Abstract
BACKGROUND:
Acute and chronic forms of lung allograft injury are associated with specific respiratory pathogens. Donor-derived cell free DNA (ddcfDNA) has been shown to be elevated with acute lung allograft injury and predictive of long-term outcomes. We examined the %ddcfDNA values at times of microbial isolation from bronchoalveolar lavage (BAL).
METHODS:
Two hundred and six BAL samples from 51 Lung Transplant Recipients (LTRs) with concurrently available plasma %ddcfDNA were analyzed along with microbiology and histopathology. Microbial species were grouped into bacterial, fungal, and viral and “higher risk” and “lower risk” cohorts based on historical association with downstream allograft dysfunction. Analyses were performed to determine pathogen category association with %ddcfDNA, independent of inter-subject variability.
RESULTS:
Presence of microbial isolates in BAL was not associated with elevated %ddcfDNA compared to samples without isolates. However, “higher risk” bacterial and viral microbes showed greater %ddcfDNA values than lower risk species (1.19% vs. 0.65%, p < 0.01), independent of inter-subject variability. Histopathologic abnormalities concurrent with pathogen isolation were associated with higher %ddcfDNA compared to isolation episodes with normal histopathology (medians 1.23% and 0.66%, p = 0.05). Assessments showed no evidence of correlation between histopathology or bronchoscopy indication and presence of higher risk vs. lower risk pathogens.
CONCLUSION:
%ddcfDNA is higher among cases of microbial isolation with concurrent abnormal histopathology and with isolation of higher risk pathogens known to increase risk of allograft dysfunction. Future studies should assess if %ddcfDNA can be used to stratify pathogens for risk of CLAD and identify pathogen associated injury prior to histopathology.
Keywords: cfDNA, lung transplantation, chronic lung allograft dysfunction, transplant infection
Background
Chronic lung allograft dysfunction (CLAD) is the leading cause of mortality after lung transplantation. Risk factors include acute complications such as acute rejection, primary graft dysfunction and pathogens.1–3 Despite advances in antimicrobial prophylaxis and treatment, respiratory pathogens remain an important risk factor for morbidity and mortality following lung transplantation. In particular, community respiratory viruses, including influenza, respiratory syncytial virus, and bacteria such as Pseudomonas aeruginosa have been associated with acute and chronic allograft dysfunction.4–8 In lung transplantation, conventional definitions of infection and colonization are often inadequate to determine pathogen-associated lung injury, as patients may lack common manifestations of respiratory infection, including fevers, leukocytosis or radiographic changes, even when positive histopathology is detected, or they may develop other allograft dysfunction despite adequate resolution of the acute infection.8–11 Furthermore, recent studies have suggested that respiratory isolation of select pathogens, such as Pseudomonas aeruginosa, may be risk factors for HLA antibody and/or CLAD irrespective of the presence of acute pneumonia,4,12 while other isolates, such as E. coli or Klebsiella species are known causes of acute infectious pneumonia, but have not been associated with CLAD.13,14 Thus, better studies are needed to define the molecular environment of the host or pathogen at time of microbial isolation to understand risk of downstream allograft injury.
Post-transplant, donor derived cell free DNA (ddcfDNA) is released from dying allograft cells into circulation. After lung transplantation, the percentage of plasma ddcfDNA (%ddcfDNA) is increased during episodes of acute cellular rejection and antibody-mediated rejection (AMR).15 Importantly, this assay has been shown to be exquisitely sensitive, often detecting injury from disease before histopathology and spirometry.16 Furthermore, early post-transplant %ddcfDNA levels correlate with subsequent allograft injury, development of CLAD, and mortality.15 In this study, we assessed whether microbial isolation from bronchoalveolar lavage (BAL) samples and biopsy results correlated with %ddcfDNA levels. We hypothesized that plasma %ddcfDNA levels would be higher in subjects with concurrent allograft injury at the time of microbial detection. We further hypothesized that microbial species that are known risk factors for downstream allograft injury (including but not limited to CLAD) would be more likely to be associated with higher %ddcfDNA levels compared to organisms which are less frequently associated with allograft injury. Herein we present our early findings.
Methods
Patients
Study subjects included lung transplant recipients (LTRs) enrolled in the Genome Transplant Dynamics Study (clinicaltrial.gov number = NCT02423070), a multicenter prospective cohort study of GRAfT (Genomic Research Alliance for Transplantation), approved by each institution’s IRB and by the central IRB at NIH. GRAfT is a consortium of the National Heart, Lung and Blood Institute and five hospitals in the Washington DC metropolitan area. Three hospitals (Johns Hopkins University, University of Maryland, and Inova Fairfax Hospital) recruited LTRs who were at least 18 years old, from July 2015 to collect serial plasma samples for %ddcfDNA assessments, alongside clinical data, pathogen isolates and histopathology results from electronic medical records. This analysis includes patients enrolled through June 2017 for which timepoints %ddcfDNA data was available.
Immunosuppression and prophylaxis
Subjects were treated according to each center’s usual immunosuppression and antimicrobial protocols. Patients received induction with high dose methylprednisone, often with basiliximab or other induction, and triple agent immunosuppression (Table S1).15,16 Institution-specific protocols for lung transplant antimicrobial prophylaxis are in supplementary table S2. Respiratory microbiology was assessed through BAL at surveillance and for-cause bronchoscopy.
Timing of bronchoscopy and %ddcfDNA sample collection
Surveillance bronchoscopy was performed at 1, 3, 6, 9, 12, and 18 months post-transplant, and during for cause evaluation of respiratory exacerbations. Bronchoscopies were considered ‘for cause’ if they were performed during a hospitalization or the subject had a concurrent decline in FEV1 of greater than 10%. Donor specific anti-HLA antibodies (HLA-DSA) surveillance varied per institution. Plasma samples for %ddcfDNA assay were collected with hospitalizations for a respiratory event and immediately prior to all bronchoscopies; this timing was selected to limit ddcfDNA leakage from the biopsy procedure. To reduce confounding from ddcfDNA release from the transplant surgery, we analyzed %ddcfDNA and microbiological data starting at post-transplant day 28.17
Categorization of microbial isolates
In this study, pathogen isolation was defined as isolation of microbes from BAL per each center’s clinical lab. As such, analysis was focused on the presence or absence of microbial isolates, rather than specific infection symptoms. Microbial isolates were categorized based on individual species and into the following categories; normal flora, bacteria, fungi, respiratory viruses and cytomegalovirus. Prior to analysis, bacterial, fungal, and viral isolates were further categorized into higher risk and lower risk categories based on previously published associations with clinical risk of allograft injury or CLAD development (Tables 1A and 1B) .8–11,13,14,18–27
Table 1A.
Pathogens Associated with Higher Risk of Allograft Injury
| Viral | Bacterial | Fungal |
|---|---|---|
| hMPV | Achromobacter spp | A. fumigatus |
| Adenovirus | S. maltophilia | C. neoformans |
| Influenza | S. aureus | Rhizopus |
| Parainfluenza | P. aeruginosa | S. brumptii |
| RSV | S. pneumonia | |
| Cytomegalovirus* |
not included in analysis of respiratory pathogens.
Table 1B.
Pathogens Associated with Lower Risk of Allograft Injury
| Viral | Bacterial | Fungal |
|---|---|---|
| Rhinovirus | Capnocytophaga spp | Aspergillus spp** |
| Enterovirus | Escherichia spp | Paecilomyces spp |
| Coronavirus* | Haemophilus spp | Penicillium spp |
| Klebsiella spp | Basidiomycete spp | |
| Cladosporium spp | ||
| Nodulisporium spp | ||
| Beauveria spp | ||
| Mycelia sterilia | ||
| M. schulzeri | ||
| Phialocephala spp | ||
| Phlebia chrysocreas | ||
| Sterile septated hyphae |
Endemic coronavirus, non COVID-19.
Aspergillus non-fumigatus spp.
To examine the general course of infections post-transplant, microbial isolates were grouped into three post-transplant time periods; early, intermediate, and late post-one year (29–120, 121–365, and >365 days post-transplant respectively) in accordance with prior studies.28,29
Histology and allograft injury criteria
All post-transplant lung pathology reports were reviewed by pathologists who were blinded to the microbiology data. Abnormal histopathology was defined by the study team based on the presence of any of the following; lymphocytic bronchiolitis or lymphoid aggregates, constrictive or obliterative bronchiolitis, other injury (i.e. inflammation), organizing pneumonia, capillaritis or endotheliitis, acute lung injury, diffuse alveolar damage, and acute cellular rejection (ACR). ACR, at a threshold of grade A1, and AMR, subclinical, possible, probable, and definite, were defined according to the criteria published by the International Society of Heart and Lung Transplantation.16,30
%ddcfDNA assay
The shotgun sequencing approach was used as previously described.17,31,32 In summary, pre-transplant blood was collected from candidates and donors to extract genomic DNA for genotyping using Illumina arrays to identify single nucleotide polymorphisms (SNPs) between transplant donor/recipient pairs. After transplantation, recipient plasma was collected for cell-free DNA isolation, library construction and shotgun sequencing (2 × 50 bp, >10 million reads per sample; HiSeQ 2500 Illumina). Using SNPs from genotype data, sequence reads were then surveyed to identify and quantify the percentage of ddcfDNA.
Statistical analysis
Descriptive analyses including demographic data of study participants and type and frequency of microbial isolates obtained from BAL were conducted. We compared %ddcfDNA levels between groups based on microbial isolation and histopathology status. As %ddcfDNA exhibited a non-normal distribution, (mean: 1.68%, median: 0.81%) non-parametric tests (Kruskal-Wallis and Mann-Whitney U) were used. To account for repeated %ddcfDNA measures within subjects and correct for inter-subject variability, the generalized estimating equation approach was used to compare %ddcfDNA between groups. %ddcfDNA was log-transformed log2(x+0.01) to normalize the distribution. We next performed bivariate analysis including variables in the model that demonstrated a p value < 0.2 in univariate models, as well as covariates with potential relevance to allograft injury: histopathology, bacterial isolates, viral isolates, and bronchoscopy indication as surveillance vs. for cause. All variables were coded as binary (positive/negative) except for bacterial and viral isolates. Viral and bacterial isolates were treated as unique covariates with three levels, no isolate, lower risk isolate, and higher risk isolate present in the BAL sample. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Demographics and infection characteristics
A total of 51 LTRs were included in the analysis (34 double lung and 17 single LTRs) (Table 2A). Median age at the time of transplant was 60 (IQR: 54 to 66), and included 27 women and 24 men. The majority (58.82%) of participants had a pre-transplant diagnosis of interstitial lung disease, with obstructive lung disease (15.69%) and cystic fibrosis (13.73%) being the next most common diagnoses.
Table 2A.
Demographic Characteristics of Study Participants
| Demographic characteristics | |
|---|---|
| Participants | 51 |
| Age at transplant (years) | 60 (54, 66) |
| Recipient gender | |
| Male | 24 (47.06) |
| Female | 27 (52.94) |
| Race | |
| White | 40 (78.43) |
| African American | 8 (15.69) |
| Other | 3 (5.88) |
| Lung transplant type | |
| Single lung | 17 (33.33) |
| Double lung | 34 (66.67) |
| Primary Diagnosis | |
| Cystic Fibrosis | 7 (13.73) |
| Obstructive lung disease | 8 (15.69) |
| ILD | 30 (58.82) |
| PVD | 1 (1.96) |
| Re-transplant | 3 (5.88) |
| Sarcoidosis | 2 (3.92) |
| Post-transplant follow up (days) | 322 (204, 392) |
Data presented as number (percentage) for categorical variables, and as median (IQR) for continuous variables.
Over the median length of follow up, 322 days post-transplant, there were 308 BAL samples with microbiological testing obtained of which 67% (n=206, [average of ~4 BAL/subject]) had a concurrent %ddcfDNA level measured (Figure S1). %ddcfDNA levels were high after transplant surgery and decayed logarithmically over time; as shown in four representative subjects (Figure S2). Respiratory pathogens were isolated in 43 of 51 (84.31%) patients. Of the 206 BAL samples analyzed, 104 (50.49%) had negative or normal flora results, and 102 (49.51%) had a specific microbe or microbes identified. Of these, 30.39% were a respiratory virus, 42.16% were bacterial pathogens, and 44.12% were fungi; 28.43% of microbe-positive samples were polymicrobial (Table 2B). 45.16% of viral isolates, 67.44% of bacterial isolates and 15.56% of fungal isolates were organisms considered to be “higher risk” for allograft injury.
Table 2B.
Pathogen Isolation and Histopathology
| Variable | |
|---|---|
| BAL samples | 206 |
| Samples with microbial isolates | |
| None/normal flora | 104/206 (50.49) |
| Single isolate | 73/206 (35.44) |
| Multiple isolates | 29/206 (14.08) |
| Respiratory virus isolated | 31/206 (15.05) |
| Higher risk pathogen | 14/31 (45.16) |
| Lower risk pathogen | 17/31 (54.84) |
| Bacteria isolated | 43/206 (20.87) |
| Higher risk pathogen | 29/43 (67.44) |
| Lower risk pathogen | 14/43 (32.56) |
| Fungi isolated | 45/206 (21.84) |
| Higher risk pathogen | 7/45 (15.56) |
| Lower risk pathogen | 38/45 (84.44) |
| Histopathology results | |
| Normal | 117 (56.80) |
| Abnormal | 53 (25.73) |
| No biopsy performed | 36 (17.48) |
BAL, bronchoalveolar lavage; ILD, interstitial lung disease; PVD, pulmonary vascular disease; Higher risk pathogen, confers higher risk of allograft injury and CLAD; Lower risk pathogen, confers lower risk of allograft injury.
Microbial isolates over time
The majority of BAL samples (46.26%) were obtained during the intermediate period (121–365 days) with 39.46% and 14.29% obtained in the early (28–120 days) and late (post-one-year) (>365 days) periods. Bacterial isolates were more common in the early post-transplant period, while fungal and viral pathogens were more common in the intermediate and late periods (Figure 1). In the non-cystic fibrosis population, the most common bacterial isolates were S. aureus (21.21%), Haemophilus spp (18.18%), and S. maltophilia (18.18%), while in the cystic fibrosis LTRs the organisms most commonly isolated were S. aureus(28.57%), P. aeruginosa (28.57%), and S. maltophilia (14.29%). The most common viral pathogens were rhinovirus (50.00%) and influenza (11.11%). Lower risk fungi (Penicillium etc) comprised 73.08% of fungal isolates, with higher risk fungi (Aspergillus, Mucor spp) isolated in 26.92% of cases. Notably, there were only three isolates of Aspergillus fumigatus (5.77%).
Figure 1.
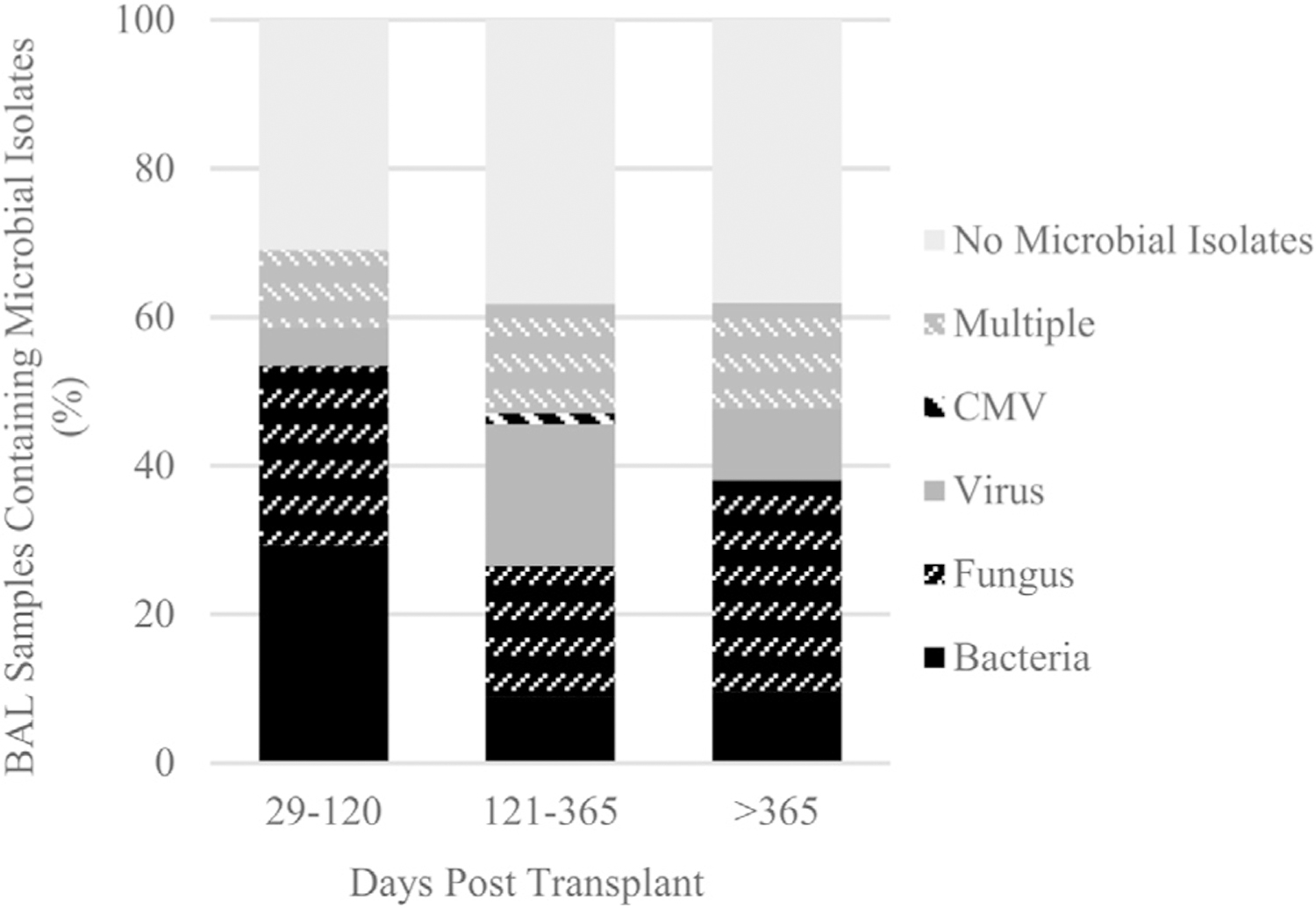
Distribution of microbial isolates over the post-transplant course during the early (29–120 DPT), intermediate (121–365 DPT), and late (>365 DPT) periods. BAL, bronchoalveolar lavage; DPT, days post transplant; CMV, cytomegalovirus; Multiple, more than one type of microbe isolated (e.g. bacteria and fungus).
Isolation and histopathology results correlate with %ddcfDNA levels
Microbial isolation alone, (any isolate positive sample compared to isolate negative) was not found to be a significant predictor of %ddcfDNA (p = 0.95), Among all bacterial (median: 0.73%), fungal (1.08%), and viral (0.99%) pathogens, no single group of isolates was associated with significantly increased levels of %ddcfDNA (p = 0.58) (Table 3). Of the 206 bronchoscopies in this study, 139 (67.47%) were conducted for surveillance and 67 (32.53%) for cause. Bronchoscopies conducted for cause were associated with higher %ddcfDNA than surveillance bronchoscopies, medians 1.30% and 0.68% respectively (p < 0.01). Among samples with positive microbial isolation, the indication for bronchoscopy (for cause vs. surveillance) was not associated with a difference in %ddcfDNA (p = 0.11).
Table 3.
Association of Microbial Isolates and Histopathology With %ddcfDNA
| Group | n (%) | %ddcfDNA | p-value | Subgroup comparison | Subgroup p-value | |
|---|---|---|---|---|---|---|
| Pathogen Type | ||||||
| Respiratory Virus | 20 (10.87) | 0.99 (0.20, 1.89) | 0.85 | Respiratory Virus | 0.57 | |
| Bacteria | 26 (14.13) | 0.73 (0.48, 1.27) | Bacterial | |||
| Fungus | 33 (17.93) | 1.08 (0.33, 2.30) | Fungus | |||
| None | 105 (57.07) | 0.73 (0.33, 1.67) | ||||
| Biopsy and Isolation | ||||||
| Biopsy result | Isolate | |||||
| Normal | Negative | 62 (36.47) | 0.74 (0.30, 1.67) | 0.13 | Normal Biopsy, Isolate Positive | 0.05* |
| Normal | Positive | 55 (32.35) | 0.66 (0.21, 1.48) | |||
| Abnormal | Negative | 27 (15.88) | 1.03 (0.45, 1.90) | Abnormal Biopsy, Isolate Positive | ||
| Abnormal | Positive | 26 (15.29) | 1.23 (0.42, 2.64) | |||
| Biopsy, Rejection, and Isolation | ||||||
| Abnormal biopsy | Isolate | |||||
| Non-ACR/AMR | Negative | 21 (12.35) | 1.03 (0.45, 1.86) | 0.98 | Non-ACR/AMR Abnormal Biopsy, Isolate Positive | 0.76 |
| Non-ACR/AMR | Positive | 20 (11.76) | 1.13 (0.49, 2.64) | |||
| ACR/AMR | Negative | 6 (3.53) | 0.97 (0.62, 4.07) | ACR/AMR, Isolate Positive | ||
| ACR/AMR | Positive | 8 (4.71) | 1.77 (0.30, 3.27) | |||
Data presented as number (percentage) for categorical variables, and as median (IQR) for continuous variables. Isolate positive, microbial isolate(s) other than normal flora were present in the BAL sample; Isolate negative, microbial isolates(s) other than normal flora not present in BAL; Abnormal biopsy, any abnormal histopathology finding; Non-ACR/AMR Abnormal biopsy, any abnormal histopathology finding other than ACR and without concurrent AMR; ACR/AMR, ACR as a biopsy finding and/or concurrent AMR; Subgroup comparison, categories included in subgroup Kruskal-Wallis or Mann-Whitney U tests.
Statistically significant (p<0.05)
Out of all transbronchial biopsies, 31.18% were found to have abnormal histopathology, with acute cellular rejection (ACR) present in 17.65% of cases and organizing pneumonia present in 11.76%. These abnormal histopathology results were associated with elevated %ddcfDNA compared to normal histopathology (medians 1.19% and 0.68%, p = 0.03) (Table 4). Abnormal histopathology was seen in 32.10% of timepoints with positive microbiology. Among samples with positive microbiology, the presence of abnormal histopathology was associated with almost two-fold higher %ddcfDNA values than those with normal histopathology, with medians of 1.23% and 0.66% respectively (p = 0.05) (Table 3). %ddcfDNA values concurrent with ACR/AMR abnormal biopsy trended higher than non-ACR/AMR cases (medians 1.77% and 1.13% respectively), but did not meet statistical significance (p = 0.76) (Table 3). Six subjects were diagnosed with AMR. Among the three with clinical AMR, one subject showed a concurrent isolation of a lower risk pathogen (rhinovirus and Klebsiella spp), and another an isolation of a higher risk pathogen (C. neoformans). One subject with sub-clinical AMR had a concurrent isolation of Haemophilus spp.
Table 4.
Univariate Predictors of %ddcfDNA
| Variable | n (%) | %ddcfDNA (IQR) |
p-value |
|---|---|---|---|
| Microbial Isolates | |||
| None/normal flora | 104 (50.48) | 0.75 (0.32, 1.73) | |
| Single isolate | 73 (35.43) | 0.92 (0.33, 1.82) | 0.76 |
| Multiple isolates | 29 (18.93) | 0.66 (0.30, 1.37) | |
| Histopathology | |||
| Abnormal | 53 (31.18) | 1.19 (0.45, 2.28) | 0.03* |
| Normal | 117 (68.82) | 0.68 (0.28, 2.28) | |
| Respiratory Virus | |||
| Isolated | 31 (15.05) | 0.56 (0.16, 1.81) | 0.32 |
| None isolated | 175 (84.95) | 0.83 (0.35, 1.73) | |
| Bacteria | |||
| Isolated | 43 (20.87) | 0.76 (0.38, 1.45) | 0.72 |
| None isolated | 163 (79.13) | 0.83 (0.30, 1.82) | |
| Fungus | |||
| Isolated | 45 (21.84) | 1.08 (0.32, 2.28) | 0.32 |
| None isolated | 161 (78.16) | 0.73 (0.30, 1.62) | |
| Higher risk pathogen | |||
| Higher risk isolated | 49 (23.78) | 1.19 (0.53, 1.96) | 0.03* |
| Lower risk isolated | 52 (25.24) | 0.65 (0.20, 1.56) | |
| No isolated | 105(50.97) | 0.73 (0.33, 1.67) | |
| Viral higher risk pathogen | |||
| Higher risk isolated | 12 (5.83) | 1.61 (0.80, 2.70) | |
| Lower risk isolated | 24 (11.65) | 0.37 (0.15, 0.93) | <0.01* |
| No viral isolates | 170 (82.52) | 0.87 (0.35 1.80) | |
| Bacterial higher risk pathogen | |||
| Higher risk isolated | 29 (14.08) | 1.00 (0.63, 1.68) | |
| Lower risk isolated | 14 (6.80) | 0.46 (0.20, 0.66) | 0.05* |
| No bacterial isolates | 163 (79.13) | 0.83 (0.30, 1.82) | |
| Fungal higher risk pathogen | |||
| Higher risk isolated | 7 (3.40) | 0.75 (0.30, 4.25) | |
| Lower risk isolated | 38 (18.45) | 1.16 (0.33, 1.93) | 0.61 |
| No fungal isolates | 161 (78.16) | 0.73 (0.30, 1.62) | |
| AMR | |||
| Positive | 7 (3.40) | 1.25 (0.13, 4.25) | 0.54 |
| Negative | 203 (98.54) | 0.80 (0.30, 1.70) | |
| CMV | |||
| Isolated | 5 (2.42) | 0.47 (0.42, 0.72) | 0.36 |
| None isolated | 201 (97.57) | 0.86 (0.30, 1.80) | |
| Bronchoscopy indication | |||
| For Cause | 67 (32.52) | 1.30 (0.40, 2.51) | <0.01* |
| Surveillance | 139 (67.48) | 0.68 (0.28, 1.41) | |
| PGD | |||
| Grade 3 | 48 (23.30) | 0.99 (0.44, 1.84) | 0.27 |
| <Grade 3 | 158 (76.70) | 0.74 (0.28, 1.68) |
%ddcfDNA presented as median (IQR); Higher risk pathogen, confers higher risk of downstream injury; Lower risk pathogen, confers lower risk of downstream injury; AMR, antibody mediated rejection; CMV, cytomegalovirus; PGD, primary graft dysfunction.
Inclusion in GEE analysis (p<0.2)
Higher risk microbial pathogens have elevated %ddcfDNA levels at time of isolation
As we did not detect a difference in %ddcfDNA levels based on overall microbial isolation, we hypothesized that the specific pathogens isolated may be important in their association with markers of injury. We thus examined whether isolation of microbes that have historically been associated with graft injury such as AMR or CLAD (higher risk) would be more injurious to the allograft, as assessed by histopathology and %ddcfDNA. Among higher risk isolates, 36.11% had concurrent abnormal histopathology compared to 28.89% among lower risk isolates (p = 0.49). However, %ddcfDNA levels were two-fold higher for higher risk pathogens compared to lower risk pathogens (medians 1.19% and 0.65%, p < 0.01). This pattern was also seen within specific groups of pathogens. Among samples with viral isolates, viruses thought to confer higher risk (such as RSV and influenza) had elevated %ddcfDNA compared to viruses considered to be lower risk (such as rhinovirus/enterovirus), with medians of 1.32% and 0.21% respectively (p < 0.01) (Figure 2A). Similarly, higher risk bacteria had elevated %ddcfDNA compared to lower risk bacteria (medians 1.00% and 0.46%, respectively [p < 0.01]) (Figure 2B).
Figure 2.
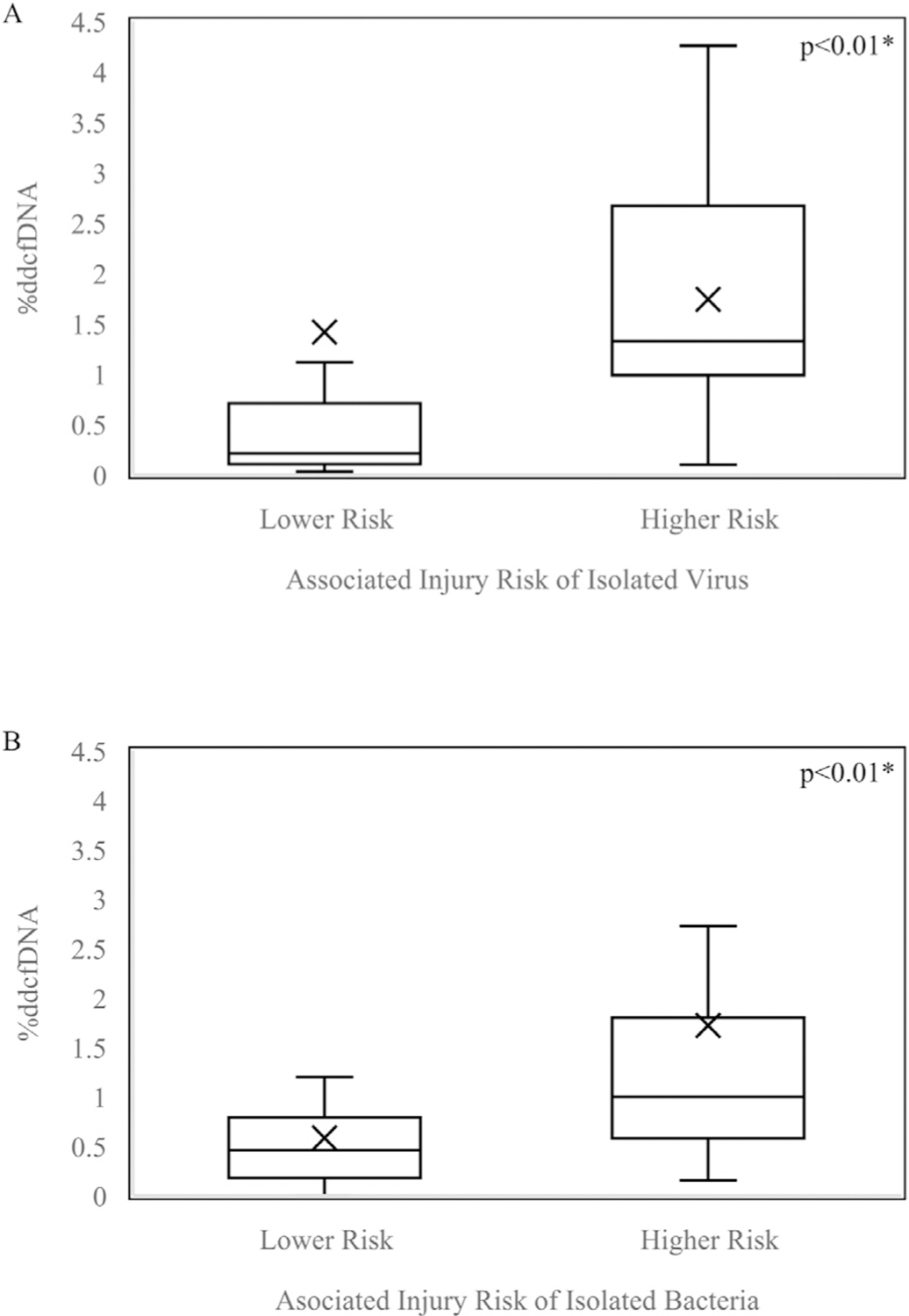
Boxplot of %ddcfDNA values among samples with higher risk vs lower risk (A) viral isolates (B) bacterial isolates. Boxes: 25th, median, and 75th percentile; whiskers: minimum and maximum (excluding outliers); x-marks: mean; dots: outliers. Higher risk, confers higher risk of downstream injury; Lower risk, confers lower risk of downstream injury. *Statistically significant (p<0.05).
We next sought to determine if these findings were independent of inter-subject variability. To address this, we used a generalized estimating equation approach to adjust for repeated measures, and showed that higher risk bacterial and viral isolates, as well as bronchoscopy indication, were independently associated with elevated %ddcfDNA levels (Figures 3 and 4). Repeated chi-square assessment between covariates of interest: histopathology, bacterial pathogen, viral pathogen, and bronchoscopy indication, showed no significant correlations (Table S3).
Figure 3.
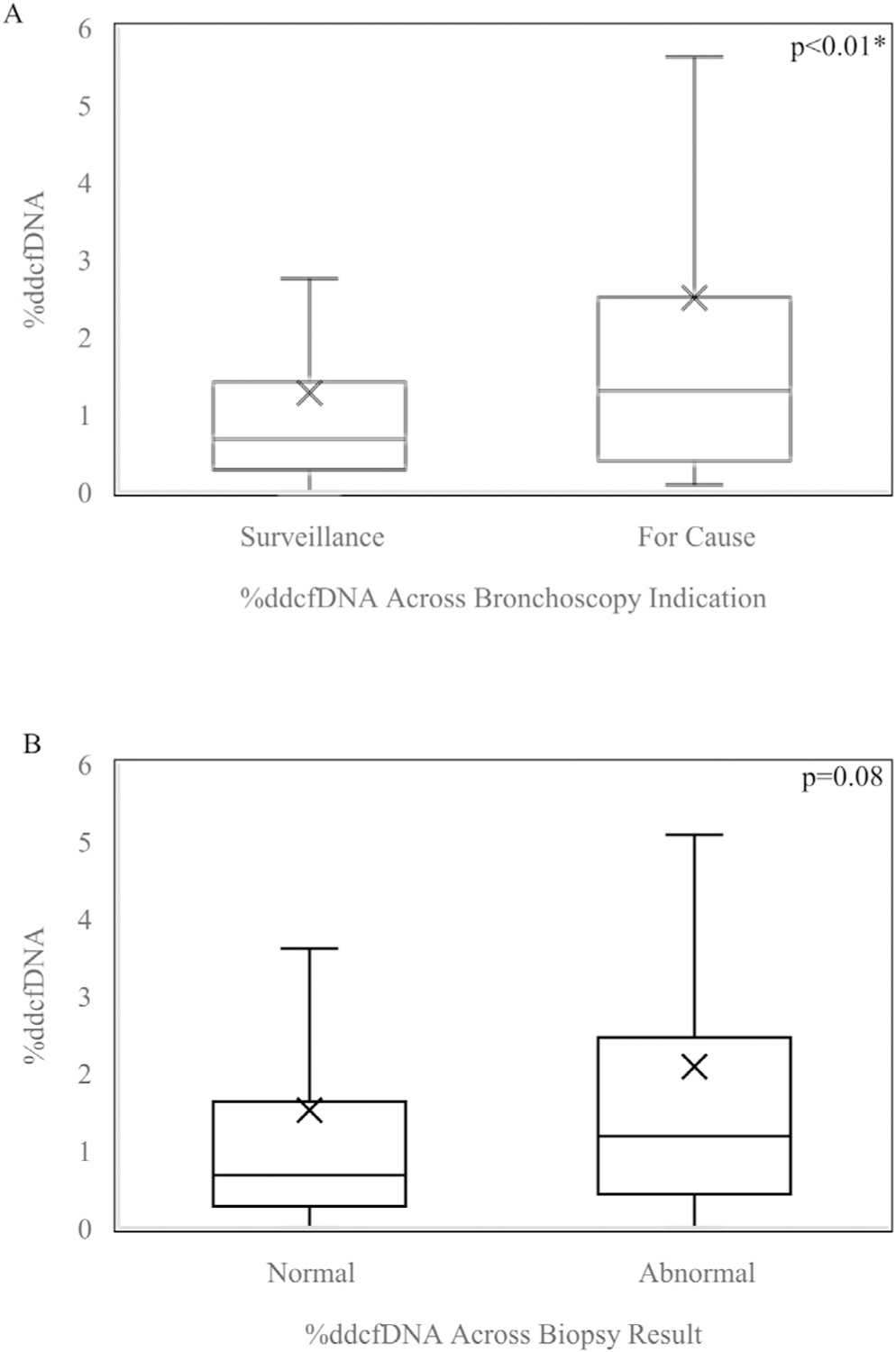
Boxplot of %ddcfDNA values among samples with (A) Surveillance vs For Cause Bronchoscopy Indication (B) Normal vs Abnormal Biopsy Result adjusted for correlation among repeated %ddcfDNA measures in the same subject. Boxes: 25th, median, and 75th percentile; whiskers: minimum and maximum; x-marks: mean. *Statistically significant (p<0.05).
Figure 4.
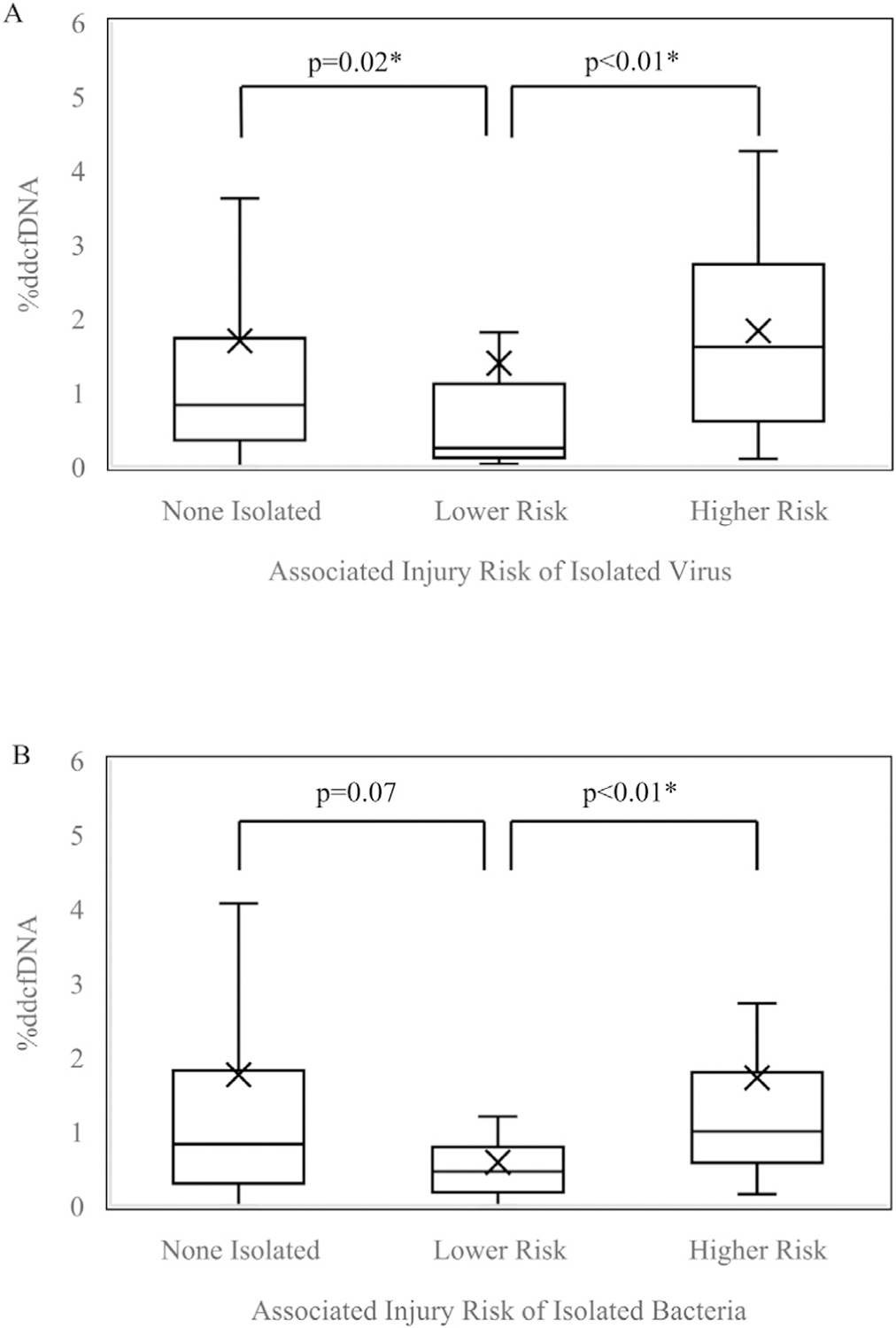
Boxplot of %ddcfDNA values among samples with higher risk vs lower risk (A) viral isolates (B) bacterial isolates adjusted for correlation among repeated %ddcfDNA measures in the same subject Boxes: 25th, median, and 75th percentile; whiskers: minimum and maximum (excluding outliers); x-marks: mean. Higher risk, confers higher risk of downstream injury; Lower risk, confers lower risk of downstream injury. *Statistically significant (p<0.05).
Discussion
Respiratory pathogens can be a significant risk factor for morbidity and mortality following lung transplantation, with studies showing that select respiratory microbial isolates associated with acute pneumonia, HLA alloimmunity and CLAD risk.1,33,34 Herein, we show that %ddcfDNA is markedly elevated in lung transplant recipients with concurrent respiratory microbial isolation and histopathologic abnormalities. Furthermore, we note that %ddcfDNA levels from Lung Transplant Recipients (LTRs) with concurrent higher risk microbial isolates in their bronchoalveolar lavage fluid is associated with elevated %ddcfDNA independent of inter-subject variations in baseline %ddcfDNA levels. These novel findings suggest that %ddcfDNA is a non-invasive marker of pathogen associated allograft injury and may detect subclinical injury that is not otherwise detected by histopathology.
Not surprisingly, simply the presence or absence of non-specific microbial isolation, irrespective of species, was not associated with %ddcfDNA. This is consistent with clinical observations of varying degrees of virulence among microbial organisms, and also may reflect the fact that this study was not designed to distinguish isolates collected from asymptomatic individuals compared to those who met the International Society of Heart and Lung Transplantation (ISHLT) definitions of pneumonia.35 We did observe that among those positive for pathogen isolation, the presence of abnormal histopathology was significantly associated with elevated %ddcfDNA as compared to those with pathogen isolation and normal histopathology. Although the sample sizes in our cohort may be underpowered for statistical significance, the magnitude of %ddcfDNA appeared to be two-fold higher in samples with both abnormal histopathology AND microbial isolation as compared to samples with only abnormal histopathology or microbial isolation, and was comparable to the magnitude of median %ddcfDNA values previously published in subjects with acute cellular rejection (ACR).16 Although our study, which had relatively low incidence of ACR and AMR, did not detect significantly higher %ddcfDNA with rejection, future studies with a larger sample size may be able to examine clinical AMR and ACR as a distinctive covariate. Future prospective studies to further examine clinical parameters are planned to determine the impact of clinical infection vs. asymptomatic infection on %ddcfDNA levels. This will be important to determine how these findings impact both concurrent and downstream allograft injury such as HLA-AMR or CLAD, both of which have been associated with select infectious risks.
When pathogens were grouped into higher and lower risk groups based on previous association with increased risk of acute or chronic allograft injury, we found that the higher risk group, which included representative species such as Pseudomonas aeruginosa, Influenza, and Aspergillus fumigatus was significantly associated with elevated %ddcfDNA, even in the absence of acute histopathologic changes.9–11,18–20 Please note that while community respiratory viruses as a group have been associated with CLAD, in our analysis, in the higher risk cohort we only included species that had individually been identified as higher risk for CLAD, such as influenza and RSV, and excluded viral species whose individual risk for CLAD was less established, such as rhinovirus.1,27,33 Similarly, while pathogens such as Klebsiella species may be isolated during acute infections, these pathogens have not been individually identified in published literature to have an association with long term allograft dysfunction, and thus were stratified into a lower risk category. This novel and striking finding, persistent among both higher risk viruses as well as clinically established higher risk bacteria, suggests a possibility that %ddcfDNA may detect early subclinical injury from microbial pathogens, and potentially identify subsets of LTR’s most at risk for downstream pathogen associated CLAD.27 Further longitudinal follow up is ongoing to determine if there is any association of this finding with a long-term clinical decline in lung function. If confirmed, this may open a pathway for additional mechanistic insights or targeted therapeutics, particularly among subjects who appear to be asymptomatically “colonized” with a given organism.
This was by design an early follow up period to examine the initial association of %ddcfDNA with microbial isolation. While the sample size was not small, a larger sample size would be needed to examine the correlation at the level of individual species and determine whether any individual pathogen is associated with a higher degree of allograft injury as measured by %ddcfDNA. We also had very few isolates of higher risk fungi, possibly due to use of prophylactic azoles during the early postoperative period, and thus were unable to analyze the impact of a higher compared to lower risk fungal pathogen on %ddcfDNA. Our relatively short time course precluded us from analyzing downstream outcomes such as CLAD, as these events tend to increase in prevalence at time points beyond the scope of this study. As other researchers have documented that a rise in %ddcfDNA in association with an infectious event may precede the development and diagnosis of AMR and CLAD, future studies with longer follow up times are ongoing.16
This early report provides initial evidence that %ddcfDNA is a molecular biomarker that correlates with concurrent histopathologic injury when microbial isolates are recovered in the lung allograft. Further observations of high values with pathogens known to pose a risk to the lung allograft, regardless of clinical histology, suggest that %ddcfDNA may be a sensitive tool to detect subclinical injury. Future research should be done to further understand the predictive value of this biomarker in the context of clinical and subclinical infection and with regards to downstream impacts on lung function.
Supplementary Material
Acknowledgments
Disclosure
K.B. has received funding from the NIH. N.P. has received research funding from Health Systems Research Institute (Thailand), NIH, Cystic Fibrosis Foundation, and Fisher Center Discovery Program, Johns Hopkins University. R. K. Avery has received study grant support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, and Takeda/Shire. P.S. has received funding from the NIH and Cystic Fibrosis Foundation.
This manuscript was funded by the National Heart, Lung, and Blood Institute, award HHSN268201800082P (I.T, A.B., J.O., S.D.N., H.V., S.A-E., P.S.). S.A-E. also received support through the Lasker Clinical Research Program, NIH Distinguished Scholar Program and Cystic Fibrosis Foundation (Grant # AGBORE20Q10)
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2021.05.012.
References
- 1.Gregson AL. Infectious triggers of chronic lung allograft dysfunction. Curr Infect Dis Rep 2016;18:21. 10.1007/s11908-016-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton CM, Iversen M, Carlsen J, et al. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J Heart Lung Transplant 2009;28:888–93. 10.1016/j.healun.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2007;175:507–13. 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 4.Botha P, Archer L, Anderson RL, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation 2008;85:771–4. 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 5.Vos R, Vanaudenaerde BM, Geudens N, Dupont LJ, Van Raemdonck DE, Verleden GM. Pseudomonal airway colonisation: risk factor for bronchiolitis obliterans syndrome after lung transplantation? Eur Respir J 2008;31:1037–45. 10.1183/09031936.00128607. [DOI] [PubMed] [Google Scholar]
- 6.Avery RK. Infections after lung transplantation. Semin Respir Crit Care Med 2006;27:544–51. 10.1055/s-2006-954612. [DOI] [PubMed] [Google Scholar]
- 7.Vu DL, Bridevaux PO, Aubert JD, Soccal PM, Kaiser L. Respiratory viruses in lung transplant recipients: a critical review and pooled analysis of clinical studies. Am J Transplant 2011;11:1071–8. 10.1111/j.1600-6143.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peghin M, Hirsch HH, Len Ó, et al. Epidemiology and immediate indirect effects of respiratory viruses in lung transplant recipients: a 5-year prospective study. Am J Transplant 2017;17:1304–12. 10.1111/ajt.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah PD, McDyer JF. Viral infections in lung transplant recipients. Semin Respir Crit Care Med 2010;31:243–54. 10.1055/s-0030-1249120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Permpalung N, Thaniyavarn T, Saullo JL, et al. Oral and inhaled ribavirin treatment for respiratory syncytial virus infection in lung transplant recipients. Transplantation 2020;104:1280–6. 10.1097/TP.0000000000002985. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni HS, Tsui K, Sunder S, et al. Pseudomonas aeruginosa and acute rejection independently increase the risk of donor-specific antibodies after lung transplantation. Am J Transplant 2020;20:1028–38. 10.1111/ajt.15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregson AL, Wang X, Injean P, et al. Staphylococcus via an interaction with the ELR+ CXC chemokine ENA-78 is associated with BOS. Am J Transplant 2015;15:792–9. 10.1111/ajt.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wojarski J, Ochman M, Medrala W, et al. Bacterial infections during hospital stay and their impact on mortality after lung transplantation: a single-center study. Transplant Proc 2018;50:2064–9. 10.1016/j.transproceed.2017.11.080. [DOI] [PubMed] [Google Scholar]
- 14.Gupta MR, Valentine VG, Walker JE, et al. Clinical spectrum of gram-positive infections in lung transplantation. Transpl Infect Dis 2009;11:424–31. 10.1111/j.1399-3062.2009.00422.x. [DOI] [PubMed] [Google Scholar]
- 15.Agbor-Enoh S, Wang Y, Tunc I, et al. Donor-derived cell-free DNA predicts allograft failure and mortality after lung transplantation. EBioMedicine 2019;40:541–53. 10.1016/j.ebiom.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agbor-Enoh S, Jackson AM, Tunc I, et al. Late manifestation of alloantibody-associated injury and clinical pulmonary antibody-mediated rejection: Evidence from cell-free DNA analysis. J Heart Lung Transplant 2018;37:925–32. 10.1016/j.healun.2018.01.1305. [DOI] [PubMed] [Google Scholar]
- 17.De Vlaminck I, Martin L, Kertesz M, et al. Noninvasive monitoring of infection and rejection after lung transplantation. Proc Natl Acad Sci USA 2015;112:13336–41. 10.1073/pnas.1517494112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridges ND, Spray TL, Collins MH, Bowles NE, Towbin JA. Adenovirus infection in the lung results in graft failure after lung transplantation. J Thorac Cardiovasc Surg 1998;116:617–23. 10.1016/S0022-5223(98)70168-0. [DOI] [PubMed] [Google Scholar]
- 19.Nȩcki M, Gawȩda M, Pandel A, et al. Microbiological status as a factor of airway complications after lung transplantation. Transplant Proc; May 2020. 10.1016/j.transproceed.2020.02.111. [DOI] [PubMed]
- 20.Wendt CH, Fox JM, Hertz MI. Paramyxovirus infection in lung transplant recipients. J Heart Lung Transplant 1995;14:479–85. [PubMed] [Google Scholar]
- 21.Ambrosioni J, Bridevaux P-O, Aubert J-D, Soccal P, Wagner G, Kaiser L. Role of rhinovirus load in the upper respiratory tract and severity of symptoms in lung transplant recipients. J Clin Virol 2015;64:1–5. 10.1016/j.jcv.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Ammerman E, Sweet SC, Storch GA, et al. Epidemiology and persistence of rhinovirus in pediatric lung transplantation. Transpl Infect Dis July 2020: e13422. 10.1111/tid.13422. [DOI] [PMC free article] [PubMed]
- 23.Orfanos S, Gomez C, Baron S, et al. Impact of gram negative bacteria airway recolonization on the occurrence of chronic lung allograft dysfunction after lung transplantation in a population of cystic fibrosis patients. BMC Microbiol 2018;18:88. 10.1186/s12866-018-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manuel O, Estabrook M. American society of transplantation infectious diseases community of practice. rna respiratory viral infections in solid organ transplant recipients: guidelines from the american society of transplantation infectious diseases community of practice. Clin Transplant 2019;33:e13511. 10.1111/ctr.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peghin M, Los-Arcos I, Hirsch HH, et al. Community-acquired respiratory viruses are a risk factor for chronic lung allograft dysfunction. Clin Infect Dis 2019;69:1192–7. 10.1093/cid/ciy1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunasekaran M, Bansal S, Ravichandran R, et al. Respiratory viral infection in lung transplantation induces exosomes that trigger chronic rejection. J Heart Lung Transplant 2020;39:379–88. 10.1016/j.healun.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayah DM, Koff JL, Leard LE, Hays SR, Golden JA, Singer JP. Rhinovirus and other respiratory viruses exert different effects on lung allograft function that are not mediated through acute rejection. Clin Transplant 2013;27:E64–71. 10.1111/ctr.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fishman JA. Infection in organ transplantation. Am J Transplant 2017;17:856–79. 10.1111/ajt.14208. [DOI] [PubMed] [Google Scholar]
- 29.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007;357:2601–14. 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 30.Levine DJ, Glanville AR, Aboyoun C, et al. Antibody-mediated rejection of the lung: a consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2016;35:397–406. 10.1016/j.healun.2016.01.1223. [DOI] [PubMed] [Google Scholar]
- 31.Agbor-Enoh S, Tunc I, De Vlaminck I, et al. Applying rigor and reproducibility standards to assay donor-derived cell-free DNA as a non-invasive method for detection of acute rejection and graft injury after heart transplantation. J Heart Lung Transplant 2017;36:1004–12. 10.1016/j.healun.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloom RD, Bromberg JS, Poggio ED, et al. Cell-free DNA and active rejection in kidney allografts. J Am Soc Nephrol 2017;28:2221–32. 10.1681/ASN.2016091034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med 2004;170:181–7. 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 34.Valentine VG, Gupta MR, Walker JE, et al. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transplant 2009;28:163–9. 10.1016/j.healun.2008.11.907. [DOI] [PubMed] [Google Scholar]
- 35.Husain S, Mooney ML, Danziger-Isakov L, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant 2011;30:361–74. 10.1016/j.healun.2011.01.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


