Abstract
Background
Pulmonary arterial venous malformation (PAVM) is an abnormal vascular malformation between pulmonary arteries and veins characterized by varying degrees of right-to-left shunts (RLS). Cryptogenic stroke (CS) due to paradoxical embolism (PE) caused by PAVM is relatively rare in the clinic.
Case Presentation
We report the case of a 54-year-old right-handed woman who presented with sudden-onset left-sided limb weakness for 2 h. A physical examination revealed normal vital signs but weakness in her left upper and lower limbs, graded as 1/5 using the Medical Research Council scale. Her National Institutes of Health Stroke Scale (NIHSS) score was 8, and her modified Rankin scale (mRS) was 4. Brain diffusion-weighted imaging showed acute infarction in the right basal ganglia and the radiation crown but brain magnetic resonance angiography found no obvious abnormality. A transcranial Doppler ultrasound with bubble study (TCD-b) found the rain curtain sign of microbubbles in the left middle cerebral artery, reflecting significant RLS. Transesophageal echocardiography (TEE) and transthoracic echocardiography (TTE) were conducted to distinguish between intra- and extracardiac shunts. A pulmonary computerized tomography angiogram (CTA) demonstrated a PAVM. We considered the patient had CS due to PE caused by PAVM. Thrombolytic therapy within the time window was performed. Then, transcatheter device occlusion of the arteriovenous fistula was successfully undertaken, and the patient carried on with rehabilitation training. At a 15-month follow-up, there were no catheter-related complications or recurrent stroke, and her NIHSS and mRS scores were both 0.
Conclusions
PAVM is an important risk factor for PE and CS and should not be ignored as a possible etiology in stroke patients without any other risk factors. CTA of the pulmonary artery is the recommended gold standard for diagnosing and locating a PAVM. Thrombolytic therapy within the time window combined with transcatheter device occlusion of arteriovenous malformation and rehabilitation training may benefit the recovery of patients with CS caused by PE resulting from PAVM.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-021-00275-y.
Keywords: Pulmonary arterial venous malformation, Cryptogenic stroke, Paradoxical embolism, Right-to-left shunt, Case report
Key Summary Points
| Pulmonary arterial venous malformation (PAVM) is a risk factor for paradoxical embolism (PE) and cryptogenic stroke (CS). |
| CS caused by PE through PAVM with limb weakness as the first symptom is relatively rare in the clinic, and PAVM should be screened as an etiology in stroke patients without any other risk factors. |
| Transcranial Doppler ultrasound with bubble study (TCD-b) is non-invasive and sensitive in detecting right-to-left shunts; if TCD-b is positive, transesophageal echocardiography or transthoracic echocardiography should be conducted to distinguish between intra- and extracardiac shunts. |
| Computerized tomography angiogram or digital subtraction angiography of the pulmonary artery is the recommended gold standard for diagnosing and locating a PAVM. |
| Our case indicated that thrombolytic therapy within the time window combined with transcatheter embolotherapy and rehabilitation training may be a benefit for the recovery of patients with CS caused by PE resulting from PAVM. |
Digital Features
This article is published with digital features, including a video, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.15179661.
Introduction
Pulmonary arterial venous malformation (PAVM), characterized by various degrees of right-to-left shunts (RLS), is an abnormal vascular malformation between pulmonary arteries and veins. A recent study revealed that patients with PAVM have an estimated incidence of 1 per 2600 [1]. Paradoxical embolism (PE) refers to arterial embolism caused by venous thrombosis into the systemic circulation through an RLS [2]. RLS can be divided into two types: intra- and extracardiac shunts. The causes of an intracardiac shunt include patent foramen ovale (PFO) and atrial septal defect (ASD), among others, while the causes of an extracardiac shunt include PAVM and aortic arch-pulmonary vein fistula, among others [3]. Although PFO is probably responsible for PE, PAVM is another less common cause of RLS resulting in stroke. PAVM can cause variously life-threatening complications, of which stroke accounts for 2.6–25.0% [4]. Cryptogenic stroke (CS) accounts for 30.0–40.0% of ischemic stroke [5]. PFO is the most common cause of CS patients < 60 years of age [6]. However, retrospective studies indicate that CS caused by PE due to a PAVM is relatively rare in the clinic [7, 8]. We report a case of CS secondary to PE from PAVM with high-flow RLS.
Case Presentation
A 54-year-old right-handed woman presented to our Emergency Department after experiencing sudden onset left-sided limb weakness. She described a 2-h episode of left upper and lower limb weakness. She had smoked for > 20 years, 20 cigarettes daily. She had no history of drinking alcohol, thrombotic vascular diseases, hypertension, hyperlipidemia, infectious diseases, heart diseases, or family hereditary diseases (e.g., hereditary hemorrhagic telangiectasia).
On initial physical examination, her vital signs were normal but she had left upper and lower limb weakness (grade 1/5 using the Medical Research Council scale). Her National Institutes of Health Stroke Scale (NIHSS) score was 8. She was infused a standard dose of intravenous recombinant tissue plasminogen activator. However, the next day’s physical examination yielded six points on the NIHSS, left upper and lower limb weakness (grade 2/5 and 3/5, respectively), and a modified Rankin scale (mRS) of 4 points.
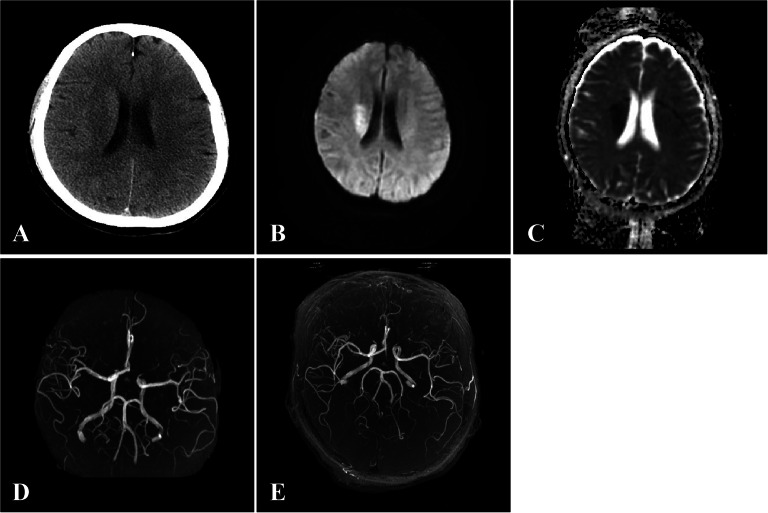
Laboratory findings revealed no obvious abnormalities in immunity, endocrine system, hypercoagulability, and infectiousness. A head computed tomography (CT) screening without contrast found no lesions (Fig. 1A). A brain diffusion-weighted imaging indicated changes on the right basal ganglia and radiation crown with acute infarction stroke (Fig. 1B and C). No obvious abnormality was found in the brain magnetic resonance angiography (Fig. 1D and E). The chest x-ray (CXR) showed no abnormal manifestations of cardiopulmonary structure. Following thrombolytic therapy, a repeat head CT demonstrated a lower signal lesion with a mild space-occupying effect in her right basal ganglia and radiation crown. We considered the possibility of cardiogenic embolism. A 24-h ambulatory electrocardiograph and echocardiography revealed no obvious abnormality (eFig.1 in Supplementary Material). However, the patient refused to undergo coronary angiography. The ultrasonography found no signs of thrombosis in the arteries and veins of her upper and lower extremities. A transcranial Doppler ultrasound with bubble study (TCD-b) found significant right-to-left shunts (RLS) (eFig.2 in Supplementary Material). According to the results of contrast transesophageal echocardiography (TEE) with Valsalva maneuver, PFO and ASD were excluded (eFig.3 in Supplementary Material). Contrast transthoracic echocardiography (TTE) revealed RLS graded as 3 (eFig.3 in Supplementary Material). A pulmonary CT angiogram (CTA) demonstrated a 1.5 × 0.8 cm PAVM in the basal segment of the right lower lobe (Fig. 2). We thus considered the patient had CS caused by PE due to PAVM.
Fig. 1.
Initial head computed tomography screening without contrast found no lesions (A). Brain diffusion-weighted imaging indicated plaque-like hyperintensity on the right basal ganglia and radiation crown (B). Apparent diffusion coefficient imaging showed low signal in the same location (C). Brain magnetic resonance angiography found no obvious abnormality (D, E)
Fig. 2.
Pulmonary computerized tomography angiogram demonstrated a 1.5 × 0.8 cm PAVM in the basal segment of the right lower lobe, revealing communication between the basal segmental feeding artery of the right inferior lobe and the draining vein of the right lower lobe
Fifteen days after intravenous thrombolysis, transcatheter device occlusion of PAVM was successfully undertaken with three coils (1 piece of 10.0 mm, 2 pieces of 8.0 mm) after 2.0% lidocaine local infiltration anesthesia (see video 1 in the online/HTML version of the manuscript). Subsequently, the patient underwent a comprehensive rehabilitation program for 2 h each day. She was discharged from the hospital on day 29 with significant improvement of her neurological deficits. At 15-month follow-up, there were no catheter-related complications or recurrent stroke, and her scores on the NIHSS and mRS were both 0. The process of the diagnosis and treatment is illustrated in eFig.4 in Supplementary Material.
Video 1: Transcatheter device occlusion of PAVM was successfully conducted with three coils (one 10.0 mm piece, two 8.0 mm pieces) after 2.0% lidocaine local infiltration anesthesia using a microcatheter (MP4 14113 KB)
Informed consent and consent for publication were provided by the patient. The study was approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (no. AF/04–07.0/10.0) and was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Discussion
Mechanisms of Cryptogenic Stroke Caused by Paradoxical Embolism Through PAVM
To the best of our knowledge, the main mechanisms of CS caused by PE through a PAVM include embolism caused by thrombosis in the fistula or peripheral venous system; embolism caused by thrombosis resulting from pulmonary artery blood directly flowing into the pulmonary vein without oxygenation; bacteria and air embolism not filtered by alveolar capillaries; and embolism caused by polycythemia [9, 10]. Moreover, the number of PAVMs and the diameter of the feeding artery are key risk factors of ischemic stroke induced by PAVM. The risk of CS is higher in patients with multiple PAVM, diffuse type of PAVM, and a feeding arterial diameter > 3.0 mm [11]. While the mortality of untreated PAVM may reach 50.0%, it can be reduced to 3.0% after treatment [12].
Clinical Presentation of Cryptogenic Stroke Resulting from PAVM
More than 80.0% of PAVMs are congenital because of defects in the development of pulmonary capillaries during the embryonic period (e.g., hereditary hemorrhagic telangiectasia), while acquired PAVM, which is associated with trauma, liver cirrhosis, fungi, tuberculosis, parasites, tumors, and surgery, is rare [13, 14]. Over 55.0% of PAVM is asymptomatic, and most of the clinical manifestations are not specific, which is related to the size and RLS of the arteriovenous fistula [15]. A previous study demonstrated that patients aged 40 to 60 years with multiple and large arteriovenous fistulae always have symptoms but PAVM with less RLS may not have any clinical symptoms [15]. Because PAVM does not affect cardiac hemodynamics, most patients remain asymptomatic and are easily misdiagnosed. Unless the shunt flow of PAVM is > 20.0% of the total systemic circulation, it may show asymptomatic hypoxemia, dyspnea, hemoptysis, chest pain, shortness of breath after activity, clubbing fingers, cyanosis, dizziness, ischemic stroke, TIA, and so on [16–18]. However, CS as a result of PE caused by PAVM is relatively rare in the clinic, as previous studies reported [7, 8]. Several studies have reported stroke caused by PAVM with limb hypoesthesia, aphasia, dysarthria, thalamic esotropia, and facial droop as the first symptoms [19–21].
Investigation and Diagnosis of Cryptogenic Stroke Associated with PAVM
Laboratory examination, including immunity, endocrine system, hypercoagulability state, infectiousness, arterial blood gas measurements, and finger oximetry, is important in screening stroke patients with suspected PAVM. The CXR has an acceptable sensitivity in detecting PAVM with a diameter of 1.0–2.0 mm [22, 23]. The typical performance of PAVM on CXR is a lobular or circular soft tissue mass which sometimes is linked to dilated vascular channels. TCD-b has been widely used in the screening of RLS, which has the advantages of non-invasiveness, good patient compliance, and 100.0% sensitivity in detecting RLS [24].
However, TCD-b cannot accurately distinguish between intra- and extracardiac shunts [24]. Contrast TEE can directly display cardiac anatomical structures such as ASD and PFO. The sensitivity and specificity of TEE with Valsalva maneuver (VM) in detecting PFO are very high [25]. Given the partial invasiveness of TEE, it is difficult for patients to cooperate to complete VM. False-negative results may occur, and the incidence of complications can reach 0.9% [26]. TTE with bubble study is recommended as a tool for screening patients with high-risk PAVM [27]. If any bubbles are detected in the left atrium, TTE is considered positive, indicating an extracardiac shunt. Furthermore, CTA or digital subtraction angiography of the pulmonary artery can help accurately diagnose and locate the PAVM. A chest CTA is a non-invasive angiography technique, which can not only find small lesions and measure the diameter of the feeding artery but also be more accurate in the diagnosis of peripheral and complex PAVM [28]. However, this examination has some radiation, and patients need to be injected with contrast agents. By far, digital subtraction angiography of the pulmonary artery is considered the gold standard for the diagnosis of PAVM and can locate and define various forms of PAVM; additionally, this examination technology with certain complications is invasive and expensive [29].
Treatment of Cryptogenic Stroke in PAVM Patients
In terms of treatment, it is advocated that active treatment should be provided to PAVM patients who have a feeding artery diameter > 3.0 mm; if conditions permit, feeding arteries with > 1.5 mm diameter should also be actively treated [11]. Some studies showed that patients with PAVM have higher recurrence rates of ischemic stroke and TIA than patients with PFO [30]. At present, the clinical treatment methods include conservative treatment, interventional therapy, and surgical treatment [31]. Asymptomatic patients can be treated conservatively, mainly to prevent respiratory tract infection and severe cough. Interventional therapy is less invasive and repeatable and can retain more lung tissue. However, multiple and extensive diffuse PAVMs are contraindications for interventional therapy. Surgery is a radical treatment, which has the advantages of being associated with high efficiency, relative safety, and low recurrence rates, but it is only suitable for a large single lesion or a single lobe of the lung [32].
Strengths and Implications
In this study, we reported a case of CS with limb weakness as the first symptom, resulting from an acute ischemic stroke. The limb weakness of the patient is an atypical presentation of PAVM, which should be cautiously considered given that misdiagnosis may be associated with greater rates of mortality and disability. TCD-b, TEE, and TTE played an essential role in detecting the RLS and ruling out PFO and ASD. CTA helped to definitively diagnose and locate of the PAVM. The patient was treated by thrombolytic therapy and transcatheter device occlusion of arteriovenous fistula, after which she continued her rehabilitation training, yielding good outcomes.
This study provides novel evidence that thrombolytic therapy within the time window combined with transcatheter device occlusion of arteriovenous malformation and rehabilitation training may be beneficial to the recovery of patients with CS caused by PE resulting from PAVM.
Conclusion
CS is a relatively rare neurological complication of PAVM. The diagnosis for CS/PVAM starts by first excluding common causes of stroke. RLS should be considered when CS occurs with limb weakness or other symptoms as the first symptom. For the diagnostic process of RLS, CXR, 24-h ambulatory electrocardiograph, and echocardiography are usually used as an office-based alternative for the screening of PAVM. TCD-b is often performed to assess RLS. If TCD-b is positive, TEE and TTE should be conducted to distinguish between intra- and extracardiac shunts. A CTA or digital subtraction angiography of the pulmonary artery is the recommended gold standard for diagnosing and locating a PAVM. Thrombolytic therapy within the time window combined with transcatheter device occlusion of arteriovenous fistula and rehabilitation training may benefit the recovery of patients with CS caused by PE due to PAVM.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patient for her participation.
Funding
This study was funded by the special project of “Lingnan modernization of traditional Chinese medicine” of the 2019 Guangdong Provincial R & D Program (no. 2020B1111100008), the Chinese Medicine Innovation Team Project of the State Administration of Traditional Chinese Medicine, and the project of the Traditional Chinese Medicine Bureau of Guangdong Province (no. 20201153). The above projects were funded the journal’s Rapid Service Fee.
Editorial Assistance
We also thank Editage (www.editage.com) for English language editing; the authors provided the funding for this assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Jie Zhan contributed to data collection gathering, designing the case report, data analysis and interpretation as well as writing the initial and subsequent drafts after they were revised by all the authors involved and submitting the final case report. Jianhua Liu, Liming Lu and Hongxia Chen contributed to data analysis and interpretation as well as critically revising the article. Cong Dong, Mei Li and Lechang Zhan contributed by data collection gathering.
Disclosures
Jie Zhan, Cong Dong, Mei Li, Lechang Zhan, Hongxia Chen, Liming Lu, and Jianhua Liu have nothing to disclose.
Compliance with Ethics Guidelines
Informed consent and consent for publication were provided by the patient. The study was approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (no. AF/04-07.0/10.0) and was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Contributor Information
Liming Lu, Email: lulimingleon@126.com.
Jianhua Liu, Email: jyhf08@sina.com.
References
- 1.Nakayama M, Nawa T, Chonan T, et al. Prevalence of pulmonary arteriovenous malformations as estimated by low-dose thoracic CT screening. Intern Med. 2012;51:1677–1681. doi: 10.2169/internalmedicine.51.7305. [DOI] [PubMed] [Google Scholar]
- 2.Guan Q, Nie ZY. Paradoxical embolism and cryptogenic stroke. Chin J Intern Med. 2014;53(02):149–150. [Google Scholar]
- 3.Guo YZ, Xing YQ. Discussion on the problems related to the diagnosis of right-to-left shunt by contrast-enhanced transcranial Doppler. Chin J Stroke. 2016;11(1):515–529. [Google Scholar]
- 4.Gossage JR, Kanj G. Pulmonary arteriovenous malformations: a state of the art review. Am J Respir Crit Care Med. 1998;158(2):643–661. doi: 10.1164/ajrccm.158.2.9711041. [DOI] [PubMed] [Google Scholar]
- 5.Smith HL, Horton BT. Arteriovenous fistula of the lung associated with polycythemia vera: report of a case in which the diagnosis was made clinically. Am Heart J. 1939;18(5):589–592. doi: 10.1016/S0002-8703(39)90882-3. [DOI] [Google Scholar]
- 6.Rengifo-Moreno P, Palacios IF, Junpaparp P, et al. Patent foramen ovale transcatheter closure vs. medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2013;34:3342–3352. doi: 10.1093/eurheartj/eht285. [DOI] [PubMed] [Google Scholar]
- 7.Hart RG, Miller VT. Cerebral infarction in young adults: a practical approach. Stroke. 1983;14(1):110–114. doi: 10.1161/01.STR.14.1.110. [DOI] [PubMed] [Google Scholar]
- 8.Adams HP, Butler MJ, Biller J, et al. Nonhemorrhagic cerebral infarction in young adults. Arch Neurol. 1986;43(8):793–796. doi: 10.1001/archneur.1986.00520080041017. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura H, Miwa K, Haruki T, et al. Pulmonary arteriovenous fistula with cerebral infarction successfully treated by video-assisted thoracic surgery. Ann Thorac Cardiovasc Surg. 2008;14(1):35–37. [PubMed] [Google Scholar]
- 10.Yeung M, Khan KA, Antecol DH, et al. Transcranial Doppler ultrasonography and transesophageal echocardiography in the investigation of pulmonary arteriovenous malformation in a patient with hereditary hemorrhagic telangiectasia presenting with stroke. Stroke. 1995;26(10):1941. doi: 10.1161/01.STR.26.10.1941. [DOI] [PubMed] [Google Scholar]
- 11.Moussouttas M, Fayad P, Rosenblatt M, et al. Pulmonary arteriovenous malformations Cerebral ischemia and neurologic manifestations. Neurology. 2000;55(7):959. doi: 10.1212/WNL.55.7.959. [DOI] [PubMed] [Google Scholar]
- 12.Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary hemorrhagic telangiectasia. J Med Genet. 2011;48(2):73–87. doi: 10.1136/jmg.2009.069013. [DOI] [PubMed] [Google Scholar]
- 13.Dong XJ, Zhou GF, Liang B, et al. Clinical analysis of imaging diagnosis and interventional treatment of pulmonary arteriovenous malformations. J Clin Radiol. 2016;35(01):131–134. [Google Scholar]
- 14.Shovlin CL. Pulmonary arteriovenous malformations. Am J Respir Crit Care Med. 2014;190(11):1217–1228. doi: 10.1164/rccm.201407-1254CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller-Hülsbeck S, Marques L, Maleux G, et al. CIRSE standards of practice on diagnosis and treatment of pulmonary arteriovenous malformations. Cardiovasc Intervent Radiol. 2019;43(3):353–361. doi: 10.1007/s00270-019-02396-2. [DOI] [PubMed] [Google Scholar]
- 16.Nacaroğlu HT, ÜnsalKarkıner CŞ, BahçeciErdem S, et al. Pulmonary vascular anomalies: a review of clinical and radiological findings of cases presenting with different complaints in childhood. Turk J Pediatr. 2016;58(3):337–342. doi: 10.24953/turkjped.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Shovlin CL, Condliffe R, Donaldson JW, et al. British Thoracic Society clinical statement on pulmonary arteriovenous malformations. Thorax. 2017;72(12):1154–1163. doi: 10.1136/thoraxjnl-2017-210764. [DOI] [PubMed] [Google Scholar]
- 18.Atshalan S, Williams LC, Tighe HC, et al. Arterial oxygen content is precisely maintained by graded erythrocytosis responses in settings of high/normal serum iron levels and predicts exercise capacity: an observational study of hypoxaemic patients with pulmonary arteriovenous malformations. Plus One. 2014;9(3):e90777. doi: 10.1371/journal.pone.0090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz MD, Palma NZ, Rocha J, et al. Pulmonary arteriovenous malformation can be associated with embolic stroke of undetermined source (ESUS) Eur J Case Rep Intern Med. 2019;6(11):001262. doi: 10.12890/2019_001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panneerselvam S, Othman BA, Kini A, et al. Pulmonary arteriovenous malformation-related embolic stroke causing thalamic esotropia. J Neuro-Ophthalmol. 2019;00:1–2. doi: 10.1097/WNO.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 21.Mehrbod N, Chitsaz A, Saadatnia M, et al. Stroke in a patient with pulmonary arteriovenous fistula: a case report study. Adv Biomed Res. 2013;2(4):1–4. doi: 10.4103/2277-9175.122499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen R. Pulmonary arteriovenous fistulae thrombosis responsible for recurrent stroke. J Neurol Neurosurg Psychiatry. 2006;77(5):707. doi: 10.1136/jnnp.2005.079764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trerotola SO, Pyeritz RE. PAVM embolization: an update. Am J Roentgenol. 2010;195:837–845. doi: 10.2214/AJR.10.5230. [DOI] [PubMed] [Google Scholar]
- 24.Manawadu D, Vethanayagam D, Saqqur M, et al. Screening for right-to-left shunts with contrast transcranial Doppler in hereditary hemorrhagic telangiectasia. Stroke. 2011;42(5):1473–1474. doi: 10.1161/STROKEAHA.110.608224. [DOI] [PubMed] [Google Scholar]
- 25.Wozniak L, Mielczarek M, Sabiniewicz R. Paradoxical brain embolism in a young man: is it only a patent foramen ovale? Neurol Neurochir Pol. 2015;49:61–64. doi: 10.1016/j.pjnns.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Jauss M, Zanette E. Detection of right-to-left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovasc Dis. 2000;10(6):490–496. doi: 10.1159/000016119. [DOI] [PubMed] [Google Scholar]
- 27.Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011;48(2):73–87. doi: 10.1136/jmg.2009.069013. [DOI] [PubMed] [Google Scholar]
- 28.Chamarthy MR, Park H, Sutphin P, et al. Pulmonary arteriovenous malformations: endovascular therapy. Cardiovasc Diagn Ther. 2018;8(3):338–349. doi: 10.21037/cdt.2017.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo GZ, Chen JB. Research advances in the diagnosis and treatment of pulmonary arteriovenous fistula. Med Recap. 2015;21(8):1460–1462. [Google Scholar]
- 30.Horner S, Niederkorn K, Gattringer T, et al. Management of right-to-left shunt in cryptogenic cerebrovascular disease: results from the observational Austrian paradoxical cerebral embolism trial (TACET) registry. Neurology. 2013;260(1):260–267. doi: 10.1007/s00415-012-6629-9. [DOI] [PubMed] [Google Scholar]
- 31.Contegiacomo A, Ciello AD, Rella R, et al. Pulmonary arteriovenous malformations: what the interventional radiologist needs to know. Radiol Med (Torino) 2019;124(10):973–988. doi: 10.1007/s11547-019-01051-7. [DOI] [PubMed] [Google Scholar]
- 32.Hull JE, Kinsey EN, Bishop WL. Mapping of the snuffbox and cubital vessels for percutaneous Arterial Venous Fistula (pAVF) in dialysis patients. J Vasc Access. 2013;14(3):245–251. doi: 10.5301/jva.5000127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.