Abstract
Diabetes mellitus (DM) is a public health problem in developing as well as developed nations. DM leads to many complications that are associated with higher morbidity and mortality worldwide. Therefore, the current study was planned to assess the prevalence and risk factors of type-2 DM in Ethiopian population. Six electronic databases such as: PubMed, Scopus, Hinari, Web of science, Google Scholar, and African Journals Online were searched for studies published in English up December 30, 2020. Newcastle–Ottawa Scale was used for quality assessment of the included studies. The data was extracted by Microsoft excel and analyzed through Stata version 16 software. The random effect meta-regression analysis was computed at 95% CI to assess the pooled prevalence and risk factors of type-2 DM. Forty observational studies were included in this systematic review and meta-analysis. The pooled prevalence of DM in Ethiopia was 6.5% (95% CI (5.8, 7.3)). The sub-group analysis revealed that the highest prevalence of DM was found in Dire Dawa city administration (14%), and the lowest prevalence was observed in Tigray region (2%). The pooled prevalence of DM was higher (8%) in studies conducted in health facility. Factors like: Age ≥ 40 years ((Adjusted Odds Ratio (AOR): 1.91 (95% CI: 1.05, 3.49)), Illiterate (AOR: 2.74 (95% CI: 1.18, 6.34)), Cigarette smoking (AOR: 1.97 (95% CI: 1.17, 3.32)), Body mass index (BMI) ≥ 25 kg/m2 (AOR: 2.01 (95 CI: 1.46, 2.27)), family history of DM (AOR: 6.14 (95% CI: 2.80, 13.46)), history of hypertension (AOR: 3.00 (95% CI: 1.13, 7.95)) and physical inactivity (AOR: 5.79 (95% CI: 2.12, 15.77)) were significantly associated with type-2 DM in Ethiopian population. In this review, the prevalence of type-2 DM was high. Factors like: Older age, illiteracy, cigarette smoking, MBI ≥ 25, family history of DM, history of hypertension and physical inactivity were an identified risk factors of type-2 DM. Therefore, health education and promotion will be warranted. Further, large scale prospective studies will be recommended to address possible risk factors of type-2 DM in Ethiopian population.
Subject terms: Health care, Risk factors
Introduction
Diabetes mellitus (DM) is a category of metabolic disorders characterized by hyperglycemia, which occurs when the pancreas stops producing enough insulin or when the body cannot use it. Diabetes causes a slew of complications that are linked to increased morbidity and mortality1,2. Diabetic Mellitus (DM) was prevalent in 9.3% of the world's population3. The mortality rate due to diabetes mellitus decreased from 2000 to 2010, then increased from 2010 to 2016 in the developed world, while the mortality rate due to DM increased in low-income countries during both periods4. The rise in the prevalence and incidence of diabetes has been attributed to changes in urban habits, which have been linked to sedentary behaviour5. In the next two decades, countries in Sub-Saharan Africa are predicted to see a significant rise in diabetes patients6. The prevalence of diabetes in Sub-Sahara Africa was reported to be lower (3%) than the global prevalence (8.5%) in 2016. However, undiagnosed diabetes mellitus in Africa (66.7%) is almost two times higher than that of developed countries 37%7,8, which contributes to the higher burden of morbidity and mortality in Africa.
Currently, Ethiopia has been challenged by the growing magnitude of non-communicable diseases (NCDs) such as diabetes. In Ethiopia, national data on the prevalence and incidence of diabetes are lacking. However, patient’s attendances and admission rates due to diabetes mellitus are rising in hospitals. In the previous 2–3 decades, there has been observable lifestyle changes with significant population growth and urbanization, which are the main risk factors repeatedly reported. In Ethiopia, according to the national WHO steps survey of 2015, the prevalence of diabetes mellitus was 3.2%9. A few other studies in Ethiopia report the prevalence of diabetes mellitus ranges from 0.5 to 6.5%10,11.
Even though in Ethiopia, one systematic review was published by Bishu.et.al; on the prevalence of diabetes mellitus and reported 2–6.5% prevalence of DM12. The previous review did not deal with socio-demographic risk factors that have a high potential effect on the development of diabetes. The review was done based on both type-1 and type-2 DM. Furthermore, the previous review did not provide evidence on the overall prevalence of DM nationwide and did not exhaustively include potential socio-demographic and personal characteristics in investigating their research. Therefore, this systematic review and meta-analysis study was aimed to assess predictors for the prevalence of type-2 diabetes mellitus in Ethiopia. The review study included previously conducted researches with different and few covariates considered as risk factors. The results obtained in the current investigation would significantly contribute to health professionals in delivering health-related education, care provision and further necessary for policy implication.
Materials and methods
Review strategies and inclusion criteria
A systematic review and meta-analysis were conducted to assess the pooled prevalence and the risk factors of type-2 DM in Ethiopia. An electronic search was conducted to retrieve studies. Published articles of cross-sectional, case–control and cohort studies were included. Databases PubMed, Hinari, Scopus, web of science, Google Scholar, and African journal online search engine were used to access the journals and articles published up to December 30, 2020. The search used the following medical subject heading terms; prevalence, risk factors, type-2 diabetes mellitus (DM) and Ethiopia separately or in combination. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was utilized to conduct this review13.
Inclusion criteria
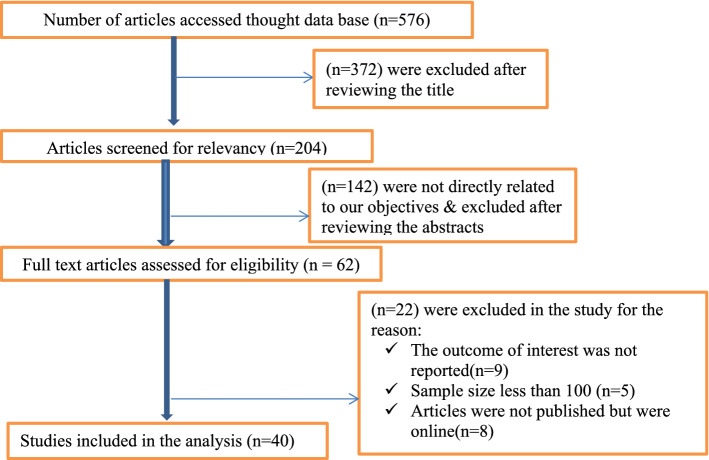
Studies conducted in Ethiopia about prevalence and predictors of type-2 diabetes mellitus with a sample size of at least 100 observations, those had been reported in English language, and articles published, and available online were included for this review study. Among many of the previously published articles, only 40 satisfied the selection criteria (Fig. 1).
Figure 1.
PRISMA flow diagram describing the selection of studies included in the systematic review and meta-analysis of prevalence and determinants of DM in Ethiopia.
Study selection and screening
All citations identified by our search strategy were exported to EndNote-X9, and duplicate articles were removed. And then, the titles and abstracts of the identified articles were screened by two independent reviewers (MAZ and AAM) and eligible studies were included for further review. The full texts of selected articles were retrieved and read thoroughly to ascertain their suitability before data extraction. In case of disagreement between the two reviewers, the discussion has been held to reach a consensus and the third reviewer as consulted. The search process was presented in the PRISMA flow chart that clearly indicates the studies included and excluded with reasons of exclusion (Fig. 1)13.
Definition of outcome measures
The primary outcome of the study was the prevalence and risk factors of DM such as sex, age, family history of DM, body mass index (BMI), education status, cholesterol level, hypertension and we developed data collection tools to collect essential data from the studies included.
Risk factor
A condition, behavior or other factor that increases a person's chances of developing a disease.
Debates mellitus
The red blood cells are separated from the sample, and the amount of glucose is measured in the remaining plasma. A plasma level of 7.8 mmol/l (200 mg/l) or greater can indicate diabetes.
Type 2 diabetes mellitus
One of the two major types of diabetes mellitus, characterized by late age of onset (30 years or older), insulin resistance, high levels of blood sugar, and little or no need for supplemental insulin.
Quality assessment for studies
The quality of meta-analysis depends on the quality of the studies included14. Two authors (MAZ and ET) had assessed the risk of bias for the studies included using the modified version Newcastle–Ottawa Scale (NOS) for cross-sectional studies15. All items of the tool were filled in for each included study with the response of yes or no, and the quality of the study was determined by summing the score given for each item which rated as low bias ≥ 7 points, moderate bias three up to six points and high bias ≤ 3 points (Table 1). To increase the validity of this review, we included studies with low and moderate risk of bias15.
Table 1.
Characteristics of the studies included and their prevalence of DM (N = 40).
| Authors | Publication year | Study area (region) | Study design | Statistical model | Sample size | Response rate (%) | Prevalence of DM (%) | SE (%) | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Alemayehu et al.16 | 2018 | Sidama | CS | OLR | 2670 | 100 | 1.9 | 0.26 | 8 points |
| Endris et al.17 | 2020 | SNNRP | CBCS | MVLR | 634 | 98.89 | 5.7 | 0.92 | 8 points |
| Toyba et al.18 | 2019 | Amhara | CBCS | MVL | 587 | 100 | 6.8 | 1.03 | 6 points |
| Shiferaw et al.19 | 2018 | Oromiya | CBCS | OLR | 402 | 100 | 6.5 | 1.23 | 8 points |
| Gizaw et al.20 | 2015 | AA | RS | Chi-square | 8048 | NS | 6.5 | 0.32 | 6 points |
| Belete et al.21 | 2019 | AA | CS | MVLR | 392 | 100 | 2.6 | 0.8 | 8 points |
| Tilahun et al.22 | 2007 | Oromiya | CS | OLR | 576 | 91.32 | 5.3 | 0.93 | 7 points |
| Mahteme et al.23 | 2016 | Amhara | CS | MVLR | 1314 | 100 | 8.3 | 0.76 | 6 points |
| Seifu et al.24 | 2020 | Sidama | CBCS | MVLR | 519 | 99.4 | 12.4 | 0.48 | 8 points |
| Alemu et al.25 | 2020 | Harari | IBCS | MVLR | 415 | 98 | 8.8 | 1.39 | 6 points |
| Yoseph et al.26 | 2013 | Oromiya | IBCS | ULR | 422 | 100 | 5 | 1.06 | 8 points |
| Temesgen and Alemu27 | 2019 | Amhara | IBCS | ULR | 422 | 96.68 | 8.8 | 1.4 | 7 points |
| Duguma et al.28 | 2020 | Oromiya | CS | MVLR | 271 | NS | 11.4 | 1.93 | 6 points |
| Abdulahi29 | 2019 | Somali | CBCS | MVLR | 525 | 100 | 8.57 | 1.22 | 6 points |
| Solomon et al.30 | 2014 | Amhara | CS | ULR | 1100 | 97.3 | 5.1 | 0.66 | 8 points |
| Addisu and Getabalew31 | 2020 | AA | IBCS | MVLR | 758 | 100 | 14.8 | 1.29 | 6 points |
| Wondemagegn et al.32 | 2017 | Amhara | CBCS | MVLR | 757 | 95.4 | 11 | 1.13 | 8 points |
| Tesfa et al.33 | 2016 | Amhara | CS | Chi-square | 385 | 95.3 | 0.34 | 0.57 | 7 points |
| Ataro et al.34 | 2018 | Harari | IBCS | MVLR | 425 | 95.9 | 7.1 | 1.24 | 8 points |
| Chanyalew and Alemayehu35 | 2017 | Oromiya | CBCS | ULR | 605 | 98.2 | 7.3 | 1.06 | 8 points |
| Tariku et al.36 | 2016 | AA | CS | MVLR | 1003 | 93.32 | 5 | 0.69 | 7 points |
| Lemba et al.37 | 2012 | Ethiopia | CS | Chi-square | 2153 | 96.4 | 6.5 | 0.53 | 8 points |
| Muche et al.38 | 2019 | Amhara | CS | ULR | 1110 | 92.5 | 13 | 1.01 | 8 points |
| Esayas et al.39 | 2011 | Oromiya | CS | Chi-square | 329 | 100 | 10 | 1.65 | 8 points |
| Getachew et al.40 | 2020 | Tigray | IBRS | Chi-square | 299,806 | 100 | 1.02 | 0.02 | 7 points |
| Abebe et al.41 | 2013 | Amhara | IBCS | Chi-square | 354,524 | NS | 4.4 | 0.03 | 6 points |
| Getasew et al.9 | 2019 | Amhara | CBCS | MVLR | 607 | 100 | 10.2 | 1.22 | 7 points |
| Tesfaye et al.42 | 2020 | Oromiya | CBCS | ULR | 915 | 95.8 | 3.1 | 0.57 | 7 points |
| Worku and Yeshaneh43 | 2017 | Ethiopia | CBCS | MVLR | 1472 | 95.5 | 3.3 | 0.46 | 8 points |
| Assefa et al.30 | 2014 | Amhara | IBCS | MVLR | 225 | 88.4 | 8.5 | 1.86 | 8 points |
| Yeromnesh et al.44 | 2015 | Tigray | IBRS | NS | 20,939 | 100 | 1.3 | 0.09 | 6 points |
| Gezahegn et al.45 | 2020 | Oromiya | IBCS | MVLR | 321 | 98.4 | 5.1 | 1.22 | 7 points |
| Desalegn et al.46 | 2015 | Harari | CS | ULR | 787 | 91 | 7 | 0.91 | 8 points |
| Fisseha and Senthil47 | 2018 | Oromiya | IBRS | NS | 22,277 | 100 | 1.43 | 0.08 | 6 points |
| Endashaw et al.48 | 2014 | AA | IBCS | MVLR | 120 | 100 | 15.8 | 3.33 | 8 points |
| Gebreegziabiher et al.10 | 2020 | Tigray | CBCS | MVLR | 321 | 98.8 | 9.3 | 1.62 | 7 points |
| Bantie et al.49 | 2019 | Amhara | CBCS | MVLR | 607 | 100 | 10.2 | 1.22 | 6 points |
| Worede et al.9 | 2017 | Amhara | CBCS | MVLR | 392 | NS | 12 | 1.64 | 6 points |
| Seyoum et al.50 | 1999 | Tigray | CBCS | NS | 890 | 97.6 | 3.7 | 0.87 | 8 points |
| Tenaye et al.51 | 2019 | DD | CS | ULR | 463 | 90.92 | 13.5 | 1.58 | 7 points |
Acronyms: AA Addis Ababa, CBCS community-based cross sectional study, CS cross-sectional study, DD Dire Dawa, DM diabetes mellitus, FBCS facility based cross sectional study, IBCS institution based cross sectional study, HBRS hospital-based retrospective study, RS retrospective study, NS not specified, OLR ordinal logistic regression, MVLR multivariate logistic regression, ULR uni-variate logistic regression.
Data extraction
Relevant data from each article was extracted by two independent reviewers (MAZ and ET). The data extraction includes; author name, year of publication, design of the study, sample size, study population, area of the study (region), response rate, the prevalence of DM, sex, age, family history of DM, BMI, alcohol consumption, cigarette smoking, hypertension, physical activity, educational status, cholesterol level, wasting circumference.
Description of studies
The literature review with the chosen search terms firstly identified 576 articles. We selected 204 articles after reviewing the proximate of the title to the predefined objectives. After further reading the full-text of each article, only 62 articles were subjected to document review. Lastly, 40 articles met the inclusion criteria for this systematic review and the meta-analysis (Fig. 1).
Data processing and analysis
After extracting the data from all eligible studies, the data were entered, and data analysis was done using (STATA version 16). The random-effects model was used for estimating the overall pooled prevalence of diabetes mellitus and its main components. In a meta-analysis, one of the relevant issues is the problem of heterogeneity among studies. To assess the consistency of studies, we use I2 test statistics52. This test examines the hypothesis of all the included studies are evaluated the same effect. Consequently, since there was heterogeneity between the original studies (I2 = 97.1%, p < 0.001), a random effect model was needed. To account for between-study variance, a random effect meta-analysis with an estimation of DerSimonian and Laird method was used53,54. The presence of publication bias is also accessed; funnel plots and tests of Begg’s were used as suggested by different scholars55.
Ethics approval and consent to participate
This research is a systematic review and a meta-analysis in which data were gathered from published articles from websites, and hence ethical approval has not been obtained from the Institutional Review Board.
Results and discussion
Review results
In this study, we have searched about 576 studies initially from September 2020 up to December 2020. The flow chart diagram represents the number of studies searched, study selection, number of studies included in the study that meet the inclusion criteria. The process of systematic literature retrieval and screening of the studies are presented in (Fig. 1). Finally, 40 studies were included in this meta-analysis and systematic review.
Description of included studies
Among those all studies included in this systematic review and meta-analysis, 14 (35%) of the studies were community-based cross-sectional, 13 (32.5%) studies were cross-sectional, 9 (22.5%) were institution based cross-sectional, and the remaining 4 (10%) studies were institution based retrospective. According to the regional locations of included studies, 22 (30%) were conducted in Amhara region, 9 (22.5%) were conducted in Oromia region, 5 (12.5%) in Addis Ababa, 4 (710%) in Tigray region, 3 (7.5%) in Harari region, 2 (5%) were conducted at the national level, 2 (5%) in Dire Dawa city administration and 1 (2.5%) studies were included in each region of SNNRP, Sidama region and Somali. But we could not find any study from Benishangul Gumuz, Afar and Gambella. From the studies which were included in this meta-analysis were used different types of statistical approach or methods to address their objectives. Among those studies about 19 (47.5%) were used multivariate logistic regression, 9 (22.5%) were used uni-variate logistic regression, 6 (15%) were used chi-square test, 3 (7.5%) were used ordinal logistic regression while the remaining 3 (7.5%) were not apply any statistical model rather they only study based on descriptive statistics, which mean that they are not study about risk factors though statistical models about the effect of them onT2DM (Table 1).
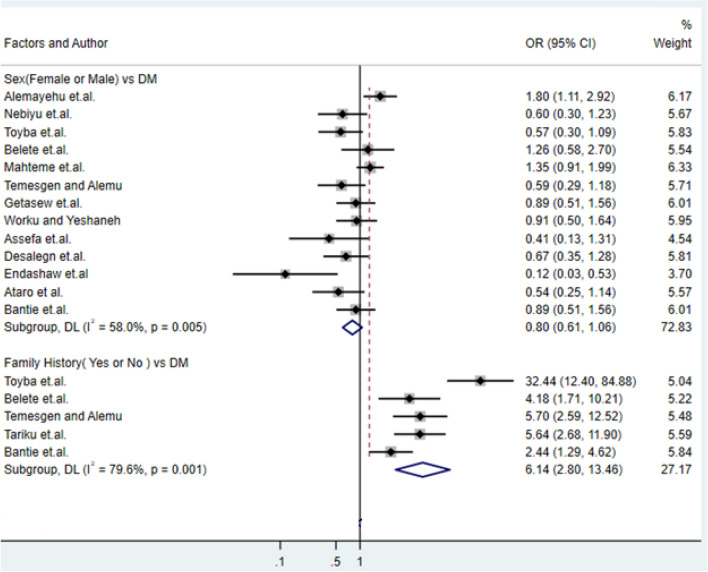
Results from Table 2, show that all studies did not used similar risk factors to study about the prevalence of T2DM. According to the associated factor sex, the highest prevalence of T2DM among the studies included were 18.4% for male and 16.2% for female which was conducted by Addisu & Getabalew while the lowest prevalence was 1.2% for both male and female which were obtained from two different study results Gezahegn et al.and Alemayehu et.al respectively.
Table 2.
Summary statistics for the prevalence of T2DM by risk factors for each study.
| Author | Associated risk factors of T2DM | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (%) | Age in year (%) | BMI (%) | WC (%) | Educational status (%) | Blood pressure (%) | Smoking (%) | Alcohol use (%) | |||||||||
| Male | Female | < 40 | ≥ 40 | < 25 | ≥ 25 | Low | High | Illiterate | Literate | < 140 | ≥ 140 | Yes | No | Yes | No | |
| Alemayehu et al. | 2.4 | 1.2 | 1.1 | 3.1 | 1.6 | 5.8 | – | – | – | 1.2 | 2.3 | – | – | – | – | |
| Endris et al. | 6.5 | 4.6 | 2.1 | 3.6 | 8.3 | 10.5 | – | – | 5.2 | 0.5 | – | – | – | – | – | – |
| Shiferaw et al. | – | – | – | – | 3.7 | 6.5 | 1.9 | 11.2 | – | – | 10.5 | 17.8 | – | – | – | – |
| Gizaw et al. | 2.3 | 1.4 | – | – | – | – | – | – | – | – | 1.7 | 2.2 | – | – | – | – |
| Belete et al. | 2.5 | 2.6 | 3 | 7.7 | 0.4 | 6.3 | 0.3 | 9.3 | 9.1 | 7.1 | 2.7 | 5.6 | 2.9 | 2.5 | 2.8 | 1.8 |
| Tilahun et al. | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Mahteme et al. | 2.1 | 1.7 | 2.4 | 3.4 | – | – | – | – | – | – | – | – | – | – | – | – |
| Seifu et al. | – | – | – | – | 2.6 | 5.5 | – | – | – | – | 4.6 | 7.7 | 6.9 | 5.4 | – | – |
| Alemu et al. | – | – | – | – | 1.2 | 7.5 | – | – | – | – | – | – | ||||
| Yoseph et al. | 3.5 | 1.3 | 1.4 | 3.6 | 1.44 | 3.5 | 0.9 | 4 | – | – | – | – | 4.3 | 0.5 | 4 | 0.9 |
| Duguma et al. | 4.4 | 7 | 5.2 | 6.3 | 7.1 | 2.9 | 5.2 | 6.3 | 8.6 | 2.2 | 2.2 | 9.2 | 10.3 | 1.1 | ||
| Abdulahi | 3.8 | 4.8 | 3 | 5.5 | 10 | 22.6 | 3.1 | 5.5 | – | – | – | – | ||||
| Solomon et al. | 1.7 | 2.6 | 0.6 | 1.2 | 1.1 | 1.5 | – | – | – | – | – | – | 1.6 | 1.4 | ||
| Addisu and Getabalew | 18.4 | 16.2 | 3.4 | 10.5 | 5.5 | 9.2 | – | – | – | – | 6.7 | 8 | 1.6 | 13.2 | 6.5 | 8.3 |
| Tariku et al. | – | – | 4.9 | 7.5 | 4.9 | 7.9 | – | – | – | – | 4.7 | 8.1 | 2.1 | 10.7 | – | – |
| Muche et al. | – | – | 8.6 | 3.2 | 1.7 | 10.1 | 2.9 | 8.9 | – | – | – | – | – | – | ||
| Getasew et al. | 2.9 | 1.6 | 0.7 | 3.9 | 1.9 | 2.6 | 2.1 | 2.5 | – | – | – | – | – | – | 3.5 | 1.2 |
| Worku and Yeshaneh | 3.4 | 3.2 | 9.7 | 6.3 | 2.4 | 19 | – | – | 3.2 | 13.3 | 5 | 10 | – | – | 2.6 | 6 |
| Assefa et al. | – | – | 3.1 | 9.1 | – | – | 5.7 | 6.5 | – | – | 3.1 | 9.1 | 1.3 | 10.9 | – | – |
| Gezahegn et al. | 1.2 | 3.7 | 1.3 | 3.8 | – | – | 2.2 | 2.8 | – | – | – | – | – | – | ||
| Desalegn et al. | 7.8 | 5.4 | 4.9 | 9.9 | 6.5 | 8.02 | 4.2 | 8.7 | – | – | – | – | 8.8 | 6.6 | ||
| Endashaw et al. | 1.9 | 4.2 | 3.6 | 2.6 | 2.9 | 3.3 | 1.6 | 4.6 | – | – | – | – | – | – | ||
| Bantie et al. | – | – | – | – | – | – | 2.1 | 3.3 | 2.5 | 3.8 | – | – | – | – | – | – |
| Ataro et al. | 10.2 | 5.7 | 3.5 | 11.1 | 6.1 | 9.3 | 5.4 | 8.3 | – | – | 5.1 | 20.4 | 13.3 | 6.6 | – | – |
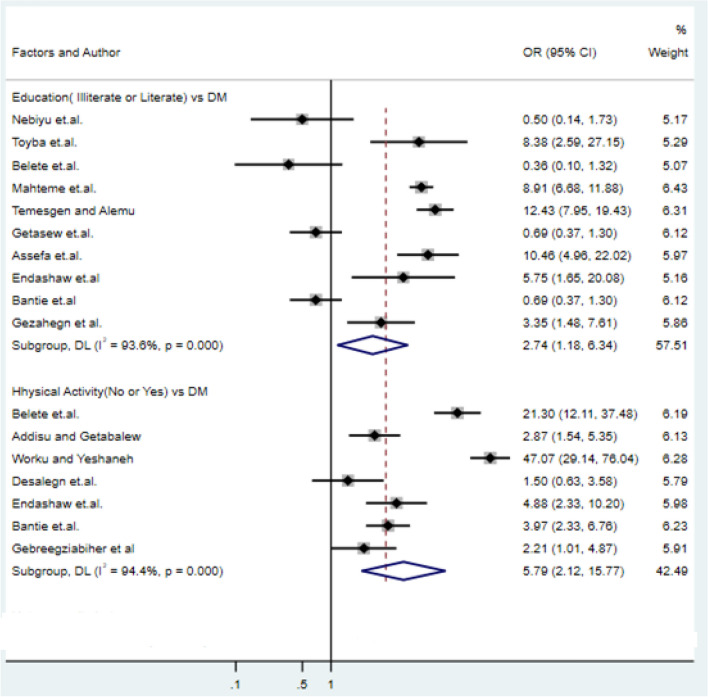
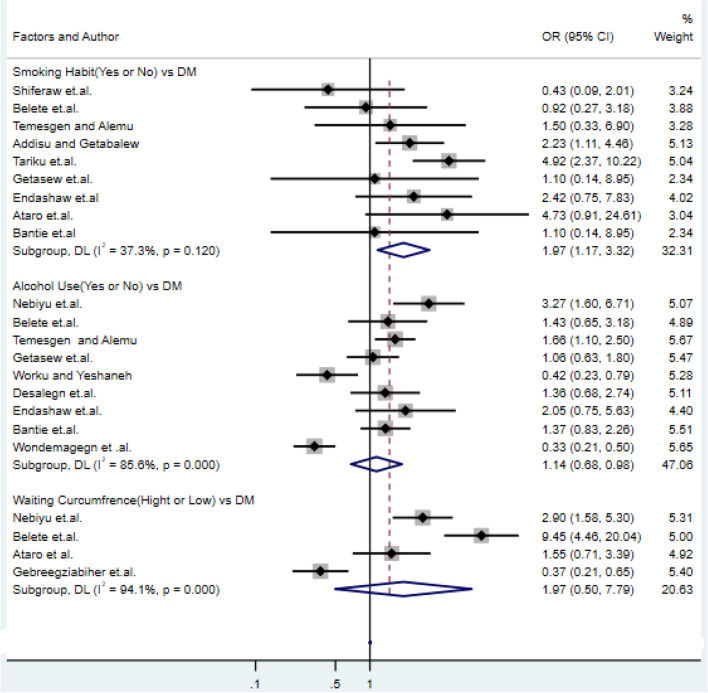
The prevalence of T2DM was higher in older age (age ≥ 40) years as compared to the elder age (age < 40 years) in almost all studies included except the study finding of Worku & Yeshaneh which was contradict4 of the other finding. The overall prevalence of T2DM was about 5.95% for age of ≥ 40 years and 3.6% for the patient whose age was less than 40 years. Similarly BMI was positively associated with TDM with the average overall prevalence of T2DM was about 3.6% for person whose BMI < 25 kg/m2 while the prevalence of T2DM was about 6.98% for persons whose BMI ≥ 25 kg/m2.The mean prevalence of T2DM was higher in illiterate case which accounts about 8.19%. The association of T2DM and alcohol consumption was, an individual who uses alcohol was more exposed to T2DM as compared to the none-user one. It is also similar in smokers. The overall mean prevalence of T2DM was 7.1% among the smoker individuals and 4.26% among alcohol users. Furthermore, blood pleasure and wasting circumference were also associated with T2DM. Among the studies included, there was the prevalence of about 9.89% T2DM among person with blood pressure of ≥ 140 mmHg while 5.46% person with blood pressure of < 140 mmHg (Table 2).
Prevalence of type-2 diabetes mellitus in the study area
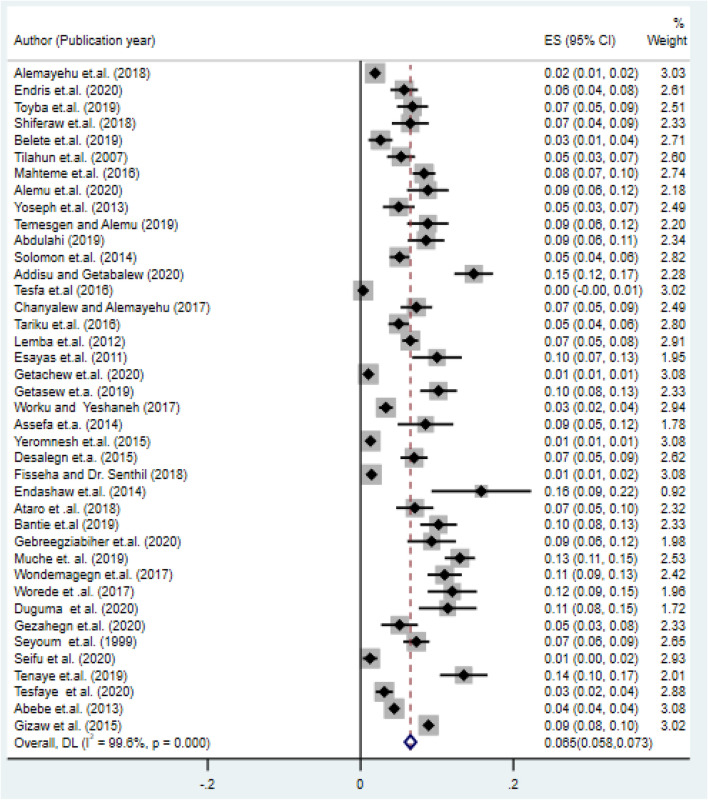
In this systematic review and meta-analysis, we observed a broader difference in the prevalence of type-2 DM among the included studies. The highest prevalence of type-2 DM was found in Addis Ababa (15.8%)16, while the lowest prevalence was confirmed in Amhara (0.34%)33. The overall pooled prevalence of type-2 diabetes in Ethiopia was 6.5% (95% CI :(5.8%, 7.3%)) (Fig. 2).
Figure 2.
Forest plot of the pooled prevalence of diabetes mellitus in Ethiopia.
Sub-group analysis of type-2 diabetes in Ethiopia
We had also conducted sub-group analyses to investigate how the prevalence of type-2 DM varies across different studies by region, study design, statistical method and data collection period. The sub-group analysis by region, the highest pooled prevalence of type-2 DM 14% (95% CI: 10%-17%) was in Dire Dawa city administration followed by 9% (95% CI: 6–11%) Addis Ababa, and the smallest were in Sidama 2% (1.1–2.4%). The sub-group analysis of type-2 DM by study design had shown the highest pooled prevalence, 8% (6–11%) for IBCS. Similarly, the highest magnitude of pooled prevalence of type-2 DM for sub-group analysis by study year was 7% (6–9%) in the studies conducted after 2016. This figure reflects that the current prevalence trend of type-2 DM has been increased as compared to the studies conducted before 2016. In the same explanation the highest prevalence of T2DM was observed from the study analyzed thought uni-variate logistic regression which was 7.4% (5.2–9.5%) followed by the chi-square test, 7% (3.1–10.8%).
Finally, since the value of I2 is large, implies that the heterogeneity on prevalence of T2DM was due to study area, study period, study design and also due to difference in statistical methodology (Table 3).
Table 3.
Sub-group analysis of studies included in the meta-analysis on the prevalence and associated factors of DM in Ethiopia.
| Sub-group | Random effects [95% CI] | Test of heterogeneity (I2) (%) |
|---|---|---|
| By region | ||
| Amhara | 8% [6–10%] | 97.3 |
| Oromiya | 6% [4–8%] | 94.4 |
| Tigray | 2% [2–3%] | 96.7 |
| Addis Ababa | 9% [5–12%] | 96.2 |
| Sidama | 2% [1.1–2.4%] | – |
| Harari | 7% [6–9%] | – |
| Somali | 9% [6–11%] | – |
| SNNRP | 6% [4–8%] | – |
| National level | 5% [2–8%] | 95.1 |
| Dire Dawa | 14% [10–17%] | – |
| By study design | ||
| CS | 7% [5–9%] | 97.1 |
| CBCS | 7% [5–9%] | 93.4 |
| IBCS | 8% [6–11%] | 92.5 |
| IBRS | 3% [2–4%] | 99.5 |
| By study period | ||
| Before 2016 | 6% [4–7%] | 99.2 |
| After 2016 | 7% [6–9%] | 97.4 |
| By statistical model | ||
| Chis-square | 7% [3.1–10.8%] | 96.6 |
| MVLR | 6.8% [4.9–8.8] | 98.6 |
| OLR | 5.5% [1.6–9.4%] | 95.9 |
| ULR | 7.4% [5.2–9.5% ] | 97.4 |
| NS | 6.8% [2.7–10.8%] | 94.5 |
Risk factors of type-2 diabetes mellitus
In this systematic review and meta-analysis, we assessed the association of different associated risk factors like sex, age, body mass index, family history, hypertension, education status, use of alcohol, smoking habit, cholesterol level, and wasting circumference with type-2 DM in Ethiopia.
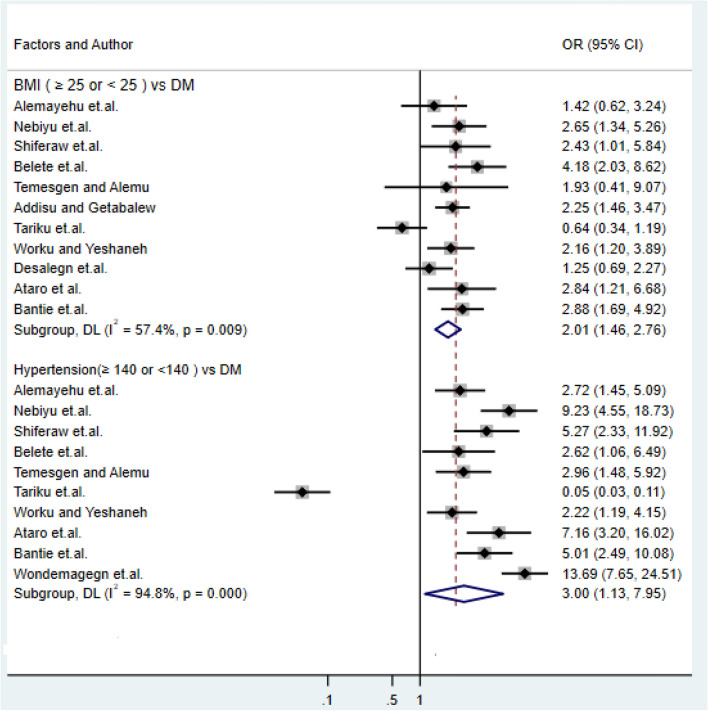
Association of body mass index and hypertension with type-2 DM
In this sub-categorical meta-analysis, eleven studies were included to examine the effect of body mass index on type-2 DM16,17,19,21,27,31,34,36,40,44,48,49. Of these, seven studies indicated a statistically significant association between body mass index and type-217,19,21,31,34,43,49 that shows a high risk of DM as body mass increased. In comparison, four studies showed there was no statistical difference in type-2DM among the normal weight and overweighed17,27,36,46. The pooled meta-regression analysis revealed a statistically significant difference in the exposure of type-2 DM among normal body mass index and obese with the odds ratio of 1.54 (95%: CI: 1.3, 1.83). Among the studies included in this meta-analysis, older age individuals were more exposed to type-2 diabetes than the younger age group, which is supported by the study1,2,5,9,17,18,20,22–24,26,28,32,33,38,51,56. In contrast, our study11,23,35,45 had got the result that contradict the other studies and concluded age had no any effect over the development of DM. Ten studies were examined the association between hypertension and DM32,34,36,43,49, and the result shows that a person with blood pressure ≥ 140 mm Hg was 2.48 times more likely to develop type-2 diabetes (OR: 2.48, 95% CI: 1.47, 4.18) (Fig. 3). Among those studies16–20,27,32, confirmed a significant statistical association between hypertension and type-2 DM.
Figure 3.
Forest plots of odds ratio for the association of body mass index and hypertension with type-2 DM.
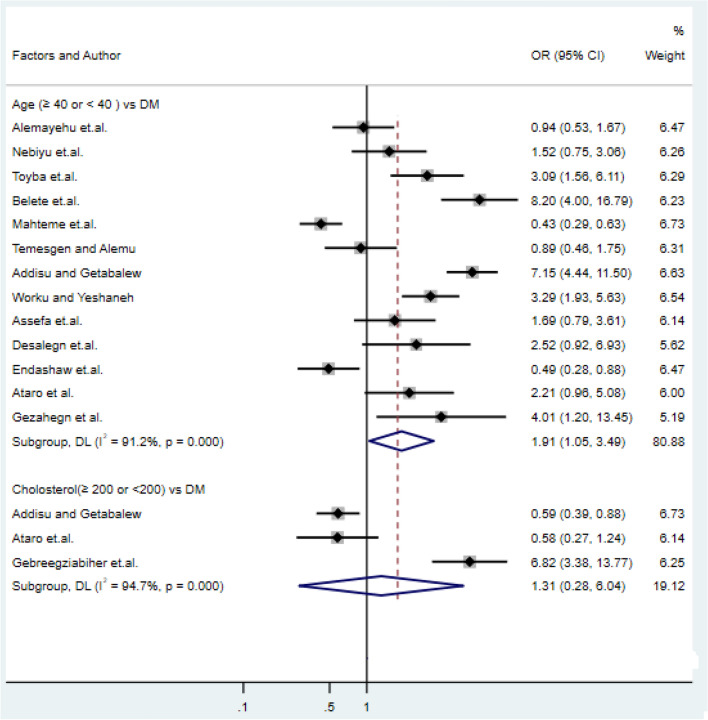
Association of age and cholesterol with type-2 DM
Thirteen studies had examined the association of age with type-2 DM21,23,27,31,34,41,42,45,46,48. Five of the studies had shown a statistically significant association of age with type-2 DM18,21,31,43,45,48 whereas the remaining seven studies have shown that age had no significant effect on type-2 DM. Consequently, the pooled meta-regression analysis showed that older age ≥ 40 years were statistical associated with type-2 DM (OR: 1.91, 95% CI: 1.05, 3.49), and cholesterol level ≥ 200 mg/dl was 1.31 times more likely to be exposed for diabetes (OR: 1.31, 95% CI: 0.28, 6.04) as compared cholesterol level < 200 mg/dl (Fig. 4) which is supported by the study of10,31.
Figure 4.
Forest plots of odds ratio for the association age and cholesterol with type-2 diabetes.
Association of education and physical exercise with type-2 DM
We use twelve studies to examine the association of education with DM and seven studies to investigate the association of physical activity with DM. Of 12 studies that examined the association of education level with type-2 DM, five studies supported that education level was associated with type-2 DM11,45,48. Among seven studies included in the analysis, six studies10,43,47–49 showed that physical activity had a significant contribution to the prevalence of type-2 DM (Fig. 5).
Figure 5.
Forest plots of odds ratio for the association of education and physical activity with type-2 diabetes.
Association of alcohol consumption, cigarette smoking and wasting circumference with type-2 DM
About the factor alcohol consumption studies32,43 with odds ratio of 1.14 (95% CI: 0.68, 0.98) and for factor cigarette smoking studies31,36 with an odds ratio of 1.97 (95% CI: 1.05, 7.79), showed alcohol consumption and cigarette smoking were significantly increase the risk of type-2 DM respectively. Moreover, another important vital factor this review that is a persistent association with type-2 DM, was wasting circumference. Wasting circumference (WC) is a diabetes risk factor that is strongly linked to other cardiovascular diseases. Study10,49 supports that an individual with high waist circumference was positively associated with DM and 1.97 times more likely to develop type-2 DM than the lower one(OR = 1.97, 95% CI: 1.05, 7.79) (Fig. 6).This result is also supported by the result of previous studies26,57,58.
Figure 6.
Forest plots of odds ratio for the association of smoking, alcohol consumption and wasting circumference with type-2 diabetes.
Association of family history of DM with type-2 DM
Furthermore, among the studies included, about the variable family history study18,21,27,36 reflect having family history DM disease will increase the risk of type-2 DM with the odds ratio of 6.14 (95% CI: 2.8, 13.46) (Fig. 7).
Figure 7.
Forest plots of odds ratio for the association of sex and family history with type-2 diabetes.
Risk of publication bias
The issue of publication bias was assessed by visual inspection of the funnel plot and using Begg’s regression test. The funnel plot showed asymmetrical, and most studies are outside of the Pseudo 95% confidence interval with Begg’s test (p value = 0.176); both the plot and the p-value shows the existence of publication bias(Fig. 8). This bias may be the inclusion of different articles with different study designs, study population, study period and area for assessment of associated factors of DM. Sub-group analysis was done to further investigate the cause of heterogeneity, and the result was presented in (Fig. S1).
Figure 8.
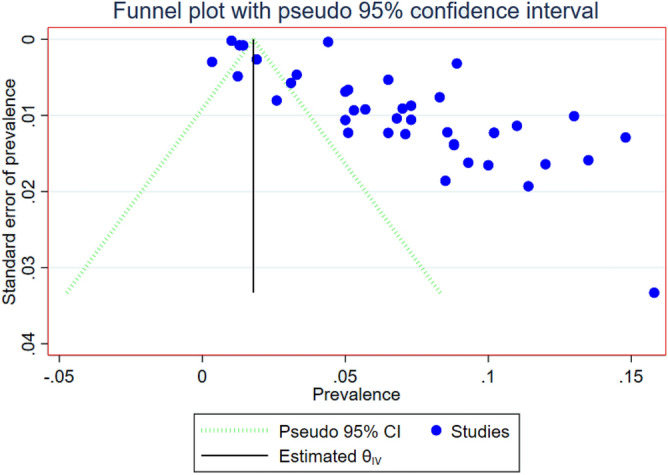
Funnel plot depicts publication bias of included studies on prevalence of DM in Ethiopia.
Discussion
Non-communicable diseases are becoming a double burden of public health problems in developing countries, including Ethiopia. Besides, the prevalence of type-2 DM is rising in developing countries in contrast to developed nations. This systematic review and meta-analysis will, update pooled estimates of type-2 DM in Ethiopia, which gives valuable information for policymakers, health planners, and the community. The prevalence of type-2 DM was high with inadequate awareness, treatment and control of type-2 DM in Ethiopia. A healthy lifestyle should be advocated to reduce the prevalence and increase awareness of risk factors, treatment, and prevention of type-2 DM in Ethiopia. The magnitude of DM prevalence in this systematic review and meta-analysis is consistent with a systematic review and meta-analysis conducted in Ghana (6.2)59, Russian (6.4%)60, New Zealand (6%)61, and Taiwan (6.7)62. However, the magnitude of DM prevalence in this systematic review and meta-analysis is higher than the study result in Nigeria (3%)56. In comparison, the magnitude is lower than the study results in Beijing, China (10.75%)63, Africa (7.2%)64, Thailand (16.8%)65, Germany (14%)66, United States (22.1%)67 and Belgium (9.4%)68. The potential reason for this difference might be due to the different lifestyle of populations, a number of studies included life expectancy difference and the study settings.
In this study, there is a minimal difference in the pooled prevalence of type-2 DM among community-based cross-sectional and institution-based studies, which was 7.3% and 8.7%, respectively. This variation could be explained by institution-based studies that include DM patients who visit health facilities for medication.
A variety of variables were linked to type-2 diabetes in this systematic review and meta-analysis. As a result, in Ethiopia, age is a strong predictor of type 2 diabetes. Hence, people over the age of 40 are twice as likely as children to develop type-2 diabetes, and are more susceptible to infectious diseases, such as heart disease, lung disease, or kidney disease. This result is in line with the study conducted in Trinidad69.
Our review study results also found that physical activity related to daily living and commuting was as effective as leisure-time exercise in reducing the risk of type-2 DM. This result was agreed with the results obtained from the study conducted in China70,71. Alcohol consumption and the resulting health effect are more complex than the mere volume of consumption measured at one point in time. Additional alcohol measurements would add weight to the validity and relevance to the alcohol measure because it is long-term consumption that tends to be of medical and public health concern. Additionally, how alcohol is consumed (i.e. with meals or bingeing on weekends) affects various health outcomes. In this study, we found alcohol consumption is positively associated with type-2 DM. This result is supported by previously conducted71,72.
In the current study, type-2 diabetes was more common among diabetic patients who were illiterate. This difference is due to the fact that diabetes self-management education is critical for reducing weight, blood pressure, and alcohol intake, and literates should have a greater understanding and ability regarding feeding style in their dietary to avoid diabetes disease. This finding is consistent with the previous studies finding46,47,49.
We confirmed in this study that higher BMI is associated with increased insulin resistance and decreased insulin sensitivity in diagnosed type-2 DM. This study shows that higher BMI is the most important factor associated with type-2 DM in Ethiopian people. The potential reason might be that excess sugar can promote weight gain, thus type-2 DM through extra calories, but has no unique diabetogenic effect at physiological levels. This finding was consistent with conclusions from Italian and Japanese population studies72,73.
Furthermore, the current investigation also indicates that family history of DM, high waist circumference and hypertension (blood pressure) ≥ 140 mm Hg are more likely exposed to type-2 DM. This idea reflects that family history, waist circumference and hypertension (blood pressure) were significant risk factors of type-2 DM disease. This conclusion is in line with the conclusion of the study conducted in Trinidad population70.
Moreover, in this systematic review and meta-analysis, some studies found contradicted results about type-2 DM predictors. There were different possible reasons for the contradiction of results conducted by various researchers about a similar factor. The reasons for the contradicting results may be sample size difference, study design difference, the difference in the study population, study area and study time. Furthermore, studies have indicated that social support and self-efficacy are determinant factors of diabetes59.
Strength and limitations of the study
Strength
This systematic examination and meta-analysis revealed the national pooled figure on DM risk factors in Ethiopia. Rising life expectancy, which is causing Ethiopia's population to age, is currently contributing to the rise in the risk of DM. As a result, the characteristics of type-2 DM prevalence as a function of population age and sex must be better understood. Owing to the unequal growth of regional economies in Ethiopia, type-2 diabetes is a modern disease that closely follows socio-economic development; it can be avoided and regulated by tailored interventions for different areas.
Finally, and perhaps most importantly, our research established several potentially useful prevention and control methods for type-2 diabetes. To minimize the prevalence of central obesity and associated type-2 diabetes, reasonable and safe diets, lifestyle improvements, cigarette smoking cessation, physical activity, and alcohol intake reduction are recommended.
Limitations
This review had not done without limitations. Firstly, the search strategy was limited to only published articles, but unpublished papers may be missed. Secondly, due to the inconsistent classification of factors for individual study, we cannot include all studies in pooled analysis quantitatively for the associated factors in this meta-analysis. This study was not without flaws. To begin with, the search strategy was limited to only the published articles; however, unpublished papers may have been overlooked. Second, we are unable to include all research in a pooled quantitative analysis for the related factors in this meta-analysis due to the inconsistent classification of factors for individual studies.
Finally, the clinical causes of type-2 diabetes mellitus were not included in this meta-analysis (HIV, TB, anemia, etc.).
Conclusion
This systematic review and meta-analysis showed the national pooled figure on the risk factors of type-2 DM in Ethiopia. At present, increasing life expectancy, which leads to Ethiopia’s aging population, contributes to Ethiopia’s aging population and contributes to the increase in diabetes risk. Therefore, characteristics of type-2 DM prevalence with the changes in the population structure of age and sex need to be better understood. Type-2 DM is a modern disease that closely follows socio-economic development; it should be prevented and controlled by personalized measures for different areas due to the uneven development of regional economies in Ethiopia.
Finally and most importantly, our study identified some potentially effective prevention and control strategies to control the fast growth of type-2 DM. Reasonable and healthy diets use, lifestyle changes, stopping cigarette smoking, physical activity, and reducing alcohol consumption are recommended to reduce the occurrence of central obesity and subsequent type-2 DM.
To decrease the overall prevalence of type-2 DM in Ethiopia, we suggest that all physicians involved in diabetes mellitus diagnosis and treatment deliver tailored diabetes health education and strict counseling on self-management. We also recommend to the colleges, universities, ministry of science and higher education and ministry of health to train and deploy diabetes health educators in all relevant health facilities and promote physical exercises and enhancing social support will strongly help to overcome diabetes problem.
Supplementary Information
Author contributions
M.A.Z. conceptualized the research problem. M.A.Z., E.T., A.A.M., A.S., T.A. developed the study design. M.A.Z. and E.T. organized the data, conducted the statistical analysis and drafted the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Data availability
Some of the data used for this study were attached with supporting material. All data were available from the corresponding author for reasonable requests.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-01256-9.
References
- 1.Kumar V, Abbas A, Aster JJP. Robbins Basic Pathology. 9. Elsevier; 2013. p. 2572013. [Google Scholar]
- 2.Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999–2011: Epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11(1):1–12. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Organization WH . Global Report on Diabetes: Executive Summary. World Health Organization; 2016. [Google Scholar]
- 5.Girach A, Manner D, Porta M. Diabetic microvascular complications: Can patients at risk be identified? A review. Int. J. Clin. Pract. 2006;60(11):1471–1483. doi: 10.1111/j.1742-1241.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- 6.Day C. Reflections from IDF-WDC 2015. Br J. Diab. 2016;16(1):37–38. doi: 10.15277/bjd.2016.064. [DOI] [Google Scholar]
- 7.Kankeu HT, Saksena P, Xu K, Evans DB. The financial burden from non-communicable diseases in low-and middle-income countries: A literature review. Health Res. Policy Syst. 2013;11(1):1–12. doi: 10.1186/1478-4505-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bickler SW, Wang A, Amin S, et al. Urbanization in sub-Saharan Africa: Declining rates of chronic and recurrent infection and their possible role in the origins of non-communicable diseases. World J. Surg. 2018;42(6):1617–1628. doi: 10.1007/s00268-017-4389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bantie GM, Wondaye AA, Arike EB, et al. Prevalence of undiagnosed diabetes mellitus and associated factors among adult residents of Bahir Dar city, northwest Ethiopia: A community-based cross-sectional study. BMJ Open. 2019;9(10):e030158. doi: 10.1136/bmjopen-2019-030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damtew E. Prevalence of Diabetes Mellitus among Active Pulmonary Tuberculosis Patients at St. Peter Specialized Hospital, Addis Ababa Ethiopia. Addis Ababa University; 2014. [Google Scholar]
- 11.Abebe SM, Andargie G, Shimeka A, et al. The prevalence of non-communicable diseases in Northwest Ethiopia: Survey of Dabat health and demographic surveillance system. BMJ Open. 2017;7(10):e015496. doi: 10.1136/bmjopen-2016-015496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishu KG, Jenkins C, Yebyo HG, Atsbha M, Wubayehu T, Gebregziabher M. Diabetes in Ethiopia: A systematic review of prevalence, risk factors, complications, and cost. Obes. Med. 2019;15:100132. doi: 10.1016/j.obmed.2019.100132. [DOI] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wannemuehler TJ, Lobo BC, Johnson JD, Deig CR, Ting JY, Gregory RL. Vibratory stimulus reduces in vitro biofilm formation on tracheoesophageal voice prostheses. Laryngoscope. 2016;126(12):2752–2757. doi: 10.1002/lary.25969. [DOI] [PubMed] [Google Scholar]
- 15.Hartling L, Milne A, Hamm MP, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J. Clin. Epidemiol. 2013;66(9):982–993. doi: 10.1016/j.jclinepi.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Zekewos A, Loha E, Egeno T, Wubshet K, Merga Z. Prevalence of diabetes mellitus and associated factors in Southern Ethiopia: A community based study. Ethiop. J. Health Sci. 2018 doi: 10.4314/ejhs.v28i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dereje N, Earsido A, Temam L, Abebe A. Prevalence and associated factors of diabetes mellitus in Hosanna Town, Southern Ethiopia. Ann. Glob. Health. 2020 doi: 10.5334/aogh.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endris T, Worede A, Asmelash D. Prevalence of diabetes mellitus, prediabetes and its associated factors in Dessie Town, Northeast Ethiopia: A community-based study. Diabetes Metab. Syndr. Obes. Targets Ther. 2019;12:2799. doi: 10.2147/DMSO.S225854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aynalem SB, Zeleke AJ. Prevalence of diabetes mellitus and its risk factors among individuals aged 15 years and above in Mizan-Aman town, Southwest Ethiopia, 2016: A cross sectional study. Int. J. Endocrinol. 2018 doi: 10.1155/2018/9317987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gizaw M, Harries A, Ade S, et al. Diabetes mellitus in Addis Ababa, Ethiopia: Admissions, complications and outcomes in a large referral hospital. Public Healh Action. 2015;5(1):74–78. doi: 10.5588/pha.14.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woldesemayat B, Amare H, Ataro Z, et al. Prevalence of diabetes mellitus and associated risk factors among adults attending at Feres Meda Health Centre, Addis Ababa, Ethiopia, 2017. Int. J. Biomed. Mater. Res. 2019;7(1):8–15. [Google Scholar]
- 22.Yemane T, Belachew T, Asaminew B. Type II diabetes mellitus in Jimma Town, southwest Ethiopia. Ethiop. J. Health Sci. 2007;17(2):107–114. [Google Scholar]
- 23.Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in South-Eastern Amhara Region, Ethiopia: A cross sectional study. PLoS ONE. 2016;11(1):e0147621. doi: 10.1371/journal.pone.0147621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seifu Y, Tsegaw D, Haji Y, Ejeso AJD. Prevalence and associated factors of diabetes mellitus among adult population in Hawassa Zuria Woreda, Sidama Region, Ethiopia. Diabetes Metab. Syndr. Obes. Targets Ther. 2020;13:4571–4579. doi: 10.2147/DMSO.S275230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebrie A, Tesfaye B, Gebru T, et al. Diabetes mellitus and its associated risk factors in patients with human immunodeficiency virus on anti-retroviral therapy at referral hospitals of Northwest Ethiopia. Diabetol. Metab. Syndr. 2020;12(1):1–8. doi: 10.1186/s13098-020-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megerssa, Y., M. Gebre, S. Birru, A. Goshu, & D. Tesfaye. Prevalence of undiagnosed diabetes mellitus and its risk factors in selected institutions at Bishoftu town, East Shoa, Ethiopia. J. Diab. Metab.S12, 008 (2013).
- 27.Fiseha T, Belete AG. Diabetes mellitus and its associated factors among human immunodeficiency virus-infected patients on anti-retroviral therapy in Northeast Ethiopia. BMC Res. Notes. 2019;12(1):1–7. doi: 10.1186/s13104-018-4038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanta Duguma WG, Mamo A, Tamiru D, Woyesa S. Diabetes mellitus and associated factors among adult HIV patients on highly active anti-retroviral treatment. HIV/AIDS (Auckland, NZ) 2020;12:657. doi: 10.2147/HIV.S279732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abas, A. H. Prevalence and associated risk factors for type 2 diabetes mellitus among adults (>= 40 years of age) in Jigjiga City, Somali region, Eastern Ethiopia (2019).
- 30.Abebe SM, Berhane Y, Worku A, Assefa A. Diabetes mellitus in North West Ethiopia: A community based study. BMC Public Health. 2014;14(1):1–8. doi: 10.1186/1471-2458-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahile AT, Bekele GEJD. Prevalence of diabetes mellitus and associated factors in Addis Ababa public health facilities, Addis Ababa, Ethiopia. Diabetes Metab. Syndr. Obes. Targets Ther. 2016;2020(13):501. doi: 10.2147/DMSO.S237995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wondemagegn AT, Bizuayehu HM, Abie DD, Ayalneh GM, Tiruye TY, Tessema MT. Undiagnosed diabetes mellitus and related factors in East Gojjam (NW Ethiopia) in 2016: A community-based study. J. Public Health Res. 2017 doi: 10.4081/jphr.2017.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habtewold TD, Tsega WD, Wale BY. Diabetes mellitus in outpatients in Debre Berhan referral hospital, Ethiopia. J. Diabetes Res. 2016 doi: 10.1155/2016/3571368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ataro Z, Ashenafi W, Fayera J, Abdosh T. Magnitude and associated factors of diabetes mellitus and hypertension among adult HIV-positive individuals receiving highly active antiretroviral therapy at Jugal Hospital, Harar, Ethiopia. HIV/AIDS (Auckland, NZ) 2018;10:181. doi: 10.2147/HIV.S176877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kassahun CW, Mekonen AG. Knowledge, attitude, practices and their associated factors towards diabetes mellitus among non diabetes community members of Bale Zone administrative towns, South East Ethiopia. A cross-sectional study. PLoS ONE. 2017;12(2):e0170040. doi: 10.1371/journal.pone.0170040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tesfaye T, Shikur B, Shimels T, Firdu N. Prevalence and factors associated with diabetes mellitus and impaired fasting glucose level among members of federal police commission residing in Addis Ababa, Ethiopia. BMC Endocr. Disord. 2016;16(1):1–9. doi: 10.1186/s12902-016-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nshisso LD, Reese A, Gelaye B, et al. Prevalence of hypertension and diabetes among Ethiopian adults. Diabetes Metab. Syndr. Clin. Res. Rev. 2012;6(1):36–41. doi: 10.1016/j.dsx.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muche AA, Olayemi OO, Gete YK. Prevalence of gestational diabetes mellitus and associated factors among women attending antenatal care at Gondar town public health facilities, Northwest Ethiopia. BMC Pregnancy Childbirth. 2019;19(1):1–13. doi: 10.1186/s12884-019-2492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gudina EK, Amade ST, Tesfamichael FA, Ram R. Assessment of quality of care given to diabetic patients at Jimma University Specialized Hospital diabetes follow-up clinic, Jimma, Ethiopia. BMC Endocr. Disord. 2011;11(1):1–9. doi: 10.1186/1472-6823-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gebremedhin G, Enqueselassie F, Deyessa N, Yifter H. Urban–rural differences in the trends of type 1 and type 2 diabetes among adults who received medical treatment from public hospitals in resource-poor community Tigray, Ethiopia. Diabetes Metab. Syndr. Obes. Targets Ther. 2020;13:859. doi: 10.2147/DMSO.S238275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abebe SM, Berhane Y, Worku A, Alemu S. Increasing trends of diabetes mellitus and body weight: A ten year observation at Gondar University Teaching Referral Hospital, Northwest Ethiopia. PLoS ONE. 2013;8(3):e60081. doi: 10.1371/journal.pone.0060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yunka TT, Mogas SB, Zawdie B, et al. The hidden burden of diabetes mellitus in an urban community of southwest Ethiopia. Diabetes Metab. Syndr. Obes. Targets Ther. 2020;13:2925. doi: 10.2147/DMSO.S269386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Animaw W, Seyoum Y. Increasing prevalence of diabetes mellitus in a developing country and its related factors. PLoS ONE. 2017;12(11):e0187670. doi: 10.1371/journal.pone.0187670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambachew Y, Kahsay S, Tesfay R, et al. Prevalence of diabetes mellitus among patients visiting medical outpatient department of Ayder Referral Hospital, Mekelle, Ethiopia: A three years pooled data. Int. J. Pharm. Sci. Res. 2015;6(02):435–439. [Google Scholar]
- 45.Gezahegn H, Ibrahim M, Mulat E. Diabetes mellitus and tuberculosis comorbidity and associated factors among Bale Zone Health Institutions, Southeast Ethiopia. Diabetes Metab. Syndr. Obes. Targets Ther. 2020;13:3879. doi: 10.2147/DMSO.S248054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayana DA, Bachu YD, Roba KT, Kebede DA. Type 2 diabetes mellitus among government employees in Harar, eastern Ethiopia: A cross-sectional study. Res. Rep. Endocr. Disord. 2015;5:71–77. [Google Scholar]
- 47.Nagaraj, F. G. D. S. Prevalance of Diabetes Mellitus Patients Attending Robe Hospital, Robe, Bale Zone, Ethiopia: A Research Article.
- 48.Ayele, B. H, Shore, H., Shunu, A., Mengesha, M. M. Diagnosed and undiagnosed Diabetes mellitus among urban adults: a population based cross-sectional study. bioRxiv. 532705 (2019). [DOI] [PMC free article] [PubMed]
- 49.Gebremedhin Gebreegziabiher TB, Tamiru D. Abnormal glucose metabolism and associated risk factors among adults in Mekelle City, Ethiopia. Diabetes Metab. Syndr. Obes. Targets Ther. 2020;13:4017. doi: 10.2147/DMSO.S280215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Worede A, Alemu S, Gelaw YA, Abebe M. The prevalence of impaired fasting glucose and undiagnosed diabetes mellitus and associated risk factors among adults living in a rural Koladiba town, northwest Ethiopia. BM Res. Notes. 2017;10(1):1–7. doi: 10.1186/s13104-016-2345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seyoum B, Kiros K, Haileselase T, Leole A. Prevalence of gestational diabetes mellitus in rural pregnant mothers in northern Ethiopia. Diabetes Res. Clin. Pract. 1999;46(3):247–251. doi: 10.1016/S0168-8227(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 52.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.IntHout J, Ioannidis JP, Borm GF. The Hartung–Knapp–Sidik–Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian–Laird method. BMC Med. Res. Methodol. 2014;14(1):1–12. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer FJD. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49(5):837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 55.Sorato MM, Tesfahun C, Lamessa D. Levels and predictors of adherence to self-care behaviour among adult type 2 diabetics at Arba Minch general hospital, Southern Ethiopia. J. Diabetes Metab. 2016;7(6):11. doi: 10.4172/2155-6156.1000684. [DOI] [Google Scholar]
- 56.Asamoah-Boaheng M, Sarfo-Kantanka O, Tuffour AB, Eghan B, Mbanya JC. Prevalence and risk factors for diabetes mellitus among adults in Ghana: A systematic review and meta-analysis. Int. Health. 2019;11(2):83–92. doi: 10.1093/inthealth/ihy067. [DOI] [PubMed] [Google Scholar]
- 57.Maina WK, Ndegwa ZM, Njenga EW, Muchemi EW. Knowledge, attitude and practices related to diabetes among community members in four provinces in Kenya: A cross-sectional study. Pan Afr. Med. J. 2010 doi: 10.4314/pamj.v7i1.69095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bouguerra R, Alberti H, Smida H, et al. Waist circumference cut-off points for identification of abdominal obesity among the Tunisian adult population. Diabetes Obes. Metab. 2007;9(6):859–868. doi: 10.1111/j.1463-1326.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- 59.Tenaye L, Mengiste B, Baraki N, Mulu E. Diabetes mellitus among adult tuberculosis patients attending tuberculosis clinics in Eastern Ethiopia. BioMed Res. Int. J. 2019;2019:1–7. doi: 10.1155/2019/7640836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ko GT, Tang JS. Waist circumference and BMI cut-off based on 10-year cardiovascular risk: Evidence for “central pre-obesity”. Obesity. 2007;15(11):2832–2839. doi: 10.1038/oby.2007.336. [DOI] [PubMed] [Google Scholar]
- 61.Dedov I, Shestakova M, Benedetti MM, et al. Prevalence of type 2 diabetes mellitus (T2DM) in the adult Russian population (NATION study) Diabetes Res. Clin. Pract. 2016;115:90–95. doi: 10.1016/j.diabres.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 62.Health. Mo. New Zealand Health Survey 2016–2017 Annual Data. https://www.health.govt.nz/publication/annualupdate-key-results-2016-17-new-zealandhealth-survey (2018).
- 63.Wang C-Y, Yu N-C, Sheu WH-H, Tsai S-T, Tai T-Y. Team care of type 2 diabetes mellitus in Taiwan. Diabetes Res. Clin. Pract. 2014;106:S309–S313. doi: 10.1016/S0168-8227(14)70735-1. [DOI] [PubMed] [Google Scholar]
- 64.Yang F, Ma Q, Liu J, et al. Prevalence and major risk factors of type 2 diabetes mellitus among adult psychiatric inpatients from 2005 to 2018 in Beijing, China: A longitudinal observational study. BMJ Open Diabetes Res. Care. 2020;8(1):e000996. doi: 10.1136/bmjdrc-2019-000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chivese T, Mahmoud W, Magodoro I, Kengne AP, Norris SA, Levitt NS. Prevalence of type 2 diabetes mellitus in women of childbearing age in Africa during 2000–2016: Protocol of a systematic review and meta-analysis. BMJ Open. 2016;6(12):e012255. doi: 10.1136/bmjopen-2016-012255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Apidechkul T. Prevalence and factors associated with type 2 diabetes mellitus and hypertension among the hill tribe elderly populations in northern Thailand. BMC Public Health. 2018;18(1):1–17. doi: 10.1186/s12889-018-5607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Müller-Wieland D, Merkel M, Hamann A, et al. Survey to estimate the prevalence of type 2 diabetes mellitus in hospital patients in Germany by systematic HbA1c measurement upon admission. Int. J. Clin. Pract. 2018;72(12):e13273. doi: 10.1111/ijcp.13273. [DOI] [PubMed] [Google Scholar]
- 68.Birabaharan, M., Strunk, A., Garg, A. & Hagmann, S. 340. Prevalence of type ii diabetes mellitus among patients living with HIV in the United States. In Paper Presented at: Open Forum Infectious Diseases (2019).
- 69.Nayak BS, Sobrian A, Latiff K, et al. The association of age, gender, ethnicity, family history, obesity and hypertension with type 2 diabetes mellitus in Trinidad. Diabetes Metab. Syndr. Clin. Res. Rev. 2014;8(2):91–95. doi: 10.1016/j.dsx.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 70.Shi L, Shu X-O, Li H, et al. Physical activity, smoking, and alcohol consumption in association with incidence of type 2 diabetes among middle-aged and elderly Chinese men. PLoS ONE. 2013;8(11):e77919. doi: 10.1371/journal.pone.0077919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Villegas R, Shu X-O, Li H, et al. Physical activity and the incidence of type 2 diabetes in the Shanghai women's health study. Int. J. Epidemiol. 2006;35(6):1553–1562. doi: 10.1093/ije/dyl209. [DOI] [PubMed] [Google Scholar]
- 72.Noale M, Maggi S, Marzari C, et al. Components of the metabolic syndrome and incidence of diabetes in elderly Italians: The Italian longitudinal study on aging. Atherosclerosis. 2006;187(2):385–392. doi: 10.1016/j.atherosclerosis.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 73.Nagaya T, Yoshida H, Takahashi H, Kawai M. Increases in body mass index, even within non-obese levels, raise the risk for type 2 diabetes mellitus: A follow-up study in a Japanese population. Diabet. Med. 2005;22(8):1107–1111. doi: 10.1111/j.1464-5491.2005.01602.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Some of the data used for this study were attached with supporting material. All data were available from the corresponding author for reasonable requests.