Abstract
Nearly all cardiovascular diseases show sexual dimorphisms in prevalence, presentation, and outcomes. Until recently, most clinical trials were carried out in males, and many animal studies either failed to identify the sex of the animals or combined data obtained from males and females. Cellular sex in the heart is relatively understudied and many studies fail to report the sex of the cells used for in vitro experiments. Moreover, in the small number of studies in which sex is reported, most of those studies use male cells. The observation that cells from males and females are inherently different is becoming increasingly clear – either due to acquired differences from hormones and other factors or due to intrinsic differences in genotype (XX or XY). Because of the likely contribution of cellular sex differences in cardiac health and disease, here, we explore differences in mammalian male and female cells in the heart, including the less-studied non-myocyte cell populations. We discuss how the heart’s microenvironment impacts male and female cellular phenotypes and vice versa, including how secretory profiles are dependent on cellular sex, and how hormones contribute to sexually dimorphic phenotypes and cellular functions. Intracellular mechanisms that contribute to sex differences, including gene expression and epigenetic remodeling, are also described. Recent single-cell sequencing studies have revealed unexpected sex differences in the composition of cell types in the heart which we discuss. Finally, future recommendations for the design and consideration of cellular sex differences in the heart are provided.
Keywords: Sex differences, cardiovascular disease, cellular sex, heart biology
1. Introduction
While sex differences in cardiovascular disease have long been documented, the basis for these observed differences in pathophysiology must be due, at least in part, to cellular sex differences. Cells in male and female hearts are inherently different and have acquired differences during puberty and adulthood that influence their function and complex interactions (Figure 1). Research is beginning to address cellular sex in many organ systems, but this aspect of biology is relatively under-studied. Here, we discuss sexual dimorphisms in the many cell types of the heart. We use “sex” to describe biology, rather than “gender,” which describes behavior. Recent reports have shown sex differences in the cellular composition of male and female hearts, including subclusters of cell populations. These observations demonstrate the challenges of unraveling the causes of larger-scale sex differences including cardiovascular disease risk, progression, and outcome [1].
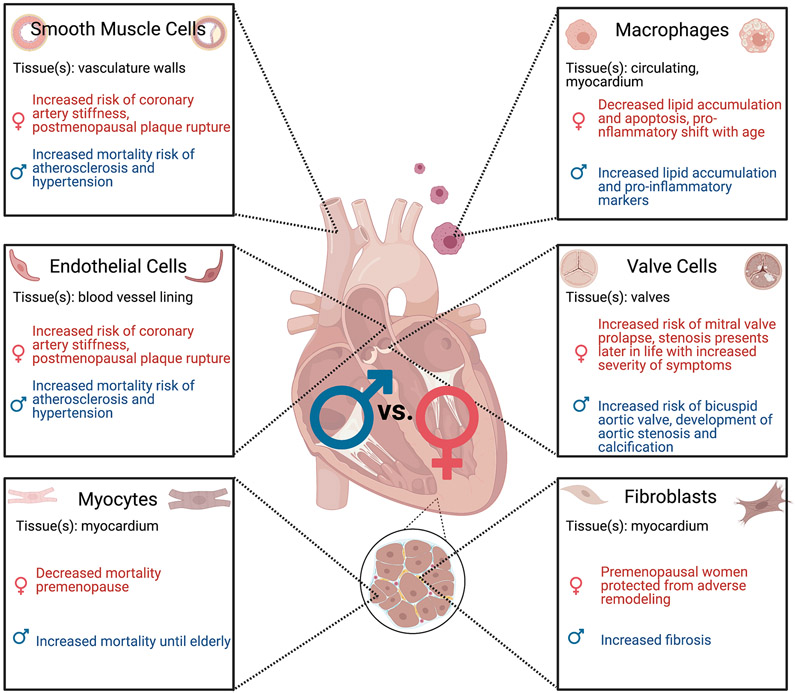
Figure 1.
Pathophysiology of cardiac diseases can be linked to differences in cellular sex of myocytes [2], endothelial cells [3-7], fibroblasts [8-10], macrophages [11-13], smooth muscle cells [3-7], and valve cells [14-19]. Figure created with BioRender.com.
Variations of the extracellular microenvironment are both a cause and an effect of sexual dimorphisms in the heart. A milieu of circulating factors, including cytokines and hormones, also contribute to cellular sex differences. In turn, these factors influence inflammation and wound repair mechanisms in a sexually dimorphic manner [20,21]. Sexual dimorphisms in extracellular matrix (ECM) exist not only due to physical differences such as height and gender normative lifestyles, but hormones and chromosomal cellular sex influence composition and homeostatic mechanisms. As such, we discuss only the influence of sex on cell phenotypes, not gender, although we acknowledge the influence of gender on heart biology is worth studying [22,23]. In response to injury and age, resident cells contribute to largely irreversible pathological matrix remodeling, while pregnancy and exercise result in adaptive remodeling that is reversible [24]. Many of these sex differences can be linked to hormonal differences, as sex hormone receptors are known to mediate fibrotic-specific ECM pathways [25], yet chromosomal genotype and epigenetic regulation can also drive cellular sex differences.
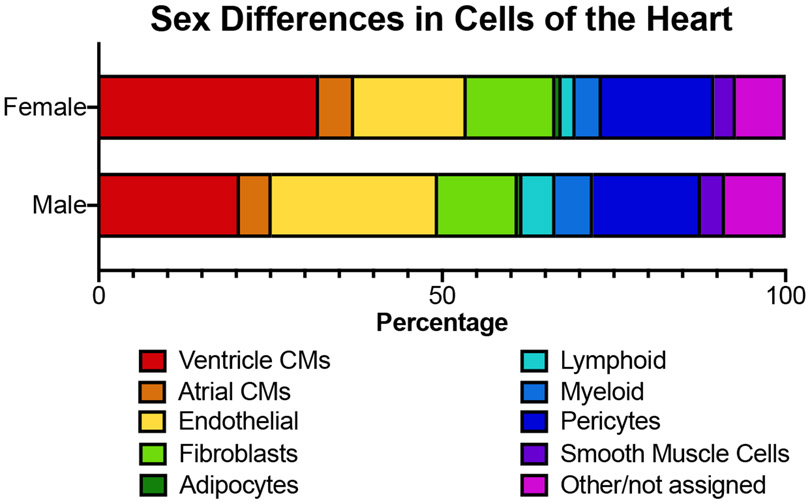
Compared to myocytes, little is known about cellular sex differences in the non-myocytes of the heart. While cardiac myocytes constitute 70% of the mass of the heart, they constitute only about 30% of the cell number. In the fetal heart, cardiac myocytes constitute a higher fraction of cells, with the proportion declining during maturation due to the greater proliferation of cardiac non-myocytes. According to the heart cell atlas [26], the most abundant cell types in the adult human heart, in descending order are: cardiac myocytes, endothelial cells, pericytes, fibroblasts, myeloid cells, lymphoid cells, smooth muscle cells, and adipocytes (Figure 2).
Figure 2.
Cell distribution in male and female human hearts based on single cell sequencing datasets from the heartcellatlas.org [26]. Healthy hearts from seven males and seven females with an age range of 40-75 years and collected from North America and the United Kingdom.
In this review article, we describe known sex differences in the cells of the mammalian heart, with an emphasis on non-myocytes. We discuss current literature regarding the extracellular and intracellular sex differences of each cell type. We briefly discuss cardiac myocytes and myeloid cells, as sex differences for both cell populations have been reviewed elsewhere [25,27-29]. There are fewer reports on cardiac smooth muscle cells, myeloid cells, and pericytes, but some seminal work is presented here. We also focus on cardiac fibroblasts, endothelial cells, and valve cells and present these cell populations in the most depth. Lymphoid cells, neural cells, and adipocytes are not discussed, as little is known about their sex differences, and they constitute small proportions of cells in the heart. We focus on baseline sex differences for each cell type, but also include findings on the dimorphic response of male and female cells to injury, age, and exercise as available. Cell types will be discussed in order of abundance in the heart. Many studies to date have made male and female comparisons for a single cell type; however, we acknowledge the complexities regarding cell-cell and cell-matrix interactions in the heart as a driving factor of sexually dimorphic phenotypes. To address these complexities, we conclude by discussing multicellular, bioengineering approaches that could direct future investigations of cellular sex in the heart and help develop in vitro models that could inform clinical studies.
2. Cells of the Heart
2.1. Cardiac Myocytes:
Clinical/pathophysiology:
Adult cardiac myocytes (CMs) produce contractile forces that pump blood through the body and constitute the majority of the cellular volume of the heart. Moreover, CM loss during cardiac injury is a major cause of heart failure since there is little proliferation of CMs post-natally [30]. Instead, there is replacement fibrosis, unlike pathological cardiac remodeling, where changes in the heart during pregnancy and exercise are largely due to CM enlargement and there are no indications of fibrosis [31]. For these and other reasons, CMs are the best-studied cell type of the heart, and many studies have identified sex differences in CM phenotypes [32-35], so we will only briefly discuss CMs.
Cell functions and populations:
CMs are known for their large size as well as their “brick-like” striated appearance. A single-cell sequencing study found that human female hearts contain a significantly higher percentage of ventricular CMs than male hearts [26], and while surprising, may partially explain why females have lower risk of cardiovascular disease as a reduced number of CMs is strongly associated with cardiac pathologies, like ischemia, heart failure, and myocardial infarction [36]. The mechanistic basis for this sex difference in CM number is unknown. However, we posit this difference is established during embryogenesis or fetal development since CMs rarely proliferate during adulthood [37,38]. Additionally, CM contractility is very much affected by sex, with male cells contracting more strongly and rapidly than female cells, while female cells relax more slowly [33,39]. In response to exercise, female hearts enlarge more than male hearts even when normalized to distance run, demonstrating sex differences in physiological CM signaling [40].
Extracellular/Intracellular:
In addition to their contractile properties, CMs are highly secretory and their secretomes change in response to stress stimuli including ER stress and ischemia [41,42]. We could find no reports, however, of sex differences in CM secretomes since most studies were carried out on neonatal or adult CMs whose sex was not reported. CM secretomes from both sexes should be characterized to gain insights into how different stimuli may impact CM secretions and overall heart health.
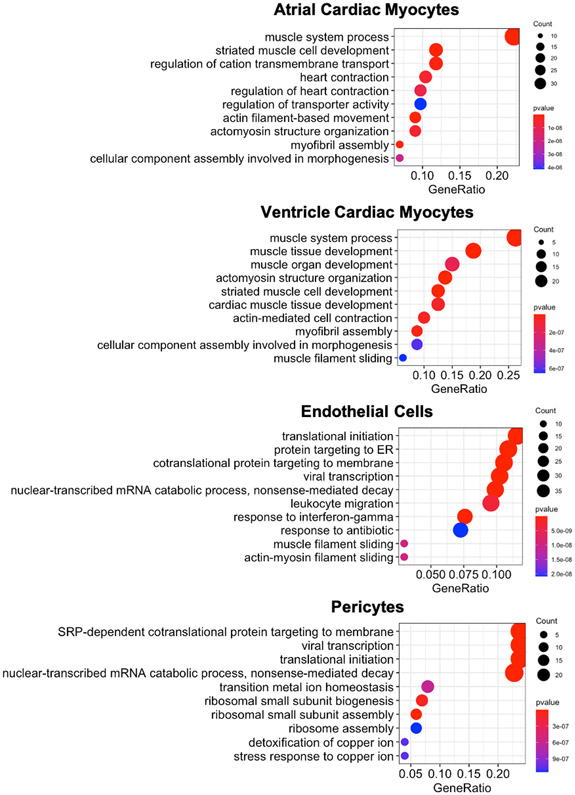
A significant number of genes are differentially regulated in female and male ventricular CMs [39]. With gene enrichment analysis, female CMs were found to upregulate genes involved with energy metabolism, while male cells upregulated genes associated with cell morphology, cell cycle, and cell movement [39]. The protein kinase A (PKA) pathway was differentially activated in female CMs and likely plays a role in mediating at least some of these differences [39]. Additionally, single-cell sequencing experiments found that muscle development and myofibril organization were enriched gene ontology (GO) terms in differentially expressed genes between male and female CMs (Figure 3) [26]. This enrichment is consistent with the known sex differences in the basic contractile function of CMs. While the differences between male and female ventricular and atrial CMs are similar (Figure 3), there are more differentially expressed genes in atrial CMs (178) compared to ventricular CMs (102) (SI Table 1). Since changes in epigenetic modifications and chromatin remodeling are associated with CM development and function [43-45], there are likely such dimorphisms in male and female cells as well. Although no studies to date have investigated epigenetic-based sexual dimorphisms in CMs, sex has been found to influence DNA methylation in myocytes from skeletal muscle [46]. Further work in this area is warranted.
Figure 3:
Gene ontology – biological processes term analysis of differentially expressed genes between male and female CMs, ECs, and pericytes. Lists of the differentially expressed genes between males and females from all cell types in the heart can be found in Supplemental Table 1. The gene ratio refers to the number of significantly different genes identified between male and female cells that fit within a specific GO term over the total number of significantly different genes identified. Essentially, the gene ratio signifies the enrichment of the GO term within the significantly differentially expressed gene set.
2.2. Endothelial Cells
Introduction
Clinical/pathophysiology:
The cardiac endothelium plays a crucial role in modulating the contractility and overall health of the myocardium. Anatomically, the cardiac endothelium can be split into three major regions: the inner linings of atrioventricular tissue, also known as the endocardial endothelium, blood vessels within the heart, also known as the myocardial endothelium, and lymphatic vessels [47]. Approximately 7-12% of total cells in the heart are endothelial cells (ECs) [26]. In the myocardial endothelium, cardiac ECs are known to contribute to sex differences in atherosclerotic vascular stiffening during coronary artery disease (CAD), which is the largest contributor to cardiovascular disease mortality and morbidity [48]. Of further note, increased arterial stiffness leads to a higher incidence of plaque formation and calcification in women relative to men [49]. Although risk factors for CAD are similar for men and women (e.g., hypertension, diabetes, high fat diets), low high-density lipoprotein levels and smoking are more predictive of coronary risk in women compared to men [50]. Pre-menopausal women are also known to have increased plaque erosion, whereas post-menopausal women experience increased plaque rupture [51]. Some of these sex differences are likely to be attributed to cellular dimorphisms between ECs.
Cell function and populations:
ECs have highly specialized functions within the endothelial linings, depending on their relative locations within the heart. In general, interactions between ECs and adjacent CMs are necessary for maintaining cardiac metabolism, growth, contractility, and rhythmicity [52]. Ten different subpopulations of ECs have been identified in male and female human hearts based on single cell sequencing [26]. Just as in CMs, there is a sex difference in the number of ECs. The hearts of male mice and humans both have a ~1.3-fold higher number of ECs relative to female hearts (Figure 2) [1,26]. Gonadectomy of mice did not reverse the sex difference in EC number [1], suggesting that the sexual dimorphic cellularity may be established independently of sex hormones. Although several reviews have discussed the roles of ECs in heart biology [3,4,52], we review the current understanding as to how biological sex may influence cardiac EC biology.
Extracellular:
Circulating Factors:
Cardiac ECs may have sex-specific secretomes since such differences have been reported in ECs from other tissues in response to stresses. For example, in response to serum starvation, male human umbilical vein endothelial cells (HUVECs) have increased apoptosis and pentraxin-related protein 3 (PTX3) levels relative to female HUVECs [5]. PTX3 primarily behaves as an acute phase response protein, and serum concentrations increase with inflammation and induce vascular endothelial dysfunction and hypertension [53]. Additionally, sex differences in endothelin-1 (ET-1) production are observed in adulthood, with men exhibiting higher plasma ET-1 levels relative to women [54]. ET-1 is a potent vasoactive peptide that is synthesized and secreted primarily by ECs; ET-1 increases cardiac contractility and heart rate [55], and may play a key role in the pathophysiology of CAD.
ECs in the heart may also regulate sexual dimorphisms in response to cardiac injuries and inflammation. Endothelial damage is exacerbated by reduced endothelial nitric oxide synthase (eNOS) activity, which causes reductions in nitric oxide availability [56]. Interestingly, eNOS gene and protein expression are increased ~2-fold in female HUVECs relative to male HUVECs [57]. Exercise has been shown to improve EC function through increased nitric oxide availability [58], but it is unclear if there are sex differences in this response. Increased autocrine EC activity is known to lead to increased cardiac hypertrophy after damage [59], which may be a partial driver of increased hypertrophy and greater left ventricular wall thickening in women due to aortic valve stenosis [60].
Cardiac ECs also have sex-specific functions in regulating recovery after myocardial infarction. Clinically, pre-menopausal women have lower rates of myocardial infarction and improved survival post-infarct, and risk for myocardial infarct increases in post-menopausal women [61]. Recent work has revealed improved recovery of the female mouse heart after myocardial ischemia, and knockout of signal transducer and activator of transcription 3 (STAT3), a transcription factor, in ECs neutralized these sex differences in myocardial recovery [62]. After ischemia, cardiac ECs show sexually dimorphic transcriptomes, with increased expression of family with sequence similarity 5, member C (FAM5C) in women after injury, which increases cardiac inflammation in women relative to men [63]. Taken together, cardiac ECs partially regulate sex dimorphisms in cardiac remodeling after injury, which may have a significant impact on how women should be treated after myocardial injury.
Extracellular Matrix:
The ECM surrounding the vascular endothelium in the heart provides a scaffold for ECs to organize into blood vessels during angiogenesis. EC adhesion to the ECM is critical for maintaining proper blood vessel stabilization, tubular formation, and vascular permeability following cardiac injury [64]. Sex hormones can impact EC behavior in vascular permeability and ECM deposition [65], but recent work suggests intrinsic sex differences in EC maintenance of vascular permeability. For example, there are sex-dependent responses of skeletal muscle ECs to cilostazol, a vasodilator, in vitro [66]. Female ECs had a significant reduction in vascular permeability in response to cilostazol treatment relative to males. The authors suggested that sex-dependent differences in collagen, fibronectin, and/or elastin may contribute to the decreased permeability observed in female ECs. Indeed, increased ECM deposition has been observed in female ECs, especially during menopause [67]. Together, both intrinsic gene expression differences and response to extracellular hormones likely regulate sexually dimorphic EC behavior, which likely explains why women are more susceptible to arterial stiffening during aging [68,69].
Intracellular:
Gene differences:
Intrinsic sex differences in gene expression of ECs are widely recognized and apparent in multiple tissues. Single cell sequencing reveals that gene expression differences in male and female ECs are related to immune cell regulation, like leukocyte migration (Figure 3). For example, Platelet and Endothelial Cell Adhesion Molecule 1 (PECAM1) is a pan-EC adhesion molecule responsible for maintaining EC intercellular junctions. Sex differences have been shown in rat ECs of the aorta and skeletal muscle, where female ECs have increased gene and protein expression for various cell adhesion markers including PECAM-1, N-cadherin (NCAD), and integrin αvβ3 and male ECs have higher levels of Vascular Cell Adhesion protein 1 (VCAM-1) [70]. Other studies have shown that 14-25% of the HUVEC transcriptome is sex-biased, based on studies comparing aortic ECs and HUVECs from boy-girl twins [71]. Open questions remain regarding sex differences are if these differences translate to cardiac ECs.
Sex differences in gene expression of endocardial ECs may also impact angiogenic behavior. Cardiac ECs express the angiogenic regulator SPARC Related Modular Calcium Binding 1 (SMOC1) to promote angiogenesis, cell migration, and proliferation across human, porcine, and murine sources [72]. Previous genetic analyses have identified expressed quantitative trait loci (eQTL) hotspots, which control sexually dimorphic gene expression, and showed a female bias for SMOC1 expression in mouse adipose tissue [73]. Another cardiac EC marker, SHROOM2, is known to regulate EC contractility, migration, and adhesion and can escape X-chromosome inactivation [74,75]. Further characterization of sexually dimorphic angiogenic behavior of cardiac ECs is warranted, based on observations in these other tissues.
Epigenetics:
Sex differences in cardiac EC chromatin remodeling have not been reported to date but may be responsible for intrinsic gene expression differences in ECs. Recent work suggests that cardiac ECs maintain open chromatin sites to actively express CM myofibril genes, previously thought to be unique to CMs [76]. However, open questions remain regarding how the common epigenetic signature between ECs and CMs leads to differential cardiac EC function relative to other EC types. We also know that cell sex influences DNA methylation and subsequent gene expression in a variety of cell types [46,77], suggesting cardiac ECs will also have sex-specific epigenetic alterations.
2.3. Pericytes
Clinical/pathophysiology:
Pericytes have been implicated in the development of disease states, including fibrosis, ossification and calcification, and atherosclerosis [78]. Specifically, pericytes contribute to fibrosis via detachment from vascular walls and differentiation into myofibroblasts [79-81]. Additionally, the loss of pericytes results in weakened capillary walls, dysfunction, and rarefaction [79]; however, studies have revealed a therapeutic potential of transplanting pericytes after cardiac injury [80], suggesting that understanding sex differences in pericyte phenotypes will aid in developing sex-specific cardiac therapies.
Cell functions and populations:
Pericytes are pleiotropic perivascular cells that are thought of as “vascular stem cells” due to their plasticity to differentiate into myofibroblasts [80] and smooth muscle cells [82] in response to injury. A majority of pericytes in the heart can be found in the coronary arteries [83]. The function of pericytes is to maintain homeostasis of the vascular wall, contribute to tissue regeneration, and promote transmigration of neutrophils [84] Studies have shown a therapeutic potential of pericyte transplantation after cardiac injury [85]. Pericyte progenitor cells can reduce scar formation and improve CM contractility, while improving heart function via microRNA (miR)-132 secretion in a myocardial infarction (MI) mouse model [86].
Extracellular/Intracellular:
Recent work recognizes the regenerative potential of cardiac pericytes, but there is a dearth of information regarding sex differences in these cells in the heart [65]. Male rats have been observed to have higher levels of miR-132 in the brain, suggesting a sexually dimorphic regulatory mechanism; however, no such studies have been performed on cardiac pericytes [87]. Sex differences were also observed in an ischemia-reperfusion (IR) injury of kidney tissue in spontaneous hypertensive rats. Results indicated that male rats had increased numbers of pericytes with respect to female rats, and that male rats experienced a greater loss of pericyte cell number post-IR [88]. Single-cell sequencing identified 106 differentially expressed genes between male and female pericytes, including smooth muscle aortic alpha-actin (ACTA2) and transgelin (TAGLN), both markers of smooth muscle cells [89], which potentially suggests that there are sex differences in differentiation into myofibroblasts or smooth muscle cells (SI Table 1, Figure 3). While pericytes are crucial for proper cardiac function and regeneration, almost nothing is known about their sex differences in the heart.
2.4. Cardiac Fibroblasts
Clinical/pathophysiology:
Cardiac fibroblasts (CFs) are critical in regulating the ECM of the healthy and diseased heart. Fibrosis is a consequence of the dysregulation of the wound healing process after cardiac injury, and therefore occurs in nearly all cardiovascular diseases, such as ischemia, hypertension, and aortic valve disease [90,91]. There are major sex differences in the prevalence and presentation of fibrosis after cardiac injury [92,93], where premenopausal women are generally protected from adverse cardiac remodeling. For example, women with left ventricle pressure overload display less myocardial collagen deposition than men [94]. Experimental studies in ovariectomized animals suggests estrogen signaling to be a key factor in the sexual dimorphic response to pressure overload [92]. These studies motivate the investigation of the role of CFs in many sexually dimorphic cardiac phenotypes.
Cell functions and populations:
Resident CFs are largely responsible for maintaining ECM homeostasis through tissue remodeling [95] and are important for maintaining proper tissue mechanics. In healthy heart tissue, CFs are largely quiescent, and characterized by expression of Transcription Factor 21 (TCF21) [96]. CFs also participate in wound healing. After cardiac injury, such as ischemia, these fibroblasts proliferate and activate to muscle-like fibroblasts called myofibroblasts [8]. Activated myofibroblasts are characterized by expression of collagens 1 and 3, alpha smooth muscle actin (αSMA), and periostin (POSTN), although other markers have been also been suggested as myofibroblast markers [8-10]. Persistent activation of myofibroblasts results in sexually dimorphic fibrosis [97]. We will review what is currently known and highlight areas which need further investigation about cellular sex in CFs.
CFs make up a large percentage of the cells in the heart, between 10-32% [26,98-100]. Interestingly, this cellular distribution is sexually dimorphic; female mice have relatively more CFs in their hearts compared to male mice [1], but this difference was eliminated with gonadectomy, which suggests hormones play a significant role in determining the CF population of male and female hearts. Recent single-cell sequencing experiments have further revealed the diversity in the CF population of the heart as well. For example, in healthy human hearts, a recent study reported seven CF subpopulations [26], while another study reported four fibroblast subpopulations [100]. These CF subpopulations correspond to distinctive functional properties, like ECM remodeling or transforming growth factor beta (TGF-β) signaling. We posit that the shifting proportions of these CF subpopulations are sexually dimorphic, since there is a differential fibrotic response in males and females. Indeed, when Angiotensin II, which causes fibrosis and hypertension, was given to mice, a pro-fibrotic subpopulation of CFs increased 2-fold in females compared to males [101].
Extracellular:
Circulating factors:
A major difference between the microenvironment of male and female CFs is hormone exposure. Female cells are exposed to higher levels of estrogen, while male cells are exposed to higher levels of androgens, and these microenvironmental differences have been shown to modulate CF phenotypes. Interestingly, CFs express the highest levels of estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), and androgen receptor (AR) of all the cells in the heart [101]. Estrogen can protect female CFs from a myofibroblast phenotype, at least in part, by downregulating collagens and matrix metalloproteases (MMPs) [102,103]. In addition, estrogen inhibits miRNAs that promote the cellular fibrotic response [104]. The mechanism for estrogen mediated protection against myofibroblast activation involves both ERα and ERβ since genetic knockout of either causes cardiac fibrosis [105,106]. Activation of the non-traditional estrogen receptor, G protein-coupled receptor 30 (GPR30), inhibits CF proliferation [107], indicating hormone receptors play major roles in controlling CF phenotypes. In addition to estrogen, androgens are also capable of modulating CFs. Testosterone protects against TGF-β-induced collagen production in male rat CFs [108].
In addition to hormones, circulating proteins differ between men and women [109] that may lead to sex differences in CFs. A recent study from our group found that in physiological microenvironments, female CFs treated with female sera from aortic valve disease patients deactivated the myofibroblast phenotype more than male CFs treated with male sera [110]. Additionally, CFs themselves secrete factors that can influence cardiac function [111]. Male CFs activated to myofibroblasts through matrix cues versus TGF-β have different secretomes, with the secretome of TGF-β-activated CFs promoting CM hypertrophy [112]. Since not all CFs secrete the same factors, it is possible that male and female CFs have different secretory profiles, offering another avenue of investigation.
Extracellular matrix:
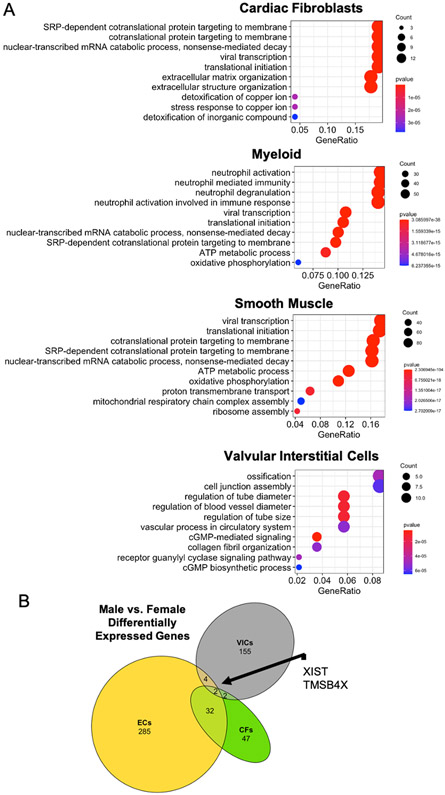
There are sex-specific differences in matrix composition that potentially influence CF phenotype, since cells are able to sense and respond to matrix cues, like stiffness or stretch [113]. For example, CFs activate to myofibroblasts when cultured on matrices with a stiffness similar to fibrotic tissue, while they maintain their quiescence on softer material that mimics healthy tissue [112]. Our group recently observed that male hearts are significantly stiffer than female rat hearts (Peter et al. JAHA Accepted). This stiffness difference might be caused by differential matrix composition. In young adult humans, female hearts contain less collagens 1 and 3 than male hearts, but the difference in ECM is reversed in hearts from aged males and females [21]. This reversal in ECM deposition with age is also observed in mice, where aged female mice display increased reactive and replacement fibrosis compared to aged male mice, likely mediated by an increase in fibroblast proliferation in females [114]. Supporting these findings, ECM organization is the top GO term difference between male and female CFs (Figure 4A). Further, sexually dimorphic regulation of matrix remodelers, including TIMPs [21,115], may contribute to the matrix differences in mechanics and/or degradation. Overall, these studies show that male and female hearts have distinctive mechanical microenvironments, likely influenced by male and female CF and vice versa, including their susceptibility to activation and proliferation in response to injury cues.
Figure 4:
A) Gene ontology – biological processes term analysis of differentially expressed genes between male and female cardiac fibroblasts, myeloid, smooth muscle, and valvular interstitial cells. Lists of the differentially expressed genes between males and females from all cell types in the heart can be found in Supplemental Table 1. The gene ratio refers to the number of significantly different genes identified between male and female cells that fit within a specific GO term over the total number of significantly different genes identified. Essentially, the gene ratio signifies the enrichment of the GO term within the significantly differentially expressed gene set. B) Comparisons of differentially expressed genes between male and female cells. ECs = endothelial cells, CFs = cardiac fibroblasts, VICs = valvular interstitial cells. Data for ECs and CFs from heartcellatlas.org. Data for VICs from microarray of porcine VICs [174].
Intracellular:
Gene/protein expression:
Recent studies indicate that there are intrinsic sex differences in gene expression of CFs (Figure 4A). Our group isolated CFs from young rats and found female CFs were more proliferative than males, but males were more myofibroblast-like than females (Peter et al. JAHA, in press). Moreover, differences in gene expression between male and female CFs have been identified via single-cell sequencing experiments [26,100,116,117]. These expression patterns likely influence the fibroblast’s ability to mitigate or promote myofibroblast activation. For example, Cysteine Rich Secretory Protein LCCL Domain Containing 2 (CRISPLD2) modulates proliferation, apoptosis, and migration in lung fibroblasts [118] and is higher in female CFs at baseline [100]. Differential gene expression in male and female CFs also controls their ability to adapt to exercise. CF expression of metallothionein 1 and 2 (two antioxidant genes) is more important for preventing exercise induced fibrosis in male mice than female mice [119]. However, not all studies report sex differences in CFs. For example, one study immunostained for fibroblast markers in young male and female mice and found no significant differences in TCF21, Platelet Derived Growth Factor Receptor Alpha (PDGFRα), or αSMA [114]. Clearly, more work is needed to determine whether these expression patterns influence sex-biased CF phenotypes.
Epigenetics:
It is unclear if genotype (XX, XY) and epigenetic remodeling influence the sexually dimorphic CF phenotype. Studies in other fibrotic models suggest that X-linked and Y-linked genes are involved in CF activation or quiescence. For example, X-linked expression of X-inactive specific transcript (XIST) has been shown to be protective against renal fibrosis [120]. Moreover, TIMP-1, a matrix remodeler, is implicated in fibrosis [121] and is capable of escaping X inactivation [74,122], which could influence female CF’s ability to regulate matrix deposition and remodeling. Epigenetic remodeling is known to influence CF proliferation and myofibroblast activation [123]. In fact, inhibition of Class I histone deacetylases (HDACs) can suppress CF proliferation and mitigate fibrotic remodeling after cardiac injury [124-126]. Other chromatin remodelers, including bromodomain-containing protein 4 (BRD4), function downstream of TGF-β to promote the expression of pro-fibrotic genes [127]. Interestingly, many of these chromatin remodelers have been found to have sexually dimorphic functions in other tissues. In the brain, BRD4-bound enhancers drive cell intrinsic sex differences in tumors and inhibitors of BRD4 have opposing effects in males and females [128]. Unfortunately, studies investigating epigenetic-based sex differences in CFs are lacking. Further epigenetic-based studies are needed to address the potential influence of inherent epigenetic differences between male and female CF proliferation and activation.
2.5. Myeloid Cells (Macrophages/Monocytes)
Clinical/pathophysiology:
The human heart has a heterogeneous population of myeloid cells, including macrophages [129] and other types of immune cells [130]. The demands of the resident and circulating myeloid cell population varies with age, inflammation state, and tissue injury in regulating repair and homeostasis. Cardiovascular disease is associated with chronic inflammation [131]. Dysregulation of the immune response prevents tissue recovery, leading to left ventricular dilation [132] or atherosclerotic plaque buildup [133]. Sex differences with respect to immunity and immune response have been reviewed and presented elsewhere in detail [134,135] including their role in cardiac tissue [136-138]. Here, we touch briefly on the role of macrophages in sex-specific cardiac disease.
Cell functions and populations:
Inflammation-mediated myeloid infiltration has been reported to be increased in males relative to females [139]. However, in a mouse model of viral myocarditis, macrophages from male mice expressed higher levels of M1(pro-inflammatory) markers while females had more M2 (anti-inflammatory) markers [140]. Additionally, M1 macrophage conditioned-medium was shown to promote an osteoblast-like phenotype in male porcine valvular interstitial cells, suggesting that macrophage polarity mediates cellular phenotypes leading to clinically relevant sex dimorphisms [141]. Another study discovered a pro-inflammatory shift accompanied by a decrease in anti-inflammatory protection in the macrophage population of aged female human hearts (>50 years old) that was not observed in males [142].
Extracellular/Intracellular:
Macrophage secretion and interaction with matrix vesicles have been linked to cardiovascular calcification [13,143,144], yet the influence of sex in this phenomenon is unexplored. Sex hormones have a well-documented contribution to sex-specific inflammation and macrophage activity [145,146]. Male macrophages have increased adrenergic receptor (AR) expression relative to females, which was reported to lead to an increase in lipid accumulation and increased risk of atherosclerosis in males [11]. Cholesterol accumulation and apoptosis were lowered in female macrophages treated with estrogen and progesterone [147,148]. Single-cell sequencing analysis of the myeloid cell population revealed that neutrophil activation is differentially regulated between male and female cells (Figure 4A).
2.6. Smooth Muscle Cells
Clinical/pathophysiology:
During atherosclerosis progression, vascular smooth muscle cells (SMCs) are the main source of plaque formation and excess ECM production, of which there are sex differences. Men are more likely to die than pre-menopausal women from atherosclerosis [149] and hypertension [150]; however, this trend is reversed with postmenopause where hypertension rates are higher in women than men.
Cell functions and populations:
The main function of SMCs is to regulate the caliber of blood vessels by relaxing or contracting [12]. In a healthy adult, SMCs adopt a quiescent or contractile phenotype to control vasodilation or constriction, respectively [151]. Functional changes in the SMC phenotype can lead to diseases, including atherosclerosis and hypertension [12,152]. However, after vessel injury, SMCs undergo a transition to a synthetic phenotype characterized by increased migration, growth, and ECM deposition. The increased incidence of atherosclerosis in males is thought to be caused in part by differences of SMC phenotype [6], specifically elevated proliferation and migration in male SMCs compared to female SMCs [7,153,154].
Extracellular/Intracellular:
Male and female SMCs express different levels of ECM remodeling proteins. In induced pluripotent stem cell (iPSC)-derived SMCs, female cells express reduced MMP-1 levels, but increased collagen 1 relative to male cells [155]. These studies indicate that SMCs are sexually dimorphic at baseline. In addition, estrogen is implicated in playing a protective role in preventing dramatic shifts in SMC phenotype. For example, estrogen decreases proliferation in female coronary arterial SMCs, but has no effect on proliferation of males or cells from ovariectomized females [156]. Estrogen also protects from excessive vascular reactivity during abnormal metabolite presence associated with hypertension [157]. However, circulating sex hormones are likely not the only factor in regulating the distinct SMC sex phenotypes. SMCs have the highest number of differently expressed genes between male and female cells from all the cell types in the heart (523) (Figure 4A, SI Table 1), including genes associated with SMC maturation/differentiation, like ACTA2, calponin (CNN1), and TAGLN [89,158]. Studies investigating SMCs from newborn rats have shown that male SMCs are more proliferative and less adhesive than female SMCs [159], suggesting that other factors are playing a role; however, little is known about sex differences in cardiac SMCs.
2.7. Valve Cells
Introduction
Clinical/pathophysiology:
Sexual dimorphisms are present in the pathophysiology of valve disease. Reports indicate that male sex is a perioperative risk factor associated with morbidity after tricuspid valve replacement [160] and is associated with decreased time between congenital pulmonary valve disruption and replacement [161]. In female patients, mitral valve prolapse is reported to occur 2.5-4% more frequently than in males. Female sex is further associated with more mitral valve leaflet thickening, and postmenopausal osteoporosis is considered a risk factor in mitral valve calcification [14,15,25]. Aortic valve stenosis (AVS) presents later in women [16], where symptoms are more severe with a lesser amount of calcification [17,18]. Interestingly, the mineralization that occurs during aortic valve calcification (AVC) develops more slowly in females relative to males [162]. Males have a 2-fold excess risk in developing AVS [18], and bicuspid valves are ~3x more likely in male patients [19]. While it is difficult to decouple cellular sex from lifestyle and mechanics from physiological differences, there is little known about how inherent cellular sex of valve cells contribute to these clinical observations.
Cell Functions and Populations:
The tricuspid, pulmonary, mitral, and aortic semilunar valves direct blood flow unidirectionally through the heart. While there is relatively similarity in the valve structures, the laminated leaflets are composed of ECM components that include collagens, elastin, laminin, fibronectin, glycosaminoglycans, and proteoglycans [163]. Valvular interstitial cells (VICs) constitute the main cell population of the leaflet, while valvular endothelial cells (VECs) line the external surfaces in contact with blood flow. Several different types of VIC phenotypes are known to exist, including progenitor endothelial/mesenchymal cells, progenitor VICs, quiescent VICs, activated VICs, and osteoblast-like VICs [164]. It is not clear whether VICs can transdifferentiate between each of these phenotypes or derive from individual progenitor or mesenchymal cells. Additionally, single cell sequencing of valve tissue identified three distinct populations of VICs, and two populations of VECs [165,166]Future work should investigate if these VIC subpopulations differ between male and female valves.
Much like the CFs discussed earlier, quiescent VICs activate to a pro-fibrotic myofibroblast phenotype, marked by increased contractility, αSMA, and ECM production [164] to maintain tissue homeostasis or in response to stimuli such as injury, pro-inflammatory biochemical cues [167] or changes in ECM composition or stiffness [168]. Alterations in VIC phenotypes and ECM can lead to valve degeneration, such as in myxomatous valve disease, sclerosis, and stenosis. Calcification, as seen in mitral and AVC, is mediated by VICs that adopt an osteoblast-like phenotype in response to mechanical and biochemical cues [141,169], leading to further stiffening of the tissue and disrupted blood flow.
Extracellular
Circulating factors:
Factors secreted from valve cells into their local environment contribute to observed sexual dimorphisms. Serum collected from male AVS patients after a transcatheter aortic valve replacement (TAVR) had increased interleukin (IL)-1β and tumor necrosis factor alpha (TNF-α), while bone morphogenetic protein (BMP)-6 was more abundant in male pre-TAVR serum relative to post-TAVR [110]. Porcine VICs exposed to the male pre-TAVR serum showed increased myofibroblast activation relative to sera collected from females, suggesting that circulating factors in male pre-TAVR serum promote an osteogenic cellular phenotype [110]. Further studies revealed IL-1β and TNF-α may regulate male VIC proliferation prior to osteoblast-like cell differentiation [170], although further study in female VICs is warranted. Additionally, male and female VICs respond differently to pro-inflammatory cues. In response to the cytokine interferon gamma (IFN-γ), human male VICs exhibit greater pro-angiogenic, pro-calcific and pro-apoptotic responses than females [171]. These IFN-γ treated male VICs also increase expression of the osteogenic markers BMP-2 and RUNX2 when compared to female VICS [172]. Reports suggest that reduced calcification in female VICs may be due to decreased alkaline phosphatase content [173], and sex-specific regulation of protein kinase B (Akt) activation [172]. While circulating sex hormones undoubtably contribute to sexual dimorphisms in vivo, in vitro studies with VICs are less clear. One report suggested that there was no direct response of VICs to sex hormones, nor of the expression of sex steroid receptors in VICs [174]. However, in a separate study, female rat VIC cell number decreased with increasing concentration of estrogen treatment relative to male cells [173].
Extracellular Matrix:
Limited data suggest sexual dimorphisms exist in the microenvironment of male and female valves with respect to the ECM composition. For example, mitral valves from healthy human females contain more of the proteoglycan, versican, relative to male tissue, and male myxomatous valves have increased proteoglycan content overall [175]. Additionally, female stenotic and rheumatic aortic human valves have increased collagen content with reduced calcification [18,176,177]. Besides whole valve tissue, sexual dimorphisms have also been observed in VIC expression profiles as well. VICs from female rats have increased glycosaminoglycan content, collagen 1, and MMP-2 expression compared to males [173]. Additionally, ECM organization and collagen fibrils are two of the most significant GO terms enriched between male and female VICs (Figure 4A), suggesting that outside-in cell signaling may be linked with cellular sex, potentially contributing to clinical differences between men and women.
Intracellular
Genetic:
Genetic and epigenetic factors contributing to sexual dimorphisms of valve cells are likely but are understudied. A microarray analysis of porcine VICs identified 183 genes that were sex-specific between male and females, particularly of pathways associated with ECM remodeling (aggrecan (ACAN), dipeptidyl peptidase 4 (DPP4)), angiogenesis (angiopoietin-like 4 (ANGPTl4)), proliferation (Calcitonin Receptor Like Receptor (CALCRL)), lipid metabolism (apolipoprotein E (APOE)), and calcification (stanniocalcin-1 (STC1), Natriuretic peptide precursor C (NPPC)) [174] (Figure 4A). Additionally, basal levels of the osteogenic gene Matrix Gla protein (MGP) and anti-apoptosis gene B-cell lymphoma 2 (BCL-2) were increased in human female calcific aortic valve tissue and VICs [172]. Interestingly, the Phosphoinositide 3-kinase (PI3K) pathway was implicated in these gene expression differences, as the use of a PI3K inhibitor LY294002 increased mineralization of female cells in vitro [172]. miRNAs are also known to play a role in AVS [178]. MiRNA-29b and miRNA-214 promote calcification of human VICs [179,180], although these data were not separated by sex. However, miRNA-29b has been found to be a predictive marker of reverse remodeling and hypertrophy in female patients after valve replacement [181]. Also, miRNA-214 was found to be male-biased in rat kidney tissue [182], suggesting a potential sex specific role of these and other miRNAs in valve disease.
Epigenetic:
Chromatin remodeling is involved in the progression of valve disease [183,184], yet how cellular sex, hormones, and sex-specific epigenetic modifications contribute to AVS and AVC remain unclear. Parallels can be drawn between the epigenetic regulation of 5-lipoxygenase (5-LO), where calcified aortic valve tissue shows decreased DNA methylation of the promoter for this proinflammatory enzyme [185], which is elsewhere linked to sex-specific inflammatory role of neutrophils [186]. In contrast, the methylation of long non-coding RNA (lncRNA) H19, known to be associated with AVC via suppression of Notch homolog 1, translocation-associated (NOTCH1) in humans [187], was not different between male and female mice [188].
3. Discussion and Forward Thinking
An increased focus on the pathophysiology of sex differences that arise in cardiac disease is a promising direction for improving patient health and treatment, yet more research is needed to understand the nuances that occur because of cellular sex, especially within individual cell types present in the heart. Based on the above-mentioned studies on cellular sex (SI Table 1), it is clear that research focused on understanding the mechanisms involved is needed if sex-dependent cardiac therapies are to be realized. Our comparisons of differentially expressed genes in male and female cells (Figure 3,4, SI Figure 1) reveal that many of these mechanisms are highly specific to each cell type and highlights the importance of studies focused on specific cell types, rather than whole cardiac tissue. Even as the scientific community is calling for increased requirements for the inclusion of the sex of animals and cells used in studies, there is still a general lack of statistical analysis between male and female samples. Future work needs to include data, even if non-significant, between the sexes.
While the focus of this review was to highlight the baseline differences between male and female cardiac cells, there are additional insights to be gained from reviewing the responses of these cells to pathological (injury, age) and physiological (pregnancy, exercise) cues. It is generally recognized that males and females experience different cardiac adaptations to some of these inputs, but how these adaptations are controlled by specific cells is under explored. It is clear there is an interplay between sex and age where the cardioprotective mechanisms associated with the female sex disappear after menopause [2]. Understanding the specific role of various cell types in this loss of protection will be important for developing postmenopausal care to women with cardiovascular disease. Even more poorly studied is the impact of pregnancy on these cell types. Sex hormones flood the body during pregnancy, in addition to transferred factors from the fetus and changing dynamics of fluid flow [189,190], influence cell phenotypes, but it is unclear if pregnancy has a transient or persistent impact on cells in the heart. Exercise also causes cardiac remodeling in a sex-specific manner. The hearts of exercised female mice increase more in size than those of males when normalized to the amount of exercise [40], but whether and how non-CM cells play a role in this sex difference is still unclear and could provide additional mechanistic insights that can be leveraged for future therapies.
Additionally, most studies to date use non-physiologic in vitro culture models to study sex differences, which neglects the extracellular matrix effects and mechanical signaling on cell-phenotype. Male and female cells have distinct microenvironments with complex interactions between neighboring cells/cell types and their local ECM [21]. The complexities of these sex-specific cellular microenvironments call for advancement in in vitro models that can recapitulate clinical findings. However, very few investigations using more complex in vitro models report cellular sex. A 2017 snapshot review of organ-on-a-chip studies reported that 62% of models did not specify cellular sex [191]. Studies on the development of biomaterial matrices are even worse; only 3.7% of publications included the sex of cells used [192]. To achieve the goal of precision, patient-specific medicine will require a fundamental understanding of the sexual dimorphisms present, not only cellular sex, but of the sex-differences in microenvironmental factors and matrix composition [192,193].
Cardiac tissue engineering necessitates complex coordination of over 20 different cell types [100], microenvironmental matrix, cellular crosstalk, fluid flow, mechanical and electrical function, and cellular sex. The bioengineering community is rapidly developing and utilizing new technologies aimed at culturing multiple cell types and mimicking cardiac tissue. One strategy for cardiac tissue engineering is recellularization of an entire cadaveric human heart with iPSC-derived CMs toward the goal of mimicking active myocardial tissue [194]. Other strategies include developing cardiac organoids to recapitulate aspects of cardiac tissue. For example, agarose organoids were engineered as a model for myocardial infarction and formed using hiPSCs, CFs, HUVECs, and mesenchymal stem cells [195]. Other examples implementing synthetic or natural-based hydrogel matrices for 3D co-culture of multiple cell types, including CFs and CMs, to regenerating models of cardiac muscle contraction and remodeling [196]. Advanced biomaterials or scar-on-a-chip methods are promising directions for studying cardiac biology and the sex differences within [197-199]. The complexity of cardiac microenvironments and the disparities between male and female cells must be addressed in developing in vitro models for cardiac tissue. Nearly all cell types of the heart are understudied regarding cellular sex and how this impacts pathophysiology; however, more research is needed to develop more personalized therapies and treatments in the future.
4. Conclusion
Sex differences in cardiac myocytes, fibroblasts, endothelial cells, valve cells, and other resident cells must drive the well-known pathophysiological differences between men and women. However, very little is known about the mechanisms underlying cellular sex phenotypes. Recent studies suggest there are differences between the sexes in cellularity, their response to extracellular cues, and gene expression and epigenetics that ultimately influence the pathophysiology of heart disease. In general, premenopausal women are protected from pathological phenotypes compared to men, which suggests that hormone signaling plays a significant role in cellular sex differences. However, intrinsic cellular sex also plays an important role, and more attention needs to be focused on this. Moreover, future work should consider additional complexities, including biomimetic in vitro systems, to probe cellular sex differences. Research could focus on harnessing these cell sex differences to better understand and develop treatment regimens for both men and women.
Supplementary Material
Supplemental Figure 1: GO term biological processes (BP) enrichment for differentially expressed genes shared by more than one cell type from Figure 4B.
Supplemental Table 2: Summary of sex differences of adult mammalian cardiac cellular sex reported and discussed within this review. h = human data, m = murine data, p = porcine data, r = rat data; *this is reviewed elsewhere in depth.
Acknowledgments
Authors acknowledge funding from NIH (RO1 HL132353, R01 HL142935, R01 GM29090, R01 117138). C.J.W. acknowledges funding from the NIH NRSA Predoctoral Fellowship (F31HL142223). M.E.S. acknowledges funding from the NIH (T32 HL007822). B.A.A. acknowledges funding from the NIH (K99 HL148542) and the Burroughs Welcome Fund Postdoctoral Enrichment Program. The graphical abstract and Figure 1 were created using BioRender.com.
Abbreviations
- ECM
extracellular matrix
- CM
cardiac myocyte
- PKA
protein kinase A
- GO
gene ontology
- EC
endothelial cells
- CAD
coronary artery disease
- PTX3
pentraxin-related protein 3
- ET-1
endothelin-1
- eNOS
nitric oxide synthase
- STAT3
signal transducer and activator of transcription 3
- PECAM1
Platelet and Endothelial Cell Adhesion Molecule 1
- NCAD
N-cadherin
- VCAM-1
Vascular Cell Adhesion protein 1
- SMOC1
SPARC Related Modular Calcium Binding 1
- eQTL
quantitative trait loci
- MI
myocardial infarction
- IR
ischemia-reperfusion
- ACTA2/αSMA
alpha smooth muscle actin
- TAGLN
transgelin
- CF
cardiac fibroblast
- POSTN
periostin
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- AR
androgen receptor
- GPR-30
G protein-coupled receptor 30
- TGF-β
transforming growth factor beta
- CRISPLD2
Cysteine Rich Secretory Protein LCCL Domain Containing 2
- TCF21
Transcription Factor 21
- PDGFRα
Platelet Derived Growth Factor Receptor Alpha
- XIST
X-inactive specific transcript
- HDAC
histone deacetylases
- BRD4
bromodomain-containing protein 4
- SMC
vascular smooth muscle cells
- IPSC
induced pluripotent stem cell
- MMP
matrix metalloprotease
- AVS
aortic valve stenosis
- AVC
aortic valve calcification
- VICs
valvular interstitial cells
- VECs
valvular endothelial cells
- TAVR
transcatheter aortic valve replacement
- IL
interleukin
- TNF-α
tumor necrosis factor
- BMP
bone morphogenetic protein
- IFN-γ
interferon gamma
- RUNX2
runt-related transcription factor 2
- HUVEC
human umbilical vein endothelial cell
- AKT
protein kinase B
- miR/miRNA
microRNA
- ACAN
aggrecan
- DPP4
dipeptidyl peptidase 4
- ANGPTL4
angiopoietin-like 4
- CALCRL
Calcitonin Receptor Like Receptor
- APOE
apolipoprotein E
- STC1
stanniocalcin-1
- NPPC
natriuretic peptide precursor C
- MGP
Matrix Gla protein
- BCL-2
B-cell lymphoma 2
- PI3K
phosphoinositide 3-kinase
- 5-LO
5-lipoxygenase
- lncRNA
long non-coding RNA
- NOTCH1
notch homolog 1, translocation-associated
- SMAD
Mothers Against DPP Homolog
Footnotes
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- [1].Squiers GT, McLellan MA, Ilinykh A, Branca J, Rosenthal NA, Pinto AR, Cardiac cellularity is dependent upon biological sex and is regulated by gonadal hormones, Cardiovasc. Res (2020). 10.1093/cvr/cvaa265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Leinwand LA, Sex is a potent modifier of the cardiovascular system, J. Clin. Invest 112 (2003) 302–307. 10.1172/jci19429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ishii Y, Langberg J, Rosborough K, Mikawa T, Endothelial cell lineages of the heart, Cell Tissue Res. 335 (2009) 67–73. 10.1007/s00441-008-0663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brutsaert DL, Cardiac endothelial-myocardial signaling: Its role in cardiac growth, contractile performance, and rhythmicity, Physiol. Rev 83 (2003) 59–115. 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- [5].Cattaneo MG, Banfi C, Brioschi M, Lattuada D, Vicentini LM, Sex-dependent differences in the secretome of human endothelial cells, Biol. Sex Differ 12 (2021) 7. 10.1186/s13293-020-00350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bačáková L, Kuneš J, Gender differences in growth of vascular smooth muscle cells isolated from hypertensive and normotensive rats, Clin. Exp. Hypertens 22 (2000) 33–44. 10.1081/CEH-100100060. [DOI] [PubMed] [Google Scholar]
- [7].Fitzpatrick LA, Ruan M, Anderson J, Moraghan T, Miller V, Gender-related differences in vascular smooth muscle cell proliferation: Implications for prevention of atherosclerosis, in: Lupus, 1999: pp. 397–401. 10.1177/096120339900800514. [DOI] [PubMed] [Google Scholar]
- [8].Baum J, Duffy HS, Fibroblasts and myofibroblasts: What are we talking about?, J. Cardiovasc. Pharmacol 57 (2011) 376–379. 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, et al. , Genetic lineage tracing defines myofibroblast origin and function in the injured heart, Nat. Commun 7 (2016) 1–14. 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bagalad B, Mohan Kumar K, Puneeth H, Myofibroblasts: Master of disguise, J. Oral Maxillofac. Pathol 21 (2017) 462–463. 10.4103/jomfp.JOMFP_146_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McCrohon JA, Death AK, Nakhla S, Jessup W, Handelsman DJ, Stanley KK, et al. , Androgen receptor expression is greater in macrophages from male than from female donors: A sex difference with implications for atherogenesis, Circulation. 101 (2000) 224–226. 10.1161/01.CIR.101.3.224. [DOI] [PubMed] [Google Scholar]
- [12].Zhuge Y, Zhang J, Qian F, Wen Z, Niu C, Xu K, et al. , Role of smooth muscle cells in cardiovascular disease, Int. J. Biol. Sci 16 (2020) 2741–2751. 10.7150/ijbs.49871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Krohn JB, Hutcheson JD, Martínez-Martínez E, Aikawa E, Extracellular vesicles in cardiovascular calcification: Expanding current paradigms, J. Physiol 594 (2016) 2895–2903. 10.1113/JP271338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sugihara N, Matsuzaki M, The influence of severe bone loss on mitral annular calcification in postmenopausal osteoporosis of elderly japanese women, Jpn. Circ. J 57 (1993) 14–26. 10.1253/jcj.57.14. [DOI] [PubMed] [Google Scholar]
- [15].Avierinos JF, Inamo J, Grigioni F, Gersh B, Shub C, Enriquez-Sarano M, Sex differences in morphology and outcomes of mitral valve prolapse, Ann. Intern. Med 149 (2008) 787–794. 10.7326/0003-4819-149-11-200812020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Saeed S, Dweck MR, Chambers J, Sex differences in aortic stenosis: from pathophysiology to treatment, Expert Rev. Cardiovasc. Ther 18 (2020) 65–76. 10.1080/14779072.2020.1732209. [DOI] [PubMed] [Google Scholar]
- [17].Myasoedova VA, Di Minno A, Songia P, Massaiu I, Alfieri V, Valerio V, et al. , Sex-specific differences in age-related aortic valve calcium load: A systematic review and meta-analysis, Ageing Res. Rev 61 (2020). 10.1016/j.arr.2020.101077. [DOI] [PubMed] [Google Scholar]
- [18].Voisine M, Hervault M, Shen M, Boilard AJ, Filion B, Rosa M, et al. , Age, Sex, and Valve Phenotype Differences in Fibro-Calcific Remodeling of Calcified Aortic Valve, J. Am. Heart Assoc 9 (2020) e015610. 10.1161/JAHA.119.015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kong WKF, Bax JJ, Michelena HI, Delgado V, Sex differences in bicuspid aortic valve disease, Prog. Cardiovasc. Dis 63 (2020) 452–456. 10.1016/j.pcad.2020.06.004. [DOI] [PubMed] [Google Scholar]
- [20].Grilo GA, Shaver PR, Stoffel HJ, Morrow CA, Johnson OT, Iyer RP, et al. , Age- and sex-dependent differences in extracellular matrix metabolism associate with cardiac functional and structural changes, J. Mol. Cell. Cardiol 139 (2020) 62–74. 10.1016/j.yjmcc.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dworatzek E, Baczko I, Kararigas G, Effects of aging on cardiac extracellular matrix in men and women, Proteomics - Clin. Appl 10 (2016) 84–91. 10.1002/prca.201500031. [DOI] [PubMed] [Google Scholar]
- [22].Chan PS, Invisible gender in medical research, Circ. Cardiovasc. Qual. Outcomes 12 (2019) 5694. 10.1161/CIRCOUTCOMES.119.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Alzahrani T, Nguyen T, Ryan A, Dwairy A, McCaffrey J, Yunus R, et al. , Cardiovascular disease risk factors and myocardial infarction in the transgender population, Circ. Cardiovasc. Qual. Outcomes 12 (2019). 10.1161/CIRCOUTCOMES.119.005597. [DOI] [PubMed] [Google Scholar]
- [24].Chung E, Leinwand LA, Pregnancy as a cardiac stress model, Cardiovasc. Res 101 (2014) 561–570. 10.1093/cvr/cvu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Regitz-Zagrosek V, Kararigas G, Mechanistic pathways of sex differences in cardiovascular disease, Physiol. Rev 97 (2017) 1–37. 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- [26].Litviňuková M, Talavera-López C, Maatz H, Reichart D, Worth CL, Lindberg EL, et al. , Cells of the adult human heart, Nature. 588 (2020) 466–472. 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Keller KM, Howlett SE, Sex Differences in the Biology and Pathology of the Aging Heart, Can. J. Cardiol 32 (2016) 1065–1073. 10.1016/j.cjca.2016.03.017. [DOI] [PubMed] [Google Scholar]
- [28].Minton K, Peritoneal sex differences, Nat. Rev. Immunol 20 (2020) 460–461. 10.1038/s41577-020-0385-3. [DOI] [PubMed] [Google Scholar]
- [29].Klein SL, Flanagan KL, Sex differences in immune responses, Nat. Rev. Immunol 16 (2016) 626–638. 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- [30].Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, et al. , Cardiomyocyte death: Mechanisms and translational implications, Cell Death Dis. 2 (2011). 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Foryst-Ludwig A, Kintscher U, Sex differences in exercise-induced cardiac hypertrophy, Pflugers Arch. Eur. J. Physiol 465 (2013) 731–737. 10.1007/s00424-013-1225-0. [DOI] [PubMed] [Google Scholar]
- [32].Stauffer BL, Sobus RD, Sucharov CC, Sex differences in cardiomyocyte connexin43 expression, J. Cardiovasc. Pharmacol 58 (2011) 32–39. 10.1097/FJC.0b013e31821b70b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Parks RJ, Howlett SE, Sex differences in mechanisms of cardiac excitation-contraction coupling, Pflugers Arch. Eur. J. Physiol 465 (2013) 747–763. 10.1007/s00424-013-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vizgirda VM, Wahler GM, Sondgeroth KL, Ziolo MT, Schwertz DW, Mechanisms of sex differences in rat cardiac myocyte response to β-adrenergic stimulation, Am. J. Physiol. - Hear. Circ. Physiol 282 (2002). 10.1152/ajpheart.2002.282.1.h256. [DOI] [PubMed] [Google Scholar]
- [35].Parks RJ, Ray G, Bienvenu LA, Rose RA, Howlett SE, Sex differences in SR Ca2+ release in murine ventricular myocytes are regulated by the cAMP/PKA pathway, J. Mol. Cell. Cardiol 75 (2014) 162–173. 10.1016/j.yjmcc.2014.07.006. [DOI] [PubMed] [Google Scholar]
- [36].Orogo AM, Gustafsson ÅB, Cell death in the myocardium: My heart won’t go on, IUBMB Life. 65 (2013) 651–656. 10.1002/iub.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yutzey KE, Cardiomyocyte proliferation, Circ. Res 120 (2017) 627–629. 10.1161/CIRCRESAHA.116.310058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Deegan DF, Karbalaei R, Madzo J, Kulathinal RJ, Engel N, The developmental origins of sex-biased expression in cardiac development, Biol. Sex Differ 10 (2019). 10.1186/s13293-019-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Trexler CL, Odell AT, Jeong MY, Dowell RD, Leinwand LA, Transcriptome and Functional Profile of Cardiac Myocytes Is Influenced by Biological Sex, Circ. Cardiovasc. Genet 10 (2017). 10.1161/CIRCGENETICS.117.001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA, Sex modifies exercise and cardiac adaptation in mice, Am. J. Physiol. - Hear. Circ. Physiol 287 (2004). 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Blackwood EA, Thuerauf DJ, Stastna M, Stephens H, Sand Z, Pentoney A, et al. , Proteomic analysis of the cardiac myocyte secretome reveals extracellular protective functions for the ER stress response, J. Mol. Cell. Cardiol 143 (2020) 132–144. 10.1016/j.yjmcc.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Stastna M, Chimenti I, Marbán E, Van Eyk JE, Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes, Proteomics. 10 (2010) 245–253. 10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Quaife-Ryan GA, Sim CB, Ziemann M, Kaspi A, Rafehi H, Ramialison M, et al. , Multicellular transcriptional analysis of mammalian heart regeneration, Circulation. 136 (2017) 1123–1139. 10.1161/CIRCULATIONAHA.117.028252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jia G, Preussner J, Chen X, Guenther S, Yuan X, Yekelchyk M, et al. , Single cell RNA-seq and ATAC-seq analysis of cardiac progenitor cell transition states and lineage settlement, Nat. Commun 9 (2018). 10.1038/s41467-018-07307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pawlak M, Kedzierska KZ, Migdal M, Nahia KA, Ramilowski JA, Bugajski L, et al. , Dynamics of cardiomyocyte transcriptome and chromatin landscape demarcates key events of heart development, Genome Res. 29 (2019) 506–519. 10.1101/gr.244491.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Davegårdh C, Hall Wedin E, Broholm C, Henriksen TI, Pedersen M, Pedersen BK, et al. , Sex influences DNA methylation and gene expression in human skeletal muscle myoblasts and myotubes, Stem Cell Res. Ther 10 (2019). 10.1186/s13287-018-1118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Brutsaert DL, Fransen P, Andries LJ, De Keulenaer GW, Sys SU, Cardiac endothelium and myocardial function, Cardiovasc. Res 38 (1998) 281–290. 10.1016/S0008-6363(98)00044-3. [DOI] [PubMed] [Google Scholar]
- [48].Mosca L, Barrett-Connor E, Kass Wenger N, Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes, Circulation. 124 (2011) 2145–2154. 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Coutinho T, Yam Y, Dwivedi G, Inácio J, Chow BJW, Sex differences in associations of arterial compliance with coronary artery plaque and calcification burden, J. Am. Heart Assoc 6 (2017). 10.1161/JAHA.117.006079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rees M, Stevenson J, Primary prevention of coronary heart disease in women, Menopause Int. 14 (2008) 40–45. 10.1258/mi.2007.007037. [DOI] [PubMed] [Google Scholar]
- [51].Yahagi K, Davis HR, Arbustini E, Virmani R, Sex differences in coronary artery disease: Pathological observations, Atherosclerosis. 239 (2015) 260–267. 10.1016/j.atherosclerosis.2015.01.017. [DOI] [PubMed] [Google Scholar]
- [52].Talman V, Kivelä R, Cardiomyocyte—Endothelial Cell Interactions in Cardiac Remodeling and Regeneration, Front. Cardiovasc. Med 5 (2018) 101. 10.3389/fcvm.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Carrizzo A, Lenzi P, Procaccini C, Damato A, Biagioni F, Ambrosio M, et al. , Pentraxin 3 induces vascular endothelial dysfunction through a P-selectin/matrix metalloproteinase-1 pathway, Circulation. 131 (2015) 1495–1505. 10.1161/CIRCULATIONAHA.114.014822. [DOI] [PubMed] [Google Scholar]
- [54].Miyauchi T, Yanagisawa M, Iida K, Ajisaka R, Suzuki N, Fujino M, et al. , Age- and sex-related variation of plasma endothelin-1 concentration in normal and hypertensive subjects, Am. Heart J 123 (1992) 1092–1093. 10.1016/0002-8703(92)90734-D. [DOI] [PubMed] [Google Scholar]
- [55].Gohar EY, Giachini FR, Pollock DM, Tostes RC, Role of the endothelin system in sexual dimorphism in cardiovascular and renal diseases, Life Sci. 159 (2016) 20–29. 10.1016/j.lfs.2016.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Förstermann U, Münzel T, Endothelial nitric oxide synthase in vascular disease: From marvel to menace, Circulation. 113 (2006) 1708–1714. 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- [57].Cattaneo MG, Vanetti C, Decimo I, Di Chio M, Martano G, Garrone G, et al. , Sex-specific eNOS activity and function in human endothelial cells, Sci. Rep 7 (2017) 1–13. 10.1038/s41598-017-10139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et al. , Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure, Circulation. 98 (1998) 2709–2715. 10.1161/01.CIR.98.24.2709. [DOI] [PubMed] [Google Scholar]
- [59].Tirziu D, Simons M, Endothelium-Driven Myocardial Growth or Nitric Oxide at the Crossroads, Trends Cardiovasc. Med 18 (2008) 299–305. 10.1016/j.tcm.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cramariuc D, Rieck ÅE, Staal EM, Wachtell K, Eriksen E, Rossebø AB, et al. , Factors Influencing Left Ventricular Structure and Stress-Corrected Systolic Function in Men and Women With Asymptomatic Aortic Valve Stenosis (a SEAS Substudy), Am. J. Cardiol 101 (2008) 510–515. 10.1016/j.amjcard.2007.09.100. [DOI] [PubMed] [Google Scholar]
- [61].Murphy E, Steenbergen C, Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury, Cardiovasc. Res 75 (2007) 478–486. 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- [62].Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, et al. , Sex differences in endothelial STAT3 mediate sex differences in myocardial inflammation, Am. J. Physiol. - Endocrinol. Metab 293 (2007) E872–E877. 10.1152/ajpendo.00251.2007. [DOI] [PubMed] [Google Scholar]
- [63].Stone G, Choi A, Meritxell O, Gorham J, Heydarpour M, Seidman CE, et al. , Sex differences in gene expression in response to ischemia in the human left ventricular myocardium, Hum. Mol. Genet 28 (2019) 1682–1693. 10.1093/hmg/ddz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Davis GE, Senger DR, Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization, Circ. Res 97 (2005) 1093–1107. 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- [65].Huxley VH, Kemp SS, Sex-specific characteristics of the microcirculation, in: Adv. Exp. Med. Biol, Springer, 2018: pp. 307–328. 10.1007/978-3-319-77932-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang J, Bingaman S, Huxley VH, Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases, Am. J. Physiol. - Hear. Circ. Physiol 298 (2010) H1146. 10.1152/ajpheart.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ogola BO, Zimmerman MA, Clark GL, Abshire CM, Gentry KM, Miller KS, et al. , Sex differences in cardiovascular and cerebrovascular physiology, disease, and signaling mechanisms: New insights into arterial stiffening: Does sex matter?, Am. J. Physiol. - Hear. Circ. Physiol 315 (2018) H1073–H1087. 10.1152/ajpheart.00132.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].DuPont JJ, Kenney RM, Patel AR, Jaffe IZ, Sex differences in mechanisms of arterial stiffness, Br. J. Pharmacol 176 (2019) 4208–4225. 10.1111/bph.14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, De Marco VG, Ramirez-Perez FI, et al. , Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females, Circ. Res 118 (2016) 935–943. 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Huxley VH, Kemp SS, Schramm C, Sieveking S, Bingaman S, Yu Y, et al. , Sex differences influencing micro- and macrovascular endothelial phenotype in vitro, J. Physiol 596 (2018) 3929–3949. 10.1113/JP276048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hartman RJG, Kapteijn DMC, Haitjema S, Bekker MN, Mokry M, Pasterkamp G, et al. , Intrinsic transcriptomic sex differences in human endothelial cells at birth and in adults are associated with coronary artery disease targets, Sci. Rep 10 (2020). 10.1038/s41598-020-69451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Awwad K, Hu J, Shi L, Mangels N, Abdel Malik R, Zippel N, et al. , Role of secreted modular calcium-binding protein 1 (SMOC1) in transforming growth factor β signalling and angiogenesis, Cardiovasc. Res 106 (2015) 284–294. 10.1093/cvr/cvv098. [DOI] [PubMed] [Google Scholar]
- [73].Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, et al. , Tissue-specific expression and regulation of sexually dimorphic genes in mice, Genome Res. 16 (2006) 995–1004. 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, et al. , Landscape of X chromosome inactivation across human tissues, Nature. 550 (2017) 244–248. 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Farber MJ, Rizaldy R, Hildebrand JD, Shroom2 regulates contractility to control endothelial morphogenesis, Mol. Biol. Cell 22 (2011) 795–805. 10.1091/mbc.E10-06-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yucel N, Axsom J, Yang Y, Li L, Rhoades JH, Arany Z, Cardiac endothelial cells maintain open chromatin and expression of cardiomyocyte myofibrillar genes, Elife. 9 (2020). 10.7554/eLife.55730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hartman RJG, Huisman SE, Den Ruijter HM, Sex differences in cardiovascular epigenetics - A systematic review, Biol. Sex Differ 9 (2018). 10.1186/s13293-018-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Murray IR, Baily JE, Chen WCW, Dar A, Gonzalez ZN, Jensen AR, et al. , Skeletal and cardiac muscle pericytes: Functions and therapeutic potential, Pharmacol. Ther 171 (2017) 65–74. 10.1016/j.pharmthera.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sickinghe AA, Korporaal SJA, Den Ruijter HM, Kessler EL, Estrogen contributions to microvascular dysfunction evolving to heart failure with preserved ejection fraction, Front. Endocrinol. (Lausanne) 10 (2019). 10.3389/fendo.2019.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen JX, Chen ST, Tao YK, Cardiac pericyte is promising target for ischemic heart diseases: Role of Notch3, Int. J. Cardiol 246 (2017) 57. 10.1016/j.ijcard.2017.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bodnar RJ, Satish L, Yates CC, Wells A, Pericytes: A newly recognized player in wound healing, Wound Repair Regen. 24 (2016) 204–214. 10.1111/wrr.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, et al. , Pericytes are progenitors for coronary artery smooth muscle, Elife. 4 (2015). 10.7554/elife.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lee LL, Chintalgattu V, Pericytes in the heart, in: Adv. Exp. Med. Biol, Springer New York LLC, 2019: pp. 187–210. 10.1007/978-3-030-11093-2_11. [DOI] [PubMed] [Google Scholar]
- [84].Ayres-Sander CE, Lauridsen H, Maier CL, Sava P, Pober JS, Gonzalez AL, Transendothelial Migration Enables Subsequent Transmigration of Neutrophils through Underlying Pericytes, PLoS One. 8 (2013). 10.1371/journal.pone.0060025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chen CW, Okada M, Proto JD, Gao X, Sekiya N, Beckman SA, et al. , Human pericytes for ischemic heart repair, Stem Cells. 31 (2013) 305–316. 10.1002/stem.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, et al. , Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132, Circ. Res 109 (2011) 894–906. 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hirsch MM, Brusco J, Vaccaro T, Margis R, Moreira JE, Gottfried C, et al. , Sex Differences and Estrous Cycle Changes in Synaptic Plasticity-related microRNA in the Rat Medial Amygdala, Neuroscience. 379 (2018) 405–414. 10.1016/j.neuroscience.2018.03.035. [DOI] [PubMed] [Google Scholar]
- [88].Crislip GR, O’Connor PM, Wei Q, Sullivan JC, Vasa recta pericyte density is negatively associated with vascular congestion in the renal medulla following ischemia reperfusion in rats, Am. J. Physiol. - Ren. Physiol 313 (2017) F1097–F1105. 10.1152/ajprenal.00261.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tawfik O, Rao D, Nothnick WB, Graham A, Mau B, Fan F, Transgelin, a Novel Marker of Smooth Muscle Differentiation, Effectively Distinguishes Endometrial Stromal Tumors from Uterine Smooth Muscle Tumors., Int. J. Gynecol. Obstet. Reprod. Med. Res 1 (2014) 26–31. [PMC free article] [PubMed] [Google Scholar]
- [90].Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC, Cardiac fibrosis: The fibroblast awakens, Circ. Res 118 (2016) 1021–1040. 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Cuspidi C, Ciulla M, Zanchetti A, Hypertensive myocardial fibrosis, Nephrol. Dial. Transplant 21 (2006) 20–23. 10.1093/ndt/gfi237. [DOI] [PubMed] [Google Scholar]
- [92].Medzikovic L, Aryan L, Eghbali M, Connecting sex differences, estrogen signaling, and microRNAs in cardiac fibrosis, J. Mol. Med 97 (2019) 1385–1398. 10.1007/s00109-019-01833-6. [DOI] [PubMed] [Google Scholar]
- [93].Singh A, Al Musa T, Treibel TA, Vassiliou VS, Captur G, Chin C, et al. , Sex differences in left ventricular remodelling, myocardial fibrosis and mortality after aortic valve replacement, Heart. 105 (2019) 1818–1824. 10.1136/heartjnl-2019-314987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].VILLARI B, CAMPBELL SE, SCHNEIDER J, VASSALLI G, CHIARIELLO M, HESS OM, Sex-dependent differences in left ventricular function and structure in chronic pressure overload, Eur. Heart J 16 (1995) 1410–1419. 10.1093/oxfordjournals.eurheartj.a060749. [DOI] [PubMed] [Google Scholar]
- [95].Porter KE, Turner NA, Cardiac fibroblasts: At the heart of myocardial remodeling, Pharmacol. Ther 123 (2009) 255–278. 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [96].Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, et al. , The bHLH transcription factor Tcf21 is required for lineagespecific EMT of cardiac fibroblast progenitors, Dev. 139 (2012) 2139–2149. 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Garate-Carrillo A, Gonzalez J, Ceballos G, Ramirez-Sanchez I, Villarreal F, Sex related differences in the pathogenesis of organ fibrosis, Transl. Res 222 (2020) 41–55. 10.1016/j.trsl.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhou P, Pu WT, Recounting cardiac cellular composition, Circ. Res 118 (2016) 368–370. 10.1161/CIRCRESAHA.116.308139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’antoni ML, Debuque R, et al. , Revisiting cardiac cellular composition, Circ. Res 118 (2016) 400–409. 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tucker NR, Chaffin M, Fleming SJ, Hall AW, Parsons VA, Bedi KC, et al. , Transcriptional and Cellular Diversity of the Human Heart, Circulation. 142 (2020) 466–482. 10.1161/CIRCULATIONAHA.119.045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].McLellan MA, Skelly DA, Dona MSI, Squiers GT, Farrugia GE, Gaynor TL, et al. , High-Resolution Transcriptomic Profiling of the Heart during Chronic Stress Reveals Cellular Drivers of Cardiac Fibrosis and Hypertrophy, Circulation. (2020) 1448–1463. 10.1161/CIRCULATIONAHA.119.045115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dworatzek E, Mahmoodzadeh S, Schriever C, Kusumoto K, Kramer L, Santos G, et al. , Sex-specific regulation of collagen i and III expression by 17β-Estradiol in cardiac fibroblasts: Role of estrogen receptors, Cardiovasc. Res 115 (2019) 315–327. 10.1093/cvr/cvy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V, 17β-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts, Cardiovasc. Res 85 (2010) 719–728. 10.1093/cvr/cvp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Queirós AM, Eschen C, Fliegner D, Kararigas G, Dworatzek E, Westphal C, et al. , Sex- and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart, Int. J. Cardiol 169 (2013) 331–338. 10.1016/j.ijcard.2013.09.002. [DOI] [PubMed] [Google Scholar]
- [105].Cheng TC, Philip JL, Tabima DM, Kumari S, Yakubov B, Frump AL, et al. , Estrogen receptor-a prevents right ventricular diastolic dysfunction and fibrosis in female rats, Am. J. Physiol. - Hear. Circ. Physiol 319 (2020) H1459–H1473. 10.1152/AJPHEART.00247.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pedram A, Razandi M, O’Mahony F, Lubahn D, Levin ER, Estrogen receptor-β prevents cardiac fibrosis, Mol. Endocrinol 24 (2010) 2152–2165. 10.1210/me.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wang H, Zhao Z, Lin M, Groban L, Activation of GPR30 inhibits cardiac fibroblast proliferation, Mol. Cell. Biochem 405 (2015) 135–148. 10.1007/s11010-015-2405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chung CC, Hsu RC, Kao YH, Liou JP, Lu YY, Chen YJ, Androgen attenuates cardiac fibroblasts activations throughmodulations of transforming growth factor-β and angiotensin II signaling, Int. J. Cardiol 176 (2014) 386–393. 10.1016/j.ijcard.2014.07.077. [DOI] [PubMed] [Google Scholar]
- [109].Lau ES, Paniagua SM, Guseh JS, Bhambhani V, Zanni MV, Courchesne P, et al. , Sex Differences in Circulating Biomarkers of Cardiovascular Disease, J. Am. Coll. Cardiol 74 (2019) 1543–1553. 10.1016/j.jacc.2019.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Aguado BA, Schuetze KB, Grim JC, Walker CJ, Cox AC, Ceccato TL, et al. , Transcatheter aortic valve replacements alter circulating serum factors to mediate myofibroblast deactivation, Sci. Transl. Med 11 (2019) 3233. 10.1126/scitranslmed.aav3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Souders CA, Bowers SLK, Baudino TA, Cardiac fibroblast: The renaissance cell, Circ. Res 105 (2009) 1164–1176. 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ceccato TL, Starbuck RB, Hall JK, Walker CJ, Brown TE, Killgore JP, et al. , Defining the Cardiac Fibroblast Secretome in a Fibrotic Microenvironment, J. Am. Heart Assoc 9 (2020) e017025. 10.1161/JAHA.120.017025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Herum KM, Choppe J, Kumar A, Engler AJ, McCulloch AD, Mechanical regulation of cardiac fibroblast profibrotic phenotypes, Mol. Biol. Cell 28 (2017) 1871–1882. 10.1091/mbc.E17-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Achkar A, Saliba Y, Fares N, Differential Gender-Dependent Patterns of Cardiac Fibrosis and Fibroblast Phenotypes in Aging Mice, Oxid. Med. Cell. Longev 2020 (2020). 10.1155/2020/8282157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Komosinska-Vassev K, Olczyk P, Winsz-Szczotka K, Kuznik-Trocha K, Klimek K, Olczyk K, Age- and gender-dependent changes in connective tissue remodeling: Physiological differences in circulating MMP-3, MMP-10, TIMP-1 and TIMP-2 level, Gerontology. 57 (2010) 44–52. 10.1159/000295775. [DOI] [PubMed] [Google Scholar]
- [116].Skelly DA, Squiers GT, McLellan MA, Bolisetty MT, Robson P, Rosenthal NA, et al. , Single-Cell Transcriptional Profiling Reveals Cellular Diversity and Intercommunication in the Mouse Heart, Cell Rep. 22 (2018) 600–610. 10.1016/j.celrep.2017.12.072. [DOI] [PubMed] [Google Scholar]
- [117].Lu T, Mar JC, Investigating transcriptome-wide sex dimorphism by multi-level analysis of single-cell RNA sequencing data in ten mouse cell types, Biol. Sex Differ 11 (2020) 61. 10.1186/s13293-020-00335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhang H, Kho AT, Wu Q, Halayko AJ, Limbert Rempel K, Chase RP, et al. , CRISPLD2 (LGL1) inhibits proinflammatory mediators in human fetal, adult, and COPD lung fibroblasts and epithelial cells, Physiol. Rep 4 (2016) e12942. 10.14814/phy2.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lighthouse JK, Burke RM, Velasquez LS, Dirkx RA, Aiezza A, Moravec CS, et al. , Exercise promotes a cardioprotective gene program in resident cardiac fibroblasts, JCI Insight. 4 (2019). 10.1172/jci.insight.92098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Yang J, Shen Y, Yang X, Long Y, Chen S, Lin X, et al. , Silencing of long noncoding RNA XIST protects against renal interstitial fibrosis in diabetic nephropathy via microRNA-93-5p-mediated inhibition of CDKN1A, Am. J. Physiol. - Ren. Physiol 317 (2019) F1350–F1358. 10.1152/ajprenal.00254.2019. [DOI] [PubMed] [Google Scholar]
- [121].Takawale A, Zhang P, Patel VB, Wang X, Oudit G, Kassiri Z, Tissue Inhibitor of Matrix Metalloproteinase-1 Promotes Myocardial Fibrosis by Mediating CD63-Integrin β1 Interaction, Hypertension. 69 (2017) 1092–1103. 10.1161/HYPERTENSIONAHA.117.09045. [DOI] [PubMed] [Google Scholar]
- [122].Anderson CL, Brown CJ, Polymorphic X-chromosome inactivation of the human TIMP1 gene, Am. J. Hum. Genet 65 (1999) 699–708. 10.1086/302556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Felisbino MB, McKinsey TA, Epigenetics in Cardiac Fibrosis: Emphasis on Inflammation and Fibroblast Activation, JACC Basic to Transl. Sci 3 (2018) 704–715. 10.1016/j.jacbts.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Nural-Guvener HF, Zakharova L, Nimlos J, Popovic S, Mastroeni D, Gaballa MA, HDAC class I inhibitor, Mocetinostat, reverses cardiac fibrosis in heart failure and diminishes CD90+ cardiac myofibroblast activation, Fibrogenes. Tissue Repair 7 (2014) 10. 10.1186/1755-1536-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Williams SM, Golden-Mason L, Ferguson BS, Schuetze KB, Cavasin MA, Demos-Davies K, et al. , Class I HDACs regulate angiotensin II-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes, J. Mol. Cell. Cardiol 67 (2014) 112–125. 10.1016/j.yjmcc.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Nural-Guvener H, Zakharova L, Feehery L, Sljukic S, Gaballa M, Anti-fibrotic effects of class I HDAC inhibitor, mocetinostat is associated with IL-6/stat3 signaling in ischemic heart failure, Int. J. Mol. Sci 16 (2015) 11482–11499. 10.3390/ijms160511482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Stratton MS, Bagchi RA, Felisbino MB, Hirsch RA, Smith HE, Riching AS, et al. , Dynamic Chromatin Targeting of BRD4 Stimulates Cardiac Fibroblast Activation, Circ. Res 125 (2019) 662–677. 10.1161/CIRCRESAHA.119.315125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kfoury N, Qi Z, Wilkinson MN, Broestl L, Berrett KC, Moudgil A, et al. , Brd4-bound enhancers drive critical sex differences in glioblastoma, BioRxiv. (2017) 199059. 10.1101/199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, et al. , The human heart contains distinct macrophage subsets with divergent origins and functions, Nat. Med 24 (2018) 1234–1245. 10.1038/s41591-018-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Swirski FK, Nahrendorf M, Cardioimmunology: the immune system in cardiac homeostasis and disease, Nat. Rev. Immunol 18 (2018) 733–744. 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- [131].Nahrendorf M, Myeloid cell contributions to cardiovascular health and disease, Nat. Med 24 (2018) 711–720. 10.1038/s41591-018-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, et al. , Impaired Infarct Healing in Atherosclerotic Mice With Ly-6Chi Monocytosis, J. Am. Coll. Cardiol 55 (2010) 1629–1638. 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Barrett TJ, Macrophages in Atherosclerosis Regression, Arterioscler. Thromb. Vasc. Biol 40 (2020) 20–33. 10.1161/ATVBAHA.119.312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Oertelt-Prigione S, The influence of sex and gender on the immune response, Autoimmun. Rev 11 (2012). 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- [135].Bird MD, Karavitis J, Kovacs EJ, Sex differences and estrogen modulation of the cellular immune response after injury, Cell. Immunol 252 (2008) 57–67. 10.1016/j.cellimm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Fairweather DL, Cooper LT, Blauwet LA, Sex and Gender Differences in Myocarditis and Dilated Cardiomyopathy, Curr. Probl. Cardiol 38 (2013) 7–46. 10.1016/j.cpcardiol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Fairweather D, Sex differences in inflammation during atherosclerosis, Clin. Med. Insights Cardiol 8 (2014) 49–59. 10.4137/CMC.S17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, et al. , Sex differences in the myocardial inflammatory response to acute injury, Shock. 23 (2005) 1–10. 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- [139].Doran SJ, Ritzel RM, Glaser EP, Henry RJ, Faden AI, Loane DJ, Sex differences in acute neuroinflammation after experimental traumatic brain injury are mediated by infiltrating myeloid cells, J. Neurotrauma 36 (2019) 1040–1053. 10.1089/neu.2018.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Li K, Xu W, Guo Q, Jiang Z, Wang P, Yue Y, et al. , Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis, Circ. Res 105 (2009) 353–364. 10.1161/CIRCRESAHA.109.195230. [DOI] [PubMed] [Google Scholar]
- [141].Grim JC, Aguado BA, Vogt BJ, Batan D, Andrichik CL, Schroeder ME, et al. , Secreted factors from proinflammatory macrophages promote an osteoblast-like phenotype in valvular interstitial cells, Arterioscler. Thromb. Vasc. Biol (2020) E296–E308. 10.1161/ATVBAHA.120.315261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].de Arellano MLB, Pozdniakova S, Kühl AA, Baczko I, Ladilov Y, Regitz-Zagrosek V, Sex differences in the aging human heart: Decreased sirtuins, proinflammatory shift and reduced anti-oxidative defense, Aging (Albany. NY) 11 (2019) 1918–1933. 10.18632/aging.101881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].New SEP, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, et al. , Macrophage-derived matrix vesicles : An alternative novel mechanism for microcalcification in atherosclerotic plaques, Circ. Res 113 (2013) 72–77. 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Jansen F, Xiang X, Werner N, Role and function of extracellular vesicles in calcific aortic valve disease, Eur. Heart J 38 (2017) 2714–2716. 10.1093/eurheartj/ehx477. [DOI] [PubMed] [Google Scholar]
- [145].Angele MK, Knöferl MW, Schwacha MG, Ayala A, Cioffi WG, Bland KI, et al. , Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage, Am. J. Physiol. - Cell Physiol 277 (1999). 10.1152/ajpcell.1999.277.1.c35. [DOI] [PubMed] [Google Scholar]
- [146].Gilliver SC, Sex steroids as inflammatory regulators, J. Steroid Biochem. Mol. Biol 120 (2010) 105–115. 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- [147].Ng MKC, Jessup W, Celermajer DS, Sex-related differences in the regulation of macrophage cholesterol metabolism, Curr. Opin. Lipidol 12 (2001) 505–510. 10.1097/00041433-200110000-00005. [DOI] [PubMed] [Google Scholar]
- [148].Cutolo M, Capellino S, Montagna P, Ghiorzo P, Sulli A, Villaggio B, Sex hormone modulation of cell growth and apoptosis of the human monocytic/macrophage cell line., Arthritis Res. Ther 7 (2005). 10.1186/ar1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Mathur P, Ostadal B, Romeo F, Mehta JL, Gender-related differences in atherosclerosis, Cardiovasc. Drugs Ther 29 (2015) 319–327. 10.1007/s10557-015-6596-3. [DOI] [PubMed] [Google Scholar]
- [150].Gillis EE, Sullivan JC, Sex Differences in Hypertension: Recent Advances, Hypertension. 68 (2016) 1322–1327. 10.1161/HYPERTENSIONAHA.116.06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Brozovich FV, Nicholson CJ, Degen CV, Gao YZ, Aggarwal M, Morgan KG, Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders, Pharmacol. Rev 68 (2016) 476–532. 10.1124/pr.115.010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Bacakova L, Travnickova M, Filova E, Matějka R, Stepanovska J, Musilkova J, et al. , The Role of Vascular Smooth Muscle Cells in the Physiology and Pathophysiology of Blood Vessels, in: Muscle Cell Tissue - Curr. Status Res. F, 2018. 10.5772/intechopen.77115. [DOI] [Google Scholar]
- [153].Moraghan T, Antoniucci DM, Grenert JP, Sieck GC, Johnson C, Miller VM, et al. , Differential response in cell proliferation to beta estradiol in coronary arterial vascular smooth muscle cells obtained from mature female versus male animals, Endocrinology. 137 (1996) 5174–5177. 10.1210/endo.137.11.8895395. [DOI] [PubMed] [Google Scholar]
- [154].Antoniucci D, Miller VM, Sieck GC, Fitzpatrick LA, Gender-related differences in proliferative responses of vascular smooth muscle cells to endothelin-1, Endothel. J. Endothel. Cell Res 8 (2001) 137–145. 10.3109/10623320109165322. [DOI] [PubMed] [Google Scholar]
- [155].Li Y, Wen Y, Green M, Cabral EK, Wani P, Zhang F, et al. , Cell sex affects extracellular matrix protein expression and proliferation of smooth muscle progenitor cells derived from human pluripotent stem cells, Stem Cell Res. Ther 8 (2017). 10.1186/s13287-017-0606-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Nakamura Y, Suzuki T, Sasano H, Estrogen actions and in situ synthesis in human vascular smooth muscle cells and their correlation with atherosclerosis, in: J. Steroid Biochem. Mol. Biol, 2005: pp. 263–268. 10.1016/j.jsbmb.2004.12.024. [DOI] [PubMed] [Google Scholar]
- [157].Barron LA, Green GCM, Khalil RA, Gender differences in vascular smooth muscle reactivity to increases in extracellular sodium salt, in: Hypertension, 2002: pp. 425–432. 10.1161/hy02t2.102779. [DOI] [PubMed] [Google Scholar]
- [158].Kennedy E, Hakimjavadi R, Greene C, Mooney CJ, Fitzpatrick E, Collins LE, et al. , Embryonic rat vascular smooth muscle cells revisited - A model for neonatal, neointimal SMC or differentiated vascular stem cells?, Vasc. Cell 6 (2014). 10.1186/2045-824X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Bačáková L, Mareš V, Lisá V, Kocourek F, Sex-dependent differences in growth and morphology of cultured vascular smooth muscle cells from newborn rats, 1997. [PubMed] [Google Scholar]
- [160].Mao B, Sun L, Zhang J, Zhou M, Zhang F, Perioperative factors associated with short- and long-Term outcomes after tricuspid valve replacement, Interact. Cardiovasc. Thorac. Surg 23 (2016) 845–850. 10.1093/icvts/ivw244. [DOI] [PubMed] [Google Scholar]
- [161].Kogon B, Plattner C, Kirshbom P, Kanter K, Leong T, Lyle T, et al. , Risk factors for early pulmonary valve replacement after valve disruption in congenital pulmonary stenosis and tetralogy of Fallot, J. Thorac. Cardiovasc. Surg 138 (2009) 103–108. 10.1016/j.jtcvs.2009.02.020. [DOI] [PubMed] [Google Scholar]
- [162].Gourgas O, Khan K, Schwertani A, Cerruti M, Differences in mineral composition and morphology between men and women in aortic valve calcification, Acta Biomater. 106 (2020) 342–350. 10.1016/j.actbio.2020.02.030. [DOI] [PubMed] [Google Scholar]
- [163].Hinton RB, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, et al. , Extracellular matrix remodeling and organization in developing and diseased aortic valves, Circ. Res 98 (2006) 1431–1438. 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- [164].Liu AC, Joag VR, Gotlieb AI, The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology, Am. J. Pathol 171 (2007) 1407–1418. 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Xu K, Xie S, Huang Y, Zhou T, Liu M, Zhu P, et al. , Cell-Type Transcriptome Atlas of Human Aortic Valves Reveal Cell Heterogeneity and Endothelial to Mesenchymal Transition Involved in Calcific Aortic Valve Disease, Arterioscler. Thromb. Vasc. Biol (2020) 2910–2921. 10.1161/ATVBAHA.120.314789. [DOI] [PubMed] [Google Scholar]
- [166].Spadaccio C, Mozetic P, Nappi F, Nenna A, Sutherland F, Trombetta M, et al. , Cells and extracellular matrix interplay in cardiac valve disease: because age matters, Basic Res. Cardiol 111 (2016) 1–22. 10.1007/s00395-016-0534-9. [DOI] [PubMed] [Google Scholar]
- [167].Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA, Valvular myofibroblast activation by transforming growth factor-β: Implications for pathological extracellular matrix remodeling in heart valve disease, Circ. Res 95 (2004) 253–260. 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- [168].Pho M, Lee W, Watt DR, Laschinger C, Simmons CA, McCulloch CA, Cofilin is a marker of myofibroblast differentiation in cells from porcine aortic cardiac valves, Am. J. Physiol. - Hear. Circ. Physiol 294 (2008). 10.1152/ajpheart.01305.2007. [DOI] [PubMed] [Google Scholar]
- [169].Rush MN, Coombs KE, Hedberg-Dirk EL, Surface chemistry regulates valvular interstitial cell differentiation in vitro, Acta Biomater. 28 (2015) 76–85. 10.1016/j.actbio.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Grim JC, Aguado BA, Vogt BJ, Batan D, Andrichik CL, Schroeder ME, et al. , Secreted factors from proinflammatory macrophages promote an osteoblast-like phenotype in valvular interstitial cells, Arterioscler. Thromb. Vasc. Biol (2020). 10.1161/ATVBAHA.120.315261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Parra-Izquierdo I, Castaøos-Mollor I, López J, Gómez C, San Román JA, Sánchez Crespo M, et al. , Lipopolysaccharide and interferon-γ team up to activate HIF-1α via STAT1 in normoxia and exhibit sex differences in human aortic valve interstitial cells, Biochim. Biophys. Acta - Mol. Basis Dis 1865 (2019) 2168–2179. 10.1016/j.bbadis.2019.04.014. [DOI] [PubMed] [Google Scholar]
- [172].Parra-Izquierdo I, Castaøos-Mollor I, López J, Gómez C, Román JAS, Crespo MS, et al. , Calcification Induced by Type i Interferon in Human Aortic Valve Interstitial Cells Is Larger in Males and Blunted by a Janus Kinase Inhibitor, Arterioscler. Thromb. Vasc. Biol 38 (2018) 2148–2159. 10.1161/ATVBAHA.118.311504. [DOI] [PubMed] [Google Scholar]
- [173].Masjedi S, Lei Y, Patel J, Ferdous Z, Sex-related differences in matrix remodeling and early osteogenic markers in aortic valvular interstitial cells, Heart Vessels. 32 (2017) 217–228. 10.1007/s00380-016-0909-8. [DOI] [PubMed] [Google Scholar]
- [174].McCoy CM, Nicholas DQ, Masters KS, Sex-related differences in gene expression by porcine aortic valvular interstitial cells, PLoS One. 7 (2012). 10.1371/journal.pone.0039980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Gupta V, Barzilla JE, Mendez JS, Stephens EH, Lee EL, Collard CD, et al. , Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves, Cardiovasc. Pathol 18 (2009) 191–197. 10.1016/j.carpath.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Simard L, Côté N, Dagenais F, Mathieu P, Couture C, Trahan S, et al. , Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis, Circ. Res 120 (2017) 681–691. 10.1161/CIRCRESAHA.116.309306. [DOI] [PubMed] [Google Scholar]
- [177].Xiao F, Zheng R, Yang D, Cao K, Zhang S, Wu B, et al. , Sex-dependent aortic valve pathology in patients with rheumatic heart disease, PLoS One. 12 (2017). 10.1371/journal.pone.0180230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Sharma S, Eghbali M, Influence of sex differences on microRNA gene regulation in disease, Biol. Sex Differ 5 (2014). 10.1186/2042-6410-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Fang M, Wang CG, Zheng C, Luo J, Hou S, Liu K, et al. , Mir-29b promotes human aortic valve interstitial cell calcification via inhibiting TGF-β3 through activation of wnt3/β-catenin/Smad3 signaling, J. Cell. Biochem 119 (2018) 5175–5185. 10.1002/jcb.26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Zheng D, Zang Y, Xu H, Wang Y, Cao X, Wang T, et al. , MicroRNA-214 promotes the calcification of human aortic valve interstitial cells through the acceleration of inflammatory reactions with activated MyD88/NF-κB signaling, Clin. Res. Cardiol 108 (2019) 691–702. 10.1007/s00392-018-1398-9. [DOI] [PubMed] [Google Scholar]
- [181].García R, Salido-Medina AB, Gil A, Merino D, Gómez J, Villar AV, et al. , Sex-Specific Regulation of miR-29b in the Myocardium Under Pressure Overload is Associated with Differential Molecular, Structural and Functional Remodeling Patterns in Mice and Patients with Aortic Stenosis, Cells. 9 (2020) 833. 10.3390/cells9040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Kwekel JC, Desai VG, Moland CL, Vijay V, Fuscoe JC, Sex differences in kidney gene expression during the life cycle of F344 rats, Biol. Sex Differ 4 (2013). 10.1186/2042-6410-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Alushi B, Curini L, Christopher MR, Grubitzch H, Landmesser U, Amedei A, et al. , Calcific Aortic Valve Disease-Natural History and Future Therapeutic Strategies, Front. Pharmacol 11 (2020). 10.3389/fphar.2020.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Gošev I, Zeljko M, Durić Ž, Nikolić I, Gošev M, Ivčević S, et al. , Epigenome alterations in aortic valve stenosis and its related left ventricular hypertrophy, Clin. Epigenetics 9 (2017). 10.1186/s13148-017-0406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Nagy E, Bäck M, Epigenetic regulation of 5-lipoxygenase in the phenotypic plasticity of valvular interstitial cells associated with aortic valve stenosis, FEBS Lett. 586 (2012) 1325–1329. 10.1016/j.febslet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- [186].Radmark O, Samuelsson B, 5-Lipoxygenase: Mechanisms of regulation, J. Lipid Res 50 (2009) S40–S45. 10.1194/jlr.R800062-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Hadji F, Boulanger MC, Guay SP, Gaudreault N, Amellah S, Mkannez G, et al. , Altered DNA Methylation of Long Noncoding RNA H19 in Calcific Aortic Valve Disease Promotes Mineralization by Silencing NOTCH1, Circulation. 134 (2016) 1848–1862. 10.1161/CIRCULATIONAHA.116.023116. [DOI] [PubMed] [Google Scholar]
- [188].Vander Roest M, Krapp C, Thorvaldsen JL, Bartolomei MS, Merryman WD, H19 is not hypomethylated or upregulated with age or sex in the aortic valves of mice, Physiol. Rep 7 (2019). 10.14814/phy2.14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Frederiksen MC, Physiologic changes in pregnancy and their effect on drug disposition, in: Semin. Perinatol, W.B. Saunders, 2001: pp. 120–123. 10.1053/sper.2001.24565. [DOI] [PubMed] [Google Scholar]
- [190].Lavie A, Ram M, Lev S, Blecher Y, Amikam U, Shulman Y, et al. , Maternal cardiovascular hemodynamics in normotensive versus preeclamptic pregnancies: A prospective longitudinal study using a noninvasive cardiac system (NICaS™), BMC Pregnancy Childbirth. 18 (2018) 229. 10.1186/s12884-018-1861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Nawroth J, Rogal J, Weiss M, Brucker SY, Loskill P, Organ-on-a-Chip Systems for Women’s Health Applications, Adv. Healthc. Mater 7 (2018). 10.1002/adhm.201700550. [DOI] [PubMed] [Google Scholar]
- [192].James BD, Guerin P, Allen JB, Let’s Talk About Sex—Biological Sex Is Underreported in Biomaterial Studies, Adv. Healthc. Mater 10 (2021). 10.1002/adhm.202001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Aguado BA, Grim JC, Rosales AM, Watson-Capps JJ, Anseth KS, Engineering precision biomaterials for personalized medicine, Sci. Transl. Med 10 (2018). 10.1126/scitranslmed.aam8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Guyette JP, Charest JM, Mills RW, Jank BJ, Moser PT, Gilpin SE, et al. , Bioengineering Human Myocardium on Native Extracellular Matrix, Circ. Res 118 (2016) 56–72. 10.1161/CIRCRESAHA.115.306874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Richards DJ, Li Y, Kerr CM, Yao J, Beeson GC, Coyle RC, et al. , Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity, Nat. Biomed. Eng 4 (2020) 446–462. 10.1038/s41551-020-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Sadeghi AH, Shin SR, Deddens JC, Fratta G, Mandla S, Yazdi IK, et al. , Engineered 3D Cardiac Fibrotic Tissue to Study Fibrotic Remodeling, Adv. Healthc. Mater 6 (2017). 10.1002/adhm.201601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Gonzalez Rodriguez A, Schroeder ME, Walker CJ, Anseth KS, FGF-2 inhibits contractile properties of valvular interstitial cell myofibroblasts encapsulated in 3D MMP-degradable hydrogels, APL Bioeng. 2 (2018) 046104. 10.1063/1.5042430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Crocini C, Walker CJ, Anseth KS, Leinwand LA, Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness, Prog. Biophys. Mol. Biol 154 (2020) 71–79. 10.1016/j.pbiomolbio.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Occhetta P, Isu G, Lemme M, Conficconi C, Oertle P, Räz C, et al. , A three-dimensional: In vitro dynamic micro-tissue model of cardiac scar formation, Integr. Biol. (United Kingdom) 10 (2018) 174–183. 10.1039/c7ib00199a. [DOI] [PubMed] [Google Scholar]
- [200].Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, et al. , Integrated analysis of multimodal single-cell data, BioRxiv. (2020). 10.1101/2020.10.12.335331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Yu G, ClusterProfiler: An universal enrichment tool for functional and comparative study, BioRxiv. (2018). 10.1101/256784. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: GO term biological processes (BP) enrichment for differentially expressed genes shared by more than one cell type from Figure 4B.
Supplemental Table 2: Summary of sex differences of adult mammalian cardiac cellular sex reported and discussed within this review. h = human data, m = murine data, p = porcine data, r = rat data; *this is reviewed elsewhere in depth.