Abstract
Acute pulmonary Exacerbations (AE) are episodes of clinical worsening in cystic fibrosis (CF), often precipitated by infection. Timely detection is critical to minimize morbidity and lung function declines associated with acute inflammation during AE. Based on our previous observations that airway protein Short Palate Lung Nasal epithelium Clone 1 (SPLUNC1) is regulated by inflammatory signals, we investigated the use of SPLUNC1 fluctuations to diagnose and predict AE in CF.
We enrolled CF participants from two independent cohorts to measure AE markers of inflammation in sputum and recorded clinical outcomes for a 1-year follow-up period.
SPLUNC1 levels were high in healthy controls (n=9, 10.7μg/mL), and significantly decreased in CF participants without AE (n=30, 5.7μg/mL, p=0.016). SPLUNC1 levels were 71.9% lower during AE (n=14, 1.6μg/mL, p=0.0034) regardless of age, sex, CF-causing mutation, or microbiology findings. Cytokines Il-1β and TNFα were also increased in AE, whereas lung function did not consistently decrease. Stable CF participants with lower SPLUNC1 levels were much more likely to have an AE at 60 days (HR: 11.49, Standard Error: 0.83, p=0.0033). Low-SPLUNC1 stable participants remained at higher AE risk even one year after sputum collection (HR: 3.21, Standard Error: 0.47, p=0.0125). SPLUNC1 was downregulated by inflammatory cytokines and proteases increased in sputum during AE.
In acute CF care, low SPLUNC1 levels could support a decision to increase airway clearance or to initiate pharmacological interventions. In asymptomatic, stable patients, low SPLUNC1 levels could inform changes in clinical management to improve long-term disease control and clinical outcomes in CF.
PLAIN LANGUAGE SUMMARY
SPLUNC1 is an abundant host defense protein found in the respiratory tract that decreases with inflammation. Individuals with cystic fibrosis experiencing clinical worsening (exacerbation) have lower levels of SPLUNC1 in their sputum. In stable cystic fibrosis patients, lower levels of SPLUNC1 may predict an upcoming respiratory illness. Therefore, SPLUNC1 may serve as a tool for early diagnosis and treatment of cystic fibrosis exacerbations.
BACKGROUND
Cystic fibrosis (CF) is a multi-system, autosomal recessive disease caused by mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene1–4. CF acute pulmonary exacerbations (AE) are generally reversible episodes of acute deterioration, associated with increased morbidity and worsening quality of life5–9. AE are frequently triggered by respiratory viruses, but also by oropharyngeal flora and bacterial respiratory pathogens such as P. aeruginosa and S. aureus10–12. Increased lung inflammation during AE, manifested as higher immune cell counts and rising concentrations of airway cytokines and proteases, contribute to tissue injury and disease progression7,13. Increased AE frequency decreases CF survivorship and accelerates lung function decline14,15. Importantly, delays in AE detection and treatment may have long-term effects on lung function recovery and response to antibiotic treatment16,17. These observations suggest that early AE detection could help improve clinical outcomes in CF.
Early or mild AE presentations can go undetected during routine visits7,18,19, exacerbating an already variable AE diagnostic approach among healthcare providers20 and impacting treatment outcomes21. Biomarkers of airway inflammation or lung function like the Forced Expiratory Volume in the first second (FEV1), are routinely used to support AE diagnosis and management18,19. However, FEV1 changes often occur as a late consequence of AE, limiting its clinical use in early detection22. Inflammatory cytokines (e.g. IL-6, IL-8, and TNFα) have also been linked to AE, but there is limited data on their ability to predict these events23–25.
Our group and others previously demonstrated that airway concentrations of host defense protein Short Palate Lung Nasal epithelium Clone 1 (SPLUNC1), are closely regulated by inflammatory signals and proteases26–29. SPLUNC1 is primarily expressed by non-ciliated epithelial and mucus cells of the upper and proximal lower respiratory tract30–32. SPLUNC1 is also present at low levels in extrapulmonary tissues, and myeloid cells30,32. SPLUNC1 has antimicrobial, immunomodulatory, and ion transport properties that are highly relevant to CF health, which may be disrupted at baseline and during AE28,33,34.
SPLUNC1 decreases within hours of exposure to inflammation, irritants, or pathogens33, and it is differentially regulated in lung disease27,28,33,35–39. In CF, previous studies have shown low levels in respiratory secretions28, but increased bronchial SPLUNC1 staining in advanced disease40. Recently, genome-wide association studies (GWAS) showed that SPLUNC1 expression was higher in stable CF patients compared to healthy controls, but lower in CF patients with more severe disease41,42. In asthma and active smokers, lower SPLUNC1 levels correlate with increased inflammation35,37,38, however, studies of its regulation in COPD have been inconclusive27,39. Beyond airway disease, SPLUNC1 dysregulation has also been reported in idiopathic pulmonary fibrosis and respiratory malignancies33,36,43. The variable relationship between SPLUNC1 regulation, inflammation, and underlying lung disease suggests that SPLUNC1 has a role as rheostat of respiratory health, whose function and regulation are context-specific.
Based on the known downregulation of SPLUNC1 by pathogens and inflammatory signals, we hypothesized that its sputum concentrations would decrease during AE, and that lower levels of SPLUNC1 would impair its host defense functions, leading to adverse clinical outcomes. Here, we show that SPLUNC1 decreases sharply as inflammation increases in AE, and that in stable patients, lower SPLUNC1 levels portend an increased AE risk. SPLUNC1 downregulation occurs shortly after exposure to cytokines and proteases, suggesting that it could detect AE at early stages, reducing diagnostic uncertainty and informing proactive interventions to decrease AE impact on CF health44–46.
MATERIALS AND METHODS
Definition of CF Exacerbation
AE were defined as the emergence of 4 of 12 signs or symptoms, prompting changes in therapy and initiation of antibiotics (modified from Fuchs’ criteria18). These criteria included: change in sinus congestion, sputum, or hemoptysis; increased cough, dyspnea, malaise, fatigue or lethargy; fever; hyporexia or weight loss; change in chest physical exam; or FEV1 decrease >10% from a previous value18. Individuals not meeting AE criteria were characterized as “CF Stable”.
Study Design
This was a two-center, prospective study of CF participants during periods of clinical stability and AE. All patients received standard-of-care therapy and CFTR modulators when they became available. Our primary objective was to define an association between AE and sputum levels of SPLUNC1. Each participant provided a sputum sample and underwent spirometry within 24 hours of sample collection. Participants were followed at quarterly outpatient clinic visits, or sooner when indicated, for up to one year (Supplemental Figure 1). Clinical information, sputum, and spirometry data were collected at each visit.
Cohort Characteristics
Discovery cohort: 44 adults with confirmed CF diagnosis from the Yale Adult CF Program were recruited from 2014 to 2016 during a) scheduled routine visits, b) unscheduled visits in which they reported AE symptoms, and c) on the first day of admission to the hospital for AE treatment. We organized study participants in two groups: 1) Stable CF participants (CF Stable): No new respiratory symptoms, presenting to clinic for scheduled follow up and, 2) AE participants (AE): Diagnosed with AE (Table 1). We also recruited 10 healthy controls (HC) to undergo sputum induction according to published protocols47. The study was approved by the Yale University Institutional Review Board and informed consent was obtained from each participant.
Table 1. Yale Cohort – Demographics.
Demographic characteristics of the Yale Adult CF Program cohort (Discovery Cohort).
| Number of Patients (n) | HC (9) | CF Stable (30) | AE (14) |
|---|---|---|---|
| Age | |||
| Age (Mean) | 33.5 | 41.1 | 32.1 |
| Age (STD) | 10.7 | 17.0 | 6.4 |
| Age (Range) | 27–45 | 20–79 | 23–43 |
| Sex | |||
| Female (n) | 1 | 17 | 8 |
| Female (%) | 11.1 | 56.7 | 57.1 |
| Male (n) | 8 | 13 | 6 |
| Male (%) | 88.9 | 43.3 | 42.9 |
| Mutation Background | |||
| F508del/F508del (n) | NA | 11 | 8 |
| F508del/F508del (%) | NA | 36.7 | 57.1 |
| F508del/other (n) | NA | 11 | 4 |
| F508del/other (%) | NA | 36.7 | 28.6 |
| Other mutations (n) | NA | 8 | 2 |
| Other mutations (%) | NA | 26.7 | 14.3 |
| FEV1 (L) | |||
| FEV1 (Mean) | NA | 2.2 | 1.9 |
| FEV1 (STD) | NA | 0.7 | 0.7 |
| FEV1 (Range) | NA | 0.57–3.3 | 0.55–2.98 |
| FEV1 (%) | |||
| FEV1 (Mean) | NA | 67.5 | 56.1 |
| FEV1 (STD) | NA | 24.3 | 24.3 |
| FEV1 (Range) | NA | 12–121 | 12–89 |
| BMI (Kg/m2) | |||
| BMI (Mean) | NA | 24.4 | 22.7 |
| BMI (STD) | NA | 4.1 | 4 |
| BMI (Range) | NA | 17.6–35.8 | 17.6–35.8 |
| Exacerbations per year (AE/y) | |||
| AE/y (Mean) | NA | 1.9 | 3.7 |
| AE/y (Range) | NA | 0–10 | 1–10 |
| CF Comorbidities | |||
| PI (n) | NA | 25 | 14 |
| PI (%) | NA | 83.3 | 100 |
| CFRD (n) | NA | 11 | 9 |
| CFRD (%) | NA | 36.7 | 64.3 |
| Microbiology | |||
| PA Colonization (n) | NA | 12 | 9 |
| PA Colonization (%) | NA | 40 | 64.3 |
| CFTR Modulators | |||
| Ivacaftor (n) | NA | 2 | 0 |
| Ivacaftor (%) | NA | 6.7 | 0 |
| Ivacaftor/Lumacaftor (n) | NA | 6 | 7 |
| Ivacaftor/Lumacaftor (%) | NA | 20 | 50 |
HC: Healthy controls; CF Stable: CF participants without exacerbation; AE: Participants with active exacerbation.
Validation cohort: 35 adult and pediatric participants with confirmed CF, previously enrolled in a prospective study of patients hospitalized for AE treatment at the University of Minnesota (UMN) were included23. All patients received standard-of-care therapy and each participant provided sputum samples and performed pulmonary function tests within 72 hours of antibiotic initiation (Table 2)48.
Table 2. University of Minnesota Cohort – Demographics.
Demographic characteristics of the University of Minnesota CF Center cohort (Validation Cohort).
| Age | CF Stable (11) | AE (24) |
|---|---|---|
| Age (Mean) | 27.1 | 30.0 |
| Age (STD) | 7.7 | 10.5 |
| Age (Range) | 14–40 | 13–57 |
| Sex | ||
| Female (n) | 7 | 12 |
| Female (%) | 63.6 | 50 |
| Male (n) | 4 | 12 |
| Male (%) | 36.4 | 50 |
| Mutation Background | ||
| F508del/F508del (n) | 6 | 14 |
| F508del/F508del (%) | 54.5 | 58.3 |
| F508del/other (n) | 5 | 9 |
| F508del/other (%) | 45.5 | 37.5 |
| Other mutations (n) | 1 | 0 |
| Other mutations (%) | 9.1 | 0 |
| FEV1 (L) | ||
| FEV1 (Mean) | 2.4 | 1.7 |
| FEV1 (STD) | 0.6 | 0.6 |
| FEV1 (Range) | 1.39–3.85 | 0.70–2.80 |
| FEV1 (%) | ||
| FEV1 (Mean) | 67.3 | 45.3 |
| FEV1 (STD) | 12.1 | 14.1 |
| FEV1 (Range) | 45.5–86.5 | 26–74 |
| BMI | ||
| BMI (Mean) | 21.8 | 21.3 |
| BMI (STD) | 2.1 | 3.1 |
| BMI (Range) | 18.1–24.6 | 13.1–25.8 |
| Exacerbations per year (AE/y) | ||
| AE/y (Mean) | 4.1 | 4.3 |
| AE/y (Range) | 1–12 | 1–10 |
| CF Comorbidities | ||
| PI (n) | 11 | 23 |
| PI (%) | 100 | 95.8 |
| CFRD (n) | 6 | 9 |
| CFRD (%) | 54.5 | 37.5 |
| Microbiology | ||
| PA Colonization (n) | 5 | 9 |
| PA Colonization (%) | 45.5 | 37.5 |
| CFTR Modulators | ||
| Ivacaftor (n) | 0 | 0 |
| Ivacaftor (%) | 0 | 0 |
| Ivacaftor/Lumacaftor (n) | 0 | 0 |
| Ivacaftor/Lumacaftor (%) | 0 | 0 |
CF Stable: CF participants without exacerbation; AE: Participants with active exacerbation.
Sputum Collection and Processing
CF participants expectorated sputum spontaneously for cultures and provided an additional study sample. Induced sputum samples were obtained from HC by induction as previously reported47,49. Sputum was diluted, filtered, centrifuged, and processed as previously reported50.
SPLUNC1 and Cytokine ELISA
A direct SPLUNC1 ELISA was developed in our laboratory to measure SPLUNC1 in sputum (details in supplemental methods). Briefly, high-binding polystyrene ELISA plates (Corning, NY, cat# 9018) were coated with sputum supernatants or recombinant human SPLUNC1 protein (rhSPLUNC1) as reference (Abnova, Taipei, Taiwan, cat# H00051297-P01). A polyclonal mouse anti-human SPLUNC1 IgG (MilliporeSigma, Burlington, MA, cat# SAB1401687) was used as detection antibody and HRP-conjugated anti-mouse IgG (Invitrogen, Carlsbad, CA, cat# G21040,) as secondary. Chromogenic tetramethylbenzidine substrate was applied (KPL, Gaithersburg, MD, cat# 5120-0047-50-76-00) and reactions were measured at optical densities of 450 and 550 nm. The assay limits of detection were 1–20,000 ng/mL. Mean intra-assay variability: 5.18% (STDEV 1.28%), Inter-assay variability: 18.44% (STDEV 12.95%).
Custom-made multiplexed cytokine ELISA assays were used to measure cytokine levels in sputum. Briefly, biotinylated capture antibodies for CXCL10, G-CSF, IFN-α2a, IFN-γ, IL-1β, IL-13, IL-29, IL-6, IL-8, MCP-1, MIP-1α, and TNF-α, were combined with an assigned “linker” for each cytokine. The linker-antibody mix was then coated onto U-plex plates and incubated overnight according to manufacturer’s specifications (U-Plex Biomarker Kit, Mesoscale Diagnostics (MSD). Rockville, MD, cat# K15235N-1). The following day, recombinant human cytokines and sputum samples were loaded onto U-Plex plates. Finally, detection antibodies for each cytokine were applied and Read Buffer T was added to each well to quantify the reaction. Plates were read on a Quickplex SQ 120 reader (MSD, cat# AI0AA-0) using MSD Discovery Workbench software version 4.0.
Western Blot
Western blots were performed as previously reported, using human neutrophil elastase (hELA2), mouse monoclonal anti-hELA2 IgG (R&D systems, cat# MAB-91671-100) and mouse polyclonal anti-human SPLUNC1 IgG28. Horseradish peroxidase-conjugated anti-mouse IgG (Invitrogen, Carlsbad, CA, cat# G21040) was used as secondary antibody. Membranes were developed using chemiluminescence and protein band densitometry was determined using ImageJ software version 1.7 (https://imagej.nih.gov/ij/index.html).
Sputum Neutrophil Elastase (NE) Activity and SPLUNC1 Degradation Assays
NE activity was determined using the 7-amino-4-methylcoumarin (MCA) assay (Peptides International, Louisville, KY, #MAA-3133), as described28. RhSPLUNC1 was incubated with recombinant human NE (rhNE, R&D systems, cat#9167-SE-020) or Pseudomonas aeruginosa elastase (LasB, a gift from Dr. Karen Agaronyan, Yale) at decreasing concentrations for 3 and 8 hours. SPLUNC1 concentrations were measured by ELISA. Starting NE concentrations (1 μM) were selected based on previous sputum NE level measurements by our group28. There were no published data on airway levels of LasB during AE to inform dose selection. However, a dose capable of inhibiting host defense peptide expression and inducing cytokine expression had been previously reported (3.75 μM)51. Based on this, we chose a starting dose of 1 μM to define minimal LasB doses capable of regulating SPLUNC1.
Regulation of Epithelial Cytokine Expression
Mouse tracheal epithelial cells (mTECs) were isolated from C57BL/6 mice and cultured at air–liquid interface (ALI) as described26. mTECs were treated with recombinant murine IL-1β (Peprotech, Rocky Hill, NJ, cat# 211-11b) or TNF-α (Peprotech, cat# 315–01A) at 10 ng/mL for 24 hours. NCI-H292 human airway epithelial cells, were treated with recombinant human IL-1β (Gibco, Gaithersburg, MD, cat# PHC0811,) or TNF-α (R&D, Minneapolis, MN, cat# 210-TA-005) at 10 ng/ml. Cellular mRNA was extracted for qPCR, and qPCR assays were performed to quantify SPLUNC1 transcriptional regulation as described26.
Statistical Analysis
Descriptive statistics were calculated for the entire participant population. Pearson, or Spearman correlations for variables that were not normally distributed, were calculated between SPLUNC1 and clinical parameters. In order to select optimal thresholds to separate groups at higher AE risk, we developed receiver-operator curves (ROC) based on the distribution of SPLUNC1, IL-1β, TNFα, G-CSF, IL-6, and IL-8 levels in the discovery cohort (Supplemental Figure 2). Using these thresholds, we applied statistical modeling (Mantel-Haenszel estimator) to predict AE-free intervals. AE-free intervals were defined as the time in days from sputum sampling in a stable patient to the time of the first AE after that visit. Finally, a Cox proportional hazards model was conducted with clinical parameters as covariates. A backward elimination strategy with a significance level to stay of 95% (a=0.05) was employed to achieve a parsimonious model. All statistical analyses were conducted using SAS 9.4 with a level of significance of 95% (α=0.05).
Please see details in Supplementary Methods.
RESULTS
SPLUNC1 is Decreased in the Sputum of Stable CF Participants
SPLUNC1 levels ranged from 4.41 to 22.24μg/mL in the sputum of healthy controls (HC). In stable CF participants, SPLUNC1 was significantly decreased, whereas total sputum protein was increased (Figure 1A, 1B). To further define the inflammatory profile of stable CF participants, we measured sputum concentrations of cytokines previously reported to be increased in CF. Of these, IFNα, IFNγ, IL1β, IL-8, IL-13, and TNFα were significantly increased in CF compared to HC (Figure 1C). There were no differences in SPLUNC1 levels of stable participants according to severity of lung function impairment, F508del genotype, use of CFTR modulators, or microbiology findings (Supplemental Tables 1–4, Supplemental Figure 3A–D). These findings indicate that SPLUNC1 is abundant in sputum and decreased in stable CF participants.
Figure 1. SPLUNC1 is Decreased in the Sputum of Stable CF Patients.
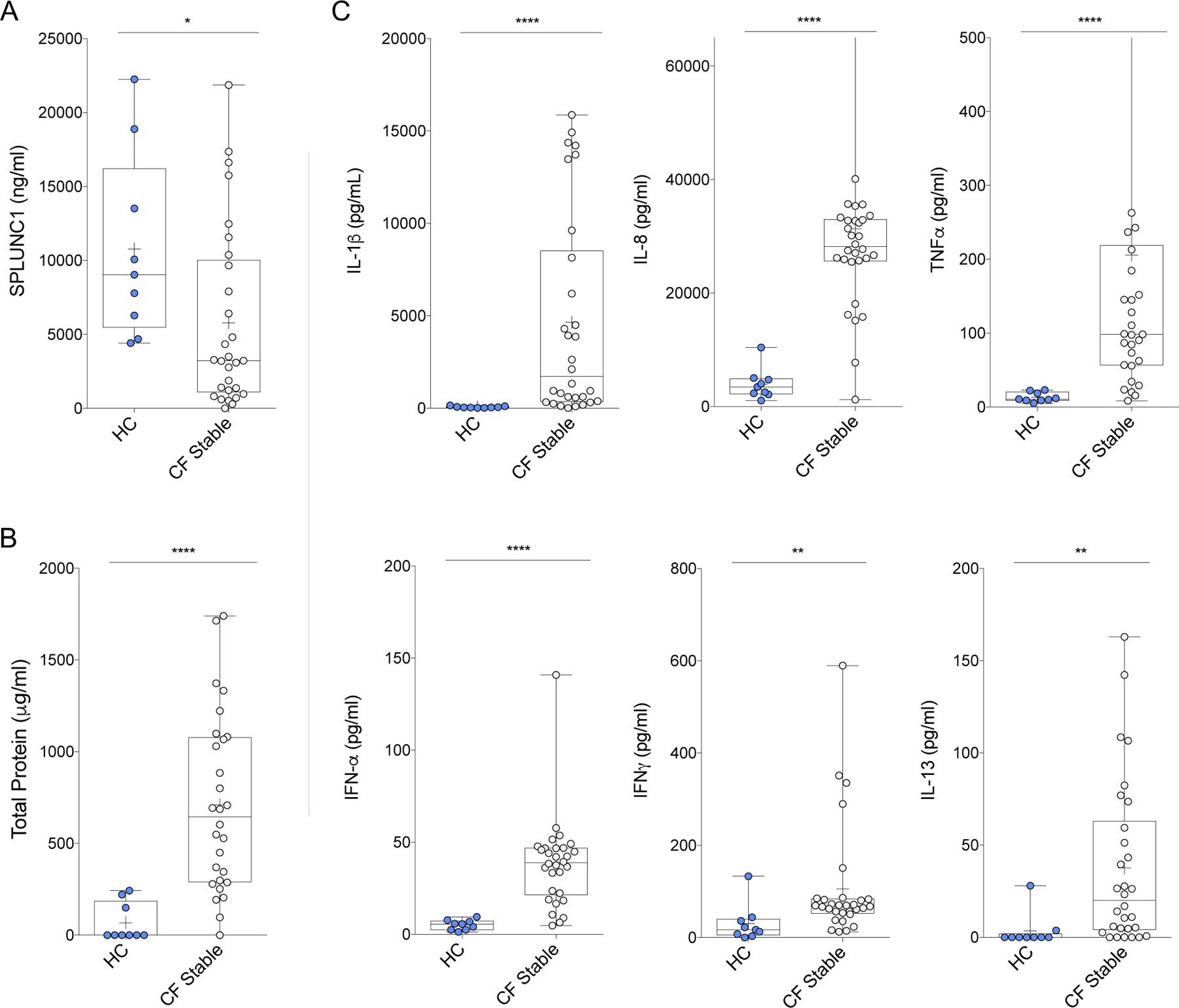
A) SPLUNC1 levels (ELISA) in sputum samples from the Yale cohort of adult CF patients without respiratory symptoms (CF Stable) and healthy controls (HC). B) Total protein in sputum (BCA assay) from the same patients. C) Inflammatory cytokine levels (ELISA) in sputum from the same patients. Additional cytokines tested without significant difference: CXCL10, G-CSF, IFNλ, IL-6, IL-13, MCP1, MIP1α. CF Samples were obtained by voluntary expectoration during clinical assessment, HC samples obtained by sputum induction with nebulized normal saline solution. + = Mean; Bar inside box: Median; Whiskers: Minimum/Maximum. Mann-Whitney Test with Bonferroni correction; * = p<0.05; ** = p<0.01; **** = p<0.0001.
SPLUNC1 Decreases Further During AE
We measured SPLUNC1 levels in sputum from stable and AE participants to determine if SPLUNC1 is a marker of AE. SPLUNC1 decreased sharply during AE in the discovery cohort (71.9% decrease) and in the validation cohort (38.6% decrease) (Figure 2A). In contrast, FEV1 did not decrease in the discovery cohort’s AE group, but was significantly lower in the validation cohort (Figure 2B).
Figure 2. SPLUNC1 is Decreased During Acute CF Exacerbations (AE).
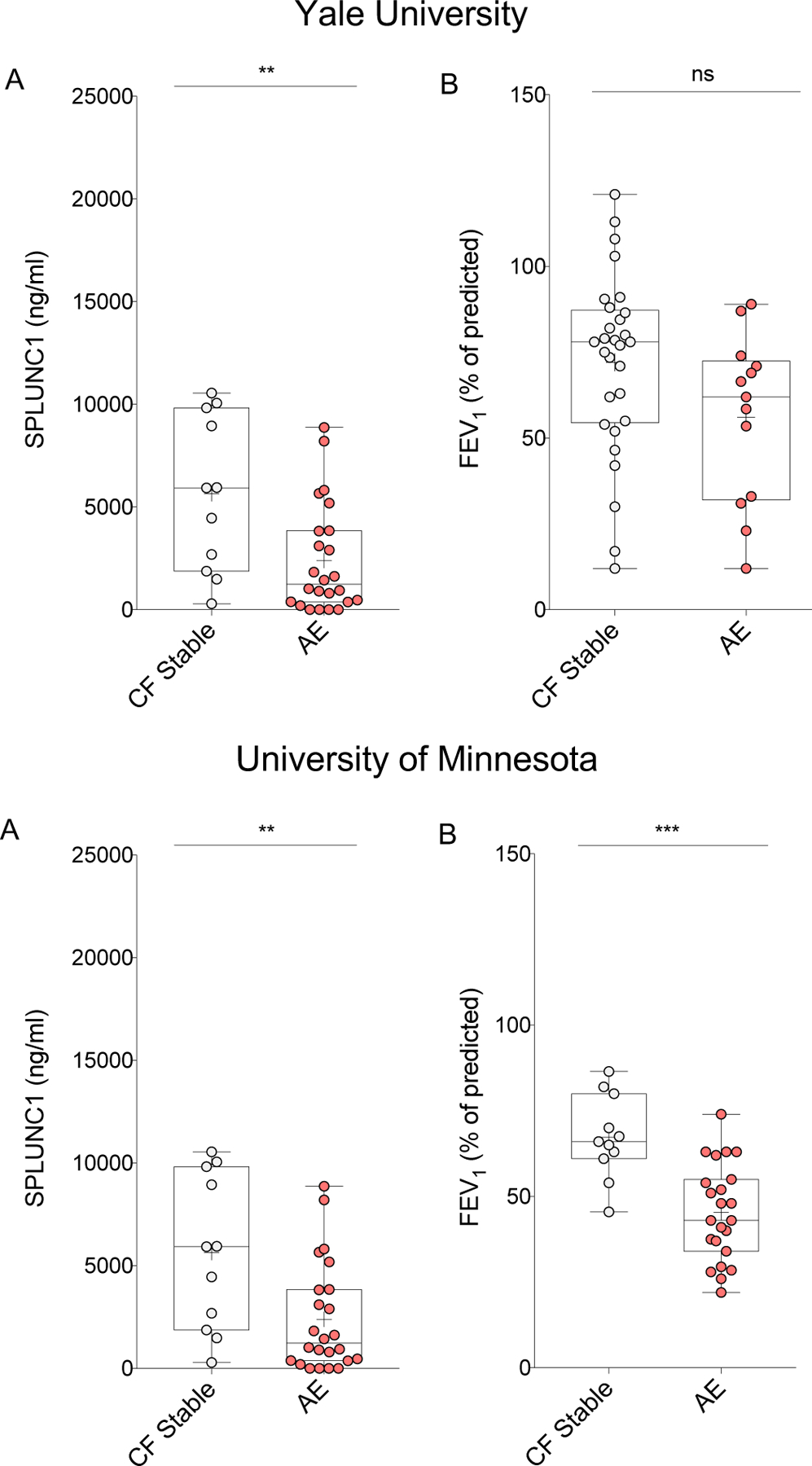
Sputum SPLUNC1 and FEV1 from two clinical cohorts including adult (Yale University, n=43) and mixed adult/pediatric (University of Minnesota, n=35) CF patients. Samples were obtained by voluntary expectoration during clinical assessment, A) SPLUNC1 quantified by ELISA, B) FEV1 (Percent of Predicted, %) obtained by spirometry during clinical assessment; CF Stable: No symptoms of AE, no antibiotic treatment. AE: Acute CF exacerbation, symptoms of AE and ongoing antibiotic therapy; FEV1: Forced Expiratory Volume in the first second; + = Mean; Bar inside box: Median; Whiskers: Minimum/Maximum. Mann-Whitney test; ** = p<0.005; *** = p<0.001; ns = not statistically significant.
Mean SPLUNC1 levels of AE participants treated with oral antibiotics (AEO) and IV antibiotics (AEIV) in the UMN cohort were lower than stable CF levels. However, there was no difference in SPLUNC1 levels between AEO and AEIV (Supplemental Figure 4). The lack of difference between these treatment groups suggests that acute drops in SPLUNC1 occur during AE regardless of its severity.
Next, we sought to define SPLUNC1 fluctuations during AE within the same individuals, relative to their stable-state reference value (individual-specific fluctuations). We compared SPLUNC1 and FEV1 (%) in paired samples from the same participants, collected during stable and AE periods. SPLUNC1 decreased during AE in the majority of paired samples from both the Yale and UMN cohorts (Figure 3A). In contrast, FEV1 decreased during AE in the in the majority UMN samples, but not in those form the Yale cohort (Figure 3B). These findings indicate that while SPLUNC1 is consistently decreased during AE, FEV1 changes during AE vary across cohorts.
Figure 3. Individual-Specific SPLUNC1 and FEV1 Decreases During AE.
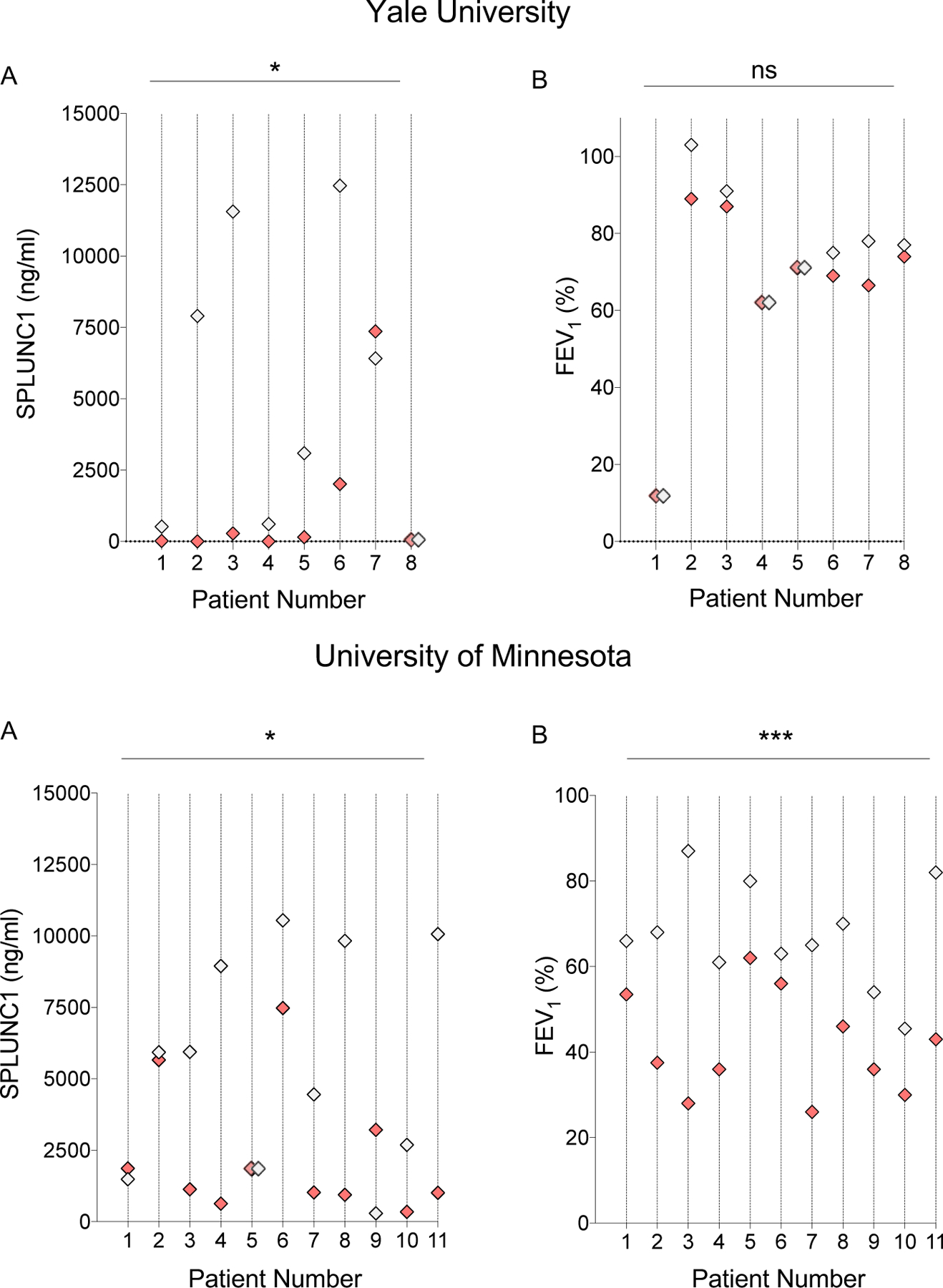
A) Paired SPLUNC1 levels in sputum samples from the same individual with and without AE (ELISA); B) Paired FEV1 measurements from the same individual with and without AE (Percent of Predicted, %) obtained by spirometry during clinical assessment; Samples from two clinical cohorts including adult (Yale University, n=8) and mixed adult/pediatric CF patients (University of Minnesota, n=11). Each vertical line and number represent a single patient that provided one Stable and one AE sample. CF Stable (Gray markers): No symptoms of AE, no antibiotic treatment. AE (Red markers): Acute CF Exacerbation, symptoms of AE and ongoing antibiotic therapy; When values were the same, these were represented by two overlapping diamonds along the patient’s line. Wilcoxon matched-pairs signed rank test; * = p<0.05, *** = p = 0.0001, ns=not statistically significant.
Low SPLUNC1 Levels Predict AE Risk in Stable CF Participants
To determine if SPLUNC1 is a predictor of AE risk, we first performed a Mantel-Haenszel survival estimator analysis for AE-free time. We separated the cohorts into high/low-SPLUNC1 groups based on a concentration threshold defined by ROC analysis comparing AE and Stable patients (Supplemental methods, Supplemental Figure 2). In stable CF participants, the SPLUNC1-low group had a median AE-free time of 43.5 days, compared to 150 days in the SPLUNC1-high group, this relationship was preserved in a subgroup analysis of patients with FEV1 >40% of predicted (Supplemental Figure 5). This suggests that higher SPLUNC1 levels are associated with longer AE-free intervals independently of stable-state FEV1.
Next, we performed Cox-proportional Hazards modeling to assess the likelihood of AE while adjusting for demographics, CFTR genotype, CF-related comorbidities, microbiology, and lung function. In the short term (60 days), participants in the SPLUNC1-low group had a significantly increased risk of AE (Hazard ratio: 11.49, p=0.003, Figure 4A), which persisted upon long-term follow up at 1 year (Hazard ratio: 3.21, p=0.013, Figure 4B).
Figure 4. SPLUNC1 Predicts AE-Free Time.
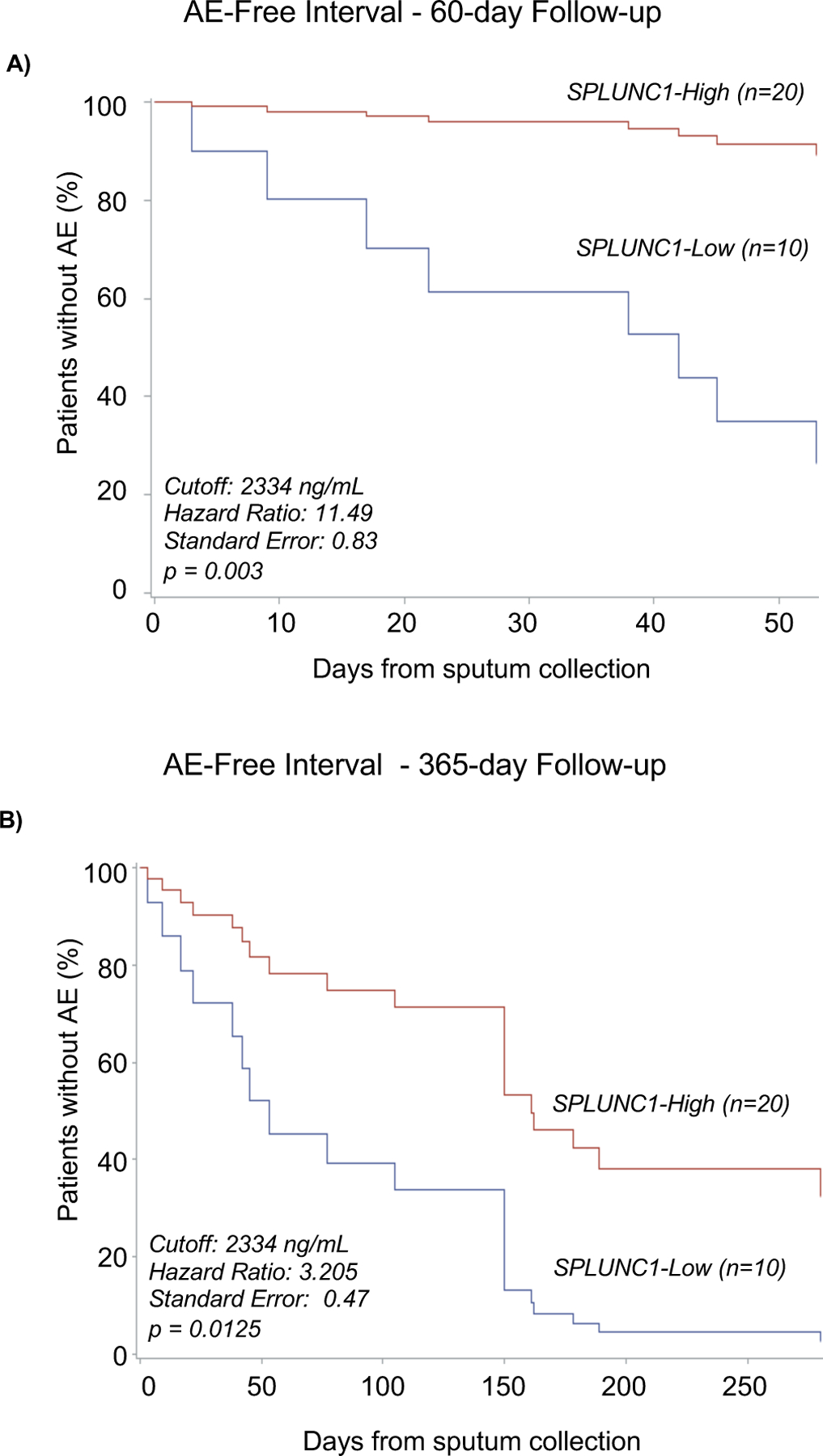
A) AE-Free time in Stable CF patients separated into SPLUNC1-High and SPLUNC1-Low groups over a 60-day follow-up period. SPLUNC1-high and –low groups were defined according to sputum concentration thresholds obtained from receiver-operator curves separating CF Stable and AE levels (Supplemental Figure 4). AE-Free time was defined as the number of days from sputum collection in Stable patients until the date of their next AE. B) AE-Free time in Stable CF patients separated into SPLUNC1-High and -Low groups over a 365-day follow up period. Cox Proportional Hazards model used to calculate AE-free intervals and adjust for age, sex, BMI, FEV1, number of F508del mutations, presence of CF-related diabetes or pancreatic insufficiency, use of CFTR modulators, and microbiology for P. aeruginosa, A. xylosoxidans, H. parainfluenzae, Methicillin-sensitive S. aureus, and Methicillin-resistant S. aureus.
In order to compare SPLUNC1 to previously reported biomarkers as predictors of AE, we defined ROC thresholds and AE-free time for G-CSF, IL1-β, IL-6, IL-8, and TNFα (Supplemental Figure 2). In a similar multivariate proportional hazards model, cytokine high/low groups based on these markers did not show an increased hazard ratio of AE at 60 days, and only high IL-1β, and TNFα were associated with an increased AE risk at 1 year of follow-up (Hazard ratios: 3.90, and 3.46 respectively, p<0.05, Supplemental Figure 6). These findings suggest that SPLUNC1 is a better predictor of AE risk in the short and long term than previously reported sputum AE markers.
Human and Bacterial Elastases Found in CF Sputum Degrade SPLUNC1
Our group and others have shown that NE degrades SPLUNC1, and that NE inhibitor Sivelestat only partially prevents SPLUNC1 degradation by CF sputum27,28. In order to understand the role of NE and bacterial elastase in decreasing SPLUNC1 during AE, we incubated rhSPLUNC1 with recombinant human neutrophil elastase (NE) or Pseudomonas aeruginosa’s Elastase B (LasB) at increasing concentrations for 3 and 8 hours. Both elastases induced a concentration-dependent decrease in full-length SPLUNC1 (Figures 5A, B). Next, we quantified NE concentrations in HC and CF sputum during stable and AE periods. NE was increased overall in CF, but it did not increase significantly from stable levels during AE (Figure 5C). Finally, to define individual-specific NE and SPLUNC1 changes, we performed Western blots of HC and CF sputum, probing for NE, followed by re-probing for SPLUNC1. Although NE was increased in CF relative to HC, NE levels were not different between Stable and AE states (Figure 5D).
Figure 5. Elastase Concentration and Activity are Increased in CF.
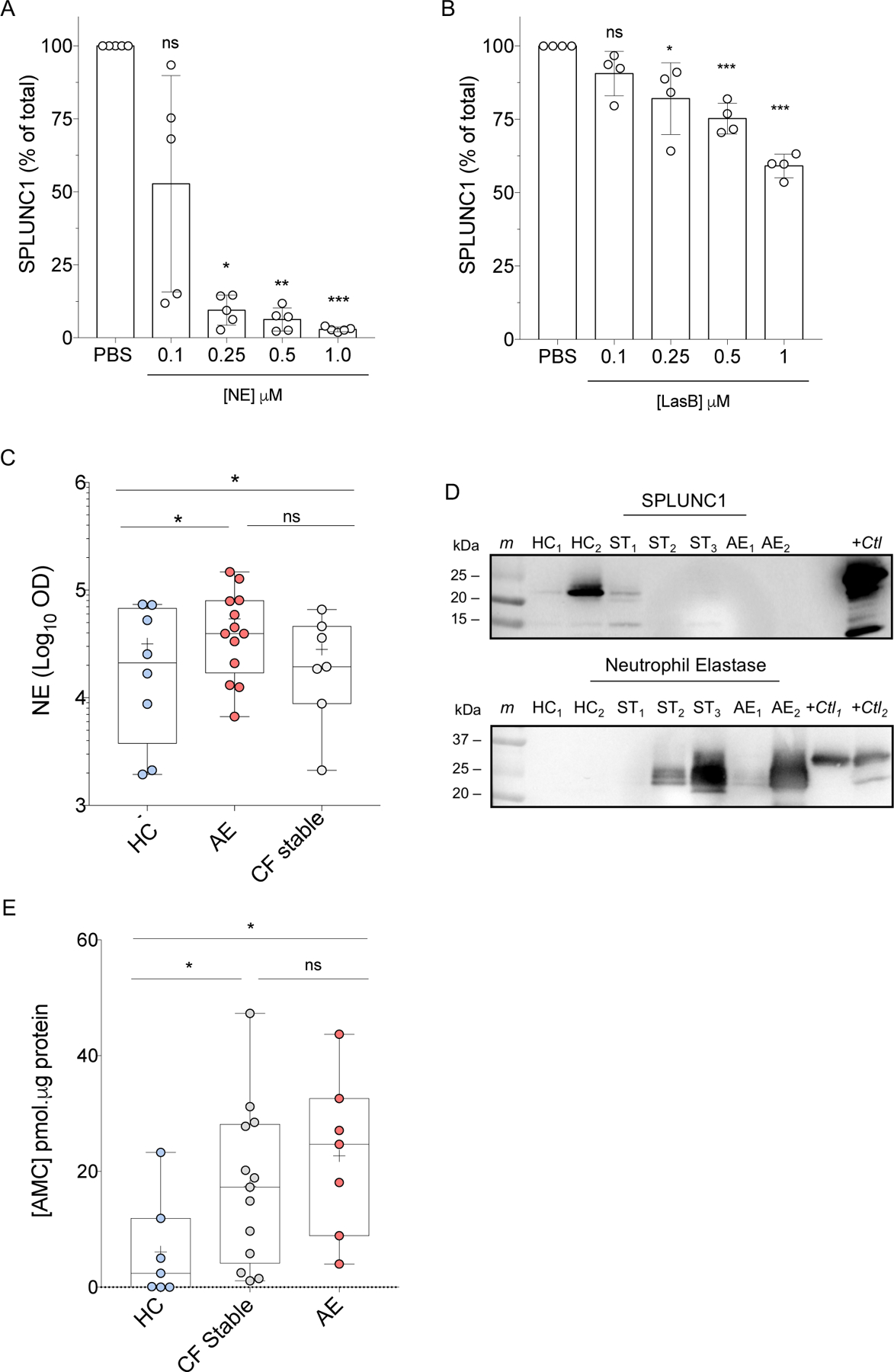
A) SPLUNC1 densitometry showing degradation by human neutrophil elastase (NE) relative to PBS control, at specified concentrations over 3 hours at 37°C. B) SPLUNC1 densitometry showing degradation by Elastase B (LasB) from P. aeruginosa relative to PBS control at specified concentrations over 8 hours at 37°C. C) NE densitometry in sputum from healthy controls (HC), Stable CF patients (CF Stable), and AE patients (AE) assessed by WB. D) Representative WB showing endogenous expression of SPLUNC1 (20–25 kD) and NE (25–30 kD) in HC and CF sputum samples from the same individuals. Membranes were probed for NE prior to stripping and re-probing for SPLUNC1. E) NE Activity in CF sputum: AMC formation from florigenic NE substrate MAA-3133 following 6 h incubation with HC, CF stable, and AE sputum at 37°C. For experiments in A and B: n = 4–5, 2 individual experiments; Mann-Whitney Test; + = Mean; Bar inside box: Median; Whiskers: Minimum/Maximum; * p < 0.05; ** = p < 0.01; *** = p < 0.005; ns: not statistically significant; HC: Healthy Control; ST: Stable CF; AE: CF exacerbation; m: marker; +ctl: recombinant protein positive control; OD: optic density.
To determine if NE activity, rather than concentration, increased during AE we measured NE-specific fluorescent cleavage products. When incubated with NE, CF sputum had much higher NE activity than HC sputum; however, there was no difference between stable and AE participants (Figure 5E).
Sputum Cytokines IL-1β and TNFα are Increased During AE
To further define the AE inflammatory profile of CF participants, we measured concentrations of thirteen cytokines in stable and AE samples. Only IL-1β and TNFα were significantly increased during AE (Figure 6A). We also sought to define a relationship between SPLUNC1 and cytokine levels in sputum during stable and AE states using Pearson’s correlation. In stable and AE groups from both cohorts, IL-1β levels inversely correlated with SPLUNC1 (Supplementary Figure 7), while CXCL10, G -CSF, IFNγ, IL-6, MCP1, MIP-1α, and TNFα did not consistently correlate with SPLUNC1 across cohorts (not shown).
Figure 6. Cytokines IL-1β and TNF-α Increase During AE & Downregulate SPLUNC1 Expression.

A) Inflammatory cytokine levels in sputum from adult CF patients without respiratory symptoms (CF Stable) and with acute CF Exacerbation (AE). Additional cytokines tested without significant difference: CXCL10, G-CSF, IFNα2, IFNγ, IFNλ, IL-6, IL-8, IL-13, MCP1, MIP1α. Mann-Whitney Test with Bonferroni correction. B) Relative SPLUNC1 mRNA expression in mouse tracheal epithelial cells (Mouse epithelium) grown at air-liquid interface and in the NCI-H292 human airway epithelial cell line (Human epithelium) treated with recombinant TNFα and IL-1β (10 ng/mL) for 24 hours (2-way ANOVA); + = Mean; Bar inside box: Median; Whiskers: Minimum/Maximum; mRNA expression quantified by qPCR. Represents 2 experiments; * = p<0.05; ** = p<0.01.
In order to determine if increased IL-1β and TNFα contributed to decreased SPLUNC1 during AE, we treated mTEC and a human airway epithelial cell line with these cytokines and measured SPLUNC1 mRNA expression. At concentrations encountered in AE sputum, both IL-1β and TNFα decreased SPLUNC1 expression by airway epithelial cells (Figure 6B). Together with our observations from NE and LasB experiments, these findings suggest that during AE, SPLUNC1 is decreased through protein degradation and cytokine-driven transcriptional downregulation.
DISCUSSION
AEs contribute to increased morbidity in CF and treatment delays are associated with poor FEV1 recovery and impaired treatment responses5–9,15–17,52,53. Yet, few biomarkers are clinically available to guide early AE interventions in order to minimize hospitalizations and improve quality of life44–46. Here, we describe a novel role for SPLUNC1 as an AE biomarker and predictor that could support clinical decision-making to improve AE outcomes.
The key finding of our study is that among CF participants, SPLUNC1 levels were considerably lower during AE compared to stable state. We propose that the mechanism for SPLUNC1 decreases in CF is multifactorial, with contributions from protease degradation and gene expression downregulation by inflammatory cytokines. Prospectively, stable patients with low SPLUNC1 had an increased likelihood of AE at 60 days and one year. These findings suggest that SPLUNC1 levels could inform the diagnosis and clinical management of AE in the short and long term.
By the time symptoms develop or FEV1 declines, airway inflammation and damage may already be underway14,15. In the face of a suspected AE with incomplete clinical criteria and normal spirometry, a low SPLUNC1 level would support a decision to increase airway clearance, adjust monitoring, or initiate pharmacological interventions when appropriate. In asymptomatic stable patients, low SPLUNC1 levels could also prompt immediate or long-term changes in clinical management.
Although symptom and spirometry monitoring at home increase AE detection, they do not prevent FEV1 decline, possibly because of the delayed nature of FEV1 changes in response to airway inflammation54. The application of clinical biomarkers is even more challenging in children, where FEV1 and cytokine abnormalities are inconsistently detected until adolescence, despite early evidence of structural lung disease44,55–57. SPLUNC1 measurements at home could detect subtle inflammatory changes that complement symptom and spirometry monitoring.
Previous studies examined the correlation between sputum biomarkers, infection, inflammation, and lung function decline in CF25,58–67. While some defined novel AE biomarkers25,68–72, to our knowledge, ours is the first study to compare a broad panel of markers in AE and stable state that includes adults and children.
Daily changes in inflammatory signals or pathogen exposures may cause small fluctuations, however, SPLUNC1 levels drop sharply during AE. We previously showed that LPS and IFN-γ have tonic suppressive effects on SPLUNC1 at baseline26. However, high-dose LPS and IFN-γ exposures decrease epithelial SPLUNC1 expression drastically, indicating a dose-dependent response. SPLUNC1’s tight regulation suggests that it is an ideal biomarker to detect early and subtle changes in lung homeostasis26.
SPLUNC1 has host protective functions relevant to CF, including regulation of airway surface liquid, antimicrobial properties, and immunomodulatory effects73–79. Therefore, SPLUNC1 decreases in CF may not only be a marker, but also a contributor to pathogenesis during AE and disease progression. Decreased SPLUNC1 in CF may impair mucociliary clearance and facilitate bacterial colonization, leading to tissue injury and exacerbated inflammation. In fact, some SPLUNC1-deficient animal models have shown increased susceptibility to infectious and non-infectious inflammation80,81. In our study, we observed increased IL-1β and TNFα during AE, but only IL-1β inversely correlated with SPLUNC1. This correlation may reflect the transcriptional effects of higher relative concentrations of IL-1β during AE when compared to TNFα (Figure 6A). Furthermore, others have shown that IL-1β may have a more potent neutrophil recruitment effect than TNFα82. Thus, the increased concentrations of IL-1β may enhance neutrophil recruitment that in turn increases SPLUNC1 degradation, strengthening the inverse correlation between SPLUNC1 and IL-1β. Our data showing increased NE levels and activity, although not different between AE and stable state, suggest that SPLUNC1 cleavage at functional sites by NE (and other proteases during AE) may disrupt its host defense functions, worsening inflammation and accelerating lung disease34,83.
Our study has some limitations. First, our study has a small sample size and predicts AE risk based on cross-sectional data. However, our findings show it was adequately powered to demonstrate differences in key observations, confirmed on a validation cohort. In the future, we would favor serial SPLUNC1 measurements to establish stable-state baselines that reflect day-to-day fluctuations. Second, there is no distinct SPLUNC1 level that separates HC from CF, or stable- from AE-state in all participants. The overlap of SPLUNC1 levels among some HC and CF patients, may be in part explained by changes in inflammation and environmental exposures between sputum samplings. However, our study highlights the value of individual biomarker variability in characterizing disease states. Third, we used IV or oral therapy as surrogates for disease severity. We appreciate that choice of therapy route is based on many factors, including access to care, AE complications, and severity of FEV1 decline84. We decided to present these data to show that SPLUNC1 can be used as a marker in AE of any severity and regardless of factors driving therapeutic decisions. Finally, our cohorts had a higher prevalence of advanced lung disease and CFTR genotypes linked to severe disease. We addressed this in our multivariate models by using a backwards elimination strategy to confirm that the predictive ability of SPLUNC1 was not affected by CFTR genotype. Larger prospective studies are needed to replicate our findings in sub-cohorts that reflect specific CFTR genotypes, comorbidities, and the impact of novel CFTR modulator combinations.
In the age of highly-effective CFTR modulator therapy, we look forward to rising life expectancy and quality of life4. We hope that measurements of non-invasive, airway-relevant biomarkers such as SPLUNC1 will become a resource to guide acute management of respiratory complications and inform our partnership with CF patients for the betterment of their long-term health.
Supplementary Material
Supplemental Figure 1. Study design (Yale discovery cohort). Forty-four adult patients with a confirmed diagnosis of CF were identified from the Yale Adult CF Program to participate in this study. These patients were recruited during their scheduled routine visits, unscheduled “sick” visits in which they reported new respiratory symptoms, and on their first day of admission to the hospital for treatment of an acute pulmonary exacerbation (AE). Our recruitment period extended from 2014–2016. We organized study participants into two groups: 1) Stable CF patients (CF Stable): Individuals without new respiratory symptoms, who presented to clinic for their scheduled quarterly follow up and, 2) Patients having an AE: Individuals with new respiratory symptoms, clinically diagnosed with AE that were prescribed treatment for AE during scheduled visit, unscheduled sick visit, or first day of hospital admission for AE. All CF patients provided spontaneously expectorated sputum samples, sputum microbiology samples, and pulmonary function tests. Healthy controls underwent sputum induction. All patients were followed for the development of AE for one year counted from the date of sputum collection.
Supplemental Figure 2. Receiver Operator Curve Development for defining SPLUNC1, IL-1β, TNFα, GCSF, IL-6, and IL-8- High and Low categories. Cutoff values (*) were selected to provide maximum sensitivity with the highest specificity possible (highlighted in gray).
Supplemental Figure 3. CF Stable SPLUNC1 levels do not vary according to FEV1, CF-causing mutation, modulator therapy, or sputum microbiology. Sputum SPLUNC1 levels in stable CF patients according to A) FEV1, B) F508del genotype, C) CFTR-modulator therapy, and D) Sputum microbiology from the Yale discovery cohort including 30 adults with CF. Samples were obtained by voluntary expectoration during clinical assessment, SPLUNC1 quantified by ELISA. FEV1 (Percent of Predicted, %) obtained by spirometry during clinical assessment; FEV1: Forced Expiratory Volume in the first second; + = Mean; Bar inside box: Median; Whiskers: Minimum/Maximum. Mann-Whitney test; ns = not statistically significant.
Supplemental Figure 4. SPLUNC1 is Decreased During CF Exacerbations Requiring Outpatient or Inpatient Antibiotic Treatment. SPLUNC1 levels in sputum samples from a clinical cohort including adult and pediatric CF patients (University of Minnesota). Samples were obtained by voluntary expectoration during clinical assessment, SPLUNC1 quantified by ELISA. CF Stable: No symptoms of AE, no antibiotic treatment. AE Outpatient (AE OP): Clinical symptoms consistent with exacerbation, treated with oral antibiotics at the time of sputum collection. AE Inpatient (AE IP): Admitted for inpatient antibiotic course, sample collected during first day of treatment. + = Mean; Bar inside box: Median; Whiskers: Minimum/Maximum; Mann-Whitney test; * = p<0.05, ** = p<0.01, ns = not statistically significant.
Supplemental Figure 5. SPLUNC1 Predicts AE-Free Time (unadjusted survival model). A) Stable CF patients were separated into SPLUNC1-high and SPLUNC1-Low groups according to a SPLUNC1 threshold of 2334 ng/mL. Time to AE was measured over 365 days from the date of sputum collection in all patients. B) Stable patients with an FEV1 >40% of predicted were separated into SPLUNC1-high and SPLUNC1-low cohorts as above. Time to exacerbation was measured for up to one year from the date of sputum collection. Mantel-Haenszel estimator was used to calculate exacerbation-free interval in each group. Values are not adjusted for clinical variables. Adjusted values from Cox proportional hazards model are presented in Figure 4. AE: CF exacerbation, HR: Hazard ratio, CI: Confidence interval.
Supplemental Figure 6. Sputum Cytokines Do Not Predict Short Term AE-Free Time. A) AE-Free time in Stable CF patients separated into IL-1β-, TNFα-, G-CSF-, IL-6-, and IL-8-High and -Low groups over a 60-day follow up period. Marker-high and –low groups were defined according to sputum concentration thresholds obtained from receiver-operator curves separating CF Stable and AE levels (Supplemental Figure 4). AE-Free time was defined as the number of days from sputum collection in Stable patients until the date of their next AE. B) AE-Free time in Stable CF patients separated into IL-1β-, TNFα-, G-CSF-, IL-6-, and IL-8-High and -Low groups over a 365-day follow up period. Cox Proportional Hazards model used to calculate AE-free intervals and adjust for age, sex, BMI, FEV1, number of F508del mutations, presence of CF-related diabetes or pancreatic insufficiency, use of CFTR correctors/modulators, and microbiology for P. aeruginosa, A. xylosoxidans, H. parainfluenzae, Methicillin-sensitive S. aureus, and Methicillin-resistant S. aureus. HR: Hazard ration; SE: Standard error.
Supplemental Figure 7. SPLUNC1 Negatively Correlates with IL-1β in Stable and AE states. Pearson correlation demonstrates an inverse relationship between IL-1β and SPLUNC1 levels in Stable (left) and AE (right) states. Sputum samples from two cohorts including adult (Yale University, Stable n=30, AE n=14) and mixed adult/pediatric (University of Minnesota, Stable n=33, AE n=32) CF participants. SPLUNC1 and IL-1β quantified by ELISA, values are square-root-transformed for analysis. r=correlation coefficient. All four correlations have a p <0.05.
Supplemental Figure 8. SPLUNC1 Western Blot does not distinguish disease states but identifies SPLUNC1 fragments. Full unedited WB showing SPLUNC1 in sputum from study participants. Sputum supernatants were loaded onto 4–15% Bis-Tris (top) or 16% Tricine gels (bottom, for improved SPLUNC1 fragment identification). Samples were electrophoresed at 130 volts. Membranes were initially probed for NE prior to stripping and re-probing for SPLUNC1. SPLUNC1 antibody: goat polyclonal hPLUNC1 antibody raised against residues Q20 - V256 of hPLUNC1 (1:3000, R&D systems), with a secondary anti-goat IgG-HRP. ST: Stable CF; AE: CF exacerbation; Black arrowheads: SPLUNC1, Red arrowheads: SPLUNC1 fragments.
Supplemental Figure 9. Full unedited Western Blots for figure 5D. Membranes were initially probed for NE prior to stripping and re-probing for SPLUNC1. A) NE antibody: Mouse monoclonal anti-hELA2 raised against residues M1–N252 (1:3000, R&D systems). B) SPLUNC1 antibody: goat polyclonal hPLUNC1 antibody raised against residues Q20 - V256 of hPLUNC1 (1:3000, R&D systems), a secondary anti-goat HRP. HC: Healthy Control; ST: Stable CF; AE: CF exacerbation; m: marker; +ctl: recombinant protein positive control.
TAKE-HOME MESSAGE.
Sputum concentrations of the secreted airway protein SPLUNC1 decrease during CF exacerbations. Lower SPLUNC1 levels in stable participants portend a significantly increased risk of exacerbation that could inform therapeutic interventions.
ACKNOWLEDGMENTS
This work was supported by The National Institutes of Health & National Heart, Lung, and Blood Institute (USA) through grants NIH R01-HL081160 and R21-AI083475 (LC), NIH T32-HL007778 and K01-HL125514-01 (CB), the Cystic Fibrosis Foundation through its Fifth Year Clinical Fellowship Award and Pilot and Feasibility Award, and the American Thoracic Society Foundation Unrestricted Research Award (CB). CF Foundation (RT); UK CF Trust (RT). We thank our patients, the medical staff at the Yale Adult Cystic Fibrosis Program, and Dr. Jonathan Koff, Director of the Adult CF Program, for their support and contributions to this project. We also thank Dr. Mehmet Kesimer from the University of North Carolina at Chapel Hill for his thoughtful review and contributions in the development of this manuscript.
Footnotes
DISCLOSURES
Dr. Tarran reports the following financial and intellectual property disclosures: Eldec Pharmaceuticals, outside the submitted work; In addition, Dr. Tarran has a patent on Peptide inhibitors of Ca2+ channels pending, a patent on PEPTIDE INHIBITORS OF SODIUM CHANNELS with royalties paid, and a patent on Regulation of sodium channels by PLUNC proteins with royalties paid. Dr. Cohn reports the following financial disclosures: Genentech, Novartis, Astra-Zeneca, GlaxoSmithKline, Regeneron, Pieris, Sanofi, all outside the submitted work. The other authors of this manuscript do not have any conflicts of interest that could be perceived to bias their work.
REFERENCES
- 1.Foundation, C. F. 2018 Annual Data Report. (Cystic Fibrosis Foundation, Bethesda, Maryland, 2019). [Google Scholar]
- 2.Rowe SM, Miller S & Sorscher EJ Cystic fibrosis. The New England journal of medicine 352, 1992–2001, doi: 10.1056/NEJMra043184 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Welsh MJ RB, Accurso F, Cutting GR. in The metabolic and molecular basis of inherited disease (ed Beaudet AL Scriver CR, Sly WS,Valle D,Childs B,Vogelstein B) 5121–5189 (McGraw-Hill, 2001). [Google Scholar]
- 4.Foundation, C. F. 2019 Annual Data Report. (Cystic Fibrosis Foundation, Bethesda, Maryland, 2020). [Google Scholar]
- 5.Flight WG et al. The effect of the weather on pulmonary exacerbations and viral infections among adults with cystic fibrosis. Int J Biometeorol 58, 1845–1851, doi: 10.1007/s00484-013-0786-0 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Flight WG et al. Incidence and clinical impact of respiratory viruses in adults with cystic fibrosis. Thorax 69, 247–253, doi: 10.1136/thoraxjnl-2013-204000 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Goss CH & Burns JL Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax 62, 360–367, doi: 10.1136/thx.2006.060889 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pribble CG, Black PG, Bosso JA & Turner RB Clinical manifestations of exacerbations of cystic fibrosis associated with nonbacterial infections. J Pediatr 117, 200–204 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wark PA et al. Viral infections trigger exacerbations of cystic fibrosis in adults and children. Eur Respir J 40, 510–512, doi: 10.1183/09031936.00202311 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Etherington C et al. The role of respiratory viruses in adult patients with cystic fibrosis receiving intravenous antibiotics for a pulmonary exacerbation. J Cyst Fibros 13, 49–55, doi: 10.1016/j.jcf.2013.06.004 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Whelan FJ & Surette MG Clinical Insights into Pulmonary Exacerbations in Cystic Fibrosis from the Microbiome. What Are We Missing? Ann Am Thorac Soc 12 Suppl 2, S207–211, doi: 10.1513/AnnalsATS.201506-353AW (2015). [DOI] [PubMed] [Google Scholar]
- 12.Parkins MD & Floto RA Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J Cyst Fibros 14, 293–304, doi: 10.1016/j.jcf.2015.03.012 (2015). [DOI] [PubMed] [Google Scholar]
- 13.van Ewijk BE, van der Zalm MM, Wolfs TF & van der Ent CK Viral respiratory infections in cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 4 Suppl 2, 31–36, doi: 10.1016/j.jcf.2005.05.011 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liou TG et al. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 153, 345–352, doi: 10.1093/aje/153.4.345 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer K et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 66, 680–685, doi: 10.1136/thx.2011.161117 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Sanders DB et al. Return of FEV1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr Pulmonol 45, 127–134, doi: 10.1002/ppul.21117 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Smith AL, Fiel SB, Mayer-Hamblett N, Ramsey B & Burns JL Susceptibility testing of Pseudomonas aeruginosa isolates and clinical response to parenteral antibiotic administration: lack of association in cystic fibrosis. Chest 123, 1495–1502, doi: 10.1378/chest.123.5.1495 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Fuchs HJ et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 331, 637–642, doi: 10.1056/NEJM199409083311003 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Stanford GE, Dave K & Simmonds NJ Pulmonary Exacerbations in Adults With Cystic Fibrosis: A Grown-up Issue in a Changing Cystic Fibrosis Landscape. Chest, doi: 10.1016/j.chest.2020.09.084 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraynack NC, Gothard MD, Falletta LM & McBride JT Approach to treating cystic fibrosis pulmonary exacerbations varies widely across US CF care centers. Pediatr Pulmonol 46, 870–881, doi: 10.1002/ppul.21442 (2011). [DOI] [PubMed] [Google Scholar]
- 21.West NE et al. Standardized Treatment of Pulmonary Exacerbations (STOP) study: Physician treatment practices and outcomes for individuals with cystic fibrosis with pulmonary Exacerbations. J Cyst Fibros 16, 600–606, doi: 10.1016/j.jcf.2017.04.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harun SN, Wainwright C, Klein K & Hennig S A systematic review of studies examining the rate of lung function decline in patients with cystic fibrosis. Paediatric respiratory reviews, doi: 10.1016/j.prrv.2016.03.002 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Laguna TA et al. Sputum club cell protein concentration is associated with pulmonary exacerbation in cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 14, 334–340, doi: 10.1016/j.jcf.2014.10.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagel SD, Kapsner R, Osberg I, Sontag MK & Accurso FJ Airway Inflammation in Children with Cystic Fibrosis and Healthy Children Assessed by Sputum Induction. American journal of respiratory and critical care medicine 164, 1425–1431, doi: 10.1164/ajrccm.164.8.2104075 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Sagel SD, Wagner BD, Anthony MM, Emmett P & Zemanick ET Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med 186, 857–865, doi: 10.1164/rccm.201203-0507OC (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britto CJ et al. Short palate, lung, and nasal epithelial clone-1 is a tightly regulated airway sensor in innate and adaptive immunity. Am J Respir Cell Mol Biol 48, 717–724, doi: 10.1165/rcmb.2012-0072OC (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang D et al. Human neutrophil elastase degrades SPLUNC1 and impairs airway epithelial defense against bacteria. PloS one 8, e64689, doi: 10.1371/journal.pone.0064689 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster MJ et al. SPLUNC1 degradation by the cystic fibrosis mucosal environment drives airway surface liquid dehydration. Eur Respir J 52, doi: 10.1183/13993003.00668-2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bingle CD & Bingle L Characterisation of the human plunc gene, a gene product with an upper airways and nasopharyngeal restricted expression pattern. Biochimica et biophysica acta 1493, 363–367 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Bartlett JA et al. PLUNC is a secreted product of neutrophil granules. J Leukoc Biol 83, 1201–1206, doi: 10.1189/jlb.0507302 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Bingle L & Bingle CD Distribution of human PLUNC/BPI fold-containing (BPIF) proteins. Biochemical Society transactions 39, 1023–1027, doi: 10.1042/BST0391023 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Erickson NA et al. Soluble mucus component CLCA1 modulates expression of leukotactic cytokines and BPIFA1 in murine alveolar macrophages but not in bone marrow-derived macrophages. Histochem Cell Biol 149, 619–633, doi: 10.1007/s00418-018-1664-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Britto CJ & Cohn L Bactericidal/Permeability-increasing protein fold-containing family member A1 in airway host protection and respiratory disease. Am J Respir Cell Mol Biol 52, 525–534, doi: 10.1165/rcmb.2014-0297RT (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walton WG et al. Structural Features Essential to the Antimicrobial Functions of Human SPLUNC1. Biochemistry 55, 2979–2991, doi: 10.1021/acs.biochem.6b00271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaefer N et al. The effect of BPIFA1/SPLUNC1 genetic variation on its expression and function in asthmatic airway epithelium. JCI Insight 4, doi: 10.1172/jci.insight.127237 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boon K et al. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF). PLoS One 4, e5134, doi: 10.1371/journal.pone.0005134 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu T et al. Identification of BPIFA1/SPLUNC1 as an epithelium-derived smooth muscle relaxing factor. Nat Commun 8, 14118, doi: 10.1038/ncomms14118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore PJ et al. Cigarette smoke modifies and inactivates SPLUNC1, leading to airway dehydration. FASEB J, fj201800345R, doi: 10.1096/fj.201800345R (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Smet EG et al. Association of innate defense proteins BPIFA1 and BPIFB1 with disease severity in COPD. Int J Chron Obstruct Pulmon Dis 13, 11–27, doi: 10.2147/COPD.S144136 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bingle L et al. Differential epithelial expression of the putative innate immune molecule SPLUNC1 in cystic fibrosis. Respir Res 8, 79, doi: 10.1186/1465-9921-8-79 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saferali A et al. Polymorphisms Associated with Expression of BPIFA1/BPIFB1 and Lung Disease Severity in Cystic Fibrosis. Am J Respir Cell Mol Biol, doi: 10.1165/rcmb.2014-0182OC (2015). [DOI] [PubMed] [Google Scholar]
- 42.Saferali A et al. Immunomodulatory function of the cystic fibrosis modifier gene BPIFA1. PLoS One 15, e0227067, doi: 10.1371/journal.pone.0227067 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bingle L et al. SPLUNC1 (PLUNC) is expressed in glandular tissues of the respiratory tract and in lung tumours with a glandular phenotype. J Pathol 205, 491–497, doi: 10.1002/path.1726 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Ranganathan SC et al. Early Lung Disease in Infants and Preschool Children with Cystic Fibrosis. What Have We Learned and What Should We Do about It? Am J Respir Crit Care Med 195, 1567–1575, doi: 10.1164/rccm.201606-1107CI (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Britto MT et al. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 121, 64–72, doi: 10.1378/chest.121.1.64 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Bhatt JM Treatment of pulmonary exacerbations in cystic fibrosis. Eur Respir Rev 22, 205–216, doi: 10.1183/09059180.00006512 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan X et al. Noninvasive analysis of the sputum transcriptome discriminates clinical phenotypes of asthma. American journal of respiratory and critical care medicine 191, 1116–1125, doi: 10.1164/rccm.201408-1440OC (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laguna TA et al. Sputum desmosine during hospital admission for pulmonary exacerbation in cystic fibrosis. Chest 136, 1561–1568, doi: 10.1378/chest.09-0217 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esther CR Jr., Peden DB, Alexis NE & Hernandez ML Airway purinergic responses in healthy, atopic nonasthmatic, and atopic asthmatic subjects exposed to ozone. Inhal Toxicol 23, 324–330, doi: 10.3109/08958378.2011.572096 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao Y et al. Multiparameter Single Cell Profiling of Airway Inflammatory Cells. Cytometry B Clin Cytom 92, 12–20, doi: 10.1002/cyto.b.21491 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saint-Criq V et al. Pseudomonas aeruginosa LasB protease impairs innate immunity in mice and humans by targeting a lung epithelial cystic fibrosis transmembrane regulator-IL-6-antimicrobial-repair pathway. Thorax 73, 49–61, doi: 10.1136/thoraxjnl-2017-210298 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aaron SD et al. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. American journal of respiratory and critical care medicine 169, 811–815, doi: 10.1164/rccm.200309-1306OC (2004). [DOI] [PubMed] [Google Scholar]
- 53.Wood RE & Leigh MW What is a “pulmonary exacerbation” in cystic fibrosis? J Pediatr 111, 841–842 (1987). [DOI] [PubMed] [Google Scholar]
- 54.Lechtzin N et al. Home Monitoring of Patients with Cystic Fibrosis to Identify and Treat Acute Pulmonary Exacerbations. eICE Study Results. Am J Respir Crit Care Med 196, 1144–1151, doi: 10.1164/rccm.201610-2172OC (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sly PD et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med 180, 146–152, doi: 10.1164/rccm.200901-0069OC (2009). [DOI] [PubMed] [Google Scholar]
- 56.Sly PD & Wainwright CE Preserving Lung Function: The Holy Grail in Managing Cystic Fibrosis. Ann Am Thorac Soc 14, 833–835, doi: 10.1513/AnnalsATS.201703-254ED (2017). [DOI] [PubMed] [Google Scholar]
- 57.Konstan MW et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr 151, 134–139, 139 e131, doi: 10.1016/j.jpeds.2007.03.006 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Sagel SD, Chmiel JF & Konstan MW Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc 4, 406–417, doi: 10.1513/pats.200703-044BR (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sagel SD, Kapsner RK & Osberg I Induced sputum matrix metalloproteinase-9 correlates with lung function and airway inflammation in children with cystic fibrosis. Pediatr Pulmonol 39, 224–232, doi: 10.1002/ppul.20165 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Kim JS, Okamoto K & Rubin BK Pulmonary function is negatively correlated with sputum inflammatory markers and cough clearability in subjects with cystic fibrosis but not those with chronic bronchitis. Chest 129, 1148–1154, doi: 10.1378/chest.129.5.1148 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Mayer-Hamblett N et al. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med 175, 822–828, doi: 10.1164/rccm.200609-1354OC (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishak A et al. BAL Inflammatory Markers Can Predict Pulmonary Exacerbations in Children With Cystic Fibrosis. Chest 158, 2314–2322, doi: 10.1016/j.chest.2020.06.044 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Wojewodka G et al. Candidate markers associated with the probability of future pulmonary exacerbations in cystic fibrosis patients. PLoS One 9, e88567, doi: 10.1371/journal.pone.0088567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolter JM, Rodwell RL, Bowler SD & McCormack JG Cytokines and inflammatory mediators do not indicate acute infection in cystic fibrosis. Clin Diagn Lab Immunol 6, 260–265 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stoltz DA, Meyerholz DK & Welsh MJ Origins of Cystic Fibrosis Lung Disease. New England Journal of Medicine 372, 1574–1575, doi: 10.1056/NEJMc1502191 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Chen JH et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143, 911–923, doi: 10.1016/j.cell.2010.11.029 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stoltz DA et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2, 29ra31, doi: 10.1126/scitranslmed.3000928 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horsley AR et al. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax 68, 532–539, doi: 10.1136/thoraxjnl-2012-202538 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Gray RD et al. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros 9, 193–198, doi: 10.1016/j.jcf.2010.01.005 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Ordonez CL et al. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med 168, 1471–1475, doi: 10.1164/rccm.200306-731OC (2003). [DOI] [PubMed] [Google Scholar]
- 71.Osika E et al. Distinct sputum cytokine profiles in cystic fibrosis and other chronic inflammatory airway disease. Eur Respir J 14, 339–346, doi: 10.1034/j.1399-3003.1999.14b17.x (1999). [DOI] [PubMed] [Google Scholar]
- 72.Paats MS et al. Cytokines in nasal lavages and plasma and their correlation with clinical parameters in cystic fibrosis. J Cyst Fibros 12, 623–629, doi: 10.1016/j.jcf.2013.05.002 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Bingle CD & Craven CJ PLUNC: a novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Human molecular genetics 11, 937–943 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Chu HW et al. Function and regulation of SPLUNC1 protein in Mycoplasma infection and allergic inflammation. J Immunol 179, 3995–4002 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Gakhar L et al. PLUNC is a novel airway surfactant protein with anti-biofilm activity. PloS one 5, e9098, doi: 10.1371/journal.pone.0009098 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gally F et al. SPLUNC1 promotes lung innate defense against Mycoplasma pneumoniae infection in mice. The American journal of pathology 178, 2159–2167, doi: 10.1016/j.ajpath.2011.01.026 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Caballero A et al. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proceedings of the National Academy of Sciences of the United States of America 106, 11412–11417, doi: 10.1073/pnas.0903609106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGillivary G & Bakaletz LO The multifunctional host defense peptide SPLUNC1 is critical for homeostasis of the mammalian upper airway. PLoS One 5, e13224, doi: 10.1371/journal.pone.0013224 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Britto CJ et al. BPIFA1 regulates lung neutrophil recruitment and interferon signaling during acute inflammation. Am J Physiol Lung Cell Mol Physiol 316, L321–L333, doi: 10.1152/ajplung.00056.2018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thaikoottathil JV et al. SPLUNC1 deficiency enhances airway eosinophilic inflammation in mice. Am J Respir Cell Mol Biol 47, 253–260, doi: 10.1165/rcmb.2012-0064OC (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wright PL et al. Epithelial reticulon 4B (Nogo-B) is an endogenous regulator of Th2-driven lung inflammation. J Exp Med 207, 2595–2607, doi: 10.1084/jem.20100786 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheikh S et al. Differing mechanisms of leukocyte recruitment and sensitivity to conditioning by shear stress for endothelial cells treated with tumour necrosis factor-alpha or interleukin-1beta. Br J Pharmacol 145, 1052–1061, doi: 10.1038/sj.bjp.0706281 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ning F et al. Structural characterization of the pulmonary innate immune protein SPLUNC1 and identification of lipid ligands. FASEB J 28, 5349–5360, doi: 10.1096/fj.14-259291 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wagener JS et al. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol 48, 666–673, doi: 10.1002/ppul.22652 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Study design (Yale discovery cohort). Forty-four adult patients with a confirmed diagnosis of CF were identified from the Yale Adult CF Program to participate in this study. These patients were recruited during their scheduled routine visits, unscheduled “sick” visits in which they reported new respiratory symptoms, and on their first day of admission to the hospital for treatment of an acute pulmonary exacerbation (AE). Our recruitment period extended from 2014–2016. We organized study participants into two groups: 1) Stable CF patients (CF Stable): Individuals without new respiratory symptoms, who presented to clinic for their scheduled quarterly follow up and, 2) Patients having an AE: Individuals with new respiratory symptoms, clinically diagnosed with AE that were prescribed treatment for AE during scheduled visit, unscheduled sick visit, or first day of hospital admission for AE. All CF patients provided spontaneously expectorated sputum samples, sputum microbiology samples, and pulmonary function tests. Healthy controls underwent sputum induction. All patients were followed for the development of AE for one year counted from the date of sputum collection.
Supplemental Figure 2. Receiver Operator Curve Development for defining SPLUNC1, IL-1β, TNFα, GCSF, IL-6, and IL-8- High and Low categories. Cutoff values (*) were selected to provide maximum sensitivity with the highest specificity possible (highlighted in gray).
Supplemental Figure 3. CF Stable SPLUNC1 levels do not vary according to FEV1, CF-causing mutation, modulator therapy, or sputum microbiology. Sputum SPLUNC1 levels in stable CF patients according to A) FEV1, B) F508del genotype, C) CFTR-modulator therapy, and D) Sputum microbiology from the Yale discovery cohort including 30 adults with CF. Samples were obtained by voluntary expectoration during clinical assessment, SPLUNC1 quantified by ELISA. FEV1 (Percent of Predicted, %) obtained by spirometry during clinical assessment; FEV1: Forced Expiratory Volume in the first second; + = Mean; Bar inside box: Median; Whiskers: Minimum/Maximum. Mann-Whitney test; ns = not statistically significant.
Supplemental Figure 4. SPLUNC1 is Decreased During CF Exacerbations Requiring Outpatient or Inpatient Antibiotic Treatment. SPLUNC1 levels in sputum samples from a clinical cohort including adult and pediatric CF patients (University of Minnesota). Samples were obtained by voluntary expectoration during clinical assessment, SPLUNC1 quantified by ELISA. CF Stable: No symptoms of AE, no antibiotic treatment. AE Outpatient (AE OP): Clinical symptoms consistent with exacerbation, treated with oral antibiotics at the time of sputum collection. AE Inpatient (AE IP): Admitted for inpatient antibiotic course, sample collected during first day of treatment. + = Mean; Bar inside box: Median; Whiskers: Minimum/Maximum; Mann-Whitney test; * = p<0.05, ** = p<0.01, ns = not statistically significant.
Supplemental Figure 5. SPLUNC1 Predicts AE-Free Time (unadjusted survival model). A) Stable CF patients were separated into SPLUNC1-high and SPLUNC1-Low groups according to a SPLUNC1 threshold of 2334 ng/mL. Time to AE was measured over 365 days from the date of sputum collection in all patients. B) Stable patients with an FEV1 >40% of predicted were separated into SPLUNC1-high and SPLUNC1-low cohorts as above. Time to exacerbation was measured for up to one year from the date of sputum collection. Mantel-Haenszel estimator was used to calculate exacerbation-free interval in each group. Values are not adjusted for clinical variables. Adjusted values from Cox proportional hazards model are presented in Figure 4. AE: CF exacerbation, HR: Hazard ratio, CI: Confidence interval.
Supplemental Figure 6. Sputum Cytokines Do Not Predict Short Term AE-Free Time. A) AE-Free time in Stable CF patients separated into IL-1β-, TNFα-, G-CSF-, IL-6-, and IL-8-High and -Low groups over a 60-day follow up period. Marker-high and –low groups were defined according to sputum concentration thresholds obtained from receiver-operator curves separating CF Stable and AE levels (Supplemental Figure 4). AE-Free time was defined as the number of days from sputum collection in Stable patients until the date of their next AE. B) AE-Free time in Stable CF patients separated into IL-1β-, TNFα-, G-CSF-, IL-6-, and IL-8-High and -Low groups over a 365-day follow up period. Cox Proportional Hazards model used to calculate AE-free intervals and adjust for age, sex, BMI, FEV1, number of F508del mutations, presence of CF-related diabetes or pancreatic insufficiency, use of CFTR correctors/modulators, and microbiology for P. aeruginosa, A. xylosoxidans, H. parainfluenzae, Methicillin-sensitive S. aureus, and Methicillin-resistant S. aureus. HR: Hazard ration; SE: Standard error.
Supplemental Figure 7. SPLUNC1 Negatively Correlates with IL-1β in Stable and AE states. Pearson correlation demonstrates an inverse relationship between IL-1β and SPLUNC1 levels in Stable (left) and AE (right) states. Sputum samples from two cohorts including adult (Yale University, Stable n=30, AE n=14) and mixed adult/pediatric (University of Minnesota, Stable n=33, AE n=32) CF participants. SPLUNC1 and IL-1β quantified by ELISA, values are square-root-transformed for analysis. r=correlation coefficient. All four correlations have a p <0.05.
Supplemental Figure 8. SPLUNC1 Western Blot does not distinguish disease states but identifies SPLUNC1 fragments. Full unedited WB showing SPLUNC1 in sputum from study participants. Sputum supernatants were loaded onto 4–15% Bis-Tris (top) or 16% Tricine gels (bottom, for improved SPLUNC1 fragment identification). Samples were electrophoresed at 130 volts. Membranes were initially probed for NE prior to stripping and re-probing for SPLUNC1. SPLUNC1 antibody: goat polyclonal hPLUNC1 antibody raised against residues Q20 - V256 of hPLUNC1 (1:3000, R&D systems), with a secondary anti-goat IgG-HRP. ST: Stable CF; AE: CF exacerbation; Black arrowheads: SPLUNC1, Red arrowheads: SPLUNC1 fragments.
Supplemental Figure 9. Full unedited Western Blots for figure 5D. Membranes were initially probed for NE prior to stripping and re-probing for SPLUNC1. A) NE antibody: Mouse monoclonal anti-hELA2 raised against residues M1–N252 (1:3000, R&D systems). B) SPLUNC1 antibody: goat polyclonal hPLUNC1 antibody raised against residues Q20 - V256 of hPLUNC1 (1:3000, R&D systems), a secondary anti-goat HRP. HC: Healthy Control; ST: Stable CF; AE: CF exacerbation; m: marker; +ctl: recombinant protein positive control.


