Abstract
Brown adipose tissue (BAT) plays a key role in energy expenditure through its thermogenic function, making its activation a popular target to reduce obesity. We recently reported that male mice housed at thermoneutrality with uncoupling protein 1 (UCP1) deficiency had increased weight gain and glucose intolerance, but eicosapentaenoic acid (EPA) ameliorated these effects. Whether female mice respond similarly to lack of UCP1 and to EPA remains unknown. We hypothesize that the effects of EPA on BAT activation are independent of UCP1 expression. We used female wild type (WT) and UCP1 knockout (KO) mice housed at thermoneutrality (30°C) as an obesogenic environment and fed them high fat (HF) diets with or without EPA for up to 14 weeks. Body weight (BW), body composition, and insulin and glucose tolerance tests were performed during the feeding trial. At termination, serum and BAT were harvested for further analyses. Mice in the KO-EPA group had significantly lower BW than KO-HF mice. In addition, KO-HF mice displayed significantly impaired glucose tolerance compared to their WT-HF littermates. However, EPA significantly enhanced glucose clearance in the KO mice compared to KO-HF mice. Protein levels of the mitochondrial cytochrome C oxidase subunits I, II, and IV were significantly lower in KO mice compared to WT. Our findings support that ablation of UCP1 is detrimental to energy metabolism of female mice in thermoneutral conditions. However, unexpectedly, EPA’s protective effects against diet-induced obesity and glucose intolerance in these mice were independent of UCP1.
Keywords: Brown adipose tissue, Eicosapentaenoic acid, Female mice, Thermoneutrality, Obesity, Uncoupling protein 1
1. Introduction
Obesity is a growing public health threat afflicting over 40% of US adults. It is linked to several comorbidities including type II diabetes, cardiovascular disease, and even some cancers [1–3]. Traditional anti-obesity strategies include dietary modification, increased physical activity, reinforcements through behavioral therapy [4], and in cases of severe obesity, medication or bariatric surgery are recommended [5, 6]. Increasingly, dietary supplements and bioactive foods are used to prevent or treat obesity [7, 8].
The unique thermogenic properties of brown adipose tissue (BAT) make its activation an intriguing target for anti-obesity research, especially in light of the discovery of functional BAT in adults [9]. The inner mitochondrial membrane of brown adipocytes contains uncoupling protein 1 (UCP1), a protein that uncouples oxidative phosphorylation from the synthesis of ATP by leaking protons across the mitochondrial membrane against their gradient [10]. This process, known as non-shivering thermogenesis, results in the production of heat and dispersion of energy, where UCP1 plays a critical role [11].
One method of activating non-shivering thermogenesis in BAT is chronic cold exposure, which stimulates norepinephrine release to activate the sympathetic nervous system [12]. Upon exposure to low temperature, cutaneous thermoreceptors such as transient receptor potential cation channel subfamily M member 8 (TRPM8) convey the sensation of cold to the hypothalamus [13]. Norepinephrine then transmits the efferent signal to BAT, activating β3-adrenergic receptors and inducing lipolysis to release free fatty acids [14]. Finally, the mitochondrial electron transport chain is activated and respiration is uncoupled via UCP1 [12, 14].
In addition to cold temperature, activators of BAT thermogenesis include exercise and certain bioactive compounds [12]. We are specifically interested in omega-3 polyunsaturated fatty acids (ω−3 PUFA), which include both docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). EPA is associated with metabolic improvements including anti-inflammatory, cardioprotective, and anti-obesity effects in rodents as well as triglyceride-lowering benefits in humans [15–18]. Previous studies from our laboratory found that EPA supplemented along with high fat (HF) diet increased UCP1 and other thermogenic markers in BAT of diet-induced obese male mice compared to mice fed HF housed in an ambient environment [3].
Considering the mechanism of BAT activation via cold temperature, an important consideration when designing studies related to obesity, and especially BAT, is the temperature at which animals are housed. For mice, thermoneutral state is reached at approximately 28–30°C [19]. However, traditionally, studies using mouse models have housed their animals at ambient temperature (22–25°C), a condition that subjects mice to chronic thermal stress and forces them to increase their metabolism in order to compensate [20]. We recently reported that EPA protected against obesity and insulin resistance independently of UCP1 in male mice at thermoneutrality [21]. Further, sexual dimorphism exists for the thermogenic role of BAT as well as diet-specific impact exists; hence, we tested whether female mice respond similarly to male mice [22]. Moreover, the mechanisms by which EPA activates BAT, including interactions with UCP1, remain incompletely understood. Herein, we evaluated the effects of EPA and UCP1 ablation on BAT in female mice living at thermoneutrality to test the hypothesis that beneficial effects of EPA on energy metabolism in female mice are independent of UCP1.
2. Materials and Methods
2.1. Genotyping
The Jackson Laboratory (Bar Harbor, ME) supplied heterozygous (HET) UCP1 C57BL/6J mice which were bred to yield homozygous WT and UCP1 knockout (KO) offspring. WT and UCP1 mutant primers were used to identify WT, KO, and HET genotypes. For the WT genotype, forward and reverse primer sequences were TCGTCATCAATAAGGGGAAAC and CTTCTTCCCTGATGCTCCAT respectively. For the KO genotype, GGTGTTTGGAGCCTGCATTGC and CTTCCTGACTAGGGGAGGAGT were used for forward and reverse sequences, respectively. DNA was isolated from the tail to be used for PCR with alkaline lysis protocol. Finally, agarose gel electrophoresis was performed to identify genotypes as described in a study by Pahlavani et al [21].
2.2. Animals and Diets
All procedures were approved by the Institutional Animal Care and Use Committee of Texas Tech University. Female WT and KO mice were housed at 30°C. Mice aged 5–6 weeks were randomly assigned to a HF diet (45% fat, 20% protein, 35% carbohydrates) with or without EPA-enriched fish oil (36 g/kg) provided by Organic Technologies (Coshocton, OH) with 10–15 animals per group. The diet used in this study is identical to our prior work [17, 21]. Mice were single-housed with 12-hour light/dark cycle and ad libitum access to food and water. Body weight was recorded once a week and food intake was calculated twice weekly for 14 weeks. Following 14 weeks on the diet, mice were sacrificed after a five-hour fasting period. Tissues including interscapular BAT were harvested, snap-frozen in liquid nitrogen, and stored at −80°C or fixed in Z-Fix, a histological fixative composed of 10% aqueous buffered zinc formalin (Anatech Ltd., Battle Creek, MI) for future analyses.
2.3. Body Composition Analysis:
We evaluated body composition (fat and lean mass) using an Echo-MRI 3–in-1 (EchoMRI LLC, Houston, TX) at 11 weeks of age.
2.4. Energy Expenditure:
We used a metabolic monitoring system (Omnitech, Columbus, OH) to evaluate total energy expenditure over 48 hours during 12:12 hour light and dark cycles. Mice had ad libitum access to food and water and were acclimated for three days prior to the measurements. The metabolic cages were housed in a room which was maintained at 28–30°C. Oxygen consumption was calculated in absolute terms relative to body weight. The VO2 were measured for 48 hours and presented as the mean VO2 over the 48-hour period.
2.5. Glucose and Insulin Tolerance Tests
Glucose tolerance test (GTT) was performed on mice at 10 weeks of age, and insulin tolerance test (ITT) was measured for 12-week-old mice. Following a 4–6-hour fasting period, blood was taken from the tail vein and glucose levels were measured using OneTouch Ultra Glucose Meter (AlphaTrak, North Chicago, IL). During GTT, mice received an intraperitoneal glucose injection (2 g/kg body weight) and then blood glucose levels were measured at 30, 60, 90, and 120 minutes following the injection. For ITT, the animals were injected with 1 U/kg body weight insulin before recording blood glucose levels at 15, 30, 45, 60, and 90 minutes. The glucose area under the curve (AUC) was calculated from baseline [23].
2.6. RNA Isolation and Quantitative Real-Time PCR
BAT samples were homogenized in QIAzol solution using a tissue homogenizer (TissueLyser LT, Qiagen, Valencia, CA), and total RNA was isolated from adipose tissue using an RNA isolation kit (Zymo Research, Irvine, CA). RNA concentration was evaluated with a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The Maxima kit (Thermo Fisher Scientific, Waltham, MA) was used to synthesize first-strand cDNA, and gene expression was performed through quantitative polymerase chain reaction (qPCR) using a thermocycler (Thermo Fisher Scientific, Waltham, MA). Primers were designed online using OligoArchitect Online (Supplementary Table 1) and purchased from Sigma-Aldrich (St. Louis, MO).
2.7. Western Blotting
Protein was extracted from BAT by lysing in modified RIPA buffer and then subjected to electrophoresis on gradient gels (Bio-Rad, Hercules, CA). Protein was then transferred to polyvinylidene fluoride membrane (Sigma-Aldrich, St louis, MO). Membranes were incubated in blocking buffer for 1 hour at room temperature and then incubated with primary antibodies for UCP1 (Thermo Fisher Scientific, Waltham, MA, # PA1–24894, mouse, 1:1000), PGC1α (Abcam, Cambridge, MA, # ab54481, mouse, 1:1000), and total OXPHOS cocktail, which detects five subunits of the individual mitochondrial OXPHOS complexes (CI, CII, CIII, CIV and CV) (Abcam, Cambridge, MA, # 110413, mouse, 1:250) overnight. After incubation with the respective secondary antibody (rabbit polyclonal and mouse polyclonal, 1:20.000), the blots were imaged using the LI-COR Imaging System (Lincoln, NE).
2.8. Mitochondrial Mass Quantification (Mitochondrial DNA)
We isolated DNA from BAT using a mammalian genomic DNA kit (Sigma-Aldrich, St. Louis, MO). Cytochrome b (Cytb), mitochondrially-encoded NADH: ubiquinone oxidoreductase core subunit 1 (Nd1), and Nd2 were used as the mtDNA primers; UCP1 and peroxisome proliferator activated receptor gamma (PPARG) were used as the genomic DNA primers. Primer sequences are presented in Supplementary Table 2. We determined the mitochondrial/nucleic DNA (mtDNA/nucDNA) ratio for each primer pair using 2×2(CTnucDNA-CTmtDNA).
2.9. Statistical Analyses
Analyses included one-way ANOVA for multiple comparisons and two-way ANOVA to measure diet X genotype interaction. For all statistical analyses, differences were considered significant at p < 0.05. All statistical tests were performed with GraphPad Prism 8 (GraphPad Software, La Jolla, CA) and data are presented as mean ± SEM.
3. Results
3.1. Effects of UCP1 Deficiency and EPA on Food Intake, Body Weight, and Body Composition
Food intake was comparable among all groups (Fig. 1A). KO-HF mice had significantly higher body weight compared to all other groups (p < 0.05; Fig. 1B), although we found no differences between either WT group or between groups fed HF. KO mice fed EPA exhibited significantly reduced final body weight compared to KO-HF mice, but final body weight did not differ between the groups (p < 0.05; Fig. 2A).
Figure 1.
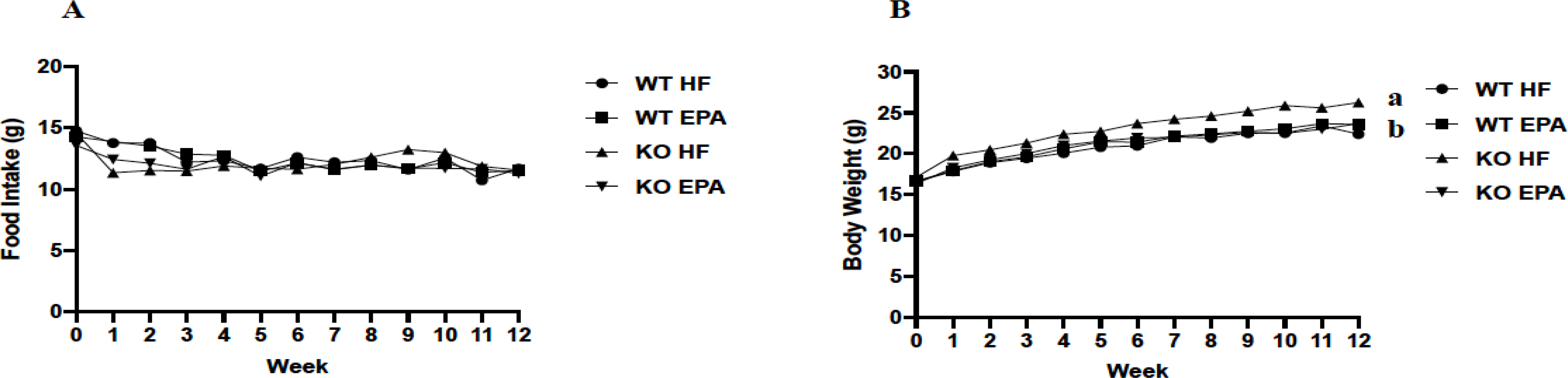
Weekly food intake (A) and weekly body weight (B) of female WT and UCP1 KO mice. A shared letter indicates no difference. p < 0.05; n = 12 WT HF, 11 WT EPA, 15 KO HF, and 15 KO EPA mice.
Figure 2.
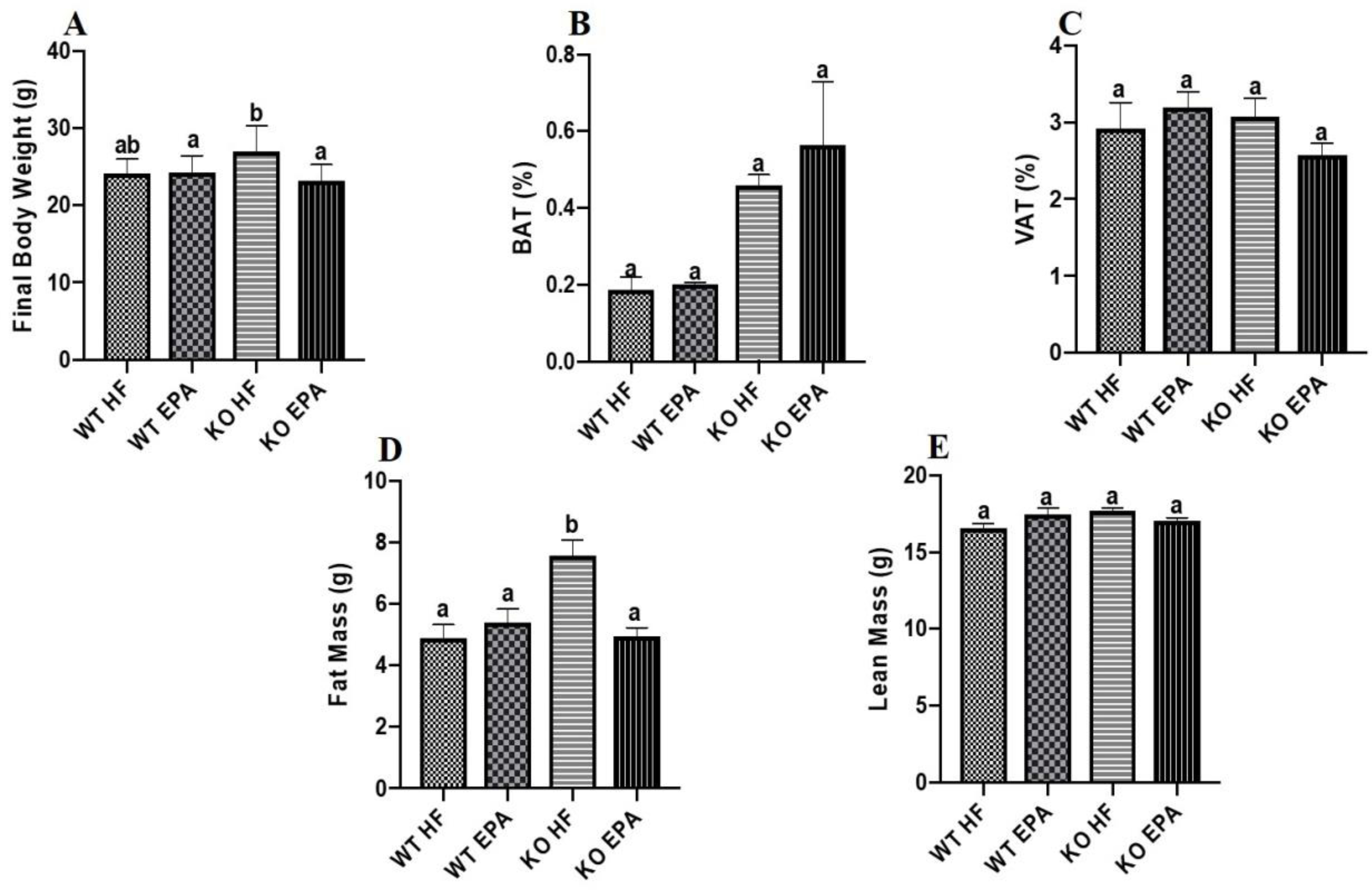
Final body weight (A), brown adipose tissue as a percentage of total body weight (B), and visceral adipose tissue as a percentage of total body weight (C), fat mass (D) and lean mass (E) of female WT and UCP1 KO mice. A shared letter indicates no difference. p < 0.05; n = 9 WT HF, 11 WT EPA, 15 KO HF, and 15 KO EPA mice.
There were no significant differences between interscapular BAT (Fig. 2B) and visceral adipose tissue (VAT)(Fig. 2C) among WT or KO groups. However, both the KO groups had higher (trending towards significance) BAT mass than the WT groups. Body fat composition was similar for both WT diet groups, and the KO-EPA group displayed significantly lower fat mass than KO-HF mice (p < 0.05; Fig. 2D). However, no difference in lean mass across the four groups was observed (Fig. 2E).
3.2. Effects of UCP1 Deficiency and EPA on Insulin and Glucose Tolerance Tests
Glucose tolerance and insulin tolerance were evaluated using GTT and ITT, respectively. In GTT, the basal levels were comparable between all groups. We observed significantly impaired glucose tolerance in KO-HF mice compared to all other groups (p < 0.05; Fig. 3A, B). Similarly, ITT revealed no significant difference in insulin sensitivity between WT groups. EPA-supplemented KO mice had significantly enhanced insulin sensitivity compared to the KO-HF group (p < 0.05; Fig. 3C, D). Results of diet X genotype interaction on final body weight, visceral fat pad, interscapular BAT weight, GTT, and ITT are shown in Supplementary Table 3.
Figure 3.
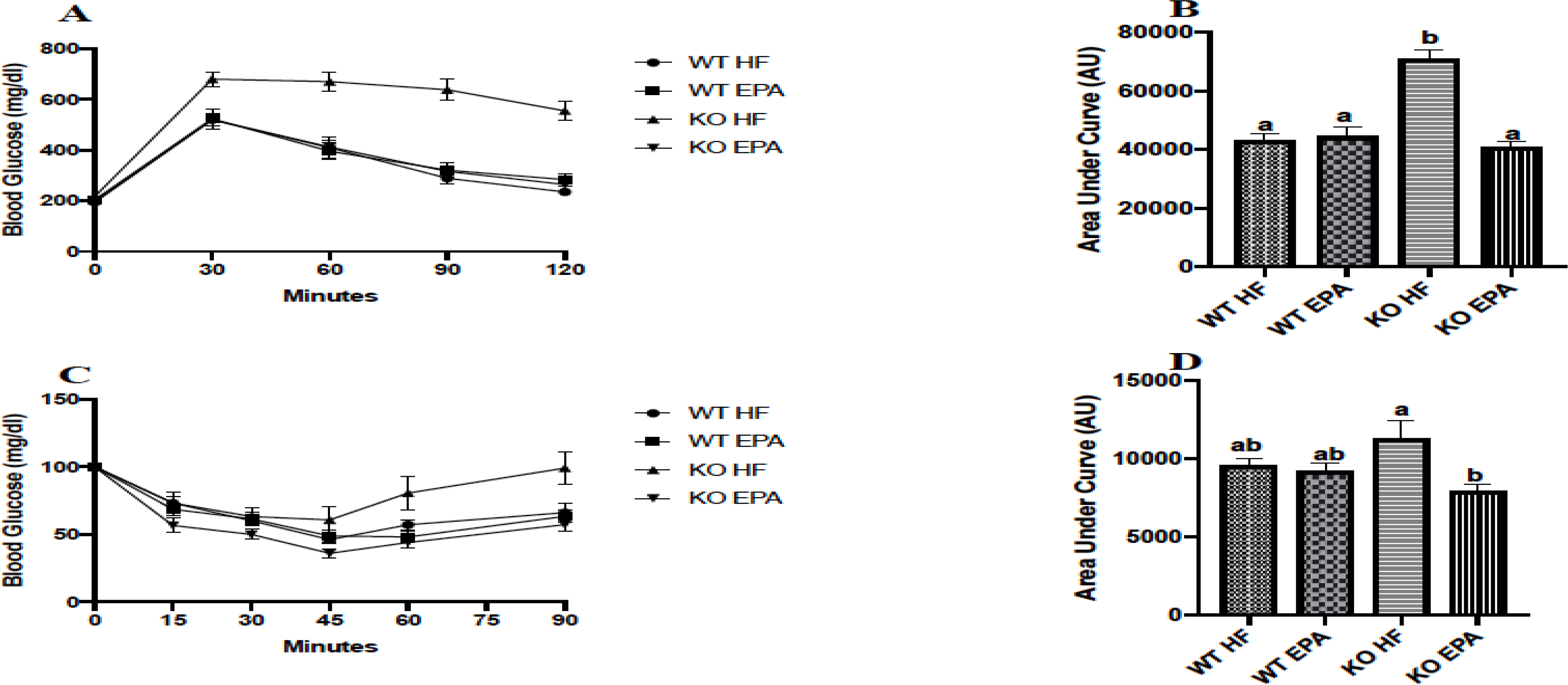
Glucose homeostasis of female WT and UCP1 KO mice evaluated via glucose tolerance test (A) with area under the curve (B) and insulin tolerance test (C) with area under the curve (D). A shared letter indicates no difference. p < 0.05; n = 9 WT HF, 10 WT EPA, 9 KO HF, and 13 KO EPA mice.
3.3. Effects of UCP1 Deficiency and EPA on BAT Gene Expression
To evaluate systematic differences among diet and genotype groups at the cellular level, we measured expression of several biomarkers of BAT via gene levels (Fig. 4 A–F) including Ucp1, Ucp2, peroxisome proliferator activated receptor gamma coactivator 1 alpha (Pgc1α), and sirtuin 1 (Sirt1). We observed no significant differences in mRNA levels of Ucp1 between the WT groups, but as expected, Ucp1 expression was almost undetected in the KO animals (Fig. 4A). Expression of Ucp2 was significantly higher in the KO-EPA mice compared to either WT group (p < 0.05; Fig. 4B). There was no difference in levels of Pgc1α between WT-HF and WT-EPA mice or the KO-HF versus KO-EPA groups, but its expression was significantly elevated in the KO mice for either diet compared to both WT groups (p < 0.05; Fig.4C). In addition, Sirt1 expression was significantly lower in the KO groups of both diets compared to WT mice (p < 0.05; Fig. 4D).
Figure 4.
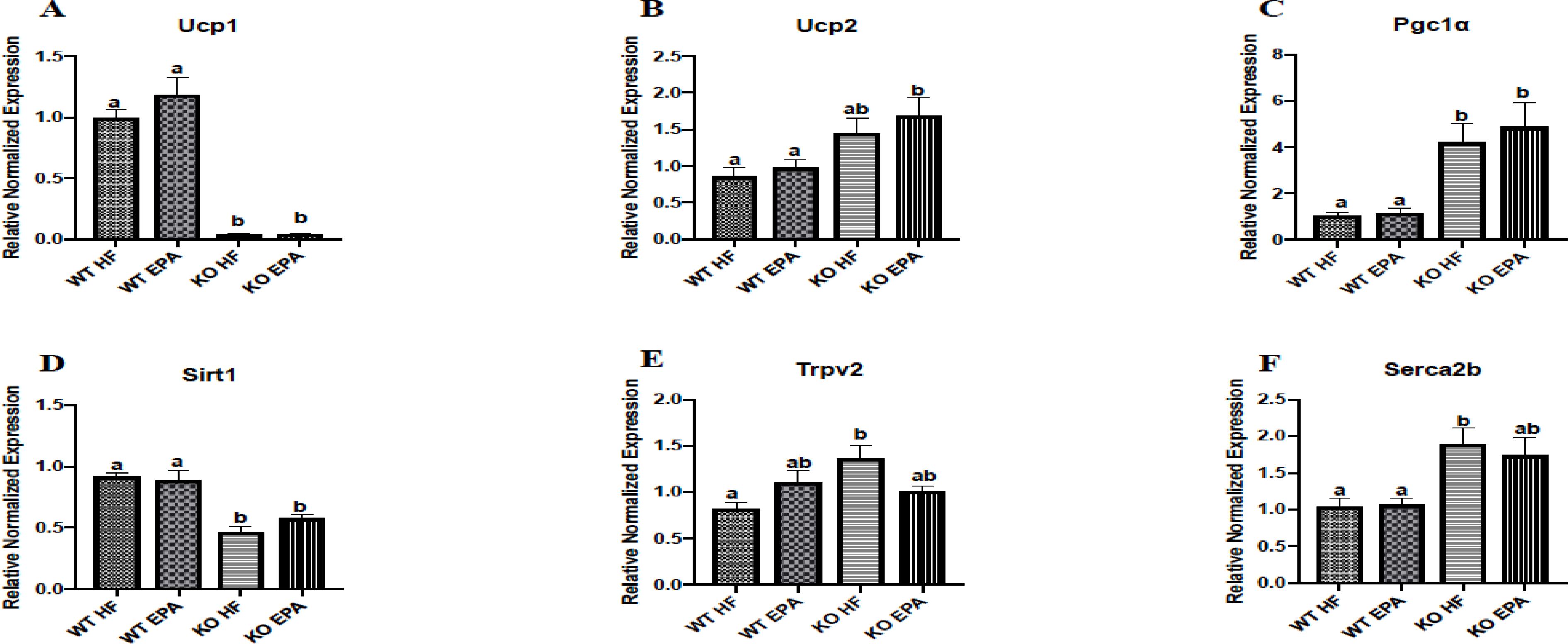
Gene expression of thermogenic, mitochondrial, and calcium cycling markers in interscapular BAT in female WT and UCP1 KO mice. A shared letter indicates no difference. p < 0.05; n = 6 mice per group.
We also evaluated expression of several mitochondrial and thermogenesis-related markers, among others, including transient receptor potential vanilloid 2 (Trpv2) (Fig 4E), sarcoplasmic/endoplasmic reticulum calcium ATPase 2b (Serca2b) (Fig 4F), Sirt2, Sirt3, peroxisome proliferator-activated receptor gamma coactivator 1-beta (Pgc1β), receptor interacting protein 140 (Rip140), mitogen-activated protein kinase (Mapk), cell death activator CIDE-A (Cidea), klotho beta (Klb), nuclear respiratory factor 1 (Nrf1), and Nrf2 (Supplementary Figure 1 and 2). We found that expression of Trpv2 was significantly higher in the KO-HF mice compared to WT-HF group (p < 0.05; Fig. 4E). In addition, there was no difference in expression of Serca2b between WT or KO genotypes, but it was expressed more highly in both KO-HF group compared to WT-HF group (p < 0.05; Fig. 4F). Further, the KO-HF mice had higher expression of Sirt2 than both WT groups (p < 0.05) (Supplementary Fig 1). Only the KO-HF group showed significantly higher expression of Rip140 compared to the WT-EPA group. There were no differences in expression of Sirt3, Pgc1β, Mapk, Cidea, Ucp3, Nrf1, or Nrf2 between any groups. Effects of diet X genotype interaction on gene expression are shown in Supplementary Table 4 and 5.
3.4. Effects of UCP1 Deficiency and EPA on mtDNA/nucDNA Ratio
For mtDNA/nucDNA, the WT-EPA group displayed a significantly lower ratio of mtDNA to nucDNA (p < 0.05) compared to the WT-HF group. Similarly, KO-EPA had a lower ratio versus KO-HF mice (p < 0.05), while no differences between the WT-HF and KO-HF or WT-EPA versus KO-EPA groups were observed. (Fig. 5A). Effects of diet X genotype interaction on mtDNA/nucDNA are shown in Supplementary Table 3.
Figure 5.
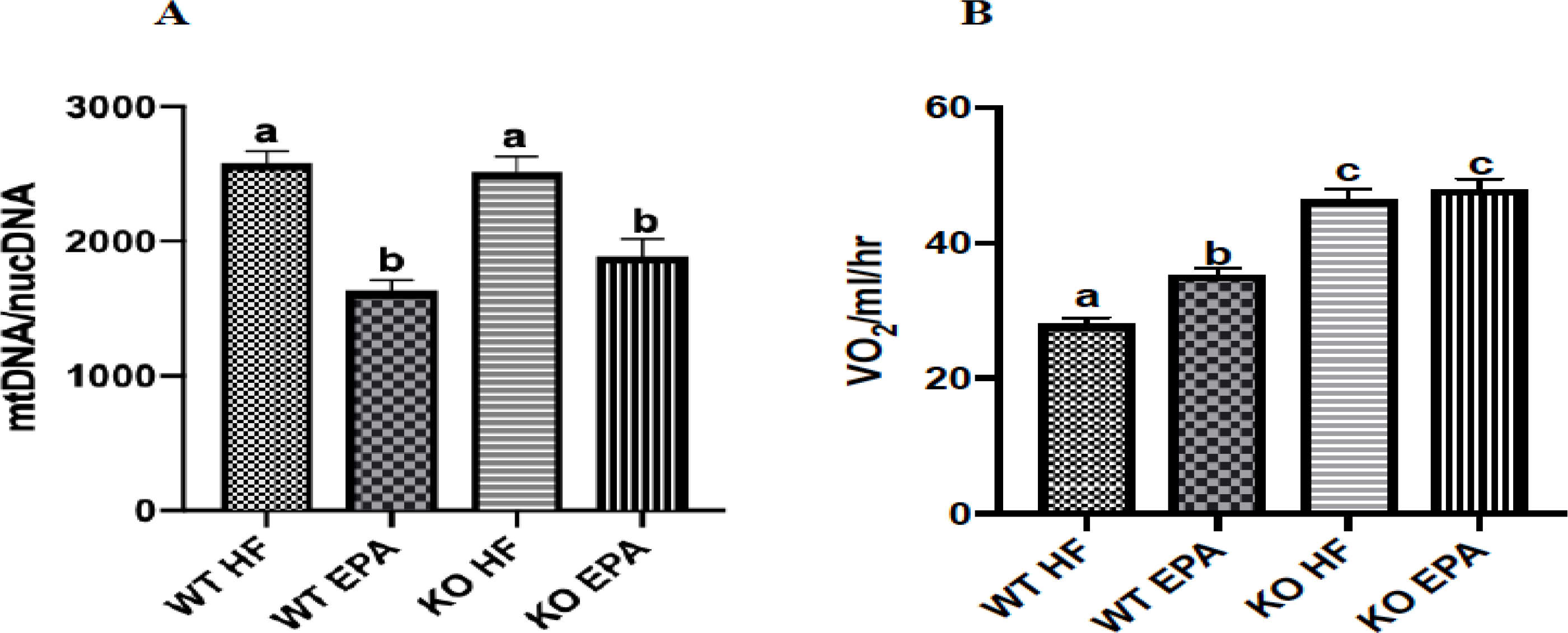
mtDNA/nucDNA ratio (n = 3 mice per group) (A) and oxygen consumption (n = 24) mice per group. (B) of WT and UCP1 KO mice. A shared letter indicates no difference. p < 0.05.
3.5. Effects of UCP1 Deficiency and EPA on Oxygen Consumption
Oxygen consumption was significantly increased in the WT-EPA group compared to WT-HF. Additionally, KO mice on either diet exhibited significantly greater oxygen consumption compared to both WT groups (p < 0.05) (Fig. 5B) with no differences within the genotype. Results of diet X genotype interaction on oxygen consumption are shown in Supplementary Table 3.
3.6. Effects of UCP1 Deficiency and EPA on Protein Content of BAT Markers
Similar to gene expression, UCP1 protein content was undetectable in the KO mice as expected (Fig. 6A). Further, no significant difference in UCP1 protein was observed within WT groups. In addition, examination of PGC1α protein levels revealed no differences between groups (Fig. 6B).
Figure 6.
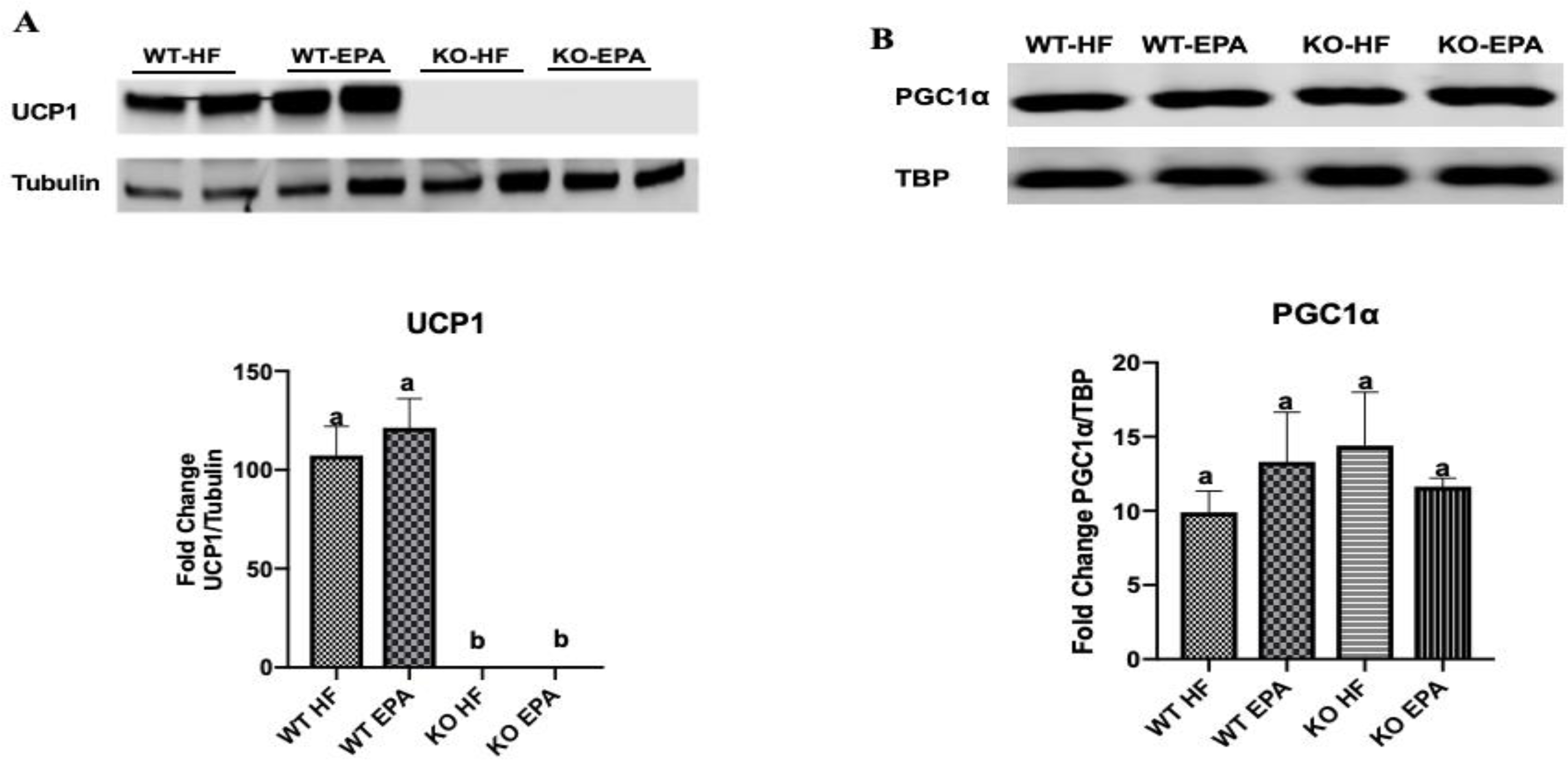
Protein content of UCP1 (n = 4 WT HF, 4 WT EPA, 3 KO HF, and 3 KO EPA mice) (A) and PGC1α (n = 3 WT HF, 5 WT EPA, 5 KO HF, and 5 KO EPA mice) (B) in interscapular BAT of WT and UCP1 KO mice. A shared letter indicates no difference. p < 0.05.
We also measured the levels of the cytochrome c oxidase subunits (COX I-V), which together make up the final component of the electron transport chain preceding ATP synthesis, complex IV (Fig. 7) [24]. Protein content of both COX I and COX II were similar between WT groups as well as KO-HF versus KO-EPA but were significantly reduced in KO mice compared to WT. There was no significant difference in protein levels of COX III between WT-HF and WT-EPA or KO-HF and KO-EPA, but in the KO-EPA groups, ablation of UCP1 significantly decreased COX III levels compared to the WT-EPA group. For COX IV, WT-EPA showed similar levels compared to WT-HF, and there were no significant differences between KO groups, but both KO groups displayed a significant decrease in protein content compared to the WT-EPA group. No differences were observed between groups for COX V content. Effects of diet X genotype interaction on protein expression are shown in Supplementary Table 4.
Figure 7.
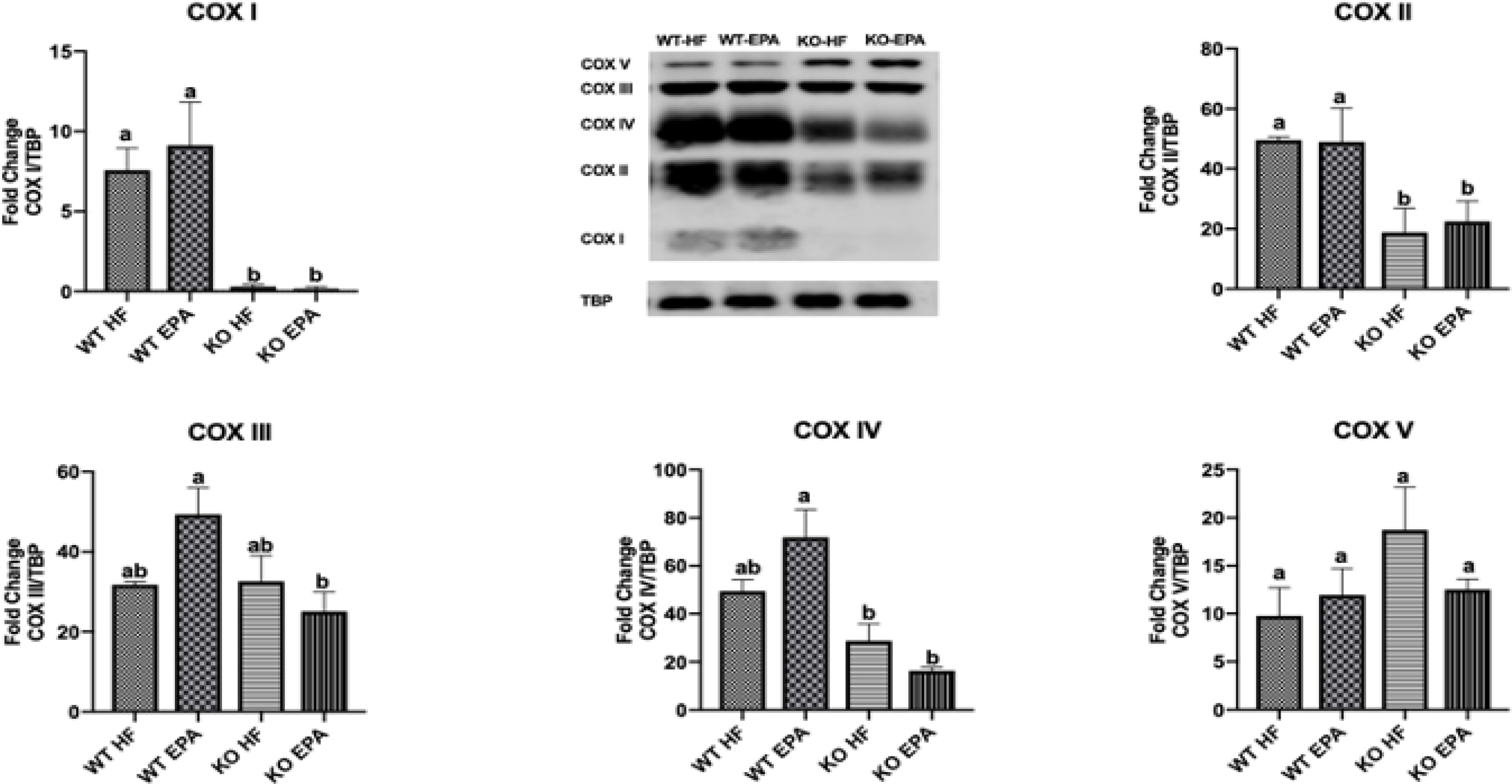
Protein content of mitochondrial OXPHOS complexes (COX I-V) in interscapular BAT of WT and UCP1 KO mice. A shared letter indicates no difference. p < 0.05; n = 3 WT HF, 5 WT EPA, 5 KO HF, and 5 KO EPA mice.
4. Discussion
Obesity is one of the greatest challenges faced by our society today, making research into the mechanisms of this disease and the development of novel anti-obesity therapies critical. In our research, we examined female mice housed at thermoneutrality to better understand how ω−3 fatty acids (EPA) reduce obesity through BAT activation in females. We focused on the metabolic effects of inactivation of UCP1, a protein responsible for the regulation of BAT thermogenesis which is located exclusively within the inner mitochondrial membrane of brown and beige adipocytes [10, 11].
Early studies hypothesized that diet-induced thermogenesis is completely mediated by UCP1 and that mice lacking this protein would be more prone to obesity [25]. However, at low temperatures, these mice fail to display the obese phenotype as expected; in fact, somewhat counterintuitively, UCP1 deletion exerts an anti-obesity effect on male mice under these conditions [25]. In contrast, this protection is reversed once UCP1 KO mice are transferred to a thermoneutral environment. Once chronic cold stress is removed, UCP1 ablation has an obesogenic effect on the animals [25, 26]. Moreover, other mechanisms have been proposed to mediate UCP-1-indepdnedent effects such as creatine metabolism [27, 28].
Most studies using mouse models house their animals at 22–23°C, a temperature which subjects mice to chronic thermal stress and forces them to increase their metabolism [20]. In the current study, we investigated the effects of UCP1 loss in a thermoneutral environment – around 30°C for mice – to provide more information about its effects under these conditions. We hypothesized that the beneficial effects of EPA would not require expression of the critical thermogenic protein UCP1. Our hypothesis was validated, and we showed that the protective effects of EPA persisted in the absence of UCP1.
Many of our findings are largely consistent with prior work, including studies reporting that UCP1 ablation exerts an obesogenic effect on male and female mice housed in both thermoneutral and ambient conditions [3, 26, 29]. Similarly, we found that female KO-HF mice consistently displayed higher overall body weight and fat percentage compared to all other groups despite having similar food intake. KO mice fed EPA had significantly reduced body fat percentage compared to the KO-HF group, consistent with studies conducted at ambient temperature which reported that EPA supplementation effectively reduced fat mass and abrogated adipocyte hypertrophy [17, 30]. Unlike this prior work, however, we found that WT mice fed either diet had comparable body fat percentage, a discrepancy that may be attributed to the fact that our study was conducted at thermoneutrality rather than ambient conditions. Modifications to methods used in previous studies offered us an opportunity to gain new insights, especially around how mice respond to temperature in the experimental setting. For instance, Winn et al. reported that female UCP1 KO mice fed a western diet showed similar weight gain and food intake compared to WT animals given the same diet [29]. However, this study focused on mice housed in an ambient environment (25°C) rather than thermoneutrality as in our study, which can cause animals to expend more energy to maintain their body temperature. In several diet-induced obesity studies, male mice gained significantly more weight than females when fed the same HF diet, and prior studies demonstrated that female C57BL/6 mice have higher insulin sensitivity than males [31–33]. It is possible that deletion of UCP1 exacerbates susceptibility to weight gain more severely in male mice than in females, which may contribute to the observed differences in weight gain and oxygen consumption between male and female UCP1 KO mice.
Cytochrome c oxidase (COX) is a protein complex that comprises complex IV of the electron transport chain and plays a key role in ATP synthesis within the mitochondria [24]. In previous studies conducted at ambient temperature and thermoneutrality, UCP1-ablated male mice fed a HF diet exhibited reduced levels of COX protein subunits I, II, and IV in BAT [21, 29]. In line with these studies, we observed significant decreases in BAT protein content of COX I and COX II in UCP1-KO mice compared to WT groups. Moreover, in EPA-fed mice, lack of UCP1 significantly decreased COX III levels, consistent with a previous study focused on female UCP1 KO mice housed at 25°C [29].
UCP1 deficiency is associated with impaired calcium cycling, leading to increased susceptibility to calcium overload [34]. Therefore, we investigated the effects of UCP1 deletion on calcium buffering at thermoneutrality by measuring gene expression of Trpv2, a calcium-permeable ion channel involved in the regulation of BAT thermogenesis and inhibition of brown adipocyte differentiation in mice [34–36]. Trpv2 is expressed in brown adipocytes, and the influx of calcium ions through Trpv2 channels promotes increased expression of thermogenic genes upon stimulation of the sympathetic nervous system [37]. Here, KO-HF mice expressed Trpv2 at a significantly higher level than WT-HF mice. Sun et al. reported that Trpv2 mRNA levels were significantly increased in BAT of WT mice given a HF diet, indicating its involvement in BAT thermogenesis during obesity [37]. However, in contrast to our study, this research was conducted at ambient temperature. In addition to increased Trpv2 expression, we found that, in both diet groups, deletion of UCP1 upregulated gene expression of Serca2b, which is also involved in calcium cycling. Similarly, it has previously been reported that in the absence of UCP1, SERCA2b mediates non-shivering thermogenesis in beige adipose tissue by uncoupling calcium movement from ATP hydrolysis [38]. However, unlike our study, this work was performed under conditions of extreme cold exposure (6°C).
Despite having similar weight gain and adipose tissue mass compared to WT mice, UCP1 ablation significantly impaired glucose clearance in HF-fed animals. This is not surprising given that BAT plays a role in insulin sensitivity and glucose homeostasis and its activity is associated with metabolic improvement in both humans and rodents [39, 40]. In regard to glucose uptake, BAT is among the most insulin-sensitive organs in rats and mice, and glucose clearing by this tissue is associated with its thermogenic program [41]. Although clinical trials have demonstrated triglyceride-reducing effects of fish oil and ω−3 PUFA, the role of these compounds with respect to insulin sensitivity and glucose clearance in humans is less clear [15, 18, 42]. Such inconsistencies in clinical findings likely stem from variations in sample size, duration of treatment, and dosage [43]. For instance, the recommended dosages for EPA and DHA range from 2–4 grams per day to provide triglyceride-lowering benefits, but average intake is significantly lower in the United States [44, 45]. In contrast, dosages of ω−3 PUFA used in animal studies often equate to a much greater percentage of total energy intake. In rodents, ω−3 PUFA including EPA are capable of improving glucose homeostasis [46, 47] through the enhancement of mitochondrial function and reduction of endoplasmic reticulum stress [48, 49]. In WT male mice housed at thermoneutrality, supplementation with EPA and DHA attenuated the detrimental effects of HF diet on glucose homeostasis [50]. Although effects of EPA on glucose tolerance were not observed in female WT mice in the current study, possibly due to sex-specific effects, we found that EPA ameliorated the adverse effects of UCP1 ablation on glucose intolerance and insulin resistance. In fact, KO-EPA mice showed comparable glucose clearance and insulin sensitivity to the WT groups despite lacking UCP1. Similarly, previous findings from our laboratory showed that for male UCP1 KO mice housed at thermoneutrality, EPA significantly improved glucose intolerance compared to KO mice fed HF [21]. Therefore, EPA may stimulate an alternative thermogenic pathway in the KO mice to exert these beneficial effects, perhaps through increases in other thermogenic markers.
Such alternative pathways have been proposed by previous studies. For male UCP1 KO mice housed at thermoneutrality, EPA significantly increased mRNA expression of the major mitochondrial biogenesis regulator Pgc1α [21]. In another study, which was conducted at ambient temperature, Pgc1α gene expression was significantly higher in female UCP1 KO mice compared to WT [21, 29], even though we saw no differences in Pgc1α protein levels. Moreover, KO-EPA mice displayed significantly upregulated expression of Ucp2 compared to WT mice. Ucp2 is thought to be involved in fatty acid metabolism, and in the studies mentioned previously, deletion of UCP1 increased Ucp2 mRNA expression compared to WT [21, 29]. Increases in Pgc1α and Ucp2 in the absence of UCP1 could be compensatory mechanisms in the KO mice, possibly induced by EPA supplementation.
Additionally, we previously reported that UCP1 KO male mice fed EPA showed a significant increase of mtDNA compared to KO-HF mice [21]. However, in the current study female KO mice demonstrated the opposite response to HF and EPA diets compared to male mice, which represents a sex-dependent effect of EPA.
Prior research from our laboratory and others found that EPA significantly increased UCP1 levels of BAT in rodents [3, 51, 52], which we did not observe in the current study. This difference can likely be attributed to the fact that most previous studies were conducted at ambient temperature, as opposed to thermoneutrality as in the current study, and cold exposure is known to dramatically increase UCP1 gene transcription [53]. In line with our findings, another study conducted at thermoneutrality demonstrated a protective effect of EPA and DHA against diet-induced obesity that was independent of UCP1 [50]. However, unlike at ambient temperature, the beneficial of EPA in WT female mice were absent at thermoneutrality, indicating temperature-dependent metabolic differences in response to EPA. In addition to the protective metabolic effects of EPA discussed in this work, other mechanisms which may contribute in part to the metabolic improvements with EPA include reduced systemic and adipose tissue inflammation, as previously reported [54, 55], and/or production of inflammation resolving lipid mediators, which we did not measure in this study [56, 57]
5. Conclusion
Collectively, our findings support that ablation of UCP1 is detrimental to energy metabolism of female mice living at thermoneutrality. Moreover, we demonstrate that EPA’s protective effects against diet-induced obesity and insulin intolerance in these animals are distinctly independent of UCP1.
These promising results should continue to drive mechanistic studies to explore adipose tissue-EPA interactions at the molecular level and identify which alternative mechanisms are driving metabolic improvements in the absence of UCP1. It would be worthwhile to determine the effects of DHA and whole fish oil on BAT metabolism in a thermoneutral environment and in the absence of UCP1. Further studies are needed to explore the effects of other ω−3 fatty acids like DHA or of fish oil as a whole in addition to EPA, and steps should be taken toward the development of clinical studies to translate our research into practice.
Supplementary Material
HIGHLIGHTS.
In a thermoneutral environment:
UCP1 deficiency in diet-induced obese female mice is detrimental to energy metabolism
Eicosapentaenoic acid (EPA) did not improve metabolic health of B6 obese females
Eicosapentaenoic acid improved metabolic functions in UCP1 deficient obese females
Protective metabolic effects of EPA in obese female mice are independent of UCP1
Acknowledgements
This work was supported by NIH (NCCIH/NIA) award# R15 AT 8879–01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS data brief. 2017:1–8. [PubMed] [Google Scholar]
- [2].Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. American Journal Of Public Health. 2013;103:1895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pahlavani M, Razafimanjato F, Ramalingam L, Kalupahana NS, Moussa H, Scoggin S, et al. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. The Journal Of Nutritional Biochemistry. 2017;39:101–9. [DOI] [PubMed] [Google Scholar]
- [4].Wirth A, Wabitsch M, Hauner H. The prevention and treatment of obesity. Deutsches Arzteblatt international. 2014;111:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bonamichi B, Parente E, Bonamichi-Santos R, Beltzhoover R, Lee J, Salles J. The Challenge of Obesity Treatment: A Review of Approved Drugs and New Therapeutic Targets. J Obes Eat Disord. 2018;04:4:2. [Google Scholar]
- [6].Cefalu WT, Bray GA, Home PD, Garvey WT, Klein S, Pi-Sunyer FX, et al. Advances in the Science, Treatment, and Prevention of the Disease of Obesity: Reflections From a Diabetes Care Editors’ Expert Forum. Diabetes Care. 2015;38:1567–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jayarathne S, Koboziev I, Park O-H, Oldewage-Theron W, Shen C-L, Moustaid-Moussa N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Preventive nutrition and food science. 2017;22:251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coats R, Martirosyan D. The effects of bioactive compounds on biomarkers of obesity. Functional Foods in Health and Disease. 2015;5:365–80. [Google Scholar]
- [9].Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. American Journal of Physiology-Endocrinology and Metabolism. 2007;293:E444–E52. [DOI] [PubMed] [Google Scholar]
- [10].Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shabalina IG, Petrovic N, de Jong JMA, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Reports. 2013;5:1196–203. [DOI] [PubMed] [Google Scholar]
- [12].Marzetti E, D’Angelo E, Savera G, Leeuwenburgh C, Calvani R. Integrated control of brown adipose tissue. Heart and metabolism : management of the coronary patient. 2016;69:9–14. [PMC free article] [PubMed] [Google Scholar]
- [13].Tajino K, Hosokawa H, Maegawa S, Matsumura K, Dhaka A, Kobayashi S. Cooling-Sensitive TRPM8 Is Thermostat of Skin Temperature against Cooling. PloS one. 2011;6:e17504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Betz MJ, Enerbäck S. Human Brown Adipose Tissue: What We Have Learned So Far. Diabetes. 2015;64:2352. [DOI] [PubMed] [Google Scholar]
- [15].Bradberry JC, Hilleman DE. Overview of omega-3 Fatty Acid therapies. P & T: A Peer-Reviewed Journal For Formulary Management. 2013;38:681–91. [PMC free article] [PubMed] [Google Scholar]
- [16].Judé S, Roger S, Martel E, Besson P, Richard S, Bougnoux P, et al. Dietary long-chain omega-3 fatty acids of marine origin: a comparison of their protective effects on coronary heart disease and breast cancers. Progress In Biophysics And Molecular Biology. 2006;90:299–325. [DOI] [PubMed] [Google Scholar]
- [17].Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, et al. Eicosapentaenoic Acid Prevents and Reverses Insulin Resistance in High-Fat Diet-Induced Obese Mice via Modulation of Adipose Tissue Inflammation. The Journal of Nutrition. 2010;140:1915–22. [DOI] [PubMed] [Google Scholar]
- [18].Nordøy A, Hansen JB, Bonaa KH, Grimsgaard S. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. The American Journal of Clinical Nutrition. 1997;66:649–59. [DOI] [PubMed] [Google Scholar]
- [19].Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Molecular Metabolism. 2018;7:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. Journal of Thermal Biology. 2012;37:654–85. [Google Scholar]
- [21].Pahlavani M, Ramalingam L, Miller EK, Scoggin S, Menikdiwela KR, Kalupahana NS, et al. Eicosapentaenoic Acid Reduces Adiposity, Glucose Intolerance and Increases Oxygen Consumption Independently of Uncoupling Protein 1. Molecular Nutrition & Food Research. 2019;0:1800821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Valle A, García-Palmer FJ, Oliver J, Roca P. Sex differences in brown adipose tissue thermogenic features during caloric restriction. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2007;19:195–204. [DOI] [PubMed] [Google Scholar]
- [23].Jawień W Searching for an optimal AUC estimation method: a never-ending task? Journal of Pharmacokinetics and Pharmacodynamics. 2014;41:655–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heinemeyer J, Braun H-P, Boekema EJ, Kouřil R. A Structural Model of the Cytochrome c Reductase/Oxidase Supercomplex from Yeast Mitochondria. Journal of Biological Chemistry. 2007;282:12240–8. [DOI] [PubMed] [Google Scholar]
- [25].Liu X, Rossmeisl M, McClaine J, Riachi M, Harper M-E, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. The Journal Of Clinical Investigation. 2003;111:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metabolism. 2009;9:203–9. [DOI] [PubMed] [Google Scholar]
- [27].Wallimann T, Tokarska-Schlattner M, Kay L, Schlattner U. Role of creatine and creatine kinase in UCP1-independent adipocyte thermogenesis. American Journal of Physiology-Endocrinology and Metabolism. 2020;319:E944–E6. [DOI] [PubMed] [Google Scholar]
- [28].Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Winn NC, Vieira-Potter VJ, Gastecki ML, Welly RJ, Scroggins RJ, Zidon TM, et al. Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. American journal of physiology Regulatory, integrative and comparative physiology. 2017;312:R74–R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pinel A, Pitois E, Rigaudiere J-P, Jouve C, De Saint-Vincent S, Laillet B, et al. EPA prevents fat mass expansion and metabolic disturbances in mice fed with a Western diet. Journal of lipid research. 2016;57:1382–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hwang L-L, Wang C-H, Li T-L, Chang S-D, Lin L-C, Chen C-P, et al. Sex Differences in High-fat Diet-induced Obesity, Metabolic Alterations and Learning, and Synaptic Plasticity Deficits in Mice. Obesity. 2010;18:463–9. [DOI] [PubMed] [Google Scholar]
- [32].Dakin RS, Walker BR, Seckl JR, Hadoke PWF, Drake AJ. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. International Journal Of Obesity. 2015;39:1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58:803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kazak L, Chouchani ET, Stavrovskaya IG, Lu GZ, Jedrychowski MP, Egan DF, et al. UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:7981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sun W, Uchida K, Tominaga M. TRPV2 regulates BAT thermogenesis and differentiation. Channels (Austin, Tex). 2016;11:94–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sun W, Uchida K, Takahashi N, Iwata Y, Wakabayashi S, Goto T, et al. Activation of TRPV2 negatively regulates the differentiation of mouse brown adipocytes. Pflügers Archiv - European Journal of Physiology. 2016;468:1527–40. [DOI] [PubMed] [Google Scholar]
- [37].Sun W, Uchida K, Suzuki Y, Zhou Y, Kim M, Takayama Y, et al. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO reports. 2016;17:383–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nature medicine. 2017;23:1454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee P, Bova R, Schofield L, Bryant W, Dieckmann W, Slattery A, et al. Brown Adipose Tissue Exhibits a Glucose-Responsive Thermogenic Biorhythm in Humans. Cell Metabolism. 2016;23:602–9. [DOI] [PubMed] [Google Scholar]
- [40].Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. The Journal of clinical investigation. 2013;123:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].H Storlien L, James D, M Burleigh K, J Chisholm D, Kraegen E. Fat feeding causes widespread in vivo insulin resistance, decrease energy expenditure, and obesity in rats 1986. [DOI] [PubMed]
- [42].Harris WS. n-3 fatty acids and serum lipoproteins: human studies. The American Journal of Clinical Nutrition. 1997;65:1645S–54S. [DOI] [PubMed] [Google Scholar]
- [43].Gao H, Geng T, Huang T, Zhao Q. Fish oil supplementation and insulin sensitivity: a systematic review and meta-analysis. Lipids in health and disease. 2017;16:131-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jain AP, Aggarwal KK, Zhang PY. Omega-3 fatty acids and cardiovascular disease. Eur Rev Med Pharmacol Sci. 2015;19:441–5. [PubMed] [Google Scholar]
- [45].Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989;2:757–61. [DOI] [PubMed] [Google Scholar]
- [46].Sener A, Zhang Y, Bulur N, Louchami K, Malaisse WJ, Carpentier YA. The metabolic syndrome of omega3-depleted rats. II. Body weight, adipose tissue mass and glycemic homeostasis. International Journal Of Molecular Medicine. 2009;24:125–9. [PubMed] [Google Scholar]
- [47].Poudyal H, Panchal SK, Diwan V, Brown L. Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Progress in Lipid Research. 2011;50:372–87. [DOI] [PubMed] [Google Scholar]
- [48].Lanza IR, Blachnio-Zabielska A, Johnson ML, Schimke JM, Jakaitis DR, Lebrasseur NK, et al. Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet. American journal of physiology Endocrinology and metabolism. 2013;304:E1391–E403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yang W, Chen X, Chen M, Li Y, Li Q, Jiang X, et al. Fish oil supplementation inhibits endoplasmic reticulum stress and improves insulin resistance: involvement of AMP-activated protein kinase. Food & Function. 2017;8:1481–93. [DOI] [PubMed] [Google Scholar]
- [50].Janovská P, Flachs P, Kazdová L, Kopecky J. Anti-Obesity Effect of n-3 Polyunsaturated Fatty Acids in Mice Fed High-Fat Diet Is Independent of Cold-Induced Thermogenesis. Physiological research / Academia Scientiarum Bohemoslovaca. 2012;62. [DOI] [PubMed] [Google Scholar]
- [51].Kim M, Goto T, Yu R, Uchida K, Tominaga M, Kano Y, et al. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Scientific reports. 2015;5:18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bargut TCL, Silva-e-Silva ACAG, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. European Journal of Nutrition. 2016;55:159–69. [DOI] [PubMed] [Google Scholar]
- [53].Ehrlich M, Ivask M, Kõks S. Cold induced Ucp1 expression in brown and white adipose tissue of WFS1-deficient mice. The FASEB Journal. 2017;31:886.6–6. [Google Scholar]
- [54].Guo XF, Sinclair AJ, Kaur G, Li D. Differential effects of EPA, DPA and DHA on cardiometabolic risk factors in high-fat diet fed mice. Prostaglandins, leukotrienes, and essential fatty acids. 2018;136:47–55. [DOI] [PubMed] [Google Scholar]
- [55].LeMieux MJ, Kalupahana NS, Scoggin S, Moustaid-Moussa N. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. The Journal of nutrition. 2015;145:411–7. [DOI] [PubMed] [Google Scholar]
- [56].González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Carracedo M, Artiach G, Arnardottir H, Bäck M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin Immunopathol. 2019;41:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


