Summary
Structural heterogeneity of nucleosomes in functional chromosomes is unknown. Here we devise the Template-, Reference- and Selection-Free (TRSF) cryo-EM pipeline to simultaneously reconstruct cryo-EM structures of protein complexes from interphase or metaphase chromosomes. The reconstructed interphase and metaphase nucleosome structures are on average indistinguishable from canonical nucleosome structures, despite DNA sequence heterogeneity, cell cycle-specific posttranslational modifications and interacting proteins. Nucleosome structures determined by a decoy-classifying method and structure variability analyses reveal the nucleosome structural variations in linker DNA, histone tails, and nucleosome core particle configurations, suggesting that the opening of linker DNA, which is correlated with H2A C-terminal tail positioning, is suppressed in chromosomes. High-resolution (3.4-3.5 Å) nucleosome structures indicate DNA sequence-independent stabilization of superhelical locations ±0~1 and ±3.5~4.5. The linker histone H1.8 preferentially binds to metaphase chromatin, from which chromatosome cryo-EM structures with H1.8 at the on-dyad position are reconstituted. This study presents structural characteristics of nucleosomes in chromosomes.
Keywords: Cryo-EM, nucleosome, chromatin, mitosis, cell cycle, histone H1, Xenopus egg extract, macroglobulin, lectin
Graphical Abstract

Introduction
The nucleosome, composed of eight core histones (H2A2, H2B2, H32, and H42) and 144~147 bp of DNA, is the fundamental structural unit of chromosomes (Olins and Olins, 2003; Richmond et al., 1984). X-ray crystal structures of nucleosomes reconstituted in vitro with nucleosome-positioning sequences revealed that DNA wraps around the core octameric histones in a left-handed direction (Luger et al., 1997; McGinty and Tan, 2015). Many structural variants of nucleosomes can also exist in vitro (Lavelle and Prunell, 2007; Zlatanova et al., 2009) (Fig. 1A left)(Arimura et al., 2012; Bancaud et al., 2007; Furuyama et al., 2013; Kato et al., 2017; Zou et al., 2018), and cryo-electron tomography (cryo-ET) analyses suggested that the canonical nucleosome structure may not be abundant in the yeast nucleus (Cai et al., 2018a; Tan et al., 2021). However, current reconstructions of in vivo nucleosomes are lower than 20 Å resolution (Cai et al., 2018b; Chicano et al., 2019; Eltsov et al., 2018; Scheffer et al., 2011), making it difficult to assess nucleosome structural heterogeneity in chromosomes.
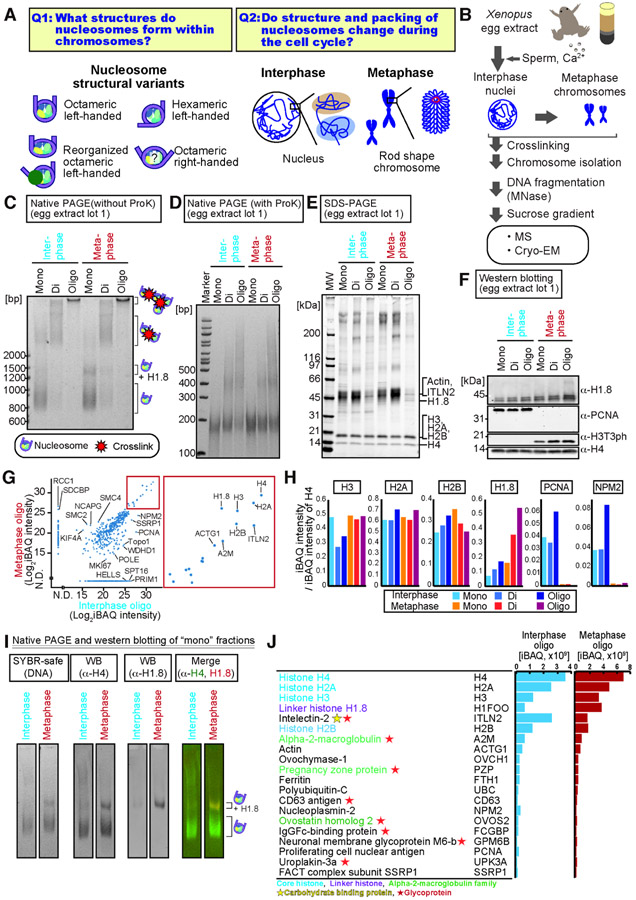
Figure 1. Isolation of nucleosomes from interphase and metaphase chromosomes.
A, Scopes of this study. B, The standard chromosomal nucleosome preparation. C, D, Native PAGE analysis of DNA (SYBR-safe) in nucleosome fractions isolated from sperm chromosomes formed in the Xenopus egg extract lot 1, without (C) or with (D) proteinase K treatment. Equal DNA amounts were loaded. E, SDS-PAGE analysis of nucleosomal proteins (gel code blue) isolated from sperm chromosomes in the egg extract lot 1. F, Western blot analysis of Figure 1E. G, Scatter plot of MS-detected proteins in the oligo nucleosome fractions from the extract lot 1. Right; a magnified view of the high signal region. H, MS signal intensities of representative proteins, normalized to H4. I, Linker histone-bound mono-nucleosomes detected by native PAGE and western blot. J, Protein abundance in the oligo-nucleosome fractions quantified by MS. Enrichment of intelectin-2 and glycoproteins (ovochymase and alpha2-macroglobulin family proteins) may reflect O-linked N-acetylglucosamine on chromosomes (Gagnon et al., 2015; Kelly and Hart, 1989).
Chromosome morphology dramatically changes upon entry into mitosis; diffuse interphase chromosomes are converted to rod-shaped chromatids (Fig. 1A right). Although the DNA loop formation by condensin drives large scale mitotic chromosome folding (Goloborodko et al., 2016; Hirano and Mitchison, 1994; Takahashi and Hirota, 2019), chromosomes depleted of condensin still exhibit mitosis-specific compaction (Gibcus et al., 2018; Samejima et al., 2018). Several lines of evidence indicate that nucleosomes are more densely packed in mitosis than in interphase. Nucleosomes assembled in vitro with histones purified from mitotic cells tend to aggregate more easily than those assembled with histones from interphase (Zhiteneva et al., 2017), and a cryo-ET study suggested that mitotic chromatin is more crowded than interphase chromatin (Cai et al., 2018a). Although a variety of mitosis-specific histone modifications are known (Wang and Higgins, 2013), it remains unclear if the nucleosome structure changes during mitotic chromatin compaction.
How nucleosomes pack in chromosome is also elusive. The 30 nm-fiber, which can be formed in vitro reconstituted poly-nucleosomes, (Dorigo et al., 2004; Ekundayo et al., 2017; Grigoryev et al., 2009; Robinson et al., 2006; Schalch et al., 2005; Song et al., 2014), cannot be detected in the interphase nuclei and mitotic chromosomes by cryo-EM and small angle X-ray scattering (Eltsov et al., 2008; Maeshima and Eltsov, 2008; Nishino et al., 2012). Recently, genome-wide nucleosome interaction mapping analyses indicate that short nucleosome stretches may locally cluster into ordered structures (Ohno et al., 2019; Risca et al., 2017). However, different folding patterns of such ordered nucleosome clusters can be predicted depending on the linker DNA angles and lengths (Buckwalter et al., 2017; Grigoryev, 2018; Koslover et al., 2010), while no reliable method to determine linker DNA angles in chromosomes is available.
Recent technological advances in single particle cryo-EM enabled high-resolution structure determination of proteins including endogenous proteins (Kastritis et al., 2017; Nakane et al., 2020; del Valle and Axel Innis, 2020; Yi et al., 2019), but, to the best of our knowledge, no structural comparisons of protein isolated from different cell cycle stages have been reported. Here, we report high-resolution structures of nucleosomes in interphase and metaphase chromosomes.
Results
Nucleosome isolation from interphase and metaphase chromosomes
We established a method to isolate nucleosomes from functional interphase and metaphase chromosomes while preserving intact protein-DNA structures for cryo-EM analysis (Fig. 1B). Xenopus egg extracts arrested at meiotic metaphase II by cytostatic factor (CSF) were released into interphase with Xenopus sperm nuclei (Murray, 1991). Nucleosomes assembled on sperm chromosomes, the nuclear envelope formed, and chromosomes were replicated. Mitotic chromosome compaction and spindle assembly were induced by adding fresh metaphase-arrested CSF extracts (Fig. 1B, S1A) (Shamu and Murray, 1992). Chromosomes were crosslinked with formaldehyde to preserve nucleosome structures, isolated via centrifugation, and then fragmented with micrococcal nuclease (MNase). The majority (60% - 90%) of DNA was solubilized from chromatin with MNase (Fig. S1B). The solubilized nucleosomes were separated by sucrose gradient centrifugation, yielding three fractions containing different sizes of DNA-protein complexes (Fig. 1C, S1D: mono, di, and oligo). DNA lengths isolated from these fractions were equivalent to those from mono-nucleosomes (150-180 bp) (Fig. 1C, 1D, S1E), indicating that nucleosomes may not be connected by linker DNAs but by inter-nucleosomal crosslinks in the di- and oligo- nucleosome fractions. Biochemical characteristics of these fractions were assessed by western blot and MS. The mitosis-specific histone H3 phosphorylation at Thr3 (H3T3ph) was observed in isolated metaphase nucleosomes but not in interphase nucleosomes (Fig. 1F) (Kelly et al., 2010). Known chromatin proteins, such as proliferating cell nuclear antigen (PCNA) and condensin subunits (CAPD2, SMC2, CAPG, SMC4) showed their expected cell cycle-dependent enrichment on nucleosome fractions (Fig. 1F-H, S1F, Table S1), confirming that cell cycle-specific biochemical characteristics of chromatin were preserved.
Unexpectedly, chromatosomes containing H1.8 (also known as B4, H1M, and H1Foo), the dominant linker histone variant in Xenopus eggs (Dworkin-Rastl et al., 1994; Wühr et al., 2014), were more prevalent in metaphase than in interphase (Fig. 1G-J, Table S1). On native polyacrylamide gel electrophoresis (PAGE) gels, the metaphase mono-nucleosome fraction showed an additional slower-migrating band corresponding to the chromatosome (Fig. 1C, S1D), which contains core histones and H1.8 (Fig. 1I). This H1.8 enrichment in metaphase nucleosome did not depend on crosslinking (Fig S1G-H). H1.8 was most enriched in the oligo- and di-nucleosome fractions (Fig. 1F, 1H, Table S1), consistent with the notion that H1 compacts chromatin (Renz et al., 1977).
Simultaneous cryo-EM reconstruction of multiple protein complexes enriched in the nucleosome fraction
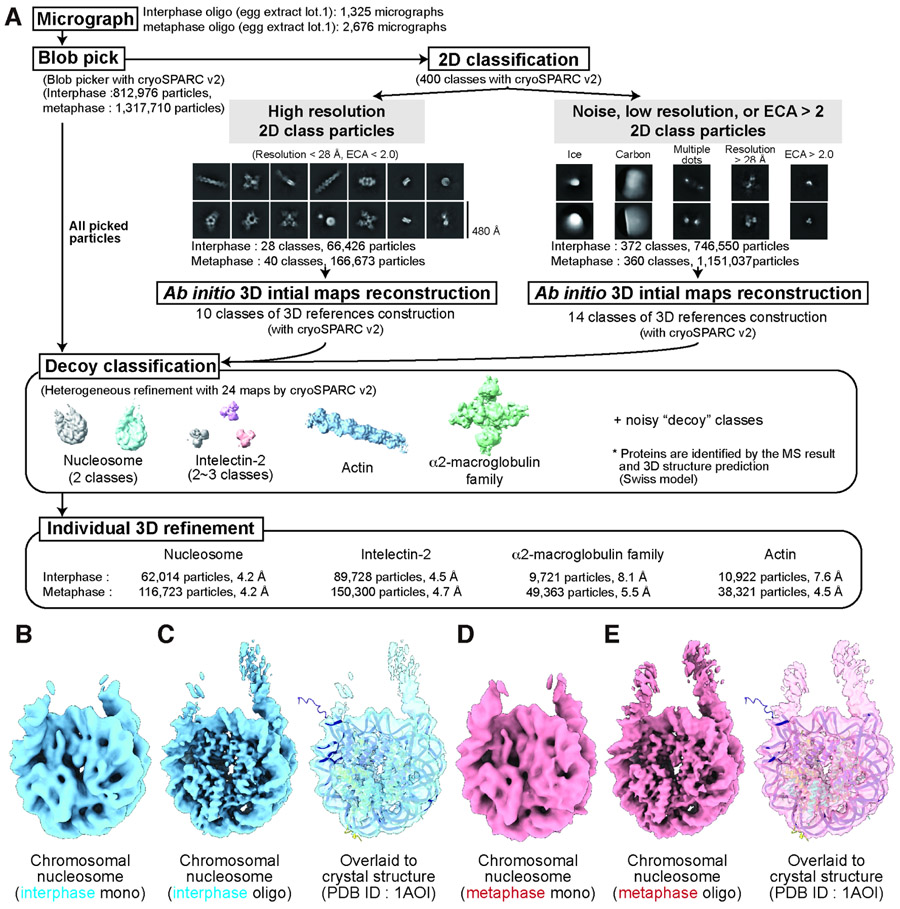
To examine structural diversity of nucleosomes formed in chromosomes, we solved cryo-EM structures of protein complexes enriched in nucleosome fractions by devising the Template-, Reference- and Selection-Free (TRSF) pipeline (Fig. 2A). This strategy aimed to preserve conformational heterogeneity and protein diversity by avoiding commonly used cryo-EM analysis procedures that entail selection bias, such as template-based particle picking, 3D classification with references of previously reported structures, and manual 2D class selection of specific targeted protein structures. Using the TRSF pipeline, we simultaneously reconstructed distinct protein complex structures (Fig. 2A middle, S3). Guided by the MS-based protein abundance (Fig. 1J, Table S1) and sequence-based 3D structure modeling (Waterhouse et al., 2018), the structures from oligo fractions were identified as an octameric mono-nucleosome, intelectin-2, alpha2-macroglobulin family protein, and actin (Fig. S3E-H). From mono fractions, two-types of intelectin-2 structures (trimer and hexamer) and an octameric mono-nucleosome were identified (Fig. S3A-D). Although resolution of the nucleosome structures from oligo fractions were much higher than mono fractions due to the higher nucleosome concentration of oligo fractions on the cryo-EM grid (Fig S2A), nucleosome structures reconstructed from both fractions were similar (Fig 2B-E). Both interphase and metaphase nucleosome structures aligned well with the canonical octameric nucleosome crystal structure (Fig. 2C, 2E) (Luger et al., 1997). While cryo-EM maps of actin and intelectin matched published EM and crystal structures, respectively (Fujii et al., 2010; Wangkanont et al., 2016), the overall structure of the maps that we identified as alpha2-macroglobulin family protein were dissimilar to the reported crystal structure of human alpha2-macroglobulin (Fig. S4) (Marrero et al., 2012). Thus, our pipeline successfully reconstructed multiple structures including a previously unsolved structure without the need for any template-picking or manual selection, which were previously required to determine multiple structures from crude samples (Kastritis et al., 2017; Kyrilis et al., 2019; Su et al., 2021; Verbeke et al., 2018, 2020). These results were reproduced with a biological replicate (lot 2; Fig S5A-F).
Figure 2. Simultaneous cryo-EM structure reconstruction of multiple protein complexes in nucleosome fractions by the TRSF pipeline.
A, TRSF cryo-EM analysis pipeline. Shown examples of 2D classification panels and 3D references were from the metaphase oligo-nucleosome fraction. B-E, Reconstructed nucleosome structures from the interphase mono fraction (B), interphase oligo fraction (C), metaphase mono fraction (D), and metaphase oligo fraction (E) of the extract lot 1, and their superposition onto the crystal structure of the canonical nucleosome (PDB ID: 1AOI).
Averaged cryo-EM structures of interphase and metaphase nucleosomes
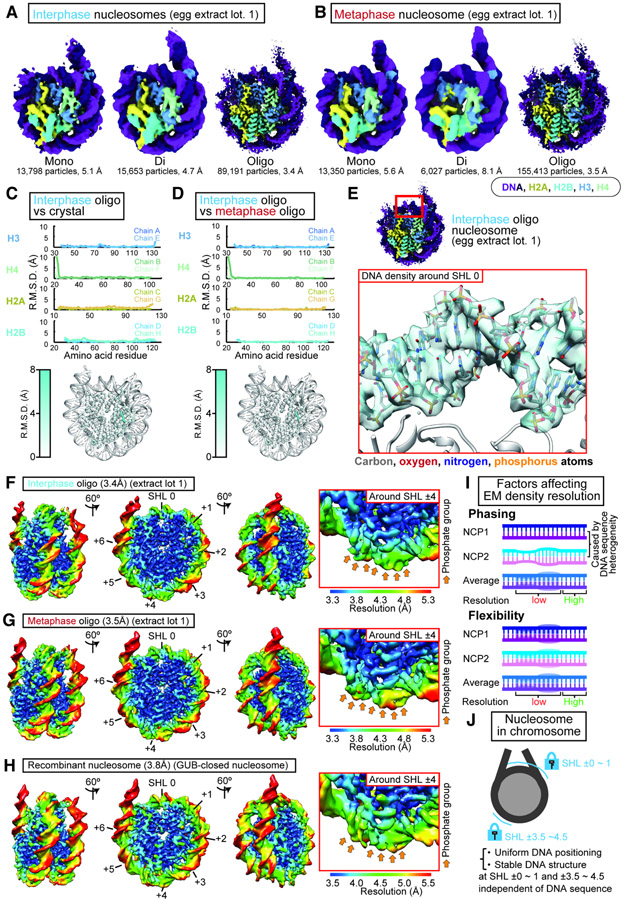
To assess structural variations of these chromosomal nucleosome structures, we next attempted to reconstruct averaged structures with higher resolution by repeating the cryo-EM processing with another pipeline combining Topaz, a convolutional neural network-based particle picking program (Bepler et al., 2019), and decoy classification (Fig. S6A). Unlike commonly used cryo-EM classification pipelines, our pipeline was designed to collect as many diverse nucleosome particle images as possible by removing only particles that were not classified as the nucleosome by the decoy classification (see method for a detail). Using this pipeline, structures of interphase and metaphase nucleosomes in the oligo-nucleosome fractions were reconstructed at a nominal 3.4 and 3.5 Å resolution, respectively (Fig. 3A, 3B, and S6B). Similar left-handed octameric nucleosome structures were reconstructed from the mono- and di-nucleosome fractions of interphase and metaphase chromosomes (Fig 3A-B) The high-resolution maps from oligo-nucleosome fractions allowed us to build atomic models in which the aromatic side chain of phenylalanine 98 of H2A.X-F1/F2, the predominant H2A isoform in egg (Shechter et al., 2009), could be distinguished from the leucine or isoleucine residues of other H2A isoforms (Fig. S7A). The atomic models for chromosomal nucleosomes are essentially identical to those of X-ray crystal structures of nucleosomes with homogenous nucleosome positioning sequences (Fig. 3C). This indicates that DNA sequence variability does not largely affect the histone octamer structure in functional chromosomes. Furthermore, no obvious cell cycle-dependent structural changes were observed in the histone octamer (Fig. 3D).
Figure 3. Averaged cryo-EM structures of interphase and metaphase nucleosomes.
A, B, Cryo-EM structures of the nucleosome core particle from interphase (A) and metaphase (B) chromosomes assembled in egg extracts lot 1. C, D, Structural comparisons between interphase nucleosomes and the canonical crystal structure of the nucleosome (PDB ID: 1AOI) (C) or between interphase and metaphase nucleosomes (D). Each histone protein chain are named separately following the definition in the nucleosome crystal structure (Luger et al., 1997). Root-mean-square deviation (R.M.S.D.) for all Cα atoms for each of the structures was calculated using PyMol and plotted (top) or mapped onto the atomic coordinates of the interphase oligo fraction nucleosome (bottom). Large values at H4 N-terminus are due to two orientations (see Fig. S9). E, Cryo-EM density of DNA around SHL 0 of nucleosome in interphase oligo fraction. F, G, H, Local resolution maps of interphase (F) and metaphase (G) nucleosomes from oligo fractions of the extract lot 1, and recombinant nucleosome (H: GUB-closed). Left, local resolution maps of the entire nucleosome. Right, local resolution maps of DNA around SHL ±3.5~4.5. Orange arrows indicate the position of phosphate group in DNA. I. Diagram of the potential factors that may affect EM density resolution. J. Graphical summary.
DNA segments at two specific superhelical locations are exceptionally ordered in chromosomal nucleosomes
Despite the heterogeneity of DNA sequences, our high-resolution metaphase and interphase nucleosome maps exhibited extraordinary homogeneity in DNA structure, such that the DNA backbone was clearly delineated (Fig. 3E). A single DNA base pair aligned at the nucleosome dyad is defined as Super Helical Location (SHL) 0 (Klug et al., 1980). Strikingly, in our high-resolution cryo-EM structures of the oligo-fraction nucleosomes, DNA segments at SHL ±3.5~4.5, as well as SHL ±0~1, exhibited higher local resolutions (Fig. 3F-G) and lower B-factor values (Fig. S7B) than those at their adjacent DNA segments did. The resolution of DNA around these segments was high enough to exhibit discrete positioning of phosphates and bases (Fig. 3E, F-G right). Two properties are likely to contribute to this high local resolution at SHL 3.5-4.5 and SHL0-1. First, the nucleotide positions of these DNA segments are strongly phased in a manner independent of DNA sequence (Fig. 3I: phasing). This was surprising since local DNA segments are known to stretch or shrink on the nucleosome to accommodate different DNA sequences or bound proteins in crystal structures (Armache et al., 2019; Chua et al., 2012; Liu et al., 2017; McGinty and Tan, 2015; Sinha et al., 2017), where different DNA conformation may be adapted depending on local bendability varied by DNA sequences (Basu et al., 2021). Second, these DNA segments must be structurally stable, since EM density may also worsen if the DNA of these regions are flexible, even if the phasing of DNA nucleotide positioning at these regions is identical among nucleosomes (Fig. 3I: Flexibility).
Similar higher local resolutions at SHL ±3.5~4.5 and SHL ±0~1 were also seen in the recombinant nucleosome with GUB nucleosome positioning DNA (Adams and Workman, 1995; An et al., 1998) (Fig. 3H, S8: “GUB-closed”). Since nucleotide positions must be phased in the homogeneous nucleosome, the result suggests that the DNA at these regions is stabilized. Supporting the high structural stability at these DNA locations, the number of DNA-histone interactions formed with basic amino acid residues on histones is particularly high in these regions (Fig. S7C-G). These data indicate that the DNA segments around SHL ±0~1 and SHL ±3.5~4.5 in chromosomal nucleosomes are strongly phased and structurally stable in a sequence-independent manner, while other regions, such as SHL ±2~3, ±5~6 and linker DNA, form relatively flexible structures and/or allow multiple DNA positioning patterns (Fig. 3J).
Qualitative identification of nucleosome structural variants
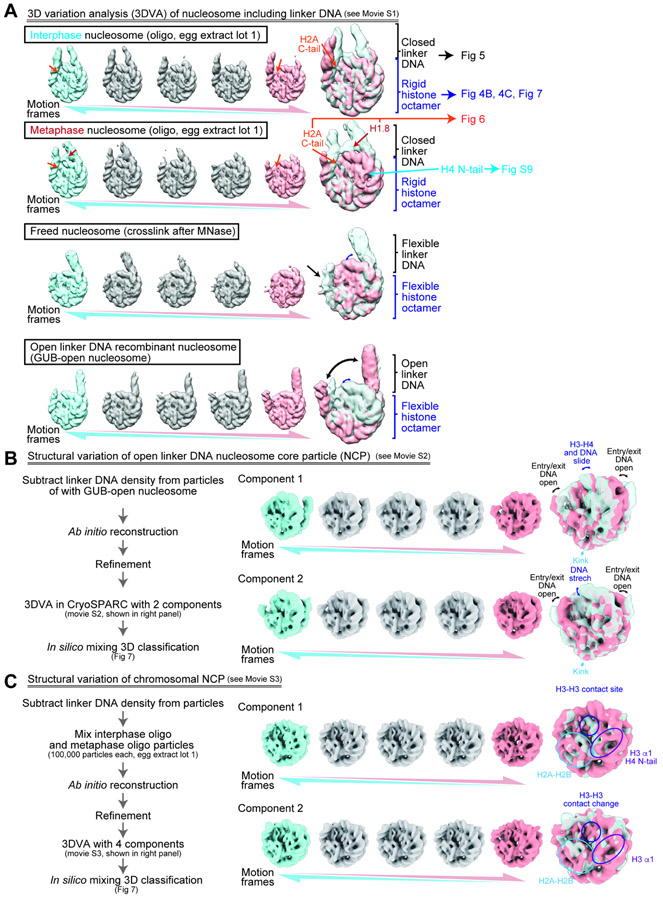
We next examined the conformational heterogeneity of nucleosome structures in chromosomes with 3D variability analysis (3DVA) in CryoSPARC (Punjani and Fleet, 2021), which models cryo-EM data into multiple series of 3D structures (motion frames), each representing a potential “trajectory” of motion (Fig. 4A, Figure360, Movie S1). Each trajectory represents a principal component of the variance present in each dataset, so each 3DVA component presented here is a representation of the major structural variations.
Figure 4. 3DVA of the nucleosomes.
A, 3DVA of the nucleosomes containing linker DNAs. Five representative structures (motion frames 3, 7, 11, 15, 19) from 3DVA component 1 class (total 20 frames) are shown. The structures of motion frames 3 and 19 were selected and overlayed for each nucleosome. EM densities for the H2A C-terminal region (orange arrows) and H1.8 (red arrows) are shown. See Movie S1 for full dataset. The identical contour level was applied for maps in each data series. B, C, NCP structural variation of GUB-open nucleosomes (B) and chromosomal nucleosomes (C).
To test if the structural variations observed in 3DVA reflect structural characteristics of nucleosomes in chromosomes rather than the fixation and freezing procedure, we determined cryo-EM structures of chromosomal nucleosomes using a modified protocol in which the same fixation and freezing procedure was applied but crosslinking was done after native nucleosomes were first released from interphase chromosomes by MNase (Fig. 4A, S8A). We called these nucleosomes “freed nucleosomes”. In addition, a more drastic structural variation including nucleosomes with wide open linker DNAs was detected when recombinant nucleosomes assembled on the GUB DNA were processed with GraFix and frozen without optimized cryoprotectant buffer (Fig. S8B). We called these structures “GUB-open nucleosomes”.
Using the 3DVA, four types of apparent structural variations were observed across different nucleosome samples (Fig. 4A, black, orange, cyan, and blue arrows, Movie S1).
Linker DNA angle variation
The linker DNA angles of interphase and metaphase nucleosomes were generally closed, relative to “freed nucleosomes”, which exhibited much larger variation in linker DNA positions (Fig. 4A, Movie S1). Quantitative assessment of this observation will be described in Figure 5.
Linker histone and H2A C-terminal tail
Extra cryo-EM density was observed in chromosomal nucleosomes, particularly when the linker DNA angle was narrowest (Fig. 4A, Movie S1, orange and red arrows). For both interphase and metaphase, the densities of the H2A C-terminal tail reached DNA at the DNA entry/exit site of the nucleosome as the DNA closed inward (Fig. 4A, Movie S1, orange arrows). In addition, as the linker DNA angle closed, an additional cryo-EM density appeared at the nucleosome dyad position, particularly in metaphase nucleosomes (Fig. 4A, Movie S1, red arrows). High resolution analysis of these extra cryo-EM densities will be described in Figure 6.
H4 N-terminal tail
Subtle structural variations around the H4 N-terminal tail were detected (Fig 4A, Movie S1, cyan arrow). Two distinct “outward” and “inward” orientations of this region have been reported. In recombinant canonical nucleosome structures, the inward orientation was clearly observed while outward orientation electron density was ambiguous (Arimura et al., 2018). The outward orientation was previously detected in nucleosomes containing histone H3 variant CENP-A and nucleosomes bound to another protein, such as Sox11 or SET8 (Ali- Ahmad et al., 2019; Arimura et al., 2019; Dodonova et al., 2020; Ho et al., 2021). Functionally, the outward orientation facilitates SET8-mediated methylation of H4K20 (Arimura et al., 2019). In the H4 N-terminal tail region of our averaged interphase and metaphase structures, cryo-EM densities were ambiguous (Fig S9B left), likely reflecting a mixture of inward and outward conformations. To clarify this, we performed a 3D classification based on the H4 tail structures, and successfully separated the outward and inward conformations (Fig S9A). This result suggests that both outward and inward H4 tail orientations are formed at comparable frequency in both interphase and metaphase nucleosomes (Fig. S9).
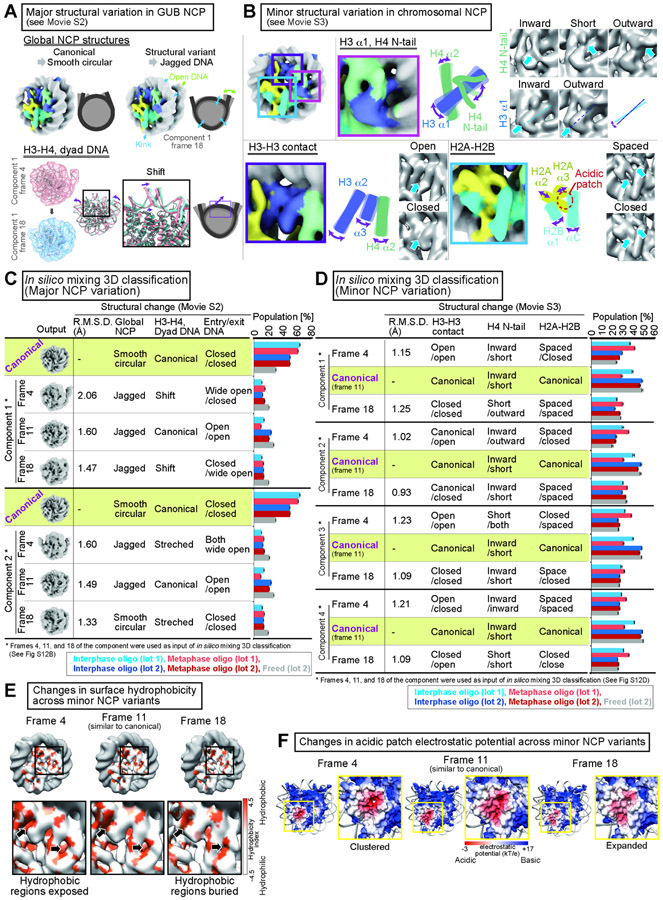
Major and minor structural variations within the NCP
The 3DVA analysis of GUB-open nucleosomes exhibited large structural rearrangements of the NCP (Fig. 4A blue arrows and brackets, Movie S1). To better visualize structural variations within the NCP, we employed the 3DVA analysis after subtracting the linker DNA densities from the original particles (Fig. 4B, 4C, Movie S2). This analysis revealed two different types of NCP structural variations; the major NCP structural variation, characterized by diverse NCP surface outlines seen in GUB NCPs (Fig. 4B, Movie S2, also see Fig. 7A), and the minor NCP structural variation, such as rearranged orientations of core histone α-helices and the H4 N-terminal tail observed in chromosomal NCPs (Fig. 4C, Movie S3, also see Fig. 7B). Population analysis of these structural variations will be described in Figure 7.
Linker DNA configurations of chromosomal nucleosomes
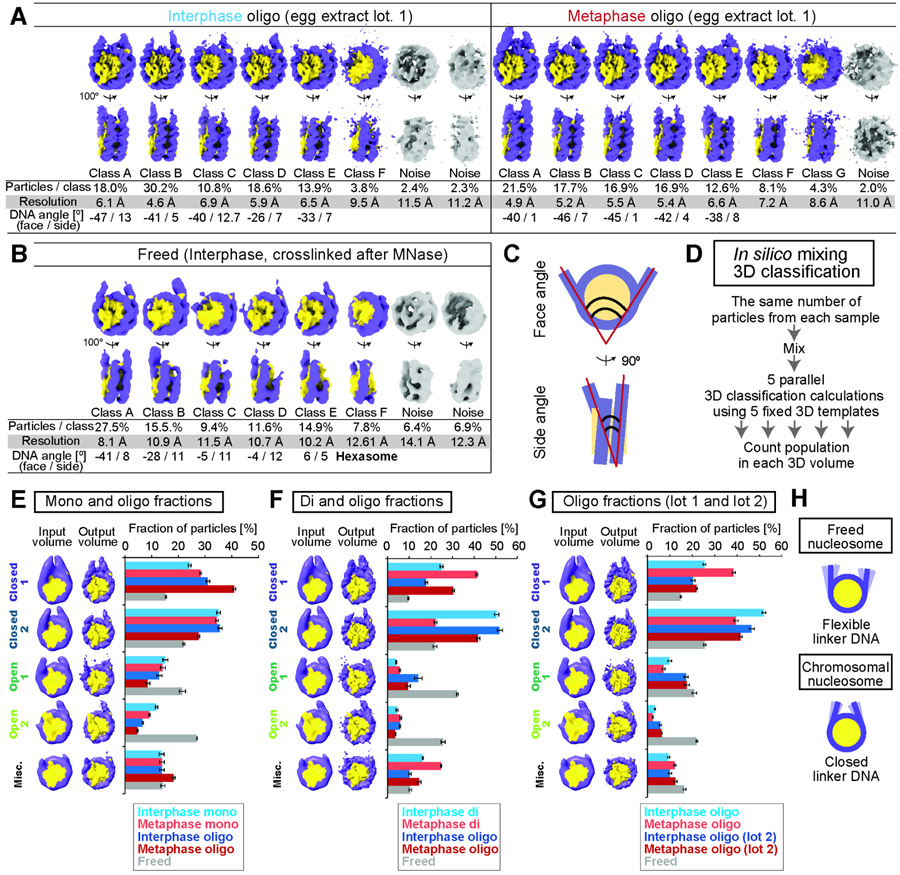
To quantitatively assess the structural variation of linker DNA configurations detected by 3DVA, we used ab initio reconstruction to generate 6-8 3D references of nucleosomes representing distinct linker DNA structural classes, and then classified all nucleosome-like particles (Fig. 5A-C, S10A-B). Linker DNAs in the majority of interphase and metaphase nucleosomes processed by our standard protocol were classified into the closed configurations, while linker DNAs in freed nucleosomes (fixed after MNase digestion of chromosomes) exhibited wider angles or could not be resolved well, likely due to their structural heterogeneity (Fig 5B). The hexasome, which lacks one H2A-H2B dimer from octameric nucleosome with partially wrapped DNA, was only observed in freed nucleosomes (Fig 5B class F).
Figure 5. The linker DNA configuration of interphase and metaphase nucleosomes.
A, 3D structure classes of nucleosomes in the oligo-nucleosome fractions of interphase chromosomes (left, extract lot 1) and metaphase chromosomes (right, extract lot 1). B, 3D structure classes of the freed nucleosome (egg extract lot2). C, Linker DNA angle definitions. D Schematic of the in silico mixing 3D classification pipeline. E-G, In silico mixing 3D classification with fixed 3D reference maps to compare the mono and oligo fractions (E), di and oligo fractions (F), and oligo fractions and their biological replicates (G). Input and output 3D maps of the in silico mixing 3D classifications were shown. Numbers and populations of particles assigned to each class are shown in Table S3. H, Graphical summary.
To quantitatively compare the linker DNA configurations between different samples, we performed in silico mixing 3D classification (Hite and MacKinnon, 2017): we combined particles from different sets of samples, and conducted a new round of classification analysis using five manually-selected 3D references with distinct linker DNA angles (Closed 1, Closed 2, Open 1, Open 2, Miscellaneous; Fig. 5D-G). Five classification replicates yielded consistent results: in all mono, di, and oligo fractions, around 60-70% of interphase or metaphase nucleosomes were assigned to two closed classes, while these proportions were reduced to 30-40% in freed mono-nucleosomes fixed after MNase digestion (Fig. 5E-G). The closed classes were also major forms in the biological replicate of oligo fractions (lot 2, Fig. 5G). These results suggest that linker DNAs are generally closed in chromosomes, but once mono-nucleosomes are released from chromosomes by MNase prior to fixation, linker DNAs can adopt more flexible open conformations, and these conformations may lead to H2A-H2B dissociation from nucleosome (Fig. 5H).
Linker histone H1.8 binds on the nucleosome dyad in metaphase chromatin
An extra dyad density reminiscent of the linker histone was observed in the metaphase nucleosome with closed linker DNA (Fig. 4A, 5A, Movie S1). Further 3D classification of Class A shown in Fig. 5A (metaphase oligo) enabled structure determination at 4.4 Å resolution (Fig. 6A, S11A-C). Since histone H1.8 preferentially cofractionated with metaphase nucleosomes (Fig. 1G-J), we built an atomic model of the H1.8-bound nucleosome and refined with our cryo-EM map. The extra EM density reasonably corresponded to our refined H1.8 atomic model (Fig. 6B). The similar structure was also reconstructed in the mono fraction in metaphase (Fig S11D). Previously, structural analyses of recombinant chromatosomes and molecular dynamics simulations had suggested that linker histones may bind to the nucleosome in multiple modes; “on-dyad”, where H1 binds at the center of the nucleosome DNA (Bednar et al., 2017; Zhou et al., 2015), and “off-dyad”, where H1 binds more than 5 bp off-center of the nucleosome DNA (Adhireksan et al., 2020; Song et al., 2014; Woods and Wereszczynski, 2020). The H1.8 position in our cryo-EM structure of the metaphase chromatosome closely corresponds to the on-dyad structure (Fig. 6C, 6D) (Bednar et al., 2017; Zhou et al., 2015).
Figure 6. Linker histone H1.8 binds on the nucleosome dyad in metaphase chromosomes.
A, Cryo-EM structure of the nucleosome class with an extra density (in red) on the dyad isolated from the oligo fraction of metaphase nucleosomes. B, Wall-eyed stereo images of the cryo-EM map superimposed on an atomic model of a H1.8-bound nucleosome (red). C, The atomic model of the H1.8-bound nucleosome superimposed on the “on-dyad” H1-bound nucleosome crystal structure (PDB ID: 5NL0). D, The cryo-EM map of the H1.8-bound nucleosome superimposed on the “off-dyad” H1 bound nucleosome cryo-EM map (emd ID: 2601). E, Reconstruction scheme of “closed” and “moderately open” linker DNA nucleosome maps. F, H2A C-tail density in the “closed” and “moderately open” linker DNA nucleosome maps. Black arrows, H2A C-tail. G, Graphical summary.
To assess the structural correlation between extra density for the H2A C-terminal tail in nucleosomes and closed linker DNAs (Fig. 4A, Movie S1), we generated 3D maps of interphase nucleosomes with closed and open linker DNAs at comparable resolutions (4.51 Å and 4.52 Å) (Fig. 6E-F). In the nucleosome structure with closed linker DNAs, the cryo-EM density corresponding to the H2A C-terminal tail was attached to the DNA, while the corresponding EM density was weaker and detached from the DNA in the nucleosome structure with open linker DNAs (Fig. 6F, black arrows), suggesting a role for the H2A C-terminal tail in influencing the linker DNA angle (Fig. 6G).
Structural variations of the NCP in chromosomes
3DVA analysis revealed major and minor NCP structural variations (Fig. 4B-C). The major structural variants are characterized by jagged NCP outlines with shifted positioning of the H3-H4 tetramer (Fig. 7A, Movie S2). To examine if the major NCP variants exist in chromosomal nucleosomes, we employed in silico mixing 3D classification for each 3DVA component using four reference 3D maps; one representing the canonical nucleosome structure (generated from composite particles of interphase and metaphase), and three representing the major nucleosome structural variants (picked from 3DVA motion frames of GUB-open NCP, which clearly exhibited major NCP rearrangements; see Methods and Fig S12A-B) (Fig. 7A, C). While more than 60% of NCPs were assigned to the major NCP structural variants in “freed nucleosomes”, only 30~40 % of the interphase and metaphase NCPs from oligo nucleosome fractions were assigned to these variants (Fig. 7C). The results suggest that formation of the major NCP structural variants is suppressed at least in clustered oligo nucleosomes.
Figure 7. Structural variation of chromosomal NCP.
A, Types of major NCP structural variations identified in 3DVA of GUB-open nucleosomes. B, Types of minor NCP structural variations identified in 3DVA of chromosomal nucleosomes. C, D, In silico mixing 3D classification of major NCP variants (C) and minor NCP variants (D). Numbers and populations of particles assigned to each class are shown in Table S3. E, Surface hydrophobicity of the chromosomal NCP structural variants. Hydrophobicity indexes were mapped on the EM maps of the representative motion frames of 3DVA component 1 of chromosomal NCP. F, Electrostatic potential of the chromosomal NCP structural variants. Surface electrostatic potentials for the atomic models for the representative motion frames of 3DVA component 3 of chromosomal NCPs are shown. The identical contour level was applied for all EM maps.
The minor NCP structural variants are characterized by rotation of H3 α1 and reorientation of the H4 N-terminal tail (Fig. 7B, magenta square), opening and closing of two H3 α-helices at the dyad (violet square), and opening and closing of the H2A-H2B acidic patch (blue square). To quantitatively analyze these minor NCP structural variants, we first performed in silico mixing 3D classification to isolate particles assigned to the canonical nucleosome class from major NCP variants (Fig S12C). We then conducted a second round of in silico mixing 3D classification against these selected particles using minor structural variants seen in chromosomal nucleosomes by 3DVA as references (Fig. 4C). This classification analysis revealed that minor NCP structural variants were prevalent in both interphase and metaphase chromosomes (Fig 7D). In the metaphase nucleosome, the amount of minor NCP structural variants was slightly increased compare to interphase (Fig 7D). In some of these variants, buried hydrophobic amino acid residues were exposed to the NCP surface (Fig. 7E), and the electrostatic potential at the H2A-H2B acidic patch was redistributed (Fig 7F), possibly affecting interaction between the NCP to other proteins and nucleosomes. These results suggest that minor NCP structural rearrangements can occur in chromosomes even in the context where the linker DNA opening and formation of major NCP structural variants are suppressed.
Discussion
Characteristics of nucleosome structures in interphase and metaphase chromosomes
Here we present sub-nanometer resolution structures of nucleosomes from interphase nuclei and metaphase chromosomes. On average, reconstructed nucleosome-like structures isolated from both interphase and metaphase chromosomes are identical to canonical left-handed octameric nucleosomes assembled on defined nucleosome positioning sequences (Fig. 2, 3) (Chua et al., 2016; Luger et al., 1997). Although it is still possible that minor populations of nucleosomes form alternative structures, we propose that our reconstructions represent the major forms in mitotic and interphase chromosomes for the following reasons. First, among the structures of five most abundant protein complexes (nucleosome, chromatosome, alpha2-macroguloblin, intelectin-2, actin filament) reconstructed by the TRSF pipeline, the only reconstructed nucleosome-like structure was the octameric left-handed nucleosome (Fig. 2, S3, S5). Second, around 60-90% of chromosomal DNA was solubilized after MNase digestion (Fig. S1B), suggesting that the solubilized nucleosomes analyzed by cryo-EM did not represent minor fractions of chromatin. Third, structures of “freed nucleosomes”, which were crosslinked after release from chromatin by MNase, were more heterogenous than those of chromosomal nucleosomes and even included the hexasome (Fig. 5), demonstrating that our procedures enabled preservation and detection of dynamic histone and DNA movements. Altogether, our analysis revealed that the major nucleosome structure in chromatin is the octameric left-handed nucleosome, and that global histone architectures are generally homogeneous.
One of the key structural features that we found in chromosomal nucleosomes is the prevalence of nucleosomes with closed linker DNA angles, which are comparable to those from compacted recombinant poly-nucleosome cryo-EM structure composed of “tetra-nucleosomal repeating units” (Fig S10C) (Song et al., 2014). It has been proposed that nucleosomes in chromatin form “nucleosome motifs”, comprised of specific oligo-nucleosome arrangements (Krietenstein and Rando, 2020; Ohno et al., 2019; Ricci et al., 2015; Risca et al., 2017). It is possible that observed nucleosomes with closed linker DNA angles reflect such nucleosome motifs.
3DVA showed that closed linker DNA angles are highly correlated with the visibility of the H2A C-terminal tail and H1.8 (Fig. 4A, 6A, 6F, Movie S1). H1.8 density was only visible on nucleosomes with the narrowest linker DNA angle, in agreement with the previous observation that H1 binding constricts the linker DNA angle (Bednar et al., 2017). Since the C-terminal tail of H2A is highly divergent among H2A variants and is a target of various modifications (Corujo and Buschbeck, 2018), it is tempting to speculate that linker DNA conformation is regulated by these H2A variants and modifications. Consistent with this idea, a recent cryo-EM study showed that the linker DNA is open in the nucleosomes reconstituted with the H2A variant H2A.Z.2.2, which has a short C-terminal tail (Zhou et al., 2020).
Local resolution analysis suggests that DNA segments at SHL −1 to +1 and SHL ± 4 are structurally rigid and uniformly positioned despite highly heterogeneous DNA sequences (Fig 3F-I). This is consistent with a single molecule force-measurement study showing that the dyad position (SHL 0) and ±40 bp from the dyad (SHL ± 4) is resistant to mechanical DNA unzipping (Hall et al., 2009), and RNA polymerase II halts around SHL ±5 and SHL ±1 on the nucleosome during transcription in vivo and in vitro (Kujirai et al., 2018; Weber et al., 2014). Since the H2A.X-F residues 15-44, interact with DNA at SHL ±4, are highly conserved among H2A variants across different species, uniform positioning of those two DNA segments may be a common feature of nucleosomes (Fig. S7G). The ATPase module of many nucleosome remodeling factors binds to SHL ±2.5 or ±6, suggesting that these factors are optimized to target the nucleosome regions that easily allow DNA repositioning (Ayala et al., 2018; Eustermann et al., 2018; Farnung et al., 2017; Han et al., 2020; He et al., 2020; Liu et al., 2017; Willhoft et al., 2018; Yan et al., 2019; Ye et al., 2019).
Implications for models of chromatin compaction during the cell cycle
Hirano hypothesized that condensin drives chromatin compaction and suppresses DNA unwinding by converting (−) crossings of linker DNAs to (+) crossings, which is inhibited in interphase by linker histones, and this inhibition released by the phosphorylation of linker histones in M phase (Hirano, 2014). However, our cryo-EM analysis did not reveal mitotic conversion of linker DNAs. The majority of interphase and metaphase nucleosomes contained closed (−) crossing linker DNAs (Fig. 5, S10), while histone H1.8 preferentially associates with the metaphase nucleosome at the on-dyad position and stabilizes (−) crossing (Fig. 5A, 6A). Our MS and cryo-EM results suggest that H1.8 in interphase may not strongly bind to nucleosome even though H1.8 accumulates in interphase nuclei (Maresca et al., 2005). This preferential association of H1.8 to mitotic nucleosomes may contribute to local chromatin compaction, since linker histone dependent chromatin compaction was reported in vitro and in vivo (Gibbs and Kriwacki, 2018; Gibson et al., 2019; Turner et al., 2018; Willcockson et al., 2021; Yusufova et al., 2021). Interestingly, our recent study suggests that H1.8 suppresses chromosome binding of condensins and DNA topoisomerase II to make chromosomes shorter and limit chromosome individualization (Choppakatla et al., 2021). Thus, stable H1.8 binding to the nucleosome is likely to be enhanced during mitosis not only for local chromatin compaction but also for a long-range DNA folding and topological organization.
Since most histone chaperones and nucleosome remodelers dissociate from mitotic chromatin (Fig S1F) (Funabiki et al., 2017; Jenness et al., 2018), one might speculate that more NCP structural variants exist in interphase than in metaphase. However, our data suggest that the formation of major NCP structural variants are suppressed in both interphase and metaphase, and the population of minor NCP structural variants was even slightly higher in metaphase than in interphase (Fig. 7). In the minor NCP structural variants, many interspaces were newly generated on the NCP surface (Fig 7E, Movie S3), mimicking a situation where nucleosome binding of CENP-C and Swi6 increased solvent accessibility of buried histone residues (Falk et al., 2015; Sanulli et al., 2019). Changes in the hydrophobicity and electrostatic potential on the nucleosome surface would potentially affect specificity and affinity of nucleosome-binding targets (Fig. 7E-F) (Skrajna et al., 2020). Exposure of hydrophobic surfaces may enhance weak, transient multivalent nucleosome-nucleosome interactions (Sanulli et al., 2019). Changes in charge distribution at the acidic patch may affect binding targets, including the H4 N-terminal tail, which is also important for nucleosome-nucleosome interactions (Chen et al., 2017). Thus, the slight population-level changes of these minor structural variants during the cell cycle may be functionally related to global changes in nucleosome-interacting proteins and the chromatin compaction mechanism. Future studies are needed to understand the mechanistic basis and functional significance of these structural variations of the nucleosome.
Limitations of the Study
We note four limitations in this study.
First, preservation of nucleosome structure variations, especially linker DNA angles, is likely affected by fixation conditions. Indeed, linker DNA conformations of the recombinant nucleosomes containing GUB DNA significantly differ between two sample preparation protocols (Fig S8B, S8K). In our standard protocol, in which nucleosomes were crosslinked by formaldehyde and frozen with optimized cryoprotectant buffer, a majority of nucleosomes had closed linker DNA conformations. In contrast, the proportion of open linker DNA conformations increased when samples were crosslinked by GraFix without magnesium and frozen on the cryo-EM grid without the cryoprotectant buffer (GUB-open nucleosomes). Even with our standard protocol, notable structural differences can be seen between lot 1 and lot 2; apparent enrichment of H4 outward orientation (Fig S9C) and nucleosome minor structural variants (Fig 7D) in metaphase seen in lot 1 was less obvious in lot 2. We noticed that spindles and nuclei clustered more frequently in lot 2 than in lot 1 (Fig S1A, S8C), potentially leading to reduced penetration of crosslinkers to nucleosomes in lot 2. In addition, MNase lots were different between these biological replicates, possibly causing subtle differences in linker DNA lengths (Fig. 1D, S8E), which might have affected H1.8 stabilization on the structure.
Second, our structural preservation may be affected by the cryo-EM grid freezing process. For example, protein adsorption to the air-water interface can cause sample denaturation (D’Imprima et al., 2019), leading to grid-to-grid variability in structure preservation. Although cryoprotectant buffer was optimized to prevent sample denaturation (Fig S2B), we cannot rule out a possibility that fragile nucleosome structural variants were selectively denatured or altered during the freezing step. However, to minimize this issue, we excluded problematic grids based on signatures such as poor reconstruction of actin filaments and prevalence of naked DNA fragments (Fig. S5G).
Third, we were able to reconstruct high resolution (< 4 Å) nucleosome structures only with the oligo-nucleosome fraction, raising the possibility that some features (i.e. DNA structural stabilization at SHL −1 to +1 and SHL ± 4; suppression of major NCP structure variant formation; two distinct orientations of the H4 N-terminal tail; association of H2A C-terminal tail to DNA) are specific to subpopulations of chromatin with clustered nucleosomes.
Fourth, we could not determine the structures of less abundant or weakly associated nucleosome-protein complexes. For example, while substantial amount of H1.8 were also detected interphase nucleosome fractions (Fig 1H: roughly 30~50% of metaphase), we could not extract the H1.8 bound nucleosome structure in cryo-EM analysis (Fig. 6). It is possible that H1.8 in interphase associates with nucleosomes weakly or at various positions, making it difficult to reconstruct the H1.8 bound nucleosome structure.
Despite these limitations, our analysis revealed several structural features of nucleosomes in chromosomes in a reproducible manner. Nucleosome structures containing diverse genomic DNA sequences are remarkably similar between interphase and metaphase; DNA linkers tend to exist in the closed conformation, yet minor NCP structural variations are commonly found. Future improvements to this strategy may capture high-resolution structures of nucleosome-nucleosome interaction and other protein-DNA complexes while they are functioning on chromosomes.
STAR Methods
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to the Lead Contact, Hironori Funabiki (funabih@rockefeller.edu)
Materials Availability
The plasmid (pUC18-GUB_176x4) generated in this study is available from the lead contact upon request.
Data and Code Availability
Cryo-EM raw data are available in the EMPIAR. EM maps are available in the EMDR. Atomic models are available in the PDB. IDs for each data are listed in star method table, Table 1 and Table 2. This paper does not report any original code. Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.
Table 1.
Data collection, model refinement and validation for simultaneous TRSF reconstruction of cryo-EM structures of multiple protein complexes. Related to Figure 2
| Micrograph name | Interphase_oligo_lot1 | Metaphase_oligo_lot 1 | Interphase_oligo _lot2 |
metaphase_oligo _lot2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deta collection and prosessing | ||||||||||
| Microscope | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | |||||
| Magnification | 28,000 | 28,000 | 28,000 | 28,000 | 28,000 | |||||
| Voltage (kV) | 200 | 200 | 200 | 200 | 200 | |||||
| Camera | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | |||||
| Electron exposure (e−/Å) | 33.1 | 38.3 | 35.3 | 34.2 | 34.2 | |||||
| Defocus range (μm) | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | |||||
| Pixel size (Å) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | |||||
| Micrographs | 1,325 | 2,676 | 1,156 | 1,044 | ||||||
| EMPIAR ID of raw micrographs | EMPIAR-10692 | EMPIAR-10691 | EMPIAR-10747 | EMPIAR-10746 | ||||||
| EM 3D maps | Octameric nucleosome |
Intelectin- 2 |
F-actin | alpha2- macroglobulin |
Octameric nucleosome |
Intelectin- 2 |
F-actin | alpha2- macroglobulin |
Octameric nucleosome |
Octameric nucleosome |
| Initial particle images (no.) | 812,976 | 1,317,710 | 320,524 | 384,740 | ||||||
| High resolution group particles(no.) | 66,426 | 166,673 | 35,700 | 55,801 | ||||||
| Low resolution group particles(no.) | 746,550 | 1,151,037 | 284,824 | 328,939 | ||||||
| particle for 3D clasification (no.) | 812,976 | 1,317,710 | 320,524 | 384,740 | ||||||
| Particle images (no.) | 62,014 | 89,728 | 10,922 | 9,721 | 116,723 | 150,300 | 38,321 | 49,363 | 40,640 | 40,822 |
| Symmetry imposed | C1 | C3 | C1 | C1 | C1 | C1 | C1 | D2 | C1 | C1 |
| Map resolution (FSC=0.143) | 4.2 | 4.5 | 7.6 | 8.07 | 4.2 | 4.7 | 4.5 | 5.5 | 4.42 | 4.32 |
| EM data resource ID | EMD-22793 | - | - | EMD-22794 | EMD-22795 | - | - | EMD-22796 | EMD-23820 | EMD-23822 |
Table 2.
Data collection, model refinement and validation for averaged nucleosome structure analysis. Related to Figure 3, 6
| Micrograph name | Interphase mono lot 1 |
Interphase di lot 1 |
Interphase oligo lot 1 |
Metaphase mono lot 1 |
Metaphase di lot 1 |
Metaphase oligo lot 1 |
Interphase, 'Freed' (Crosslinked after MNase) lot 2 |
Interphase oligo lot 2 |
Metaphase oligo lot 2 |
GUB-closed nucleosome |
GUB-open nucleosome |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deta collection and prosessing | ||||||||||||||
| Microscope | FEI Titan Krios | FEI Titan Krios | FEI Talos Arctica | FEI Titan Krios | FEI Titan Krios | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | |||
| Magnification | 25,000 | 20,000 | 28,000 | 25,000 | 20,000 | 28,000 | 28,000 | 28,000 | 28,000 | 28,000 | 28,000 | |||
| Voltage (kV) | 300 | 300 | 200 | 300 | 300 | 200 | 200 | 200 | 200 | 200 | 200 | |||
| Camera | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | |||
| Electron exposure (e−/Å) | 45.0 | 55.5 | 33.1 | 44.7 | 54.0 | 38.3 | 35.3 | 37.3 | 31.1 | 34.2 | 34.2 | |||
| Defocus range (μm) | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | |||
| Pixel size (Å) | 1.33 | 1.6 | 1.5 | 1.33 | 1.6 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | |||
| Micrographs | 1,622 | 1,270 | 1,325 | 1,637 | 1,347 | 2,676 | 2,049 | 1,375 | 1,044 | |||||
| EMPIAR ID of raw micrographs | EMPIAR-10742 | EMPIAR-10744 | EMPIAR-10692 | EMPIAR-10743 | EMPIAR-10745 | EMPIAR-10691 | EMPIAR-10750 | EMPIAR-10747 | EMPIAR-10746 | EMPIAR-10749 | EMPIAR-10748 | |||
| EM 3D map. Name | Nucleosome | Nucleosome | Nucleosome | Nucleosome | Nucleosome | Nucleosome | Nucleosome + H1.8 |
"Free" nucleosome |
Nucleosome | Nucleosome | Nucleosome + H1.8 |
GUB-closed nucleosome |
GUB-open nucleosome |
|
| Initial particle images (no.) | 147,921 | 213,608 | 571,558 | 116,669 | 167,734 | 898,553 | 259,060 | 178,949 | 194,837 | 203,052 | 199,125 | |||
| Particles for nucleosome ab-initio reconstruction (no.) | 13,434 | 21,650 | 85,994 | 11,325 | 9,515 | 154,208 | 25,123 | 61,931 | 50,002 | 86,720 | 75,171 | |||
| Particles for 'decoy' ab-initio reconstruction (no.) | 134,487 | 100,946 | 485,564 | 105,344 | 102,722 | 744,345 | 133,700 | 117,018 | 144,835 | 116,332 | 123,954 | |||
| particle for 3D clasification (no.) | 147,921 | 122,596 | 571,558 | 116,669 | 112,237 | 898,553 | 158,823 | 178,949 | 194,837 | 203,052 | 199,125 | |||
| Particle images (no.) | 13,798 | 15,653 | 89,191 | 13,350 | 6,027 | 155,413 | 27,383 | 20,835 | 52,163 | 52,941 | 6,399 | 76,391 | 62,823 | |
| Symmetry imposed | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | |
| Map resolution (FSC=0.143) | 5.12 | 4.74 | 3.38 | 5.64 | 8.1 | 3.5 | 4.42 | 5.44 | 3.54 | 3.77 | 5.6 | 3.77 | 4.52 | |
| CTF rifinement | ✓ (Relion 3.1) | ✓
(Relion 3.1) |
✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | - | ✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | - | ✓ (Relion 3.1) | ✓ (Relion 3.1) | |
| Particle polishing | ✓ (Relion 3.1) | ✓
(Relion 3.1) |
✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | - | ✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | - | ✓ (Relion 3.1) | ✓ (Relion 3.1) | |
| Postprocess Pixel size | 1.3 | 1.66 | 1.47 | 1.3 | 1.66 | 1.47 | - | 1.47 | 1.47 | 1.47 | - | 1.47 | 1.47 | |
| EM data resource ID | EMD-22797 | EMD-22798 | EMD-22790 | EMD-22800 | EMD-22801 | EMD-22791 | EMD-22792 | EMD-23826 | EMD-23819 | EMD-23821 | - | EMD-23823 | EMD-23824 | |
| Atomic coordinate name | - | - | Nucleosome | - | - | Nucleosome | Nucleosome + H1.8 | - | - | - | - | - | - | - |
| Pixel size | 1.47 | 1.47 | 1.47 | |||||||||||
| Model resolution (Å) | 3.38 | 3.5 | 4.4 | |||||||||||
| Initial model used (code) | 6DZT, 5NL0 | 6DZT, 5NL0 | 5NL0 | |||||||||||
| Map sharpening | RELION 3.1 (Postprocess) | RELION 3.1 (Postprocess) | CryoSPARC, bsoft (blocres/blocafilt) | |||||||||||
| Map sharpening B factor (Å2) | −60.3386 | −30.7959 | - | |||||||||||
| Model composition | ||||||||||||||
| Non-hydrogen atoms | 12,169 | 12,408 | 13,710 | |||||||||||
| Protein residues | 755 | 760 | 842 | |||||||||||
| Nucleotide | 302 | 312 | 344 | |||||||||||
| Model vs. Data | ||||||||||||||
| CC (mask) | 0.81 | 0.8 | 0.73 | |||||||||||
| CC (box) | 0.74 | 0.73 | 0.83 | |||||||||||
| CC (peak) | 0.7 | 0.69 | 0.72 | |||||||||||
| CC (volume) | 0.79 | 0.78 | 0.76 | |||||||||||
| R.m.s. deviations | ||||||||||||||
| Bond lengths (Å) | 0.008 | 0.008 | 0.008 | |||||||||||
| BoBond angles (°) | 0.691 | 0.753 | 0.867 | |||||||||||
| Validation | ||||||||||||||
| MolProbity score | 2.42 | 2.56 | 2.96 | |||||||||||
| Clashscore | 6.86 | 9.71 | 30.6 | |||||||||||
| Ramachandran plot | ||||||||||||||
| Favored (%) | 94.99 | 95.3 | 93.93 | |||||||||||
| Allowed (%) | 5.01 | 4.57 | 6.07 | |||||||||||
| Disallowed (%) | 0 | 0.13 | 0 | |||||||||||
| Protein data bank ID | 7KBD | 7KBE | 7KBF | |||||||||||
Experimental Model and Subject Details
Xenopus laevis sperm and eggs were collected by the method described previously (Murray, 1991). Sperm were collected from the matured (7.5-9 cm) male Xenopus laevis (Nasco, LM00715). Eggs were collected from the matured (9+ cm) female Xenopus laevis (Nasco, LM00535). Frogs were housed in the water tanks with temperature controlled at 16-20 °C at the Rockefeller University Comparative Bioscience Center. Prior to ovulation, 11 frogs were transferred at the certified satellite animal facility with temperature control in the Funabiki lab. Animal husbandry and protocol (#20031) approved by the Institutional Animal Care and Use Committee at the Rockefeller University were followed.
Method details
Xenopus egg extracts and chromatin formation
Cytostatic factor (CSF) metaphase-arrested Xenopus laevis egg extracts were prepared with the method described previously (Murray, 1991). To prevent spontaneous cycling of egg extracts, 0.1 mg/ml cycloheximide was added to the CSF extract. For interphase chromosome preparation, Xenopus laevis sperm nuclei (final concentration 2000/μl) were added to 8 ml of CSF extracts, which were incubated for 90 min at 20 °C after adding 0.3 mM CaCl2, which releases CSF extracts into interphase. To monitor spindle assembly, Alexa594-labeled-bovine brain tubulin (final concentration 19 nM) was added to the extract during the incubation. For metaphase sperm chromosome preparation, cyclin B Δ90 (final concentration 24 μg/ml) and 4 ml fresh CSF extract was added to 8 ml of the extract containing interphase sperm nuclei prepared with the method described above. The extracts were incubated for 60 min at 20 °C, during which each tube was gently mixed every 10 min.
Nucleosome isolation from Xenopus egg extracts chromosomes
To crosslink the Xenopus egg extracts chromosomes, nine times volume of ice-cold buffer XL (80 mM PIPES-KOH [pH 6.8], 15 mM NaCl, 60 mM KCl, 30 % glycerol, 1 mM EGTA, 1 mM MgCl2, 10 mM β-glycerophosphate, 10 mM sodium butyrate, 2.67 % formaldehyde) was added to the interphase or metaphase extract with chromosomes, which was further incubated for 60 min on ice. These fixed samples were layered on 3 ml of buffer SC (80 mM HEPES-KOH [pH 7.4], 15 mM NaCl, 60 mM KCl, 1.17 M sucrose, 50 mM glycine, 0.15 mM spermidine, 0.5 mM spermine, 1.25x cOmplete EDTA-free Protease Inhibitor Cocktail (Roche), 10 mM β-glycerophosphate, 10 mM sodium butyrate, 1 mM EGTA, 1 mM MgCl2) in 14 ml centrifuge tubes (Falcon, #352059) and spun at 3,300 (2,647 rcf) rpm at 4 °C for 40 min using JS 5.3 rotor in Avanti J-26S (Beckman Coulter). Chromatin was collected from the bottom of the centrifuge tube. Collected chromatin was further spun through buffer SC as described above. Chromatin was collected from the bottom of the centrifuge tube, and then this step was repeated once again. Only at the 1st round of centrifugation over sucrose cushion, chromatin trapped at the boundary between the extract and buffer SC was also collected and applied for 2nd round centrifugation over sucrose cushion. The isolated interphase nuclei and metaphase chromosomes were associated with white aggregations and spindle microtubules, respectively. To remove them, chromosomes were gently pipetted with a 2 ml pipette. These chromosomes were pelleted by centrifugation at 5,492 rpm (2,500 rcf) using SX241.5 rotor in Allegron X-30R (Beckman Coulter). The chromosome pellets were resuspended with 200 μL of buffer SC. To digest chromatin, 1.5 U/μL (0.1 U/μL for the egg extract lot 2) of MNase (Worthington Biochemical Corporation) and CaCl2 were added to 7.4 mM, and the mixture was incubated at 4 °C for 6 h. MNase reaction was stopped by adding 900 μL MNase stop buffer (15 mM HEPES-KOH [pH 7.4], 150 mM KCl, 5 mM EGTA, 10 mM β-glycerophosphate, 10 mM sodium butyrate, 5 mM DTT). The soluble fractions released by MNase were isolated by taking supernatants after centrifugation at 13,894 rpm (16,000 rcf) at 4 °C for 30 min using SX241.5 rotor in Allegron X-30R (Beckman Coulter). The supernatants were collected and layered onto the 10-22 % linear sucrose gradient solution with buffer SG (15 mM HEPES-KOH [pH 7.4], 50 mM KCl, 10-22 % sucrose, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 10 μg/ml chymostatin, 10 mM sodium butyrate, 10 mM β-glycerophosphate, 1 mM EGTA) and spun at 32000 rpm (max 124,436 rcf) and 4 °C for 13 h using SW55Ti rotor in Optima L80 (Beckman Coulter). The samples were fractionated from the top of the sucrose gradient.
To prepare “freed nucleosome”, the interphase nucleosomes that was crosslinked after MNase, chromosome-containig Xenopus egg extracts were diluted with the buffer XL without formaldehyde and incubated for 60 min on ice. The soluble chromatin fraction after stopping the MNase reaction using the same method as described above was concentrated to 60 μL using Amicon Ultra centrifugal filter 100K (Millipore Sigma; 100K filter was chosen to avoid concentration of MNase). The concentrated nucleosomes were crosslinked by adding 540 μL buffer XL and incubated on ice for 1 h. The crosslinking reaction was stopped by diluted with 1.8 ml buffer Q (15 mM HEPES-KOH [pH 7.4], 15 mM NaCl, 60 mM KCl, 1 mM EGTA, 1 mM MgCl2, 10 mM β-glycerophosphate, 10 mM sodium butyrate, 500 mM glycine). Quenched sample was layered onto the 10-22 % linear sucrose gradient solution with buffer SG and spun at 27,000 rpm (max 129,657 rcf) at 4 °C for 20 h using SW40Ti rotor in Optima L80 (Beckman Coulter). The centrifuged samples were fractionated from the top of the sucrose gradient.
Native and SDS-PAGE analysis of nucleosomes
For the native PAGE for nucleosome (Fig 1C, S8D, S8G), nucleosome fractions containing 100 ng DNA were to load onto a 0.5x TBE 6 % native PAGE gel. For the native PAGE for nucleosomal DNA (Fig 1D, S8E, S8H), nucleosome fractions containing 100 ng DNA were mixed with 1 μL of 10 mg/ml RNaseA (Thermo Scientific) and incubated at 55 °C for 30 min. To deproteinize and reverse-crosslink DNA, RNaseA treated samples were then mixed with 1 μL of 19 mg/ml Proteinase K solution (Roche) and incubated at 55 °C for overnight. Samples were loaded to 0.5x TBE 6 % native PAGE. Native PAGE gels were stained by SYBR-safe or SYTO-60 to detect DNA. For SDS-PAGE analysis (Figure 1E, S8F), nucleosome fractions containing 200 ng DNA were loaded to a 4-20 % gradient gel.
Western blotting of native PAGE to detect H1.8-bound nucleosome
Fifteen μL of the interphase-mono and metaphase-mono fractions just after sucrose gradient was applied onto 6 % native PAGE with x0.5 TBE, and DNA was stained by SYBR-safe (Invitrogen). After acquiring image, the native PAGE gel was soaked in transfer buffer (25 mM Tris, 192 mM glycine and 20% methanol) and blotted onto the nitrocellulose membrane (GE) using TE42 Tank Blotting Units (Hoefer) at 15 V, 4 °C for 4 h. Histone H4 was detected by 2.4 μg/ml of mouse monoclonal antibody (CMA400) provided by H. Kimura (Hayashi-Takanaka et al., 2015). H1.8 was detected by 1μg/ml of rabbit polyclonal antibody against full-length Xenopus laevis H1.8 (Jenness et al., 2018). As secondary antibodies, IRDye 680LT goat anti-rabbit IgG (Li-Cor 926-68021; 1:10,000) and IR Dye 800CW goat anti-mouse IgG (Li-Cor 926-32210; 1:15,000) were used. The images were taken with Odyssey Infrared Imaging System (Li-Cor).
Nucleosome dialysis and concertation for Cryo-EM and MS
Nucleosome contained sucrose gradient fractions were collected and dialyzed against 600 ml dialysis buffer EM (10 mM HEPES-KOH [pH 7.4] 30 mM KCl, 1 mM EGTA, 0.3 μg/ml leupeptin, 0.3 μg/ml pepstatin, 0.3 μg/ml chymostatin, 1 mM sodium butyrate, 1 mM β-glycerophosphate) using Tube-o-dialyzer 15 kDa (G-Biosciences) at 4 °C. The samples were further dialyzed twice against the fresh dialysis buffer. The samples were concentrated using Amicon Ultra centrifugal filters 10K (Millipore Sigma). Absorbance at wavelength 260 nm were 2.705, 1.342, 3.756, 2.564, 2.06, 5.244, 1.389, 2.479, and 2.915 for interphase mono nucleosome (lot 1), interphase di nucleosome (lot 1), interphase oligo nucleosome (lot 1), metaphase mono nucleosome, metaphase di nucleosome (lot 1), metaphase oligo nucleosome (lot 1), interphase oligo nucleosome (lot 2), metaphase oligo nucleosome (lot 2), and freed nucleosome (lot 2) respectively. The isolated nucleosomes were stored at 4 °C.
Mass spectrometry
The samples containing 800 ng DNA were incubated at 95 °C for 30 min to reverse crosslink. DNA amounts were estimated by the 260 nm absorbance. Samples were applied to SDS-PAGE (4-20% gradient gel) (Bio-Rad). The Gel was stained by Coomassie Brilliant Blue G-250 (Thermo Fisher). Gel bands were excised by scalpel and cut into pieces of approximately 2 mm x 2 mm. Destaining, in-gel digestion and extraction was performed as described (Shevchenko et al., 2007). Briefly, bands were excised with scalpel and de-stained using 30 % acetonitrile, 100 mM ammonium bicarbonate in water. Gel pieces were dehydrated using 100 % acetonitrile. Disulfide bonds were reduced with dithiothreitol, and cysteines were alkylated using iodoacetamide. Proteins were digested by hydrating the gel pieces in a solution of sequencing grade trypsin and endopeptidase LysC in 50 mM ammonium bicarbonate. Digestion proceeded overnight at 37 °C. Peptides were extracted into 70 % acetonitrile, 5 % formic acid for three iterations. Extracted peptides were purified using in-house constructed micropurification C18 tips. Purified peptides were analyzed by LC-MS/MS using a Dionex3000 HPLC equipped with a NCS3500RS nano- and microflow pump coupled to a Q-Exactive HF mass spectrometer (Thermo Scientific). Peptides were separated by reversed phase chromatography (solvent A: 0.1% formic acid in water, solvent B: 80% acetonitrile, 0.1% formic acid in water) across a 70-min gradient. Spectra were recorded in positive ion data-dependent acquisition mode, fragmenting the 20 most abundant ions within each duty cycle. MS1 spectra were recorded with a resolution of 60 k and AGC target of 3e6. MS2 spectra were recorded with a resolution of 30 k and ACC target of 2e5. Spectra were queried against a Xenopus laevis database (Wühr et al., 2014) (3413 sequences) concatenated with common contaminants using MASCOT through Proteome Discoverer v.1.4 (Thermo Scientific). Oxidation of M and acetylation of protein N-termini were set as variable modifications and carbamidomethylation of C was set as static modification. A false discovery rate of 1% was applied. A large number of identified peptides map to multiple highly similar proteins. iBAQ values of these peaks were summed before drawing the figures (Schwanhüusser et al., 2011).
Western blotting
Nucleosome fractions containing 200 ng DNA were applied for SDS-PAGE with 4-20 % gradient SDS-PAGE gel (Bio-rad). Proteins were transferred to the nitrocellulose membrane (GE) from the SDS-PAGE gel using TE42 Tank Blotting Units (Hoefer) at 15 V, 4 °C for 4 h. As primally antibodies, 1 μg/ml of Mouse monoclonal H3T3ph antibody 16B2 (Kelly et al., 2010) and 1 μg/ml of mouse monoclonal PCNA antibody (Santa Cruz SC-56) were used. For H4 and H1.8 detection, antibodies described above were used as primally antibodies. As secondary antibodies, IRDye 680LT goat anti-rabbit IgG (Li-Cor 926-68021; 1:10,000) and IR Dye 800CW goat anti-mouse IgG (Li-Cor 926-32210; 1:15,000) were used. The images were taken with Odyssey Infrared Imaging System (Li-Cor).
Histone preparation for recombinant nucleosome
All histones were purified with the method described previously (Zierhut et al., 2014). Briefly, bacterial expressed X. laevis H2A, H2B, H3.2, and H4 were purified from inclusion bodies. Histidine-tagged histones (H2A, H3.2, and H4) or untagged H2B expressed in bacteria were resolubilized from the inclusion bodies by incubation with the 6 M guanidine HCl. For Histidine-tagged histones, the resolubilized histidine-tagged histones were purified with Ni affinity chromatography using Ni-NTA beads (Qiagen). For untagged H2B, resolubilized histones were used for H2A-H2B dimer formation without further purification. To reconstitute H3–H4 tetramer and H2A–H2B dimer, denatured histones were mixed at a concentration of ~45 μM and dialyzed to refold histones with removing the guanidine. Histidine-tags were removed by TEV protease treatment, and H3–H4 tetramer and H2A–H2B dimer were isolated with gel-filtration chromatography on a HiLoad 16/60 Superdex 75 pg column (GE Healthcare). Fractions containing (H3–H4 tetramer and H2A–H2B dimer) were concentrated and stored at −80 °C.
DNA preparation for recombinant nucleosome
Four tandem 176 bp GUB DNA sequences (An et al., 1998) were cloned into pUC18 vector (pUC18-GUB_176x4). The E.coli DH5a that possessing the plasmid was cultured in 1.5x TBG-M9-YE medium with 25 μg/ml carbenicillin at 37 °C for overnight. The plasmid was purified using plasmid plus giga kits (Qiagen) following standard protocol. The 176 bp GUB DNA was cut out from the purified plasmid with EcoRV (New England Biolabs). The plasmid backbone was removed by polyethylene glycol precipitation using PEG-6000. The 176 bp GUB DNA was recovered from the supernatant of the polyethylene glycol precipitation fraction and further purified with gel-filtration chromatography on a HiLoad 16/60 Superdex 75 pg column (GE Healthcare) using TE buffer (10 mM Tris-HCl [pH 7.5] and 0.1 mM EDTA). DNA containing fractions were collected and stored at −20 °C.
The DNA sequence of the 176 bp GUB DNA is the following. 5’- ATCCC TCTAG ACGGA GGACA GTCCT CCGGT TACCT TCGAA CCACG TGGCC GTCTA GATGC TGACT CATTG TCGAC ACGCG TAGAT CTGCT AGCAT CGATC CATGG ACTAG TCTCG AGTTT AAAGA TATCC AGCTG CCCGG GAGGC CTTCG CGAAA TATTG GTACC CCATG GAAGA T-3’
Recombinant nucleosome reconstitution
Approximately 1.6 μM of purified 176 bp GUB DNA was mixed with 3.2 μM H3-H4 reconstituted histone dimers and 3.5 μM H2A-H2B reconstituted dimers with the salt dialysis method described previously (Zierhut et al., 2014). The sample mixture was transferred into a dialysis cassette and placed into a high salt buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 2M NaCl, 5 mM β-mercaptoethanol, and 0.01% Triton X-100). Using a peristaltic pump, the high salt buffer was exchanged with low salt buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 50 mM NaCl, 5 mM β-mercaptoethanol, 0.01% Triton X-100) at roughly 2 ml/min for overnight at 4 °C. In preparation for cryo-EM image collection, the dialysis cassette containing the sample was then placed in a buffer containing 10 mM HEPES-HCl (pH 7.4) and 30 mM KCl, and dialyzed for 48 h at 4 °C. The sample was then incubated at 55 °C for 2 h and centrifuged at 4 °C, 5000 rpm for 5 min in 5415D centrifuge (Eppendorf) to remove aggregates.
Recombinant nucleosome with open linker DNA conformation (GUB-open) purification
The reconstituted nucleosomes were purified and fixed using the GraFix method. Briefly, a sucrose gradient was created using 2.3 ml of a 20 % sucrose solution containing 15 mM HEPES [pH 7.4] and 4 % paraformaldehyde aqueous solution (EM Grade) (Electron Microscopy Sciences) and 2 ml of a 10% sucrose solution containing 15 mM HEPES [pH 7.4]. Reconstituted nucleosome samples were overlaid onto the sucrose gradient and spun at 32000 rpm for 20 h at 4 °C using SW55Ti rotor in Optima L80 (Beckman Coulter). Fractions containing the fixed samples were collected and dialyzed in 15 mM HEPES (pH 7.4) for 48 h at 4 °C with one fresh exchange of buffer halfway through, using a Tube-O-DIALYZER (50 kDa) (G-Biosciences). Samples were concentrated using an Amicon Ultra 100K centrifugation filter (Millipore) to final concentration of 247 ng/μl DNA.
Recombinant nucleosomes with closed linker DNA conformation (GUB-closed) purification
Reconstituted nucleosome with GUB DNA was dialyzed against the XB-CSF buffer (10 mM HEPES-KOH [pH 7.4], 100 mM KCl, 1 mM EGTA, 1 mM MgCl2, 0.1 mM CaCl2, 1.7 % sucrose) and concentrated to 60 μL using Amicon Ultra centrifugal filters 100K (Millipore Sigma). The concentrated nucleosome was crosslinked by diluted with 540 μL buffer XL and incubated on ice for 1 h. The crosslinking reaction was stopped by diluting with 1.8 ml buffer Q. Quenched sample was layered onto the 10-22 % linear sucrose gradient solution with buffer SG and spun at 27000 rpm (max 129,657 rcf) and 4 °C for 20 h using SW40Ti rotor in Optima L80 (Beckman Coulter). The centrifuged samples were fractionated from the top of the sucrose gradient. Nucleosome-containing fractions were collected and dialyzed against 600 ml dialysis buffer EM using Tube-o-dialyzer 15 kDa (G-Biosciences) at 4 °C. The samples were concentrated using Amicon Ultra centrifugal filters 10K (Millipore Sigma) to a final concentration of 448 ng/μl DNA.
Cryoprotectant buffer optimization
Unlike previously reported recombinant nucleosome with 601 DNA (Chua et al., 2016), nucleosomes from Xenopus egg extract chromosomes were thoroughly denatured during cryo-EM grid freezing without cryoprotectant (Fig S2B left). In those girds, many free DNAs that may be released from broken nucleosomes are observed. This suggest that chromosomal nucleosomes with native DNA sequence are fragile than nucleosome with strong positioning sequence. To reduce the structural denaturization during cryo-EM grid freezing, cryoprotectant buffer were screened and optimized to the buffer containing 1% trehalose and 0.1% 1,6,-hexanediol. Under this condition, although a small amount of free DNAs are still observed, most of nucleosome-like structure remained intact (Figure S2B right).
Cryo-EM grid preparations and data collection
For the nucleosomes purified from Xenopus egg extracts and GUB-closed nucleosomes, 2 μl of sample was mixed with 0.5 μl of cryoprotectant buffer (10 mM HEPES-KOH [pH 7.4], 30 mM KCl, 1 mM EGTA, 0.3 μg/ml Leupeptin, 0.3 μg/ml Pepstatin, 0.3 μg/ml Chymostatin, 1 mM Sodium Butyrate, 1 mM β-glycerophosphate, 5% trehalose, 0.5% 1,6,-hexanediol) just before loading the sample on the cryo-EM grid. 2.5 μl of the sample was frozen onto a glow discharged Quantifoil Gold R 1.2/1.3 300 mesh grid (Quantifoil). For GUB-open nucleosome, 2.5 μl of the samples was frozen onto a glow discharged Quantifoil Gold R 1.2/1.3 300 mesh grid (Quantifoil) without adding cryoprotectant buffer. The samples were frozen under 100% humidity, 20 sec incubation, and 5 sec blotting time using the Vitrobot Mark IV (FEI). Grids were imaged on a Talos Arctica (FEI) installed with a K2 Camera (GATAN) and a field emission gun operating at 200 kV or Titan Krios (FEI) installed with a K2 Camera (GATAN) and a 300 kV field emission gun. All data were collected as 50 frames/movie in super resolution mode. The conditions for the data collection were listed in Table 1 and 2.
TRSF cryo-EM structure reconstruction
Briefly, particles were picked from micrographs (Fig. S2A) using the CryoSPARC blob picker and were then split into two groups based on the resolution and effective classes assigned (ECA) value of the 2D classification (Fig. 2A: 2D classification) (Punjani et al., 2017). Multiple 3D maps were generated from both groups by CryoSPARC ab initio reconstruction (Punjani et al., 2017), resulting in two types of maps: 3D maps derived from particles with high-resolution features, and “decoy” maps derived from the particles that were noisy, low-resolution, or ambiguous. All picked particles and 3D maps were then used for multireference 3D classification by CryoSPARC heterogenous refinement (Fig 2A: decoy classification, Fig S3A-B, S3E-F). Compared to other workflows, our pipeline uniquely combines three main strategies that allowed for unbiased assessment of conformational diversity and in silico purification of our extremely heterogeneous sample.
First, we avoided using template-based particle picking, which can miss particles that do not resemble the template. Instead, particles were picked by CryoSPARC blob picker, which does not require a template map (Fig 2A: blob pick). 2D class averaging revealed diverse particle views and protein heterogeneity, confirming the success of this particle picking approach (Fig 2A: 2D classification).
Second, when reference maps for 3D classification were generated, we avoided introducing a human selection bias. In a common classification pipeline, one might subjectively select 2D classes that are similar to the expected structure of a target protein, and then generate a 3D initial map using these selected 2D classes (Bell et al., 2016; Vargas et al., 2014). Alternatively, one might use previously-determined cryo-EM or crystal structures as references for 3D classification (Bell et al., 2016; Grigorieff, 2016; de la Rosa-Trevín et al., 2016; Punjani et al., 2017; Scheres, 2012). While these common methods may work for structural analysis of homogenous samples, they may exclude un-recognizable structures in heterogenous samples. In contrast, employing CryoSPARC’s ab initio reconstruction (Punjani et al., 2017), our pipeline successfully generated multiple independent 3D structures simultaneously from heterogeneous particles that were selected based on the resolution and ECA of the 2D classes, rather than recognizable visual features. This allowed us to remove biases commonly generated by manual particle selection and use of externally derived 3D references.
Finally, both 3D reference generation and 3D classification were performed using all picked particles (Fig 2A: decoy classification). Common pipelines use multiple copies of a single 3D map for 3D classification (Lyumkis et al., 2013; Scheres et al., 2007). While these pipelines are suited for classifying minor structural variations of the target protein, they may miss rare or radically altered structures. Instead, we used twenty-four distinct 3D references, all of which were data-derived and thus encompassed the innate heterogeneity of the sample. These include ten “target” classes to resolve heterogeneous high-resolution particles and fourteen “decoy” classes to filter out noise and low-quality protein particles (Fig 2A: decoy classification, Fig S3A-B, S3E-F). This decoy classification approach is reminiscent of “random-phase” 3D classification (Gong et al., 2016) and multireference 3D classification with structurally dissimilar cryo-EM maps as decoys (Nguyen et al., 2019). However, using data-derived decoys instead is useful to tailor 3D references to the specific noise present in the dataset without introducing any external biases (Lilic et al., 2020).
Specifically, both for the interphase oligo fraction and metaphase oligo fraction, data were processed with the identical procedure. Movie frames were motion corrected and dose weighted with a binning factor of 2 using MOTIONCOR2 (Zheng et al., 2017) with RELION3.0 (Scheres, 2012). Particles were picked by blob picker (minimum particle diameter = 50 Å, maximum particle diameter = 300 Å, minimum separation diameters = 100 Å) and extracted (extraction box size = 320 pixels, Fourier crop to 160 pixels) using CryoSPARC v2.15 (Punjani et al., 2017). Extracted particles were applied for 2D classification with 400 classes using CryoSPARC v2.15. Using 2D classification results, particles were split to the lower resolution group and the higher resolution group. The 2D classes whose resolution were higher than 28 Å (did not considered for the samples with egg extract lot 2) and effective classes assigned (ECA) value better than 2 (1.6 for the samples with egg extract lot 2) were assigned as the higher resolution group. 2D classes containing obvious ice signals, carbon signals, and strong small dots were manually removed from the higher resolution group and reassigned as the lower resolution group. 3D initial models were generated with ab initio reconstruction of CryoSPARC v2.15 (10 classes for the higher resolution group, 14 classes for the lower resolution group, maximum resolution = 20 Å, class similarity = 0). 3D classifications were performed using all eighteen 3D classes generated by Ab-initio reconstruction and all picked particles using the heterogeneous refinement of CryoSPARC v2.15 (Refinement box size = 160 voxels). After the 3D classification, particles for each reasonable structure class were re-extracted with the original pixel size with optimized box sizes for each particle. Nucleosome classes, intelectin classes, actin classes, and alpha2-macroglobulin classes were re-extracted with 256, 100, 320, 320 box sizes, respectively. New 3D references were prepared with the re-extracted particles by ab initio reconstruction and further refined with homogenous refinement or non-uniform refinement using CryoSPARC v2.15. For the interphase actin and intelectin classes, multiple models were generated by ab initio reconstruction (Class similarity = 0) and further “decoy” classifications were performed. For the mono fractions, the same pipeline was used with several modifications: micrographs were denoise with Topaz denoise to improve blob pick accuracy, maximum particle diameter of blob pick = 200 Å, initial extraction box size = 256 pixels (Fourier crop to 128 pixels), higher resolution group threshold; < 20Å resolution, < 2.0 ECA. Final extraction box size = 200 pixels. Chimera and ChimaraX were used for 3D map visualization (Pettersen et al., 2004).
Actin cryo-EM structure analyses as a quality control of the EM grids
For the EM grids of oligo nucleosome fractions that contain decent amount of actin filament, we used the actin filament cryo-EM structure as an internal control for assessing the sample denaturization and grid qualities. Actin particles were picked by Topaz v0.2.3 using actin particles separated from the simultaneous cryo-EM structures reconstruction as training models (Bepler et al., 2019). For each EM grids for oligo fractions of the chromosomal nucleosomes, 7500 particles were randomly selected and extracted (box size = 320 pixels). Using identical input 3D map, maps were refined for each EM grids separately with homogenous refinement of CryoSPARC v2.15 (without helical refinement). Grids in which the filament actin resolution surpassed 5 Å were qualified and used for this study.
Cryo-EM image processing for the averaged nucleosome structure determination
While our TRSF reconstruction pipeline (Fig. 2) excelled at reducing particle selection bias, the particle picking process inevitably missed many nucleosome particles on the micrographs: a 100 Å minimum inter-particle distance was needed to prevent redundant picking of large proteins by CryoSPARC blob picker, but this excluded many clustered nucleosome particles.
Unlike the TRSF pipeline, this Topaz and decoy classification-based pipeline was exclusively focused on analyzing nucleosome-like structures. However, we expect that structural diversity within these nucleosome-like particles was still preserved for three reasons. First, although Topaz initially required manual selection of particles for training, it failed to remove even lectin particles (Fig. S6A: decoy classification), suggesting that the selection was not stringent enough to exclude nucleosome structural variants. Second, 3D decoy classification was performed with all picked particles since manual particle curation could propagate bias. Third, only one nucleosome 3D map was used for decoy classification to include all nucleosome-like particles within one reconstruction, while multiple decoy classes were used to exclude unrelated particles (Fig. S6A: decoy classification). As we will describe later, both octasome and hexasome particles were assigned to the nucleosome class, suggesting that the output maps of this pipeline retain structural diversity of the nucleosome in chromosomes.
Specifically, All Cryo-EM images of interphase mono-, interphase di-, interphase oligo-, freed-, metaphase mono-, metaphase di-, metaphase oligo-, and in vitro reconstituted nucleosome were processed with the same procedure (Fig S6A). Movie frames were motion-corrected and dose-weighted with a binning factor of 2 using MOTIONCOR2 (Zheng et al., 2017)with RELION3.0 or 3.1 (Scheres, 2012). Particles were picked by Topaz v0.2.3 with 500~2000 manually picked nucleosome-like particles as training models (Bepler et al., 2019). Picked particles were extracted using CryoSPARC v2.15 (extraction box size = 200 or 256 pixels) (Punjani et al., 2017). Extracted particles were applied for 2D classification with 200 classes using CryoSPARC v2.15. Using 2D classification result, particles were split to the nucleosome-like groups and the non-nucleosome-like groups. For the interphase di- and metaphase di- data, intelectin 2D classes were removed, and remaining particles were applied for second round of 2D classification, because abundant intelectin-2 particles inhibited the formation of nucleosome-like 2D classes. Four of 3D initial models were generated for both groups with ab initio reconstruction of CryoSPARC v2.15 (Class similarity = 0). One nucleosome like model was selected and used as a given model of 3D classification with all four of the “decoy” classes generated from non-nucleosome-like group. After the first round 3D classification, the particles that were assigned to the “decoy” classes were removed, and remained particles were applied for the second round 3D classification with the same setting with the first round. These steps were repeated until 90~95 % of particles were classified as a nucleosome-like class. Repeat times of this step were described in Table S2. Using RELION 3.1 beta, CTF refinement, Bayesian polishing, and postprocessing were performed. Local resolution was calculated with the Blocres and Blocfilt in the Bsoft package (Cardone et al., 2013). Chimera and ChimaraX were used for 3D map visualization (Pettersen et al., 2004).
For the 3D classification to analyze linker DNA structure variety shown in Fig 5, S8K, and S10A-B, six to eight 3D references were generated with ab initio reconstruction of CryoSPARC v2.15 using purified nucleosome-like particles listed in Table S2 (Class similarity = 0.9). 3D classifications was performed using all newly generated 3D references and purified particles using the heterogeneous refinement of CryoSPARC v2.15.
For the atomic model building, initial atomic models of linker DNA containing nucleosomes were built by combining linker DNA moiety of gH1.0 binding nucleosome (PDB ID: 5NL0) and nucleosome moiety of ScFv binding nucleosome (PDB ID: 6dzt) and by replacing amino acids sequence to that of Xenopus laevis core histones using Coot (Bednar et al., 2017; Emsley and Cowtan, 2004; Zhou et al., 2019). The initial atomic model was docked onto the cryo-EM map postprocessed by RELION 3.1 beta and refined using Coot and PHENIX (Liebschner et al., 2019).
3D Variability Analysis (3DVA)
For the 3D structure dynamics analysis of nucleosome containing linker DNA shown in Fig 4A, the purified nucleosome particles used for 3D classification analysis (listed in Table S2) were used for the 3DVA in CryoSPARC v2.15 (Punjani and Fleet, 2021). Numbers of 3DVA components and filter resolution were set by user. After several round of tryout, numbers of 3DVA components were manually optimized to 2, which can explain most of the representative protein movements of chromosomal nucleosome without making redundant component. For the interphase nucleosomes crosslinked after MNase, filter resolution was set to 12 Å, whereas for other samples, filter resolution was set to 8 Å. All output 3D maps were loaded with Chimera (Pettersen et al., 2004) and movies were created from the maps.
For the 3DVA of NCPs shown in Fig 4B, C, linker DNA densities of each nucleosome were removed from original particles listed in Table S2 using particle subtraction in cryoSPARC v2.15.
For GUB-open nucleosomes, the new initial 3D map was generated with linker DNA subtracted particles by ab initio reconstruction of cryoSPARC v2.15. The 3D map was further refined with homogeneous refinement of cryoSPARC v2.15. Particle stack and mask generated by NU-refinement were used for 3DVA of cryoSPARC v2.15 (number of modes = 2, Filter resolution = 8Å).
For chromosomal NCPs, 100,000 particles were selected from interphase or metaphase nucleosome particles whose linker DNAs had been subtracted. Particles were mixed, and new initial 3D map was generated by ab initio reconstruction of cryoSPARC v2.15. Particle stack and mask generated by homogeneous refinement were used for 3DVA of cryoSPARC v2.15 (number of modes = 4, Filter resolution = 8Å).
To calculate the surface hydrophobicity and electrostatic potential and global R.M.S.D., an atomic model was generated for each NCP structural variant. Using PHENIX package, the atomic model of interphase oligo nucleosome was docked on to the 3D maps of NCP structural variants generated by 3DVA. Docked atomic models were refined with rigid body refinement in PHENIX. Global R.M.S.D. of Cα and P atoms were calculated by PyMol. To show the surface hydrophobicity (Fig. 7E), hydropathy indexes (Kyte and Doolittle, 1982) were assigned to each amino acid residue and mapped on the original EM maps by Chimera. To show the electrostatic potential (Fig. 7F), surface electrostatic potentials were calculated by APBS and PDB2PQR (Baker et al., 2001; Dolinsky et al., 2004) using refined atomic models for each NCP structural variant, and the colored surface electrostatic potentials were drawn by Chimera.
In silico mixing 3D classification for assessing linker DNA angle
For the in silico mixing 3D classification for analyzing linker DNA structure variation shown in Fig 5 and S6, five different linker DNA angle 3D maps were selected as the fixed templates for all samples (“closed 1” : metaphase oligo class A (Fig 5A), “closed 2” : metaphase oligo class D (Fig 5A), “open 1” : interphase oligo class E (Fig 5A), “open 2” : interphase crosslink after MNase class E (Fig 5B), and “misc.” : metaphase oligo class F (Fig 5A)). As decoy maps, 4 noise classes (shown in Fig. S6A: decoy classification) were used. Because of the original pixel size discrepancy, in silico mixing 3D classification for mono nucleosome fractions (original image pixel size = 1.33 Å/pixel), di nucleosome fractions (original image pixel size =1.6 Å/pixel), and others (original image pixel size = 1.5 Å/pixel) were performed separately. For the mono nucleosome fractions, from the purified particle set of each interphase and metaphase nucleosome sample (listed in Table S2), 17000 particles were selected randomly and extracted (original image pixel size= 1.33Å/pixel, extraction box size = 300 pixels, Fourier crop to 150 pixels). Particles of mono nucleosome fractions were mixed with 17,000 particles randomly selected from the purified particle set (listed in Table S2) of interphase oligo nucleosome (egg extract lot1), metaphase oigo nucleosome (egg extract lot1), and freed nucleosomes (original image pixel size = 1.5Å/pixel, extraction box size = 266 pixels, Fourier crop to 150 pixels). For the di nucleosome fractions, 12,000 particles of interphase or metaphase nucleosomes were extracted (original image pixel size= 1.6 Å/pixel, extraction box size = 180 pixels, Fourier crop to 150 pixels). Particles of di nucleosome fractions were mixed with the 12,000 particles randomly selected from the purified particle set (listed in Table S2) of interphase oligo nucleosomes (egg extract lot1), metaphase oligo nucleosomes (egg extract lot1), and freed nucleosomes (original image pixel size = 1.5 Å/pixel, extraction box size = 192 pixels, Fourier crop to 150 pixels). For oligo nucleosome fractions, 25,000 particles of interphase nucleosomes (egg extract lot1), metaphase nucleosomes (egg extract lot1), interphase nucleosomes (egg extract lot2), metaphase nucleosomes (egg extract lot2), and freed nucleosomes were selected from the purified particle set (listed in Table S2), extracted (image pixel size = 1.5 Å/pixel, extraction box size = 200 pixels), and mixed. After mixing these particles, 3D classification was performed five times using all five 3D maps and merged particle set using heterogeneous refinement of CryoSPARC v2.15 (refinement box size =200, initial resolution = 32 Å). The particles assigned to each class were counted (Table S3) and plotted.
In silico mixing 3D classification for assessing H4 N-terminal tail orientations
The analysis pipeline was depicted in Fig. S9A. Since each nucleosome particle has two H4 N-terminal tails and structural discrepancy between these tails deterred the 3D classification, one out the two H4 N-terminal tails was mathematically subtracted before in silico mixing 3D classification. In addition, to use the structural information of both side of the H4 tails, particles were symmetrically doubled prior to the H4 tail density subtraction. From each interphase oligo (egg extract lot1), metaphase oligo (egg extract lot1), interphase oligo (egg extract lot2), metaphase oligo (egg extract lot2), and recombinant nucleosome (GUB-closed), 50,000 particles were selected were mixed. Using mixed particles, 3D map of the nucleosome was refined applying C2 symmetry with homogeneous refinement of CryoSPARC v2.15. The particles were then symmetrically doubled using symmetry expansion of CryoSPARC v2.15. Density around the one side of the H4 N-terminal tail and both side of the linker DNA were subtracted from each particle using particle substruction of CryoSPARC v2.15.
To prepare 3D templates for the in silico mixing 3D classification, 3D map was refined using density subtracted particles with homogeneous refinement of CryoSPARC v2.15 (C1 symmetry). The H4 N-terminal tail density was masked, and the H4 N-terminal tail structural variants were generated by of CryoSPARC v2.15 (simple mode, number of modes = 2, Filter resolution = 6Å, number of frames = 20). The 3D maps representing ‘inward’ or ‘outward’ H4 tail orientation were selected as 3D templates for the in silico mixing 3D classification.
In silico mixing 3D classifications were performed using twelve of 3D templates (4 of the ‘inward’ H4 tail maps, 4 of the ‘outward’ H4 tail maps, and 4 of decoy maps) and 500,000 of the density subtracted particles with heterogeneous refinement of CryoSPARC v2.15 (C1 symmetry, refinement box size =200, initial resolution = 16 Å, number of initial random assignment iterations = 0). The in silico mixing 3D classification was performed five times. The particles assigned to each class were counted (Table S3) and plotted.
After the in silico mixing 3D classification, particles assigned to the 4 of ‘inward’ H4 tail maps and the 4 of the ‘outward’ H4 tail maps ware mixed, respectively. 3D maps of the nucleosomes with ‘inward’ and ‘outward’ H4 tail were refined with homogeneous refinement of CryoSPARC v2.15 (C1 symmetry). For both of the ‘inward’ and ‘outward’ map, particles were split back to the original image group. Using these particle sets, 3D maps of the nucleosomes were refined with homogeneous refinement of CryoSPARC v2.15 (C1 symmetry).
In silico mixing 3D classification for assessing NCP structural variation
The analysis pipeline for the in silico mixing 3D classification of major NCP variation shown in Fig. 7C was depicted in Fig. S12A. Three 3D maps (frame 4, 11, and 18) for each component were selected from 3DVA of GUB-open NCP, which had generated 21 motion frames per each component. In the 3DVA, largest structural differences were found between motion frames 1 and 21, and they were structurally most deviated from the averaged GUB NCP structure, while the structure of the motion frame 11 was most similar to that of the averaged GUB NCP. We chose frames 4 and 18, instead of frames 1-3 and 19-21, for reference maps because frames 1 and 21 contain many noisy densities (e.g., gaps in helices and densities outside of the NCP). As a 3D reference of canonical nucleosome structure in chromosome, a refined 3D map generated from 100,000 each of linker DNA subtracted interphase oligo nucleosome, metaphase oligo nucleosome particle was used. As decoy maps, 4 noise classes shown in Fig. S6A were used. As input particles for in silico mixing 3D classification,25,000 each of linker DNA subtracted interphase oligo nucleosome (egg extract lot 1), metaphase oligo nucleosome particle (egg extract lot 1), interphase oligo nucleosome (egg extract lot 2), metaphase oligo nucleosome particle (egg extract lot 2), and freed nucleosome was were randomly selected and mixed (Total 125,000 particles). Using these 3D reference maps and particles, 3D classification was performed five times using heterogeneous refinement of CryoSPARC v2.15 (refinement box size =200, initial resolution = 20 Å, number of initial random assignment iterations = 0). To confirm the accuracy of 3D classification, output 3D maps were compared to the input maps of the in silico mixing 3D classification, and the parameters of the in silico mixing 3D classification were optimized for output 3D maps to preserve the feature of input maps (Fig S12B). For each 3DVA component, the in silico mixing 3D classification was performed five times. The particles assigned to each class were counted (Table S3) and plotted.
The analysis pipeline for the in silico mixing 3D classification of minor NCP variation shown in Fig. 7D was depicted in Fig. S12C. Prior to the in silico mixing 3D classification using minor NCP variant maps, the particles of major NCP variant were removed by performing the in silico mixing 3D classification using 1 canonical NCP map, 6 major NCP variant maps (GUB-open, frame 4, 11, and 18 from component 1 and 2), and 4 decoy maps as input 3D maps (Fig. S12C). As input particles same 175,000 mixed particles for assessing the major NCP variation were used. Using these 3D reference maps and particles, 3D classification was performed using heterogeneous refinement of CryoSPARC v2.15 (refinement box size =200, initial resolution = 20 Å, number of initial random assignment iterations = 0). Using the 73,682 particles assigned to the canonical nucleosome, in silico mixing 3D classification of minor NCP variation were performed. As input maps for in silico mixing 3D classification, three 3D maps (frame 4, 11, and 18) of each component generated by 3DVA of chromosomal NCP were selected. The 3DVA of chromosomal NCP (using metaphase and interphase oligo nucleosome particles) generated 21 motion frames per each component. Frames 11 of each component are most similar to the canonical nucleosome structure in chromosome, while we chose frames 4 and 18 of components 1-4 to represent NCP structural variants, as the rationale explained above for the GUB nucleosome in silico mixing 3D classification. As decoy maps, 4 noise classes shown in Fig. S6A were used. Using these 3D reference maps and particles, 3D classification was performed five times using heterogeneous refinement of CryoSPARC v2.15 (refinement box size =200, initial resolution = 13 Å, number of initial random assignment iterations = 0). To confirm the accuracy of 3D classification, output 3D maps were compared to the input maps of the in silico mixing 3D classification, and the parameters of the in silico mixing 3D classification were optimized for output 3D maps to preserve the feature of input maps (Fig S12D). For each 3DVA component, the in silico mixing 3D classification was performed five times. The particles assigned to each class were counted (Table S3) and plotted.
Reconstruction of “closed” and “moderately open” linker DNA nucleosome maps (Fig. 6F)
Using the interphase nucleosome particles shown in Table S2, twenty classes of 3D maps were generated by 3DVA of cryoSPARC v2.15 (cluster mode, number of modes = 2, Filter resolution = 8Å, number of frames = 20). To generate the “closed” and “open” linker DNA nucleosome maps at the comparable resolution, five “closed” linker DNA classes (20,738 particles) and five “moderately open” linker DNA classes (19,120 particles) were selected, and initial 3D maps were generated by ab initio reconstruction of cryoSPARC v2.15. Initial 3D models were further refined with homogeneous refinement in cryoSPARC v2.15.
Cryo-EM image processing for the H1.8-bound nucleosome structure determination
41,416 particles assigned to the class that has the extra density of the metaphase oligo fraction were applied for homogeneous refinement (Fig S11A). Refined particles were further purified with the heterogeneous refinement using an H1-bound class and an H1-unbound class as decoys. The purified particles were further refined and split to four of 3D classes using the ab initio reconstruction of (4 classes, class similarity = 0.9) and heterogeneous refinement of CryoSPARC v2.15. Two classes that had reasonable extra density were selected and refined with homogeneous refinement. The final resolution was determined as 4.42Å with the gold stand FSC threshold (FSC = 0.143).
For the atomic model building, H1 segment of the gH1.0-bound nucleosome structure (PDB ID: 5NL0) was replaced with the H1.8 structure model built using SWISS-MODEL: homology modeling tool (Waterhouse et al., 2018). The initial atomic model was docked onto the cryo-EM map locally filtered by Bsoft (Blocres and Blocfilt) and refined using Coot and PHENIX (Cardone et al., 2013; Emsley and Cowtan, 2004; Liebschner et al., 2019). Chimera and ChimaraX were used for 3D map visualization (Pettersen et al., 2004).
Quantification and Statistical Analysis
In silico mixing 3D classification analyses were performed five times for each analysis (Fig 5E, 5F, 5G, 7C, 7D, and S9C). The relative populations of the particles assigned to each class were counted for each round of classification analysis. Average and standard deviation of populations of five independent in silico mixing 3D classification analyses were calculated using Excel (Microsoft). Number of the particles for each class used for each analysis and their classification results are reported in Table S3.
Supplementary Material
Figure360. Introduction video for 3DVA. Related to Figure 4A.
Movie S1. The 3DVA of the interphase oligo (lot 1), metaphase oligo (lot 1), freed (lot 2), and GUB-open nucleosome. Related to Figure 4, 5, 6, 7.
Table S1. Mass spectrometry analysis results. Related to Figure 1
Sheet 1. Raw data of the Mass spectrometry analysis of the mono, di, and oligo fractions of interphase and metaphase chromosome.
Sheet 2. Modified data with summing the iBAQ values of peptides that separately mapped to highly similar protein isoforms.
Table S3. Number of particles in each 3D class after in silico mixing 3D classification. Related to Star methods.
Figure S1-S12, Table S2
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-histone H4 mouse monoclonal antibody | (Hayashi-Takanaka et al., 2015) | CMA400 |
| Anti-histone H1.8 (Xenopus laevis) mouse monoclonal antibody | (Jenness et al., 2018) | RU1974 |
| Anti-histone H3T3ph mouse monoclonal antibody | (Kelly et al., 2010) | 16B2 |
| IRDye 680LT goat anti-rabbit IgG | LI-COR | 926-68021 |
| IR Dye 800CW goat anti-mouse IgG | LI-COR | 926-32210 |
| Biological samples | ||
| Xenopus laevis sperm nuclei | Nasco Isolated from male Xenopus laevis | LM00715 (male Xenopus laevis)) |
| Xenopus laevis egg | Nasco Laid by female Xenopus laevis | LM00535 (female Xenopus laevis) |
| Alexa594-labeled-tubulin | (Hyman et al., 1991) Isolated from bovine brain | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Chorionic gonadotropin human | Sigma | CG10-1VL |
| Pregnant Mare Serum Gonadotropin (PMSG) | Prospec | HOR-272 |
| Cycloheximide | Sigma | C-7698 |
| CaCl2 | Sigma Aldrich | C7902-500G |
| Cyclin B Δ90 | (Glotzer et al., 1991) | N/A |
| Cysteine | Sigma | C7352-1KG |
| PIPES | Alfa Aesar | A16090 |
| NaCl | Millipore Sigma | SX0420-5 |
| Glycerol | Alfa Aesar | 36646 |
| EGTA | Sigma Aldrich | E4378-250G |
| MgCl2 | Acros organic | 197530010 |
| β-glycerophosphate | Acros organic | 410991000 |
| Sodium butyrate | Aldrich | 303410-100G |
| Formaldehyde | Fisher bioreagents | BP531-25 |
| HEPES | Akron biotech | AK1069-1000 |
| Sucrose | Fisher chemical | S5-3 |
| KCl | Fisher chemical | P217-3 |
| Spermidine | Sigma | S-2501 |
| Spermine | Sigma | S1141-5G |
| cOmplete EDTA-free Protease Inhibitor Cocktail | Roche | 11873580001 |
| MNase | Worthington Biochemical Corporation | LS004798 |
| Leupeptin | Millipore Sigma | El8 |
| Pepstatin | Sigma | P5318-25MG |
| Chymostatin | Millipore Sigma | El6 |
| RNaseA | Thermo Scientific | EN0531 |
| Proteinase K solution | Roche | 03115828001 |
| SYBR-safe | Invitrogen | S33102 |
| 4-20 % gradient SDS-PAGE gel | BioRad | 4561096 |
| SYTO-60 | Invitrogen | S11342 |
| Tris | Sigma | T1503-5KG |
| Glycine | Sigma | G7126-5KG |
| Amicon Ultra centrifugal filter 100K | Millipore Sigma | UFC510024 |
| Methanol | Fisher chemical | A452SK-4 |
| Tube-o-dialyzer 15 kDa | G-Biosciences | 786-618 |
| Amicon Ultra centrifugal filters 10K | Millipore Sigma | UFC501024 |
| Coomassie Brilliant Blue G-250 | Calbiochem | 3340 |
| Xenopus laevis H2A, H2B, H3.2, H4 | (Zierhut et al., 2014) | N/A |
| Ni-NTA beads | Qiagen | 30210 |
| TEV protease (S219V) | (Tropea et al., 2009) | N/A |
| Carbenicillin | Alfa Aesar | J61949 |
| EcoRV (New England Biolabs) | New England Biolabs | R0195S |
| PEG-6000 | Fluka | 81253 |
| Triton X-100 | BioRad | 161-0407 |
| Methanol | Fisher chemical | A452SK-4 |
| Paraformaldehyde aqueous solution | Electron Microscopy Sciences | 157-8 |
| Tube-O-DIALYZER (100 kDa) (G-Biosciences) | G-Biosciences | 786-619 |
| Trehalose | Sigma | T0167-100G |
| 1,6,-hexanediol | Alfa Aesar | A12439 |
| Deposited data | ||
| Recombinant poly-nucleosome (emd2601) (Song et al., 2014) | EMDR (Song et al., 2014) | emd2601 |
| Recombinant Xenopus laevis canonical nucleosome | PDB (Luger et al., 1997) | 1AOI |
| Recombinant gH1.0 binding nucleosome | PDB (Bednar et al., 2017) | 5NL0 |
| Recombinant ScFv binding canonical nucleosome | PDB (Zhou et al., 2019) | 6DZT |
| Cryo-EM map of nucleosome in interphase oligo fraction (lot 1, TRSF) | This paper; EMDR | EMD-22793 |
| Cryo-EM map of alpha-2-macroglobulin family protein in interphase oligo fraction (lot 1, TRSF) | This paper; EMDR | EMD-22794 |
| Cryo-EM map of nucleosome in metaphase oligo fraction (lot 1, TRSF) | This paper; EMDR | EMD-22795 |
| Cryo-EM map of alpha-2-macroglobulin family protein in metaphase oligo fraction (lot 1, TRSF) | This paper; EMDR | EMD-22796 |
| Cryo-EM map of nucleosome in interphase mono fraction (lot 1, averaged) | This paper; EMDR | EMD-22797 |
| Cryo-EM map of nucleosome in interphase di fraction (lot 1, averaged) | This paper; EMDR | EMD-22798 |
| Cryo-EM map of nucleosome in interphase oligo fraction (lot 1, averaged) | This paper; EMDR | EMD-22790 |
| Cryo-EM map of nucleosome in metaphase mono fraction (lot 1, averaged) | This paper; EMDR | EMD-22800 |
| Cryo-EM map of nucleosome in metaphase di fraction (lot 1, averaged) | This paper; EMDR | EMD-22801 |
| Cryo-EM map of nucleosome in metaphase oligo fraction (lot 1, averaged) | This paper; EMDR | EMD-22791 |
| Cryo-EM map of freed nucleosome (crosslinked after MNase, averaged) | This paper; EMDR | EMD-23826 |
| Cryo-EM map of nucleosome of interphase oligo fraction (lot 2, averaged) | This paper; EMDR | EMD-23819 |
| Cryo-EM map of nucleosome of metaphase oligo fraction (lot 2, averaged) | This paper; EMDR | EMD-23821 |
| Cryo-EM map of GUB-closed nucleosome (averaged) | This paper; EMDR | EMD-23823 |
| Cryo-EM map of GUB-open nucleosome (averaged) | This paper; EMDR | EMD-23824 |
| Cryo-EM map of H1-bound nucleosome of metaphase oligo fraction (lot 1) | This paper; EMDR | EMD-22792 |
| Atomic coordinates of nucleosome of interphase oligo fraction (lot 1, averaged) | This paper; PDB | 7KBD |
| Atomic coordinates of nucleosome of metaphase oligo fraction (lot 1, averaged) | This paper; PDB | 7KBE |
| Atomic coordinates of H1 bound nucleosome of metaphase oligo fraction (lot 1) | This paper; PDB | 7KBF |
| Raw micrographs of interphase mono fraction (egg extract lot 1) | This paper; EMPIAR | EMPIAR-10742 |
| Raw micrographs of interphase di fraction (egg extract lot 1) | This paper; EMPIAR | EMPIAR-10744 |
| Raw micrographs of interphase oligo fraction (egg extract lot 1) | This paper; EMPIAR | EMPIAR-10692 |
| Raw micrographs of metaphase mono fraction (egg extract lot 1) | This paper; EMPIAR | EMPIAR-10743 |
| Raw micrographs of metaphase di fraction (egg extract lot 1) | This paper; EMPIAR | EMPIAR-10745 |
| Raw micrographs of metaphase oligo fraction (egg extract lot 1) | This paper; EMPIAR | EMPIAR-10691 |
| Raw micrographs of ‘freed’fraction (Crosslinked after Mnase, interphase) | This paper; EMPIAR | EMPIAR-10750 |
| Raw micrographs of interphase oligo fraction (egg extract lot 2) | This paper; EMPIAR | EMPIAR-10747 |
| Raw micrographs of metaphase oligo fraction (egg extract lot 2) | This paper; EMPIAR | EMPIAR-10746 |
| Raw micrographs of GUB-closed nucleosome | This paper; EMPIAR | EMPIAR-10749 |
| Raw micrographs of GUB-open nucleosome | This paper; EMPIAR | EMPIAR-10748 |
| Recombinant DNA | ||
| pUC18-GUB_176x4 | This paper | N/A |
| Software and algorithms | ||
| MASCOT through Proteome Discoverer v.1.4 | Thermo Scientific | N/A |
| MOTIONCOR2 | (Zheng et al., 2017) | https://emcore.ucsf.edu/ucsf-software |
| RELION3 | (Scheres, 2012) | https://github.com/3dem/relion |
| CryoSPARC v2.15 | (Punjani et al., 2017) | https://cryosparc.com |
| Chimera | (Pettersen et al., 2004) | https://www.cgl.ucsf.edu/chimera/ |
| ChimaraX | (Pettersen et al., 2004) | https://www.rbvi.ucsf.edu/chimerax/ |
| Topaz v0.2.3 | (Bepler et al., 2019) | https://github.com/tbepler/topaz |
| Bsoft | (Cardone et al., 2013) | https://lsbr.niams.nih.gov/bsoft/ |
| Coot | (Emsley and Cowtan, 2004) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| PHENIX | (Liebschner et al., 2019) | https://phenix-online.org/documentation/reference/refinement.html |
| APBS | (Baker et al., 2001) | https://www.cgl.ucsf.edu/chimera/docs/ContributedSoftware/apbs/apbs.html |
| PDB2PQR | (Dolinsky et al., 2004) | https://www.cgl.ucsf.edu/chimera/docs/ContributedSoftware/apbs/pdb2pqr.html |
| SWISS-MODEL | (Waterhouse et al., 2018) | https://swissmodel.expasy.org |
| RING 2.0 webserver | (Piovesan et al., 2016) | http://old.protein.bio.unipd.it/ring/ |
| Pyem | (Asarnow et al., 2019) | https://github.com/asarnow/pyem |
| ImageJ | (Schneider et al., 2012) | https://imagej.nih.gov/ij/ |
| Python | Python Software Foundation | https://www.python.org |
| Jupyter Notebook | Project Jupyter | https://jupyter.org |
| Microsoft Excel | Microsoft | https://www.microsoft.com/en-us/microsoft-365/excel |
| Other | ||
| Xenopus laevis MS database | (Wühr et al., 2014) | https://scholar.princeton.edu/wuehr/protein-concentrations-xenopus-egg |
| Centrifuge rotor | Beckman Coulter | SX241.5 |
| Ultra centrifuge rotor | Beckman Coulter | SW55Ti |
| Ultra centrifuge rotor | Beckman Coulter | SW40Ti |
| Centrifuge rotor | Beckman Coulter | JS 5.3 |
| Centrifuge | Beckman Coulter | Allegron X-30R |
| Ultra centrifuge | Beckman Coulter | Optima L80 |
| Centrifuge | Beckman Coulter | Avanti J-26S |
| Imaging System | Li-cor | Odyssey Infrared Imaging System |
| LC-MS/MS system | Thermo Scientific | Dionex3000 HPLC, NCS3500RS nano- and microflow pump, Q-Exactive HF mass spectrometer |
| Plunge freezer | FEI | Vitrobot Mark IV |
| Plasma Cleaner | Gatan | Solarus II |
| Cryo-EM | FEI | Talos Arctica TEM |
| Cryo-EM | FEI | Titan Krios TEM |
| Cryo-EM detector | GATAN | K2 Camera |
Acknowledgments
We are grateful to Seth Darst, James Chen, Elizabeth Campbell, Gregory Alushin, Hiroshi Suzuki, Mark Ebrahim, Johanna Sotiris, and Honkit Ng for their technical advice and assistance for the Cryo-EM. We thank Hiroshi Kimura and Pavan Choppakatla for sharing reagents, and Soeren Heissel for MS analysis. We thank Haruka Oda and Coral Zhou for their technical advice for chromatin isolation from Xenopus egg extract. We thank Seth Darst, James Chen, Gregory Alushin, Viviana Risca, Hitoshi Kurumizaka, Tomoya Kujirai, Risa Fujita, Kayo Nozawa, Yoshimasa Takizawa, Christian Zierhut, Simona Giunta, Isabel Wassing, Pavan Choppakatla, and Ryan Kim for their comments to the manuscript. This work was conducted with the help of High Performance Computing resource center, Proteomics Resource Center, Electron Microscopy Resource Center, and the Evelyn Gruss Lipper Cryo-Electron Microscopy Resource Center at the Rockefeller University. This work was funded by National Institutes of Health Grants R35GM132111 to H.F.. Y.A. was supported by Japan Society for the Promotion of Science Overseas Research Fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
H.F. is affiliated with Graduate School of Medical Sciences, Weill Cornell Medicine, and Cell Biology Program, the Sloan Kettering Institute. The authors declare that no competing financial interests exist.
References
- Adams CC, and Workman JL (1995). Binding of disparate transcriptional activators to nucleosomal DNA is inherently cooperative. Mol. Cell. Biol 15, 1405–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhireksan Z, Sharma D, Lee PL, and Davey CA (2020). Near-atomic resolution structures of interdigitated nucleosome fibres. Nat. Commun 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali- Ahmad A, Bilokapić S, Schäfer IB, Halić M, and Sekulić N (2019). CENP - C unwraps the human CENP - A nucleosome through the H2A C- terminal tail . EMBO Rep. 20, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An W, Leuba SH, Van Holde K, and Zlatanova J (1998). Linker histone protects linker DNA on only one side of the core particle and in a sequence-dependent manner. Proc. Natl. Acad. Sci. U. S. A 95, 3396–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura Y, Tachiwana H, Oda T, Sato M, and Kurumizaka H (2012). Structural analysis of the hexasome, lacking one histone H2A/H2B dimer from the conventional nucleosome. Biochemistry 51. [DOI] [PubMed] [Google Scholar]
- Arimura Y, Ikura M, Fujita R, Noda M, Kobayashi W, Horikoshi N, Sun J, Shi L, Kusakabe M, Harata M, et al. (2018). Cancer-associated mutations of histones H2B, H3.1 and H2A.Z.1 affect the structure and stability of the nucleosome. Nucleic Acids Res. 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura Y, Tachiwana H, Takagi H, Hori T, Kimura H, Fukagawa T, and Kurumizaka H (2019). The CENP-A centromere targeting domain facilitates H4K20 monomethylation in the nucleosome by structural polymorphism. Nat. Commun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armache JP, Gamarra N, Johnson SL, Leonard JD, Wu S, Narlikar GJ, and Cheng Y (2019). Cryo-EM structures of remodeler-nucleosome intermediates suggest allosteric control through the nucleosome. Elife 8, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarnow D, Palovcak E, and Cheng Y (2019). asarnow/pyem: UCSF pyem v0.5. [Google Scholar]
- Ayala R, Willhoft O, Aramayo RJ, Wilkinson M, McCormack EA, Ocloo L, Wigley DB, and Zhang X (2018). Structure and regulation of the human INO80-nucleosome complex. Nature 556, 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Sept D, Joseph S, Holst MJ, and McCammon JA (2001). Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci 98, 10037 LP – 10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancaud A, Wagner G, Conde e Silva N, Lavelle C, Wong H, Mozziconacci J, Barbi M, Sivolob A, Le Cam E, Mouawad L, et al. (2007). Nucleosome Chiral Transition under Positive Torsional Stress in Single Chromatin Fibers. Mol. Cell 27, 135–147. [DOI] [PubMed] [Google Scholar]
- Basu A, Bobrovnikov DG, Qureshi Z, Kayikcioglu T, Ngo TTM, Ranjan A, Eustermann S, Cieza B, Morgan MT, Hejna M, et al. (2021). Measuring DNA mechanics on the genome scale. Nature 589, 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednar J, Garcia-Saez I, Boopathi R, Cutter AR, Papai G, Reymer A, Syed SH, Lone IN, Tonchev O, Crucifix C, et al. (2017). Structure and Dynamics of a 197 bp Nucleosome in Complex with Linker Histone H1. Mol. Cell 66, 384–397.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JM, Chen M, Baldwin PR, and Ludtke SJ (2016). High resolution single particle refinement in EMAN2.1. Methods 100, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bepler T, Morin A, Rapp M, Brasch J, Shapiro L, Noble AJ, and Berger B (2019). Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JM, Norouzi D, Harutyunyan A, Zhurkin VB, and Grigoryev SA (2017). Regulation of chromatin folding by conformational variations of nucleosome linker DNA. Nucleic Acids Res. 45, 9372–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Chen C, Tan ZY, Huang Y, Shi J, and Gan L (2018a). Cryo-ET reveals the macromolecular reorganization of S. pombe mitotic chromosomes in vivo. Proc. Natl. Acad. Sci. U. S. A 115, 10977–10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Böck D, Pilhofer M, and Gan L (2018b). The in situ structures of mono-, di-, and trinucleosomes in human heterochromatin. Mol. Biol. Cell 29, 2450–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone G, Heymann JB, and Steven AC (2013). One number does not fit all: Mapping local variations in resolution in cryo-EM reconstructions. J. Struct. Biol 184, 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yang R, Korolev N, Liu CF, and Nordenskiöld L (2017). Regulation of Nucleosome Stacking and Chromatin Compaction by the Histone H4 N-Terminal Tail–H2A Acidic Patch Interaction. J. Mol. Biol 429, 2075–2092. [DOI] [PubMed] [Google Scholar]
- Chicano A, Crosas E, Otón J, Melero R, Engel BD, and Daban J (2019). Frozen- hydrated chromatin from metaphase chromosomes has an interdigitated multilayer structure. EMBO J. 38, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppakatla P, Dekker B, Cutts EE, Vannini A, Dekker J, and Funabiki H (2021). Linker histone H1.8 inhibits chromatin-binding of condensins and DNA topoisomerase II to tune chromosome length and individualization. eLife 2021;10:e68918 DOI: 10.7554/eLife.68918 https://pubmed.ncbi.nlm.nih.gov/34406118/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EYD, Vasudevan D, Davey GE, Wu B, and Davey CA (2012). The mechanics behind DNA sequence-dependent properties of the nucleosome. Nucleic Acids Res. 40, 6338–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EYD, Vogirala VK, Inian O, Wong ASW, Nordenskiöld L, Plitzko JM, Danev R, and Sandin S (2016). 3.9 Å structure of the nucleosome core particle determined by phase-plate cryo-EM. Nucleic Acids Res. 44, 8013–8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corujo D, and Buschbeck M (2018). Post-translational modifications of H2A histone variants and their role in cancer. Cancers (Basel). 10, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Imprima E, Floris D, Joppe M, Sánchez R, Grininger M, and Kühlbrandt W (2019). Protein denaturation at the air-water interface and how to prevent it. Elife 8, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodonova SO, Zhu F, Dienemann C, Taipale J, and Cramer P (2020). Nucleosome-bound SOX2 and SOX11 structures elucidate pioneer factor function. Nature 580, 669–672. [DOI] [PubMed] [Google Scholar]
- Dolinsky TJ, Nielsen JE, McCammon JA, and Baker NA (2004). PDB2PQR: an automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorigo B, Schalch T, Kulangara A, Duda S, Schroeder RR, and Richmond TJ (2004). Nucleosome arrays reveal the two-start organization of the chromatin fiber. Science 306, 1571–1573. [DOI] [PubMed] [Google Scholar]
- Dworkin-Rastl E, Kandolf H, and Smith RC (1994). The maternal histone H1 variant, H1M (B4 protein), is the predominant H1 histone in Xenopus pregastrula embryos. Dev. Biol 161, 425–439. [DOI] [PubMed] [Google Scholar]
- Ekundayo B, Richmond TJ, and Schalch T (2017). Capturing Structural Heterogeneity in Chromatin Fibers. J. Mol. Biol 429, 3031–3042. [DOI] [PubMed] [Google Scholar]
- Eltsov M, MacLellan KM, Maeshima K, Frangakis AS, and Dubochet J (2008). Analysis of cryo-electron microscopy images does not support the existence of 30-nm chromatin fibers in mitotic chromosomes in situ. Proc. Natl. Acad. Sci. U. S. A 105, 19732–19737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltsov M, Grewe D, Lemercier N, Frangakis A, Livolant F, and Leforestier A (2018). Nucleosome conformational variability in solution and in interphase nuclei evidenced by cryo-electron microscopy of vitreous sections. Nucleic Acids Res. 46, 9189–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, and Cowtan K (2004). Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Eustermann S, Schall K, Kostrewa D, Lakomek K, Strauss M, Moldt M, and Hopfner K (2018). remodelling by the INO80 complex. Nature 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk SJ, Guo LY, Sekulic N, Smoak EM, Mani T, Logsdon GA, Gupta K, Jansen LET, Van Duyne GD, Vinogradov SA, et al. (2015). CENP-C reshapes and stabilizes CENP-A nucleosomes at the centromere. Science 348, 699 LP – 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnung L, Vos SM, Wigge C, and Cramer P (2017). Nucleosome-Chd1 structure and implications for chromatin remodelling. Nature 550, 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Iwane AH, Yanagida T, and Namba K (2010). Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature 467, 724–728. [DOI] [PubMed] [Google Scholar]
- Funabiki H, Jenness C, and Zierhut C (2017). Nucleosome-Dependent Pathways That Control Mitotic Progression. Cold Spring Harb. Symp. Quant. Biol 82, 173–185. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Codomo CA, and Henikoff S (2013). Reconstitution of hemisomes on budding yeast centromeric DNA. Nucleic Acids Res. 41, 5769–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon J, Daou S, Zamorano N, Iannantuono NVG, Hammond-Martel I, Mashtalir N, Bonneil E, Wurtele H, Thibault P, and Affar EB (2015). Undetectable histone O-GlcNAcylation in mammalian cells. Epigenetics 10, 677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs EB, and Kriwacki RW (2018). Linker histones as liquid-like glue for chromatin. Proc. Natl. Acad. Sci. U. S. A 115, 11868–11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibcus JH, Samejima K, Goloborodko A, Samejima I, Naumova N, Nuebler J, Kanemaki MT, Xie L, Paulson JR, Earnshaw WC, et al. (2018). A pathway for mitotic chromosome formation. Science 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BA, Doolittle LK, Schneider MWG, Jensen LE, Gamarra N, Henry L, Gerlich DW, Redding S, and Rosen MK (2019). Organization of Chromatin by Intrinsic and Regulated Phase Separation. Cell 179, 470–484.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, and Kirschner MW (1991). Cyclin is degraded by the ubiquitin pathway. Nature 349, 132–138. [DOI] [PubMed] [Google Scholar]
- Goloborodko A, Imakaev MV, Marko JF, and Mirny L (2016). Compaction and segregation of sister chromatids via active loop extrusion. Elife 5, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Qian H, Zhou X, Wu J, Wan T, Cao P, Huang W, Zhao X, Wang X, Wang P, et al. (2016). Structural insights into the Niemann-Pick C1 (NPC1)-mediated cholesterol transfer and ebola infection. Cell 165, 1467–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorieff N (2016). Frealign: An Exploratory Tool for Single-Particle Cryo-EM (Elsevier Inc.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryev SA (2018). Chromatin Higher-Order Folding: A Perspective with Linker DNA Angles. Biophys. J 114, 2290–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryev SA, Arya G, Correll S, Woodcock CL, and Schlick T (2009). Evidence for heteromorphic chromatin fibers from analysis of nucleosome interactions. Proc. Natl. Acad. Sci. U. S. A 106, 13317–13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MA, Shundrovsky A, Bai L, Fulbright RM, Lis JT, and Wang MD (2009). High-resolution dynamic mapping of histone-DNA interactions in a nucleosome. Nat. Struct. Mol. Biol 16, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Reyes AA, Malik S, and He Y (2020). Cryo-EM structure of SWI/SNF complex bound to a nucleosome. Nature 579, 452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takanaka Y, Maehara K, Harada A, Umehara T, Yokoyama S, Obuse C, Ohkawa Y, Nozaki N, and Kimura H (2015). Distribution of histone H4 modifications as revealed by a panel of specific monoclonal antibodies. Chromosom. Res 23, 753–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wu Z, Tian Y, Yu Z, Yu J, Wang X, Li J, Liu B, and Xu Y (2020). Structure of nucleosome-bound human BAF complex. Science 367, 875–881. [DOI] [PubMed] [Google Scholar]
- Hirano T (2014). Condensins and the evolution of torsion-mediated genome organization. Trends Cell Biol. 24, 727–733. [DOI] [PubMed] [Google Scholar]
- Hirano T, and Mitchison TJ (1994). A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell 79, 449–458. [DOI] [PubMed] [Google Scholar]
- Hite RK, and MacKinnon R (2017). Structural Titration of Slo2.2, a Na+-Dependent K+ Channel. Cell 168, 390–399.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CH, Takizawa Y, Kobayashi W, Arimura Y, Kimura H, and Kurumizaka H (2021). Structural basis of nucleosomal histone H4 lysine 20 methylation by SET8 methyltransferase. Life Sci. Alliance 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Steffen P, Wordeman L, and Mitchison T (1991). Preparation of modified tubulins. In Molecular Motors and the Cytoskeleton, (Academic Press; ), pp. 478–485. [DOI] [PubMed] [Google Scholar]
- Jenness C, Giunta S, Müller MM, Kimura H, Muir TW, and Funabiki H (2018). HELLS and CDCA7 comprise a bipartite nucleosome remodeling complex defective in ICF syndrome. Proc. Natl. Acad. Sci. U. S. A 115, E876–E885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastritis PL, O’Reilly FJ, Bock T, Li Y, Rogon MZ, Buczak K, Romanov N, Betts MJ, Bui KH, Hagen WJ, et al. (2017). Capturing protein communities by structural proteomics in a thermophilic eukaryote. Mol. Syst. Biol 13, 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato D, Osakabe A, Arimura Y, Mizukami Y, Horikoshi N, Saikusa K, Akashi S, Nishimura Y, Park S-Y, Nogami J, et al. (2017). Crystal structure of the overlapping dinucleosome composed of hexasome and octasome. Science 356. [DOI] [PubMed] [Google Scholar]
- Kelly WG, and Hart GW (1989). Glycosylation of chromosomal proteins: Localization of O-linked N-acetylglucosamine in Drosophila chromatin. Cell 57, 243–251. [DOI] [PubMed] [Google Scholar]
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, and Funabiki H (2010). Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 330, 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A, Rhodes D, Smith J, Finch JT, and Thomas JO (1980). A low resolution structure for the histone core of the nucleosome. Nature 287, 509–516. [DOI] [PubMed] [Google Scholar]
- Koslover EF, Fuller CJ, Straight AF, and Spakowitz AJ (2010). Local geometry and elasticity in compact chromatin structure. Biophys. J 99, 3941–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krietenstein N, and Rando OJ (2020). Mesoscale organization of the chromatin fiber. Curr. Opin. Genet. Dev 61, 32–36. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Ehara H, Fujino Y, Shirouzu M, Sekine S. ichi, and Kurumizaka H (2018). Structural basis of the nucleosome transition during RNA polymerase II passage. Science 362, 595–598. [DOI] [PubMed] [Google Scholar]
- Kyrilis FL, Meister A, and Kastritis PL (2019). Integrative biology of native cell extracts: A new era for structural characterization of life processes. Biol. Chem 400, 831–846. [DOI] [PubMed] [Google Scholar]
- Kyte J, and Doolittle RF (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol 157, 105–132. [DOI] [PubMed] [Google Scholar]
- de la Rosa-Trevín JM, Quintana A, del Cano L, Zaldívar A, Foche I, Gutiérrez J, Gómez-Blanco J, Burguet-Castell J, Cuenca-Alba J, Abrishami V, et al. (2016). Scipion: A software framework toward integration, reproducibility and validation in 3D electron microscopy. J. Struct. Biol 195, 93–99. [DOI] [PubMed] [Google Scholar]
- Lavelle C, and Prunell A (2007). Chromatin Polymorphism and the Nucleosome Superfamily. Cell Cycle 6, 2113–2119. [DOI] [PubMed] [Google Scholar]
- Liebschner D, Afonine PV, Baker ML, Bunkoczi G, Chen VB, Croll TI, Hintze B, Hung LW, Jain S, McCoy AJ, et al. (2019). Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. Sect. D Struct. Biol 75, 861–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilic M, Chen J, Boyaci H, Braffman N, Hubin EA, Herrmann J, Müller R, Mooney R, Landick R, Darst SA, et al. (2020). The antibiotic sorangicin A inhibits promoter DNA unwinding in a Mycobacterium tuberculosis rifampicin-resistant RNA polymerase. Proc. Natl. Acad. Sci 117, 30423 LP – 30432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li M, Xia X, Li X, and Chen Z (2017). Mechanism of chromatin remodelling revealed by the Snf2-nucleosome structure. Nature 544, 440–445. [DOI] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, Sargent DF, and Richmond TJ (1997). Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260. [DOI] [PubMed] [Google Scholar]
- Lyumkis D, Brilot AF, Theobald DL, and Grigorieff N (2013). Likelihood-based classification of cryo-EM images using FREALIGN. J. Struct. Biol 183, 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima K, and Eltsov M (2008). Packaging the genome: The structure of mitotic chromosomes. J. Biochem 143, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca TJ, Freedman BS, and Heald R (2005). Histone H1 is essential for mitotic chromosome architecture and segregation in Xenopus laevis egg extracts. J. Cell Biol 169, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero A, Duquerroy S, Trapani S, Goulas T, Guevara T, Andersen GR, Navaza J, Sottrup-Jensen L, and Gomis-Rüth FX (2012). The crystal structure of human α2-macroglobulin reveals a unique molecular cage. Angew. Chemie - Int. Ed 51, 3340–3344. [DOI] [PubMed] [Google Scholar]
- McGinty RK, and Tan S (2015). Nucleosome structure and function. Chem. Rev 115, 2255–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AW (1991). Cell cycle extracts. Methods Cell Biol. 36, 581–605. [PubMed] [Google Scholar]
- Nakane T, Kotecha A, Sente A, McMullan G, Masiulis S, Brown PMGE, Grigoras IT, Malinauskaite L, Malinauskas T, Miehling J, et al. (2020). Single-particle cryo-EM at atomic resolution. Nature 587, 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AH, Thomsen ARB, Cahill TJ, Huang R, Huang LY, Marcink T, Clarke OB, Heissel S, Masoudi A, Ben-Hail D, et al. (2019). Structure of an endosomal signaling GPCR–G protein–β-arrestin megacomplex. Nat. Struct. Mol. Biol 26, 1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino Y, Eltsov M, Joti Y, Ito K, Takata H, Takahashi Y, Hihara S, Frangakis AS, Imamoto N, Ishikawa T, et al. (2012). Human mitotic chromosomes consist predominantly of irregularly folded nucleosome fibres without a 30-nm chromatin structure. EMBO J. 31, 1644–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Ando T, Priest DG, Kumar V, Yoshida Y, and Taniguchi Y (2019). Sub-nucleosomal Genome Structure Reveals Distinct Nucleosome Folding Motifs. Cell 176, 520–534.e25. [DOI] [PubMed] [Google Scholar]
- Olins DE, and Olins AL (2003). Chromatin history: Our view from the bridge. Nat. Rev. Mol. Cell Biol 4, 809–814. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera - A visualization system for exploratory research and analysis. J. Comput. Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Piovesan D, Minervini G, and Tosatto SCE (2016). The RING 2.0 web server for high quality residue interaction networks. Nucleic Acids Res. 44, W367–W374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punjani A, and Fleet DJ (2021). 3D variability analysis: Resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol 213, 107702. [DOI] [PubMed] [Google Scholar]
- Punjani A, Rubinstein JL, Fleet DJ, and Brubaker MA (2017). CryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296. [DOI] [PubMed] [Google Scholar]
- Renz M, Nehls P, and Hozier J (1977). Involvement of histone H1 in the organization of the chromosome fiber. Proc. Natl. Acad. Sci. U. S. A 74, 1879–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci MA, Manzo C, García-Parajo MF, Lakadamyali M, and Cosma MP (2015). Chromatin fibers are formed by heterogeneous groups of nucleosomes in vivo. Cell 160, 1145–1158. [DOI] [PubMed] [Google Scholar]
- Richmond TJ, Finch JT, Rushton B, Rhodes D, and Klug A (1984). Structure of the nucleosome core particle at 7 resolution. Nature 311, 532–537. [DOI] [PubMed] [Google Scholar]
- Risca VI, Denny SK, Straight AF, and Greenleaf WJ (2017). Variable chromatin structure revealed by in situ spatially correlated DNA cleavage mapping. Nature 541, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJJ, Fairall L, Huynh VAT, and Rhodes D (2006). EM measurements define the dimensions of the “30-nm” chromatin fiber: Evidence for a compact, interdigitated structure. Proc. Natl. Acad. Sci. U. S. A 103, 6506–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K, Booth DG, Ogawa H, Paulson JR, Xie L, Watson CA, Platani M, Kanemaki MT, and Earnshaw WC (2018). Functional analysis after rapid degradation of condensins and 3D-EM reveals chromatin volume is uncoupled from chromosome architecture in mitosis. J. Cell Sci. 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanulli S, Trnka MJ, Dharmarajan V, Tibble RW, Pascal BD, Burlingame AL, Griffin PR, Gross JD, and Narlikar GJ (2019). HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature 575, 390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalch T, Duda S, Sargent DF, and Richmond TJ (2005). X-ray structure of a tetranucleosome and its implications for the chromatin fibre. Nature 436, 138–141. [DOI] [PubMed] [Google Scholar]
- Scheffer MP, Eltsov M, and Frangakis AS (2011). Evidence for short-range helical order in the 30-nm chromatin fibers of erythrocyte nuclei. Proc. Natl. Acad. Sci. U. S. A 108, 16992–16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SHW (2012). RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol 180, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SHW, Gao H, Valle M, Herman GT, Eggermont PPB, Frank J, and Carazo JM (2007). Disentangling conformational states of macromolecules in 3D-EM through likelihood optimization. Nat. Methods 4, 27–29. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhüusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, and Selbach M (2011). Global quantification of mammalian gene expression control. Nature 473, 337–342. [DOI] [PubMed] [Google Scholar]
- Shamu CE, and Murray AW (1992). Sister chromatid separation in frog egg extracts requires DNA topoisomerase II activity during anaphase. J. Cell Biol. 117, 921–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter D, Chitta RK, Xiao A, Shabanowitz J, Hunt DF, and Allis CD (2009). A distinct H2A.X isoform is enriched in Xenopus laevis eggs and early embryos and is phosphorylated in the absence of a checkpoint. Proc. Natl. Acad. Sci. U. S. A 106, 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havliš J, Olsen JV, and Mann M (2007). In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc 1, 2856–2860. [DOI] [PubMed] [Google Scholar]
- Sinha KK, Gross JD, and Narlikar GJ (2017). Distortion of histone octamer core promotes nucleosome mobilization by a chromatin remodeler. Science 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrajna A, Goldfarb D, Kedziora KM, Cousins EM, Grant GD, Spangler CJ, Barbour EH, Yan X, Hathaway NA, Brown NG, et al. (2020). Comprehensive nucleosome interactome screen establishes fundamental principles of nucleosome binding. Nucleic Acids Res. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Chen P, Sun D, Wang M, Dong L, Liang D, Xu RM, Zhu P, and Li G (2014). Cryo-EM study of the chromatin fiber reveals a double helix twisted by tetranucleosomal units. Science 344, 376–380. [DOI] [PubMed] [Google Scholar]
- Su CC, Lyu M, Morgan CE, Bolla JR, Robinson CV, and Yu EW (2021). A ‘Build and Retrieve’ methodology to simultaneously solve cryo-EM structures of membrane proteins. Nat. Methods 18, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, and Hirota T (2019). Folding the genome into mitotic chromosomes. Curr. Opin. Cell Biol 60, 19–26. [DOI] [PubMed] [Google Scholar]
- Tan ZY, Cai S, Noble AJ, Chen JK, Shi J, and Gan L (2021). Heterogeneous non-canonical nucleosomes predominate in yeast cells in situ. BioRxiv 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea JE, Cherry S, and Waugh DS (2009). Expression and Purification of Soluble His6-Tagged TEV Protease. In High Throughput Protein Expression and Purification: Methods and Protocols, Doyle SA, ed. (Totowa, NJ: Humana Press; ), pp. 297–307. [DOI] [PubMed] [Google Scholar]
- Turner AL, Watson M, Wilkins OG, Cato L, Travers A, Thomas JO, and Stott K (2018). Highly disordered histone H1–DNA model complexes and their condensates. Proc. Natl. Acad. Sci. U. S. A 115, 11964–11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle AH, and Axel Innis C (2020). Prospects for antimicrobial development in the cryo-EM era – A focus on the ribosome. FEMS Microbiol. Rev 44, 793–803. [DOI] [PubMed] [Google Scholar]
- Vargas J, Álvarez-Cabrera AL, Marabini R, Carazo JM, and Sorzano COS (2014). Efficient initial volume determination from electron microscopy images of single particles. Bioinformatics 30, 2891–2898. [DOI] [PubMed] [Google Scholar]
- Verbeke EJ, Mallam AL, Drew K, Marcotte EM, and Taylor DW (2018). Classification of Single Particles from Human Cell Extract Reveals Distinct Structures. Cell Rep. 24, 259–268.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke EJ, Zhou Y, Horton AP, Mallam AL, Taylor DW, and Marcotte EM (2020). Separating distinct structures of multiple macromolecular assemblies from cryo-EM projections. J. Struct. Biol 209, 107416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, and Higgins JMG (2013). Histone modications and mitosis: Countermarks, landmarks, and bookmarks. Trends Cell Biol. 23, 175–184. [DOI] [PubMed] [Google Scholar]
- Wangkanont K, Wesener DA, Vidani JA, Kiessling LL, and Forest KT (2016). Structures of Xenopus embryonic epidermal lectin reveal a conserved mechanism of microbial glycan recognition. J. Biol. Chem 291, 5596–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, De Beer TAP, Rempfer C, Bordoli L, et al. (2018). SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CM, Ramachandran S and Henikoff S (2014). Nucleosomes are context-specific, H2A.Z-Modulated barriers to RNA polymerase. Mol. Cell 53, 819–830. [DOI] [PubMed] [Google Scholar]
- Willcockson MA, Healton SE, Weiss CN, Bartholdy BA, Botbol Y, Mishra LN, Sidhwani DS, Wilson TJ, Pinto HB, Maron MI, et al. (2021). H1 histones control the epigenetic landscape by local chromatin compaction. Nature 589, 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willhoft O, Ghoneim M, Lin CL, Chua EYD, Wilkinson M, Chaban Y, Ayala R, McCormack EA, Ocloo L, Rueda DS, et al. (2018). Structure and dynamics of the yeast SWR1-nucleosome complex. Science 362. [DOI] [PubMed] [Google Scholar]
- Woods DC, and Wereszczynski J (2020). Elucidating the influence of linker histone variants on chromatosome dynamics and energetics. Nucleic Acids Res. 48, 3591–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wühr M, Freeman RM, Presler M, Horb ME, Peshkin L, Gygi SP, and Kirschner MW (2014). Deep proteomics of the xenopus laevis egg using an mRNA-derived reference database. Curr. Biol 24, 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Wu H, Li X, Gao N, and Chen Z (2019). Structures of the ISWI–nucleosome complex reveal a conserved mechanism of chromatin remodeling. Nat. Struct. Mol. Biol 26, 258–266. [DOI] [PubMed] [Google Scholar]
- Ye Y, Wu H, Chen K, Clapier CR, Verma N, Zhang W, Deng H, Cairns BR, Gao N, and Chen Z (2019). Structure of the RSC complex bound to the nucleosome. Science 366, 838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, Verbeke EJ, Chang Y, Dickinson DJ, and Taylor DW (2019). Electron microscopy snapshots of single particles from single cells. J. Biol. Chem 294, 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufova N, Kloetgen A, Teater M, Osunsade A, Camarillo JM, Chin CR, Doane AS, Venters BJ, Portillo-Ledesma S, Conway J, et al. (2021). Histone H1 loss drives lymphoma by disrupting 3D chromatin architecture. Nature 589, 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, and Agard DA (2017). MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiteneva A, Bonfiglio JJ, Makarov A, Colby T, Vagnarelli P, Schirmer EC, Matic I, and Earnshaw WC (2017). Mitotic post-translational modifications of histones promote chromatin compaction in vitro. Open Biol. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BR, Jiang J, Feng H, Ghirlando R, Xiao TS, and Bai Y (2015). Structural Mechanisms of Nucleosome Recognition by Linker Histones. Mol. Cell 59, 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BR, Yadav KNS, Borgnia M, Hong J, Cao B, Olins AL, Olins DE, Bai Y, and Zhang P (2019). Atomic resolution cryo-EM structure of a native-like CENP-A nucleosome aided by an antibody fragment. Nat. Commun 10, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Dai L, Li C, Shi L, Huang Y, Guo Z, Wu F, Zhu P, and Zhou Z (2020). Structural basis of nucleosome dynamics modulation by histone variants H2A.B and H2A.Z.2.2. EMBO J. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierhut C, Jenness C, Kimura H, and Funabiki H (2014). Nucleosomal regulation of chromatin composition and nuclear assembly revealed by histone depletion. Nat. Struct. Mol. Biol 21, 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatanova J, Bishop TC, Victor JM, Jackson V, and van Holde K (2009). The Nucleosome Family: Dynamic and Growing. Structure 17, 160–171. [DOI] [PubMed] [Google Scholar]
- Zou T, Hashiya F, Wei Y, Yu Z, Pandian GN, and Sugiyama H (2018). Direct Observation of H3–H4 Octasome by High-Speed AFM. Chem. - A Eur. J 24, 15998–16002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure360. Introduction video for 3DVA. Related to Figure 4A.
Movie S1. The 3DVA of the interphase oligo (lot 1), metaphase oligo (lot 1), freed (lot 2), and GUB-open nucleosome. Related to Figure 4, 5, 6, 7.
Table S1. Mass spectrometry analysis results. Related to Figure 1
Sheet 1. Raw data of the Mass spectrometry analysis of the mono, di, and oligo fractions of interphase and metaphase chromosome.
Sheet 2. Modified data with summing the iBAQ values of peptides that separately mapped to highly similar protein isoforms.
Table S3. Number of particles in each 3D class after in silico mixing 3D classification. Related to Star methods.
Figure S1-S12, Table S2
Data Availability Statement
Cryo-EM raw data are available in the EMPIAR. EM maps are available in the EMDR. Atomic models are available in the PDB. IDs for each data are listed in star method table, Table 1 and Table 2. This paper does not report any original code. Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.
Table 1.
Data collection, model refinement and validation for simultaneous TRSF reconstruction of cryo-EM structures of multiple protein complexes. Related to Figure 2
| Micrograph name | Interphase_oligo_lot1 | Metaphase_oligo_lot 1 | Interphase_oligo _lot2 |
metaphase_oligo _lot2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deta collection and prosessing | ||||||||||
| Microscope | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | |||||
| Magnification | 28,000 | 28,000 | 28,000 | 28,000 | 28,000 | |||||
| Voltage (kV) | 200 | 200 | 200 | 200 | 200 | |||||
| Camera | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | |||||
| Electron exposure (e−/Å) | 33.1 | 38.3 | 35.3 | 34.2 | 34.2 | |||||
| Defocus range (μm) | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | |||||
| Pixel size (Å) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | |||||
| Micrographs | 1,325 | 2,676 | 1,156 | 1,044 | ||||||
| EMPIAR ID of raw micrographs | EMPIAR-10692 | EMPIAR-10691 | EMPIAR-10747 | EMPIAR-10746 | ||||||
| EM 3D maps | Octameric nucleosome |
Intelectin- 2 |
F-actin | alpha2- macroglobulin |
Octameric nucleosome |
Intelectin- 2 |
F-actin | alpha2- macroglobulin |
Octameric nucleosome |
Octameric nucleosome |
| Initial particle images (no.) | 812,976 | 1,317,710 | 320,524 | 384,740 | ||||||
| High resolution group particles(no.) | 66,426 | 166,673 | 35,700 | 55,801 | ||||||
| Low resolution group particles(no.) | 746,550 | 1,151,037 | 284,824 | 328,939 | ||||||
| particle for 3D clasification (no.) | 812,976 | 1,317,710 | 320,524 | 384,740 | ||||||
| Particle images (no.) | 62,014 | 89,728 | 10,922 | 9,721 | 116,723 | 150,300 | 38,321 | 49,363 | 40,640 | 40,822 |
| Symmetry imposed | C1 | C3 | C1 | C1 | C1 | C1 | C1 | D2 | C1 | C1 |
| Map resolution (FSC=0.143) | 4.2 | 4.5 | 7.6 | 8.07 | 4.2 | 4.7 | 4.5 | 5.5 | 4.42 | 4.32 |
| EM data resource ID | EMD-22793 | - | - | EMD-22794 | EMD-22795 | - | - | EMD-22796 | EMD-23820 | EMD-23822 |
Table 2.
Data collection, model refinement and validation for averaged nucleosome structure analysis. Related to Figure 3, 6
| Micrograph name | Interphase mono lot 1 |
Interphase di lot 1 |
Interphase oligo lot 1 |
Metaphase mono lot 1 |
Metaphase di lot 1 |
Metaphase oligo lot 1 |
Interphase, 'Freed' (Crosslinked after MNase) lot 2 |
Interphase oligo lot 2 |
Metaphase oligo lot 2 |
GUB-closed nucleosome |
GUB-open nucleosome |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deta collection and prosessing | ||||||||||||||
| Microscope | FEI Titan Krios | FEI Titan Krios | FEI Talos Arctica | FEI Titan Krios | FEI Titan Krios | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | FEI Talos Arctica | |||
| Magnification | 25,000 | 20,000 | 28,000 | 25,000 | 20,000 | 28,000 | 28,000 | 28,000 | 28,000 | 28,000 | 28,000 | |||
| Voltage (kV) | 300 | 300 | 200 | 300 | 300 | 200 | 200 | 200 | 200 | 200 | 200 | |||
| Camera | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | Gatan K2 Summit | |||
| Electron exposure (e−/Å) | 45.0 | 55.5 | 33.1 | 44.7 | 54.0 | 38.3 | 35.3 | 37.3 | 31.1 | 34.2 | 34.2 | |||
| Defocus range (μm) | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | −1 ~ −3 | |||
| Pixel size (Å) | 1.33 | 1.6 | 1.5 | 1.33 | 1.6 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | |||
| Micrographs | 1,622 | 1,270 | 1,325 | 1,637 | 1,347 | 2,676 | 2,049 | 1,375 | 1,044 | |||||
| EMPIAR ID of raw micrographs | EMPIAR-10742 | EMPIAR-10744 | EMPIAR-10692 | EMPIAR-10743 | EMPIAR-10745 | EMPIAR-10691 | EMPIAR-10750 | EMPIAR-10747 | EMPIAR-10746 | EMPIAR-10749 | EMPIAR-10748 | |||
| EM 3D map. Name | Nucleosome | Nucleosome | Nucleosome | Nucleosome | Nucleosome | Nucleosome | Nucleosome + H1.8 |
"Free" nucleosome |
Nucleosome | Nucleosome | Nucleosome + H1.8 |
GUB-closed nucleosome |
GUB-open nucleosome |
|
| Initial particle images (no.) | 147,921 | 213,608 | 571,558 | 116,669 | 167,734 | 898,553 | 259,060 | 178,949 | 194,837 | 203,052 | 199,125 | |||
| Particles for nucleosome ab-initio reconstruction (no.) | 13,434 | 21,650 | 85,994 | 11,325 | 9,515 | 154,208 | 25,123 | 61,931 | 50,002 | 86,720 | 75,171 | |||
| Particles for 'decoy' ab-initio reconstruction (no.) | 134,487 | 100,946 | 485,564 | 105,344 | 102,722 | 744,345 | 133,700 | 117,018 | 144,835 | 116,332 | 123,954 | |||
| particle for 3D clasification (no.) | 147,921 | 122,596 | 571,558 | 116,669 | 112,237 | 898,553 | 158,823 | 178,949 | 194,837 | 203,052 | 199,125 | |||
| Particle images (no.) | 13,798 | 15,653 | 89,191 | 13,350 | 6,027 | 155,413 | 27,383 | 20,835 | 52,163 | 52,941 | 6,399 | 76,391 | 62,823 | |
| Symmetry imposed | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 | |
| Map resolution (FSC=0.143) | 5.12 | 4.74 | 3.38 | 5.64 | 8.1 | 3.5 | 4.42 | 5.44 | 3.54 | 3.77 | 5.6 | 3.77 | 4.52 | |
| CTF rifinement | ✓ (Relion 3.1) | ✓
(Relion 3.1) |
✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | - | ✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | - | ✓ (Relion 3.1) | ✓ (Relion 3.1) | |
| Particle polishing | ✓ (Relion 3.1) | ✓
(Relion 3.1) |
✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | - | ✓ (Relion 3.1) | ✓ (Relion 3.1) | ✓ (Relion 3.1) | - | ✓ (Relion 3.1) | ✓ (Relion 3.1) | |
| Postprocess Pixel size | 1.3 | 1.66 | 1.47 | 1.3 | 1.66 | 1.47 | - | 1.47 | 1.47 | 1.47 | - | 1.47 | 1.47 | |
| EM data resource ID | EMD-22797 | EMD-22798 | EMD-22790 | EMD-22800 | EMD-22801 | EMD-22791 | EMD-22792 | EMD-23826 | EMD-23819 | EMD-23821 | - | EMD-23823 | EMD-23824 | |
| Atomic coordinate name | - | - | Nucleosome | - | - | Nucleosome | Nucleosome + H1.8 | - | - | - | - | - | - | - |
| Pixel size | 1.47 | 1.47 | 1.47 | |||||||||||
| Model resolution (Å) | 3.38 | 3.5 | 4.4 | |||||||||||
| Initial model used (code) | 6DZT, 5NL0 | 6DZT, 5NL0 | 5NL0 | |||||||||||
| Map sharpening | RELION 3.1 (Postprocess) | RELION 3.1 (Postprocess) | CryoSPARC, bsoft (blocres/blocafilt) | |||||||||||
| Map sharpening B factor (Å2) | −60.3386 | −30.7959 | - | |||||||||||
| Model composition | ||||||||||||||
| Non-hydrogen atoms | 12,169 | 12,408 | 13,710 | |||||||||||
| Protein residues | 755 | 760 | 842 | |||||||||||
| Nucleotide | 302 | 312 | 344 | |||||||||||
| Model vs. Data | ||||||||||||||
| CC (mask) | 0.81 | 0.8 | 0.73 | |||||||||||
| CC (box) | 0.74 | 0.73 | 0.83 | |||||||||||
| CC (peak) | 0.7 | 0.69 | 0.72 | |||||||||||
| CC (volume) | 0.79 | 0.78 | 0.76 | |||||||||||
| R.m.s. deviations | ||||||||||||||
| Bond lengths (Å) | 0.008 | 0.008 | 0.008 | |||||||||||
| BoBond angles (°) | 0.691 | 0.753 | 0.867 | |||||||||||
| Validation | ||||||||||||||
| MolProbity score | 2.42 | 2.56 | 2.96 | |||||||||||
| Clashscore | 6.86 | 9.71 | 30.6 | |||||||||||
| Ramachandran plot | ||||||||||||||
| Favored (%) | 94.99 | 95.3 | 93.93 | |||||||||||
| Allowed (%) | 5.01 | 4.57 | 6.07 | |||||||||||
| Disallowed (%) | 0 | 0.13 | 0 | |||||||||||
| Protein data bank ID | 7KBD | 7KBE | 7KBF | |||||||||||