Abstract
Purpose of review
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic has overwhelmed the global community, negatively impacting patient health and research efforts; associated neurological manifestations are a significant cause of morbidity. This review outlines the worldwide epidemiology of neurologic manifestations of different SARS-CoV-2 clinical pediatric phenotypes, including acute coronavirus disease 2019 (COVID-19), multisystem inflammatory syndrome in children (MIS-C) and postacute sequelae of COVID-19 (PASC). We discuss strategies to develop adaptive global research platforms for future investigation into emerging pediatric neurologic conditions.
Recent findings
Multicenter, multinational studies show that neurological manifestations of acute COVID-19, such as smell/taste disorders, headache, and stroke, are common in hospitalized adults (82%) and children (22%), associated with increased mortality in adults. Neurological manifestations of MIS-C are reported in up to 20% of children, including headache, irritability, and encephalopathy. Data on PASC are emerging and include fatigue, cognitive changes, and headache. Reports of neurological manifestations in each phenotype are limited by lack of pediatric-informed case definitions, common data elements, and resources.
Summary
Coordinated, well resourced, multinational investigation into SARS-CoV-2-related neurological manifestations in children is critical to rapid identification of global and region-specific risk factors, and developing treatment and mitigation strategies for the current pandemic and future health neurologic emergencies.
Keywords: coronavirus disease 2019, epidemiology, multisystem inflammatory syndrome in children, pediatric, severe acute respiratory syndrome coronavirus-2
INTRODUCTION
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the virus that causes acute coronavirus disease 2019 (COVID-19) and the postinfectious hyperinflammatory shock syndrome, multisystem inflammatory syndrome in children (MIS-C), was declared the source of a global pandemic on March 11, 2020 by the World Health Organization (WHO) [1]. [2] (Fig. 1). Neurological manifestations of SARS-CoV-2 infection were recognized early as a significant cause of mortality and morbidity, similar to influenza, Zika, and other emerging virus outbreaks [3,4]. This review will outline the worldwide epidemiology and clinical manifestations of acute COVID-19, MIS-C, and postacute sequelae of SARS-CoV-2 (PASC) and their respective neurological manifestations in children.
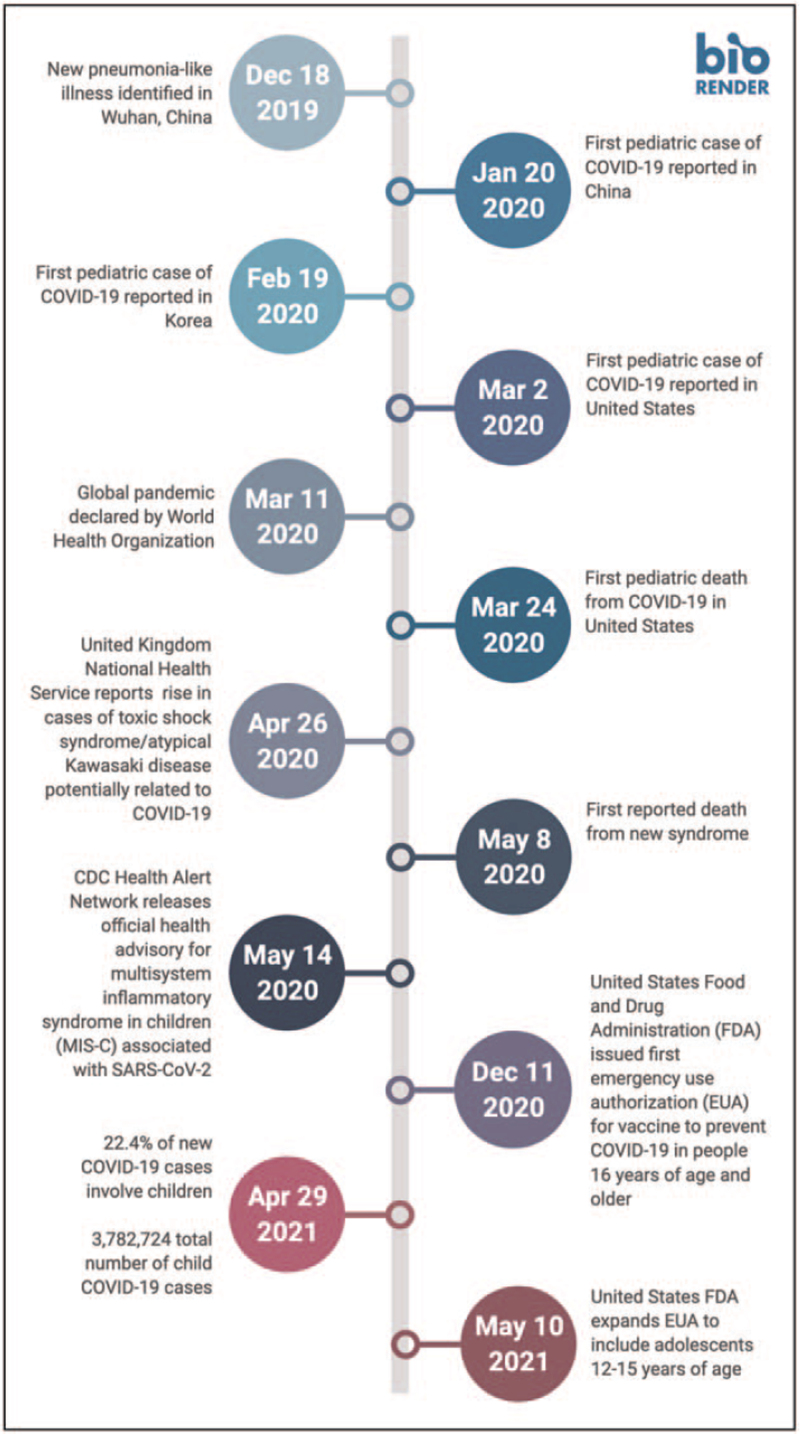
FIGURE 1.
Timeline of SARS-CoV-2 Pandemic. CDC, Centers for Disease Control and Prevention; EUA, emergency use authorization; FDA, Food and Drug Administration; MIS-C, multisystem inflammatory syndrome in children. Adapted from ‘Timeline (8 Segments, Vertical)’, by BioRender.com (2020).
Box 1.
no caption available
PEDIATRIC SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS-2 EPIDEMIOLOGY
As of July 12, 2021, the WHO estimates that there have been 186,638,285 confirmed cases of COVID-19 reported worldwide, including 4,035,037 deaths. The WHO estimates that children under 14 years old have thus far accounted for 5,486,503 of reported COVID-19 cases (2.5%) and 2,717 of reported deaths (0.07%) [5▪▪]. The United Nations Children's Fund (UNICEF) estimates that children under 20 years old account for 13% of reported cases (10–15% in high and upper middle income countries [HIC], vs 10–11% in lower middle and low income [LMIC] countries), and 0.3% of reported deaths (0.1% in HIC, 1.1% in LMIC) [6]. The American Academy of Pediatrics (AAP) and the Children's Hospital Association estimate that children account for 14.2% of total reported cases in the United States (US) [7]. The differences in these estimates are likely related to access to testing and reporting strategies in different regions and countries.
In addition to case counts and mortality rates, the SARS-CoV-2 pandemic has had other significant impacts on overall child health. Modeling estimates from LMICs suggest there has been an increase in child mortality from causes other than SARS-CoV-2 infection due to widespread disruption of healthcare systems [8]. Projections from UNICEF estimate that as a result of the pandemic, 140 million children are living in poverty in developing countries [9▪▪], 463 million children have not had access to remote learning during school closures, 80 million children under the age of 1 have not received routine vaccinations [10], and 1.8 billion children are at risk for violence, exploitation and abuse due to disruption of violence prevention and response services [11].
Severe acute respiratory syndrome coronavirus-2 variants
As of July 2, 2021, the WHO has designated four notable SARS-CoV-2 variants of concern (VOC) in widespread global circulation, including: Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2). Although there are many more variants in circulation, the VOCs listed above have each been associated with a change in public health significance, such as increased transmissibility, increased virulence, change in clinical presentation, decreased effectiveness of public health and social measures or available diagnostics, vaccines, and therapeutics [12].
Severe acute respiratory syndrome coronavirus-2 clinical phenotypes in children
Acute coronavirus disease 2019
Clinical signs and symptoms of acute COVID-19 range in severity. Up to 33% of infected individuals may be asymptomatic [13]. The remainder of children, similar to adults, have symptoms ranging from mild upper respiratory symptoms (fever, chills, cough) to more severe symptoms of hypoxemia, acute respiratory distress syndrome, and shock. Ten percent of children experience more severe illness with acute COVID-19 [14,15], particularly among Black children [16▪], and those with reported underlying medical conditions [17,18].
Multisystem inflammatory syndrome in children
First described in April 2020, MIS-C is a postinfectious hyperinflammatory syndrome that occurs in <1% of children [19] following confirmed SARS-CoV-2 infection [20]. Previously referred to as atypical Kawasaki disease, pediatric MIS (PMIS), and pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS), MIS-C is a distinct clinical and immunologic entity from COVID-19 [21▪]. MIS-C manifests primarily with gastrointestinal, mucocutaneous, and cardiovascular symptoms including myocardial dysfunction and shock; respiratory symptoms are less common. About 60% of children with MIS-C require intensive care unit admission. In addition, while children with acute COVID-19 have evidence of active SARS-CoV-2 infection by polymerase chain reaction (PCR), children with MIS-C more often have negative PCR but evidence of past infection by serology [22,23▪▪].
As of June 28, 2021, the Centers for Disease Control and Prevention (CDC) reported a total of 4,196 cases of MIS-C in the US, including 37 deaths [24]. Affected children are more likely to be older school age or adolescent (compared to younger age), Black or Hispanic (compared to White) [25,26▪▪], and unlike acute COVID-19, children with MIS-C are most often previously healthy [26▪▪]. Similar epidemiology has been described in the United Kingdom (UK) [27], Europe [28,29], Canada, and South Africa [30]. Notably, there have been disproportionately fewer MIS-C reports in Asian countries; reasons for this difference are not yet clear [31].
NEUROLOGICAL MANIFESTATIONS OF SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS-2
Multiple human coronaviruses (HCoV) have previously been associated with neurological manifestations [32]. Encephalitis [33] and acute disseminated encephalomyelitis (ADEM) [34] have been reported in association with HCoV-OC43. Headache, seizures, and cerebrovascular disease have been reported in association with SARS [35,36]. Finally, ADEM, Guillain-Barré syndrome (GBS), Bickerstaff encephalitis, intracerebral hemorrhage, vasculopathy, encephalopathy, and seizures have been reported in association with Middle Eastern Respiratory Syndrome (MERS) [35,37–39].
Acute coronavirus disease 2019 and multisystem inflammatory syndrome in children
Neurological manifestations in acute COVID-19 are reported globally in up to 82% of hospitalized adults [40▪,41,42]. The most commonly reported neurological manifestations are acute encephalopathy (up to 49%) and headache (37%); hospitalized adults with a neurological manifestation have been reported to have 6-fold higher odds of mortality [40▪]. Smell/taste disorders (anosmia, dysgeusia) have also been reported in 48–80% of all adults with acute COVID-19 [43,44], and ischemic stroke has been reported in 0.4–2.7% adults hospitalized or presenting to an ED [42,45–48]. There have been case reports of peripheral neurologic disorders (including GBS) [49–52], meningoencephalitis [53,54], ADEM and acute hemorrhagic necrotizing encephalopathy [49,55], seizures [56–58], and posterior reversible encephalopathy syndrome [52].
In children, there are fewer data regarding the prevalence of neurological manifestations of acute COVID-19 or MIS-C. Clinical neurologic, neurophysiologic, and neuroimaging data have not been routinely reported in large published epidemiologic cohorts, and cohorts often do not differentiate between acute COVID-19 and MIS-C. One potential reason for this lack of neurologic data is that some of the more subtle neurologic symptoms such as smell/taste disorders, headache, altered mental status, weakness, or paresthesia, may be difficult to identify in young children, and children may not recognize or volunteer these symptoms unless specifically asked. This ascertainment bias limits the ability of retrospective studies to adequately estimate the burden of neurologic manifestations among children.
The largest epidemiological study to date to examine neurological manifestations in hospitalized children included 1695 children with acute COVID-19 and MIS-C from 61 US hospitals. They found neurological manifestations in 22% of children with acute COVID-19 and 20% of children with MIS-C [59▪▪]. Children with underlying neurological conditions had a higher prevalence of neurological manifestations (42% vs 22% previously healthy children) in the overall mixed cohort. The clinical spectrum of neurological manifestations reported was similar to that seen in adults. About a quarter of children with neurological manifestations presented with altered mental status or confusion, and seizures were more commonly a presenting symptom in younger children (∼38% in children <5 years). Among a subset of 43 children with ‘life-threatening’ neurologic manifestations in the mixed cohort, 26% died and 40% of survivors had new neurologic deficits at discharge [59▪▪].
In the broader published pediatric literature, neurological manifestations such as smell/taste disorders, headache, seizures, and stroke appear to predominate in acute COVID-19, whereas headache, encephalopathy, and MRI changes suggesting neuroinflammation predominate in MIS-C. Examples of neurological manifestations in children reported to date are summarized by clinical phenotype in Table 1[60,61,62▪▪,63▪,64,65▪,66,67▪,68–75,76▪,77,78▪,79,80▪▪]. As these studies represent different pediatric cohorts (e.g. critically ill vs hospitalized patients), caution should be taken in extrapolating this data.
Table 1.
Prevalence of neurologic manifestations reported with children hospitalized with SARS-CoV-2 infection by clinical phenotypea
| Mixed cohort (Acute COVID-10 and MIS-C) | Acute COVID-19 | MIS-C |
| Any neurologic sign or symptom | ||
| 3/33 (9% Peru) [60]13/90 (14% Chile) [61]51/1334 (4% UK) [62▪▪] | 186/577 (32% US) [26▪▪]239/1079 (22% US) [59▪▪]2/48 (8% USA/Canada) [63▪]2/27 (7% France) [64] | 30/99 (30% New York, US) [19]13/186 (7% US) [23▪▪]218/539 (40% US) [26▪▪]126/616 (20% US) [59▪▪]24/46 (52% UK) [65▪]14/36 (39% Turkey) [66]4/27 (15% UK) [67▪] |
| Myalgias/muscular symptoms | ||
| 3/33 (9% Peru) [60]8/90 (9% Chile) [61]1/10 (10% Mexico) [68] | 1/24 (4% Spain) [69] | 17/186 (9% US) [23▪▪]7/43 (16% Spain) [69]17/45 (38% Iran) [70] |
| Headache | ||
| 5/33 (15% Peru) [60]1/9 (11% India) [71] | 3/24 (13% Spain) [69]4/27 (15% UK) [62▪▪]4/100 (4% Italy) [72]0/21 (0% India) [73] | 29/99 (29%, New York, USA) [19]24/46 (52% UK) [65▪]3/36 (8% Turkey) [66]3/27 (11% UK) [67▪]13/44 (30% Spain) [69]10/25 (40% UK) [62▪▪]5/20 (25% India) [73]61/268 (25% UK/Ireland) [74]27/56 (48% Brazil) [75] |
| Acute encephalopathy/acute agitation | ||
| 24/46 (54% UK) [65▪]6/9 (66% India) [71] | 7/1079 (0.6% US) [59▪▪]2/27 (7% France) [64]3/27 (11% Spain) [69]14/27 (52% UK) [62▪▪]1/21 (5% India) [73] | 2/99 (2%, New York, USA) [19]8/616 (1% US) [59▪▪]24/46 (52% UK) [65▪]7/36 (19% Turkey) [66]4/27 (15% UK) [67▪]2/45 (4% Spain) [69]22/25 (88% UK) [62▪▪]11/20 (55% India) [73]13/268 (5% UK/Ireland) [74] |
| Meningitis and/or encephalitis | ||
| 2/10 (20%; optic neuritis), 1/10 (10%; Anti-NMDA encephalitis, Mexico) [68] | 2/1079 (0.2% US) [59▪▪] | 6/616 (1% US) [59▪▪] |
| Seizures | ||
| 6/9 (66% India) [71] | 0/27 (0% Spain) [69]8/27 (30% UK) [62▪▪]5/168 (3% Italy) [76▪]3/62 (5% Saudi Arabia) [77] | 1/46 (2% UK) [65▪]1/45 (2% Spain) [69]4/25 (16% UK) [62▪▪]3/268 (1% UK/Ireland) [74]11/20 (55% India) [73] |
| Stroke | ||
| 8/971 (0.8% multicenter) [78▪]2/10 (20% Mexico) [68] | 9/1079 (0.8% US) [59▪▪] | 3/616 (0.5% US) [59▪▪] |
| Cerebral edema/intracranial hypertension | ||
| None | 2/1079 (0.2% US) [59▪▪] | 2/616 (0.3% US) [59▪▪]4 cases (Philadelphia, US) [79] |
| Myelopathy | ||
| 3/10 (30% Mexico; GBS) [68] | 3/1079 (0.3% US; GBS) [59▪▪]5/27 (18.5% UK; GBS) [62▪▪] | 1/616 (0.2% US; GBS) [59▪▪] |
| Brain MRI abnormalities | ||
| 7/38 (18% international; ADEM-like pattern, myelitis, neuritis, splenial lessons) [80▪▪] | 11/25 (44% UK; abnormal imaging) [62▪▪]6/12 (50% international; autoimmune presentations), 4/12 (33%, thromboischemic), 4/12 (33% infectious sequalae) [80▪▪] | 7/16 (44% UK; splenial changes, microhemorrhages, subcortical white matter lesions) [65▪]4/27 (15% UK; splenial lesions) [67▪]17/23 (74% UK; abnormal imaging) [62▪▪]4/11 (64% international; splenial lesions, ADEM-like lesions) [80▪▪] |
This table demonstrates the spectrum of neurological manifestations reported. This list is not all-inclusive of the current literature. Caution should be taken in comparing one study to another as each study has a different study population (e.g. all patients with neurological manifestations, all hospitalized patients), study designs, and study definitions. ADEM, acute disseminated encephalomyelitis; MIS-C, multisystem inflammatory syndrome in children; NMDA, N-methyl-D-aspartate; UK, United Kingdom; US, United States.
There are several significant knowledge gaps in the epidemiology of pediatric SARS-CoV-2 neurological manifestations. First, cases of acute COVID-19 and MIS-C are not always clearly differentiated. Second, lack of common data elements and definitions for neurologic signs and symptoms make prevalence estimates difficult to interpret and evaluate in meta-analyses. For example, the term ‘encephalopathy’ is nonspecific; it may include altered mental status, irritability, lethargy, or some combination of the three. Several upcoming studies, including the pediatric arm of the GCS-NeuroCOVID (NCT04379089) may help address this limitation [81] (Table 2). Third, despite limits placed on the conduct of research during the pandemic, increased research resources and preexisting research networks in HIC compared to LMIC led to publication biases, limiting our understanding of the scope of neurological manifestations and outcomes in less resourced regions. Finally, there are limited data regarding the impact of variants, vaccination, and disparities in access to vaccination, on the prevalence of neurological manifestations.
Table 2.
Ongoing/upcoming studies examining neurologic manifestations of SARS-CoV-2
| Study (Country) | Design | Primary Outcomes | Neurology-Pertinent Secondary Outcomes |
| COVID-19: Pediatric Research Immune Network on SARS-CoV-2 and MIS-C (PRISM)Trial number: NCT 04588363 (United States) | • Study Design: Prospective observational multisite cohort• Population: Acute COVID-19 and/or MIS-C• Inclusion criteria: <21 years old• Exclusion criteria: none | • Proportion of patients with death, rehospitalization, or complication | • Neurologic sequelae up to 1-year postillness• Health-related quality of life up to 1-year postillness |
| Global Consortium to Study Neurological dysfunction in COVID-19 (GCS-NeuroCOVID)Trial number: NCT 04379089 (Multinational) | • Study Design: Prospective observational multisite registry• Population: Acute COVID-19 and/or MIS-C• Inclusion criteria: <18 years old• Exclusion criteria: none | • Overall prevalence of neurological manifestations among hospitalized COVID-19 and/or MIS-C patients• Health-related quality of life 1-month postdischarge | • In-hospital, 30 and 90-day mortality• Discharge modified Rankin score |
| Neurocognitive Impairment in Patients With COVID-19Trial number: NCT04359914 (Germany) | • Study Design: Observational case control study• Population: Cohorts of adults and children with (cases) and without (controls) SARS-CoV-2 infection• Inclusion criteria: Patients admitted to a hospital and SARS-CoV-2 confirmed (cases) or excluded (controls) within 48 h of admission• Exclusion criteria: none | • Incidence of delirium/neurocognitive impairment in adult and pediatric patients with COVID-19• Change in neuroaxonal injury biomarker levels in patients with COVID-19• Neurocognitive 3-months outcome in patients with COVID-19 | • 90-day modified Rankin scale |
| CORONERVE:Neurological complications of COVID-19(United Kingdom) | • Study design: Multisite observational study• Population: Children with acute onset neurological syndrome including encephalitis, encephalopathy, meningitis, demyelination, myelitis, cerebrovascular, cerebellar, Guillain-Barre syndrome, etc. in eligible population• Inclusion criteria: Child 0–18 years of age with suspected or proven COVID-19 infection and acute neurological syndrome as above• Exclusion criteria: No evidence of active or recent COVID-19 Infection. | • To determine the demographic, clinical, laboratory and radiographic features of patients presenting with acute neurological syndromes during or within 6 weeks of virologically proven coronavirus infection | • Proportion receiving immune and associated therapies• Clinical outcomes of patients which each neurological syndrome relative to baseline features and treatments received |
| NEPSycon-COVID: Assessment of Neurological, Epidemiological, Psychiatric and Psychosocial Consequences during the COVID-19 PandemicTrial number: CTRI/2020/08/027490 (India) | • Study design: Prospective observational study• Population: All neurological patients [1–70 years] visiting the neurological emergency with the following criteria.• Inclusion criteria:a. Patients of neurological disorders with previously described symptoms related to COVID-19 infection (e.g. stroke, acute encephalitis, seizure, Guillain-Barre syndrome, ADEM)b. Patients of neurological disorders precipitated or preceded by respiratory symptoms in the last 2–4 weeksc. Patients of neurological symptoms (not fitting the criteria of a/b) but found to have lymphopenia / thrombocytopenia /thrombotic states / elevated D-dimer levels• Exclusion criteria: neurological disorders of other etiology and lack of consent | • Death | • Proportions of patients with each neurological diagnosis in the group with neurological disease• Admission to critical (intensive/high dependency) care unit• Time to discharge from hospital• Functional outcome at discharge (or 30 days from admission, if still in hospital) |
| Perinatal Covid-19 Infection, NO Pathway, and Minipuberty (miniNO-COVID)Trial number: NCT04952870 (France) | • Study Design: Single site prospective observational case-control study• Population: Newborn infants (24 to 41 weeks gestational age) or young infants (< 3 months)• Inclusion criteria:Group 1: Antenatal or perinatal COVID-19 infectionGroup 2: Severe cardiorespiratory diseases requiring inhaled NO treatmentGroup 3: Control group without perinatal COVID-19 infection and no inhaled NO treatment [matched]• Exclusion criteria: <24 weeks gestational age, severe brain lesions: bilateral/extensive periventricular leukomalacia, intracranial hemorrhage grade 3 or 4 | • The follicle stimulating hormone (FSH) plasma concentrations measured at the postnatal age of 3 months | • Rate of negative hearing and olfactive tests• Developmental scores (ASQ-3, ASQ-2E, Bayley III)• Time of mutual gaze interactions (vs noninteractive periods) measured by eye-tracking glasses (mother and children) at 9 months |
| Brain imaging in baby studyTrial number: NCT04443179 (United Kingdom) | • Study Design: Prospective observational case control study• Population: Community sampling of eligible cohorts listed below• Inclusion criteria: Pregnant mothers with and without confirmed COVID-19; Infants with and without an immediate family history of Autism Spectrum, neurodevelopmental conditions; based in England, UK• Exclusion criteria: based on further screening• Note: This is not a COVID-19 specific study. COVID-19 cohort was added to an existing study. | • Neurodevelopmental outcomes of children at 3–4 years of age | |
| CLoCk: Children & young people with Long Covid studyTrial number: ISRCTN34804192 (United Kingdom) | • Study design: A longitudinal cohort analytic study with online questionnaire (3, 6, 12 and 24 months post-COVID test)• Population: SARS-CoV-2 positives aged 11–17 years compared with age, sex and region matched SARS-CoV-2 test negative controls• Inclusion criteria: Those that have had a COVID-19 test between 01/09/2020 and 28/02/2021.• Exclusion criteria: None | • Physical symptoms: ISARIC Pediatric COVID-19 questions• Emotional and mental health: Strength and Difficulties Questionnaire• Quality of life/functioning: EQ-5D-Y• Fatigue: Chalder Fatigue Questionnaire• Wellbeing: Warwick Edinburgh Mental Wellbeing Scale (WEMWBS, short version)• Loneliness: adapted UCLA 4 items |
SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; COVID-19, coronavirus disease 2019.
Postacute severe acute respiratory syndrome coronavirus-2 infection
Postacute sequelae of SARS-CoV-2 infection among survivors are increasingly recognized, and sometimes referred to as Long COVID or PASC. In a systematic review of English-language cohort studies that included 9,751 adults following acute COVID-19 hospitalization, over 70% reported the persistence of one or more new symptoms such as fatigue (median: 40%), dyspnea (median: 36%), anxiety (median: 22%), anosmia and/or dysgeusia (median: 16%), depression (median: 15%), and cognitive deficits (median: 18%), including memory loss (median: 28%) and concentrating difficulties (median: 22%) [82]. Similar symptoms have been reported six months after acute infection in about 50% of adults with milder disease that did not require acute hospitalization [83].
Children who have had either acute COVID-19 infection and/or MIS-C treated as outpatients or inpatients may also have postacute sequelae of infection [84,85,86▪,87▪], though estimates of prevalence vary. In a study of 129 children with prior SARS-CoV-2 infection in Italy, 58% had persistent symptoms at a mean of 163 (±114) days follow-up. Risk factors included symptomatic infection and hospitalization. The most commonly reported symptoms were insomnia (18%), respiratory symptoms (15%), nasal congestion (12%), fatigue (11%) and concentration issues (10%) [84]. However, in the UK, <1% of children <17 years old self-reported symptoms of long COVID [88]. Hospitalized children and their families are also at risk of postintensive care/hospital syndrome (PICS) [89], compounding the virus's effects on functional health domains, health-related quality of life, future development, and participation. Ongoing multicenter studies will provide further information about the long-term sequelae and outcomes associated with acute COVID-19 and MIS-C in pediatrics (Table 2).
SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS-2 VACCINES AND NEUROLOGIC ADVERSE EVENTS
In the US and the UK, the first vaccine approved for emergency use in people ≥16 years in December 2020, was Pfizer-BioNTech's mRNA vaccine [90,91]. This approval was extended to US adolescents 12–15 years of age in May 2021 [92] with ongoing trials for younger children to determine dosing, safety, and efficacy. Multiple other vaccines using mRNA, viral vector, and inactivated virus-based platforms have been introduced since. As of July 12, 2021, over 3 billion vaccines doses had been administered worldwide [5▪▪]. Impact of vaccination and VOC emergence on the frequency and presentation of neurological manifestations remains to be determined.
As of July 2021, there have been no reports of neurologic adverse events following vaccination in children. In adults, GBS variants have been reported following viral vector-based vaccine administration (Oxford-AstraZeneca, Johnson & Johnson's Janssen), but this is rare [93,94]. Currently, a history of GBS is not considered a contraindication for receiving a SARS-CoV-2 vaccine. In addition, while there was early concern of acute onset peripheral facial nerve palsy following mRNA-based vaccine administration (Pfizer-BioNTech, Moderna) [95,96], subsequent evaluation of 320 million vaccination administrations from the WHO database demonstrated no increase over baseline population incidence following vaccination [97]. These data highlight the importance of reporting and monitoring potential vaccine adverse effects through national and international systems, such as the CDC's Vaccine Adverse Event Reporting System, to determine if there are true associations between specific vaccines and potential neurologic adverse events.
KEY LESSONS FOR THE NEXT PANDEMIC
The emergence of multiple novel pathogens in the 21st century has reinforced the critical need for rapid, prospective, global epidemiologic and clinical data collection when new infectious threats present [3,35,37–39]. Although national and international public health systems in HIC have developed excellent mechanisms to monitor case counts and mortality, most of our understanding of neurological manifestations has initially come from HIC case reports and case series [98]. Prospective global data on neurological manifestations and outcomes of the various SARS-CoV-2 clinical phenotypes are only now beginning to emerge, 17 months into the pandemic, with pediatric data lagging. Investment in research to examine the impact SARS-CoV-2 infection has on the developing brain in the context of the individual and family is greatly needed.
Although the pandemic has highlighted examples of clinical research of good quality despite constraints, it has also highlighted gaps in existing international research infrastructure [99▪]. Standardized reporting of clinical data, as used in the ISARIC/WHO clinical characterization protocol [16▪] and by the Overcoming COVID-19 investigators [23▪▪,26▪▪,59▪▪], provided timely epidemiological, clinical, and prognostic information during the pandemic for children. Large scale interventional trials in adults (e.g., SOLIDARITY, RECOVERY, ACTT, REMAP-CAP) were instrumental in reducing uncertainty at the bedside and improving outcomes [100–103]. However, such large-scale interventional trials in children have not yet emerged.
Single-center studies are locally impacted by patient population demographics (e.g., age, comorbidities, social determinants of health) and by the frequency of neurologic manifestations. There is thus a critical need for multicenter, multinational collaboration to better understand neurologic manifestations of pediatric diseases. Clinical research networks in pediatrics (e.g., PALISI, PCCSSG, ESPNIC, ANZICS-PSG, LaRed, PACCMAN, CPCCRN), neurology (e.g., PNCRG, NeuroNEXT), and infectious disease (ISARIC) worldwide have produced innovations in epidemiology, pathobiology, and therapeutic efficacy in a variety of disorders. These networks could be leveraged to create a resource previously unavailable to transform pediatric neurologic care worldwide.
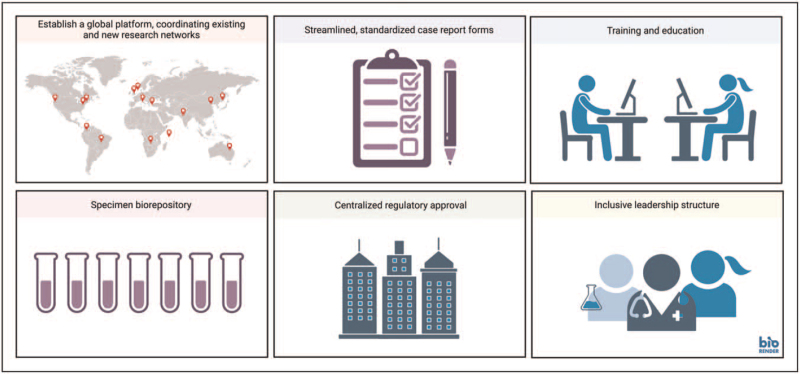
We propose establishing an inclusive, global neurology research network platform in advance of the next pandemic to facilitate rapid execution of stream-lined and cost-effective neurology-focused pediatric protocols. This platform should incorporate key principles of a Learning Health System, scaled for global application [104]. Necessary steps include: (a) sufficient, equity-oriented funding for platform infrastructure to facilitate participation, (b) coordination among existing and newly created clinical research networks; (c) streamlined, standardized case report form with predefined common data elements; (d) training/education bundles; (e) biorepository with standards for sample collection, transfer, and storage; (f) preexisting and centralized ethical/regulatory approval; and (g) inclusive (e.g., clinical, research, funder, family/patient) and effective leadership structure (Fig. 2). This platform could facilitate swift understanding of the epidemiological scope of the neurologic problem, generate hypotheses, evaluate potential risk factors and outcomes, and support direct public health efforts. When therapeutic interventions are proposed, this platform may provide the springboard for efficient clinical trials using innovative methodologies such as randomized registry trials and a master protocol to test multiple interventions.
FIGURE 2.
Preparing for the Next Pandemic. Adapted from ‘Global Presence (World Map)’, by BioRender.com (2020).
CONCLUSION
Neurological manifestations of SARS-CoV-2 infection in children are common, heterogenous, and distinct in each clinical phenotype (acute COVID-19, MIS-C, and PASC). Their potential impact on overall pediatric SARS-CoV-2 morbidity and mortality has yet to be determined. A strategy to create an equitable, global neurology-focused platform for future emergent deployment to capture high-quality data and specimens is critically needed to care for and protect children with neurologic manifestations of disease across the globe.
Acknowledgements
Figures created with BioRender.com.
Financial support and sponsorship
NIH K23 NS094069 (J.L.M.)
Neurocritical Care Society INCLINE grant and NIH (NINDS) R01 NS096714 (E.L.F.)
Financial Disclosure Statement: The authors have no financial relationships relevant to this article to disclose.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Alicia M. Alcamo and Jennifer L. McGuire are co-first authors.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed 2020; 91:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson CP, Busl KM. Neurologic manifestations of severe respiratory viral contagions. Crit Care Explor 2020; 2:e0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carod-Artal FJ. Neurological complications of Zika virus infection. Expert Rev Anti Infect Ther 2018; 16:399–410. [DOI] [PubMed] [Google Scholar]
- 5▪▪. WHO. WHO Coronavirus (COVID-19) Dashboard [Internet]. Geneva: World Health Organization; 2020 [cited July 12, 2021]. Available from: https://covid19.who.int. [Google Scholar]; This website is continually updated to demostrate the global epidemiological impact of SARS-CoV-2.
- 6. UNICEF. Children in monetary poor households and COVID-19: Technical Note [Internet]. UNICEF; November 2020 [cited July 12, 2021]. Available from: https://data.unicef.org/resources/children-in-monetary-poor-households-and-covid-19/. [Google Scholar]
- 7. Children and COVID-19: State Data Report. A joint report from the American Academy of Pediatrics and the Children's Hospital Association. [Internet]. 2021 [cited July 12, 2021]. Available from: https://downloads.aap.org/AAP/PDF/AAP%20and%20CHA%20-%20Children%20and%20COVID-19%20State%20Data%20Report%206.24%20FINAL.pdf. [Google Scholar]
- 8.Roberton T, Carter ED, Chou VB, et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Global Health 2020; 8:e901–e908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪▪. UNICEF. COVID-19 confirmed cases and deaths: age- and sex-disaggreated data [Internet]. UNICEF; May 2021 [cited July 12, 2021]. Available from: https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-dashboard/. [Google Scholar]; This website is continually updated to demostrate the global epidemiological impact of SARS-CoV-2 by age and sex.
- 10. UNICEF. Immunization coverage: Are we losing ground? [Internet]. UNICEF; July 2020 [cited July 12, 2021]. Available from: https://data.unicef.org/resources/immunization-coverage-are-we-losing-ground/. [Google Scholar]
- 11. UNICEF. Protecting children from violence in the time of COVID-19 [Internet]. UNICEF; August 2020 [cited July 12, 2021]. Available from: https://data.unicef.org/resources/protecting-children-from-violence-in-the-time-of-covid-19-brochure/. [Google Scholar]
- 12. WHO. Tracking SARS-CoV-2 variants [Internet]. Geneva: WHO; [cited July 12, 2021]. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. [Google Scholar]
- 13.Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann Intern Med 2021; 174:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in china. Pediatrics 2020; 145:e20200702. [DOI] [PubMed] [Google Scholar]
- 15.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr 2020; 179:1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪.Swann OV, Holden KA, Turtle L, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. The BMJ 2020; 370:m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes clinically differences between the pediatric clinical phenotypes of SARS-CoV-2 infection (acute COVID-19 infection and MIS-C).
- 17.Williams N, Radia T, Harman K, et al. COVID-19 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review of critically unwell children and the association with underlying comorbidities. Eur J Pediatr 2021; 180:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis 2021; 103:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Diorio C, Henrickson SE, Vella LA, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Investig 2020; 130:5967–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows the immunological differences between the pediatric clinical phenotypes of SARS-CoV-2 infection (acute COVID-19 infection and MIS-C).
- 22.Belay ED, Abrams J, Oster ME, et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr 2021; 175:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪▪.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is one of the largest multicenter epidemiological studies to describe MIS-C.
- 24. CDC. Multisystem Inflammatory Syndrome (MIS) [Internet]. CDC; [cited July 12, 2021]. Available from: https://www.cdc.gov/mis-c/cases/index.html. [Google Scholar]
- 25.Lee EH, Kepler KL, Geevarughese A, et al. Race/ethnicity among children with COVID-19-associated multisystem inflammatory syndrome. JAMA Netw open 2020; 3:e2030280–e12030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪▪.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021; 325:1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is one of the largest multicenter epidemiolgoical studies to describe the differences between the pediatric clinical phenotypes of SARS-CoV-2 infection (acute COVID-19 infection and MIS-C).
- 27.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. ECDC. Rapid Risk Assessment: Paediatric inflammatory multisystem sydnrome and SARS-CoV-2 infection in children [Internet]. European Centre for Disease Prevention and Control May 2020 [cited July 12, 2021]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-risk-assessment-paediatric-inflammatory-multisystem-syndrome-15-May-2020.pdf. [Google Scholar]
- 30.Webb K, Abraham DR, Faleye A, et al. Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc Health 2020; 4:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Tang Y, Shi Y, et al. Why multisystem inflammatory syndrome in children has been less commonly described in Asia? Transl Pediatr 2020; 9:873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bender SJ, Weiss SR. Pathogenesis of murine coronavirus in the central nervous system. J Neuroimmune Pharmacol 2010; 5:336–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morfopoulou S, Brown JR, Davies EG, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med 2016; 375:497–498. [DOI] [PubMed] [Google Scholar]
- 34.Yeh EA, Collins A, Cohen ME, et al. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics 2004; 113:e73–e76. [DOI] [PubMed] [Google Scholar]
- 35.Ng Kee Kwong KC, Mehta PR, Shukla G, et al. COVID-19, SARS and MERS: a neurological perspective. J Clin Neurosci 2020; 77:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zegarra-Valdivia JA, Chino-Vilca BN, Tairo-Cerron T, et al. Neurological components in coronavirus induced disease: a review of the literature related to SARS, MERS, and COVID-19. Neurol Res Int 2020; 2020:6587875. [Google Scholar]
- 37.Algahtani H, Subahi A, Shirah B. Neurological complications of middle east respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med 2016; 2016:3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection 2015; 43:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol 2017; 13:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪.Chou SHY, Beghi E, Helbok R, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19 – a report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Network Open 2021; 4:e2112131. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is one of the largest, multicenter, multinational studies evaluating neurological manifesations in adult patients with acute COVID-19 infection.
- 41.Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol 2020; 7:2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saniasiaya J, Islam MA, Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID-19): a meta-analysis of 27,492 patients. Laryngoscope 2021; 131:865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 277:2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klok FA, Kruip M, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020; 191:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol 2020; 77:1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez-Fernandez F, Sandoval Valencia H, Barbella-Aponte RA, et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain 2020; 143:3089–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothstein A, Oldridge O, Schwennesen H, et al. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke 2020; 51:e219–e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 2020; 143:3104–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-barre syndrome associated with SARS-CoV-2. N Engl J Med 2020; 382:2574–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abu-Rumeileh S, Abdelhak A, Foschi M, et al. Guillain-Barre syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol 2021; 268:1133–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin E, Lantos JE, Strauss SB, et al. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York City. AJNR Am J Neuroradiol 2020; 41:2001–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 2020; 94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 Encephalitis. Brain Behav Immun 2020; 87:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reichard RR, Kashani KB, Boire NA, et al. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol 2020; 140:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danoun OA, Zillgitt A, Hill C, et al. Outcomes of seizures, status epilepticus, and EEG findings in critically ill patient with COVID-19. Epilepsy Behav 2021; 118:107923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emami A, Fadakar N, Akbari A, et al. Seizure in patients with COVID-19. Neurol Sci 2020; 41:3057–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dono F, Nucera B, Lanzone J, et al. Status epilepticus and COVID-19: a systematic review. Epilepsy Behav 2021; 118:107887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪▪.Larovere KL, Riggs BJ, Poussaint TY, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol 2021; 78:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is one of the first multicenter studies to evaluate neurological manifesations in children with SARS-CoV-2 infection, differentiating between acute COVID-19 and MIS-C.
- 60.Llaque-Quiroz P, Prudencio-Gamio R, Echevarria-Lopez S, et al. Clinical and epidemiological characteristics of children with COVID-19 in a pediatric hospital in Peru. Rev Peru Med Exp Salud Publica 2020; 37:689–693. [DOI] [PubMed] [Google Scholar]
- 61.Sandoval F, Julio K, Mendez G, et al. Neurologic features associated with SARS-CoV-2 infection in children: a case series report. J Child Neurol 2021; 36:853–866. [DOI] [PubMed] [Google Scholar]
- 62▪▪.Ray STJ, Abdel-Mannan O, Sa M, et al. Neurological manifestations of SARS-CoV-2 infection in hospitalised children and adolescents in the UK: a prospective national cohort study. Lancet Child Adolesc Health 2021; 5:631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]; This cohort study evalutes neurological manifesations in children and adolescent in SARS-CoV-2 infection.
- 63▪.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 2020; 174:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]; This was one of the first multicenter, multinational reports of the impact of acute COVID-19 in pediatrics.
- 64.Oualha M, Bendavid M, Berteloot L, et al. Severe and fatal forms of COVID-19 in children. Arch Pediatr 2020; 27:235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65▪.Penner J, Abdel-Mannan O, Grant K, et al. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health 2021; 5:473–482. [DOI] [PubMed] [Google Scholar]; This study is a longitudinal cohort of children with MIS-C to evaluate the presence of persistent symptoms at 6 months following discharge.
- 66.Alkan G, Sert A, Oz SKT, et al. Clinical features and outcome of MIS-C patients: an experience from Central Anatolia. Clin Rheumatol 2021; 40:4179–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67▪.Hacohen Y, Abdel-Mannan O, Eyre M, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol 2020; 77:1440–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes neuroimaging differences in the different SARS-CoV-2 clinical phenotypes.
- 68.Sanchez-Morales AE, Urrutia-Osorio M, Camacho-Mendoza E, et al. Neurological manifestations temporally associated with SARS-CoV-2 infection in pediatric patients in Mexico. Childs Nerv Syst 2021; 37:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Salido A, de Carlos Vicente JC, Belda Hofheinz S, et al. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care 2020; 24:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mamishi S, Movahedi Z, Mohammadi M, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol Infect 2020; 148:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh A, Saini I, Meena SK, et al. Demographic and clinical profile of mortality cases of COVID-19 in children in New Delhi. Indian J Pediatr 2021; 88:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parri N, Lenge M, Buonsenso D, et al. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med 2020; 383:187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupta Dch S, Chopra Md N, Singh Md A, et al. Unusual clinical manifestations and outcome of multisystem inflammatory syndrome in children (MIS-C) in a tertiary care hospital of North India. J Trop Pediatr 2021; 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flood J, Shingleton J, Bennett E, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS): Prospective, national surveillance, United Kingdom and Ireland. Lancet Region Health 2021; 3:100075–1100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lima-Setta F, Magalhaes-Barbosa MC, Rodrigues-Santos G, et al. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J Pediatr 2021; 97:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76▪.Garazzino S, Montagnani C, Donà D, et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill 2020; 25:pii=2000600. 10.2807/1560-7917.ES.2020.25.18.2000600 [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of preliminary results from an Italian multicenter study, reporting epidemiological, clinical and therapeutic aspects of SARS-CoV-2 in pediatrics.
- 77.Alnajjar AA, Dohain AM, Abdelmohsen GA, et al. Clinical characteristics and outcomes of children with COVID-19 in Saudi Arabia. Saudi Med J 2021; 42:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78▪.Beslow LA, Linds AB, Fox CK, et al. Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann Neurol 2021; 89:657–665. [DOI] [PubMed] [Google Scholar]; This is a large, multinational study that evaluates pediatric stroke risk associated with SARS-CoV-2 infection.
- 79.Becker AE, Chiotos K, McGuire JL, et al. Intracranial hypertension in multisystem inflammatory syndrome in children. J Pediatr 2021; 233:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80▪▪.Lindan CE, Mankad K, Ram D, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health 2021; 5:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study evaluates neuroimaging abnormalities in children with SARS-CoV-2 infection.
- 81.Frontera J, Mainali S, Fink EL, et al. Global consortium study of neurological dysfunction in COVID-19 (GCS-NeuroCOVID): study design and rationale. Neurocrit Care 2020; 33:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19. JAMA Netw Open 2021; 4:e2111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021; 27:1607–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr 2021; 110:2208–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Int J Paediatr 2021; 110:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86▪.Say D, Crawford N, McNab S, et al. Postacute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health 2021; 5:e22–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study specifically looks at PASC symptoms in children that had mild or asymptomatic acute COVID-19 infection.
- 87▪.Sterky E, Olsson-Åkefeldt S, Hertting O, et al. Persistent symptoms in Swedish children after hospitalisation due to COVID-19. Acta Paediatr 2021; 110:2578–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study begins to highlight the presence of PASC and describes symptoms in children following acute COVID-19 infection.
- 88. Ayoubkhani, D. a. P., P. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK [Internet]. 2021 [cited July 19, 2021]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/alldatarelatingtoprevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk. [Google Scholar]
- 89.Manning JC, Pinto NP, Rennick JE, et al. Conceptualizing post intensive care syndrome in children-The PICS-p framework. Pediatr Crit Care Med 2018; 19:298–300. [DOI] [PubMed] [Google Scholar]
- 90. FDA. Pfizer-BioNTech COVID-19 Vaccine [Internet]. United States: FDA; [cited July 12, 2021]. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine. [Google Scholar]
- 91. Agency, M. a. H. P. R. Vaccine BNT162b2 – CONDITIONS OF AUTHORISATION UNDER REGULATION 174 [Internet]. 2020 [cited July 19, 2021]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/991392/Pfizer_BioNTech_Conditions.pdf. [Google Scholar]
- 92. FDA. Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight Against Pandemic [Internet]. United States: FDA; 2021 [cited July 12, 2021]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use. [Google Scholar]
- 93.Allen CM, Ramsamy S, Tarr AW, et al. Guillain-barre syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol 2021; 90:315–318. [DOI] [PubMed] [Google Scholar]
- 94.Marquez Loza AM, Holroyd KB, Johnson SA, et al. Guillain-barre syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality. Neurology 2021; 96: [DOI] [PubMed] [Google Scholar]
- 95.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020; 383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Renoud L, Khouri C, Revol B, et al. Association of facial paralysis with mRNA COVID-19 vaccines: a disproportionality analysis using the World Health Organization Pharmacovigilance Database. JAMA Intern Med 2021; 181:1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gupta M, Wahl B, Adhikari B, et al. The need for COVID-19 research in low- and middle-income countries. Glob Health Res Policy 2020; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99▪.Angus DC, Gordon AC, Bauchner H. Emerging lessons from COVID-19 for the US clinical research enterprise. JAMA 2021; 325:1159–1161. [DOI] [PubMed] [Google Scholar]; This editorial highlights challenges associated with research during the COVID-19 pandemic and highlights ways to streamline research efforts for future pandemics.
- 100.Consortium WHOST, Pan H, Peto R, et al. Repurposed antiviral drugs for Covid-19 – interim WHO Solidarity Trial Results. N Engl J Med 2021; 384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – final report. N Engl J Med 2020; 383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: The REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 2020; 324:1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Olsen, L. A., Aisner, D. and McGinnis, J. M. The Learning Healthcare System: Workshop Summary. Washington (DC): 2007. [PubMed] [Google Scholar]