Abstract
BACKGROUND:
Six hours was historically regarded as the limit of acceptable ischemic time for lung allografts. However, broader sharing of donor lungs often necessitates use of allografts with ischemic time >6 hours. We characterized the association between ischemic time ≥8 hours and outcomes after lung transplantation using a contemporary cohort from a high-volume institution.
METHODS:
Patients who underwent primary isolated bilateral lung transplantation between 1/2016–5/2020 were included. Patients bridged to transplant with extracorporeal membrane oxygenation or mechanical ventilation, and ex-vivo perfusion cases were excluded. Recipients were stratified by total allograft ischemic time <8 hours (standard) versus ≥8 hours (long). Perioperative outcomes and post-transplant survival were compared between groups.
RESULTS:
Of 358 patients, 95 (26.5%) received long ischemic time (≥8 hours) lungs. Long ischemic time recipients were more likely to be male and have donation after circulatory death donors than standard ischemic time recipients. On unadjusted analysis, long and standard ischemic time recipients had similar survival, and similar rates of grade 3 primary graft dysfunction at 72 hours, extracorporeal membrane oxygenation post-transplant, acute rejection within 30 days, reintubation, and post-transplant length of stay. After adjustment, long and standard ischemic time recipients had comparable risks of mortality or graft failure.
CONCLUSIONS:
In a modern cohort, use of lung allografts with “long” ischemic time ≥8 hours was associated with acceptable perioperative outcomes and post-transplant survival. Further investigation is required to better understand how broader use impacts post-lung transplant outcomes and the implications for smarter sharing under an evolving national allocation policy.
INTRODUCTION
Lung transplantation (LTx) is the gold-standard therapy for patients with end-stage lung disease, offering a survival benefit compared to remaining on the waitlist.1,2 In the US, LTx is increasingly performed with a record 2,562 transplants performed in 2018.3 Nevertheless, transplant candidates continue to outnumber available donor lungs and up to 30% of candidates die or are removed from the waitlist prior to transplantation.3
Broader geographic sharing may increase lung availability, particularly for high-acuity patients.4 However, this allocation strategy remains shrouded by long-standing concern regarding potential associations between prolonged ischemic times (IT), ischemia-reperfusion injury, and compromised post-transplant survival.5,6 To mitigate these risks, six hours has traditionally been regarded as the upper limit of acceptable lung IT.5,7 However, historical studies present unclear conclusions regarding the effect of IT >6 hours on early allograft function and post-transplant survival.5,6,8,9 Alternatively, recent analyses of the United Network for Organ Sharing (UNOS) and International Society for Heart and Lung Transplantation (ISHLT) registries found no association between IT >6 hours and primary graft failure or long-term survival,10,11 and emerging evidence suggests that lungs with IT ≥4 hours may offer superior five-year survival than those with shorter ITs.12
In November 2017, US lung allocation policy was urgently revised, incorporating new provisions to remove donation service areas (DSAs) from the allocation schema. This led to increased lung availability and improved access to LTx among high-acuity candidates, in accordance with the 1998 Final Rule.4,13 This new policy replaced DSA with a 250-nautical mile radius around the donor hospital as the first unit of lung allocation,13 increasing procurement travel distances and ITs.14,15 To support broad and equitable sharing, use of lung allografts with IT >6 hours is often necessary. New evidence is required to understand how use of these lungs may impact post-transplant outcomes in the modern era. In this study, we characterized the association between lung IT ≥8 hours and outcomes after LTx using a contemporary cohort from a high-volume institution. We hypothesized that long ITs do not predict greater morbidity or mortality after LTx.
METHODS
Data sources and study population
We conducted a single-center retrospective cohort study using institutional and UNOS data. Patients who underwent primary isolated bilateral LTx at Duke University Hospital between January 1, 2016 and May 31, 2020 were included. To limit potential confounding of prolonged ITs by operative complexity and use of lung preservation technologies other than cold storage, patients with history of prior LTx, multiorgan or single LTx recipients, those bridged to LTx using extracorporeal membrane oxygenation (ECMO) or mechanical ventilation, and those who received lungs placed on ex-vivo lung perfusion (EVLP) were excluded (Figure S1). This study was approved by our Institutional Review Board (Pro00103325).
Study design
Recipients were stratified by lung IT <8 hours (standard) versus ≥8 hours (long) based on total IT of the second implanted lung. An 8-hour cutoff was selected to represent “long” IT based on clinical judgment and experience among LTx surgeons at our institution. Recipient, operative, donor characteristics and post-transplant outcomes were compared between IT strata.
The primary outcome was survival to death or graft failure. Secondary outcomes included rejection-free and chronic lung allograft dysfunction (CLAD)-free survival, 30-day reintervention (surgical, bronchoscopic, radiologic), 30-day hospital or intensive care unit (ICU) readmission, post-transplant length of stay (LOS), grade 3 primary graft dysfunction (PGD3) at 72 hours, need for postoperative ECMO, postoperative date of extubation, tracheostomy within 7 days, reintubation or dialysis during the transplant hospitalization, 30-day biopsy-proven acute rejection, and 90-day mortality. Graft failure was defined by death or retransplantation; causes included primary graft failure (early), and CLAD or other etiologies (late). CLAD, PGD3, and acute rejection were defined according to ISHLT guidelines.16–18 Bronchoscopic reintervention was defined by need for an intervention such as bronchial dilation or stenting beyond routine post-transplant surveillance.
Statistical analysis
Recipient, operative, donor characteristics and perioperative outcomes were compared between IT strata using Wilcoxon rank-sum tests for continuous variables and Chi-squared and Fisher’s exact tests for categorical variables. Unadjusted survival was estimated using the Kaplan-Meier method and compared between groups using log-rank tests. To further investigate the impact of long IT on post-transplant outcomes, a subgroup analysis was performed among recipients of long IT lungs, stratified by donor type (donation after brain death [DBD] vs donation after circulatory death [DCD]). Perioperative outcomes and post-transplant survival were compared between DBD and DCD donor strata; in these strata, survival analysis was restricted to one-year post-transplant due to a small number of patients who received DCD donor lungs.
Adjusted survival to death or graft failure was modeled using multivariable Cox regression. Covariates were selected a priori based on guidance from Scientific Registry of Transplant Recipients models,19 clinical judgment, and availability within the dataset. In addition to IT, the final model included recipient age, cardiac output, lung allocation score, six-minute walk distance, arterial pCO2, body mass index, history of thoracic surgery other than LTx, percentage of predicted forced expiratory volume in one second, donor age, and donor/recipient weight ratio.
As a sensitivity analysis, the Cox model was used to investigate the relationship between IT and survival to death or graft failure modeling IT as a continuous variable. The association between IT and log hazard of death or graft failure was modeled assuming linearity and using restricted cubic splines. Models were compared using Akaike information criterion values. The proportional hazard assumption was tested using Schoenfeld residuals. Unadjusted survival to death or graft failure was also compared among recipients of lungs with IT <6 hours, 6–8 hours, 8–10 hours, and >10 hours to examine the impact of incrementally increasing IT >6 hours. A two-sided p-value less than 0.05 was considered statistically significant. All analyses were performed using R version 3.6.1 (Vienna, Austria).
RESULTS
Recipient, operative, and donor characteristics
Of 358 LTx recipients, 95 (26.5%) and 263 (73.5%) received long and standard IT lungs, respectively. Compared to standard IT recipients, long IT recipients were more likely to be male (69.5% vs 54.4%, p=0.01). Additional recipient characteristics were similar between groups (Table 1).
Table 1.
Recipient characteristics
| Characteristic | Standard Ischemia (<8 Hours) N = 263 | Long Ischemia (≥8 Hours) N = 95 | P-value |
|---|---|---|---|
| Age (years) | 60.0 [51.0, 67.0] | 58.0 [42.5, 66.0] | 0.2 |
| Sex | 0.01 | ||
| Female | 120 (45.6%) | 29 (30.5%) | |
| Male | 143 (54.4%) | 66 (69.5%) | |
| Race | 0.7 | ||
| Caucasian/White | 222 (84.4%) | 83 (87.4%) | |
| Black or African American | 34 (12.9%) | 9 (9.5%) | |
| Other | 7 (2.7%) | 3 (3.2%) | |
| Ethnicity (Hispanic) | 3 (1.1%) | 2 (2.1%) | 0.6 |
| Panel reactive antibody at transplant (%) | |||
| Class I | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.8 |
| Class II | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.7 |
| Etiology of respiratory failure | 0.1 | ||
| Alpha-1-antitrypsin deficiency | 10 (3.8%) | 3 (3.2%) | |
| Acute respiratory distress syndrome/pneumonia | 1 (0.4%) | 0 (0%) | |
| Autoimmune interstitial lung disease | 21 (8.0%) | 3 (3.2%) | |
| Bronchiectasis | 3 (1.1%) | 3 (3.2%) | |
| Chronic obstructive pulmonary disease/emphysema | 49 (18.6%) | 13 (13.7%) | |
| Cystic fibrosis | 34 (12.9%) | 19 (20.0%) | |
| Eisenmenger syndrome | 0 (0%) | 2 (2.1%) | |
| Hypersensitivity pneumonitis | 10 (3.8%) | 2 (2.1%) | |
| Idiopathic pulmonary fibrosis | 99 (37.6%) | 39 (41.1%) | |
| Pulmonary hypertension | 6 (2.3%) | 4 (4.2%) | |
| Other interstitial lung disease | 11 (4.2%) | 1 (1.1%) | |
| Other | 19 (7.2%) | 6 (6.3%) | |
| Disease group | 0.2 | ||
| A | 66 (25.1%) | 22 (23.2%) | |
| B | 9 (3.4%) | 6 (6.3%) | |
| C | 34 (12.9%) | 19 (20.0%) | |
| D | 154 (58.6%) | 48 (50.5%) | |
| Status at time of transplant | 0.9 | ||
| Inpatient/hospitalized | 26 (9.9%) | 10 (10.5%) | |
| Outpatient | 237 (90.1%) | 85 (89.5%) | |
| History of previous thoracic surgery | 28 (10.6%) | 10 (10.5%) | >0.9 |
| Lung allocation score at time of transplant | 43.3 [36.3, 51.0] | 42.9 [37.4, 52.8] | 0.5 |
| Most recent 6-minute walk distance (feet) | 1400 [1130, 1640] | 1390 [1150, 1620] | 0.8 |
| Cardiac output (L/min) | 5.20 [4.60, 6.00] | 5.30 [4.58, 6.50] | 0.3 |
| Arterial pCO2 at transplant (mmHg) | 46.0 [41.0, 52.0] | 47.0 [41.5, 51.0] | 0.7 |
| Smoking history | 141 (53.6%) | 42 (44.2%) | 0.1 |
| Body mass index at transplant (kg/m 2 ) | 25.0 [22.1, 27.1] | 24.8 [20.1, 26.9] | 0.3 |
| Pre-transplant pulmonary rehabilitation | 258 (98.1%) | 90 (94.7%) | 0.09 |
| Pre-transplant pulmonary function | |||
| Forced expiratory volume in 1 second (% predicted) | 33.0 [22.0, 52.0] | 34.5 [22.0, 50.8] | 0.9 |
| Forced vital capacity (% predicted) | 47.0 [38.0, 59.0] | 47.0 [34.3, 60.8] | 0.8 |
Presented as median (interquartile range) for continuous variables and frequency (proportion) for categorical variables.
Among all LTx, median allograft IT was 7.22 hours (interquartile range 6.20–8.00; full range 3.90–20.0). Among long IT lungs, median IT was 8.70 hours versus 6.52 hours among standard IT lungs (p<0.01). The proportion of long IT LTx increased over time (Figure 1). Long IT LTx were more likely to be performed on cardiopulmonary bypass (CPB) than standard IT LTx (31.6% vs 17.1%, p<0.01). Long IT lungs were more likely to come from DCD donors than standard IT lungs (20.0% vs 5.3%, p<0.01) (Table 2).
Figure 1.
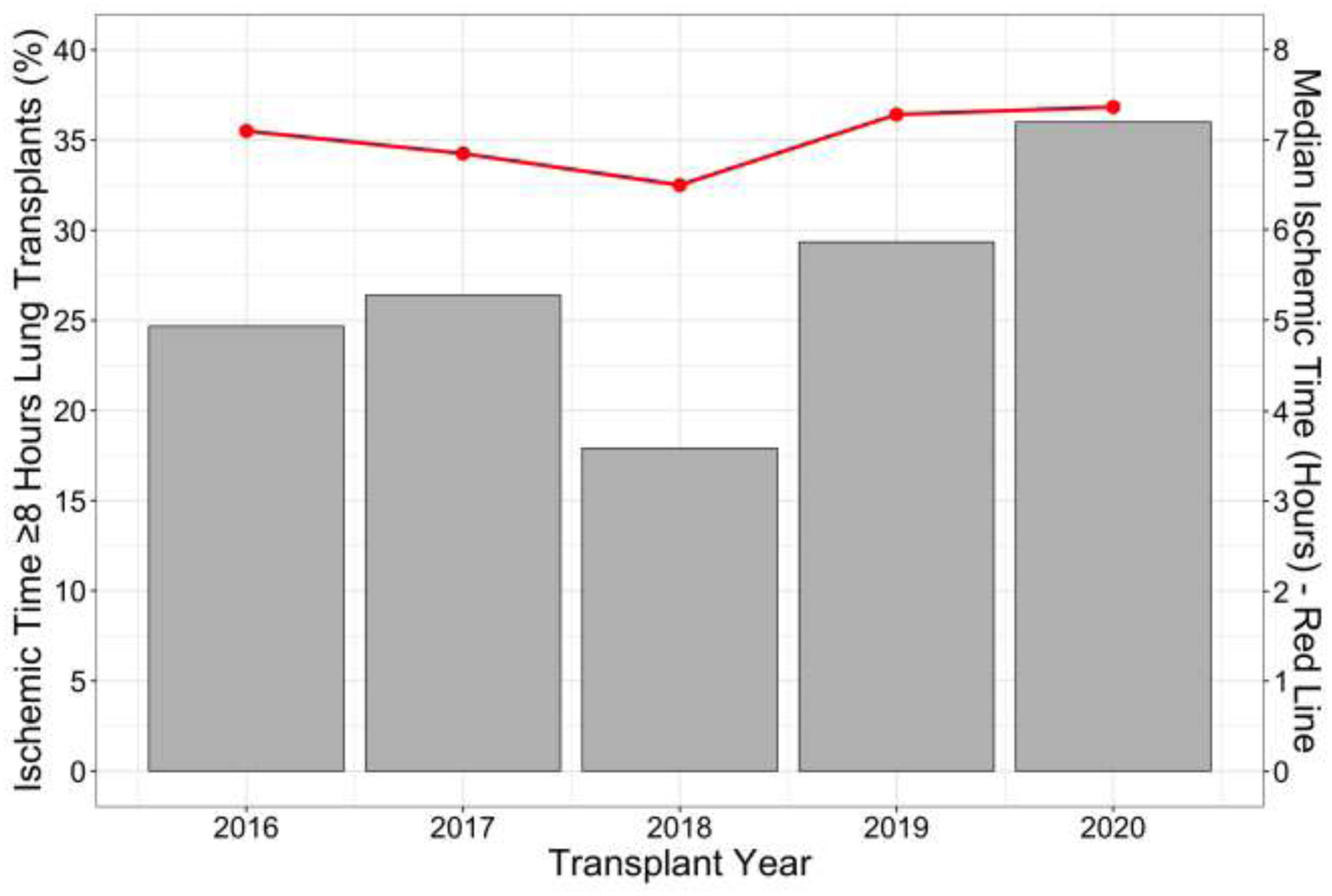
Trend in lung allograft ischemic time and proportion of ischemic time ≥8 hours lung transplants over the study period.
Table 2.
Operative characteristics
| Characteristic | Standard Ischemia (<8 Hours) N = 263 | Long Ischemia (≥8 Hours) N = 95 | P-value |
|---|---|---|---|
| Ischemic time (hours) a | |||
| First implanted lung | 5.08 [4.53, 5.72] | 7.02 [6.57, 7.58] | <0.01 |
| Second implanted lung | 6.52 [5.88, 7.26] | 8.70 [8.28, 9.30] | <0.01 |
| Cardiopulmonary bypass used | 45 (17.1%) | 30 (31.6%) | <0.01 |
| Bypass time (minutes) | 228 [176, 293] | 247 [169, 315] | 0.4 |
| Timing of cardiopulmonary bypass initiation b | 0.5 | ||
| Prior to allograft implantation | 40 (88.9%) | 25 (83.3%) | |
| After implantation of first lung | 5 (11.1%) | 5 (16.7%) | |
| Reason for cardiopulmonary bypass use b | 0.2 | ||
| Pulmonary hypertension | 16 (35.6%) | 14 (46.7%) | |
| Concurrent cardiac operation planned | 7 (15.6%) | 1 (3.3%) | |
| Hemodynamic instability prior to native lung explant | 8 (17.8%) | 2 (6.7%) | |
| Desaturation after implantation of first donor lung | 4 (8.9%) | 4 (13.3%) | |
| Other | 10 (22.2%) | 9 (30.0%) | |
| Donor type | <0.01 | ||
| Donation after brain death donor | 249 (94.7%) | 76 (80.0%) | |
| Donation after circulatory death donor | 14 (5.3%) | 19 (20.0%) | |
| Intraoperative transfusion requirement (units) | |||
| Packed red blood cells | 1.00 [0.00, 3.00] | 2.00 [0.00, 3.00] | 0.2 |
| Fresh frozen plasma | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.500] | 0.4 |
| Cryoprecipitate | 0.00 [0.00, 1.00] | 0.00 [0.00, 2.00] | 0.01 |
| Platelets | 0.00 [0.00, 0.00] | 0.00 [0.00, 1.00] | 0.08 |
| Donor/recipient weight ratio | 1.09 [0.909, 1.30] | 1.15 [0.988, 1.34] | 0.2 |
| Serologies | |||
| Donor Epstein-Barr virus positive | 245 (93.2%) | 81 (85.3%) | 0.04 |
| Donor cytomegalovirus positive | 163 (62.0%) | 56 (58.9%) | 0.6 |
| Recipient cytomegalovirus positive | 153 (58.2%) | 49 (51.6%) | 0.3 |
| Cytomegalovirus mismatch (D+/R−) | 66 (25.1%) | 25 (26.3%) | 0.8 |
Presented as median (interquartile range) for continuous variables and frequency (proportion) for categorical variables.
Standard versus long lung allograft ischemia was defined based on the total ischemic time of the second implanted lung.
Among patients who underwent lung transplantation on full cardiopulmonary bypass.
Compared to standard IT lungs, long IT lungs were procured from donors located farther from the transplant center (median 318 vs 212 nautical miles, p<0.01) (Figure S2). Additional donor characteristics were similar between groups (Table 3).
Table 3.
Donor characteristics
| Characteristic | Standard Ischemia (<8 Hours) N = 263 | Long Ischemia (≥8 Hours) N = 95 | P-value |
|---|---|---|---|
| Age (years) | 34.0 [27.0, 47.0] | 35.0 [25.0, 44.5] | 0.3 |
| PHS increased risk | 83 (31.6%) | 27 (28.4%) | 0.6 |
| Smoking history ≥20 pack-years | 22 (8.4%) | 8 (8.4%) | >0.9 |
| PaO2/FiO2 ratio | 432 [366, 495] | 422 [340, 489] | 0.3 |
| Cause of death | 0.7 | ||
| Anoxia | 92 (35.0%) | 28 (29.5%) | |
| Cerebrovascular accident/stroke | 73 (27.8%) | 31 (32.6%) | |
| Head trauma | 90 (34.2%) | 34 (35.8%) | |
| Other | 8 (3.0%) | 2 (2.1%) | |
| Mechanism of death | 0.09 | ||
| Asphyxiation | 5 (1.9%) | 6 (6.3%) | |
| Blunt injury | 53 (20.2%) | 19 (20.0%) | |
| Cardiovascular | 22 (8.4%) | 9 (9.5%) | |
| Drug intoxication | 62 (23.6%) | 10 (10.5%) | |
| Gunshot wound | 37 (14.1%) | 15 (15.8%) | |
| Intracranial hemorrhage/stroke | 75 (28.5%) | 31 (32.6%) | |
| Natural cause | 1 (0.4%) | 0 (0%) | |
| Seizure | 1 (0.4%) | 2 (2.1%) | |
| Other | 7 (2.7%) | 3 (3.2%) | |
| Blood infection | 25 (9.5%) | 10 (10.5%) | 0.8 |
| Pulmonary infection | 172 (65.4%) | 64 (67.4%) | 0.6 |
| Extended-criteria donor a | 155 (58.9%) | 60 (63.2%) | 0.5 |
| Distance from transplant center (nautical miles) | 212 [116, 415] | 318 [158, 498] | <0.01 |
Presented as median (interquartile range) for continuous variables and frequency (proportion) for categorical variables.
Extended-criteria donors were defined as those with any of the following characteristics: age>55 years, smoking history ≥20 pack-years, PaO2/FiO2 ratio ≤300, donation after circulatory death, or US Public Health Service increased risk for disease transmission classification.
Perioperative outcomes
Recipients of long and standard IT lungs had similar rates of PGD3 at 72 hours, post-transplant ECMO, reintubation, acute rejection within 30 days, and post-transplant LOS. Compared to standard IT recipients, long IT recipients were more likely to require reintervention within 30 days (31.6% vs 18.3%, p<0.01). There was a trend toward a higher rate of intubation >48 hours post-transplant among long IT recipients (30.5% vs 21.3%, p=0.07) (Table 4).
Table 4.
Perioperative outcomes
| Characteristic | Standard Ischemia (<8 Hours) N = 263 | Long Ischemia (≥8 Hours) N = 95 | P-value |
|---|---|---|---|
| Reintervention within 30 days | 48 (18.3%) | 30 (31.6%) | <0.01 |
| Surgical | 47 (17.9%) | 29 (30.5%) | |
| Re-exploration for bleedinga | 33 (70.2%) | 24 (82.8%) | |
| Re-exploration for infectiona | 4 (8.5%) | 2 (6.9%) | |
| Incision and drainagea | 4 (8.5%) | 1 (3.4%) | |
| Othera | 5 (10.6%) | 2 (6.9%) | |
| Bronchoscopic | 2 (0.8%) | 1 (1.1%) | |
| Radiologic | 2 (0.8%) | 2 (2.1%) | |
| Grade 3 primary graft dysfunction at 72 hours b | 38/262 (14.5%) | 16/92 (17.4%) | 0.5 |
| Ungradablec | 1/263 (0.4%) | 3/95 (3.2%) | |
| Post-operative extracorporeal membrane oxygenation | 28 (10.6%) | 16 (16.8%) | 0.1 |
| Postoperative date of initiation | 0.00 [0.00, 3.00] | 0.00 [0.00, 0.00] | 0.5 |
| Postoperative date of decannulation | 10.5 [4.75, 15.3] | 6.50 [4.00, 10.3] | 0.3 |
| Duration of support (days) | 8.00 [4.00, 13.0] | 5.50 [4.00, 8.50] | 0.4 |
| Prolonged continuation of support initiated intraoperativelyd | 19 (67.9%) | 13 (81.3%) | 0.3 |
| Extubated in >48 hours | 56 (21.3%) | 29 (30.5%) | 0.07 |
| Tracheostomy within 7 days | 47 (17.9%) | 23 (24.2%) | 0.2 |
| Reintubated during transplant hospitalization | 55 (20.9%) | 25 (26.3%) | 0.3 |
| Renal replacement therapy during transplant hospitalization | 33 (12.5%) | 10 (10.5%) | 0.6 |
| Intensive care unit readmission within 30 days | 36 (13.7%) | 11 (11.6%) | 0.6 |
| Hospital readmission within 30 days | 65 (24.7%) | 19 (20.0%) | 0.3 |
| Post-transplant length of stay (days) | 21.0 [15.0, 39.0] | 25.0 [17.5, 36.0] | 0.2 |
| Acute rejection within 30 days | 43 (16.3%) | 15 (15.8%) | 0.9 |
| Mortality within 90 days | 10 (3.8%) | 3 (3.2%) | >0.9 |
Presented as median (interquartile range) for continuous variables and frequency (proportion) for categorical variables.
Among patients who underwent surgical reintervention within 30 days.
A total of 354/358 patients were included in the analysis of grade 3 primary graft dysfunction at 72 hours, excluding 4 patients for whom primary graft dysfunction could not be graded (see footnote c). Patients who were on extracorporeal membrane oxygenation support and had radiographic evidence of pulmonary edema at 72 hours post-transplant were classified as having grade 3 primary graft dysfunction per International Society for Heart and Lung Transplantation guidelines.
In accordance with International Society for Heart and Lung Transplantation guidelines, patients who were on extracorporeal membrane oxygenation support and had no radiographic evidence of pulmonary edema at 72 hours post-transplant were designated as “ungradable” and excluded from the analysis of 72-hour primary graft dysfunction.
Among patients who required postoperative extracorporeal membrane oxygenation support.
In a subgroup analysis of long IT recipients, DBD versus DCD donor status did not impact overall, rejection-free, or CLAD-free survival up to one-year post-transplant (Figure S3). Recipients of DCD donor lungs were more likely to require ICU readmission within 30 days than recipients of DBD donor lungs (31.6% vs 6.6%, p<0.01) (Table S1).
Post-transplant survival
On unadjusted analysis, survival to death or graft failure was similar between long and standard IT groups (Figure 2A, log-rank p=0.99). Specifically, 1- and 3-year survival were approximately 87.9% (95% confidence interval [CI] 81.0–95.4%) and 77.1% (95% CI 66.7–89.1%), respectively, among recipients of long IT lungs compared to 89.6% (95% CI 85.8–93.5%) and 76.1% (95% CI 70.0–82.7%), respectively, among recipients of standard IT lungs. Rejection-free and CLAD-free survival were similar between groups (Figures 2B and 2C). After adjustment for donor and recipient factors, long IT was not independently associated with increased risk of mortality or graft failure compared to standard IT (hazard ratio 0.83, 95% CI 0.46–1.51, p=0.5) (Table S2).
Figure 2. Kaplan-Meier survival analysis stratified by lung allograft ischemic time <8 hours versus ≥8 hours.
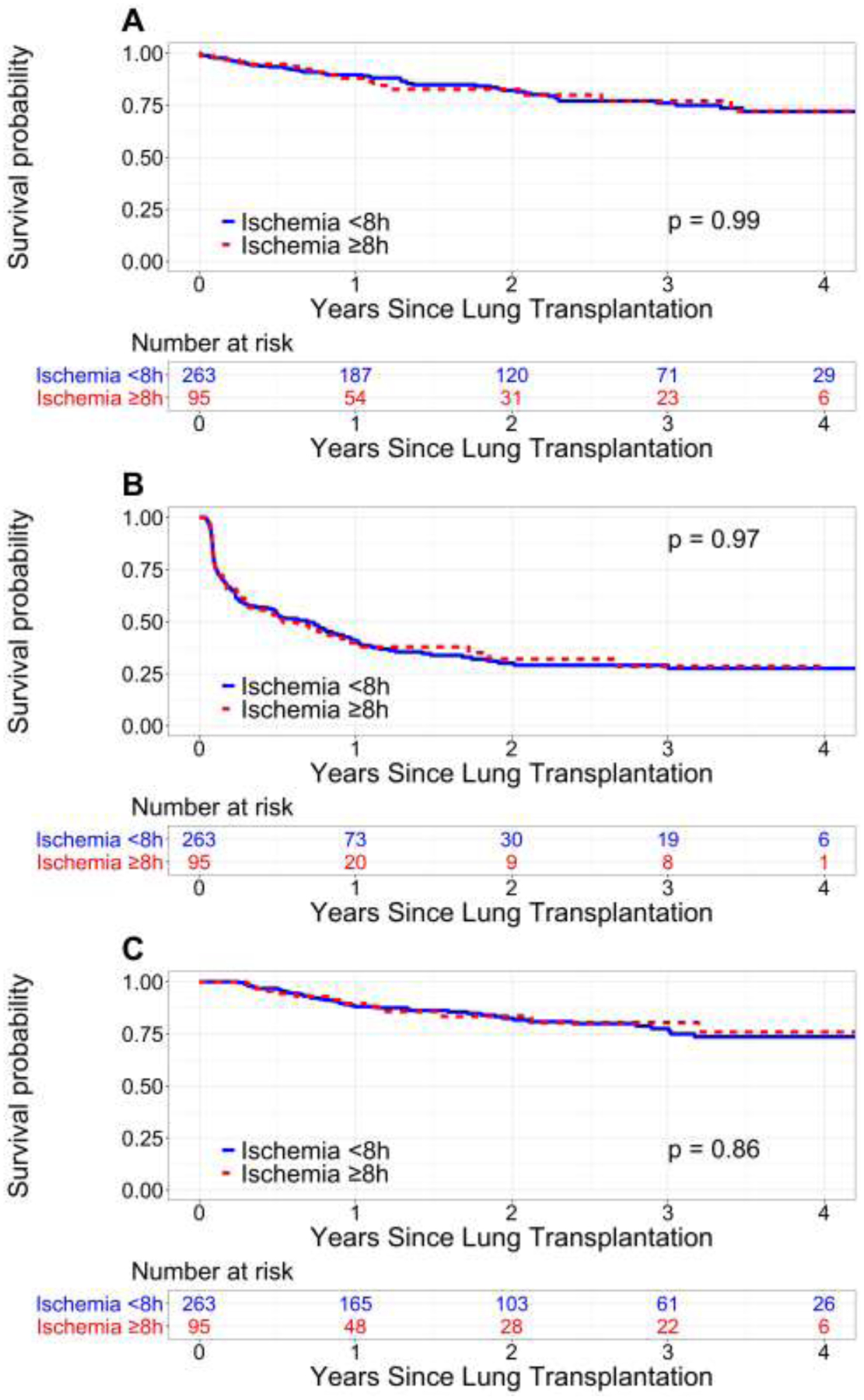
(A) Patient or graft survival. (B) Rejection-free survival. (C) Chronic lung allograft dysfunction-free survival.
Sensitivity analyses
In our sensitivity analysis, linear modeling of IT versus log hazard of death or graft failure yielded a slightly better model fit than use of restricted cubic splines (Figure 3). No violation of the proportional hazard assumption was noted for either model. The risk of death or graft failure was approximately constant for ITs up to 20 hours; conclusions were similar with use of restricted cubic splines (data not shown). When IT was categorized into increments above 6 hours, there was no difference in overall survival among recipients of lungs with IT <6 hours, 6–8 hours, 8–10 hours, and >10 hours (Figure 4).
Figure 3. Logarithm of the hazard ratio of death or graft failure according to lung allograft ischemic time.
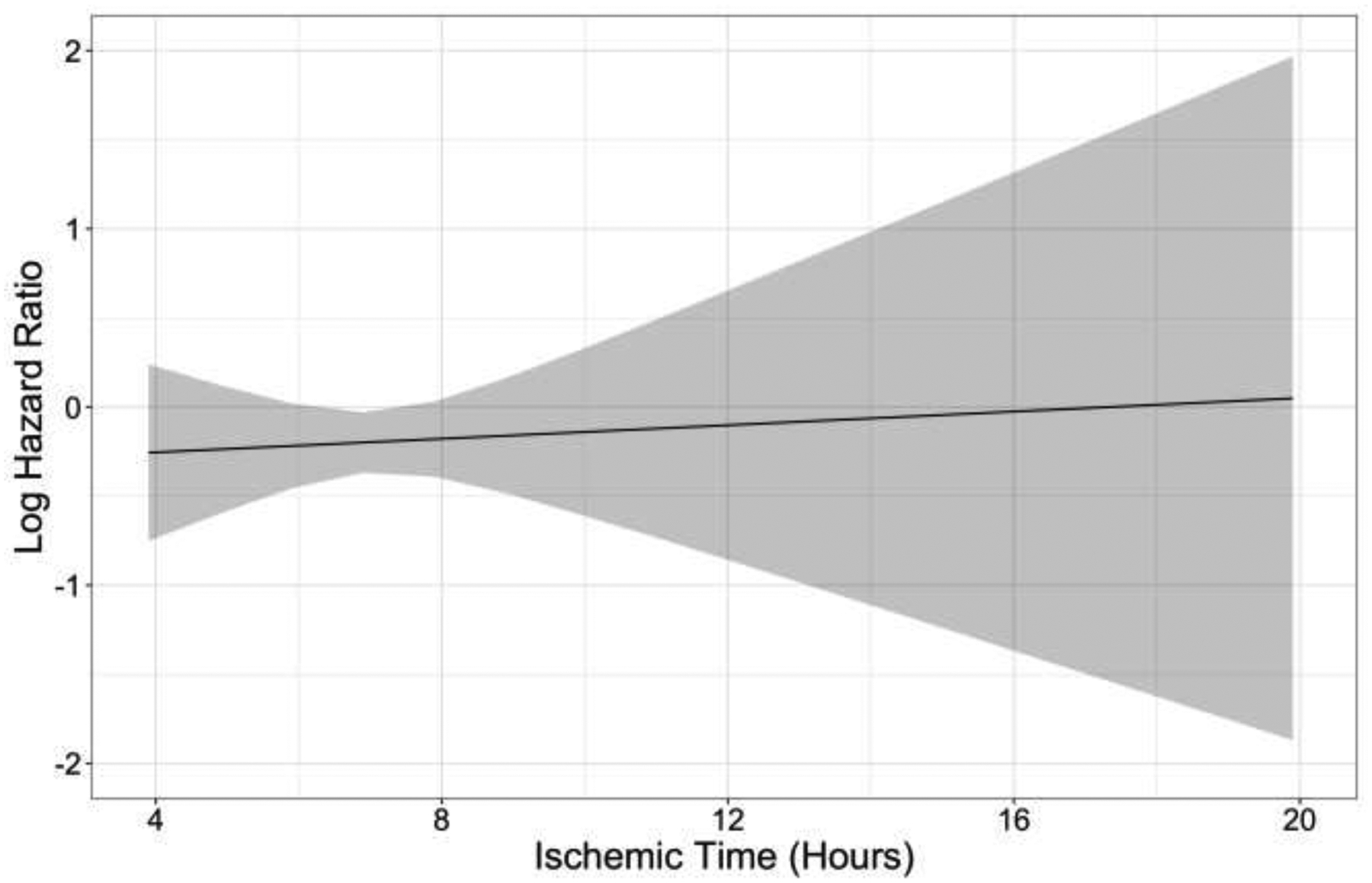
Estimation was performed using a linear model (solid line) with a 95% confidence interval (gray area).
Figure 4.
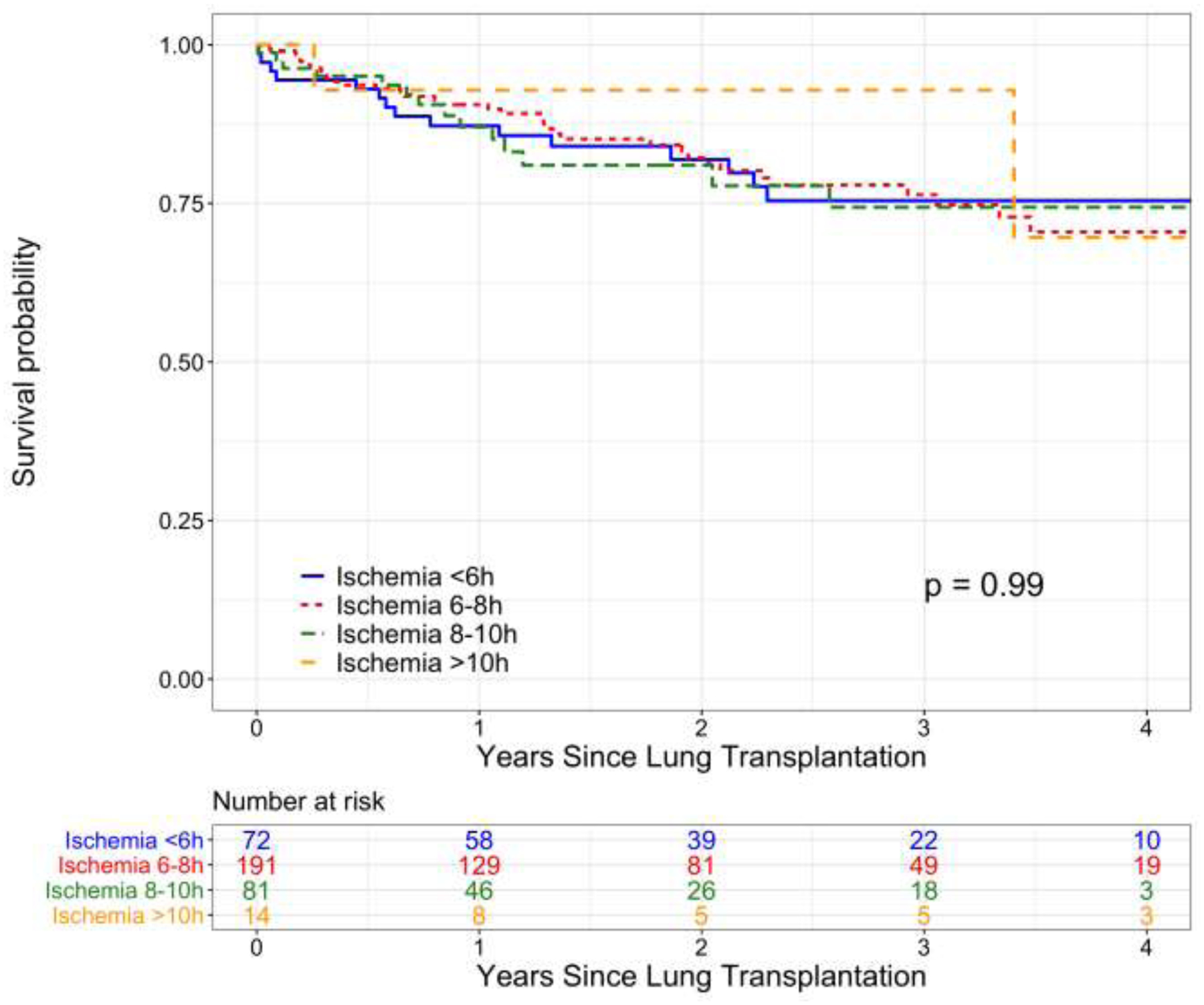
Kaplan-Meier survival analysis of survival to death or graft failure stratified by lung allograft ischemic time <6 hours, 6–8 hours, 8–10 hours, or >10 hours.
DISCUSSION
For over 20 years, six hours was regarded as the upper limit of acceptable lung IT.5–8 However, broad and equitable allocation frequently necessitates use of allografts with ITs exceeding this threshold.14,15 In this study, we characterized our contemporary experience using lungs with long IT (≥8 hours). We found that long ITs were associated with similar perioperative outcomes and post-LTx survival compared to standard ITs. These findings suggest that routine use of lungs with prolonged ITs may support acceptable outcomes, and facilitate broader use.
Concern for increased risk of ischemia-reperfusion injury represents a central deterrent to using lungs with IT >6 hours.5,6 Physiologically, ischemia is associated with oxidative stress, disruption of cellular homeostasis, cell death, and inflammation, all of which contribute to allograft injury.20–22 Nonetheless, the literature remains divided regarding the relationship between IT and risk of PGD, the primary clinical manifestation of lung ischemia-reperfusion injury.21,23,24 While earlier work suggests that increasing IT may be an independent risk factor for PGD and poor oxygenation early post-LTx,6,23 more recent studies found no association between prolonged ischemia and PGD3 at 48 or 72 hours or primary graft failure.10,24 In our study, rates of PGD3 at 72 hours were similar among standard and long IT recipients. Rates of post-transplant ECMO, reintubation, and tracheostomy were also similar between groups, providing further evidence to support comparable early allograft function regardless of IT more or less than 8 hours. Long IT recipients were more likely to require reoperation for postoperative bleeding, likely due to increased use of CPB in this group25; however, CPB was infrequently used for post-implantation desaturations, further highlighting acceptable allograft oxygenation despite long IT. Prevention of PGD3 is essential due to its association with post-transplant morbidity and mortality, including prolonged mechanical ventilation, LOS, and CLAD.17,21,24,26 Our findings suggest that use of lungs with long IT offers acceptable early outcomes, without significantly increasing the risk of severe PGD.
Acceptable long-term outcomes are also necessary to support broader use of lungs with IT >6 hours. Several studies found an association between IT >6 hours and compromised post-LTx survival,6,27 while others found no difference in survival among recipients of allografts with ITs above and below this threshold.5,8 Recently, large registry studies found similar long-term survival with IT ≥6 hours and <6 hours, despite differential survival at 30 days.10,11 In our study, use of long IT lungs was not associated with compromised overall, rejection-free, or CLAD-free survival up to 4 years post-transplant. While ISHLT data also suggest that CLAD incidence is unaffected by prolonged allograft ischemia,11 the relationship between IT and acute rejection remains unclear. While available evidence supports potentially increased risk of acute rejection with prolonged ischemia due to allograft immunogenicity resulting from ischemia-reperfusion injury,11,20,22 our findings suggest that prolonged ischemia beyond 6 hours does not increase the incidence of acute rejection up to 4 years post-transplant. Our findings suggest that use of long IT lungs offers acceptable long-term safety; however, further study is warranted to clarify what duration of IT begins to affect post-LTx outcomes.
There is historical reluctance to extend lung IT beyond 6 hours. In the late 1980s, median IT was approximately 4 hours, and fewer than 10% exceeded 6 hours; in 2015, however, median IT was over 5 hours, with nearly 30% of those exceeding 6 hours.11 These data suggest that a six-hour threshold may already be obsolete, despite a paucity of strong evidence examining the safety of using lungs with ITs above this cutoff. At our institution, median IT for bilateral LTx performed between January 2016 and May 2020 was 7.22 hours overall, and 6.52 hours among lungs with standard IT (<8 hours). While willingness to tolerate longer durations of ischemia may vary among LTx centers,28 our findings support the recent published trend,11 in which use of lungs with ITs >6 hours may reflect routine practice in the modern era.
In modern cohorts, EVLP offers an alternative to cold storage with accumulating evidence to support similar outcomes between these preservation strategies.29–32 In particular, portable EVLP devices may facilitate extended lung preservation without prolonged cold ischemia.33 EVLP has been used in both standard and “marginal” lung allografts, including those from DCD donors.29,32,33 Increasing data suggests that LTx outcomes may be unaffected by DCD versus DBD donor status, with or without EVLP.34,35 Nevertheless, prolonged cold ischemia may differentially affect DCD and DBD donor lungs, potentially increasing risk of mortality after DCD LTx.36 In our study, a higher proportion of DCD donor lungs had long versus standard ITs; however, among long IT recipients, short-term outcomes were similar between DCD and DBD donor groups. Despite a higher rate of ICU readmissions among recipients of long IT DCD versus DBD donor lungs, this corresponded to an absolute difference of one patient (6 DCD versus 5 DBD), likely representing statistical artifact due to a small denominator (N=19) in the DCD subgroup. As EVLP allografts were excluded from our study, our findings suggest that neither DCD alone nor DCD with prolonged ischemia necessarily represent indications for EVLP. Since EVLP is not uniformly available across institutions and is more resource intensive than cold storage, our findings may facilitate broader use of DCD donor lungs. However, further investigation is required to understand how use of DCD donor lungs with prolonged ITs affects long-term outcomes across institutions, and when EVLP may be more beneficial for these grafts.
To our knowledge, ours is the first study to examine the relationship between longer ITs (≥8 hours) and post-LTx outcomes. In combination with limited evidence supporting acceptable short-term survival with 10 and 12 hours of ischemia,10 our findings suggest that the limit of tolerable lung IT remains to be determined. While Thabut and colleagues demonstrated an exponential relationship between IT and risk of mortality,6 our data suggests that in a modern cohort with standardized preservation techniques, the risk of mortality or graft failure remains approximately constant with ITs measured up to 20 hours. Despite this encouraging trend, only 14 (3.9%) allografts in our study had IT >10 hours, limiting our ability to evaluate the relationship between ITs >10 hours and risk of mortality or graft failure. Therefore, the approximately constant risk of mortality or graft failure for ITs up to 20 hours should be interpreted with caution, as very few cases are represented.28 Rather, the range of ITs in our study may represent proof of concept that lung ITs may be extended beyond historical limits, presenting an opportunity to extend provision of LTx to a greater proportion of waitlisted patients.
There are several limitations to our study. As we examined a series of LTx performed at a single, high-volume institution, our experience may not generalize to other programs. However, use of institutional data allowed for granular examination of perioperative outcomes that is not possible with national databases. At our institution, patients who cannot be weaned from the ventilator post-transplant undergo early tracheostomy placement. While tracheostomy within 7 days was included to capture perioperative complications within our practice, an alternative outcome such as tracheostomy prior to discharge may elucidate differences between standard and long IT recipients, and better align with LTx practices more broadly. Additionally, we excluded bridged and redo LTx cases to limit confounding of long ITs by increased operative complexity and duration; this may further limit generalizability of our findings, and further study is required to understand appropriate use of long IT lungs for these patient subgroups. As recent data to inform the safety of using lungs with IT >6 hours remains sparse, our findings may nonetheless provide helpful new evidence to support provisions for smarter lung sharing without emphasizing ITs as a barrier to organ acceptance. Our 8-hour definition of “long ischemia” was based on clinical judgement and experience among LTx surgeons at our institution and may not reflect broad consensus regarding standard versus prolonged ITs. In our sensitivity analysis modeling IT as a continuous variable, we did not identify an inflection point in risk of death or graft failure that may have been better suited to our data. Given the small number of patients with IT >10 hours and the limited duration of post-transplant follow-up in our cohort, we may have been underpowered to observe such a change in trajectory; however, contrast between our findings and those of Thabut and colleagues,6 despite similarly-sized patient cohorts, suggest that contemporary LTx patients no longer experience a dramatic increase in risk of death with increasing IT. Further investigation in a national cohort with longer follow-up duration is warranted to better understand the current relationship between IT and risk of adverse post-transplant outcomes. Finally, the modest number of long IT LTx in our study likely limited our power to detect between-group differences in binary variables, necessitating critical evaluation of all relationships regardless of statistical significance.
CONCLUSIONS
In this single-center analysis, we found that use of lungs with IT ≥8 hours and <8 hours were associated with similar perioperative outcomes and survival up to 4 years post-transplant. Amidst a trend of increasing ITs in an era of broader lung sharing, our findings provide promising new evidence to support the safety of using lungs with long ITs in routine practice. Further investigation in a national cohort is warranted to better understand how broader use of these allografts impacts post-LTx survival and implications for an evolving national allocation policy.
Supplementary Material
ACKNOWLEDGMENTS
SEH is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR002555. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- CI
confidence interval
- CLAD
chronic lung allograft dysfunction
- CPB
cardiopulmonary bypass
- DBD
donation after brain death
- DCD
donation after circulatory death
- DSA
donation service area
- ECMO
extracorporeal membrane oxygenation
- EVLP
ex-vivo lung perfusion
- ICU
intensive care unit
- ISHLT
International Society for Heart and Lung Transplantation
- IT
ischemic time
- LOS
length of stay
- LTx
lung transplantation
- PGD3
grade 3 primary graft dysfunction
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
The authors report no conflicts of interest.
REFERENCES
- 1.Vock DM, Durheim MT, Tsuang WM, et al. Survival Benefit of Lung Transplantation in the Modern Era of Lung Allocation. Ann Am Thorac Soc. 2017;14(2):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thabut G Estimating the Survival Benefit of Lung Transplantation: Considering the Disease Course during the Wait. Ann Am Thorac Soc. 2017;14(2):163–164. [DOI] [PubMed] [Google Scholar]
- 3.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Lung. Am J Transplant. 2020;20(s1):427–508. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services. Organ Procurement and Transplantation Network Final Rule. Fed Regist. 1999;64(202):56650–56661. [PubMed] [Google Scholar]
- 5.Fiser SM, Kron IL, Long SM, et al. Influence of Graft Ischemic Time on Outcomes Following Lung Transplantation. J Hear Lung Transplant. 2001;20(12):1291–1296. [DOI] [PubMed] [Google Scholar]
- 6.Thabut G, Mal H, Cerrina J, et al. Graft Ischemic Time and Outcome of Lung Transplantation: A Multicenter Analysis. Am J Respir Crit Care Med. 2005;171:786–791. [DOI] [PubMed] [Google Scholar]
- 7.Kirk AJB, Colquhoun IW, Dark JH. Lung Preservation: A Review of Current Practice and Future Directions. Ann Thorac Surg. 1993;56(4):990–1000. [DOI] [PubMed] [Google Scholar]
- 8.Gammie JS, Stukus DR, Pham SM, et al. Effect of Ischemic Time on Survival in Clinical Lung Transplantation. Ann Thorac Surg. 1999;68:2015–2020. [DOI] [PubMed] [Google Scholar]
- 9.Snell GI, Rabinov M, Griffiths A, et al. Pulmonary allograft ischemic time: an important predictor of survival after lung transplantation. J Hear Lung Transplant. 1996;15(2):160–168. [PubMed] [Google Scholar]
- 10.Grimm JC, Valero III V, Kilic A, et al. Association Between Prolonged Graft Ischemia and Primary Graft Failure or Survival Following Lung Transplantation. JAMA Surg. 2015;21287(6):547–553. [DOI] [PubMed] [Google Scholar]
- 11.Chambers DC, Yusen RD, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report — 2017; Focus Theme: Allograft ischemic time. J Hear Lung Transplant. 2017;36(10):1047–1059. [DOI] [PubMed] [Google Scholar]
- 12.Chambers DC, Zuckermann A, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th adult lung transplantation report — 2020; focus on deceased donor characteristics. J Hear Lung Transplant. 2020;39(10):1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.OPTN/UNOS Executive Committee. Broader Sharing of Adult Donor Lungs. Published 2017. https://optn.transplant.hrsa.gov/media/2314/broader_sharing_lungs_20171124.pdf
- 14.Puri V, Hachem RR, Frye CC, et al. Unintended consequences of changes to lung allocation policy. Am J Transplant. 2018;19:2164–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goff RR, Wilk A. Monitoring of the Lung Allocation Change, 2 Year Report; Removal of DSA as a Unit of Allocation. Published 2020. https://optn.transplant.hrsa.gov/media/3661/item_25_thoracic_committee_20200212.pdf
- 16.Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment―A consensus report from the Pulmonary Council of the ISHLT. J Hear Lung Transplant. 2019;38(5):493–503. [DOI] [PubMed] [Google Scholar]
- 17.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading—A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Hear Lung Transplant. 2017;36(10):1097–1103. [DOI] [PubMed] [Google Scholar]
- 18.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection. J Hear Lung Transplant. 2007;26(12):1229–1242. [DOI] [PubMed] [Google Scholar]
- 19.SRTR Risk Adjustment Model Documentation: Posttransplant Outcomes. Published 2020. https://www.srtr.org/reports-tools/posttransplant-outcomes/
- 20.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia – Reperfusion – induced Lung Injury. Am J Respir Crit Care Med. 2003;167:490–511. [DOI] [PubMed] [Google Scholar]
- 21.Porteous MK, Diamond JM, Christie JD. Primary graft dysfunction: lessons learned about the first 72 hours after lung transplantation. Curr Opin Organ Transplant. 2015;20(5):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christie JD. Lung Allograft Ischemic Time: Crossing the Threshold. Am J Respir Crit Care Med. 2005;171:2004–2005. [DOI] [PubMed] [Google Scholar]
- 23.Kuntz CL, Hadjiliadis D, Ahya VN, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant. 2009;23:819–830. [DOI] [PubMed] [Google Scholar]
- 24.Diamond JM, Lee JC, Kawut SM, et al. Clinical Risk Factors for Primary Graft Dysfunction after Lung Transplantation. Am J Respir Crit Care Med. 2013;187(5):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ius F, Kuehn C, Tudorache I, et al. Lung transplantation on cardiopulmonary support: Venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2012;144(6):1510–1516. [DOI] [PubMed] [Google Scholar]
- 26.Diamond JM, Arcasoy S, Kennedy CC, et al. Report of the International Society for Heart and Lung Transplantation Working Group on Primary Lung Graft Dysfunction, part II: Epidemiology, risk factors, and outcomes — A 2016 Consensus Group statement of the International Society for Heart and Lung Tr. J Hear Lung Transplant. 2017;36(10):1104–1113. [DOI] [PubMed] [Google Scholar]
- 27.Ghaidan H, Fakhro M, Lindstedt S. Impact of allograft ischemic time on long-term survival in lung transplantation: a Swedish monocentric study. Scand Cardiovasc J. 2020;54(5):322–329. [DOI] [PubMed] [Google Scholar]
- 28.Hayes D Jr, Hartwig MG, Tobias JD, Tumin D. Lung Transplant Center Volume Ameliorates Adverse Influence of Prolonged Ischemic Time on Mortality. Am J Transplant. 2017;17:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeung JC, Krueger T, Yasufuku K, et al. Outcomes after transplantation of lungs preserved for more than 12 h: a retrospective study. Lancet Respir Med. 2017;5(2):119–124. [DOI] [PubMed] [Google Scholar]
- 30.Jawitz OK, Raman V, Becerra D, et al. Lung Transplantation After Ex Vivo Lung Perfusion: Early Outcomes From a US National Registry. Ann Surg. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Divithotawela C, Cypel M, Martinu T, et al. Long-term Outcomes of Lung Transplant With Ex Vivo Lung Perfusion. JAMA Surg. 2020;154(12):1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cypel M, Yeung JC, Liu M, et al. Normothermic Ex Vivo Lung Perfusion in Clinical Lung Transplantation. N Engl J Med. 2011;364(15):1431–1440. [DOI] [PubMed] [Google Scholar]
- 33.Loor G, Warnecke G, Villavicencio MA, et al. Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND): a single-arm, pivotal trial. Lancet Respir. 2019;7(11):975–984. [DOI] [PubMed] [Google Scholar]
- 34.van Suylen V, Luijk B, Hoek RAS, et al. A Multicenter Study on Long-Term Outcomes After Lung Transplantation Comparing Donation After Circulatory Death and Donation After Brain Death. Am J Transplant. 2017;17(10):2679–2686. [DOI] [PubMed] [Google Scholar]
- 35.Cypel M, Levvey B, Raemdonck D Van, et al. International Society for Heart and Lung Transplantation Donation After Circulatory Death Registry Report. J Hear Lung Transplant. 2015;34(10):1278–1282. [DOI] [PubMed] [Google Scholar]
- 36.Puri V, Scavuzzo M, Guthrie T, et al. Lung Transplantation and Donation After Cardiac Death: A Single Center Experience. Ann Thorac Surg. 2009;88(5):1609–1615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


