Abstract
The current coronavirus disease outbreak of 2019 (COVID-19) has led to a global pandemic. The principal cause of mortality in COVID-19 is represented lung injury with the development of acute respiratory distress syndrome (ARDS). In patients with COVID-19 infection, liver injury or liver dysfunction has been reported. It may be associated with the general severity of the disease and serve as a prognostic factor for ARDS development. In COVID-19, the spectrum of liver damage may range from direct SARS-CoV-2 viral proteins, inflammatory processes, hypoxemia, the antiviral drugs induced hepatic injury and the presence of the preexisting liver disease. We highlight in this review important topics such as the epidemiological features, potential causes of liver injury, and the strategies for management and prevention of hepatic injury in COVID-19 patients.
Key indexing terms: COVID-19, Pathogenesis, Liver transaminases, Liver injury, Cytokine storm
Introduction
Corona Virus Disease (COVID)-19 is a respiratory viral infection induced by a recently emerged coronavirus, Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2).1 Upon 14 July 2021, the records counted around 188,975,095 confirmed cases of COVID-19, and 4,070,387 deaths, worldwide.2 On the same date in Egypt, the statistics referred to 16,412 deaths caused by COVID-19 and around 283,320 confirmed infections with more than 219,525 recovered cases.2 The main target organ of COVID-19 is the lung and represents the most causal agent for morbidity and mortality. However, neurologic, renal, hepatobiliary, cardiac, and gastrointestinal tract implications are increasingly being recognized.3 The percentage of liver injury cases in patients with COVID-19 ranged from 14% to 53%.4 It was detailed that the frequency of mortality was greater in patients with hypertension (48%), taken after by 21% in diabetics, 14% in patients with cardiovascular sickness, 10% in those with a persistent lung infection, and 4% each for danger, cerebrovascular diseases and chronic kidney infection.5 Moreover, the mortality rate in patients with pre-existing liver disease was 0–2%.6
Even though elevated liver proteins were detailed as an extra pulmonary clinical sign, and nearly one-half of patients experienced grades of hepatic damage,7, 8, 9 liver damage in patients with SARS diseases was shown within the mild and moderate rise of alanine and/or aspartate aminotransferases (ALT and AST) with a few degrees of hypoalbuminemia and hyperbilirubinemia through the early stage of the infection.10 , 11 The causal agent for liver test marker abnormalities in COVID-19 is not yet interpreted. There's a lack of knowledge about if the pre-existing liver illnesses affected the severity of the infection. Many antiviral drugs and steroids are utilized to treat the disease with modest or high severity and may induce liver toxicity in COVID-19 patients. The systemic immune response may worsen the infection progression, ending with liver damage.1 Massive quantities of pro-inflammatory cytokines in serum (counting TNF-α, IL-6, and IL-1β) were found in most severe infections, showing cytokine storm disorder related to the infection severity.12 Up to date, the availability of COVID-19 vaccines is complicated.13 , 14 Attempts are being made for the improvement of certain and compelling prophylactic vaccination.14 Many epitopes of SARS-CoV-2 have been testified targeting of the immunity via these epitopes can confer sufficient protection facing this epidemic and give exploratory stages for the improvement of prophylactic vaccines.13 , 15
This review sheds light on the potential mechanisms of liver injury in COVID-19 infected patients and offers a detailed description of how to treat liver injury during COVID-19 infection. The improvement of the therapeutic routes for pre-existing liver disease patients associated with SARS-CoV-2 is a must for better COVID-19 and liver disease outcomes.
MOLECULAR STRUCTURE OF SARS CoV2
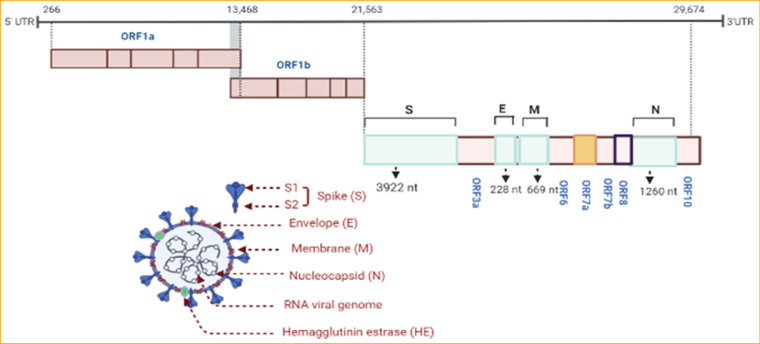
SARS-CoV-2 virion, positive-sense single-stranded RNA, has a massive genome (29.9 kb) like the other CoVs.16 The nucleocapsid here is surrounded by spike protein (S). Viral RNA is comprised of the phosphorylated nucleocapsid (N) protein. SARS-CoV-2 holds two types of spike protein (S); the ordinary spike glycoprotein trimmer (the familiar spike in all CoVs) and the hemagglutinin esterase (HE) (known in some CoVs).17 On the envelope (E) protein, the S protein is embedded alternately with the membrane (M) protein.18 The viral genome here has 5´ and 3´ terminal sequences (265 nt at the 5´ terminal and 229 nt at the 3´terminal region), as known in all β-CoVs, with a gene order 5´replicase open reading frame (ORF) 1ab-S-envelope(E)-membrane(M)-N-3´ (Fig. 1 ). The predicted S, ORF3a, E, M, and N genes of SARS-CoV-2 are 3822, 828, 228, 669, and 1260 nt in length, respectively.18
Figure 1.
Genome organization of SARS CoV-2 and its encoded proteins. The orf1ab gene constitutes two-thirds of the genome, encodes a total of 16 non-structural proteins (NSPs) within the pp1ab gene. The other third of SARS CoV-2 includes four genes that encode four structural proteins (S, M, E, N), and six accessory genes that encode six accessory proteins (orf3a, orf6, orf7a, orf7b, orf8, and orf10).
SARS- CoV2 HOST RECEPTORS IN LIVER TISSUE
The respiratory tract is not the unique tropism for SARS-CoV-2; it is also founded in the kidneys, heart, liver and surprisingly, the brain. The ACE2 protein displays within the gastrointestinal (GI) tract and within the colon, the biliary system, and the liver at high levels.19 Moreover, SARS-CoV2-interacts with three receptors in liver tissue, the expression fluctuats among cell types. ACE2, for illustration, is expressed in cholangiocytes, along with hepatocytes. TMPRSS2 is expressed in hepatocytes, cholangiocytes, erytroid cells, and sinusoidal endothelial cells. All cell types can represent FURIN.20 These receptors' existence indicates the emergence of liver disease in patients with COVID-19, the direct viral cytopathic effect. Several studies revealed the relationship between COVID-19 and hepatic manifestation. Therefore, the apoptosis and ballooning degeneration along with the bile duct injury may be the consequence of viral infection, with in situ hybridization and electron microscopy (EM) demonstrating viral particles within the liver. Moreover, Wang et al.21 showed that 3.9% of COVID-19 patients had chronic liver disease, with 4.3% mortality. A previous study reported that 11% of COVID-19 patients had pre-existing chronic liver disease (Xu and colleagues).22 Wang et al.23 confirmed that SARS-CoV-2 could harm liver cells by the direct cytopathic effect. Earlier studies have shown that about 60% of SARS patients have hepatic damage, and reverse transcription-polymerase chain reaction (RT-PCR) has identified positive SARS-COV in hepatic tissues.10
TRAJECTORY OF LIVER FUNCTION TESTS IN COVID 19 PATIENTS
Chen et al.7 was the first study that reported abnormal liver tests in patients with COVID-19. Abnormal alanine transaminase/aspartate transaminase (ALT/AST) levels and slightly elevated bilirubin levels indicate liver damage.8 , 24, 25, 26 In severe cases, the albumin is lessened and the level of albumin ranges from 26.3 to 30.9 g/L.7 An additional study from Wuhan on 113 patients observed that ALT, AST, γ-glutamyl transferase (GGT), alkaline phosphatase and bilirubin levels were significantly higher in deceased patients than others. Elevated AST (>40 U/L) was observed in 25 (16%) recovered patients and in 59 (52%) deceased patients, similarly abnormal ALT (>41 U/L) was noticed in 30 (19%) recovered patients and in 30 (27%) deceased patients. Comparably, hypoalbuminemia (<32 g/L) was reported in 22 (14%) recovered patients compared with 74 (65%) deceased patients. Serum bilirubin ranged from 8.4 µmol and 12.6 µmol in the recovered and deceased patients, respectively.5 Also, Chen et al.5 reported 13 (5%) patients with COVID-19 progressed to acute liver injury during the infection, of them 10 (76.9%) died. These findings were limited by the small number of patients, but it had a crucial message on patients with COVID-19 and hepatic injury. A recent study showed that patients with augmented aminotransferases (AST and/or ALT above 40 U/L), had elevated inflammatory indexes (high C-reactive protein [CRP], procalcitonin [PCT], ferritin, lactate dehydrogenase [LDH], GGT, lactate and d-dimer).27 In severe and critical cases of COVID-19, associated with poor outcomes, the level of liver enzymes is substantially higher. Huang et al. 24 found that the AST elevation was observed in 8 out of 13 (62%) ICU patients and 7 out of 28 (25%) patients in the non-ICU setting. Furthermore, in patients receiving lopinavir/ritonavir therapy, a higher percentage of enzyme elevation was observed (56.1% vs. 25%).25 Zhang et al.1 observed that in 54% of COVID-19 patients, the GGT was augmented. Zhou et al.28 reported that the increased level of ALT and a reduction in platelet and albumin were associated with a high mortality rate. Moreover, in a group comprised of 1099 severe patients with COVID-19, abnormal hepatic function tests (AST, ALT and total bilirubin) were reported in 22.2%, 21.3% and 10.5% of the cases, respectively.29 Furthermore, coagulation abnormalities have been associated with thrombocytopenia in critically ill COVID-19 infected patients.21 Liver test abnormalities can be found in patients with COVID-19. This finding is associated with a more extended hospital stay and a more severe clinical course. The exact physio pathological mechanisms are not completely understood, with potential roles for direct viral lesions in hepatic/cholangiocytes cells, inflammatory damage, hypoxic/shock-related circulatory compromise, and drug toxicity all under consideration.
HISTOLOGIC CHARACTERISTICS OF THE LIVER IN COVID-19 INFECTED PATIENTS
Hepatocyte deterioration and focal necrosis of the lobules, detected through liver histopathology, were observed in the examined postmortem liver of a COVID-19 infected patient. Inflammatory cells such as neutrophils, lymphocytes, and monocytes also infiltrated the portal triad. Obstruction of the hepatic sinus with micro-thrombosis was noted. Although, the histopathologic signs of liver failure or damage to the bile duct had not been recognized yet.30
Additionally, peripheral blood testing determined a considerable decline of CD4 and CD8 cells, whereas they were in hyper activation status. Also, an excessive concentration of pro-inflammatory CCR6+ Th17 CD4 T cells was observed and large amounts of cytotoxic granules were found in CD8 T cells that could lead to hepatocellular disorder.31 Tian et al.32 observed that the liver biopsies from four post mortem COVID-19 patients showed mild lobular lymphocytic infiltration and necrosis.
Moreover, from one patient and using RT-PCR, SARS-CoV-2 genomic RNA was separated from liver tissue. Wang et al.,23 using electron microscopy (EM) imaging, described viral shape in hepatocytes analogous to SARS‐CoV‐2 virions in the liver samples of two deceased COVID‐19 patients. So, the histopathological alterations described in these patients may be triggered by direct cytopathic consequences of SARS‐CoV‐2.23 Wichmann et al.33 performed autopsies on many pre-existing cardiac diseases of COVID-19 patients and discovered hepatomegaly, persistent inflammation and fat alteration.
MECHANISMS OF LIVER INJURY IN COVID-19 INFECTION
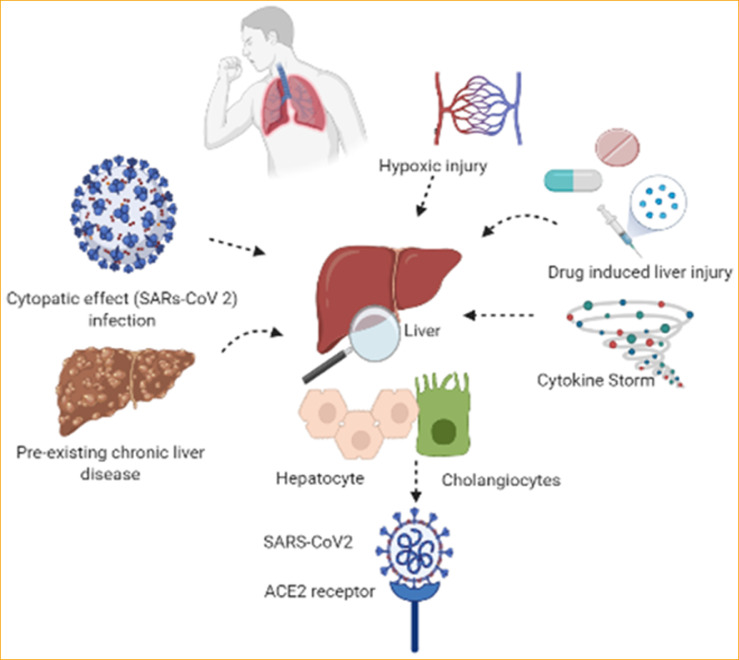
Data reported by case studies affirmed that liver damage is frequently observed in COVID-19; however the magnitude and underlying mechanisms are up to this time unclear. Fig. 2 depicts all the possible pathophysiological observations that will be addressed below.
Figure 2.
Potential mechanisms of hepatic injury in SARS-CoV-2 infection.
Direct viral effect on the liver
In many systemic infections, the liver orchestrates a vital role in host-microbe protection by collecting the systemic and portal circulation. Yang et al.34 revealed the direct cytopathic effect of SARS-CoV2 on the liver and other factors as seen in sepsis.
Many publications mentioned that SARS-CoV-2 might enter the liver cells via ACE2 receptor, which is expressed in the liver and bile duct cells.35 Recent data showed ACE2 expression in 2.6% of hepatocytes and 59.7% of cholangiocytes, the level of ACE2 expression in cholangiocytes was like that in type 2 alveolar cells of the lungs, interpreting that SARS-CoV-2 may directly attach to ACE2 found on cholangiocytes and causing liver dysfunction.36 As the suggestions that ACE2 expression from bile duct cells is greater than that of hepatic cells, the bile duct epithelial cells have a master role in liver regeneration and immune response.37 Cholangiocytes have a role in liver physiology (regeneration and adaptive immune response mechanisms) and accordingly the disturbance of cholangiocyte function can lead to hepatobiliary damage. In an ex vivo study, Zhao et al.38 found that SARS-CoV-2 infection interrupts the barrier and the transportation of bile acid via cholangiocytes using the dysregulation of genes incorporated in tight junction formation and carriage of bile acid. This could be due to the direct viral cytopathogenic effect on target cells that express ACE2 and TMPRSS2.
Moreover, gene ontology (GO) analysis in the same study revealed an alteration of genes involved in cell death. SARS-CoV-2 infection triggers many factors as: cell apoptosis factors (CD40 molecule [CD40], serine/threonine kinase 4 [STK4] and caspase recruitment domain family member 8 [CARD8]). The latter conclusion suggested that SARS-CoV-2 infection triggers cell death of patients with cholangiocytes.38 Therefore, it is clear that SARS-CoV-2 infection is the causal agent for direct cholangiocyte injury and subsequent bile acid accumulation ending liver damage in COVID-19 patients. However, the exact mechanisms of this proposed direct damage pathway are yet to be clarified. Hepatocellular necrosis, fatty degeneration, cellular infiltration, increase in ballooned hepatocytes, and mitotic cells were observed in the liver biopsy of SARS patients, referring to incorporating SARS-CoV-2 in the induction of liver apoptosis.4 , 10 Considering the above-mentioned findings, the idea that the liver damage in COVID-19 patients might result from direct cholangiocyte injury and subsequent bile acid accumulation caused by the virus infection.
Systemic cytokine storm
Cytokine storm is a hyperinflammatory response of the host body triggered by viral infection, which recruits a persistent activation and generation of lymphocytes and macrophages that will produce massive quantities of inflammatory cytokine. Inflammatory cytokine storm gives an order to pulmonary and non-pulmonary organs failure as (kidneys, liver, and cardiac muscle). Several data have been reported the correlation between the liver damage and severe pneumonia in COVID-19 patients via the inflammatory storm.39 COVID-19 cases with severe pneumonia demonstrated stimulation of inflammatory biomarkers involving CRP, inflammatory cytokines (eg. IL-2, IL-6, IL-7, IL-18, TNFα, interferon-γ, and ferritin),24 neutrophils and lymphocytes.1 , 24
Severe hypercytokinemia could provoke a cascade of actions that ends with tissue damage and multiorgan failure, especially in the liver.40 Hepatic inflammation, including the innate immune cell stimulation and cytokine generation, is a master cause for liver injury.41 , 42 IL-6 secreted into the liver during the initial stages of inflammation induces an enormous number of proteins,43 including CRP, fibrinogen, haptoglobin, alpha-antitrypsin and serum amyloid A (SAA). On the contrary, IL-6 was reported to lessen albumin, fibronectin, and transferrin generation.44 A correlation between lymphopenia and liver injury was remarked in some cases with CRP ≥ 20 mg/L levels and count of lymphocyte <1.1 × 109/L as an independent risk parameter predicting liver injury. Lymphopenia in COVID-19 studies was observed in more than 70% of patients and those with lesser lymphocyte counts are more susceptible to worse consequences.45 This information represents a correlation between inflammatory responses and liver injury in severe COVID-19 patients.
Effect of the drug on liver functions
A variety of antiviral drugs, steroids and antibiotics are utilized in treatment of COVID-19 patients. The liver plays a master function in the metabolism of all these drugs; accordingly, these drugs may cause hepatotoxicity. So far, there is no proof that drugs cause liver injury in severe COVID-19 patients. The initial presentation of COVID-19 is manifested usually by fever, cough, dyspnea and fatigue. Therefore, the patients occasionally consume antipyretic drugs. The latter pills mostly contain acetaminophen and are known to initiate direct hepatocyte toxicity.46 In the meantime, various antiviral (remdesivir, lopinavir/ritonavir, oseltamivir, arbidol and ribavirin), antibiotic (macrolids), antimalaria/ (hydroxychloroquine), immunomodulation (corticosteroids, tocilizumab) and antipyretic (acetaminophen) therapies are approved for COVID-19 severe cases.1 These drugs might cause abnormality in liver function. Besides, hemolysis triggered by ribavirin could stimulate tissue hypoxia, which may elevate serum liver enzyme. Many studies revealed that hepatic injury in COVID-19 patients may be due to the consumption of antiviral drugs like arbidol, oseltamivir, lopinavir, and ritonavir applied for the treatment of COVID-19 critical cases.25 , 47 Cai et al.47 reported that ritonavir and lopinavir are significant contributors to liver test abnormalities in COVID-19 patients. Also, in the previous study, liver injury was augmented up to four-fold post to lopinavir and ritonavir consumption. Furthermore, remdesivir is an antiviral nucleoside analog that is extensively used in coronaviruses treatment; remdesivir was first applied to treat patients with COVID-19 in the USA48 after showing in vitro antiviral activity anti SARS-CoV-2.49 Remdesivir achieved more than 60% clinical recovery in severe COVID-19 patients. In vitro study, remdesivir was shown to be lowly hepatocytes cytotoxic.50 However, there have been reports of adverse effects, including rising amounts of hepatic enzymes, diarrhea, rash, abnormal kidney function, and hypotension.51 Moreover, Wang et al.52 described elevated transaminase rates in patients treated with redeliver or placebo; patients with cirrhosis and/or with baseline AST/ALT >5 × upper limit were excluded. Also, the usage of tocilizumab for limiting cytokine release syndrome is correlated with augmented AST/ALT levels and the possibility of toxic hepatitis.53
The hydroxychloroquine (HCQ) and chloroquine (CQ) mechanism of action is to inhibit the virus's entrance by targeting the endosomal pathway.54 During the COVID-19 pandemic CQ and HCQ have been chosen as candidates for treatment of COVID-19 due to their anti-SARS-CoV-2 properties and safety for treatment of malaria and autoimmune disease.55 However, contradictory results are reported in clinical trials of COVID-19 with CQ or HCQ treatment. Several studies revealed that CQ and HCQ have no clinical efficacy in the treatment of COVID-19.56, 57, 58 To date, the effectiveness of CQ and HCQ as an antiviral drug against SARS-CoV-2 is still debatable. Recently, CQ and HCQ may cause cardiotoxicity and a high rate of mortality in severe COVID-19 infected patients. Hence, for treating severe COVID-19 patients, HCQ and CQ should be administered cautiously based on the current treatment guidelines.
Furthermore, the antibiotics used for COVID-19 treatment have shown an association with the increased prevalence of liver test abnormalities in the regression model; however, the association was not significant.47 Moreover, corticosteroids positively correlated with high ALT/AST ranges in COVID-19 patients,59 also associated with steatosis.60 Accordingly, cirrhotic patients should be treated prudently with anti-inflammatory drugs.61
Hypoxic injury
Ischemic hepatitis, also named, hypoxic hepatitis, is occasionally observed in severe patients and declares a sign of cardiac, respiratory, or circulatory failure, causing passive congestion or lessened perfusion of the liver.62 , 63 There is a compensatory decline in peripheral and splanchnic blood flow in systemic stress conditions, leading to a deterioration in hepatic blood flow and thereby ending with hepatocellular hypoxia.64 Animal models65 show that hypoxia can cause hepatic cell death and infiltration of inflammatory cells, with lipid accumulation and an increase in oxygen reactive species.66 ALT/AST elevations mark hypoxic liver damage due to oxygen imbalance. Additionally, Kupffer cells can stimulate cytokines due to ischemia and recruit polymorph nuclear leukocytes.67 The last phenomenon progresses with elevated levels of transaminases, elevated LDH levels, which can recover as hypoxia is adjusted.62 Therefore, hypoxemia may be one of the physio pathological causes of liver damage in patients with COVID-19.
Impact of pre-existing liver disease on COVID 19 prognosis
Patients of any age with the following described liver diseases are at high risk of progressive infection from SARs-CoV2 virus.
Chronic viral hepatitis
No evidence clarifies synergism chronic viral hepatitis (hepatitis B &C) with SARS-CoV-2. Nevertheless, it is well-known that SARS-COV patients suffering from viral hepatitis are at risk of more liver injury. This was due to heightened hepatitis virus replication during SARS-COV Infection.68 In a recent study from Wuhan, 9% of patients had underlying liver disease of cirrhosis or hepatitis.26 Zou et al.69 have recently reported that the patients with SARS-CoV-2 and hepatitis B co-infection with liver injury are prone to a worse prognosis. Lin et al.70 observed that most COVID-19 cases suffering hepatitis B co-infection had abnormal total bilirubin, AST, and ALT alterations. A retrospective analysis of more than three hundred COVID‐19 patients noticed that twelve patients had a co-infection with hepatitis B and two patients had a co-infection with hepatitis C .71 In another study of baseline liver biochemical parameters in 324 cases in China, the HBsAg‐positivity ranges in COVID‐19 infected cases raised to 6.5%.72 So, the synergism of the pre-existing liver infection with disease prognosis and outcomes in COVID-19 need a comprehensive study to be evaluated.
Chronic liver disease
Liver cirrhosis now affects 112 million people worldwide, resulting in 2 million deaths each year due to hepatic decompensation and hepatocellular carcinoma (HCC).73 In several recently published series, high mortality rates of COVID-19 were reported among cirrhotic patients.74 , 75 In addition, it was noted that the Child-Pugh baseline score was significantly related to mortality. Lung injury is the most common cause of death among COVID-19 patients. As a potential driver of continuous lung injury, liver dysfunction is involved. Indeed, in patients with bacterial chest sepsis, the significance of liver failure is well recognized.76 The lethal combination of cirrhosis and SARS-CoV-2 may be attributed to immune alteration caused by viral infection and coagulation disorders. Ascites or encephalopathy worsening, immune dysfunction in viral infection, increased burden of venous thromboembolic disease, and coexisting lung disease have all contributed to pulmonary dynamics dysregulation. According to Marjot et al.77 patients with cirrhosis have a 32% mortality rate compared to 8% for those without cirrhosis and the mortality rate increased in patients with cirrhosis in relation to the Child-Pugh class (A [19%], B [35%], C [51%]). Respiratory failure was the foremost reason of death (71%) .
Alcohol associated liver disease (ALD) and COVID
The impact of COVID-19 in patients with alcoholic liver disease or in patients with alcoholic hepatitis is very limited.75 , 78 Nevertheless, studies of patients with cirrhosis showed increased mortality in patients with alcohol-related cirrhosis, as in other patients with cirrhosis.75 , 78 Patients with alcohol-related cirrhosis often have associated comorbidities, like obesity, diabetes mellitus and chronic kidney disease, which also increase the risk of COVID-19 complications.79 Kim et al.,80 reported in a study of 867 patients with chronic liver disease and diagnosed with COVID-19 infection that ALD was autonomously linked with an increased risk of poor survival and COVID-19 mortality rate. The inflammatory state caused by danger-associated molecular patterns (DAMPs) is associated with ALD, which leads to the production of pro-inflammatory cytokines by distinct immune cells.75 , 81 In patients with ALD, it was postulated that the superimposed cytokine storm caused by SARS-CoV-2 could aggravate the increased inflammatory process, resulting in worse outcomes.82
Non-alcoholic fatty liver disease (NAFLD)
Obesity is a risk factor for an acute COVID-19 infection.83 The adipose tissue may serve as a viral reservoir and an immunological core for the inflammation.84 Diabetes and hypertension as elements of the metabolic syndrome are extensively detected in patients with severe COVID-19.85 As non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver disease ([NAFLD], or metabolic dysfunction-associated fatty liver disease) are strictly related to these metabolic comorbidities, detecting patients with NAFLD progression to a more severe outcome of COVID-19 has a great clinical importance.86 In a retrospective study of 202 COVID-19 patients, Ji et al.87 stated that around 35% of COVID-19 patients were comorbid with NAFLD, and patients having NAFLD had an augmented risk of severe COVID-19, higher likelihood of liver test abnormalities during hospitalization, and extended viral shedding times in comparing with COVID-19 patients without NAFLD. On the other side, allelic variants correlated with NASH progression did not show any association with the severity of COVID-19 infection.88 Moreover, the relevant genes for SARS-CoV-2 infection did not show dysregulation in liver tissues from patients having NAFLD.89
Liver transplant recipients
During the COVID-19 pandemic, liver transplantation was challenging because many hospitals had to practically prevent or vastly reduce their transplantation programs due to an abrupt decrease in the number of donors and the switch of many care facilities into COVID-19 units. Managment of post liver transplant recipients during the COVID-19 pandemic is a hard challenge for clinicians due to the circumscribed available information and the vital need to continue immunosuppressive drugs in these patients, placing them at risk for more serious courses of COVID-19 infection and potential sustained viral shedding. Qin et al.90 reported the first case of SARS-CoV-2 infection in a hepatocellular carcinoma patient who underwent liver transplantation, and found an increased viral burden with increased immunosuppressant dose. Bhoori et al.91 revealed that immunosuppressive drugs did not affect the incidence of COVID-19 severity. Early reports from Italy reported low mortality rates less than 5% in transplant recipients,92 but subsequent analyses reported mortality rates around 25% in liver and other solid organ transplant recipients.93 , 94 Results of a prospective European study from 19 transplant centers95 involving 57 liver transplant (LT) recipients with confirmed SARS-CoV-2 infection were recently published. The overall and in-hospital case-fatality rates for patients with severe COVID-19 infection were 12% and 17%, respectively, which are consistent with the expected mortality rate. There was an underlying history of cancer in five of the 7 patients who died. The available evidence does not support the concept that transplantation or specific immunosuppressive treatments have a substantial influence on the possibility of disease severity, but those with underlying cancer may require particular consideration.96
Recently, several vaccines against COVID-19 have been registered and proved their efficacy in healthy individuals. However, careful evaluation for vaccination of immunocompetent patients remain necessary due to the potential risk of immune imbalance related to their disease or the immunosuppressive treatment. Boyarsky et al.97 reported an adequate humoral response after the full immunization regimen of mRNA vaccine among solid organ transplant recipients and a poor response was associated with the use of antimetabolite immunosuppression.
COVID-19 and HCC
Because of chemotherapy and disease treatments, cancer patients are frequently immunosuppressed. In a Chinese study of 1590 cancer patients with COVID-19 among 575 hospitals, it was observed that patients with underlying cancer were at higher risk of contracting SARS-CoV-2 Infection and progresses to a severe outcome. They also showed progressive outcomes than patients without cancer.98 Most HCC patients have chronic liver disease and as a result, they are categorized as a high-risk group and are suspected of having worse outcomes. EASL recommends postponing loco regional treatments wherever possible and gradually remove anti-cancer immunological therapy.99
Diagnostic work up of the patient and treatment
The risk of drug-induced liver injury is heightened in COVID-19 patients with pre-existing elevated transaminase levels. Hence, antipyretic consumption, antiviral or herbal drugs must be under physician monitoring to prevent drug-induced liver injury. The elevation of ALT/AST >5 times the normal amount should be followed by drugs cessation. Also, the extensive usage of corticosteroids (methylprednisolone) can reactivate chronic hepatitis B. Therefore, patients that have positive HBsAg must be treated with antiviral drugs. Also, there is a recommendation for screening for the hepatitis B core antibody and, if positive, treating patients with antivirals along with steroid therapy. Moreover, it was observed that the introduction of lopinavir combined with ritonavir could accelerate liver damage in hepatitis C or hepatitis B infected patients100; thus, the usage of the drugs mentioned above in COVID‐19 treatment in patients suffering from liver disease is not recommended.
Management of recipients after liver transplantation in the COVID-19 pandemic is a complex challenge for clinicians due to the necessity for immunosuppressive drugs in these patients and the shortage of data available that puts them at risk for more aggressive courses of COVID-19 infection and possible persistent viral shedding. Lessening immunosuppression doses to the minimally acceptable level appears reasonable in liver transplanted patients; especially, in case of lymphopenia or severe infection.99 In addition, physicians have to take into account the drug-drug interactions in the transplantation setting. Particularly, immunosuppressive drugs and ritonavir-boosted antiviral drugs display related interactions through CYP34A, which augments calcineurin and mTOR inhibitors' levels. Accordingly, remdesivir or chloroquine-based regimens seem to be safe, while boosted protease inhibitors should be avoided.
In order to avoid getting sick with or spreading COVID-19 infection, patients post-liver transplantation or suffering pre-existing liver disease must stick to the same precautionary measures followed by people with other medical conditions. Therefore, it is recommended to treat COVID-19 patients with liver injury using drugs that can inhibit systemic inflammation and care for liver functions. Furthermore, the influence of drugs on liver injury should be frequently monitored and evaluated throughout the treatment of COVID-19. It is recommended that acetaminophen be considered and that non-steroidal anti-inflammatory medicines should be avoided during cirrhosis. The administration of the antiviral agents in COVID-19 infected patients with decompensated liver disease should be regarded.
Conclusions
Abnormal Liver tests are reasonably more frequent in severe patients with COVID-19 infection. Liver function abnormalities require clinical monitoring, continuous observations and, possibly, specific treatment. Liver test biomarkers (especially AST and GGT) as well as viral hepatitis markers should be regularly observed and monitored through hospitalization. More consideration should be paid to minimize liver damage in patients with pre-existing liver disease. Cautious usage of antiviral therapies in liver disease patients and drug-drug interactions in post-liver transplanted patients must be considered. Furthermore, reduction of immunosuppression in liver transplant patients may be considered in the presence of moderate COVID-19 infection and in patients with lymphopenia, fever, or worsening pneumonia. More studies to interpret the pathogenic mechanisms of liver injury in severe COVID-19 patients are urgently needed. Upon these studies, researchers can postulate preventive strategies and effective therapies in COVID-19 infected patients with preexisting liver disease.
CRediT authorship contribution statement
Dr. Reham M. Dawood: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. Ghada Maher Salum: Resources, Software, Writing – review & editing. Mai Abd El-Meguid: Resources, Software, Data curation, Validation, Writing – review & editing.
Declaration of Competing Interest
All authors have none to declare.
Acknowledgments
The authors received no financial support to produce this manuscript.
References
- 1.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; 2021. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019;382(8):727–733. doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chau T.N., Lee K.C., Yao H., et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J., Liu J., Zhao X., et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020;71(15):706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Li S., Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12(3) doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang W., Yang Y., Rao Y., Rao X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 2020;5:18. doi: 10.1038/s41541-020-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawood R.M., El-Meguid M.A., Salum G.M., El-Wakeel K., Shemis M., El Awady M.K. Bioinformatics prediction of B and T cell epitopes within the spike and nucleocapsid proteins of SARS-CoV2. J Infect Public Health. 2020 doi: 10.1016/j.jiph.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Y., Yang H., Ji W., et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4) doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu F., Zhao S., Yu B., et al. Complete genome characterisation of a novel coronavirus associated with severe human respiratory disease in Wuhan, China. bioRxiv. 2020:2020.2001.2024.919183.
- 19.Xu H., Zhong L., Deng J., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirola C.J., Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40(8):2038–2040. doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X.W., Wu X.X., Jiang X.G., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Liu S., Liu H., et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73(4):807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan Z., Chen L., Li J., et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medetalibeyoglu A., Catma Y., Senkal N., et al. The effect of liver test abnormalities on the prognosis of COVID-19. Ann Hepatol. 2020;19(6):614–621. doi: 10.1016/j.aohep.2020.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q., Wang R.S., Qu G.Q., et al. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36(1):21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian S., Xiong Y., Liu H., et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wichmann D., Sperhake J.P., Lutgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z., Xu M., Yi J.Q., Jia W.D. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4(1):60–63. [PubMed] [Google Scholar]
- 35.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar P., Sharma M., Kulkarni A., Rao P.N. Pathogenesis of liver injury in coronavirus disease. J Clin Exp Hepatol. 2019;10(6):641–642. doi: 10.1016/j.jceh.2020.05.006. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banales J.M., Huebert R.C., Karlsen T., Strazzabosco M., LaRusso N.F., Gores G.J. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16(5):269–281. doi: 10.1038/s41575-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao B., Ni C., Gao R., et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020;53(1):19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald B., Kubes P. Innate immune cell trafficking and function during sterile inflammation of the liver. Gastroenterology. 2016;151(6):1087–1095. doi: 10.1053/j.gastro.2016.09.048. [DOI] [PubMed] [Google Scholar]
- 42.Dawood R.M., El-Meguid M.A., Salum G.M., El Awady M.K. Key players of hepatic fibrosis. J Interferon Cytokine Res. 2020;40(10):472–489. doi: 10.1089/jir.2020.0059. [DOI] [PubMed] [Google Scholar]
- 43.Dawood R.M., Salum G.M., Abd El-Meguid M., Shemis M., Abdel Aziz A.O., El Awady M.K. Recipient interleukin 6 gene polymorphism and expression predict HCV recurrence post liver transplantation. Gene. 2020;754 doi: 10.1016/j.gene.2020.144887. [DOI] [PubMed] [Google Scholar]
- 44.Heinrich P.C., Castell J.V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L., Li S., Xu M., et al. Risk factors related to hepatic injury in patients with corona virus disease 2019. medRxiv. 2020:2020.2002.2028.20028514.
- 46.Deng S.Q., Peng H.J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai Q., Huang D., Yu H., et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoehl S., Rabenau H., Berger A., et al. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382(13):1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren T.K., Jordan R., Lo M.K., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grein J., Ohmagari N., Shin D., et al. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genovese M.C., Kremer J.M., van Vollenhoven R.F., et al. Transaminase levels and hepatic events during tocilizumab treatment: pooled analysis of long-term clinical trial safety data in rheumatoid arthritis. Arthritis Rheumatol. 2017;69(9):1751–1761. doi: 10.1002/art.40176. [DOI] [PubMed] [Google Scholar]
- 54.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 55.Hickley N.M., Al-Maskari A., McKibbin M. Chloroquine and hydroxychloroquine toxicity. Arch Ophthalmol. 2011;129(11):1506–1507. doi: 10.1001/archophthalmol.2011.321. [DOI] [PubMed] [Google Scholar]
- 56.Mahévas M., Tran V.-.T., Roumier M., et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalised for COVID-19 infection and requiring oxygen: results of a study using routinely collected data to emulate a target trial. medRxiv. 2020:2020.2004.2010.20060699.
- 57.Magagnoli J., Narendran S., Pereira F., et al. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19. Med. 2020;1(1):114–127. doi: 10.1016/j.medj.2020.06.001. e113. (New York, NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang W., Cao Z., Han M., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei F., Liu Y.M., Zhou F., et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72(2):389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nardo A.D., Schneeweiss-Gleixner M., Bakail M., Dixon E.D., Lax S.F., Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41(1):20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parohan M., Yaghoubi S., Seraj A. Liver injury is associated with severe Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of retrospective studies. medRxiv. 2020:2020.2004.2009.20056242. [DOI] [PMC free article] [PubMed]
- 62.Waseem N., Chen P.H. Hypoxic hepatitis: a review and clinical update. J Clin Transl Hepatol. 2016;4(3):263–268. doi: 10.14218/JCTH.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lightsey J.M., Rockey D.C. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol. 2017;33(3):158–163. doi: 10.1097/MOG.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 64.Dunn G.D., Hayes P., Breen K.J., Schenker S. The liver in congestive heart failure: a review. Am J Med Sci. 1973;265(3):174–189. doi: 10.1097/00000441-197303000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Cheung K.S., Hung I.F.N., Chan P.P.Y., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosser B.G., Gores G.J. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108(1):252–275. doi: 10.1016/0016-5085(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 68.Peiris J.S., Chu C.M., Cheng V.C., et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zou X., Fang M., Li S., et al. Characteristics of liver function in patients with SARS-CoV-2 and chronic HBV coinfection. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin Y., Yuan J., Long Q., et al. Patients with SARS-CoV-2 and HBV co-infection are at risk of greater liver injury. Genes Dis. 2020 doi: 10.1016/j.gendis.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang S., Han P., Xiao F. Manifestations of liver injury in 333 hospitalized patients with coronavirus disease 2019. Chin J Dig. 2020;40(3) [Google Scholar]
- 72.Qian Z.P., Mei X., Zhang Y.Y., et al. [Analysis of baseline liver biochemical parameters in 324 cases with novel coronavirus pneumonia in Shanghai area] Zhonghua Gan Zang Bing Za Zhi. 2020;28(3):229–233. doi: 10.3760/cma.j.cn501113-20200229-00076. [DOI] [PubMed] [Google Scholar]
- 73.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 74.Qi X., Liu Y., Wang J., et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2021;70(2):433–436. doi: 10.1136/gutjnl-2020-321666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iavarone M., D'Ambrosio R., Soria A., et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73(5):1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah B.A., Ahmed W., Dhobi G.N., Shah N.N., Khursheed S.Q., Haq I. Validity of pneumonia severity index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J Chest Dis Allied Sci. 2010;52(1):9–17. [PubMed] [Google Scholar]
- 77.Marjot T., Moon A.M., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74(3):567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moon A.M., Webb G.J., Aloman C., et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73(3):705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kushner T., Cafardi J. Chronic liver disease and COVID-19: alcohol use disorder/alcohol-associated liver disease, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, autoimmune liver disease, and compensated cirrhosis. Clin Liver Dis. (Hoboken) 2020;15(5):195–199. doi: 10.1002/cld.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim D., Adeniji N., Latt N., et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarin S.K., Choudhury A., Lau G.K., et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS study (APASL COVID-19 liver injury spectrum study) Hepatol Int. 2020;14(5):690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cai Q., Chen F., Wang T., et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen. China. Diabetes Care. 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 84.Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (Silver Spring) 2020;28(7):1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eslam M., Sanyal A.J., George J. International consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):e1991. doi: 10.1053/j.gastro.2019.11.312. 1999-2014. [DOI] [PubMed] [Google Scholar]
- 87.Ji D., Qin E., Xu J., et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2021;73(2):451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valenti L., Jamialahmadi O., Romeo S. Lack of genetic evidence that fatty liver disease predisposes to COVID-19. J Hepatol. 2020;73(3):709–711. doi: 10.1016/j.jhep.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Biquard L., Valla D., Rautou P.E. No evidence for an increased liver uptake of SARS-CoV-2 in metabolic-associated fatty liver disease. J Hepatol. 2020;73(3):717–718. doi: 10.1016/j.jhep.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qin J., Wang H., Qin X., et al. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatology. 2020;72(4):1491–1493. doi: 10.1002/hep.31257. [DOI] [PubMed] [Google Scholar]
- 91.Bhoori S., Rossi R.E., Citterio D., Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transplant. 2020;26(6):832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 93.Belli L.S., Duvoux C., Karam V., et al. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5(8):724–725. doi: 10.1016/S2468-1253(20)30183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee B.T., Perumalswami P.V., Im G.Y., Florman S., Schiano T.D., Group C.S. COVID-19 in liver transplant recipients: an initial experience from the US epicenter. Gastroenterology. 2020;159(3):1176–1178. doi: 10.1053/j.gastro.2020.05.050. e1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Becchetti C., Zambelli M.F., Pasulo L., et al. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69(10):1832–1840. doi: 10.1136/gutjnl-2020-321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Belli L.S., Duvoux C., Karam V., et al. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5(8):724–725. doi: 10.1016/S2468-1253(20)30183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boettler T., Newsome P.N., Mondelli M.U., et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2(3) doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Motor S., Alp H., Senol S., et al. Comparison of the chronic effects of ribavirin and caffeic acid phenethyl ester (CAPE) on pancreatic damage and hepatotoxicity. Int J Clin Exp Med. 2014;7(4):1005–1013. [PMC free article] [PubMed] [Google Scholar]