Abstract
Background
COVID-19 disease has been associated with several cardiovascular complications that rarely occur in the acute phase of the disease.
Case report
A 13-year-old pediatric patient with congenital sideroblastic anemia associated with YARS2 mutation presenting with COVID-19 infection and worsening pericardial effusion followed by a respiratory failure refractory to supplemental oxygen therapy leading to cardiac arrest.
Discussion
This case highlights the rapid deterioration that can occur in children with serious hematologic disorders in the context of COVID-19 especially when complicated with pericardial effusion.
Conclusion
The importance of pericardiocentesis early in order to allow better ventilation in any significant pericardial effusion case associated with COVID-19 infection and the need for prompt care escalation to centers where ECMO is available.
Keywords: Pericardial effusion, COVID-19, Sideroblastic anemia, YARS2, ECMO
1. Introduction
Sideroblastic anemia is characterized by the presence of iron containing ring sideroblasts in the bone marrow. [1] These erythroid precursors can be found in both inherited and acquired causes of sideroblastic anemia. Congenital forms of this condition can be divided into syndromic and non-syndromic forms and are triggered by a germline mutation in either a nuclear or a mitochondrial gene. [2,3] Variants in YARS2; a nuclear gene encoding a mitochondrial tyrosyl-tRNA synthetase that attaches tyrosine to its cognate tRNA; have been linked to myopathy, lactic acidosis, and sideroblastic anemia 2 (MLASA2), a syndromic form of inherited sideroblastic anemias. [3].
Pericardial effusion and neutropenia have been reported in patients exhibiting YARS2 variants and there has been mentions in the literature discussing the incidence of hypertrophic cardiomyopathy in this specific population. [[3], [4], [5]] To our knowledge, there are no reported cases describing worsening pericardial effusions in a Coronavirus disease 2019 (COVID-19) positive pediatric patient, in the context of a mitochondrial respiratory chain disorder.
COVID-19 is an emerging global pandemic manifesting through respiratory illness with multiple accounts of cardiovascular complications. In the context of multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19, pulmonary hypertension, myocarditis, and cardiac arrhythmias have all been reported; however, these complications rarely occur in the acute phase of the disease. In addition, the data is still limited regarding the management for these patients. [6].
2. Case Report
We describe a thirteen-year-old boy with congenital sideroblastic anemia with YARS2 mutation on chronic transfusion presenting with COVID-19 infection and pericardial effusion. He was admitted to our institution with shortness of breath, palpitations, non-productive cough, pallor, and lethargy of 8 days duration. On admission, he was afebrile, normotensive, with mild respiratory distress. Oxygen saturation was 96% in the upright position, and 91% in the supine position. This was accompanied with worsening dyspnea and orthopnea.
He was started on 1 L/min of supplemental oxygen by nasal cannula. Initial laboratory evaluation was consistent with anemia (Hemoglobin 6.4 g/dl), leukopenia (White blood cell count 2.97 × 103 g/dl), severe neutropenia (Absolute Neutrophil Count 120), and thrombocytopenia (Platelet count 81 x 10 ^3/μ). C-reactive protein was unremarkable (0.4 mg/dl), his ferritin was elevated (8606 ng/dl) despite daily oral iron chelation with JadeNu; and his initial D-dimer (0.44 μg/ml) as well as his fibrinogen (186 mg/dl) were both within normal range. He had a negative Epstein-Barr virus (EBV), Cytomegalovirus (CMV), and Mycoplasma IgM antibody titers. Purified protein derivative (PPD) was negative as well. Due to his respiratory concerns upon presentation, a nasopharyngeal swab was sent for COVID-19 testing by real time PCR (RT-PCR) and the result was positive. Serology for SARS-COV 2 infection was positive for IgM titers. After consulting infectious diseases, Remdesivir 5 mg/kg/dose once per day and therapeutic dose Enoxaparin 1 mg/kg/dose every 12hrs were started.
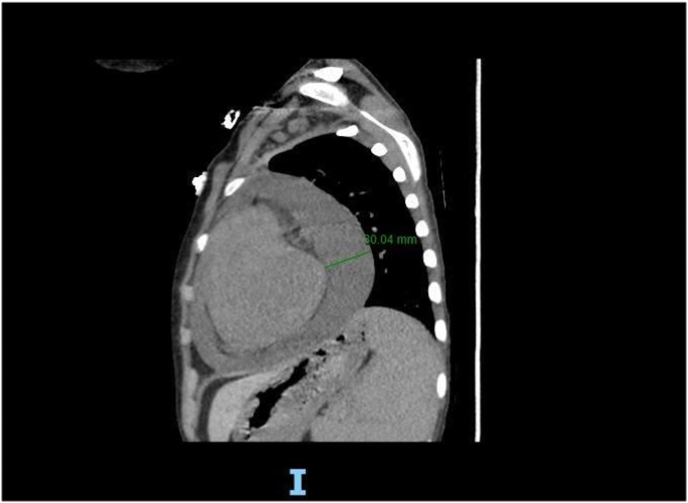
Electrocardiogram (EKG) was done and showed sinus tachycardia and diffuse ST elevations. Chest radiograph showed confluent infiltrates at the left mid and lower lung fields suggestive of an infectious process and an enlarged cardiac silhouette. He received two packed red blood cell (RBCs) transfusions and was started on antibiotics given his neutropenia and concern for pneumonia. The initial regimen as recommended by the infectious disease team was Meropenem 1g every 8hrs and Teicoplanin 300 mg every 12hrs. Chest computed tomography (CT) showed lobular consolidations and areas of ground-glass opacities at the left upper lobe with a moderate pericardial effusion reaching a maximal thickness of 3 cm (Fig. 1). The heart was also enlarged with a left shift of the cardiac silhouette.
Fig. 1.
CT chest: Moderate pericardial effusion reaching a maximal thickness of 3 cm, atelectatic changes at the left base associated with lobular mosaic pattern of attenuation at the left upper lobe.
Echocardiography at that time confirmed a moderate to large circumferential lobulated pericardial effusion with mild dilation of the pulmonary artery associated with normal left ventricular systolic function, normal right ventricle size and function. The interventricular septum showed slight bowing to the left. Estimated pulmonary artery pressure was ∼40 mm Hg consistent with pulmonary hypertension (PH). The pediatric cardiology team was consulted and the patient was started on medical management for pericarditis with pericardial effusion. He was started also on a non-steroidal anti-inflammatory drug, colchicine at 0.5 mg orally every 24 hours, and intravenous solumedrol at 1mg/kg every 12 hours. Pericardiocentesis was discussed but deferred at that time as the patient was hemodynamically stable, did not have signs of tamponade, and had an increased risk of periprocedural bleed due to his thrombocytopenia and anticoagulant therapy. Pulmonary vasodilation was also discussed for PH therapy.
A day later, the patient started deteriorating. He developed worsening acute hypoxemic, hypercapnic, respiratory failure refractory to supplemental oxygen therapy. He was quickly transferred to the COVID intensive care unit. He was intubated and mechanical ventilation was initiated on volume control mode with a tidal volume (TV) of 8 cc/kg, initial positive end-expiratory pressure (PEEP) of 6 cm H2O, and fraction of inspired oxygen (FiO2) of 100% with minimal improvement in his oxygen saturation. An arterial blood gas showed a PH of 7.29, PCO2 78, PO2 117 and HCO3 37.5. Ventilation settings were adjusted accordingly, however hypoxemia and hypercapnia persisted.
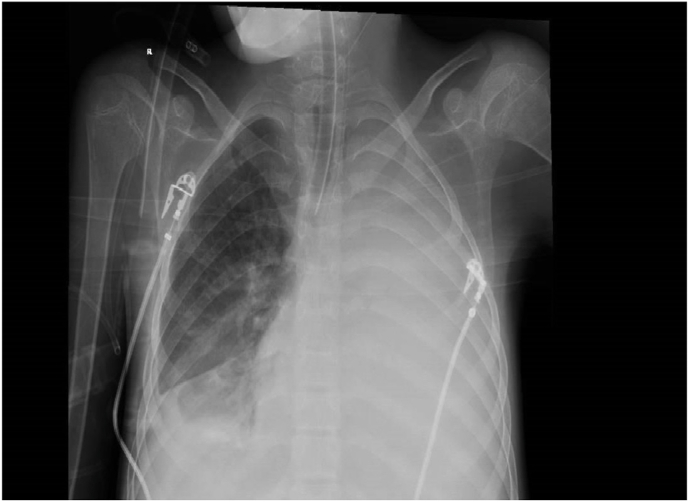
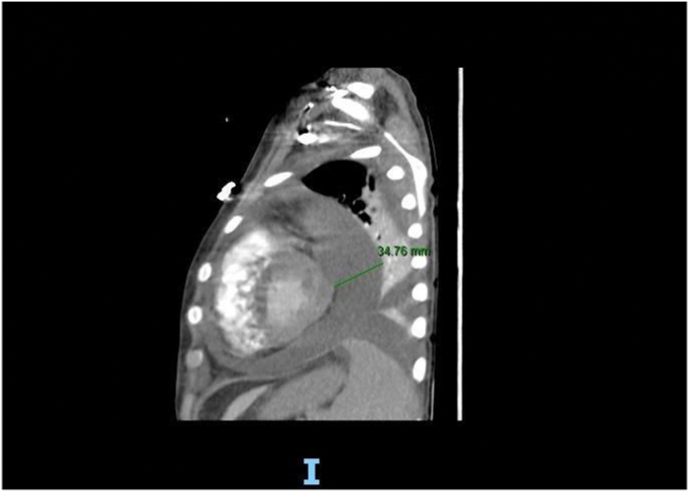
Chest radiograph (Fig. 2) revealed diffuse white out lung on the left side along with minimal pleural effusion at the lower lobe. Repeated CT scan (Fig. 3) and serial Echocardiography showed persistent pericardial effusion with worsening in his pulmonary hypertension (55 mmHg) but no hemodynamic or cardiac function changes. Tocilizumab (IL-6216 pg/ml) was added to the therapy plan. Inhaled Nitric oxide was considered but deferred due to concern about acute kidney injury. Although extracorporeal membrane oxygenation (ECMO) was discussed, this was not available in our center and transfer was impossible at that time. On the same day, the patient went into cardiac arrest after a rapid drop in oxygen saturation and bradycardia followed by a cessation of electrical and mechanical activity of the heart.
Fig. 2.
CXR: Complete whiteout of the left hemithorax, limited pulmonary expansion on the right right-sided pleural effusion filling the fissure with underlying segmental atelectasis.
Fig. 3.
CT chest: Marked Dilatation of the main pulmonary trunk; Interval circumferential increase in the size of pericardial effusion reaching 34 mm in maximal thickness.
3. Discussion
We describe here a patient with congenital YARS2 sideroblastic anemia with rapidly progressive respiratory failure in the context of COVID-19 infection and pericardial effusion. COVID-19 in children is usually mild with only 9% requiring intubation and mechanical ventilation. [7] Although, pericardial effusion was presumably rare compared to other COVID-19 related cardiac disorders such as pericarditis and myocarditis, multiple case series supported pericardial involvement with effusion in COVID-19 cases. [[8], [9], [10]].
Our patient's presentation is remarkable as despite the aggressive medical therapy, he unfortunately passed away before more invasive therapies such as ECMO and pericardiocentesis could be implemented. His evolving pericardial effusion and pulmonary hypertension were an important factor of mortality. The role of pericardiocentesis in similar cases is uncertain in children. Amin et al. describe a case where successful resolution of respiratory symptoms were observed in a patient with a large pericardial effusion after pericardial tap. [11] Unfortunately, this was not possible in our case in a timely manner. Also, our patient deteriorated quickly and his hypoxemic respiratory failure was refractory despite high settings on mechanical ventilation. ECMO was not available in our center, and he could not have been promptly transferred to another center for care escalation.
Our case highlights the severity and rapid deterioration that can occur in immunosuppressed children with chronic diseases in the context of COVID-19 respiratory illness especially when pericardial effusion is present. It also emphasizes the need for prompt care escalation to centers where ECMO is available. In our case, the large effusion was compressing the left lung causing a near complete collapse and preventing adequate ventilation. It is also important to consider the need for pericardiocentesis early in order to allow better ventilation.
Financial disclosure
The authors have indicated no financial relationships relevant to this article to disclose.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Sources of support
The authors declare no sources of support including grants, fellowships or gifts of materials.
Declaration of competing interest
All authors declare that they have no conflict of interest.
References
- 1.Bergmann A.K., Campagna D.R., McLoughlin E.M., et al. Systematic molecular genetic analysis of congenital sideroblastic anemia: evidence for genetic heterogeneity and identification of novel mutations. Pediatr. Blood Cancer. 2010;54(2):273–278. doi: 10.1002/pbc.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tesarova M., Vondrackova A., Stufkova H., et al. Sideroblastic anemia associated with multisystem mitochondrial disorders. Pediatr. Blood Cancer. 2019;66(4) doi: 10.1002/pbc.27591. [DOI] [PubMed] [Google Scholar]
- 3.Riley L.G., Heeney M.M., Rudinger-Thirion J., et al. The phenotypic spectrum of germline YARS2 variants: from isolated sideroblastic anemia to mitochondrial myopathy, lactic acidosis and sideroblastic anemia 2. Haematologica. 2018;103(12):2008–2015. doi: 10.3324/haematol.2017.182659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley L.G., Menezes M.J., Rudinger-Thirion J., et al. Phenotypic variability and identification of novel YARS2 mutations in YARS2 mitochondrial myopathy, lactic acidosis and sideroblastic anaemia. Orphanet J. Rare Dis. 2013;8:193. doi: 10.1186/1750-1172-8-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahni R., Wedatilake Y., Cleary M.A., Lindley K.J., Sibson K.R., Rahman S. A distinct mitochondrial myopathy, lactic acidosis and sideroblastic anemia (MLASA) phenotype associates with YARS2 mutations. Am. J. Med. Genet. 2013;161A(9):2334–2338. doi: 10.1002/ajmg.a.36065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Gonzalez M., Castellano-Martinez A., Cascales-Poyatos H.M., Perez-Reviriego A.A. Cardiovascular impact of COVID-19 with a focus on children: a systematic review. World J Clin Cases. 2020;8(21):5250–5283. doi: 10.12998/wjcc.v8.i21.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swann O.V., Holden K.A., Turtle L., Pollock L., Fairfield C.J., Drake T.M., et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. Br. Med. J. 2020 Aug 27:370. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauer François, Dagrenat Charlotte, Couppie Philippe, Jochum Gaelle, Leddet Pierre. Pericardial effusion in patients with COVID-19: case series. European Heart Journal - Case Reports. October 2020;4(Issue FI1):1–7. doi: 10.1093/ehjcr/ytaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond T.T., Das A., Manzuri S., Ehrett S., Guleserian K., Brenes J. Pediatric COVID-19 and pericarditis presenting with acute pericardial tamponade. World J Pediatr Congenit Heart Surg. 2020 Nov;11(6):802–804. doi: 10.1177/2150135120949455. Epub 2020 Sep 10. PMID: 32909890; PMCID: PMC7484599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amoozgar Behzad, Kaushal Varun, Mubashar Umair, Sen Shuvendu, Yousaf Shakeel, Yotsuya Matthew. Symptomatic pericardial effusion in the setting of asymptomatic COVID-19 infection. Medicine: September. 2020;99(37) doi: 10.1097/MD.0000000000022093. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin H., Gyawali B., Chaudhuri D. A large pericardial effusion culminating in left lung collapse. Cureus. 2019;11(7):e5287. doi: 10.7759/cureus.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]