Abstract
Objective
Excess dietary fat and sodium are both associated with obesity and metabolic dysfunction. In mice, high sodium (NaCl) has been shown to block high fat (HF) diet-induced weight gain. Here we investigated the impact of high fat/high sodium (HF/NaCl) diet on metabolic function in the absence of obesity.
Methods
Wild type mice were administered chow, NaCl (4%,), HF, and HF/NaCl diets. Metabolic analysis was performed by measuring fasted blood glucose and insulin levels and by GTT and ITT.
Results
After 10 wk on diets, male and female mice on HF diet gained weight and HF/NaCl mice had significantly reduced weight gain similar to chow fed mice. In the absence of obesity, HF/NaCl mice had significantly elevated fasting blood glucose and impaired glucose control during GTT. Both NaCl and HF/NaCl mice had decreased pancreas and β-cell mass. Administration of sodium in drinking water did not protect mice from HF diet-induced weight gain and obesity. Further analysis revealed that longer administration of HF/NaCl for 20 wk resulted in significant weight gain and insulin resistance.
Conclusions
Our data demonstrate that despite early inhibitory effects on fat deposition and weight gain, HF/NaCl diet does not prevent the metabolic consequences of HF diet consumption.
Keywords: sodium, high fat, glucose intolerance, obesity, inflammation
INTRODUCTION
Obesity is one of the most important risk factors for the development of metabolic dysfunction such as insulin resistance and type 2 diabetes mellitus. The Western diet, which includes highly processed foods containing a high percentage of saturated fats, sugars and salt, contributes to obesity and metabolic disease not only through increased caloric content, but also though systemic effects that induce inflammation and dysregulation of metabolic function. The mechanisms by which these nutrient components interact to alter metabolism and fat deposition is complex and remains incomplete.
Excess sodium intake is associated with high blood pressure and other cardiovascular consequences, and the vast majority of scientific literature strongly supports the reduction of sodium to decrease the risk of cardiovascular disease and stroke (1, 2, 3). There is also a body of literature associating sodium with insulin resistance and metabolic disease. In human studies, high sodium intake has been shown to be associated with insulin resistance and metabolic syndrome (4, 5, 6, 7). Similarly, in animal models, high salt administration has been reported to decrease glucose tolerance and insulin sensitivity (8). Consistent with these findings, a low sodium diet can increase insulin sensitizing adipokines while decreasing adipose tissue inflammation in rodents (9).
The mechanisms by which sodium affects insulin sensitivity and metabolic function is multifactorial and not fully understood. One potential mechanism is through activation of the renin-angiotensin system where this has been shown to be associated with insulin resistance and metabolic dysfunction in humans (10, 11). A similar effect is thought to occur where by short term, highly restricted, low sodium increases insulin resistance through RAS activation (12). In contrast, mineralocorticoid receptor blockade was shown to enhance adiponectin and decrease adipose tissue inflammation (13). In other models, dietary sodium has been shown to have important pathophysiological effects through regulation of immune cell phenotypes. Sodium has been shown to drive Th17 differentiation, which exacerbates inflammatory diseases like multiple sclerosis (14), and it also enhances proinflammatory phenotypes in monocytes and macrophages (15, 16, 17). Overall, these cellular effects translate into poor clinical outcomes in animal models of disease (14, 18).
In addition to its effects on insulin sensitivity and metabolic function, sodium has also been shown to affect fat deposition and adiposity. In humans, high sodium intake is associated with obesity (19, 20). However, the mechanistic understanding of this is confounded by conflicting reports in animal models. In one study, high sodium intake was found to increase fat depot mass without affecting body weight (21), where as others indicate that sodium actually decreases body weight and fat depot mass (22, 23). In the context of DIO, sodium has been shown to dose-dependently prevent weight gain with the addition of 4% NaCl being sufficient in one study to completely block DIO (24, 25).
Although high fat/high sodium (HF/NaCl) was shown to dramatically prevent weight gain and adiposity, the impact that HF/NaCl have on glucose regulation in the absence of obesity is unknown. In the present study, we examine the metabolic consequences of HF/NaCl diet administration in mice. Here we tested the effect of HF/NaCl diet on glucose control and insulin sensitivity to determine if chronic administration of HF/NaCl affects glucose homeostasis and metabolic function.
METHODS
Animals
Wild type, male and female C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and allowed to acclimate for 1 week prior to all studies. Mice were multi-housed in static cages in a temperature controlled SPF room (21–23°C) with a light:dark cycle of 12:12 h (lights on at 6 A.M.). Prior to experiments, mice were normalized by body weights and randomly assigned to treatment groups. Mice were maintained on standard laboratory chow or on sodium and fat adjusted diets (described below) and water ad libitum. Estimation of required sample size for all experiments were based on A priori power analysis calculations using expected standard deviations based on previous experiments and published literature. All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (8th Edition) and were approved by the Institutional Animal Care and Use Committee of the University of Michigan.
Rodent Diets
Standard laboratory chow diet was 5L0D (LabDiet). A 4% high sodium diet was prepared in house by adding an additional 3% NaCl to powder chow (5001, LabDiet) and forming pellets. High fat diet (D12492) and high fat/high sodium diet (D06111102) were and (Research Diets). Calorie and sodium composition of all diets are provided in Table S1. For high sodium experiments administered in the water, mice were given 2% NaCl water ad libitum.
Body Composition and Indirect Calorimetry
See supplemental methods.
Food Consumption
Food intake was repeatedly measured in both standard static cages and metabolic cages over 2 or 3 day intervals.
Glucose and Insulin Tolerance Tests
For glucose tolerance tests (GTT), mice were fasted for 6 h (5:00 A.M.–11:00 A.M.) before receiving an intraperitoneal injection of glucose. Glucose dose (1.25 mg/g lean mass) was determined from lean body mass to avoid confounding effects from obesity. Blood glucose was measured at 0, 15, 30, 60, and 120 min after glucose injection using a Contour (Bayer) glucometer. For insulin measurements, plasma was collected at 0, 30 and 60 min after glucose injection and plasma insulin was assayed using an Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem). For insulin tolerance tests, mice were injected with insulin (0.5 U/kg lean mass) (Humulin R; Lilly) and blood glucose was measured at 0, 15, 30, 60, and 120 min.
Gene expression analysis
See supplemental methods.
β-Cell Mass
Cohorts of male mice were used for these experiments. The entire pancreas was excised, weighed, and fixed in 4% formaldehyde overnight. Tissue was then processed and embedded in paraffin and 5 serial sections were cut throughout the pancreas approximately 100 μm apart. Sections were immunostained for insulin and then scanned and analyzed. The total pancreas area and the insulin positive regions were measured using ImageJ software (ver. 1.52) to determine the β-cell percentage, and the β-cell percent was then multiplied by the pancreas weight to determine the β-cell mass.
Statistical analysis
See supplemental methods.
RESULTS
Incorporation of sodium into high fat diet suppresses diet-induced obesity in both male and female mice.
Wild type mice were placed on chow, high sodium (NaCl), high fat (HF), and high fat high sodium (HF/NaCl) diets for 10 weeks. Consistent with what has been previous reported in male mice, incorporation of 4% sodium into 60% high fat diet effectively prevented weight gain during 10 weeks on high fat diet (Figure 1A–B). We extended this finding to females and found that sodium also significantly prevented HF diet-induced weight gain in female mice (Figure 1B). There was a significant reduction in total fat mass as well as fat deposition in select adipose tissue depots in both male (Figure 1C) and female mice (Figure 1D). No statistically significant differences were detected in lean body mass or fluid percent as a result of sodium administration (Figure 1E–F). Mice on HF and HF/NaCl diets had similar food and calorie intake, and mice on NaCl and HF/NaCl diets had expected increases in water consumption (Figure S1).
Figure 1. HF/NaCl diet prevents weight gain in male and female mice.
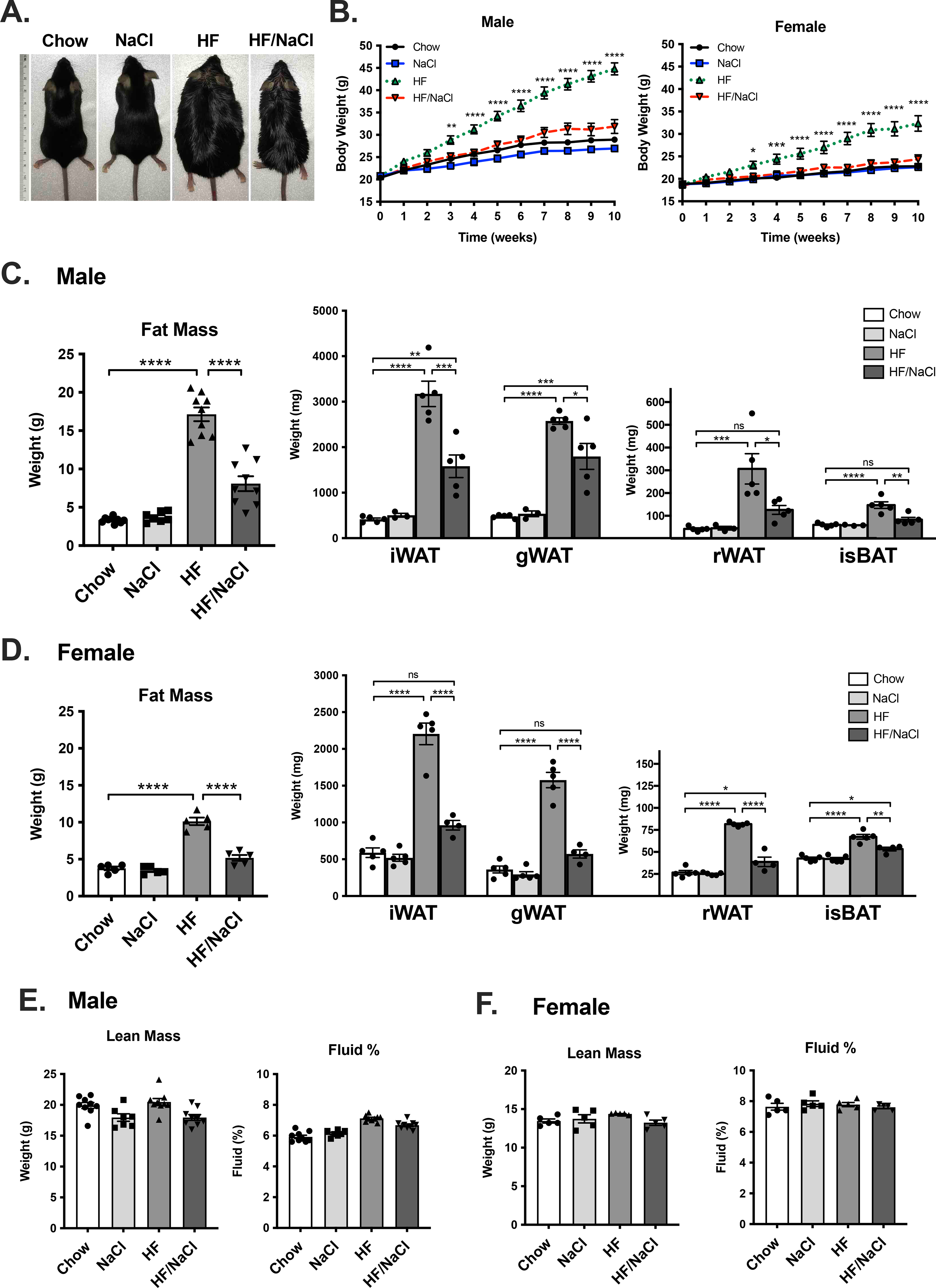
(A) Photograph of mice after 10 wk on diet. (B) Body weight in male and female mice. Total fat mass from body composition analysis and fat depot mass in (C) male, and (D) female mice after 10 wk on diet. Lean body mass and fluid % in (E) male (F) female mice at wk 10. Data represented as mean ± SEM. N = 4–9 per group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Similar to what has been reported previously, HF diet significantly reduces the respiratory exchange ratio and results in a change in energy substrate utilization from glucose to fatty acids. These differences occur rapidly by 1 wk (Figure S2A) and remain constant at 8 wk (Figure S2B). No differences were detected between HF and HF/NaCl treated mice in males (Figure S2A–B) and females (Figure S2C). No significant changes were detected in energy expenditure when normalized to LBM. When normalized to total body weight, HF diet treated mice had significantly lower energy expenditure compared to HF/NaCl mice (Figure S2).
Incorporation of high sodium into high fat diet causes glucose intolerance in the absence of obesity.
Although HF/NaCl mice are protected from high fat-induced weight gain, they have similar food consumption compared to HF diet treated mice and are therefore exposed to similar caloric composition. To determine if HF/NaCl diet has an effect on glucose homeostasis in the absence of obesity, we measured glucose levels during treatment. Both HF and HF/NaCl diet significantly increased fasted blood glucose levels in female mice after 2 wk on diets (Figure 2A). After 8 wk on diets, both HF and HF/NaCl significantly increased fasted blood glucose in male and female mice after (Figure 2B). Only HF diet resulted in significantly elevated fed blood glucose levels after 8 wk on diets (Figure 2C).
Figure 2. Mice on HF/NaCl diet have elevated blood glucose.
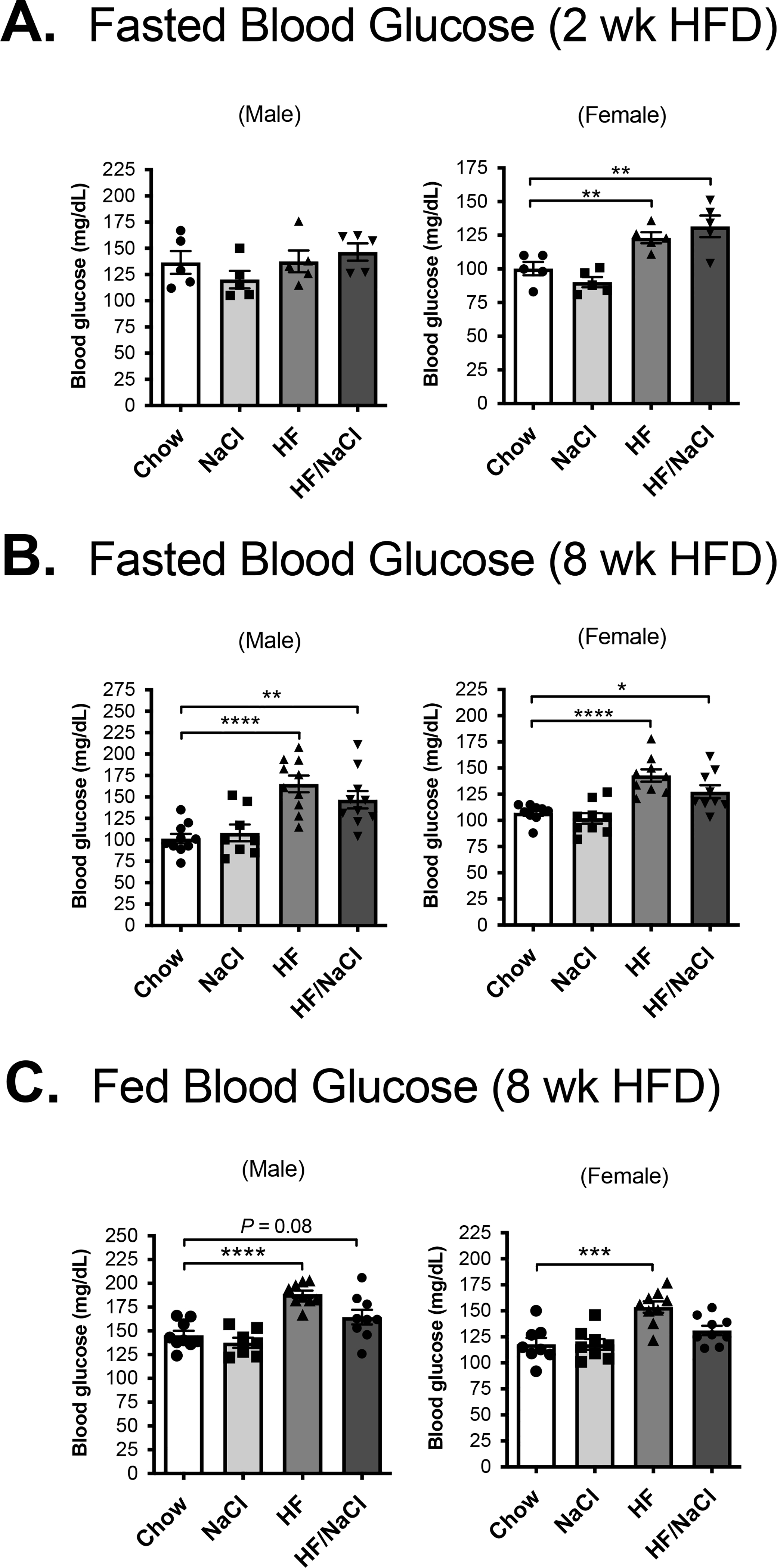
Fasted blood glucose levels at (A) 2 wk and (B) 8 wk on diet. (C) Fed blood glucose at 8 wk. Data represented as mean ± SEM. N = 5–9 per group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We next tested whether sodium also affected glucose homeostasis during GTTs. After 2 wk on sodium and fat adjusted diets where mice have not gained significant weight, both HF and HF/NaCl treatment significantly elevated blood glucose after glucose injection in male mice (Figure 3A). After 8 weeks on diets where HF diet mice had significant adiposity and HF/NaCl were normal weight, a similar effect in GTT was detected where both HF and HF/NaCl mice had significantly elevated blood glucose after GTT when compared to chow an NaCl treated mice (Figure 3B). In female mice, only HF/NaCl diet resulted in significantly elevated blood glucose levels during GTT after both 2 wk and 8 wk on diets (Figure 3C–D).
Figure 3. HF/NaCl diet induces glucose intolerance in the absence of obesity.
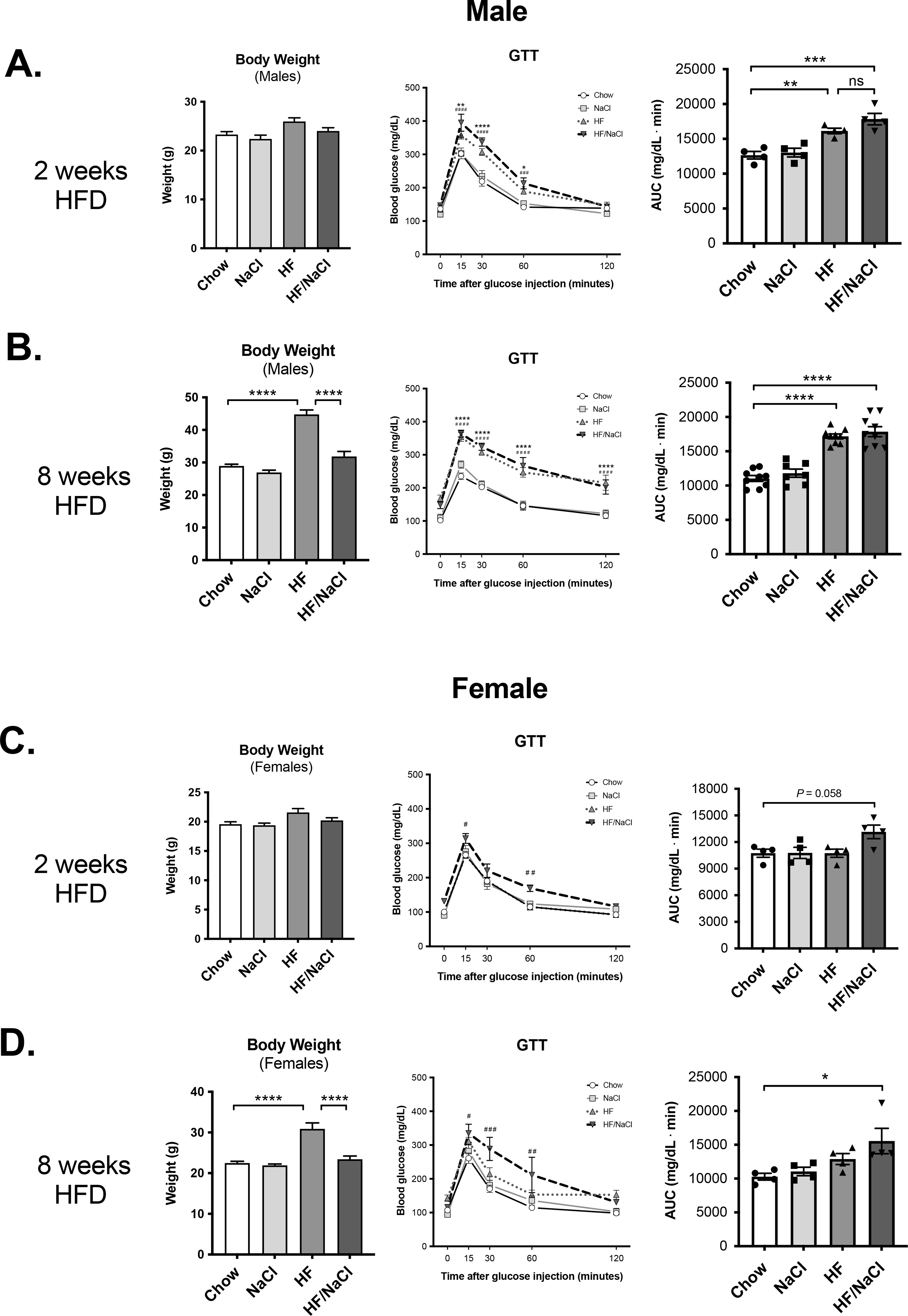
Body weight and GTT at 2 wk on diet in (A) male and, (C) female mice. Body weight and GTT at 8 wk on diet in (B) male and (D) female mice. Data represented as mean ± SEM. N = 4–9 per group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. * Chow v. HF, # Chow v. HF/NaCl.
To determine if HF/NaCl diet resulted in impaired insulin response in the absence of obesity, we measured plasma insulin levels. After 2 wk on diets, none of the treatments resulted in increased fasted insulin levels. By 8 wk on diets, only mice on a HF diet had significantly elevated fed and fasted insulin levels (Figure 4A). We performed ITTs on the mice at 8 wk on diets and found that again only HF diet treated male mice had impaired insulin responses with increased blood glucose after insulin injection (Figure 4B). Although females had significantly high blood glucose during the ITT, the percent reduction in blood glucose was similar (Figure 4C). This likely reflects the reduced obesity response seen in female mice during shorter length of HF diet treatment.
Figure 4. HF, but not HF/NaCl diet induces insulin resistance at 8 wk on diet.
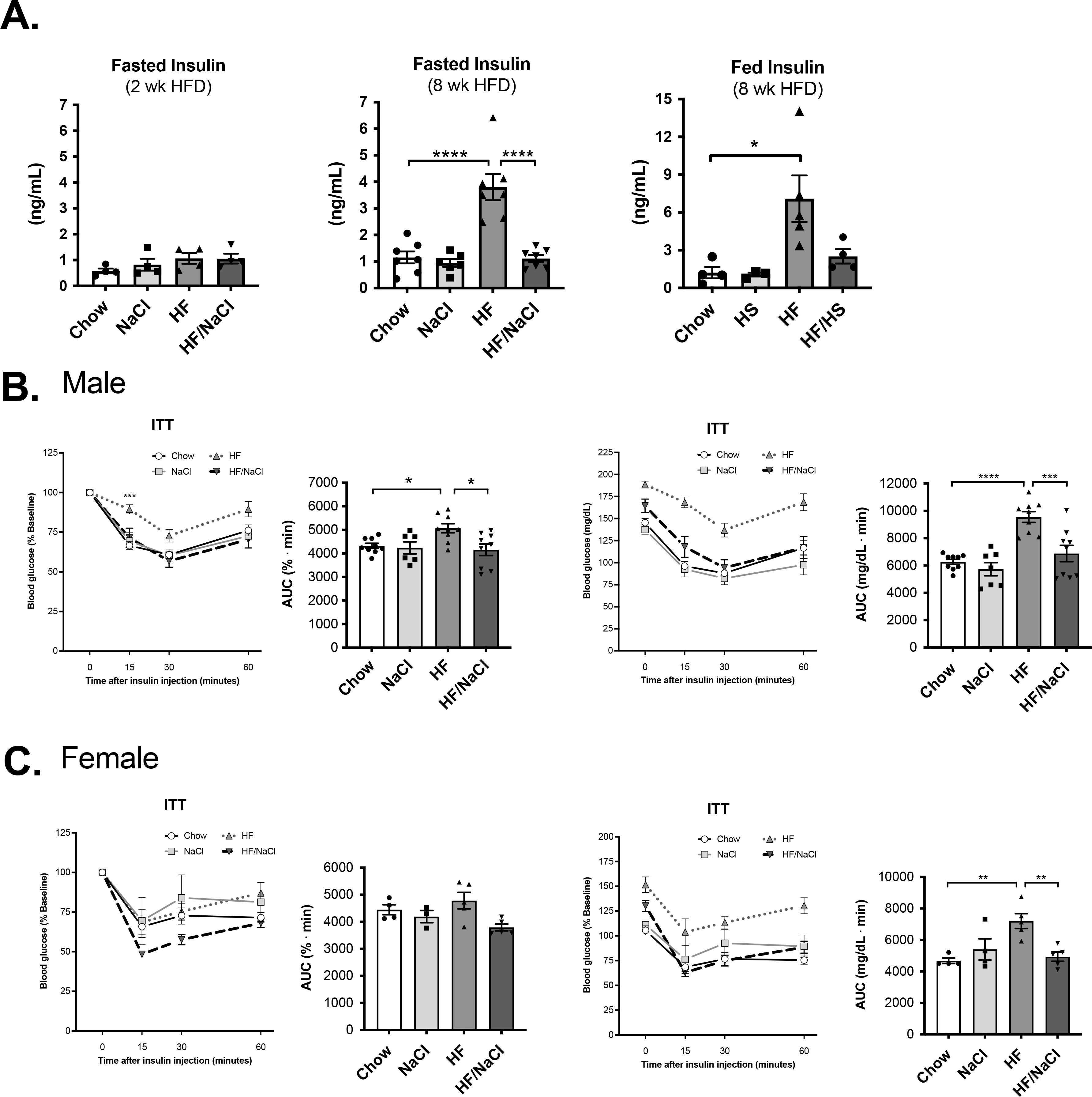
(A) Fasted and fed insulin levels at 2 and 8 wk on diet. ITT at 8 wk on diets in (B) male, and (C) female mice. Data represented as mean ± SEM. N = 4–9 per group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Upon dissection, histological assessment of gonadal white adipose tissue revealed increased adipocyte hypertrophy in HF diet treated mice and comparatively normal adipocyte size in HF/NaCl treated mice (Figure 5A–B). Similarly, hepatosteatosis was present in mice on HF diet whereas mice on HF/NaCl had normal liver histology. To assess whether changes in adipose depot function contribute to impaired glucose homeostasis in mice on HF/NaCl diet, we analyzed adipokine and inflammatory cytokine gene expression in gWAT depots after 10 wk on diets. The expression of inflammatory genes TNFα and IL-1β were modestly increased in HF diet treated mice, but not in mice on HF/NaCl diet (Figure 5B). Adiponectin expression was significantly decreased only with HF diet treatment although PPARγ was significantly decreased in mice on both a HF and HF/NaCl diet (Figure 5B).
Figure 5. Adipose tissue inflammation during HF diet.
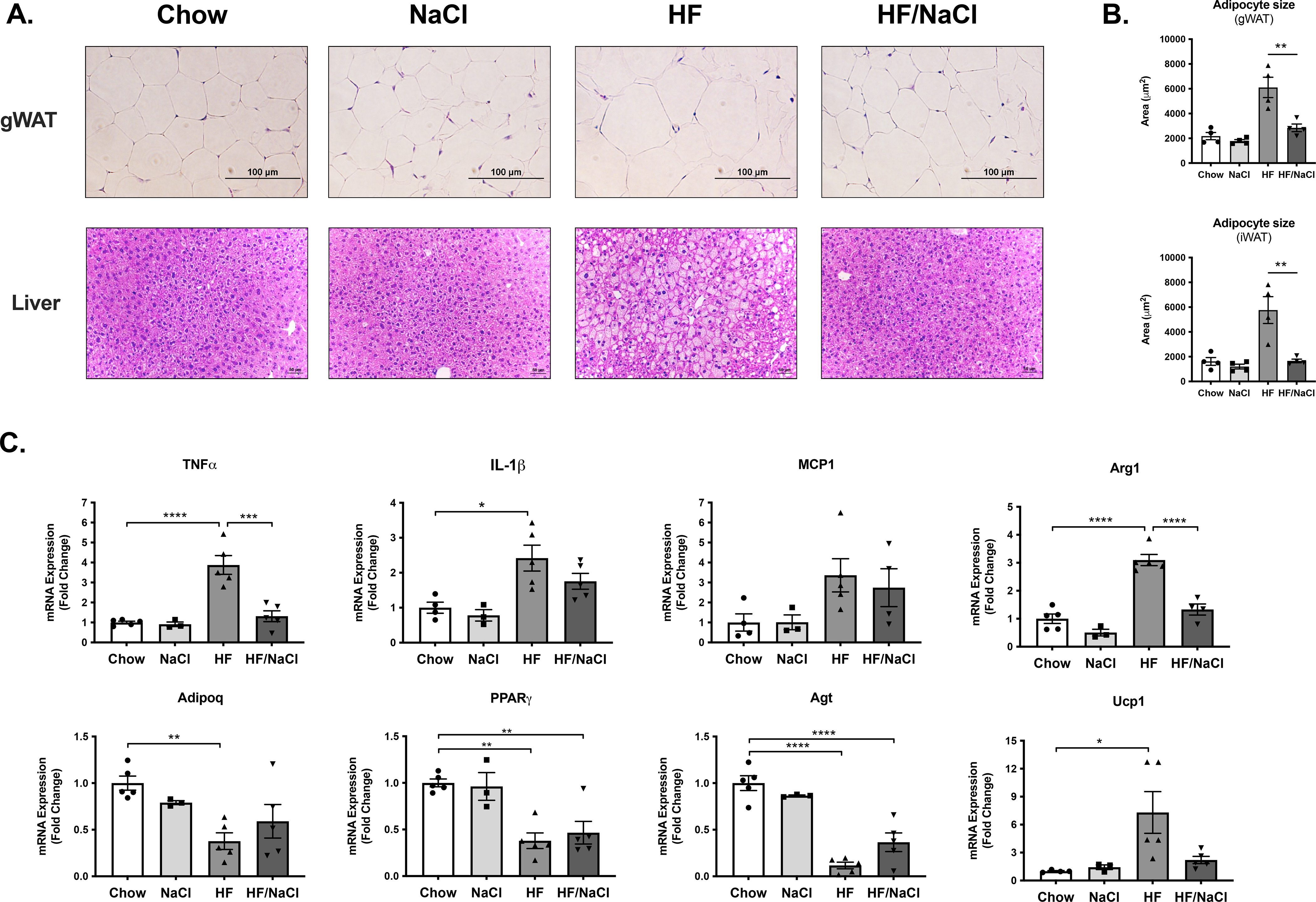
(A) Histology of gWAT and liver from male mice after 10 wk on diets (B) Adipocyte size (cross sectional area μm2) (C) Gene expression analysis measured by qRT-PCR of inflammatory cytokines and adipokines from gWAT at 10 wk on diets. Data represented as mean ± SEM. N = 3–5. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Mice on HF/NaCl diet have decreased pancreas and β-cell mass
To determine if HF/NaCl diet has an effect on metabolic capacity and glucose control through effects on pancreas function we first measured pancreas mass. Treatment with HF/NaCl diet for 10 weeks resulted in a small, but statistically significant reduction in pancreas mass in both male and female mice (Figure 6A–B). Female mice on NaCl diet also had a reduction in pancreas mass. We next assessed β-cell mass in male mice after 10 weeks on diets. Mice on HF diet had compensatory increases in β-cell mass compared to chow control mice (Figure 6C). Mice on NaCl and HF/NaCl diets had a statistically significant decrease in β-cell mass compared to both chow control and HF treated mice.
Figure 6. Decreased pancreas and β-cell mass in mice on HF/NaCl diet.
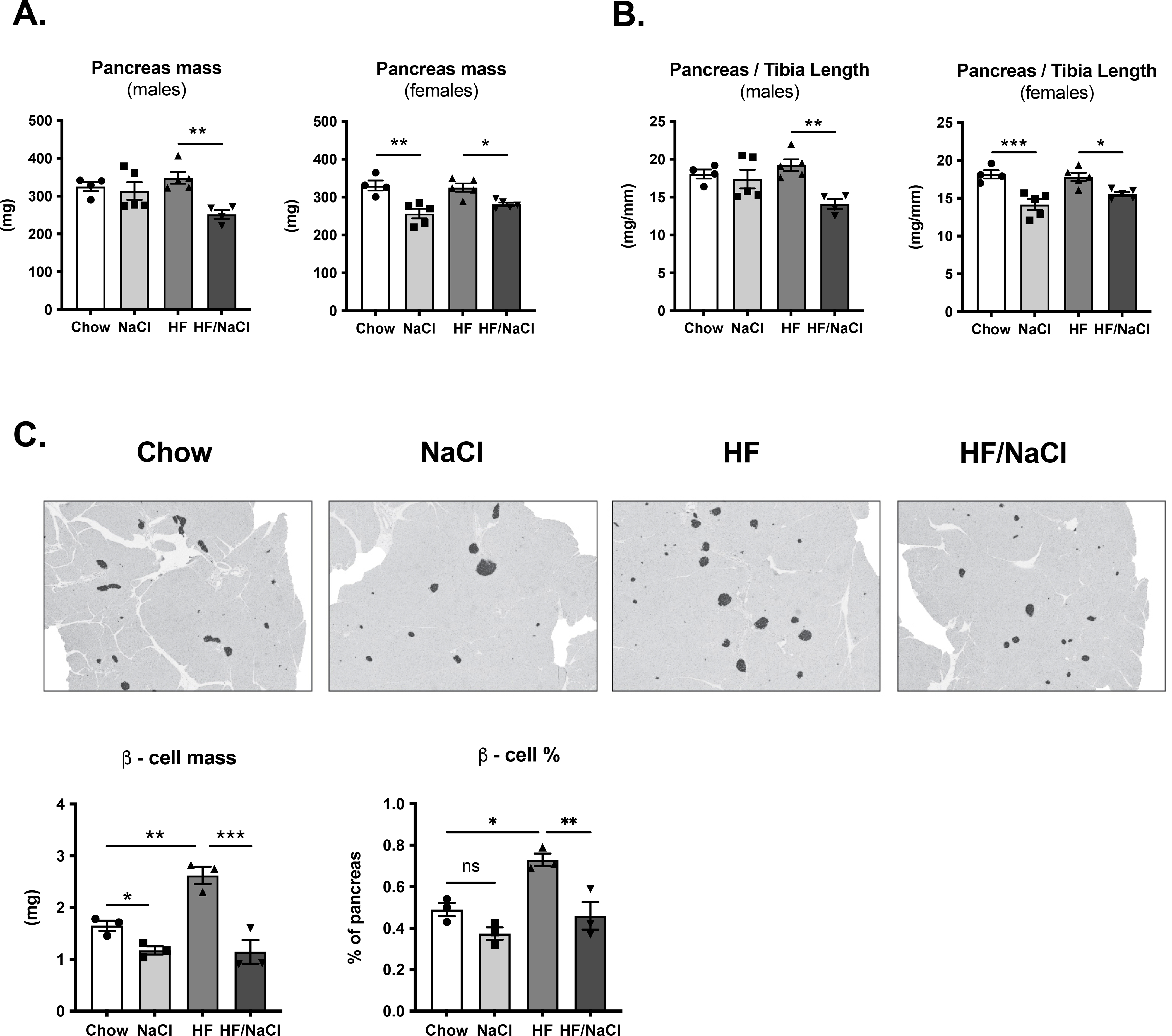
(A) Pancreas mass in male and female mice at 10 wk on diet (B) Pancreas mass normalized to tibia length at 10 wk on diet (C) Photomicrographs of pancreas with immunohistochemical staining for insulin and quantification of β-cell mass from male mice. Data represented as mean ± SEM. N = 3–5. *P < 0.05, **P < 0.01, ***P < 0.001.
High sodium administered in water does not prevent high fat diet induced obesity and insulin resistance.
We next assessed whether high sodium would also prevent DIO when administered in the drinking water. We selected a 2% NaCl water dose based on pilot experiments where we tested multiple concentrations of NaCl water (1%, 1.5%, 2%) and measured water consumption to estimate NaCl intake. The 2% NaCl water was determined to provide an equivalent NaCl intake, therefore wild type mice were placed on chow or high fat (HF) diets and sodium was administered as 2% NaCl water. Food consumption, kcal intake, water consumption and total NaCl intake were equivalent in HF/NaCl treatment whether NaCl was administered in diet or in water (Figure 7A). In the absence of HF diet, administration of NaCl in water did result in a significant increase in water consumption and NaCl intake compared to NaCl administration in the diet.
Figure 7. High salt administered in water does not prevent weight gain in male mice.
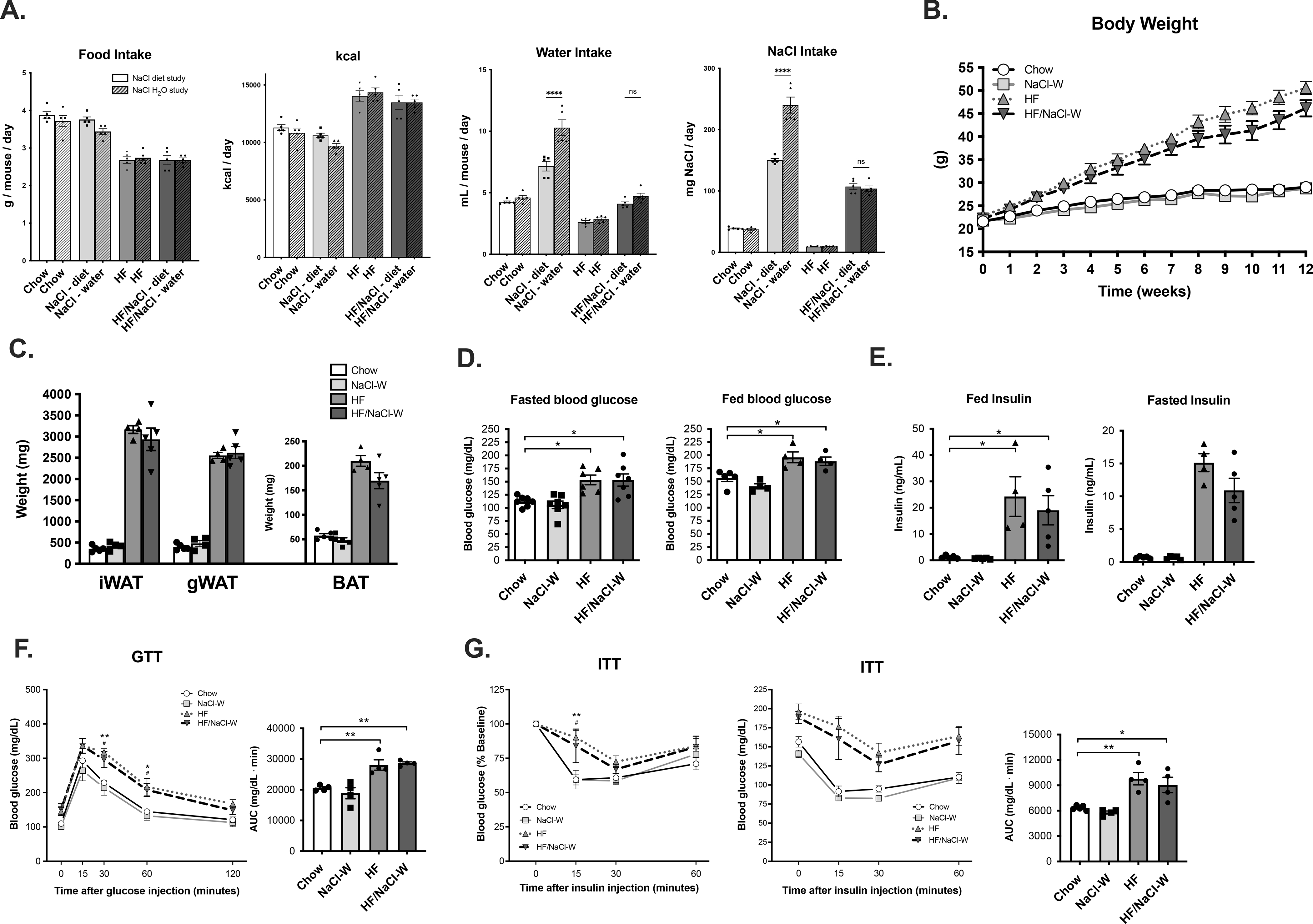
(A) food consumption, kcal intake, water consumption and NaCl intake (B) weight gain of male mice on diets (C) fat depot mass at 12 wk (D) fasted and fed blood glucose at 12 wk on diets (E) fed and fasted insulin at 12 2k on diet (F) GTT at 12 wk on diet and (G) ITT at 12 wk on diet. Data represented as mean ± SEM. N = 4–7 per group. *P < 0.05, **P < 0.01. * Chow v. HF, # Chow v. HF/NaCl.
Interestingly, administration of high sodium in drinking water did not prevent HF diet-induced weight gain (Figure 7B). Both HF and HF/NaCl-W mice had significantly increased fat deposition in major fat depots although no differences were detected between the two treatments (Figure 7C). Both HF and HF/NaCl-W treatment resulted in significantly increased fasted and fed blood glucose (Figure 7D) and significantly elevated fed and fasted insulin levels (Figure 7E). We next challenged mice with GTT and ITT to test glucose tolerance and insulin sensitivity. Both HF and HF/NaCl-W resulted in significantly increased glucose levels during both GTTs (Figure 7F) and ITTs (Figure 7G), although no differences were present as a result of increased sodium.
Incorporation of sodium into high fat diet only protects from obesity and insulin resistance during short-term feeding.
Since incorporation of sodium in HF diet protected mice from weight gain and development of insulin resistance, we tested whether administration of HF/NaCl diet to obese mice would result in weight loss and normalization of insulin sensitivity. Mice were treated with chow, NaCl, HF, and HF/NaCl diets, and after 10 wk, obese mice on HF diet were switched to HF/NaCl diet. The switch to HF/NaCl diet resulted in an initial decrease in weight, but mice eventually resumed gaining weight (Figure 8A). Mice on HF/NaCl diet also gained a significant amount of weight from week 10 until week 20 and were obese by the end of the 20 week treatment. There were no differences in food consumption when obese mice were on HF or HF/NaCl diets (Figure 8B). As expected, HF and HF/NaCl treated mice had increased fasted blood glucose levels, and only obese HF treated mice had increased fasted insulin after 10 wk on diets (Figure 8C). At 10 weeks post diet switch, both HF to HF/NaCl and HF/NaCl treatments resulted in increased fasted insulin levels (Figure 8D). When subjected to GTT, both mice on HF to HF/NaCl and HF/NaCl diet for 20 weeks had increased blood glucose (Figure 8E). After 20 wk, mice on HF/NaCl diet also had significantly reduced insulin sensitivity during ITT (Figure 8F).
Figure 8. Dietary switch from HF to HF/NaCl does not reverse weight gain in male mice.
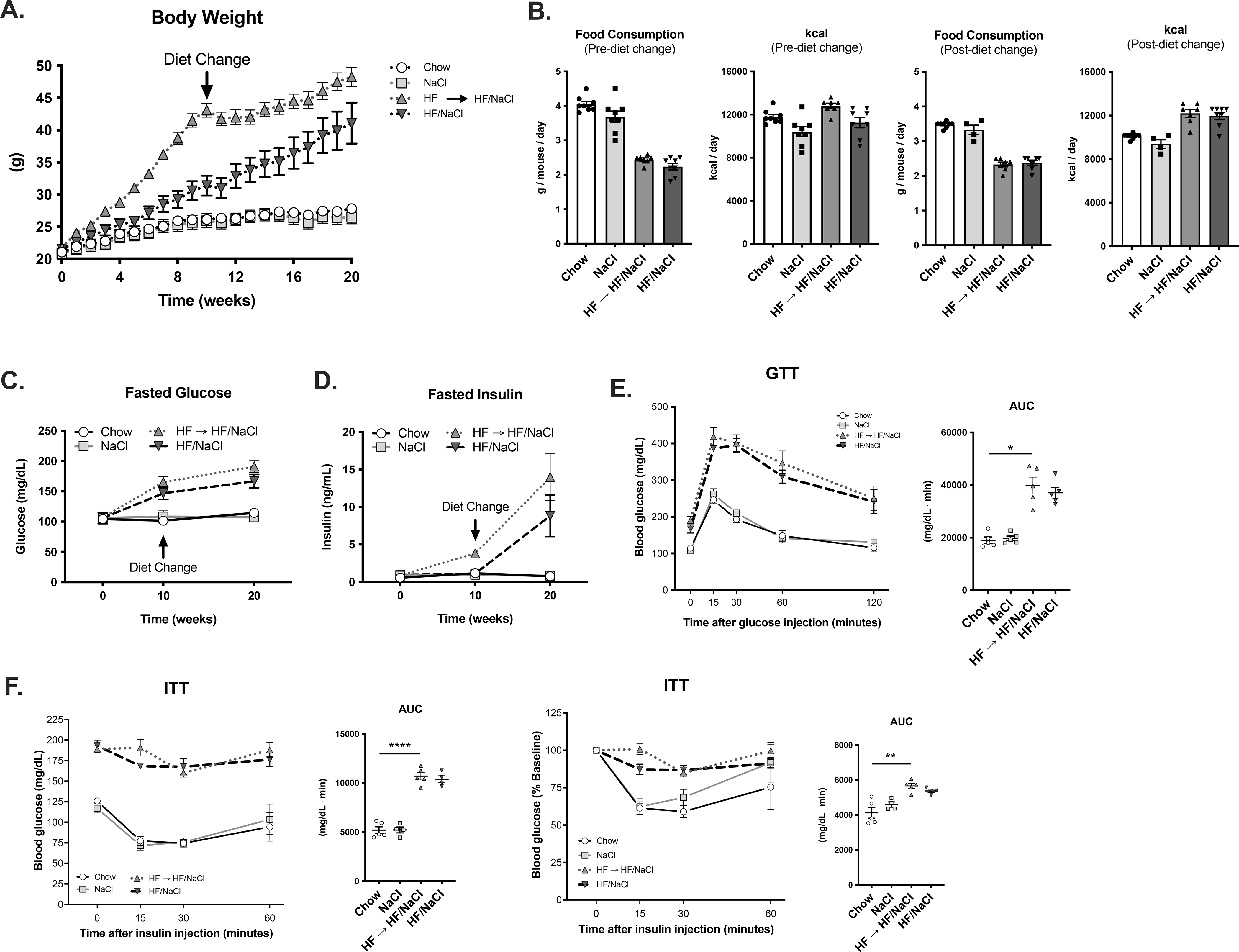
(A) Body weight of male mice (B) Food consumption and kcal intake before and after diet switch. Time course of (C) fasted blood and (D) fasted insulin. (E) GTT and (F) ITT at 20 wk after diet switch. Data represented as mean ± SEM. N = 4–9 per group. *P < 0.05, **P < 0.01, ****P < 0.0001.
DISCSUSSION
Excess sodium has been implicated in the development of obesity and metabolic dysfunction, and more recently, studies have shown that sodium can dose-dependently suppress high fat diet-induced weight gain. In the present study, we examined the metabolic consequence of HF/NaCl in the absence of obesity. Similar to what was reported previously (25), we found that HF/NaCl diet effectively prevented weight gain in male and female mice. However, when we extended the diet administration and body weight analysis to 20 wk, HF/NaCl mice had significant adiposity. This indicates that the suppressive effects on weight gain are only transient.
Mice on HF diet had decreased energy expenditure when normalized to body weight. This is consistent to what has been reported in the literature and it is thought that this significantly contributes to observed obesity changes. However, white adipose tissue often has lower metabolic activity than other tissues, and therefore normalization to body weight or lean body mass may not accurately reflect the correct expenditure rate. While it is possible that the significant increase in energy expenditure in mice on HF/NaCl diet at the later 8 wk analysis could be responsible for the transient inhibition of weight gain, more detailed and rigorous analysis will be required to elucidate this mechanism (26).
Further analysis of early administration (10 wk) revealed that despite the lack of weight gain and obesity, mice on HF/NaCl diet had significantly elevated fasting blood glucose and GTTs after 2 wk administration. This early effect on glucose levels by HF diet is consistent with literature where it has been shown that nearly maximal glucose intolerance and insulin resistance can be achieved within 3 days of HF diet administration (27). We did not detect any differences in blood glucose during ITT in HF/NaCl mice compared to chow, suggesting that HF/NaCl mice still maintain normal insulin sensitivity. These results indicate that the lack of obesity in HF/NaCl mice does not completely protect them from the metabolic consequences of HF diet.
In female mice, the HF/NaCl diet also resulted in a modest increase in glucose intolerance compared to HF diet controls. It is well known that female mice have a delayed and blunted response to HF diet, and female mice do not exhibit the same degree of insulin resistance compared with male mice (28). Increased glucose intolerance in female mice treated with HF/NaCl diet may indicate a sexually dimorphic effect with respect to salt sensitivity and glucose regulation, however an overwhelming majority of studies have found that female mice actually have decreased salt sensitivity with respect to blood pressure elevation (29, 30). This is in contrast to humans where there is evidence that women are more salt sensitive than men (31, 32, 33). In addition, it has been found that women have an increased taste for salt and are more likely to excessively consume salt than men. (34, 35). Similarly, female rodents have been reported to consume more salt even though they are less salt sensitive to hypertension (36, 37). There is insufficient evidence in animal models to draw strong conclusions on whether a sexual dimorphism exists with regard to salt sensitivity, glucose regulation, and insulin resistance.
High sodium administration in water has been reported to cause impaired glucose control in mice in the absence of high fat diet (8). However, we did not detect any signs of glucose intolerance or insulin resistance in our NaCl diet treated group. A previous study that showed salt can induce insulin resistance used Balb/c mice, which are known to have significantly different responses in a variety of animal models possibly due to differences in immune cell phenotypes. However, when we assessed the effect of NaCl in balb/c mice, we also did not detect any signs glucose intolerance or insulin resistance (data not shown). This could be due to differences in the microbiota which can arise in different animal vivaria. Animal microbiota has been shown to affect glucose control and insulin resistance (38), and it is also known that housing conditions can significantly affect the microbiota and can alter the phenotypes in many disease models.
It is also possible that sodium-induced changes in gut microbiota could be important in the observed weight gain inhibition and glucose intolerance phenotype. Several studies have shown that high sodium diet can elicit significant changes in the intestinal microbiota and cause dysbiosis through alteration of Lactobacillus other bacterial species. These high sodium-induced changes in microbiota have been found to exacerbate numerous animal models of disease including colitis, EAE, hypertension, and autism (39, 40, 41). Wilck and colleagues found that decreased Lactobacillus resulted in enhanced Th17 cell activation, which exacerbated EAE and hypertension. Importantly, Th17 activation also contributes to obesity-associated inflammation and type 2 diabetes (42). High sodium diet has also been found to induce changes in fecal short chain fatty acids (43, 44). Short chain fatty acids can alter host metabolism, and total levels of SCFA correlate with insulin sensitivity, therefore sodium-induced changes could have important implications in both glucose control and obesity.
Although NaCl diet alone did not result in impaired glucose control, both NaCl and HF/NaCl mice had decreased pancreas mass and decreased β-cell mass. This may indicate that impaired glucose control in mice on HF/NaCl diet is in part due to reduced insulin secretion as a result of decreased β-cell mass. This would also be consistent with our results showing increased glucose intolerance with apparently normal insulin sensitivity during ITT. However, the literature on the effects of high and low sodium intake on β-cell mass and insulin secretion is limited. In humans, sodium restriction has actually been shown to be associated with decreased insulin secretion (45).
The presence of glucose intolerance may also suggest that significant fat absorption is occurring and that other metabolic factors may be contributing to inhibition of weight gain. Weidemann et al. found that HF/NaCl mice had significantly decreased fat absorption using fecal steatocrit measurements, and they concluded that suppression of absorption was through RAS activation. Our early metabolic analysis at 1 wk on diets using CLAMS did indicate that HF/NaCl had similar energy substrate utilization as mice on HF diet whereby HF resulted in increased fatty acid oxidation and reduced glucose oxidation. While it is possible that macronutrient composition and not quantity could account for our observed glucose intolerance, we also did not detect differences in absorption using bomb calorimetry (data not shown).
Administration of an equivalent dose of sodium in drinking water did not suppress HF diet-induced body weight gain. HF/NaCl-W mice had elevated fasting insulin and glucose levels and also had increased GTT and ITT at similar levels as the HF diet group. If suppression of weight gain is through systemic RAS activation, it would be expected that NaCl administered in water should have a similar effect. A previous report found that a concentration of NaCl diet (4.3%) similar to what we used, inhibited weight gain in rats on a normal, low fat diet (23). Interestingly, administration of NaCl in water (1%) that provided a similar NaCl intake did not result in inhibition of body weight gain. Although we did not detect any differences in body weight gain when NaCl was administered during low fat, chow diet, our result in HF diet administration is very similar. In this previous study, it was found that when NaCl was administered in the diet versus water, rats had decreased water intake and reduced energy retention, and it was suggested that this effect is due to decreased energy efficiency as a result of changes in electrolyte concentration. However, in our study the NaCl intake and water intake were not different whether NaCl was administered in diet or water. This may suggest a different mechanism, although a more thorough analysis of calorie absorption and energy efficiency is necessary.
Although mice on HF/NaCl diet have delayed weight gain, they did eventually begin to gain weight which resulted in obesity and insulin resistance after 20 wk of administration. Given that high sodium has direct proinflammatory effects on immune cells like macrophages and lymphocytes, it could be hypothesized that the addition of high sodium may further enhance metabolic or cardiovascular complications after mice have reached an equivalent degree of obesity. This could potentially lead to more severe metabolic and cardiovascular consequences than HF diet alone.
The suppressive effects of salt on weight gain in rodents contrasts what has been observed in humans. In our study and most studies in the literature, high salt has inhibitory effects on weight gain in rodents (22, 23, 24, 25). In contrast, almost all human data has shown that high sodium intake is associated with weight gain or obesity (19, 20). These comparisons are difficult to interpret because the levels of NaCl intake in humans may not be sufficient to achieve a similar effect. Humans consume an average of 3600 mg sodium (9144 mg NaCl) per day which is approximately 130 mg/kg. In comparison, mice on a high NaCl diet consume approximately 100 mg NaCl per day, which is roughly 4000 mg/kg. These concentrations of NaCl would most certainly not be palatable in most humans.
In summary, several major observations have emerged from our analysis of a HF/NaCl diet: 1) non-obese mice on HF/NaCl have impaired glucose control, 2) high sodium decreases pancreas and β-cell mass, 3) prolonged administration of HF/NaCl diet (>12 weeks) leads to obesity, 4) administration of HF/NaCl diet to obese animals does not reverse weight gain, 5) administration of high sodium in drinking water does not prevent HF diet-induced weight gain.
Supplementary Material
STUDY IMPORTANCE.
What is already known?
Excess sodium intake is associated with increased risk of cardiovascular disease, obesity and inflammation
Animal models have shown that high sodium can inhibit adiposity in mice
What does this study add?
High sodium causes impaired glucose control during high fat diet in the absence of obesity
Inhibitory effects of sodium on high-fat diet induced weight gain are transient and are dependent on the route of sodium intake
How might these results change the direction of research or the focus of clinical practice?
These findings demonstrate an important interaction between high fat diet, sodium intake and glucose regulation, and they indicate that a thorough understanding of this interaction is critical in our understanding of metabolic dysfunction.
Acknowledgments
Funding: This study was supported by National Institutes of Health grant F31NS108617 (T.M.V.), by National Institutes of Health grant T32-HL125242, Crohns and Colitis Foundation award 609962 (R.A.F.), and by P30 grants DK020572 (MDRC), DK089503 (MNORC), and 1U2CDK110678-01 (Mi-MMPC).
Footnotes
Disclosures: The authors declared no conflict of interest.
References
- 1.He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet 2011;378: 380–382. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014;371: 624–634. [DOI] [PubMed] [Google Scholar]
- 3.Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, Campbell N, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation 2012;126: 2880–2889. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann IS, Cubeddu LX. Salt and the metabolic syndrome. Nutr Metab Cardiovasc Dis 2009;19: 123–128. [DOI] [PubMed] [Google Scholar]
- 5.Baudrand R, Campino C, Carvajal CA, Olivieri O, Guidi G, Faccini G, et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol (Oxf) 2014;80: 677–684. [DOI] [PubMed] [Google Scholar]
- 6.Vedovato M, Lepore G, Coracina A, Dodesini AR, Jori E, Tiengo A, et al. Effect of sodium intake on blood pressure and albuminuria in Type 2 diabetic patients: the role of insulin resistance. Diabetologia 2004;47: 300–303. [DOI] [PubMed] [Google Scholar]
- 7.Lima NK, Tozetto DJ, Lima LG, Nobre F, Moriguti JC, Ferriolli E, et al. Salt and insulin sensitivity after short and prolonged high salt intake in elderly subjects. Braz J Med Biol Res 2009;42: 738–743. [DOI] [PubMed] [Google Scholar]
- 8.Boini KM, Hennige AM, Huang DY, Friedrich B, Palmada M, Boehmer C, et al. Serum- and glucocorticoid-inducible kinase 1 mediates salt sensitivity of glucose tolerance. Diabetes 2006;55: 2059–2066. [DOI] [PubMed] [Google Scholar]
- 9.Baudrand R, Lian CG, Lian BQ, Ricchiuti V, Yao TM, Li J, et al. Long-term dietary sodium restriction increases adiponectin expression and ameliorates the proinflammatory adipokine profile in obesity. Nutr Metab Cardiovasc Dis 2014;24: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab 2010;95: 1986–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronconi V, Turchi F, Appolloni G, di Tizio V, Boscaro M, Giacchetti G. Aldosterone, mineralocorticoid receptor and the metabolic syndrome: role of the mineralocorticoid receptor antagonists. Curr Vasc Pharmacol 2012;10: 238–246. [DOI] [PubMed] [Google Scholar]
- 12.Garg R, Williams GH, Hurwitz S, Brown NJ, Hopkins PN, Adler GK. Low-salt diet increases insulin resistance in healthy subjects. Metabolism 2011;60: 965–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation 2008;117: 2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013;496: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenstedt EF, Verberk SG, Kroon J, Neele AE, Baardman J, Claessen N, et al. Salt increases monocyte CCR2 expression and inflammatory responses in humans. JCI Insight 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang WC, Zheng XJ, Du LJ, Sun JY, Shen ZX, Shi C, et al. High salt primes a specific activation state of macrophages, M(Na). Cell Res 2015;25: 893–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Zhang L, Ji WJ, Yuan F, Guo ZZ, Pang B, et al. Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyte-platelet aggregates in humans: implications in end organ inflammation. PLoS One 2013;8: e60332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest 2015;125: 4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi SS, Firestone MJ, Beasley JM. Independent associations of sodium intake with measures of body size and predictive body fatness. Obesity (Silver Spring) 2015;23: 20–23. [DOI] [PubMed] [Google Scholar]
- 20.Song HJ, Cho YG, Lee HJ. Dietary sodium intake and prevalence of overweight in adults. Metabolism 2013;62: 703–708. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca-Alaniz MH, Brito LC, Borges-Silva CN, Takada J, Andreotti S, Lima FB. High dietary sodium intake increases white adipose tissue mass and plasma leptin in rats. Obesity (Silver Spring) 2007;15: 2200–2208. [DOI] [PubMed] [Google Scholar]
- 22.Cui H, Yang S, Zheng M, Liu R, Zhao G, Wen J. High-salt intake negatively regulates fat deposition in mouse. Sci Rep 2017;7: 2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drori D The effect of sodium chloride ingestion on food intake and on fat deposition in male rats. Br J Nutr 1976;35: 195–200. [DOI] [PubMed] [Google Scholar]
- 24.DeClercq VC, Goldsby JS, McMurray DN, Chapkin RS. Distinct Adipose Depots from Mice Differentially Respond to a High-Fat, High-Salt Diet. J Nutr 2016;146: 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weidemann BJ, Voong S, Morales-Santiago FI, Kahn MZ, Ni J, Littlejohn NK, et al. Dietary Sodium Suppresses Digestive Efficiency via the Renin-Angiotensin System. Sci Rep 2015;5: 11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, et al. A guide to analysis of mouse energy metabolism. Nat Methods 2011;9: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 2011;60: 2474–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One 2012;7: e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayorh MA, Socci RR, Eatman D, Wang M, Thierry-Palmer M. The role of gender in salt-induced hypertension. Clin Exp Hypertens 2001;23: 241–255. [DOI] [PubMed] [Google Scholar]
- 30.Dahl LK, Knudsen KD, Ohanian EV, Muirhead M, Tuthill R. Role of the gonads in hypertension-prone rats. J Exp Med 1975;142: 748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ, et al. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol 2004;94: 222–227. [DOI] [PubMed] [Google Scholar]
- 32.Chen J Sodium sensitivity of blood pressure in Chinese populations. Curr Hypertens Rep 2010;12: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shukri MZ, Tan JW, Manosroi W, Pojoga LH, Rivera A, Williams JS, et al. Biological Sex Modulates the Adrenal and Blood Pressure Responses to Angiotensin II. Hypertension 2018;71: 1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michikawa T, Nishiwaki Y, Okamura T, Asakura K, Nakano M, Takebayashi T. The taste of salt measured by a simple test and blood pressure in Japanese women and men. Hypertens Res 2009;32: 399–403. [DOI] [PubMed] [Google Scholar]
- 35.Rhee MY, Kim JH, Kim YS, Chung JW, Bae JH, Nah DY, et al. High sodium intake in women with metabolic syndrome. Korean Circ J 2014;44: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krecek J, Novakova V, Stibral K. Sex differences in the taste preference for a salt solution in the rat. Physiol Behav 1972;8: 183–188. [DOI] [PubMed] [Google Scholar]
- 37.Flynn FW, Schulkin J, Havens M. Sex differences in salt preference and taste reactivity in rats. Brain Res Bull 1993;32: 91–95. [DOI] [PubMed] [Google Scholar]
- 38.Cani PD. Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol 2019;15: 69–70. [DOI] [PubMed] [Google Scholar]
- 39.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017;551: 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afroz KF, Reyes N, Young K, Parikh K, Misra V, Alvina K. Altered gut microbiome and autism like behavior are associated with parental high salt diet in male mice. Sci Rep 2021;11: 8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miranda PM, De Palma G, Serkis V, Lu J, Louis-Auguste MP, McCarville JL, et al. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018;6: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ip B, Cilfone NA, Belkina AC, DeFuria J, Jagannathan-Bogdan M, Zhu M, et al. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFalpha production. Obesity (Silver Spring) 2016;24: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bier A, Braun T, Khasbab R, Di Segni A, Grossman E, Haberman Y, et al. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu L, Zhu S, Peng X, Li K, Peng W, Zhong Y, et al. High Salt Elicits Brain Inflammation and Cognitive Dysfunction, Accompanied by Alternations in the Gut Microbiota and Decreased SCFA Production. J Alzheimers Dis 2020;77: 629–640. [DOI] [PubMed] [Google Scholar]
- 45.Luther JM, Byrne LM, Yu C, Wang TJ, Brown NJ. Dietary sodium restriction decreases insulin secretion without affecting insulin sensitivity in humans. J Clin Endocrinol Metab 2014;99: E1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


