Abstract
Introduction
We developed a new treatment method that combines tympanoplasty with transplantation of autologous cultured nasal mucosal epithelial cell sheets to regenerate the mucosa of patients with adhesive otitis media, which has been difficult to treat effectively. We verified whether this procedure could be performed safely and measured its therapeutic efficacy.
Methods
Autologous nasal mucosal epithelial cell sheets were manufactured at a good manufacturing practice-compliant cell processing facility using autologous nasal mucosal tissue. We performed tympanoplasty and transplanted the cell sheets into the middle ear cavity in six patients with adhesive otitis media.
Results
The manufactured autologous cultured epithelial cell sheets met the predetermined quality standards and were successfully transplanted safely in all cases. Computed tomography findings after cell sheet transplantation showed that aeration in the tympanic cavity was maintained or restored in five of the six patients (83.3%). Four of the six (66.7%) patients had postoperative air-bone gap within 20 dB, which is considered a postoperative success in tympanoplasty for chronic middle ear disease.
Conclusions
The results of this clinical study suggest that tympanoplasty with cell sheet transplantation can be used to treat adhesive otitis media by reliably preventing re-adhesion of the tympanic membrane.
Keywords: Cell sheet, Cell sheet transplantation, Mucosal regeneration, Adhesion, Tympanoplasty, Adhesive otitis media
1. Introduction
Adhesive otitis media is a condition in which the tympanic membrane is depressed and adheres to the fibrous scar tissue caused by chronic inflammation in the inner wall of the middle ear cavity, resulting in the loss of aeration in the tympanic cavity. As one of the most common forms of intractable otitis media, adhesive otitis media is the most difficult to treat because there is no way to simply release and maintain the adhesions of the tympanic membrane to date. Although there have been many attempts to prevent re-adhesion of the tympanic membrane after surgery, it is extremely difficult to prevent this re-adhesion and improve and maintain hearing [[1], [2], [3]].
We hypothesized that by regenerating the damaged middle ear mucosa and reducing chronic inflammation in the early postoperative period, we could prevent re-adhesion of the tympanic membrane in adhesive otitis media. We have developed a new technique for middle ear surgery that involves the transplantation of autologous cultured nasal mucosal epithelial cell sheets to regenerate the middle ear mucosa after surgery (Fig. 1). Previously, we reported a new regenerative therapy called cell sheet transplantation in patients with cholesteatoma, with no adverse events or complications. In the study, we confirmed that cell sheet transplantation into the middle ear, especially the mastoid, facilitated mucosa regeneration and restored aeration of the mastoid [4]. Here, we present the clinical results of this new therapy, optimized particularly for patients with adhesive otitis media. In this study, we examined the effect of cell sheet transplantation on the prevention of tympanic membrane adhesions and increased aeration of the tympanic cavity in patients with adhesive otitis media.
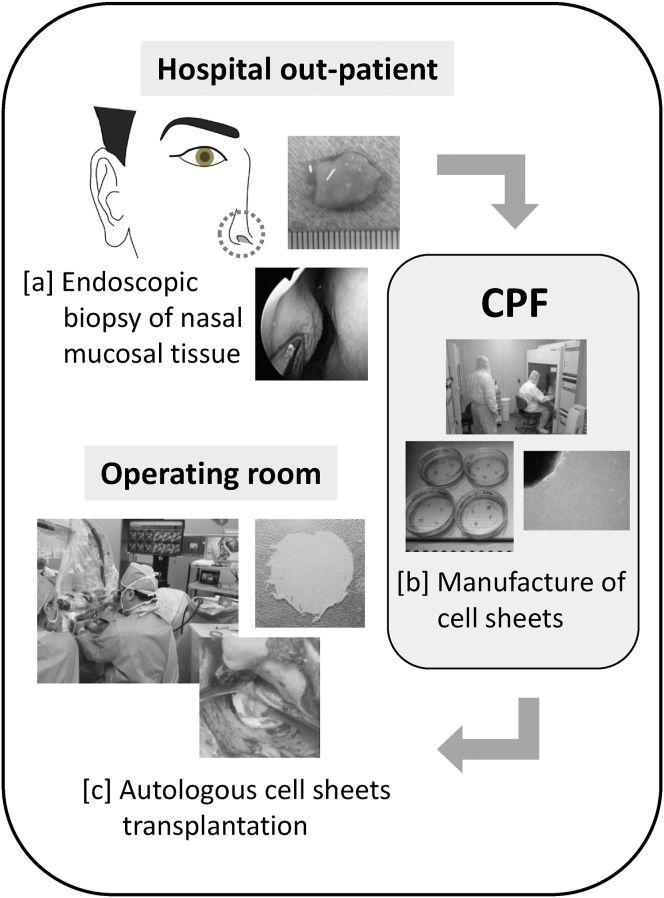
Fig. 1.
Scheme of the present clinical study: Transplantation of autologous cell sheets produced from nasal mucosa into the middle ear. Inferior nasal turbinate mucosa tissue of approximately 10 × 10 mm in size was collected by endoscopy in the outpatient department of the hospital. The harvested nasal mucosa was immediately transported to the cell processing facility (CPF). Autologous human nasal epithelial cell sheets are prepared by aseptic manipulation in the CPF. The cell sheets manufactured were transported to the operating room and transplanted.
This study was one of a series of regenerative medicine projects in which cultured cells were transplanted into the human middle ear for the first time. This is one of the few reports of human subject research for cell transplantation therapy in the field of otorhinolaryngology.
2. Methods
2.1. Subjects
Patient profiles are shown in Table 1. The clinical subjects were six patients (five males, one female) diagnosed with adhesive otitis media between May 2015 and January 2019. The age of the patients at the time of transplantation ranged from 38 to 62 (mean 45.3) years, and the observation period after transplantation ranged from 9 to 36 (mean 16.3) months. Two patients (cases 2 and 4) had a history of tympanoplasty, whereas tympanoplasty performed in the study was the first for the other four patients.
Table 1.
Summary of the patients with adhesive otitis media treated with cell sheet transplantation.
| Patient No. | Age/Gender | Affected side | Adhesion | Type of surgery | Preoperative hearing level (dB) | Site of cell sheet transplantation |
Type of ossiculoplasty | |
|---|---|---|---|---|---|---|---|---|
| Backside of cartilage | Tympanic cavity | |||||||
| 1 | 52 IF | Right | Total | Initial | 61.7 | + | + | Columella on the footplate |
| 2 | 62/M | Right | Total | Revision | 61.3 | + | + | Columella on the footplate |
| 3 | 38/M | Right | Partial | Initial | 40.0 | + | - | Columella on the stapes |
| 4 | 40 /M | Right | Partial | Revision | 58.8 | + | - | Columella on the stapes |
| 5 | 38/M | Left | Partial | Initial | 47.5 | + | - | Columella on the stapes |
| 6 | 42/M | Right | Partial | Initial | 42.5 | + | - | Columella on the stapes |
The primary end points of the study were safety of this new treatment (occurrence of adverse effects and abnormal changes in blood test values, 1 month after transplantation) and efficacy of the new treatment based on postoperative tympanic membrane assessment, computed tomography (CT) results, and pure tone audiometry at least 6 months after transplantation. Postoperative hearing improvement was determined by assessing hearing levels at least 6 months after surgery, based on the American Academy of Otolaryngology-Head and Neck Surgery guidelines [5]. Pure tone averages were calculated using the average responses to four-tones (0.5, 1, 2, and 3 kHz) as recommended by the American Academy of Otolaryngology.
In accordance with the “Guidelines for Clinical Research Using Human Stem Cells,” we submitted a protocol for review by the Ministry of Health, Labor, and Welfare Health Policy Bureau and received official approval (no. 0719:5) on July 19, 2013. Moreover, this clinical study was conducted in accordance with the relevant guidelines and approved by the Certified Committee for Regenerative Medicine at St. Marianna University School of Medicine and Kanto-Shinetsu Regional Bureau of MHLW (no. PB3170011).
2.2. Preparation of nasal mucosal epithelial cell sheets
All cell sheets were manufactured in a good manufacturing practice-compliant cell-processing facility (CPF) located at Jikei University School of Medicine. Inferior turbinate mucosa sample was collected by endoscopy from each patients included in this study in the outpatient department of the hospital. The submucosa of the inferior turbinate was anesthetized with xylocaine (AstraZeneca, Osaka, Japan) to retrieve 10 × 10-mm specimen (Fig. 1a), and then the collected tissue was placed in a transport solution and transported to a CPF. At the CPF, epithelial tissues were collected using a surgical knife and cultured in keratinocyte culture medium (KCM) [4,6] supplemented with autologous serum for approximately 14 d (explant culture). The KCM consisted of Dulbecco's modified Eagle's medium (DMEM; Gibco, Thermo Fisher, MA, USA) and DMEM with Ham's F-12 medium (Gibco) containing 0.3 mM saxizon (Takeda Yakuhin Kogyo, Osaka, Japan), 140.0 mU/mL insulin (Humulin, Novo Nordisk, Bagsvaerd, Denmark), 2.0 nM triiodothyronine (MP Biomedicals, CA, USA), 0.2 mM epidermal growth factor (Higeta-Shoyu, Tokyo, Japan), 1.0 nM cholera toxin (Wako Pure Chemicals, Osaka, Japan), 100 U/mL penicillin, 69 mM streptomycin (Wako Pure Chemicals), and 0.4 mg/mL amphotericin B (Bristol-Myers Squibb, NY, USA), at a ratio of 1:1. After primary culture, the obtained epithelial cells were seeded on a temperature-responsive culture dish and cultured in KCM supplemented with autologous serum for approximately 12 d (passage culture) to produce cultured nasal mucosal epithelial cell sheets (Fig. 1b).
We performed sterility, mycoplasma, viral (HBV, HCV, HIV, HTLV-1), syphilis, endotoxin, and Mycoplasma pneumoniae DNA quantification tests using culture supernatant. In addition, we observed cell proliferation under a microscope and confirmed the condition of the cultured epithelial sheets based on cell morphology and stratification. Furthermore, we conducted a detachment test using cultured epithelial sheets on the day before scheduled shipping to determine the number of cells, cell viability, and cell purity (the pan-cytokeratin-positive cell rate measured by flow cytometry [MACSQuant® Analyzer, Miltenyi Biotec, Bergisch Gladbach, Germany]).
The results of various quality control tests were meticulously checked before the scheduled day of transplantation to ensure that the transplanted cell sheets met predetermined criteria. The cell sheets manufactured in the CPF were then transported to the operating room and transplanted (Fig. 1c).
2.3. Tympanoplasty with cell sheet transplantation
In middle ear surgeries, the first step is typically to create and elevate the tympanomeatal flap. Adhesions to the ossicles or promontory are removed, and the ossicular chain is examined.
For all cases in this study, the cell sheet was combined with cartilage to form a hybrid tympanic membrane. The cell sheet was placed on the harvested auricular cartilage fragments used to reconstruct the tympanic membrane (Fig. 2a and b). The cartilage was combined with the cell sheet to reconstruct the tympanic membrane (Fig. 2c). To prevent readhesion of the tympanic membrane, the cell sheet was transplanted on the reverse side of the newly formed tympanic membrane.
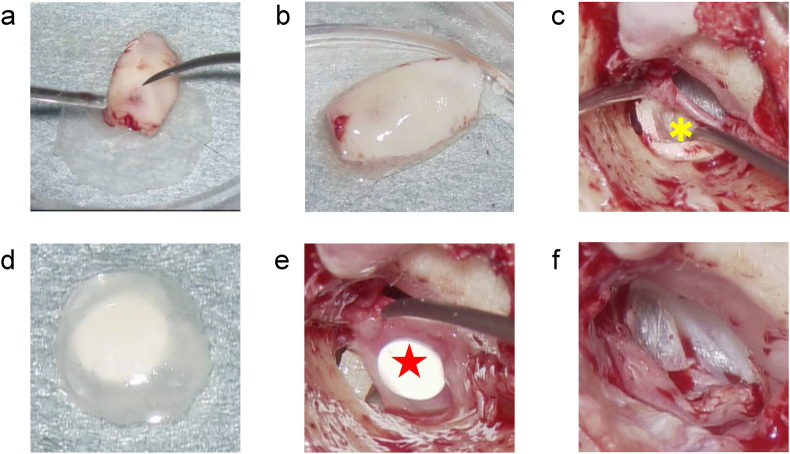
Fig. 2.
Step-wise procedure of cell sheet transplantation for right adhesive otitis media. (a) Using a surgical needle under a microscope, the cell sheet was placed to cover the harvested auricular cartilage fragments used to reconstruct the tympanic membrane. (b) The cartilage was combined with the cell sheet for reconstructing the tympanic membrane. (c) The tympanic membrane was reconstructed with cartilage that was laminated with the cell sheet. The tympanic membrane was reconstructed by inserting the cartilage so that the surface of the cell sheet was facing the tympanic cavity. Yellow (∗) is the cartilage with cell sheet aligned. (d) For cell sheet transplantation onto the bony surface of the tympanic cavity, the cell sheet was placed on a silicon plate that acted as a support. (e) Red (★) is a cell sheet placed on the silicon plate. The cell sheet is placed on the inner surface of the silicon plate. The silicon plate is used as a support to carry the cell sheet into the tympanic cavity. The cell sheet is released from the silicon plate, and only the silicon plate is removed, thereby successfully transplanting the cell sheet into the tympanic cavity. (f) The tympanomeatal flap was returned to the original position, and the cell sheet transplantation into the middle ear was complete.
After carefully observing the tympanic cavity, the cell sheet was transplanted into areas of the cavity where the normal mucosa was missing. A silicone plate was used as a carrier to deliver the cell sheet into the tympanic cavity (Fig. 2d and e). The cell sheets were transplanted onto the exposed bony surface of the tympanic cavity where the mucosa had eroded. With regard to the method of ossicular reconstruction, the columella was attached to the stapes (reconstruction between tympanic membrane and stapes head) in cases with intact superstructure of the stapes, and the columella was attached to the foot plate (reconstruction between tympanic membrane and stapes footplate) in cases with missing superstructure of the stapes. To complete cell sheet transplantation into the middle ear, the tympanomeatal flap was returned to its original position (Fig. 2f).
3. Results
3.1. Characterization of nasal mucosal epithelial cell sheets
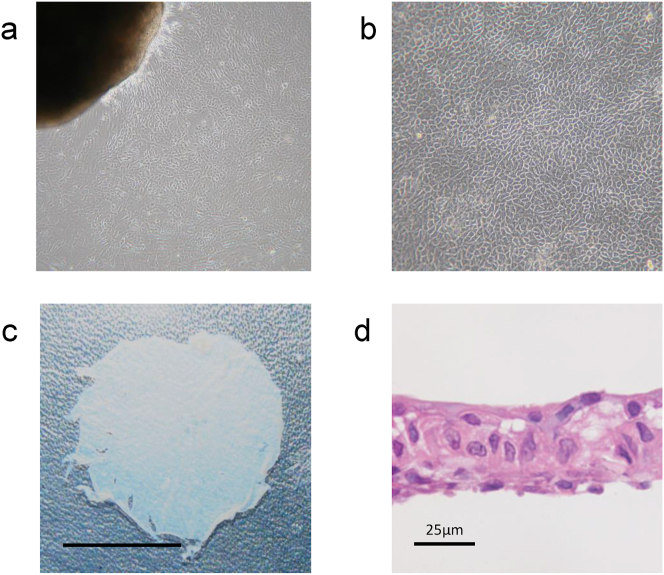
In the explant culture, epithelial cells were observed to proliferate from the margins of the tissue fragments starting approximately on day 5 (Fig. 3a). In the passage culture, epithelial cells continued to proliferate stably on the temperature-responsive cell culture dish and maintained a polygonal cobblestone-like morphology (Fig. 3b). Cells grown on the temperature-responsive cell culture dish could be collected in sheets (Fig. 3c). Fig. 3d shows cell sheets fixed in 10% neutral buffered formalin, embedded in 3-μm-thick paraffin sections, stained with hematoxylin and eosin, and confirmed to have a structure consisting of multiple epithelial cell layers.
Fig. 3.
Cultivation of nasal mucosal epithelial cells and manufactured cell sheet. (a) In explant culture, epithelial cells were observed to proliferate from the tissue fragments. (b) It was observed that the epithelial cells stably proliferated on the temperature-responsive culture dish while maintaining the cobblestone-like morphology even in the passage culture. (c) After 12 d of culture on a temperature-responsive culture dish, the cultured cells could be harvested as one continuous sheet by lowering the culture temperature. Scale bar = 5 mm. (d) The cell sheet to be transplanted was confirmed to be a layered structure consisting of epithelial cells. Scale bar = 25 μm.
In quality tests using cell sheet culture supernatants before transplantation, all manufactured cell sheets satisfied the predetermined standard values. All manufactured autologous epithelial cell sheets met the predetermined quality criteria (Table 2).
Table 2.
Results of the quality control tests of the cell sheets.
| Patient number |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Cell number (cells/sheet) | 1.2×105 | 1.3×105 | 4.6×105 | 2.3×105 | 1.5×105 | 7.1×105 |
| Cell survival rate (average %) | 92.3% | 88.9% | 98.3% | 97.4% | 93.9% | 97.6% |
| PCK-positive cells (%) | 94.2% | 92.0% | 97.8% | 97.9% | 90.5% | 94.9% |
| Mycoplasma | Negative | Negative | Negative | Negative | Negative | Negative |
| Endotoxin (EU/ml) | 3.97 | 0.16 | 0.08 | 0.01 | 0.06 | 0.2 |
| Bacteria and fungi | Negative | Negative | Negative | Negative | Negative | Negative |
| Virus (HBV, HCV, HIV-1, HTLV-1 ) | Negative | Negative | Negative | Negative | Negative | Negative |
3.2. Clinical results of cell sheet transplantation for patients with adhesive otitis media
In all cases, the cell sheets were successfully and safely transplanted. A summary of the postoperative course is shown in Table 3. The mean hearing levels of the patients in this study were 52.0 (preoperative) and 34.5 dB (postoperative). The postoperative air-bone gap (A-B gap) was within 10 dB for one ear, 11–20 dB for three ears, and >31 dB for two ears. The number of patients with postoperative A-B gap within 20 dB, which is considered postoperative success in tympanoplasty for chronic middle ear disease, was four out of six (66.7%).
Table 3.
Summary of postoperative results of patients with adhesive otitis media who were treated with cell sheet transplantation.
| Patient No. | Postoperative aeration of tympanic cavity | Postoperative hearing level (dB) | Postoperative air-bone gap (dB) | Complications | Months of follow-up |
|---|---|---|---|---|---|
| 1 | - | 56.7 | 38.7 | None | 12 |
| 2 | + | 57.5 | 34.2 | None | 27 |
| 3 | + | 27.5 | 16.3 | None | 8 |
| 4 | + | 22.5 | 15.0 | None | 20 |
| 5 | + | 25.0 | 16.3 | None | 19 |
| 6 | + | 17.5 | 7.5 | None | 12 |
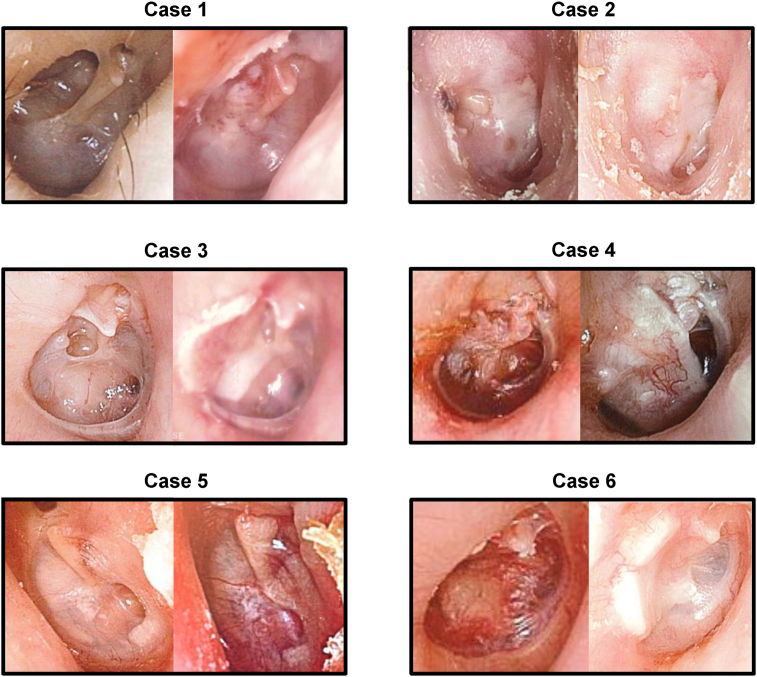
Photographs of preoperative and postoperative tympanic membranes are shown in Fig. 4. For each case, the left panel shows the findings of the preoperative tympanic membrane and the right panel shows the postoperative findings.
Fig. 4.
Tympanic membrane findings after cell sheet transplantation in all cases. For each case, the left and right panels show the morphological findings of the tympanic membrane before and after cell sheet transplantation, respectively. All these tympanic membrane findings are noted after the reconstruction of the tympanic membrane with cartilage.
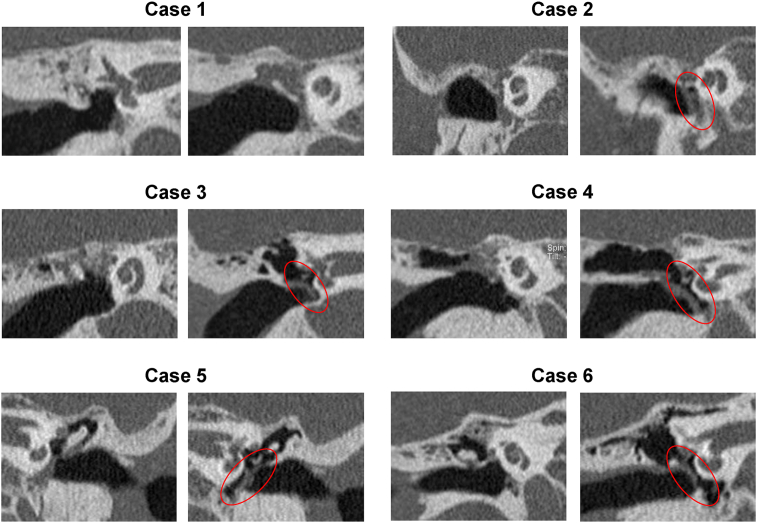
The preoperative and postoperative CT images of all cases are shown in Fig. 5. All the images are coronal views, and, for each case, the left and right panels show scans from before and after cell sheet transplantation, respectively. In all cases, preoperative CT showed adherent tympanic membranes and loss of tympanic cavity aeration. Postoperative CT showed that aeration in the tympanic cavity was maintained or restored in five of the six cases (83.3%). Except in Case 1, aeration was observed in all tympanic cavities (the area circled in red) after cell sheet transplantation.
Fig. 5.
Computed tomography (CT) findings after cell sheet transplantation in all the cases. All temporal bone CT findings are observed in a coronal view, and for each case, the left panel indicates the view before, whereas the right panel indicates the view after cell sheet transplantation. In all the cases, preoperative CT showed that the tympanic membrane was adherent and the aeration in the tympanic cavity was lost. In five out of six cases, aeration was observed in the tympanic cavity (the area circled in red) after cell sheet transplantation.
3.3. Adverse events and complications
The patients were observed after tissue harvest to create cell sheets and after cell sheet transplantation. After hemostasis was achieved, no rebleeding occurred in sites where the nasal mucosa was harvested, and harvest sites showed good mucosal epithelialization. None of the patients showed abnormal changes in blood test values after transplantation, and no adverse events or complications were observed.
4. Discussion
Indications for surgery for adhesive otitis media include stopping otorrhea, preventing complications (inner ear damage, migration to cholesteatoma), and improving hearing for conductive hearing loss. However, with surgeries for adhesive otitis media, the bony surface of the middle ear is often exposed when the epithelium is detached and removed. Even after detaching the adhesions, the middle ear mucosa is difficult to preserve owing to the presence of fibrous scar tissue caused by chronic inflammation that leads to the adhesions. Furthermore, postoperative mucosal regeneration cannot be expected, resulting in a high rate of re-adhesion of the tympanic membrane after surgery. As a result, postoperative hearing improvement in cases of adhesive otitis media is considerably less than that in cases with other middle ear diseases. Therefore, surgery is seldom recommended. Although there have been some innovations in surgery for adhesive otitis media, even with short-term improvement after surgery, the tympanic membrane almost always re-adheres after a long period. Although there have been many attempts to prevent re-adhesion of the tympanic membrane after surgery [[1], [2], [3]], it is extremely difficult to prevent re-adhesion and improve hearing. Currently, there is no way to simply release the tympanic membrane from adhesions and maintain this status.
We hypothesized that if the damaged middle ear mucosa can be regenerated after surgery at an early stage, re-adhesion of the tympanic membrane in adhesive otitis media can be prevented. Regenerative medicine using cultured cells has attracted attention in recent years. With the aim to regenerate damaged middle ear mucosa soon after surgery, we previously created three-dimensional artificial middle ear mucosa using cultured cells and transplanted it into a rabbit model of middle ear mucosal damage [7,8]. Subsequently, with the rapid progress of regenerative medicine using cell sheet engineering with temperature-responsive culture dishes [[9], [10], [11]], we focused on the application of this cell sheet technology to the middle ear [12]. It is difficult to collect the middle ear mucosa with minimal invasion as a cell source for cell sheet fabrication, and patients with chronic otitis media, such as adhesive otitis media, often have bilateral lesions. Therefore, we focused on the nasal mucosa as a source for cell sheet production, which can be collected in a minimally invasive outpatient procedure and is anatomically continuous and histologically similar to the middle ear mucosa. Inferior nasal turbinate mucosa was selected because it is the safest to collect clinically and is suitable for culture [13].
With this rationale, we developed an autologous cultured nasal mucosal epithelial cell sheet for early mucosal regeneration on the exposed middle ear bony surface after surgery. As a preclinical study, we transplanted cultured nasal mucosal epithelial cell sheets into a model of middle ear mucosal damage using domestic rabbits and found that these sheets were effective in regenerating the middle ear mucosa [14]. We also succeeded in producing nasal mucosal cell sheets in humans [6,13] and performed cell sheet transplantation in five patients with chronic otitis media in a previous clinical study, with no adverse events in any of the cases [4].
In this clinical study, none of the patients showed adverse effects or events after cell sheet transplantation. The CT findings after cell sheet transplantation showed that aeration in the tympanic cavity was maintained or restored in 83.3% of the cases. Additionally, 66.7% of the cases had a postoperative A-B gap of 20 dB or less, which is considered a postoperative success in tympanoplasty for chronic middle ear disease. These results are promising, considering that the surgical outcome of patients with adhesive otitis media is so poor that surgery itself is rarely performed by any institution. Based on our finding that tympanic cavities recovered a high percentage of aeration after cell sheet transplantation, we speculate that the cell sheets may have a regenerative effect on the mucosa as well as suppress the inflammatory scar tissue that causes re-adhesions after adhesion detachment. It is also considered that one of the major causes of most intractable otitis media, including adhesive otitis media, is the impairment of the gas exchange function of the middle ear. We also considered the possibility that the regenerated middle ear mucosa could restore the gas exchange function and contribute to the maintenance of eustachian tube function. In order to completely cure intractable otitis media, it would be more ideal if the middle ear mucosa could be regenerated to restore the gas exchange function and maintain the eustachian tube function. We speculated that the gas entering the middle ear cavity through the regenerated middle ear mucosa might release the negative pressure in the middle ear cavity and reduce the burden on the eustachian tube.
In Case 1, the tympanic membrane after cell sheet transplantation was considered morphologically improved relative to the membrane pretransplantation; however, aeration in the tympanic cavity was not restored. In addition to severe tympanic membrane adhesion in Case 1, the cell sheet may have been insufficiently effective because the transplantation technique is in an early stage of clinical research. Even if adequate cell sheets are manufactured, the cell sheets will not be effective unless they can be transplanted to the appropriate site. In contrast, although Case 2 had a history of multiple surgeries, the tympanic membrane was fully adhesive and its lesions were severe, and a satisfactory improvement in hearing was not observed after transplantation, The CT results showed morphological improvement as the tympanic cavity contained a small amount of air after cell sheet transplantation, which partially prevented re-adhesion of the tympanic membrane.
In the case of mild tympanic membrane adhesions, we found that cell sheet transplantation for the mucosal defect alone was sufficient if a part of the normal mucosa could be preserved. Therefore, it is necessary to carefully check the margin between the normal mucosa and diseased tissue, detach the adhesion, and remove only the lesion.
By improving the cell sheet and verifying the effect of cell sheet transplantation, we believe that we will be able to prevent re-adhesion of the tympanic membrane associated with mucosal regeneration even in cases with severe tympanic membrane adhesion, such as Cases 1 and 2. A previous study showed that cell sheets are effective for early middle ear mucosal regeneration [4], and we believe that further analysis of the site of cell sheet transplantation (based on pathological condition) and effectiveness of the therapy using an adhesive otitis media model will enable improvements in the therapeutic effects of preventing re-adhesion of the tympanic membrane associated with mucosal regeneration. Because surgery is not currently recommended for most patients with adhesive otitis media, the results of this study mark great progress toward developing an effective therapy with sustained improvements for these cases.
Although previous studies have suggested the efficacy of cell sheet transplantation into the middle ear, the fate and mechanism of action of the cell sheets after transplantation have remained unresolved. It is difficult to elucidate how the cultured nasal mucosal cells will change depending on the surrounding environment after transplantation; however, it is possible that the cell sheets themselves will promote regeneration and induce cells from the surrounding environment through the paracrine effect. The paracrine effect of cell sheets has been recognized in other fields [[15], [16], [17]], and past animal experiments suggest that cell sheets may survive for approximately 8 weeks after transplantation [14]. We believe that it is necessary to revisit basic research to improve efficacy and understand post-transplantation cell dynamics to optimize this technique for regenerative medicine.
In summary, combining autologous cultured nasal mucosal epithelial cell sheet transplantation technology and tympanoplasty offers an effective treatment for adhesive otitis media. In addition, because adhesions between tissues were prevented in this study and both mucosal regeneration and inflammation suppression may have affected this, this technique has potential to be applied in various ways to other parts of the body.
5. Conclusions
The results of this study suggest the possibility of a new treatment method for adhesive otitis media, which is one of the most difficult middle ear diseases to cure. We believe that our results are also promising more broadly, as they provide new insights for potential applications to prevent adhesions in other parts of the body. Clinical applications for regenerative medicine using cell sheet technology have been made in various areas [[18], [19], [20], [21], [22], [23]]. However, regenerative medicine using human somatic cells is lagging in the field of otorhinolaryngology. This is one of the few reports of cell transplantation therapy in the field of otorhinolaryngology. We hope to revitalize regenerative medicine research in this field by promoting this cell sheet therapy as a building block. As there are limitations in verifying the efficacy of this therapy only through clinical research, we are planning to conduct clinical trials in the future. We also aim to commercialize the nasal mucosal epithelial cell sheet and establish its use in new surgical methods that can prevent re-adhesion of tympanic membranes. Finally, we hope that the proposed therapy will become a new treatment method for patients suffering from recurrence and prolongation of intractable adhesive otitis media.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
K.Y. and T.M. conceptualized and designed the study. All authors researched, collated, and wrote this paper.
Declaration of competing interest
Masayuki Yamato is an equity holder of CellSeed Inc., and Tokyo Women's Medical University currently receives research funding from CellSeed, Inc. Masayuki Yamato is also an advisor for commercial efforts at Helios and NIPPI (Japan).
Acknowledgments
The authors thank Kanako Taya and Yuka Izawa (Department of Otorhinolaryngology, Jikei University School of Medicine, Tokyo) for assistance in fabricating cell sheets and proofreading the manuscript. This work was supported by the Japan Society for the Promotion of Science (KAKENHI Grant Numbers 16K11198, 18K09332, 18K09357, 18K16907, 19K18785 and 19H03806), Japan Agency for Medical Research and Development (Grant numbers 18bk0104051 and 19bk0104086) and Jikei University Strategic Prioritizing.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Suzuki J., Yanagihara N., Kadera K. The partially implantable middle ear implant, case reports. Adv Oto-Rhino-Laryngol. 1987;37:178–184. doi: 10.1159/000414138. [DOI] [PubMed] [Google Scholar]
- 2.McGhee M.A., Dornhoffer J.L. The effect of gelfilm in the prevention of fibrosis in the middle ear of the animal model. Am J Otol. 1999;20:712–716. [PubMed] [Google Scholar]
- 3.Ng M., Linthicum F.H., Jr. Long-term effects of Silastic sheeting in the middle ear. Laryngoscope. 1992;102:1097–1102. doi: 10.1288/00005537-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto K., Yamato M., Morino T., Sugiyama H., Takagi R., Yaguchi Y. Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. NPJ Regen Med. 2017;2:6. doi: 10.1038/s41536-017-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc Committee on hearing and equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. Otolaryngol Head Neck Surg. 1995;113:186–187. doi: 10.1016/S0194-5998(95)70103-6. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto K., Morino T., Kasai Y., Komori M., Yamato M., Kojima H. Preclinical assessment of transplantable human nasal mucosal epithelial cell sheets. Regen Ther. 2021;18:59–65. doi: 10.1016/j.reth.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Wada K., Tanaka Y., Kojima H., Inamatsu M., Yoshizato K., Moriyama H. In vitro reconstruction of a three-dimensional middle ear mucosal organ and its in vivo transplantation. Acta Otolaryngol. 2006;126:801–810. doi: 10.1080/00016480500507385. [DOI] [PubMed] [Google Scholar]
- 8.Yaguchi Y., Wada K., Uchimizu H., Tanaka Y., Kojima H., Moriyama H. Middle ear mucosa regeneration by grafting of artificial mucosa. Acta Otolaryngol. 2007;127:1038–1044. doi: 10.1080/00016480701200285. [DOI] [PubMed] [Google Scholar]
- 9.Okano T., Yamada N., Sakai H., Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly (N-isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 10.Yamato M., Utsumi M., Kushida A., Konno C., Kikuchi A., Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7:473–480. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- 11.Yamato M., Sekine H., Yang J., Sekiya S., Haraguchi Y., Shimizu T. Cell sheet engineering for regenerative medicine: from the viewpoint of inflammation. Inflamm Regen. 2007;27:156–164. doi: 10.2492/inflammregen.27.156. [DOI] [Google Scholar]
- 12.Yaguchi Y., Murakami D., Yamato M., Hama T., Yamamoto K., Kojima H. Middle ear mucosal regeneration with three-dimensionally tissue-engineered autologous middle ear cell sheets in rabbit model. J Tissue Eng Regen Med. 2016;10:E188–E194. doi: 10.1002/term.1790. [DOI] [PubMed] [Google Scholar]
- 13.Hama T., Yamamoto K., Yaguchi Y., Murakami D., Sasaki H., Yamato M. Autologous human nasal epithelial cell sheet using temperature-responsive culture insert for transplantation after middle ear surgery. J Tissue Eng Regen Med. 2017;11:1089–1096. doi: 10.1002/term.2012. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K., Hama T., Yamato M., Uchimizu H., Sugiyama H., Takagi R. The effect of transplantation of nasal mucosal epithelial cell sheets after middle ear surgery in a rabbit model. Biomaterials. 2015;42:87–93. doi: 10.1016/j.biomaterials.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Narita T., Shintani Y., Ikebe C., Kaneko M., Campbell N.G., Coppen S.R. The use of scaffold-free cell sheet technique to refine mesenchymal stromal cell-based therapy for heart failure. Mol Ther. 2013;21:860–867. doi: 10.1038/mt.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shudo Y., Miyagawa S., Nakatani S., Fukushima S., Sakaguchi T., Saito A. Myocardial layer-specific effect of myoblast cell-sheet implantation evaluated by tissue strain imaging. Circ J. 2013;77:1063–1072. doi: 10.1253/circj.cj-12-0615. [DOI] [PubMed] [Google Scholar]
- 17.Umezawa T., Higa K., Serikawa M., Yamamoto M., Matsunaga S., Shimazaki J. Proliferative activity of skeletal myoblast sheet by paracrine effects of mesenchymal stem cells. J Oral Biosci. 2016;58:158–166. doi: 10.1016/j.job.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 19.Ohki T., Yamato M., Ota M., Takagi R., Murakami D., Kondo M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588. doi: 10.1053/j.gastro.2012.04.050. e2. [DOI] [PubMed] [Google Scholar]
- 20.Sawa Y., Miyagawa S., Sakaguchi T., Fujita T., Matsuyama A., Saito A. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today. 2012;42:181–184. doi: 10.1007/s00595-011-0106-4. [DOI] [PubMed] [Google Scholar]
- 21.Sato M., Yamato M., Mitani G., Takagaki T., Hamahashi K., Nakamura Y. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen Med. 2019;4:4. doi: 10.1038/s41536-019-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanzaki M., Takagi R., Washio K., Kokubo M., Yamato M. Bio-artificial pleura using an autologous dermal fibroblast sheet. NPJ Regen Med. 2017;2:26. doi: 10.1038/s41536-017-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanzaki M., Takagi R., Washio K., Kokubo M., Mitsuboshi S., Isaka T. Bio-artificial pleura using autologous dermal fibroblast sheets to mitigate air leaks during thoracoscopic lung resection. NPJ Regen Med. 2021;6:2. doi: 10.1038/s41536-020-00113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.