atherosclerosis Risk in Communities (ARIC) Study. Journal of Hypertension in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niiranen TJ, Kalesan B, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF, et al. Aortic-Brachial Arterial Stiffness Gradient and Cardiovascular Risk in the Community: The Framingham Heart Study. Hypertension 2017; 69:1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong MK, Schultz MG, Picone DS, Sharman JE. Aortic-to-brachial artery stiffness gradient is not blood pressure independent. J Hum Hypertens 2019; 33:385–392. [DOI] [PubMed] [Google Scholar]
- 17.Yu S, McEniery CM. Central Versus Peripheral Artery Stiffening and Cardiovascular Risk. Arterioscler Thromb Vasc Biol 2020; 40:1028–1033. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Atherosclerosis Risk in Communities (ARIC) study: Design and Objectives. The ARIC Investigators. American Journal of Epidemiology 1989; 129:687–702. [PubMed] [Google Scholar]
- 20.Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol 2003; 91:1519–1522, A1519. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens 2009; 27:2022–2027. [DOI] [PubMed] [Google Scholar]
- 22.Meyer ML, Tanaka H, Palta P, Patel MD, Camplain R, Couper D, et al. Repeatability of Central and Peripheral Pulse Wave Velocity Measures: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens 2016; 29:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuoka O, Otsuka K, Murakami S, Hotta N, Yamanaka G, Kubo Y, et al. Arterial stiffness independently predicts cardiovascular events in an elderly community -- Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomed Pharmacother 2005; 59 Suppl 1:S40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turin TC, Kita Y, Rumana N, Takashima N, Kadota A, Matsui K, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality in the general population: findings from the Takashima study, Japan. Hypertens Res 2010; 33:922–925. [DOI] [PubMed] [Google Scholar]
- 25.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015; 66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.London GM, Pannier B. Arterial functions: how to interpret the complex physiology. Nephrol Dial Transplant 2010; 25:3815–3823. [DOI] [PubMed] [Google Scholar]
- 27.Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem 1983; 29:1075–1080. [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18:499–502. [PubMed] [Google Scholar]
- 29.Warnick GR, Mayfield C, Benderson J, Chen JS, Albers JJ. HDL cholesterol quantitation by phosphotungstate-Mg2+ and by dextran sulfate-Mn2+-polyethylene glycol precipitation, both with enzymic cholesterol assay compared with the lipid research method. Am J Clin Pathol 1982; 78:718–723. [DOI] [PubMed] [Google Scholar]
- 30.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012; 126:2890–2909. [DOI] [PubMed] [Google Scholar]
- 31.Dudgeon P A Comparative Investigation of Confidence Intervals for IndependentVariables in Linear Regression. Multivariate Behav Res 2016; 51:139–153. [DOI] [PubMed] [Google Scholar]
- 32.Bia D, Valtuille R, Galli C, Wray S, Armentano R, Zocalo Y, et al. Aortic-Radial Pulse Wave Velocity Ratio in End-stage Renal Disease Patients: Association with Age, Body Tissue Hydration Status, Renal Failure Etiology and Five Years of Hemodialysis. High Blood Press Cardiovasc Prev 2017; 24:37–48. [DOI] [PubMed] [Google Scholar]
- 33.Picone DS, Schultz MG, Climie RE, Srikanth V, Sharman JE. Aortic-to-brachial stiffness gradient and kidney function in type 2 diabetes. J Hypertens 2016; 34:1132–1139. [DOI] [PubMed] [Google Scholar]
- 34.Kimoto E, Shoji T, Shinohara K, Inaba M, Okuno Y, Miki T, et al. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes 2003; 52:448–452. [DOI] [PubMed] [Google Scholar]
- 35.Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res 2012; 5:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 2006; 114:597–605. [DOI] [PubMed] [Google Scholar]
- 37.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2007; 115:2722–2730. [DOI] [PubMed] [Google Scholar]
- 38.Isnard RN, Pannier BM, Laurent S, London GM, Diebold B, Safar ME. Pulsatile diameter and elastic modulus of the aortic arch in essential hypertension: a noninvasive study. J Am Coll Cardiol 1989; 13:399–405. [DOI] [PubMed] [Google Scholar]
- 39.Gaddum NR, Keehn L, Guilcher A, Gomez A, Brett S, Beerbaum P, et al. Altered dependence of aortic pulse wave velocity on transmural pressure in hypertension revealing structural change in the aortic wall. Hypertension 2015; 65:362–369. [DOI] [PubMed] [Google Scholar]
- 40.Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 2010; 31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifkova R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015; 241:507–532. [DOI] [PubMed] [Google Scholar]
- 42.van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, et al. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol 2014; 63:1739–1747. [DOI] [PubMed] [Google Scholar]
- 43.Fortier C, Agharazii M. Arterial Stiffness Gradient. Pulse (Basel) 2016; 3:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


