Abstract
Background:
Continuous positive airway pressure (CPAP) in preterm infants is initially beneficial, but animal models suggest longer term detrimental airway effects towards asthma. We used a neonatal CPAP mouse model and human fetal airway smooth muscle (ASM) to investigate the role of extracellular calcium sensing receptor (CaSR) in these effects.
Methods:
Newborn wild type and smooth muscle-specific CaSR−/− mice were given CPAP for 7 days via a custom device (mimicking CPAP in premature infants), and recovered in normoxia for another 14 days (representing infants at 3–4 years). Airway reactivity was tested using lung slices, and airway CaSR quantified. Role of CaSR was tested using NPS2143 (inhibitor) or siRNA in WT mice. Fetal ASM cells stretched cyclically with/without static stretch mimicking breathing and CPAP were analyzed for intracellular Ca2+ ([Ca2+]i) responses, role of CaSR and signaling cascades.
Results:
CPAP increased airway reactivity in WT but not CaSR−/− mice, increasing ASM CaSR. NPS2143 or CaSR siRNA reversed CPAP effects in WT mice. CPAP increased fetal ASM [Ca2+]I, blocked by NPS2143, and increased ERK1/2 and RhoA suggesting two mechanisms by which stretch increases CaSR.
Conclusions:
These data implicate CaSR in CPAP effects on airway function with implications for wheezing in former preterm infants.
INTRODUCTION
Airway hyperreactivity is a common feature in asthma and wheezing disorders that represent major long-term morbidities in former preterm infants (1–3). Following premature birth and consequent immaturity of the pulmonary and respiratory systems, such infants are often administered supplemental O2 and/or some form of ventilatory support. While invasive mechanical ventilation was previously common, non-invasive nasal continuous positive airway pressure (CPAP) has become the primary mode of support for preterm infants with respiratory distress syndrome (3, 4). In this regard, early use of CPAP has been shown to be beneficial in promoting alveolar development, but longer term effects are not clear. In previous studies, using a neonatal mouse model of CPAP, we demonstrated that early CPAP results in sustained, long-term increase in airway reactivity even following discontinuation of CPAP and growth in normal oxygen concentrations (5). What is less clear are the underlying mechanisms that contribute to CPAP-induced airway hyperreactivity.
As throughout life, early postnatal bronchial airways undergo cyclical stretch via tethering parenchyma with added, externally imposed forces of CPAP. Bronchial airways of premature infants may be particularly susceptible to CPAP given greater compliance compared to term neonate or adult (6, 7) resulting in more stretch for the same CPAP. Thus, in the context of prematurity, signaling mechanisms in epithelium or ASM activated by stretch of CPAP may substantially influence airway structure and function. Previous data show stretch is important for in utero airway development, including for airway smooth muscle (ASM) (8, 9) and thus some level of stretch might be beneficial while “too much” stretch may be detrimental. There is currently little information on the role of developing ASM in responses of fetal/neonatal airways to stretch (5, 8–10). Given that the numbers of conducting airways are fixed at birth, factors that detrimentally influence ASM in the late fetal/early postnatal period can have long-lasting influence. Studies in immature rabbits suggest their airway walls have greater percentage of ASM with less cartilage compared to adult (11, 12). Accordingly, CPAP is likely to have greater effect on ASM in premature infants. Conversely, given the important of ASM in airway structure and function (13–17), altered ASM mass, contractility, and proliferation should all have greater contribution to airway tone. Accordingly, in this study we explored mechanisms in ASM that could contribute to CPAP effects.
An important aspect of fetal development is that the extracellular environment which can modulate airway growth. Fetal airways in fact have higher extracellular Ca2+ ([Ca2+]o) of ~1.7 mM compared to adult airways (~1.2 mM) (18, 19). Accordingly, it is possible that [Ca2+]o itself contributes to ASM in health and disease, and given that CPAP increases airway smooth muscle expression (MacFarlane et al., 2020), we hypothesized Ca2+ could thus mediate or modulate CPAP effects.
[Ca2+]o is normally sensed by the extracellular Ca2+ sensing receptor (CaSR) (20–22). A member of the Class C family of GPCRs that includes metabotropic GABA-B and glutaminergic receptors (23, 24) (23), the CaSR is well-known for its role in calciotropic tissues such as parathyroid gland, kidney or bone towards sensing and regulating [Ca2+]o (20–22) making it the target of activators (calcimimetics) for treating hyperparathyroidism, and negative allosteric modulators (calcilytics such as NPS2143) for genetic hypocalcemia (25, 26). We and others have now shown CaSR in non-calciotropic tissues and its role in regulating [Ca2+]I, gene expression, cellular proliferation and production of ECM proteins (18, 20, 21, 27–29) (28, 30). CaSR can couple to multiple G-proteins (Gq/11 or Gi/o) (27) and via Gq/11 activate PLCβ-IP3 to increase [Ca2+]i. In ASM and in pulmonary artery, CaSR can also modulate Ca2+ influx (31) (30). Furthermore, via DAG-PKC or Gi/o, CaSR can modulate MAP kinase signaling (32) contributing to longer-term effects such as proliferation and ECM production relevant to airway remodeling. We previously showed that CaSR promotes adult ASM proliferation via PLC (30) and ERK1/2 (28). In this regard, CaSR may be particularly relevant in developing ASM that is expected to undergo greater proliferation towards lung growth and more dependent on plasma membrane [Ca2+]i regulatory pathways. We have previously shown that CaSR is involved in adult ASM in the context of adult asthma and contributes to airway hyperreactivity (28). CaSR is expressed in embryonic lung mesenchyme (18, 33). Furthermore, we recently showed that developing human ASM expresses CaSR that regulates [Ca2+]i in the context of ASM contractility, and promotes cell proliferation (30). Accordingly, in the present study, we explored the hypothesis that the CaSR contributes to CPAP effects in the context of enhanced contractility.
We used converging approaches to explore CaSR in the developing airway in the context of CPAP. We applied a neonatal mouse model of CPAP and the precision cut lung slice technique that we previously established as relevant to exploring stretch in the context of immaturity. Both wildtype (WT) and smooth muscle-specific constitutional CaSR knockout (KO; CaSR−/−) mice were used, along with NPS2143 and siRNA to inhibit CaSR. Separately, we explored human fetal ASM (fASM) from lungs of 18–22 week fetuses, a developmental period of rapid bronchial growth and proximate to neonatal survival in the ICU where CPAP would be applied. Compared to adult ASM, mechanisms of [Ca2+]i regulation are less-studied and there are currently no data on stretch effects. We previously characterized suitability of isolated fASM cells in exploring [Ca2+]i (34).
METHODS
Mice:
Time-pregnant wildtype (WT) mice (C57BL/6J) were purchased from a commercial vendor (Charles River, Wilmington, MA). Mice negative for CaSR in smooth muscle (CaSR−/−) were bred and reared in the Case Western Reserve animal facility (transfer from Dr. Chang’s lab at UCSF; Case IACUC 2015-0066). These CaSR−/− mice were also on a C57BL/6 background, and the process for generating these smooth muscle specific CaSR KO mice has been previously published (28).
The day after birth (P1), both male and female mouse pups were randomized to be treated with or without CPAP (in 21% O2) for the first week of postnatal life as described previously (5), i.e. until P7. Mice were then allowed to survive at 21% O2 without any further CPAP interventions for two more weeks. At three weeks (P21), mice were euthanized and the lungs prepared for either measurements of airway reactivity using the precision lung slice preparation or immunohistochemical analysis of CaSR, αSM-actin expression and signaling proteins. All procedures were carried out in accordance with the National Institute of Health (NIH) guidelines for care and use of laboratory animals and were approved by the Animal Care and Use Committee at Case Western Reserve University.
Neonatal CPAP:
Following the day of birth (P0), litters were equally divided, with both male and female pups randomly assigned to receive CPAP (6 cmH2O) or control (0 cmH2O) starting the following day (P1) for the first 7 postnatal days as previously described (5, 35). Pups and dams were maintained in a temperature controlled room during a 12:12hr, light:dark cycle and provided food and water ad lib.
Details of the neonatal CPAP apparatus and technique have been previously published (5). Briefly, each day unanesthetized neonatal mice were separated from their dams and fitted with a custom made mask to administer CPAP while spontaneously breathing and resting on a temperature controlled heat pad (Gaymar T/pump, Orchard Park, NY). An entry port in the mask was used to deliver humidified air, which passed through to an exit port connected to a downstream manometer. An adjustable leak in the tubing between mask and manometer enabled continuous flow through the mask, while also allowing fine adjustment of the back-pressure to the mask and thus, to achieve a desired level of CPAP of 6cm H2O. A custom-made system allowed delivery of CPAP to multiple mice simultaneously. CPAP was administered for 2 hrs on the first day to minimize the duration the pups were separated from the mother, but was increased to 3 hrs/session for the following 6 consecutive days (7 days total). Control mice were also separated from dams, and underwent the same procedures of mask fitting, heat pad, and identical airflow, but did not receive CPAP (i.e. 0 cm H2O). After each session of CPAP, the mask was removed and the pups were returned to the mother to resume normal rearing.
At the end of the CPAP exposure period, mice were allowed an additional 2 weeks of un-interrupted maternal care at which time the pups were prepared for assessment of airway reactivity to methacholine challenge using the in vitro precision-cut living lung slice preparation. Additional mice were used for rtPCR on whole lung homogenates, and immunohistochemistry.
Lung slice preparation:
This technique has also been previously described (5). At P21, WT and CaSR−/− mice were euthanized via anesthetic overdose (intraperitoneal ketamine 100 mg/kg and xylazine 10mg/kg). The trachea was cannulated (0.58 mm PE tubing, Clay Adams, Sparks, MD), chest cavity opened, and ~0.8ml liquefied agarose (Invitrogen, Carlsbad, CA; #A9539; 38°C) gently injected to inflate the lungs. The preparation was then refrigerated en bloc for 30 minutes to allow the agarose to gel following which 300μm lung slicers were cut using a vibratome (VT1000, Leica Microsystems, Wetzler, Germany). Slices were immersed in DMEM + Pen/Strep (Life Technologies, Carlsbad, CA) and allowed to recover overnight an incubator (5% CO2; 37°C).
The following day, lung slices were rinsed in HBSS and placed in an in vitro recording chamber for live imaging using a microscope (DMLFS, Leica Microsystems, Wetzler, Germany) and continuous perfusion (7ml/min) with HBSS. A digital video camera (Rolera Fast, QImaging, Surrey, Canada) was used to identify individual airways under 5X magnification. After baseline perfusion, slices were exposed to increasing doses of methacholine and changes in airway lumen area recorded. The extent of airway constriction in response to increasing doses of methacholine (0.25, 0.5, 1, 2, 4, and 8μM; Sigma Aldrich, St Louis, Mom #1396364) was determined at the end of a 2 minute period of exposure at each dose. ImageJ analysis software was used to calculate the luminal area (in pixels) and extent of airway constriction assessed with greater decrease in lumen area interpreted as greater reactivity.
Individual airways were chosen at random and the response to methacholine was performed on one airway/lung section, although measurements were typically collected from 1–2 sections per animal. Thus, treatment groups consisted typically of 1–2 airways/animal, 2–3 animals per litter, 2–3 litters per group. Airways with a baseline area above 0.05 mm2 were excluded for analysis since we showed previously they are unaffected by CPAP.
For assessment of airway responsiveness in the presence of NPS2143 (Tocris, Minneapolis, MN; #3626) individual lung slices from WT mice were pre-incubated in a tissue culture incubator in either 10μM NPS2143, or vehicle (0.4% DMSO) for one hour. The slice was then transferred to a petri dish containing the same solution for imaging. The slice was allowed to equilibrate for 5 min prior to assessment to the maximum (8μM) methacholine challenge. The NPS dose was selected based on our previous in vivo study using adult mice (28).
CaSR and aSM-actin immunoreactivity:
P21 WT and CaSR−/− mice were euthanized via anesthetic overdose and lungs inflated with a 4% paraformaldehyde at a pressure of 25cm H2O via tracheal cannulation. The lungs were then paraffin embedded and 5μm thick sections were cut and mounted onto glass slides. Immunostaining was done using standard techniques. Slides were de-paraffinized, rehydrated in PBS, and incubated in blocking buffer (1% BSA in PBS). Slides were then incubated overnight at 4°C in CaSR antibody (1:100, ProteinTech, Rosemont, IL; #19125-1-AP) and actin antibody directly conjugated to Cy3 (1:100; Sigma, #C6198). The following day, sections were rinsed in PBS, and then incubated in goat anti-rabbit conjugated to Alexa 488 (1:200, Thermo Fisher Scientific, #A-11008). Slides were finally rinsed in PBS and coverslipped with Vectashield mounting media (Vector Laboratories, Ontario, Canada) And imaged using a Leica DMLP microscope (Leica Microsystems, Buffalo Grove, IL), and a Qimaging Retiga EXi camera and Qcapture pro software (Teledyne photometrics, Tucson, AZ). Images were analyzed using ImageJ as described below.
rtPCR:
Whole lung tissue was removed and frozen for later analysis of mRNA expression using qRT-PCR. RNA was extracted from the frozen lung tissue using RiboZol (VWR, Radnor, PA; #N580) digestion, phenol chloroform extraction, and quantified using Nanodrop spectroscopy. cDNA was then generated using qScript cDNA synthesis kit (Quanta Biosciences) through reverse transcription. Real time qPCR was performed with Taqman probes for CaSR (catalog# Mm00443375_m1), and compared to GAPDH (Thermo Fisher) as the housekeeping gene using PerfeCTa qPCR Fastmix, UNG, ROX (Quanta Biosciences; #95077). Duplicates were run for each sample. The Fold-changes were calculated using 2-ˆΔΔCT method and StepOne software v2.3 (Applied Biosystems). Statistical comparisons were performed using the ΔCT values.
siRNA administration:
We performed additional experiments to test whether blocking CaSR via targeted siRNA treatment would reverse airway reactivity. Lung slices from P21 day old mice were separated between 3 wells (~3–4 slices/well) containing DMEM + Pen/strep cocktail and each well was then randomly chosen to receive either 4nM of CaSR siRNA (Thermo Fisher, Waltham, MA; Silencer siRNAID 161711; Cat#AM16708), scramble siRNA (Thermo Fisher,Silencer negative control Cat#AM4611), or transfection control (Ctrl; lipofectamine). The siRNA concentration was based on vendor recommendation (5 nM) and pilot studies using 0.5–20 nM with maximal functional effect (changes in lung slice contractility) noted at 4–5 nM.
Individual slices were incubated for 48 hrs at 37°C in tissue culture, and then underwent assessment of airway reactivity as described above, or immunohistochemical analysis of smooth muscle CaSR expression using confocal microscopy. The treatment groups included mice from both control and CPAP (i.e. 6 groups total). For the immunohistochemical analysis, following siRNA administration, slices were fixed overnight in 4% paraformaldehyde and immunostained for CaSR and actin as described above.
Airway CaSR and aSM-actin quantification:
Image analysis was performed after loading the airway images into ImageJ and splitting the red and green channels. Background correction was done at the same level for all images. The airway was then isolated within the image. Positive staining for CaSR and αSM-actin, was identified by adjusting the threshold equally on all images, and then quantifying the area of positive staining. The area of positive staining was then normalized to airway lumen circumference. Analysis of CaSR expression was limited to CaSR expressed in smooth muscle, and was achieved by identifying αSM-actin positive areas in the section and using those as the region of interest (ROI) for CaSR positive staining. For analysis of both CaSR and αSM-actin in thick sections after siRNA exposure, three z-stack images captured using confocal microscopy and located in the center of the airway were analyzed and averaged to provide one data point per airway.
Human Fetal ASM:
As previously described (16, 30, 34, 36) tracheobronchial samples of de-identified 18–22 week fetuses following demise (StemCell Express, Arlington, MA) were enzymatically digested and the isolated cells cultured in DMEM/F12 (Life Technologies, Rockville, MD) supplemented with 10% FBS, Pen+Strep (Life Technologies). Cells within five passages of subculture were used for experiments, performed in serum-starved conditions (0.5% FBS). The study was considered exempt by the Mayo Institutional Review Board since maternal or fetal identifiers were not available and isolated cells were stored with unique identifiers unrelated to their source.
Cell stretch:
fASM cells were grown in 6-well plates with flexible membranes, and the plates placed in a cell stretcher system (FlexCell FX-6000T) such that the membranes were stretched over a rigid post following negative pressure applied to the sealed unit in which the plates were placed. Cells underwent oscillatory stretch of 5% at 1Hz (mimicking breathing) for 48h without (Control) or with an additional 5% static stretch (mimicking CPAP). Experiments were then performed for [Ca2+]i imaging or signaling pathways.
[Ca2+]i imaging
Previously described techniques for fura-2 based fASM [Ca2+]i imaging were used (30). fASM cells from different groups were plated on 8-well glass-bottomed imaging chambers (Thermo Fisher Scientific,#155411) and experiments performed in Hanks Balanced Salt Solution (HBSS) where [Ca2+]o could be varied. As the intent was to explore the CaSR, which is sensitive to [Ca2+]o, cells were deprived of Ca2+ for 12h before experimentation while on the FlexCell. Cells were treated for 1 h with either medium (vehicle) or 1 uM NPS2143 (calcilytic, Tocris) (28, 30). They were loaded with 5 uM fura-2/AM for 30 min in 0 mM [Ca2+]o and then washed. Imaging was done on an inverted microscope (Nikon Eclipse Ti-U) with perfusion used to alter [Ca2+]o or add agonists as necessary. Baseline, peak, and amplitude of [Ca2+]i responses were recorded and analyzed.
Signaling Pathways:
In human fASM cells exposed to stretch vs. not, Western analysis was performed for two major signaling pathways relevant to CaSR signaling, p42/44 (Cell Signal, #9102 for total, #9101 for phospho, Rabbit), and RhoA (Cell Signal, #21175, Rabbit) (28).
Data Analysis:
For the mouse studies, statistical comparison of responses to methacholine between control and different levels of CPAP treated groups was made using a three-way, repeated measures ANOVA (SigmaPlot, Systat Software Inc. San Jose, CA). Comparison for IHC and rtPCR between control and 6 cmH2O CPAP was performed using a t-test. Differences were considered significant at p<0.05. fASM cells were from four or more fetuses. For cell imaging studies, at least 20 cells/protocol/sample were sampled. “N” values represents numbers of samples. Statistical analysis was performed using individual Student t-test or ANOVA with Bonferroni correction for multiple comparisons. Statistical significance was tested at the p < 0.05 level. Statistical analyses were performed using GraphPad Prism 7.03 software. All data are expressed as mean ± SD.
RESULTS
CPAP and CaSR in WT mice:
In WT mice, neonatal CPAP (6 cmH2O) for 7 days resulted in a long-term increase in airway and whole lung CaSR 2 weeks later. Immunofluorescence of lung sections (Fig 1A) showed increased expression of smooth muscle protein (Fig 1B) as well as of CaSR (Fig 1B) within airways although the ratio of CaSR/smooth muscle actin was only slightly increased (Fig 1C). PCR analysis of whole lung homogenate 2 weeks after CPAP treatment ended also showed increased CaSR mRNA compared to untreated control mice (Fig. 1D), corroborated by CaSR protein analysis (Fig. 1E).
Figure 1:
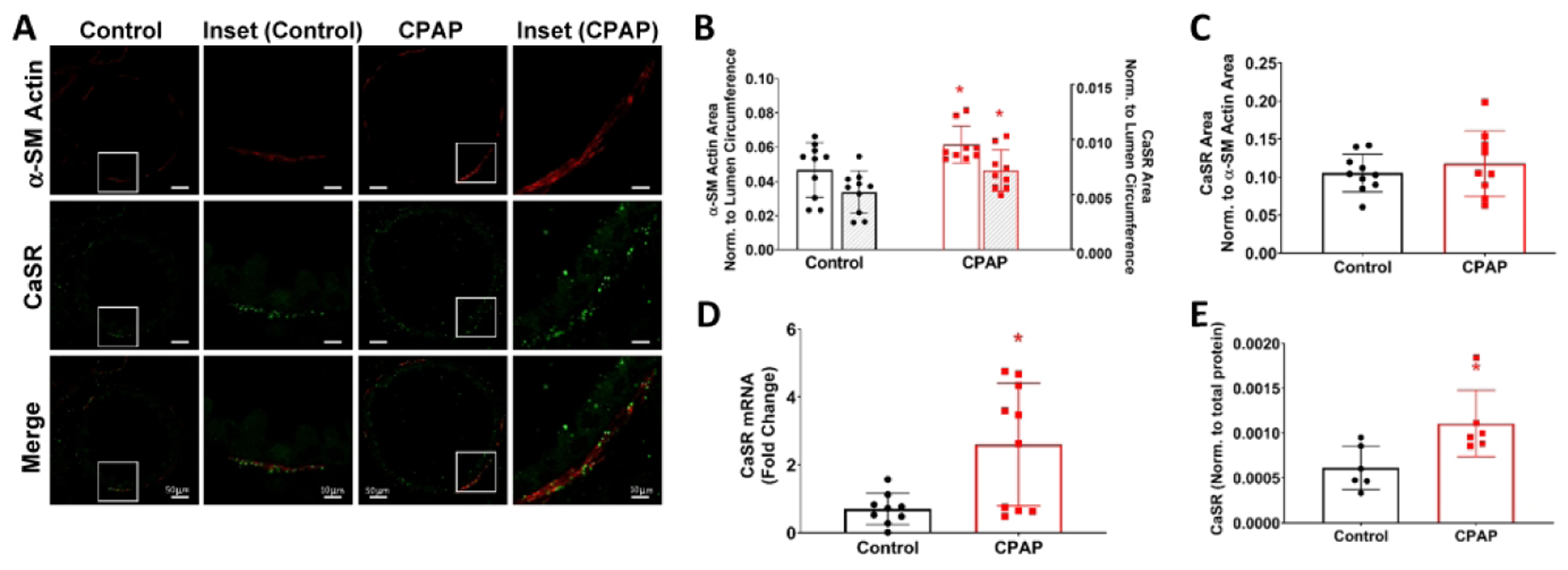
Effects of daily continuous positive airway pressure (CPAP; 6cmH2O, 3hr/day, postnatal days P1-7) on long-term changes in airway and lung calcium sensing receptor (CaSR) expression. Immunofluorescence (IF) for αSM-actin (red) and CaSR (green) in P21 day old male mice (A), two weeks after CPAP treatment ended showed increased immunoreactivity for αSM-actin and CaSR (B), although the amount of CaSR normalized to αSM-actin area was not affected by CPAP (C). Representative images of lung sections from control and CPAP treated mice are shown, including high resolution images of the white box from the CPAP mouse in the rightmost column of A. Separately, whole-lung RT-PCR (D) and Western (E) showed significant increase in CaSR levels. *indicates significant difference (p<0.05; t-test) between Ctrl and CPAP. N=7–10 airways from 4–5 mice/group.
Airway reactivity to methacholine, CPAP and CaSR:
The increased airway CaSR expression (Fig. 1) in WT mice following neonatal CPAP was associated with a long-term increase in airway reactivity (Fig. 2A), as indicated by the more pronounced decrease in airway lumen area with increasing doses of methacholine applied to the precision cut lung slices.
Figure 2:
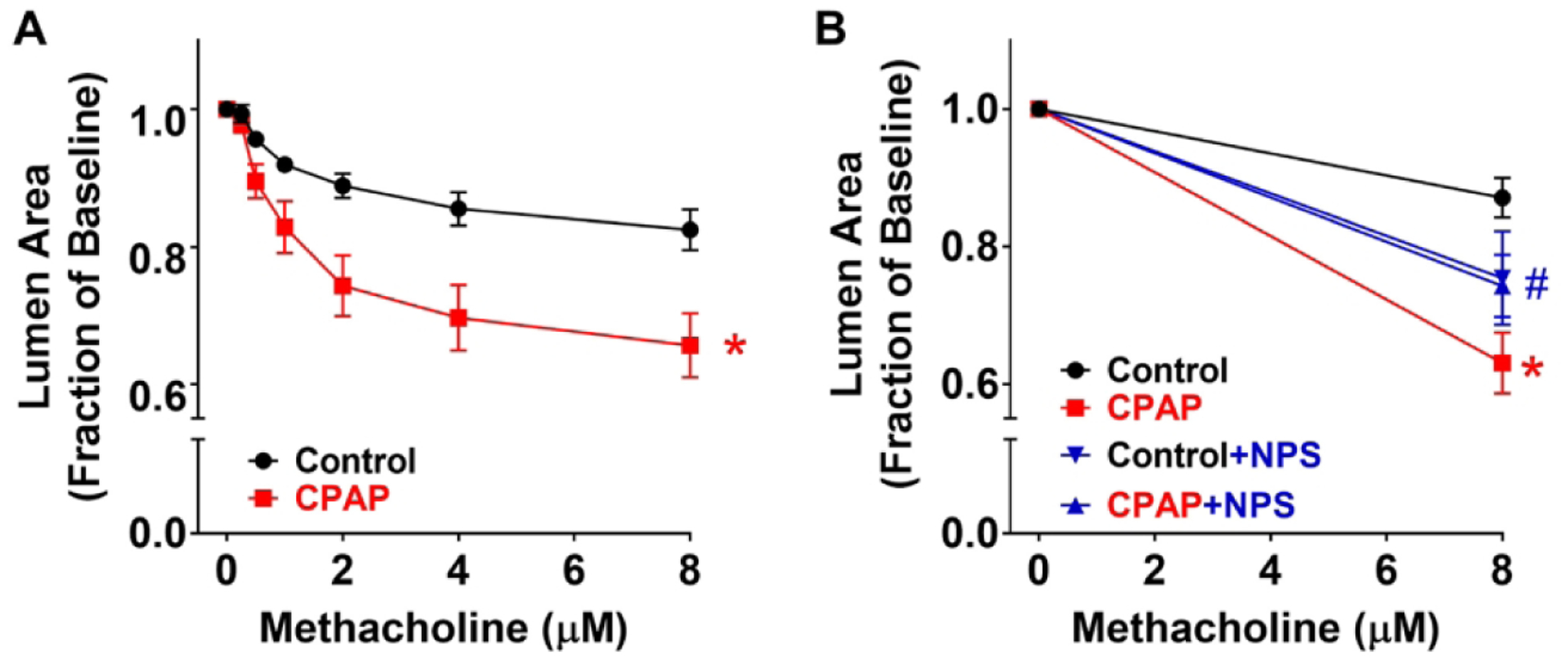
Effect of CPAP and CaSR inhibition on airway responses to methacholine challenge in P21 day old WT mice. In precision cut lung sections from mice exposed to 7 days of CPAP, there was higher reactivity to methacholine compared to control mice at P21 days (A). However, in slices incubated with the CaSR inhibitor, NPS 2143 for 1 hr prior, such CPAP-induced differences were substantially blunted (B). Values are expressed as fraction of baseline lumen size. The smaller lumen size at increasing concentration to methacholine signifies increased AW reactivity.* indicates significant difference (p<0.05) between Ctrl and CPAP, # indicates significant NPS effect (p<0.05) (three-way ANOVA with repeated measures). N=7–10 airways from 4–5 mice/group.
Based on the finding of increased CaSR, we explored the impact of altered CaSR function or expression. First, in lung slices of WT animals, inhibition of CaSR with NPS2143 significantly reduced CPAP-induced airway contractility, approximately halfway between control and CPAP effects at the maximum methacholine concentration (Fig 2B). Interestingly, in control animals, acute administration of NPS slightly increased airway contractility. Second, in the absence of airway CaSR expression (ie. smooth muscle-specific CaSR−/− mice), airway contractility following CPAP was significantly less than CPAP-exposed WT mice (Fig 3A). Interestingly, in CaSR−/− mice not exposed to CPAP, airways showed slightly higher contractility compared to WT controls: an effect similar to NPS above. Absence of CaSR within the smooth muscle layer of airways was verified by immunofluorescence (Fig 3B). Finally, CaSR siRNA transfection of lung slices from WT mice resulted in substantially blunted effect of CPAP compared to scrambled siRNA transfected slices from CPAP mice (Fig 4A). Efficacy of siRNA transfection was shown by immunofluorescence (Fig 4B) and quantified (Fig 4C). Scramble siRNA had no effect on CPAP-induced airway hyperreactivity. Human fASM and Stretch
Figure 3:
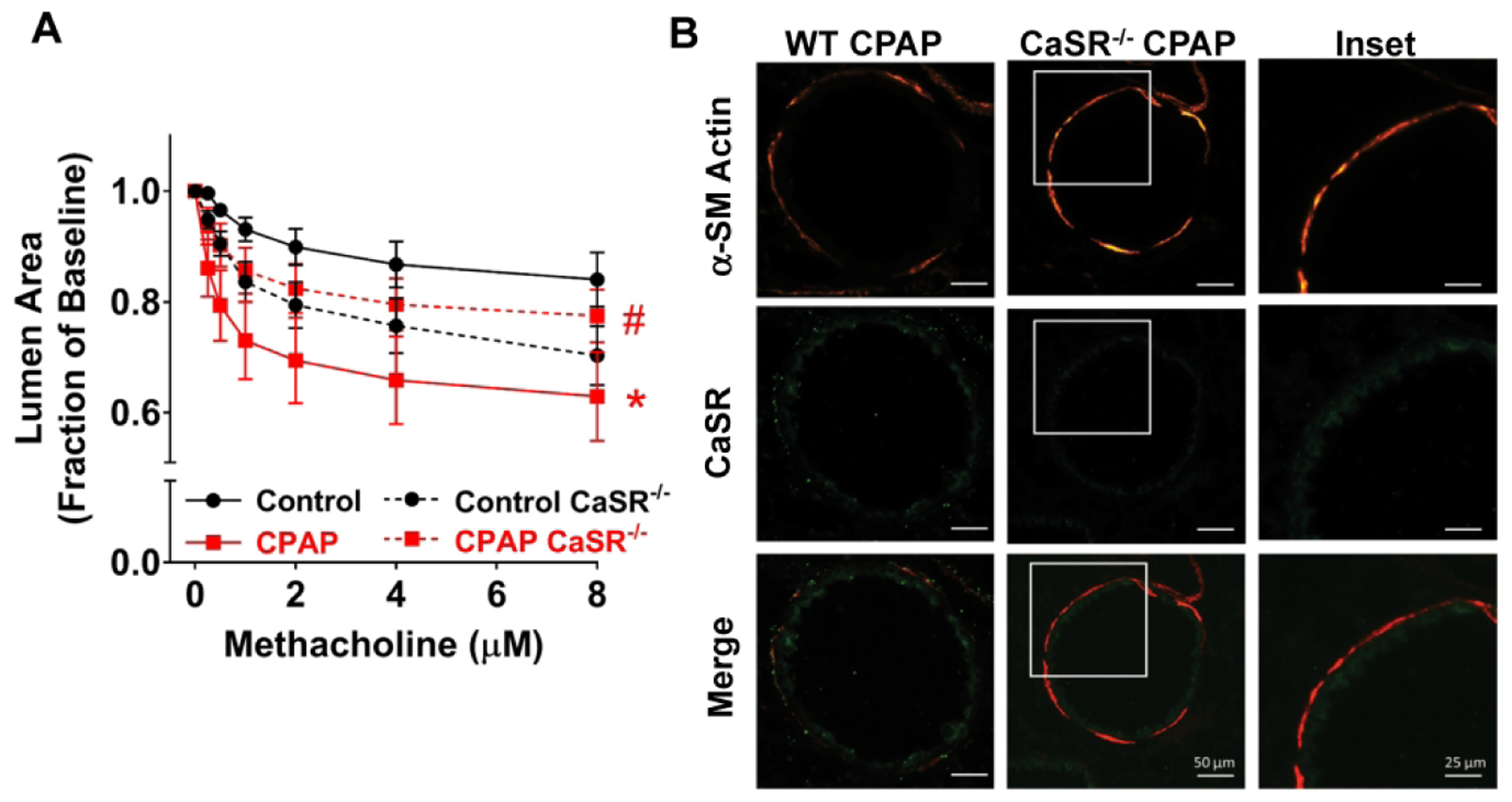
Effect of CPAP in CaSR knockout mice. In mice constitutively lacking smooth muscle CaSR (CaSR−/−), neonatal CPAP resulted in less hyperreactivity compared to WT mice (A). Lack of CaSR within the smooth muscle layer was verified via αSM-actin and CaSR immunoreactivity in P21 day old mice (B). Higher resolution images of the CaSR−/− mouse (white square) is also provided. * indicates significant difference (p<0.05 t-test) between Ctrl and CPAP, # indicates significant CaSR KO effect (p<0.05; t-test). N=7–10 airways from 4–5 mice/group.
Figure 4:
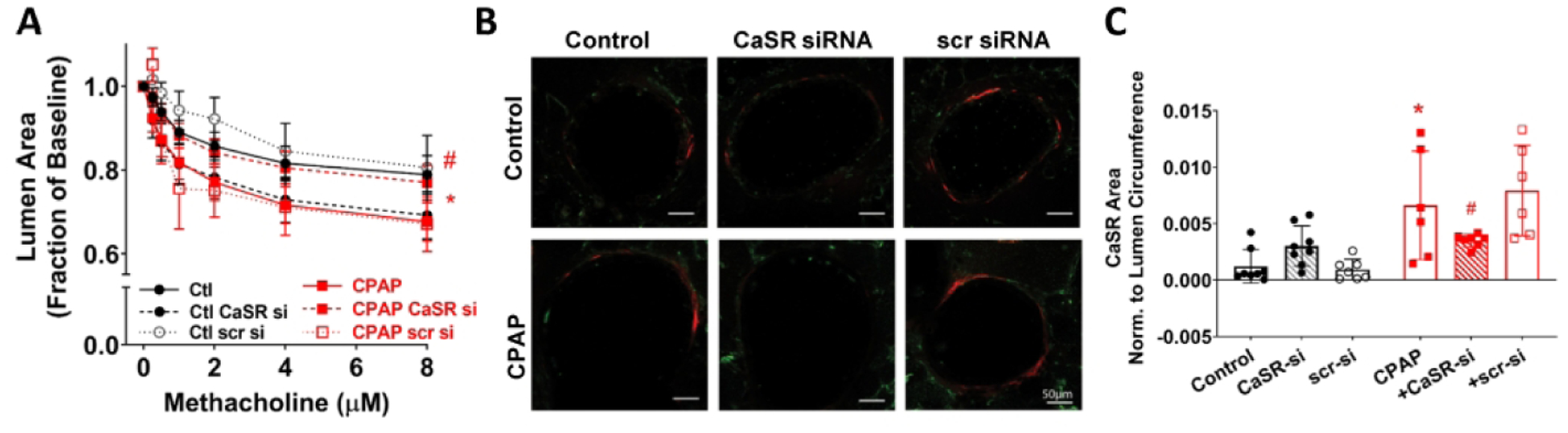
Effect of CaSR siRNA on CPAP-enhanced airway reactivity. In lung slices of wildtype mice, 48 hr exposure to CaSR siRNA resulted in reduced airway hyperreactivity compared to scrambled siRNA controls (A). Efficacy of CaSR siRNA was verified via αSM-actin and CaSR immunoreactivity in P21 day old mice (B), as summarized in (C). * indicates significant difference (p<0.05) between Ctrl and CPAP, # indicates significant CaSR siRNA effect (p<0.05). N=7–10 airways from 4–5 mice/group (three-way ANOVA with repeated measures).
Human fASM cells that underwent 5% static stretch for 48h mimicking CPAP showed a significantly higher baseline [Ca2+]i level (Fig 5A, B) and peak response to histamine (Fig 5A, C) in the presence of 0.5 or 1 mM [Ca2+]o. Addition of 1 μM NPS2143 (calcilytic) blocked the enhancing effect of static stretch on [Ca2+]i (Fig 5A–C).
Figure 5:
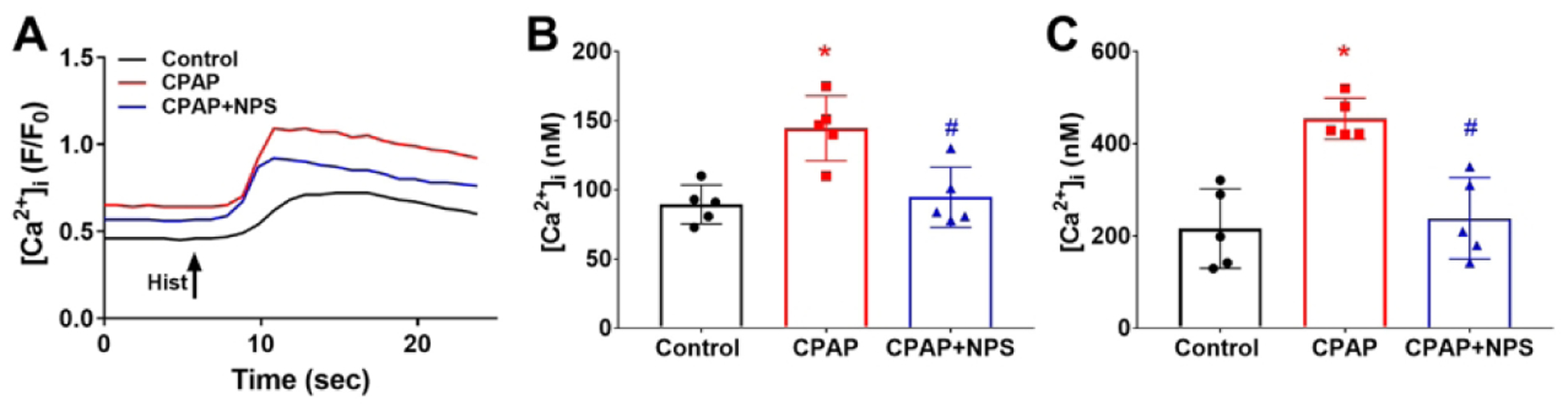
Effect of mechanical stretch on [Ca2+]i responses in human fetal airway smooth muscle (fASM) cells. fASM cells were exposed to 48h of static 5% stretch mimicking CPAP, on a background of 5% oscillation mimicking breathing. Such stretch enhanced subsequent baseline (A, B) and histamine induced [Ca2+]i responses (A, C). CaSR inhibition with NPS2143 blunted CPAP effects. * indicates significant difference (p<0.05; t-test) between Ctrl and stretch, # indicates significant NPS effect (p<0.05; t-test). N=4–5 cell lines with data averaged from at least 20 cells per line.
CaSR in human fASM
We previously showed that CaSR is localized to airway smooth muscle in ~22wk human fetuses and in isolated fASM cells (30). This is consistent with current findings of smooth muscle CaSR in mouse airways (Fig 1). Also, consistent with CPAP effects in mice (Fig 1), in fASM cells, exposure to static stretch for 48h increased CaSR expression (Fig 6). Western blot analyses of stretched fASM showed increased ERK1/2 and RhoA compared to non-static stretched fASM (Fig 6).
Figure 6:
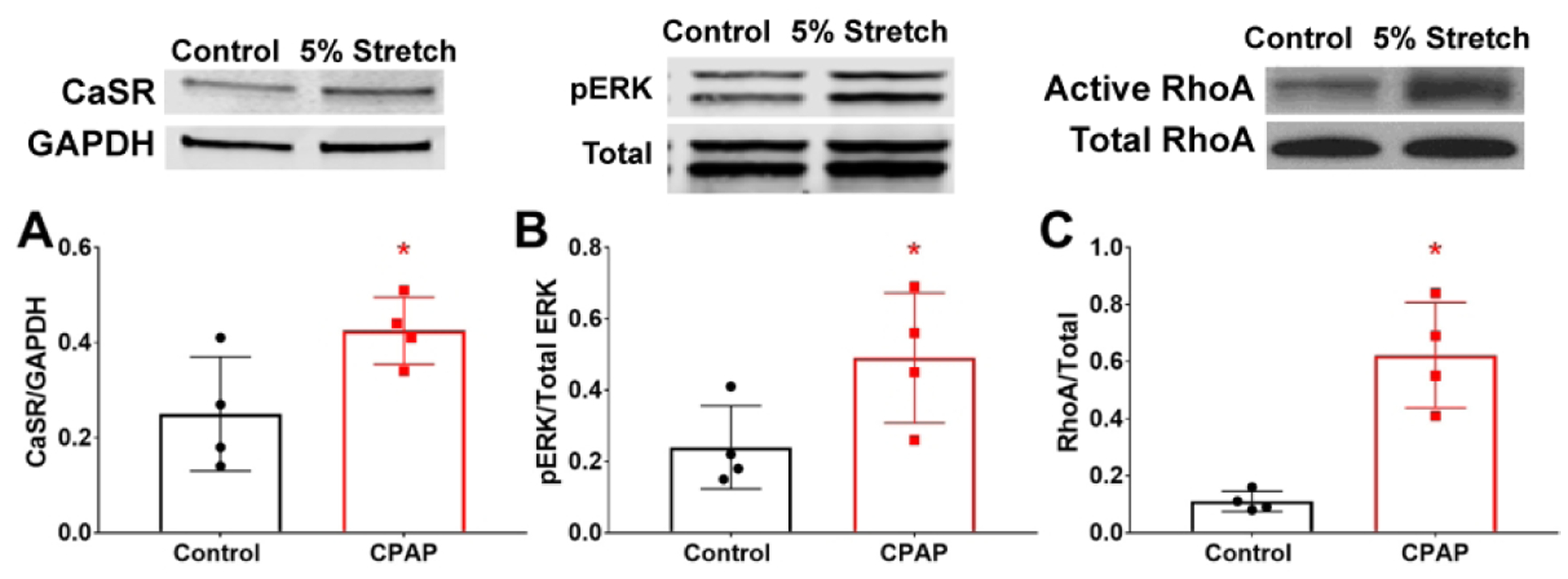
CaSR and mechanical stretch in human fASM cells. Exposure to static stretch increased CaSR expression in fASM cells (A), and activated p42/44 ERK (B) and RhoA (C). * indicates significant difference (p<0.05; t-test) between Ctrl and stretch (p<0.05; t-test). N=4–5 cell lines.
DISCUSSION
Our study demonstrates that in developing human ASM, a functional CaSR is involved in the effects of mechanical stretch in the context of CPAP on Ca2+ and contractility responses to bronchoconstrictor agonist. Data showing functional CaSR in fASM is consistent with our previous reports in adult and developing ASM (28, 30). The relevance of CaSR particularly in developing ASM lies in its response to known relatively higher, physiologically relevant [Ca2+]o concentrations of the fetal lung (18, 19) that likely drive lung morphogenesis (37), and the ability of CaSR to elevate [Ca2+]i and contractility (30). The relevance of CaSR in stretch-exposed fASM lies in the potential to target this mechanism to alleviate detrimental long-term aspects of CPAP in the immature lung in spite of its early benefits in maintenance of oxygen and in alveolar development.
In the current study, we used a previously-validated neonatal mouse model that is advantageous towards understanding stretch and airway distension effects on postnatal airway development in humans. Here, the newborn mouse lung is at the saccular stage of development (38) and thus comparable to a human infant at 26–28 weeks gestation, i.e. a stage of prematurity and premature birth when CPAP may be implemented in the neonatal ICU. In this regard, an important distinction would be the effects of CPAP on bronchial vs. parenchymal/alveolar compartments. Clinically, the benefit of CPAP in enhancing alveolar distension and thus aiding oxygenation in premature infants is well-recognized. However, the effects of CPAP on bronchial airways per se hav not been examined. Furthermore, the longer-term impact of CPAP on bronchial or alveolar compartments is not known. From a model perspective, we recently confirmed using MRI of neonatal mouse lungs that clinically-relevant CPAP results in lung inflation (Mayer, MacFarlane, Prakash, unpublished observations). In this regard, given that alveologenesis is incomplete at this age, and the alveolar compartment is therefore higher resistance, much of the pressure of CPAP is likely to be transmitted to the already-developed bronchiolar airways, the target of our studies. Accordingly, the persistence of airway hyperreactivity in this mouse model for a further 2 weeks post-CPAP becomes translationally relevant to understanding postnatal influences of CPAP. Here, the 3-week old mouse is optimal given that in terms of lung development, it approximates a 3–4 year old child and thus a time when wheezing and asthma manifest in former preterm infants. Thus our findings of persistent increased airway reactivity are relevant towards distinguishing early benefits of CPAP for alveolar expansion and oxygenation vs. longer-term detrimental effects on bronchial airways per se. Similar effects on airway reactivity have been found in neonatal rats in a more “severe” model where animals are anesthetized daily, intubated and mechanically ventilated with or without high intermittent positive pressure (39). These models while differing in invasiveness and extent of stretch, collectively demonstrate that daily distension of the airways and lung during the early neonatal period can have long-term unintended detrimental consequences on airway structure/function. Furthermore, the relevance of our model for bronchial airways per se is highlighted by findings in our previous study (5, 35) where we found that daily CPAP (as implemented in this study) results in minimal to no changes to the alveolar compartment even at 3 weeks but does have detrimental impact on the bronchial airways.
In our study, we also confirmed our previous finding that CPAP causes a longer term increase in ASM (10). Previous studies including our own have shown increased ASM and metabolic activity of immature lungs stimulated by stretch (40) (10) and would certainly be consistent with accelerated lung growth in the early phase (38, 41) but conversely showing that an exaggerated and sustained changes in ASM structure/function can occur with CPAP that would lead to thicker, more reactive airways, that would contribute to wheezing/asthma.
Compared to adult ASM, there is less information regarding mechanisms and changes in [Ca2+]i or contractility, partly reflecting lack of age-appropriate models, particularly in humans. We have previously showed that fASM cells are a good model to understand contractility and remodeling in the developing airway given that these cells express the same machinery as adult ASM, and are responsive to agonists such as ACh or histamine (34).
We have previously established expression of CaSR and its influence on [Ca2+]i in fASM (30). A member of the Class C family of GPCRs that includes metabotropic GABA-B and glutaminergic receptors (23, 24) (23), the CaSR is well-known for sensing and regulating [Ca2+]o in calciotropic tissues such as parathyroid gland, kidney or bone (20–22), with activators (calcimimetics) being used to treat hyperparathyroidism, and negative allosteric modulators (calcilytics) for genetic hypocalcemia (25, 26). There is now substantial evidence for CaSR in non-calciotropic tissues with roles including regulation of [Ca2+]I, gene expression, cellular proliferation and production of ECM (18, 20, 21, 27–29) (28, 30). Such pleiotropic effects occur through the multiple pathways by which CaSR can function. CaSR can couple to multiple G-proteins (Gq/11 or Gi/o) (27) and via Gq/11 activate PLCβ-IP3 to increase [Ca2+]i. Furthermore, via DAG-PKC or Gi/o, CaSR can modulate MAP kinase (32). In adult ASM, CaSR promotes proliferation via PLC (30) and ERK1/2 (28). Given that extracellular Ca2+ is higher in developing lung, and that developing ASM is expected to undergo greater proliferation towards overall growth, CaSR may be particularly relevant.
The results of the present study now link stretch/CPAP effects on contractility of developing ASM to the CaSR. Our data first show that CPAP in neonatal mouse and stretch in human fASM both enhance CaSR expression. Thus, even if there were no additional effects of CPAP/stretch, in vivo increased CaSR would allow for greater responsiveness to a higher [Ca2+]o in developing lung and thus enhance [Ca2+]i and contractility or other downstream CaSR effects. We further show that CPAP/stretch effects actually involve CaSR and the airway/ASM responsiveness to agonist, which is suppressed by the negative allosteric modulator NPS2143 or by the absence of CaSR in the KO mouse model. Our signaling data also show that CaSR may be involved in longer-term effects via upregulation of MAPK or RhoA that are important at least in adult ASM (42). Accordingly, CaSR becomes an appealing target for alleviating the detrimental effects of stretch. Here the efficacy of NPS2143 is encouraging given ongoing clinical trials using calcilytics in the context of adult asthma (43). A potential caveat is the observation that in non-stretched groups, we saw some worsening of Ca2+ or contractility with NPS2143, the reasons for which are unclear. In blood vessels and in HEK293 expression systems, CaSR can show a biphasic response to extracellular calcium, but there is no prior data on whether altering CaSR levels or NPS2143 results in a biphasic response that could potentially underlie the worsening effect of NPS2143. CaSR is known to heterodimerize with other Class C GPCRs, and it is possible that altering CaSR levels or CaSR activation changes the sensitivity of its heterodimer partners with downtstream effects on PLC or other signal transduction pathways. These issues remain to be explored. Regardless, given that NPS2143 is likely to be administered only in the context of prematurity or CPAP, the worsening effects we see may or may not be clinically relevant.
The present study did not specifically explore the upstream mechanisms by which CPAP induces changes in CaSR or its downstream effects. Interestingly, pathways including RhoA/ROCK and ERKs and even cAMP/PKA, that are activated by CaSR are also sensitive to stretch. Therefore, it is entirely possible that initial stretch activates these pathways allowing for their sustained activation via CaSR. In other cell types including in the lung, stretch can also activate Wnt/β-catenin and other mechanosensitive pathways such as Yap/Taz or transcription factors (e.g. Egr1) (44–46). There are currently no data on developing ASM per se, but the roles of Wnt/β-catenin or Yap/Taz have been recognized in human ASM or fibroblasts in the context of asthma or pulmonary fibrosis (44–47). Future studies should consider these potentially targetable pathways in addition to CaSR in alleviating the effects of CPAP.
CONCLUSIONS:
In conclusion, using an age-appropriate neonatal mouse model, we find that clinically relevant levels of CPAP pressure applied in patterns reflecting the human NICU experience result in persistent increase of bronchial airway reactivity involving the CaSR. Further work is needed to understand the upstream mechanistic pathways contributing to both increased CaSR expression and its activity, and to the airway hyperreactivity to determine potential longer-term consequences of neonatal CPAP in preterm infants.
Impact:
Neonatal CPAP increases airway reactivity to bronchoconstrictor agonist
CPAP increases smooth muscle expression of the extracellular calcium-sensing receptor (CaSR)
Inhibition or absence of CaSR blunts CPAP effects on contractility
These data suggest a causal/contributory role for CaSR in stretch effects on the developing airway
These data may impact clinical recognition of the ways that CPAP may contribute to wheezing disorders of former preterm infants
Acknowledgements:
We would like to acknowledge the commitment from Morgan Hazard for assistance with CPAP exposure.
Funding:
Funded by the National Heart, Lung and Blood Institute (Bethesda, MD) Grants R01HL056470 (YSP, RJM, PMM), R01HL138402 (PMM, CMP, YSP), NIH grant S10-OD024996, NHLBI PO1HL107147 and the Department of Pediatrics, Rainbow Babies and Children’s Hospital, Cleveland, Ohio. This study was also funded in part by generous financial contributions from William and Lois Briggs.
Footnotes
Ethics approval: All procedures were carried out in accordance with the National Institute of Health (NIH) guidelines for care and use of laboratory animals and were approved by the Animal Care and Use Committee at Case Western Reserve University.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Baraldi E, Carraro S, Filippone M Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early Hum Dev 85, S1–3 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Jaakkola JJ, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol 118, 823–830 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Joshi S, et al. Exercise-induced bronchoconstriction in school-aged children who had chronic lung disease in infancy. J Pediatr 162, 813–818 e811 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Laughon MM, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med 183, 1715–1722 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer CA, Martin RJ, MacFarlane PM Increased airway reactivity in a neonatal mouse model of continuous positive airway pressure. Pediatr Res 78, 145–151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFawn PK, Mitchell HW Bronchial compliance and wall structure during development of the immature human and pig lung. The European respiratory journal 10, 27–34 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Tepper RS, Wiggs B, Gunst SJ, Pare PD Comparison of the shear modulus of mature and immature rabbit lungs. Journal of applied physiology 87, 711–714 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, et al. Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. The Journal of clinical investigation 106, 1321–1330 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitterman JA The effects of mechanical forces on fetal lung growth. Clinics in perinatology 23, 727–740 (1996). [PubMed] [Google Scholar]
- 10.MacFarlane PM, et al. CPAP protects against hyperoxia-induced increase in airway reactivity in neonatal mice. Pediatr Res, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramchandani R, et al. Differences in airway structure in immature and mature rabbits. Journal of applied physiology 89, 1310–1316 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Ramchandani R, Shen X, Gunst SJ, Tepper RS Comparison of elastic properties and contractile responses of isolated airway segments from mature and immature rabbits. Journal of applied physiology 95, 265–271 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Prakash YS Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol 305, L912–933 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakash YS Emerging concepts in smooth muscle contributions to airway structure and function: implications for health and disease. Am J Physiol Lung Cell Mol Physiol 311, L1113–L1140 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson MA, et al. cAMP-mediated secretion of brain-derived neurotrophic factor in developing airway smooth muscle. Biochim Biophys Acta 1853, 2506–2514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogel ER, et al. Moderate hyperoxia induces extracellular matrix remodeling by human fetal airway smooth muscle cells. Pediatr Res 81, 376–383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faksh A, et al. Effects of antenatal lipopolysaccharide and postnatal hyperoxia on airway reactivity and remodeling in a neonatal mouse model. Pediatr Res 79, 391–400 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riccardi D, Brennan SC, Chang W The extracellular calcium-sensing receptor, CaSR, in fetal development. Best Pract Res Clin Endocrinol Metab 27, 443–453 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs CS, Kronenberg HM Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18, 832–872 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Brennan SC, et al. Calcium sensing receptor signalling in physiology and cancer. Biochim Biophys Acta 1833, 1732–1744 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Riccardi D, Kemp PJ The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol 74, 271–297 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Goltzman D, Hendy GN The calcium-sensing receptor in bone--mechanistic and therapeutic insights. Nat Rev Endocrinol 11, 298–307 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Patel BS, Ravix J, Pabelick C, Prakash YS Class C GPCRs in the airway. Curr Opin Pharmacol 51, 19–28 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brauner-Osborne H, Wellendorph P, Jensen AA Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets 8, 169–184 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Hannan FM, Olesen MK, Thakker RV Calcimimetic and calcilytic therapies for inherited disorders of the calcium-sensing receptor signalling pathway. Br J Pharmacol, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemeth EF, Van Wagenen BC, Balandrin MF Discovery and Development of Calcimimetic and Calcilytic Compounds. Prog Med Chem 57, 1–86 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Conigrave AD, Ward DT Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab 27, 315–331 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Yarova PL, et al. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci Transl Med 7, 284ra260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schepelmann M, et al. The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am J Physiol Cell Physiol 310, C193–204 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roesler AM, et al. Calcium sensing receptor in developing human airway smooth muscle. J Cell Physiol 234, 14187–14197 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith KA, et al. Calcium-Sensing Receptor Regulates Cytosolic [Ca (2+) ] and Plays a Major Role in the Development of Pulmonary Hypertension. Front Physiol 7, 517 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kifor O, et al. Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am J Physiol Renal Physiol 280, F291–302 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Brennan SC, et al. The extracellular calcium-sensing receptor regulates human fetal lung development via CFTR. Sci Rep 6, 21975 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartman WR, et al. Oxygen dose responsiveness of human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303, L711–719 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyburn B, et al. The Effect of Continuous Positive Airway Pressure in a Mouse Model of Hyperoxic Neonatal Lung Injury. Neonatology 109, 6–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel ER, et al. Perinatal oxygen in the developing lung. Can J Physiol Pharmacol 93, 119–127 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jesudason EC Airway smooth muscle: an architect of the lung? Thorax 64, 541–545 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Backstrom E, Hogmalm A, Lappalainen U, Bry K Developmental stage is a major determinant of lung injury in a murine model of bronchopulmonary dysplasia. Pediatr Res 69, 312–318 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Fukunaga T, et al. Prolonged high intermittent positive-pressure ventilation induces airway remodeling and reactivity in young rats. Am J Physiol 275, L567–573 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Liu M, et al. Stimulation of fetal rat lung cell proliferation in vitro by mechanical stretch. Am J Physiol 263, L376–383 (1992). [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Garbutt V, McBride JT Strain-induced growth of the immature lung. J Appl Physiol (1985) 81, 1471–1476 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Freeman MR, et al. Brain-derived neurotrophic factor and airway fibrosis in asthma. Am J Physiol Lung Cell Mol Physiol 313, L360–L370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrigan CJ Calcilytics: a non-steroidal replacement for inhaled steroid and SABA/LABA therapy of human asthma? Expert Rev Respir Med 14, 807–816 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Tschumperlin DJ, Ligresti G, Hilscher MB, Shah VH Mechanosensing and fibrosis. J Clin Invest 128, 74–84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tschumperlin DJ Mechanotransduction. Compr Physiol 1, 1057–1073 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Liu F, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308, L344–357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumawat K, Gosens R WNT-5A: signaling and functions in health and disease. Cell Mol Life Sci 73, 567–587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


