Abstract
Background
The accuracy of urine dipsticks to detect increased albuminuria is uncertain. We aimed to assess the diagnostic accuracy of urine dipsticks for detecting albuminuria.
Methods
A systematic review of studies that assessed the diagnostic accuracy of urine dipstick testing for detecting albuminuria has been conducted (using as reference standard the albuminuria in a 24-hour sample or the albumin-to-creatinine ratio) in Scopus, PubMed, and Google Scholar. The risk of bias of the included studies has been assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. Whenever possible, we performed meta-analyses for sensitivity and specificity. The certainty of the evidence has also been assessed using the Grading of Recommendations Assessment, Development, and Evaluation methodology.
Results
A total of 14 studies have been included in this review, having assessed all albumin-to-creatinine ratio (ACR) as assessed standard. Each study used different dipstick types. The resulting pooled sensitivity and specificity for each cutoff point were as follows: for ACR >30 mg/g (13 studies): 0.82 (95% confidence interval: 0.76–0.87) and 0.88 (0.83–0.91); for ACR 30–300 mg/g (7 studies): 0.72 (0.68–0.77) and 0.82 (0.76–0.89); and for ACR >300 mg/g (7 studies): 0.84 (0.71–0.90) and 0.97 (0.95–0.99), respectively. An overall high risk of bias, an important heterogeneity in all pooled analysis, and a very low certainty of the evidence have been found.
Conclusions
Pooled sensitivity and specificity of urine dipsticks have been calculated for different ACR cutoff points. However, the dipstick types differed across studies, and the certainty of the evidence was very low. Thus, further well-designed studies are needed to reach more confident estimates and to assess accuracy differences across dipstick types.
Registration
PROSPERO (CRD42019124637).
Keywords: Albuminuria, Sensitivity and specificity, Renal insufficiency, Chronic
Albuminuria; Sensitivity and specificity; Renal insufficiency, Chronic.
1. Introduction
For the diagnosis of chronic kidney disease (CKD), guidelines recommend performing an initial albuminuria testing using either the albumin-to-creatinine ratio (ACR) or the protein-to-creatinine ratio (PCR). When ACR and PCR are not available, some guidelines recommend the use of semiquantitative methods (urine dipsticks) that can measure albuminuria or express the result as ACR and the subsequent confirmation of the positive dipstick results with a quantitative laboratory test [1, 2]. However, no consensus regarding the use of urine dipsticks have been reached, and some guidelines do not support it [3].
Urine dipsticks represent a feasible point-of-care test that could be used in areas where other laboratory analyses are not available. However, its sensitivity for albuminuria detection is a bit concerning, since false negatives have been proposed to appear in the presence of ketones, glucose, blood, pigments, vitamins, or antibiotics [4, 5, 6], and a manual reading may incur operator-dependent faults [7].
Two previous systematic reviews have assessed the use of urine dipsticks for detecting albuminuria in the context of performing a CKD diagnosis. One of them performed its literature search in December 2013 and evaluated the diagnostic accuracy of point-of-care (POC) tests (either semiquantitative and quantitative) in people at risk of CKD, including nine studies that assessed the accuracy of urine dipsticks, reporting a pooled sensitivity and specificity of 0.76 and 0.93, respectively, for ACR >30 mg/g [8]. The other study is a Cochrane systematic review, which in September 2014 has searched for randomized controlled trials that assessed the effects of using urine dipstick testing for CKD diagnosis, and no other study was found [9].
Given the need for updated systematizations of the evidence to perform adequate decision-making, we aimed to perform a systematic review on the diagnostic accuracy of urine dipstick testing for detecting albuminuria.
2. Materials and methods
This systematic review was conducted following the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy and the Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA) reporting guidelines [10, 11]. The protocol was registered at PROSPERO (CRD42019124637, available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=124637).
2.1. Data sources and searches
The search process had two steps: 1) Systematic searches have been performed in PubMed (via MEDLINE), Scopus, and Google Scholar in July 2020 (search terms are available in Table 1). 2) Furthermore, the references of each of the studies included in Step 1 were reviewed to find more eligible studies. The publication date has no restriction.
Table 1.
Search strategy.
| Search engine | Date | Term | Results |
|---|---|---|---|
| Pubmed | July 07, 2020 | ((“Reagent Strips”[Mesh] OR strip[tiab] OR strips[tiab] OR dipstick∗[tiab] OR point-of-care[TIAB] OR kit[TIAB]) AND (“Proteinuria”[Mesh] OR proteinuria[tiab] OR “Albuminuria”[Mesh] OR albuminuria[tiab] OR microalbuminuria[tiab]) AND (Sensitivity[tiab] OR Specificity[tiab] OR “Sensitivity and Specificity”[Mesh] OR “Predictive Value of Tests”[Mesh] OR “predictive value”[tiab] OR “Area Under Curve”[Mesh] OR “area under curve”[tiab] OR auc[tiab] OR “ROC Curve”[Mesh] OR roc[tiab] OR “receiver operating characteristic”[tiab] OR “receiver operating characteristics”[tiab] OR accuracy[tiab] OR predict∗[tiab])) NOT (letter [pt] OR editorial [pt] OR news [pt] OR historical article [pt] OR case reports [pt] OR letter[TI] OR comment∗[TI] OR animal∗[TI] OR “Animals, Laboratory”[Mesh] OR “Animal Experimentation”[Mesh] OR “Rodentia”[Mesh] OR rats[TI] OR rat[TI] OR mouse[TI] OR mice[TI] OR cat[ti] OR cats[ti] OR dog[ti] OR dogs[ti]) | 716 |
| Scopus | July 07, 2020 | TITLE-ABS-KEY ((“Reagent Strips” OR strip OR strips OR dipstick) AND (proteinuria OR ∗albuminuria) AND (Sensitivity OR Specificity OR “predictive value” OR “Area Under Curve” OR auc OR “ROC Curve” OR “receiver operating characteristic” OR accuracy OR predict∗)) AND NOT (KEY (“Laboratory animals” OR “Animal Experimentation” OR Rodentia) OR TITLE (animal∗ OR rodentia OR dog OR dogs OR cat OR cats OR mice OR mouse OR rat OR rats)) | 759 |
| Google Scholar | July 07, 2020 | Strip dipstick albuminuria sensitivity specificity | 100 |
2.2. Studies selection and data extraction
Observational studies that met the following inclusion criteria have been included:
-
1.
Evaluated the diagnostic accuracy of urine dipstick for albuminuria or ACR assessment.
-
2.
Used a quantitative laboratory method for the assessment of the reference standard: either urine albumin (e.g., turbidimetry, nephelometry, or radioimmunoassay) or urine creatinine (e.g., Jaffe's Method).
-
3.
If the reference standard was albuminuria, studies should have used 24-hour urinary samples.
-
4.
Assessed the dipstick accuracy for any of the following cutoff values: ACR >30 mg/g or albuminuria >30 mg/l, ACR >300 mg/g or albuminuria >300 mg/l, or ACR between 30 and 300 mg/g or albuminuria between 30 and 300 mg/l, due to their clinical for CKD diagnosis or stratification according to clinical guidelines [1, 2].
-
5.
Were written in English, Spanish, Portuguese, French, German, or Japanese.
After identifying the articles from the search strategy, duplicates were removed using the EndNote software. Then, each title and abstract were screened using the Rayyan QCRI application [12], and the potentially includable studies were assessed and reviewed in full text. Two independent reviewers conducted each step. Any discrepancy was discussed and solved by a third party.
2.3. Data extraction and quality assessment
For each study, information on each of the following main items was recorded: population, urine dipstick, urine specimen, reference standard, diagnostic accuracy measurements for each cutoff value, and funding. For the population, the age, the setting (general population, screening, primary care, and outpatient), the albuminuria prevalence (albuminuria >30 mg/dl or ACR >30 mg/g), and the number of patients have been collected. The following urine dipstick variables have also been collected: brand, type of albumin and creatinine measurement (colors or trace/+), and lecture (manually or automatically). Regarding the urine specimen, the time of collection (early morning, single random, or 24-hours), the number of measurements, and the number of samples have been collected. Concerning the reference standard test, the time of urine collection (early morning, single random, or 24-hours), the biochemical marker (albuminuria or ACR), and the laboratory method used to identify albumin and creatinine (turbidimetry, nephelometry, radioimmunoassay, Jaffe's, and enzymatic methods, etc.) have been recorded. Finally, the following diagnostic accuracy measurements have been collected: true positives, true negatives, false positives, and false negatives, which are needed to perform the meta-analyses. Two independent reviewers conducted each step of the data extraction. Any discrepancy was discussed and solved by a third party.
For the study quality assessment, two independent authors assessed the risk of bias using the revised tool for the quality assessment of diagnostic accuracy studies-2 (QUADAS-2) [13]. Discrepancies were solved by a consensus between the authors. The QUADAS-2 tool consists of four domains with two to three signaling questions for each domain. For the QUADAS-2 evaluation, criteria were used as reported elsewhere [13]. However, some specific criteria for this review have been established:
For the “index test” domain, the following values were considered as pre-specified thresholds for the index test: ACR >30 mg/g or albuminuria >30 mg/l, ACR >300 mg/g or albuminuria >300 mg/l, and ACR between 30 and 300 mg/g or albuminuria between 30 and 300 mg/l [1].
For the “reference standard” domain for creatinine, the test was considered as likely to correctly classify the target condition when isotope dilution mass spectrometry was used [14, 15, 16], while for protein and albumin, any technique has been considered (e.g., turbidimetry, nephelometry, and radioimmunoassay). In both cases, 24-hour urinary samples were considered as the reference standard [1, 2].
For the “flow and timing” domain, an appropriate interval between index test and reference standard was considered when the collection of the index and reference samples were performed simultaneously. Also, sample processing for both tests (index and reference) was necessary to be performed within the first 8 h after sample collection, or if any of those samples were processed after 8 h from collection, it was stated that such sample was preserved refrigerated [17, 18, 19].
Furthermore, a summary of the findings of our study was reported using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology [20, 21].
2.4. Data synthesis and analysis
A meta-analyses was performed to obtain pooled sensitivities and specificities, along with their 95% confidence intervals (95% CIs). Random-effects models were performed given the high heterogeneity of the studies’ results. Heterogeneity was assessed using a chi-squared test and the I2 statistic. According to a recommendation stated on the PRISMA-DTA, a publication bias has not been evaluated due to the lack of a defined method that should be used in systematic reviews of diagnostic test accuracy studies [11]. Each analysis was performed in Stata v14.0 software (StataCorp LP, College Station, TX, USA).
Furthermore, the number of false-positive and false-negative results has been estimated, from a fictitious population of 1,000 individuals. To estimate the prevalence of ACR >30 mg/g, ACR between 30 and 300 mg/g, and ACR >300 mg/g, the median prevalence from the included studies that have measured all these three cutoff values has been calculated.
3. Results
3.1. Study selection
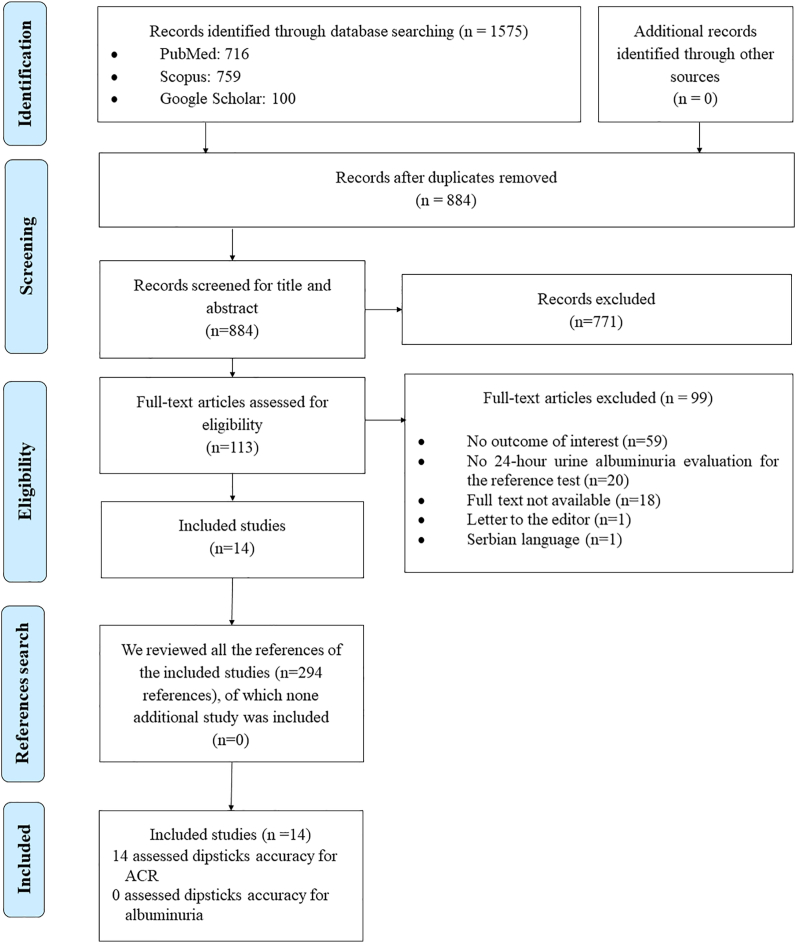
We found 1,575 records during database searching. After duplicate removal, 884 titles and abstracts have been screened, from which 113 underwent a full-text review, and finally 14 studies were included. Similarly, the 294 references of these manuscripts have been examined, and none have met the inclusion criteria. All 14 included studies have been assessed for the accuracy of urine dipsticks for ACR. No study was found that has assessed the accuracy of urine dipsticks for albuminuria directly (Figure 1 and Table 2).
Figure 1.
Flow diagram (study selection).
Table 2.
Studies that were evaluated in full text and were excluded.
| N | Author | Year | Title | Exclusion reason |
|---|---|---|---|---|
| 1 | Sultana | 2018 | Dipstick Method versus Spot Urinary Protein Creatinine Ratio for Evaluation of Massive Proteinuria in Childhood Nephrotic Syndrome | No outcome of interest |
| 2 | Yanagisawa | 2018 | Prevalence of Chronic Kidney Disease and Poor Diagnostic Accuracy of Dipstick Proteinuria in Human Immunodeficiency Virus-Infected Individuals: A Multicenter Study in Japan | No outcome of interest |
| 3 | Ratnayake | 2017 | Screening for chronic kidney disease of uncertain etiology in Sri Lanka: usability of surrogate biomarkers over dipstick proteinuria | No outcome of interest |
| 4 | Koeda | 2016 | Comparison between urine albumin-to-creatinine ratio and urine protein dipstick testing for prevalence and ability to predict the risk for chronic kidney disease in the general population (Iwate-KENCO study): A prospective community-based cohort study | No outcome of interest |
| 5 | Chang | 2016 | The efficacy of semiquantitative urine protein-to-creatinine (P/C) ratio for the detection of significant proteinuria in urine specimens in health screening settings | No outcome of interest |
| 6 | Lopez De Leon | 2015 | Strong correlation between protein reagent strip and protein-to-creatinine ratio for detection of renal dysfunction in HIV-infected patients: a cross-sectional study | No outcome of interest |
| 7 | Lim | 2014 | Diagnostic accuracy of urine dipstick for proteinuria in older outpatients | No outcome of interest |
| 8 | Masimango | 2014 | Prevalence of microalbuminuria and diagnostic value of dipstick proteinuria in outpatients from HIV clinics in Bukavu, the Democratic Republic of Congo | No outcome of interest |
| 9 | Wahbeh | 2014 | Spot urine protein-to-creatinine ratio compared with 24-hour urinary protein in patients with kidney transplant | No outcome of interest |
| 10 | Kumar | 2013 | Comparison of urinary protein: Creatinine index and dipsticks for detection of microproteinuria in diabetes mellitus patients | No outcome of interest |
| 11 | Bello | 2012 | Multiple versus single and other estimates of baseline proteinuria status as predictors of adverse outcomes in the general population | No outcome of interest |
| 12 | Viana | 2012 | Prediction of cardiovascular events, diabetic nephropathy, and mortality by albumin concentration in a spot urine sample in patients with type 2 diabetes | No outcome of interest |
| 13 | Chotayaporn | 2011 | Comparison of proteinuria determination by urine dipstick, spot urine protein creatinine index, and urine protein 24 h in lupus patients | No outcome of interest |
| 14 | White | 2011 | Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community | No outcome of interest |
| 15 | Panek | 2011 | Screening for proteinuria in kidney transplant recipients | No outcome of interest |
| 16 | Lin | 2011 | The characteristics of new semiquantitative method for diagnosing proteinuria by using random urine samples | No outcome of interest |
| 17 | Collier | 2009 | A study of the relationship between albuminuria, proteinuria, and urinary reagent strips | No outcome of interest |
| 18 | Guy | 2009 | Diagnostic accuracy of the urinary albumin: creatinine ratio determined by the CLINITEK Microalbumin and DCA 2000 + for the rule-out of albuminuria in chronic kidney disease | No outcome of interest |
| 19 | Haysom | 2009 | Diagnostic accuracy of urine dipsticks for detecting albuminuria in indigenous and non-indigenous children in a community setting | No outcome of interest |
| 20 | Krol | 2009 | Early detection of chronic kidney disease: results of the PolNef study | No outcome of interest |
| 21 | Afolabi | 2009 | Prevalence of chronic kidney disease in a Nigerian family practice population | No outcome of interest |
| 22 | Biswas | 2009 | Quantitation of proteinuria in nephrotic syndrome by spot urine protein creatinine ratio estimation in children | No outcome of interest |
| 23 | Guy | 2009 | Use of a first-line urine protein-to-creatinine ratio strip test on random urines to rule out proteinuria in patients with chronic kidney disease | No outcome of interest |
| 24 | Siedner | 2008 | Diagnostic accuracy study of urine dipstick in relation to 24-hour measurement as a screening tool for proteinuria in lupus nephritis | No outcome of interest |
| 25 | Abo-Zenah | 2008 | Prevalence of increased albumin excretion rate in young Saudi adults | No outcome of interest |
| 26 | Garcia | 2006 | [Urinary dipsticks must not be used to detect diabetes-induced incipient nephropathy] | No outcome of interest |
| 27 | Lane | 2006 | Can spot urine protein/creatinine ratio replace 24 h urine protein in usual clinical nephrology? | No outcome of interest |
| 28 | Gai | 2006 | Comparison between 24-h proteinuria, urinary protein/creatinine ratio and dipstick test in patients with nephropathy: Patterns of proteinuria in dipstick-negative patients | No outcome of interest |
| 29 | Cortes-Sanabria | 2006 | Utility of the Dipstick Micral test II in the screening of microalbuminuria of diabetes mellitus type 2 and essential hypertension | No outcome of interest |
| 30 | Zeller | 2005 | Diagnostic significance of transferrinuria and albumin-specific dipstick testing in primary care patients with elevated office blood pressure | No outcome of interest |
| 31 | Naka | 2005 | Usefulness of protein/creatinine ratio in spot urine using test strips | No outcome of interest |
| 32 | Zeller | 2005 | Value of a standard urinary dipstick test for detecting microalbuminuria in patients with newly diagnosed hypertension | No outcome of interest |
| 33 | Hoy | 2004 | Albuminuria: marker or target in indigenous populations | No outcome of interest |
| 34 | Morishita | 2004 | Estimation of quantitative proteinuria using a new dipstick in random urine samples | No outcome of interest |
| 35 | Agarwal | 2004 | Quantitation of proteinuria by spot urine sampling | No outcome of interest |
| 36 | Parikh | 2004 | Rapid microalbuminuria screening in type 2 diabetes mellitus: Simplified approach with Micral test strips and specific gravity | No outcome of interest |
| 37 | Osta | 2003 | [Evaluation of two rapid tests for the determination of microalbuminuria and the urinary albumin/creatinine ratio] | No outcome of interest |
| 38 | Croal | 2003 | Evaluation of the Bayer Multistix PRO 10LS Point-of-Care Urine Test | No outcome of interest |
| 39 | Meinhardt | 2003 | Microalbuminuria in diabetes mellitus–Efficacy of a new screening method in comparison with timed overnight urine collection | No outcome of interest |
| 40 | Baskar | 2003 | Uncertain clinical utility of contemporary strategies for microalbuminuria testing | No outcome of interest |
| 41 | Agarwal | 2002 | Dipstick proteinuria: Can it guide hypertension management? | No outcome of interest |
| 42 | Wallace | 2001 | Multisite evaluation of a new dipstick for albumin, protein, and creatinine | No outcome of interest |
| 43 | Shihabi | 2000 | Clinical evaluation of a new strip test for proteinuria on Clinitek® urinalysis systems | No outcome of interest |
| 44 | Lum | 2000 | How effective are screening tests for microalbuminuria in random urine specimens? | No outcome of interest |
| 45 | Davidson | 1999 | Relationship between dipstick positive proteinuria and albumin:creatinine ratios | No outcome of interest |
| 46 | Pugia | 1999 | Screening school children for albuminuria, proteinuria, and occult blood with dipsticks | No outcome of interest |
| 47 | Gerber | 1998 | Assessment of a new dipstick test in screening for microalbuminuria in patients with hypertension | No outcome of interest |
| 48 | Leong | 1998 | The use of semiquantitative urine test-strip (Micral-Test) for microalbuminuria screening in patients with diabetes mellitus | No outcome of interest |
| 49 | Minetti | 1997 | Accuracy of the urinary albumin titrator stick “Micral-Test” in kidney-disease patients | No outcome of interest |
| 50 | Pugia | 1997 | Comparison of urine dipsticks with quantitative methods for microalbuminuria | No outcome of interest |
| 51 | Gilbert | 1997 | Detection of microalbuminuria in diabetic patients by urinary dipstick | No outcome of interest |
| 52 | Adamson | 1993 | Screening strategies in the detection of microalbuminuria in insulin-dependent diabetic patients | No outcome of interest |
| 53 | Gilbert | 1992 | Semiquantitative determination of microalbuminuria by urinary dipstick | No outcome of interest |
| 54 | Kouri | 1991 | Microalbuminuria. Invalidity of simple concentration-based screening tests for early nephropathy due to urinary volumes of diabetic patients | No outcome of interest |
| 55 | Abitbol | 1990 | Quantitation of proteinuria with urinary protein/creatinine ratios and random testing with dipsticks in nephrotic children | No outcome of interest |
| 56 | Sawicki | 1989 | Comparison of Methods for Determination of Microalbuminuria in Diabetic Patients | No outcome of interest |
| 57 | Ralston | 1988 | Screening for proteinuria in a rheumatology clinic: comparison of dipstick testing, 24 h urine quantitative protein, and protein/creatinine ratio in random urine samples | No outcome of interest |
| 58 | Hemmingsen | 1981 | Diagnostic value of a test-strip in detecting increased urinary excretion of albumin, igg and Î22-microglobulin in patients with suspected proteinuria | No outcome of interest |
| 59 | James | 1978 | Proteinuria: accuracy and precision of laboratory diagnosis by dipstick analysis | No outcome of interest |
| 60 | Delanghe | 2017 | Sensitive albuminuria analysis using dye-binding based test strips | No 24-hour urine albuminuria evaluation for the reference test |
| 61 | Asberg | 2016 | Using probit regression to disclose the analytical performance of qualitative and semiquantitative tests | No 24-hour urine albuminuria evaluation for the reference test |
| 62 | Turin | 2014 | Kidney function, albuminuria, and life expectancy | No 24-hour urine albuminuria evaluation for the reference test |
| 63 | Sarafidis | 2008 | A comparative evaluation of various methods for microalbuminuria screening | No 24-hour urine albuminuria evaluation for the reference test |
| 64 | Sam | 2003 | The significance of trace proteinuria | No 24-hour urine albuminuria evaluation for the reference test |
| 65 | Penders | 2002 | Quantitative evaluation of urinalysis test strips | No 24-hour urine albuminuria evaluation for the reference test |
| 66 | Pugia | 2001 | Albuminuria and proteinuria in hospitalized patients as measured by quantitative and dipstick methods | No 24-hour urine albuminuria evaluation for the reference test |
| 67 | Soonthornpun | 2000 | The Utility of Conventional Dipsticks for Urinary Protein for Screening of Microalbuminuria in Diabetic Patients | No 24-hour urine albuminuria evaluation for the reference test |
| 68 | Pegoraro | 1997 | Simplified screening for microalbuminuria | No 24-hour urine albuminuria evaluation for the reference test |
| 69 | Jensen | 1996 | The Micral test for diabetic microalbuminuria: predictive values as a function of prevalence | No 24-hour urine albuminuria evaluation for the reference test |
| 70 | Webb | 1996 | The use of the Micral-Test strip to identify the presence of microalbuminuria in people with insulin-dependent diabetes mellitus (IDDM) participating in the EUCLID study | No 24-hour urine albuminuria evaluation for the reference test |
| 71 | Bloomgarden | 1996 | Urine reagent stick protein determination: Utility in individuals with diabetes mellitus | No 24-hour urine albuminuria evaluation for the reference test |
| 72 | Gossain | 1996 | Utility of micral-test strips in screening for microalbuminuria | No 24-hour urine albuminuria evaluation for the reference test |
| 73 | de Grauw | 1995 | Screening for microalbuminuria in type 2 diabetic patients: the evaluation of a dipstick test in general practice | No 24-hour urine albuminuria evaluation for the reference test |
| 74 | Agardh | 1993 | A new semiquantitative rapid test for screening for microalbuminuria | No 24-hour urine albuminuria evaluation for the reference test |
| 75 | Tiu | 1993 | Comparison of six commercial techniques in the measurement of microalbuminuria in diabetic patients | No 24-hour urine albuminuria evaluation for the reference test |
| 76 | Jury | 1992 | Assessment of Micral-Test microalbuminuria test strip in the laboratory and in diabetic outpatients | No 24-hour urine albuminuria evaluation for the reference test |
| 77 | Marshall | 1992 | Micral-Test strips evaluated for screening for albuminuria | No 24-hour urine albuminuria evaluation for the reference test |
| 78 | Spooren | 1992 | Micral-Test: a qualitative dipstick test for micro-albuminuria | No 24-hour urine albuminuria evaluation for the reference test |
| 79 | Bangstad | 1991 | New semiquantitative dipstick test for microalbuminuria | No 24-hour urine albuminuria evaluation for the reference test |
| 80 | Oyebisi | 2018 | Prevalence and Pattern of Chronic Kidney Disease and its Associated Risk Factors in a Rural Community in South Western Nigeria | Full text not available |
| 81 | Ali | 2017 | Role of micral-test for the detection of microalbuminuria | Full text not available |
| 82 | Aziz | 2015 | Correlation of urine biomarkers: Microalbuminuria and spot urine protein among diabetic patients. Application of spot urine protein in diabetic kidney disease, nephropathy, proteinuria estimation, diagnosing, and monitoring | Full text not available |
| 83 | Shah | 2015 | Usefulness of spot urine protein creatinine ratio in the diagnosis of childhood nephrotic syndrome | Full text not available |
| 84 | Krairittichai | 2011 | Accuracy of urine dipstick test for microalbuminuria in type 2 diabetes mellitus patients | Full text not available |
| 85 | Chan | 2005 | Can the urine dipstick test reduce the need for microscopy for assessment of systemic lupus erythematosus disease activity? | Full text not available |
| 86 | Tsujikawa | 2005 | Evaluation of novel test strip to measure albumin and creatinine in urine | Full text not available |
| 87 | Le Floch | 2001 | Interest of clinitek® microalbumin in screening for microalbuminuria: Results of a multicentre study in 302 diabetic patients | Full text not available |
| 88 | Ng | 2000 | Evaluation of a rapid screening test for microalbuminuria with a spot measurement of urine albumin-creatinine ratio | Full text not available |
| 89 | Ujjin | 2000 | Evaluation of microalb immunoturbidimetric test for albuminuria screening | Full text not available |
| 90 | Pugia | 1998 | Comparison of instrument-read dipsticks for albumin and creatinine in urine with visual results and quantitative methods | Full text not available |
| 91 | Fernandez | 1998 | Rapid screening test evaluation for microalbuminuria in diabetes mellitus | Full text not available |
| 92 | Jazayeri | 1998 | Urine protein dipstick measurements: A screen for a standard, 24-hour urine collection | Full text not available |
| 93 | Jensen | 1993 | Screening of microalbuminuria with the Micral-Test. A semiquantitative urinary dipstick | Full text not available |
| 94 | Schaufelberger | 1992 | [Evaluation of a strip test (Micral-test) for the semiquantitative assessment of microalbuminuria in clinical practice] | Full text not available |
| 95 | Poulsen | 1992 | Evaluation of a dipstick test for microalbuminuria in three different clinical settings, including the correlation with urinary albumin excretion rate | Full text not available |
| 96 | Allen | 1991 | Dipstick analysis of urinary protein. A comparison of Chempstrip-9 and Multistix-10SG | Full text not available |
| 97 | Coonrod | 1989 | Assessment of AlbuSure and its usefulness in identifying IDDM subjects at increased risk for developing clinical diabetic nephropathy | Full text not available |
| 98 | Poulsen | 1995 | Evaluation of a new semiquantitative stix for microalbuminuria | Letter to the editor |
| 99 | Dajak | 2012 | [Evaluation of methods for rapid microalbuminuria screening in kidney disease patients] | Serbian language |
3.2. Study characteristics
Of the 14 included studies, five took place in Korea [22, 23, 24, 25, 26], four in the United Kingdom [8, 27, 28, 29], one in Italy [30], one in South Africa [31], one in Japan [32], one in Spain [33], and one in China [34]. Regarding the population, eight studies were performed in persons with comorbidities [8, 23, 24, 27, 28, 29, 31, 32], of which six were performed in persons with diabetes [23, 24,27, 28, 31, 32], one in persons with diabetes and/or CKD [29], and one in persons with CKD [8]. Furthermore, one study was performed in the general population [25], and one in general population or diabetic patients without CKD [30]; also, the remaining four studies did not define their study population [22, 26, 33, 34].
Regarding the assessed dipstick, the most used brand was Clinitek (Bayer and Siemens Medical Solutions Diagnostics) in six studies [24, 27, 29, 30, 31, 35] and was followed by URiSCAN (YD Diagnostics Corp.) in three studies [22, 23, 26]. Noting that each study used a different type of dipstick brand is important (Clinitek, Microalbustix, URiSCAN, Urisys, Uropaper, Siemens).
Thirteen out of 14 studies performed an automatic reading of the dipstick [22, 23, 24, 25, 26, 27, 29, 30, 31, 32, 33, 34, 35], and the remaining study performed a manual lecture [28]. Also, eight studies took a random urine sample [22 ,23 ,25, 26, 29, 32, 34, 35], four took an early morning sample [24, 27, 28, 30], and two did not define it [31, 33]. As for the reference standard, all studies have evaluated ACR, eight have collected random urine [22, 23, 25, 26, 29, 32, 34, 35], four have collected early morning sample [24, 27, 28, 30], and three did not define their sample [26, 31, 33] (Tables 3 and 4).
Table 3.
Characteristics and risk of bias of the included studies.
| Author (year) | Population/Setting | Sample size | Age | Dipstick that assessed ACR |
Reference test (ACR) |
Funding received | Risk of bias |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brand (Manufacturer) | Result | Lecture | Type of urine specimen | Type of urine specimen | Patient selection | Index test | Reference standard | Flow and timing | |||||
| Parsons 1999 (UK) [29] | Diabetic and/or kidney failure patients/outpatient | 144 | Not defined | Clinitek (Bayer plc, Newbury) | Colors | Automatic | Random | Random | Industry | High | Unclear | High | Unclear |
| Croal 2001 (UK) [27] | Diabetic patients/outpatient | 252 | Not defined | Clinitek 50 (Bayer Diagnostics, Tarrytown, USA) | Not defined | Automatic | Early morning | Early morning | Government | High | Unclear | High | Unclear |
| Graziani 2009 (Italy) [30] | General population and diabetic patients/primary care | 460 | Not defined | Clinitek Microalbumin (Siemens Medical Solutions Diagnostics, Mishawaka, IN, USA) | Colors | Automatic | Early morning | Early morning | Industry | Low | Unclear | High | Low |
| Lloyd 2011 (South Africa) [31] | Diabetic Patients/outpatient | 204 | Not defined | Clinitek® (Siemens® Medical Solutions Diagnostics, formerly Bayer) | Not defined | Automatic | Not defined | Not defined | Industry | High | High | High | Low |
| Nagrebetsky 2013 (UK) [28] | Diabetic patients/outpatient | 87 | Mean: 68 yr | Microalbustix (Siemens Healthcare Diagnostics Ltd, Frimley, UK) | Colors | Manual | Early morning | Early morning | Government | High | Low | High | High |
| McTaggart 2012 (UK) [35] | Patients with or at risk of CKD/primary care | 619 | Mean: 66.5 yr | Clinitek Microalbumin 9 reagent strips (Siemens Medical Solutions Diagnostics) | Trace and + | Automatic | Random | Random | Industry | High | Low | High | Low |
| Cho 2014 (Korea) [22] | Not defined/not defined | 1,040 | Not defined | URiSCAN Super cassette ACR (YD Diagnostics Corp., Korea) | Trace and + | Automatic | Random | Random | Not mentioned | Unclear | Unclear | High | Low |
| Park 2017 (Korea) [25] | General population/National Survey | 20,759 | Mean: 46.6 yr | Urisys 2400 cassette strip (Roche, Mannheim, Germany) | Trace and + | Automatic | Random (early morning if possible) | Random (early morning if possible) | Government | Low | Unclear | High | High |
| Nah 2017 (Korea) [24] | Prediabetic (preDM) and diabetic (DM) patients/primary care | 501 | Median: 60 yr (preDM); 64 yr (DM) | Clinitek Microalbumin 2 reagent strips (Siemens, New York, NY, USA) | Colors | Automatic | Early morning | Early morning | Not mentioned | High | Unclear | High | Low |
| Lim 2018 (Korea) [26] | Not defined/not defined | 1,020 | Not defined | URiSCAN 2 ACR Strip (YD diagnostics, Yongin, Korea) and CLINITEK Microalbumin 2 Strip (Siemens, New York, NY, USA) | Trace and + | Automatic | Random | Random | Government | Unclear | Unclear | High | High |
| Shiwa 2018 (Japan) [32] | Diabetic patients/outpatient | 291 | Mean: 64.3 yr | Uropaper αIII (Eiken; Eiken Chemical, Tokyo, Japan) | Trace and + | Automatic | Random | Random | Not mentioned | High | Unclear | High | Low |
| Salinas 2018 (Spain) [33] | Not defined/primary care | 9,148 | Mean: 63 yr | Brand not defined (Sysmex, Kobe, Japan) | Not defined | Automatic | Not defined | Not defined | Not funded | High | Unclear | High | Unclear |
| Kim 2020 (Korea) [23] | Diabetic patients/outpatient | 1,881 (development cohort); 431 (validation cohort) | Median: 66 yr (development cohort); 63 yr (validation cohort) | URiSCAN 2ACR strip (YD-Diagnostics Co., Yongin, Korea) | Colors | Automatic | Random | Random | Government | High | Unclear | High | Low |
| Yang 2020 (China) [34] | Not defined/not defined | 1029 | Not defined | Siemens Novus with Pro12 dipsticks (Amesdata Biotech Co.) | Not defined | Automatic | Random | Random | Not mentioned | Unclear | Unclear | High | Low |
ACR: albumin-creatinine ratio; CKD: chronic kidney disease; preDM: prediabetes mellitus; DM: diabetes mellitus; yr: years; UK: the United Kingdom; +: expressed as 1+/2+/3+/4 + according to cutoff value.
Table 4.
Study characteristics of individual studies.
| Author (year) | Country | Population/Setting | Sample size | Age | Albuminuria prevalence (%) | Dipstick |
Comparison |
Outcome | Cutoff value | Funding | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brand | Marker | Type of urine specimen | Result | Lecture | Laboratory test |
Marker | Type of urine specimen | ||||||||||
| Albumin test | Creatinine test | ||||||||||||||||
| Parsons (1999) [29] | United Kingdom | Diabetic or kidney failure patients/outpatient | 144 | Not defined | 55.56 | Clinitek (Bayer plc, Newbury) | ACR | Random | Colors | Automatic (Cinitek-50, Bayer plc, Newbury) | Latex particle inmunoinhibition assay | Jaffe's method | ACR | Random | S, E, and PPV | 30 mg/g | Grant from Bayer |
| Croal (2001) [27] | United Kingdom | Diabetic patients/outpatient | 252 | Not defined | 20.63 | Clinitek 50 (Bayer Diagnostics, Tarrytown, USA) | ACR | Early morning | Not defined | Automatic (Bayer Clinitek 50 urine chemistry analyzer, Bayer Diagnostics, Tarrytown, USA) | Nephelometry | Not defined | ACR | Early morning | S, E, PPV, and NPV | 30 mg/g | Scottish Office Department of Health |
| Graziani (2009) [30] | Italy | General population and diabetic patients/primary care | 460 | Not defined | 18.15 | Clinitek Microalbumin (Siemens Medical Solutions Diagnostics, Mishawaka, IN, USA) | ACR | Early morning | Colors | Automatic (Clinitek Status, Siemens Medical Solutions Diagnostics, Mishawaka, IN, USA) | Nephelometry | Alkaline picrate method | ACR | Early morning | S, E, PPV, and NPV | 30 and 300 mg/g | Strips and Instrument provided by Siemens |
| Lloyd (2011) [31] | South Africa | Diabetic patients/outpatient | 204 | Not defined | 36.27 | Clinitek® | ACR | Not defined | Not defined | Automatic (Clinitek® status reluctance photometer) | Immunone-phelometry | Jaffe's method | ACR | Not defined | S and E | 3.4 mg/mmol (∼30 mg/g) | Instruments and reagents provided by Siemens and HemoCue |
| Nagrebetsky (2013) [28] | United Kingdom | Diabetic patients/outpatient | 87 | Mean: 68 yr | 10.34 | Microalbustix (Siemens Healthcare Diagnostics Ltd, Frimley, UK) | ACR | Early morning | Colors | Manually | Immunotur-bidimetry | Jaffe's method | ACR | Early morning | S and E | 3.4 mg/mmol (∼30 mg/g) | National Institute for Health Research, United Kingdom |
| McTaggart (2012) [35] | United Kingdom | Patients with or at risk of CKD/primary care | 619 | Mean: 66.5 yr | 20.19 | CLINITEK Microalbumin 9 reagent strips | ACR | Random | Trace and + | Automatic (CLINITEK Status Analyzer) | Immunotur-bidimetry | Enzymatic methods | ACR | Random | S, E, PPV, NPV, and LR | 30 mg/g | Grant by Siemens |
| Cho (2014) [22] | Korea | Not defined/not defined | 1040 | Not defined | 47.12 | URiSCAN Super cassette ACR | ACR | Random | Trace and + | Automatic (URiSCAN Super Plus, YD Electronics Co., Ltd., Korea) | Immunotur-bidimetry | Jaffe's method | ACR | Random | S, E, PPV, NPV, and correlation | 30 and 300 mg/g | Not mentioned |
| Park (2017) [25] | Korea | General population/National Survey | 20759 | Mean: 46.6 yr | 8.57 | Urisys 2400 cassette strip (Roche, Mannheim, Germany) | ACR | Random (early morning if possible) | Trace and + | Automatic (Urisys 2400 automated analyzer, Roche, Mannheim, Germany) | Immunotur-bidimetry | Colorimetric assay and Jaffe's method | ACR | Random (early morning if possible) | S, E, PPV, and NPV | 30 and 300 mg/g | Grant by Kangwon National University Hospital |
| Nah (2017) [24] | Korea | Prediabetic and diabetic patients/primary care | 501 | Median: 60 yr (preDM); 64 yr (DM) | 21.96 | CLINITEK Microalbumin 2 reagent strips (Siemens, New York, NY, USA) | ACR | Early morning | Colors | Automatic (CLINITEK Advantus Analyzer, Siemens) | Turbidimetric Immunoassay | Enzymatic methods | ACR | Early morning | S, E, PPV, and NPV | 30 and 300 mg/g | Not mentioned |
| Lim (2018) [26] | Korea | Not defined/not defined | 1020 | Not defined | 50.98 (URiSCAN); 49.24 (CLINITEK) | URiSCAN 2 ACR Strip and CLINITEK Microalbumin 2 Strip | ACR | Random | Trace and + | Automatic (URiSCAN New Pro, YD diagnostics, Yongin, Korea and Clinitek status plus, Siemens) | Immunoturb-idimetry | Jaffe's method | ACR | Random | S, E, PPV, NPV, and LR | 30 and 300 mg/g | Ministry of Health & Welfare, Republic of Korea |
| Shiwa (2018) [32] | Japan | Diabetic patients/outpatient | 291 | Mean: 64.3 yr | 29.55 | Uropaper αIII (Eiken; Eiken Chemical, Tokyo, Japan) | ACR | Random | Trace and + | Automatic | Not defined | Not defined | ACR | Random | S, E, and ROC curve | 30 and 300 mg/g | Not mentioned |
| Salinas (2018) [33] | Spain | Not defined/primary care | 9148 | Mean: 63 yr | 13.98 | Sysmex, Kobe, Japan | ACR | Not defined | Not defined | Automatic (UC-3500, Sysmex, Kobe, Japan) | Immunotur-bidimetry | Jaffe's method | ACR | Not defined | S, E, PPV, NPV, and LR | 30 and 300 mg/g | Not funded |
| Kim (2020) [23] | Korea | Diabetic patients/outpatient | 1881 (development cohort); 431 (validation cohort) | Median: 66 yr (development cohort); 63 yr (validation cohort) | 42.53 (development cohort); 25.75 (validation cohort) | URiSCAN 2ACR strip | ACR | Random | Colors | Automatic (URiSCAN1 2ACR system, YD-Diagnostics Co., Yongin, Korea) | Immunotur-bidimetry | Jaffe's method | ACR | Random | S and E | 30 and 300 mg/g | Ministry of Health & Welfare, Republic of Korea |
| Yang (2020) [34] | China | Not defined/not defined | 1029 | Not defined | 50.94 | Siemens Novus with Pro12 dipsticks | ACR | Random | Not defined | Automatic (Siemens Novus) | Immunotur-bidimetry | Not defined | ACR | Random | S, E, PPV, and NPV | 30 and 300 mg/g | Not mentioned |
ACR: albumin-creatinine ratio; preDM: prediabetes mellitus; DM: diabetes mellitus; +: expressed as 1+/2+/3+/4 + according to cutoff values; S: sensitivity; E: specificity; PPV: positive predictive value; NPV: negative predictive value; ROC: receiver operating characteristic; LR: likelihood ratio.
3.3. Results of meta-analyses
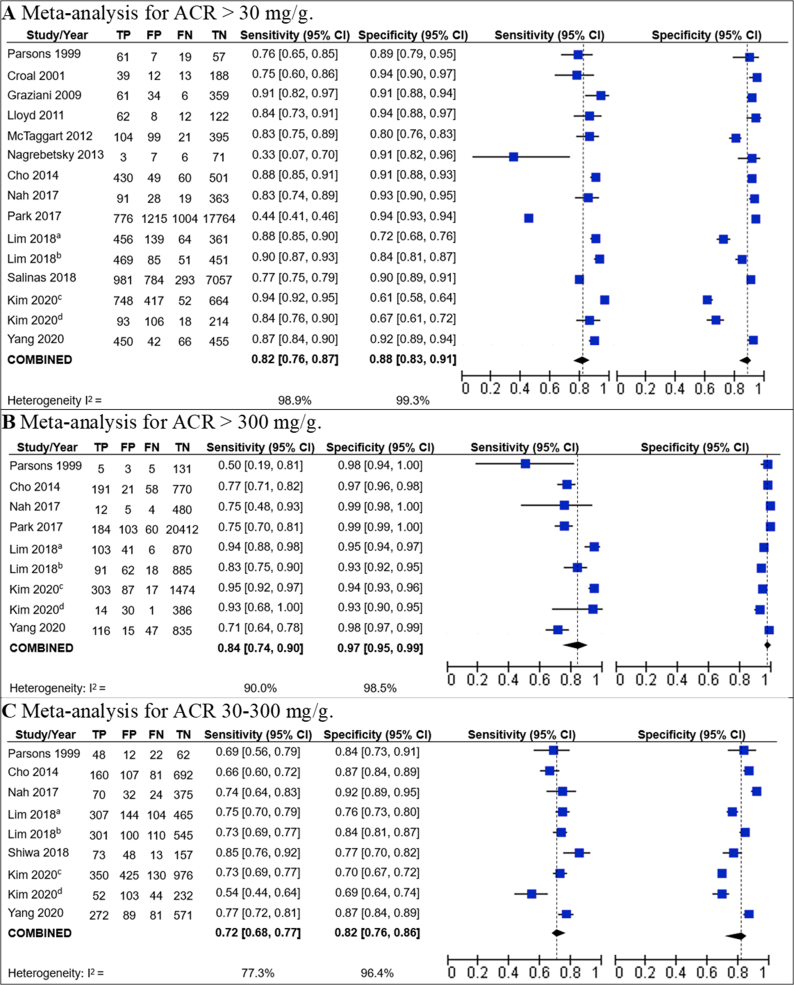
Twelve studies were included for the meta-analyses that assessed dipstick accuracy for the cutoff point of ACR >30 mg/g [22, 23, 24, 25, 26, 27, 29, 30, 31, 33, 34, 35], and the pooled estimate gave a sensitivity and specificity of 0.82 (95% CI 0.76–0.87) and 0.88 (95% CI 0.83–0.91), respectively. Regarding the cutoff point of ACR 30–300 mg/g, seven studies were included [22, 23, 24, 26, 29, 32, 34], and the pooled estimate gave a sensitivity and specificity of 0.72 (95% CI 0.68–0.77) and 0.82 (95% CI 0.76–0.86), respectively. Regarding the cutoff point of ACR >300 mg/g, seven studies were included [22, 23, 24, 25, 26, 29, 34], and the pooled estimate gave a sensitivity and specificity of 0.84 (95% CI 0.74–0.90) and 0.97 (95% CI 0.95–0.99) (Figure 2).
Figure 2.
Forest plots on sensitivity and specificity for urine dipstick testing ACR. aThis observation corresponds to the results for the URiSCAN 2 ACR Strip dipstick brand reported by Lim 2018. bThis observation corresponds to the results for the CLINITEK Microalbumin 2 Strip dipstick brand reported by Lim 2018. cThis observation corresponds to the “development cohort” from Kim 2020. dThis observation corresponds to the “validation cohort” from Kim 2020.
Kim 2020 is mentioned twice in the meta-analyses: one for its developmental cohort and the other for its validation cohort [23]. Also, Lim 2018 is mentioned twice: one for each dipstick used, one for the use of CLINITEK Microalbumin 2 Strip dipstick brand, and the other for the use of URiSCAN 2 ACR Strip dipstick brand [26].
All of these meta-analyses showed high heterogeneity (I2 being higher than 70% for all of the meta-analyses). For the dipstick's sensitivity for ACR >30 mg/g, heterogeneity was found to be mainly generated by two studies: Nagrebetsky 2013 and Park 2017. Nagrebetsky 2013 is the only study that used a manual lecture of the dipstick result, which may explain its low sensitivity [28]. However, the study by Park 2017 does not have a special characteristic that could explain the lower sensitivity with respect to the rest of studies, although it was the only study that used the Urisys 2400 cassette strip recorded from a device (Urisys 2400 automated analyzer, Roche, Mannheim, Germany) for the diagnosis of albuminuria [25].
A sensitivity analyses have been performed excluding the only study that used a manual lecture of the dipstick (Nagrebetsky 2013), for the ACR >30 mg/g cutoff point. The resulting sensitivity and specificity were: 0.83 (95% CI: 0.78–0.88, I2 = 99%) and 0.87 (95% CI: 0.82–0.91, I2 = 99%).
3.4. Risk of bias
In the patient selection domain, most of the studies (9/14) had a high risk of bias, and 3/14 had unclear risk bias. The most frequent limitation was that studies did not avoid inappropriate exclusion. For the index test domain, most of the studies were considered to have an unclear risk of bias (11/14) due to a lack of information regarding the interpretation of the index test. For the reference standard domain, all studies were found to have a high risk of bias because the reference tests used were not the gold standard (turbidimetry, nephelometry, or radioimmunoassay for albuminuria; isotope dilution mass spectrometry for creatinine), and whether the interpretation of the results of the index and reference tests was independent was not clear. With regard to the flow and timing domain, 3/14 studies were found to have a high risk of bias and 3/14 had an unclear risk of bias because not all patients were included in the analysis or different standard tests were used. Additionally, the intervals between the index test and the reference standard were not clear for some studies (Table 3).
3.5. Summary of findings
A Summary of Findings (SoF) table has been performed. All the pooled sensitivities and specificities had a very low certainty of the evidence, due to a high or very high risk of bias, inconsistency, and imprecision.
To estimate the impact that the pooled sensitivity and specificity would have as regards false-positive and false-negative cases, these were estimated a fictitious population of 1,000 individuals. For these estimations, different prevalences of ACR >30 mg/g, between 30 mg/g and 300 mg/g, and >300 mg/g were assumed and calculated using the median prevalence of studies that showed information for the prevalence of all these three cutoff values [22, 23, 24, 26, 29, 34] (Table 5).
Table 5.
Summary of findings to evaluate the certainty of the evidence, using the GRADE methodology.
| Cutoff point | Number of studies (participants) | Summary of sensitivity (95% CI) | Summary of specificity (95% CI) | Certainty of the evidenceb | Consequences in a fictitious population of 1000 patientsa |
||
|---|---|---|---|---|---|---|---|
| Assumed prevalence | Underdiagnosed or false negatives (95% CI) | Overdiagnosed or false positives (95% CI) | |||||
| ACR >30 mg/g | 13 (38,582) | 0.82 (0.76–0.87) | 0.88 (0.83–0.91) | Sensitivity: very low1,2,3 Specificity: very low1,2 |
48.2% | 87 (63 a 116) | 62 (47 a 88) |
| ACR 30–300 mg/g | 7 (7,377) | 0.72 (0.68–0.77) | 0.82 (0.76–0.86) | Sensitivity: very low1,4 Specificity: very low1,2 |
30.2% | 85 (69 a 97) | 126 (98 a 168) |
| ACR >300 mg/g | 7 (27,596) | 0.84 (0.74–0.90) | 0.97 (0.95–0.99) | Sensitivity: very low1,2 Specificity: very low1,2 |
10.5% | 17 (11 a 27) | 27 (9 a 45) |
ACR: albumin-creatinine ratio; CI: confidence intervals.
This fictitious population is assumed to have a prevalence of ACR >30 mg/g of 48.2 %, a prevalence of ACR between 30 mg/g and 300 mg/g of 30.2%, and a prevalence of ACR >300 mg/g of 10.5%. Median of the prevalence of studies that have reported ACR cutoff point >30, 30–300, and >300 mg/g (Parsons et al., Cho et al., Nah et al., Lim et al., Kim et al. and Yang et al.).
Explanation of the certainty of the evidence: 1. Very high risk of bias, 2. very high inconsistency, 3. high imprecision, 4. high inconsistency.
4. Discussion
4.1. Comparison with previous studies
One previous systematic review was found that assessed the diagnostic accuracy of POC tests (either semiquantitative or quantitative) in people at risk of CKD. It performed its literature search in December 2013 and meta-analyzed nine studies that assessed urine dipsticks, obtaining a pooled sensitivity and specificity of 0.76 and 0.93, respectively, for ACR >30 mg/g [8]. Five of the nine studies included by such review have been included in our study. The other four studies were excluded since two of them evaluated cutoff values different than ours [36, 37], one did not have its full text available [38], and one was published as a letter to the editor [39].
Moreover, other eight studies have been included that assessed ACR >30 mg/g, for a total of 13 meta-analyzed studies, finding that a urine dipstick test has a sensitivity and specificity of 0.82 (95% CI: 0.76–0.87) and 0.88 (95% CI: 0.83–0.91), respectively; while the cited systematic review found a sensitivity and specificity of 0.76 (95% CI: 0.63–0.86) and 0.93 (95% CI: 0.84–0.97), respectively. As seen, confidence intervals overlapped considerably. Furthermore, while the cited systematic review reported their result uniquely for the ACR >30 mg/g cutoff value, the ACR between 30 mg/g and 300 mg/g and the ACR >300 mg/g have also been included due to their clinical relevance.
4.2. Implications for clinical practice
Urine dipsticks represent a time-saving POC screening test in resource-limited settings that lack laboratory analysis [40]. However, to adequately decide whether to use urine dipsticks, or in cases which use it, stakeholders should consider its sensitivity and specificity, along with the expected number of false positives and false negatives for their study population.
For detecting ACR >30 mg/g, dipsticks showed a limited sensitivity (0.82, 95% CI: 0.76–0.87) and specificity (0.88, 95% CI: 0.83–0.91). A suboptimal accuracy could be expected, due to the effect of some interfering compounds (drugs, vitamins, urine preservatives, and detergents), urine pH, and storage deficiencies [40].
As shown in the SoF table, for a 1,000-patient population with a prevalence of ACR >30 mg/g of 48.2%, the pooled accuracy was translated into 87 false-negative results and 62 false-positive results. Each one of these groups has a different impact. Since CKD Clinical guidelines recommend that patients with positive dipstick results are assessed using quantitative methods before establishing the CKD diagnosis [1, 2], false positives could be corrected in this step, although causing preoccupation and expenses for these patients. Conversely, false-negative results could be more problematic, since these cases could mean a loss of opportunity for performing a timely diagnosis and management of CKD. Repeated dipstick assessments could diminish the false-negatives rates. However, KDIGO and NICE guidelines do not state any recommendation regarding the frequency of ACR testing. However, the Australian CKD guidelines recommend annual screening of urine ACR in people at risk of CKD [41].
4.3. Impact in progression
The detection of ACR >300 mg/g is also relevant in assessing CKD progression. In CKD patients, the KDIGO guideline recommends annual albuminuria tests or every 1–3 months in patients with a high risk of CKD progression [1]. Furthermore, the NICE guidelines establish a frequency of monitoring the glomerular filtration rate (GFR) per year depending on ACR and GFR in people with or at risk of CKD [2]. For a population of 1,000 persons with an ACR >300 mg/g prevalence of 10.5% (i.e., 105 persons), the use of dipsticks has been estimated to end in 17 false negatives and 27 false positives. Analyzing how repeated dipstick assessment yielded negative results remains relevant, and confirmation with quantitative methods in positive ones would improve these results.
Performing economic analyses that could guide decision-making regarding urine dipsticks’ use for a specific country or region is also important. These analyses should perform sensitivity analyses for different types of patients, with different frequencies of dipstick use, and with different decision trees. Only one economic analysis performed for Korean diabetic patients with a GFR >60 ml/min per 1.73 m2 and a negative urine dipstick result has been found. For this analysis, the authors considered a sensitivity and specificity of 0.84 and 0.67, respectively, for ACR >30 mg/g, values from one of the studies included in our systematic review [23]. In this model, testing the annual progression with a semiquantitative tool saved 16.7% (339.6 USD) of costs per diabetic patient for 10 years compared with doing it with a quantitative test [23].
4.4. Limitations and strengths
This study has some limitations that must be considered to adequately interpret our results: 1) Subgroup analyses according to the operator (laboratory vs. clinical) were not performed due to the lack of precise data on this variable given in the articles, according to the dipstick brand due to the high variability, or according to the lecture (manually/automatic) since only one study used manual lecture. 2) Sensitivity and specificity may improve after indexing dipstick results for urine concentration [42, 43]; however, dipstick results have not been indexed due to a lack of reporting data in the included studies. 3) ACR results were not adjusted by muscle mass, and albuminuria reference tests are not well standardized in the included studies, a flaw that may guide to obtain imprecise results. 4) Our results had a very low certainty of the evidence, so future well-designed studies are needed to reach a conclusion and to explain the heterogeneity found in our results. 5) The accuracy of different dipstick brands and types could not be assessed since each study used a different type of dipstick.
However, this study considered a broad inclusion criteria for the population (general population and people at risk or with CKD). Furthermore, a meta-analyses for the three cutoff values (ACR >30, 30–300, >300 mg/g) have been performed with clinical relevance according to current clinical guidelines.
5. Conclusion
A total of 14 studies were found and assessed for sensitivity and specificity of urine dipsticks for ACR assessment, and no study was found that assessed urine dipsticks for albuminuria assessment. Dipstick types used were different across studies. The sensitivity and specificity of dipsticks have been pooled for ACR >30 mg/g, ACR 30–300 mg/g, and ACR >300 mg/g. However, all meta-analyses showed high heterogeneity, and the certainty of the evidence was very low for all the results. Thus, further well-designed studies are needed to reach more confident estimates and to assess accuracy differences across dipstick types. Until then, clinical practitioners should be cautious when interpreting dipstick results.
Declarations
Author contribution statement
Jhonatan R. Mejia, Jose Ernesto Fernandez-Chinguel: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Gandy Dolores-Maldonado, Naysha Becerra-Chauca, Sergio Goicochea-Lugo: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Percy Herrera-Añazco: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jessica Hanae ZafraTanaka, Alvaro Taype-Rondan: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The protocol is registered at PROSPERO (CRD42019124637) available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=124637. The datasets used and/or analyzed during the current study are available at dx.doi.org/10.6084/m9.figshare.13350923.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Dr. Virgilio Failoc-Rojas for his collaboration with data collection.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney inter S. 2013;3:1–150. [Google Scholar]
- 2.National Institute for Health and Clinical Excellence (Great Britain) National Institute for Health and Care Excellence (UK). National Clinical Guideline Centre; London: 2015. National Institute for Health and Care Excellence: Clinical Guidelines. Chronic Kidney Disease in Adults: Assessment and Management Clinical Guideline [CG182] 2015. [Google Scholar]
- 3.Scottish Intercollegiate Guidelines Network . 2008. Guideline 103. Diagnosis and Management of Chronic Kidney Disease. [Google Scholar]
- 4.Wilson P., Clarke F.V., Cutler R.R., Merrett J.O., Jenks P. Usefulness of urine dipstick tests. False negative results may occur in the absence of antibiotics, ketones, and glucose. BMJ. 1996;313(7063):1009–1010. doi: 10.1136/bmj.313.7063.1009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson E., Thompson D. Routine urinalysis. Lancet. 1977;2(8033):361–362. doi: 10.1016/s0140-6736(77)91529-x. [DOI] [PubMed] [Google Scholar]
- 6.Lee W., Kim Y., Chang S., Lee A.J., Jeon C.H. The influence of vitamin C on the urine dipstick tests in the clinical specimens: a multicenter study. J. Clin. Lab. Anal. 2017;31(5) doi: 10.1002/jcla.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rumley A. Urine dipstick testing: comparison of results obtained by visual reading and with the Bayer CLINITEK 50. Ann. Clin. Biochem. 2000;37(Pt 2):220–221. doi: 10.1258/0004563001899041. [DOI] [PubMed] [Google Scholar]
- 8.McTaggart M.P., Newall R.G., Hirst J.A., Bankhead C.R., Lamb E.J., Roberts N.W. Diagnostic accuracy of point-of-care tests for detecting albuminuria: a systematic review and meta-analysis. Ann. Intern. Med. 2014;160(8):550–557. doi: 10.7326/M13-2331. [DOI] [PubMed] [Google Scholar]
- 9.Krogsbøll L.T., Jørgensen K.J., Gøtzsche P.C. Screening with urinary dipsticks for reducing morbidity and mortality. Coch. Database Syst. Rev. 2015;(1) doi: 10.1002/14651858.CD010007.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks J.J., Wisniewski S., Davenport C. In: Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 100. Deeks J.J., Bossuyt P.M., Gatsonis C., editors. The Cochrane Collaboration; 2013. Guide to the contents of a Cochrane diagnostic test accuracy protocol. [Google Scholar]
- 11.Salameh J.-P., Bossuyt P.M., McGrath T.A., Thombs B.D., Hyde C.J., Macaskill P. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ. 2020;370:m2632. doi: 10.1136/bmj.m2632. [DOI] [PubMed] [Google Scholar]
- 12.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Levey A.S., Coresh J., Greene T., Marsh J., Stevens L.A., Kusek J.W. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 15.Myers G.L., Miller W.G., Coresh J., Fleming J., Greenberg N., Greene T. Recommendations for improving serum creatinine measurement: a report from the laboratory working group of the national kidney disease education program. Clin. Chem. 2006;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 16.Piéroni L., Bargnoux A.-S., Cristol J.-P., Cavalier E., Delanaye P. Did creatinine standardization give benefits to the evaluation of glomerular filtration rate? EJIFCC. 2017;28(4):251–257. [PMC free article] [PubMed] [Google Scholar]
- 17.Spierto F.W., Hannon W.H., Gunter E.W., Smith S.J. Stability of urine creatinine. Clin. Chim. Acta. 1997;264(2):227–232. doi: 10.1016/s0009-8981(97)00080-6. [DOI] [PubMed] [Google Scholar]
- 18.Giampietro O., Penno G., Clerico A., Cruschelli L., Cecere M. How and how long to store urine samples before albumin radioimmunoassay: a practical response. Clin. Chem. 1993;39(3):533–536. [PubMed] [Google Scholar]
- 19.Herrington W., Illingworth N., Staplin N., Kumar A., Storey B., Hrusecka R. Effect of processing delay and storage conditions on urine albumin-to-creatinine ratio. Clin. J. Am. Soc. Nephrol. 2016;11(10):1794–1801. doi: 10.2215/CJN.13341215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schünemann H.J., Mustafa R.A., Brozek J., Steingart K.R., Leeflang M., Murad M.H. GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2020;122:142–152. doi: 10.1016/j.jclinepi.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Schünemann H.J., Mustafa R.A., Brozek J., Steingart K.R., Leeflang M., Murad M.H. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. J. Clin. Epidemiol. 2020;122:129–141. doi: 10.1016/j.jclinepi.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Cho M.-C., Ji M., Kim S.Y., Choe W., Lee W., Chun S. Evaluation of the URiSCAN super cassette ACR semiquantitative urine dipstick for microalbuminuria screening. J. Clin. Lab. Anal. 2014;28(4):281–286. doi: 10.1002/jcla.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y., Park S., Kim M.H., Song S.H., Lee W.M., Kim H.S. Can a semi-quantitative method replace the current quantitative method for the annual screening of microalbuminuria in patients with diabetes? Diagnostic accuracy and cost-saving analysis considering the potential health burden. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0227694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nah E.H., Cho S., Kim S., Cho H.I. Comparison of urine albumin-to-creatinine ratio (ACR) between ACR strip test and quantitative test in prediabetes and diabetes. Ann Lab Med. 2017;37(1):28–33. doi: 10.3343/alm.2017.37.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J.I., Baek H., Kim B.R., Jung H.H. Comparison of urine dipstick and albumin:creatinine ratio for chronic kidney disease screening: a population-based study. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim S., Yu H.J., Lee S., Park H., Kwon M.J., Woo H.Y. Evaluation of the URiSCAN 2 ACR Strip to estimate the urine albumin/creatinine ratios. J. Clin. Lab. Anal. 2018;32(3) doi: 10.1002/jcla.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croal B.L., Mutch W.J., Clark B.M., Dickie A., Church J., Noble D. The clinical application of a urine albumin:creatinine ratio point-of-care device. Clin. Chim. Acta. 2001;307(1-2):15–21. doi: 10.1016/s0009-8981(01)00450-8. [DOI] [PubMed] [Google Scholar]
- 28.Nagrebetsky A., Jin J., Stevens R., James T., Adler A., Park P. Diagnostic accuracy of urine dipstick testing in screening for microalbuminuria in type 2 diabetes: a cohort study in primary care. Fam. Pract. 2013;30(2):142–152. doi: 10.1093/fampra/cms057. [DOI] [PubMed] [Google Scholar]
- 29.Parsons M., Newman D.J., Pugia M., Newall R.G., Price C.P. Performance of a reagent strip device for quantitation of the urine albumin: creatinine ratio in a point of care setting. Clin. Nephrol. 1999;51(4):220–227. [PubMed] [Google Scholar]
- 30.Graziani M.S., Gambaro G., Mantovani L., Sorio A., Yabarek T., Abaterusso C. Diagnostic accuracy of a reagent strip for assessing urinary albumin excretion in the general population. Nephrol. Dial. Transplant. 2009;24(5):1490–1494. doi: 10.1093/ndt/gfn639. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd M., Kuyl J., Jaarsveld H.V. Evaluation of point-of-care tests for detecting microalbuminuria in diabetic patients. S. Afr. Fam. Pract. 2011;53:281–286. [Google Scholar]
- 32.Shiwa T., Nishimura M., Kato M. The effectiveness of the semi-quantitative assessment of microalbuminuria using routine urine dipstick screening in patients with diabetes. Intern. Med. 2018;57(4):503–506. doi: 10.2169/internalmedicine.9069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salinas M., López-Garrigós M., Flores E., Lugo J., Leiva-Salinas C. Urinary albumin strip assay as a screening test to replace quantitative technology in certain conditions. Clin. Chem. Lab. Med. 2018;57(2):204–209. doi: 10.1515/cclm-2018-0546. [DOI] [PubMed] [Google Scholar]
- 34.Yang C.-J., Chen D.-P., Wen Y.-H., Lai N.-C., Ning H.-C. Evaluation the diagnostic accuracy of albuminuria detection in semi-quantitative urinalysis. Clin. Chim. Acta. 2020;510:177–180. doi: 10.1016/j.cca.2020.06.036. [DOI] [PubMed] [Google Scholar]
- 35.McTaggart M.P., Price C.P., Pinnock R.G., Stevens P.E., Newall R.G., Lamb E.J. The diagnostic accuracy of a urine albumin-creatinine ratio point-of-care test for detection of albuminuria in primary care. Am. J. Kidney Dis. 2012;60(5):787–794. doi: 10.1053/j.ajkd.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Davidson E.M., Croal B.L. Introduction of an albumin-to-creatinine ratio point-of-care device: analytic, clinical, and cost-effectiveness aspects. Point Care. 2003;2(2) [Google Scholar]
- 37.Guy M., Newall R., Borzomato J., Kalra P.A., Price C. Diagnostic accuracy of the urinary albumin: creatinine ratio determined by the CLINITEK Microalbumin and DCA 2000+ for the rule-out of albuminuria in chronic kidney disease. Clinica chimica acta; Int. J. Clin. Chem. 2009;399(1-2):54–58. doi: 10.1016/j.cca.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Le Floch J.P., Marre M., Rodier M., Passa P. Interest of Clinitek Microalbumin in screening for microalbuminuria: results of a multicentre study in 302 diabetic patients. Diabetes Metab. 2001;27(1):36–39. [PubMed] [Google Scholar]
- 39.Pickersgill A.J., McInnes E.A., Wiener K. Clinitek Microalbumin assay. Diabet. Med. 2001;18(11):937–939. doi: 10.1046/j.0742-3071.2001.00590.x. [DOI] [PubMed] [Google Scholar]
- 40.Kavuru V., Vu T., Karageorge L., Choudhury D., Senger R., Robertson J. Dipstick analysis of urine chemistry: benefits and limitations of dry chemistry-based assays. Postgrad. Med. 2020;132(3):225–233. doi: 10.1080/00325481.2019.1679540. [DOI] [PubMed] [Google Scholar]
- 41.Toussaint N. 2012. KHA-CARI Guideline: Screening for Early Chronic Kidney Disease. [Google Scholar]
- 42.Agarwal R., Panesar A., Lewis R.R. Dipstick proteinuria: can it guide hypertension management? Am. J. Kidney Dis. 2002;39(6):1190–1195. doi: 10.1053/ajkd.2002.33389. [DOI] [PubMed] [Google Scholar]
- 43.Constantiner M., Sehgal A.R., Humbert L., Constantiner D., Arce L., Sedor J.R. A dipstick protein and specific gravity algorithm accurately predicts pathological proteinuria. Am. J. Kidney Dis. 2005;45(5):833–841. doi: 10.1053/j.ajkd.2005.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The protocol is registered at PROSPERO (CRD42019124637) available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=124637. The datasets used and/or analyzed during the current study are available at dx.doi.org/10.6084/m9.figshare.13350923.