Abstract
Introduction
Transthyretin amyloidosis (ATTR amyloidosis) is a clinically heterogeneous disease caused by mutations in the transthyretin (TTR) gene or aggregation of wild-type transthyretin (ATTRwt). In Spain, there are two large endemic foci of ATTR amyloidosis caused by the Val30Met variant, with additional cases across the country; however, these data may be incomplete, as there is no centralized patient registry. The Transthyretin Amyloidosis Outcomes Survey (THAOS) is an ongoing, global, longitudinal, observational survey of patients with ATTR amyloidosis, including both inherited and wild-type disease, and asymptomatic patients with TTR mutations. This analysis aimed to gain a deeper understanding of the clinical profile of patients with ATTR amyloidosis in Spain.
Methods
This was a descriptive analysis of the demographic and clinical characteristics of symptomatic patients enrolled at six sites geographically dispersed throughout Spain (data cutoff: January 6, 2020). Patient data at enrollment, including genotype, demographics, and clinical presentation for symptomatic patients, were recorded. Patients were grouped by predominant phenotype based on clinical measures at enrollment: predominantly cardiac, predominantly neurologic, or mixed (cardiac and neurologic).
Results
There were 379 patients (58.0% male; 63.3% symptomatic) enrolled in the six THAOS sites in Spain. Predominant genotypes were the Val30Met mutation (69.1%) or ATTRwt (15.6%). Predominant phenotype distribution was neurologic (50.4%), mixed (35.8%), and cardiac (13.8%) for all symptomatic patients (n = 240); neurologic (67.8%), mixed (21.2%), and cardiac (11.0%) for symptomatic Val30Met (n = 146); and mixed (64.9%), cardiac (22.8%), and neurologic (12.3%) for symptomatic ATTRwt (n = 57). Symptomatic patients reported a range of ATTR amyloidosis signs and symptoms at enrollment, with autonomic neuropathy and sensory neuropathy common in all phenotypes.
Conclusions
These results from THAOS highlight the phenotypic heterogeneity associated with ATTR amyloidosis in Spain and the importance of comprehensive neurologic and cardiac evaluations in all patients with ATTR amyloidosis.
Trial registration
ClinicalTrials.gov: NCT00628745.
Keywords: Amyloidosis, Cardiac, Polyneuropathy, Spain, Transthyretin
Key Summary Points
| Why carry out this study? |
| Transthyretin amyloidosis is a heterogeneous disease caused by mutations in the transthyretin (TTR) gene (ATTRv amyloidosis) or the aggregation of wild-type TTR (ATTRwt amyloidosis). |
| In Spain there are two large endemic foci of ATTRv amyloidosis (Majorca and Huelva), and additional cases occur across the country; however, these data may be incomplete, as there is no centralized patient registry. |
| This study was carried out to gain a deeper understanding of the clinical profile of patients with ATTR amyloidosis in Spain. |
| What was learned from the study? |
| Over half of the patients with ATTR amyloidosis enrolled in the Transthyretin Amyloidosis Outcomes Survey (THAOS) in Spain were from non-endemic sites, suggesting wide dispersion of the disease across the country. |
| Furthermore, results of this analysis highlight the multisystemic nature and phenotypic heterogeneity associated with ATTR amyloidosis in Spain and the importance of a comprehensive multidisciplinary approach for all patients with ATTR amyloidosis. |
Introduction
Transthyretin amyloidosis (ATTR amyloidosis) is a progressive, and life-threatening, heterogeneous disease caused by mutations in the transthyretin (TTR) gene (ATTRv amyloidosis) or the aggregation of wild-type TTR (ATTRwt amyloidosis), resulting in the deposition of amyloid fibrils in peripheral nerves and vital organs, leading mainly to polyneuropathy and/or cardiomyopathy [1].
Transthyretin amyloid polyneuropathy is a common clinical presentation of ATTRv amyloidosis, and is most frequently associated with the Val30Met (p.Val50Met) variant, the most common TTR mutation worldwide [1]. It is considered endemic in Portugal and Sweden, with foci in Japan, Brazil, and certain regions of Spain [2].
More than 140 ATTRv genotypes have been identified (http://amyloidosismutations.com/attr.html), and four main phenotype groups largely associated with TTR mutation or time of disease onset have been described: Val30Met early onset (≤ 50 years); Val30Met late onset (> 50 years); non-Val30Met mixed phenotype, and non-Val30Met cardiac phenotype (e.g., Val122Ile [p.Val142Ile]) [3].
While TTR mutations may be associated with a specific phenotype [3, 4], the clinical presentation may also be influenced by other factors such as a patient’s country of origin. In Portuguese patients with the Val30Met variant, symptoms typically emerge in the third decade (early-onset disease), while in patients with the Val30Met variant from other European countries, symptoms emerge in the seventh decade (late-onset disease), and often with a mixed phenotype [5, 6]. Other factors such as age, sex, fibril type, and maternal inheritance may also influence phenotype and age of onset [7].
In Spain there are two large endemic foci of ATTRv amyloidosis (Majorca and Huelva), and additional cases occur across the country; however, these data may be incomplete, as there is no centralized patient registry. In Majorca, the largest focus in Spain, the prevalence has been estimated to be 5/100,000 inhabitants [8, 9], considered the fifth largest focus worldwide. In Majorca, the mean age at onset has previously been reported as 46 years [8]; epidemiological data of ATTRv amyloidosis in other areas of Spain is scarce.
An improved understanding of the regional characteristics of patients with ATTR amyloidosis can help optimize clinical assessment and disease management. The aim of this analysis was to gain a deeper understanding of the clinical profile of patients with ATTR amyloidosis in Spain enrolled in the Transthyretin Amyloidosis Outcomes Survey (THAOS). Established in 2007, THAOS is the largest ongoing, global, longitudinal, observational survey of patients with ATTR amyloidosis, both inherited and wild-type disease, and asymptomatic patients with TTR mutations (ClinicalTrials.gov: NCT00628745) [10].
Methods
The design of THAOS has been published previously [10]. Symptomatic patients enrolled in six sites geographically dispersed throughout Spain participated in this analysis (data cutoff: January 6, 2020). These sites included Huelva, Barcelona, San Sebastian, Madrid, and Palma de Majorca. Clinical characteristics of patients, by each phenotype, were assessed.
All study sites received ethical or institutional review board approval prior to patient enrollment, and each patient provided written informed consent. The study followed the Good Pharmacoepidemiology Practice guidelines and the principles of the Declaration of Helsinki.
Patient demographics of sex, geographical location, race/ethnicity, age at enrollment and symptom onset, and time from symptom onset to diagnosis were examined. Patients were classified into one of three phenotypes based on symptoms at enrollment in THAOS [4]. Patients with the predominantly cardiac phenotype were those (1) with abnormal electrocardiogram (ECG) due to rhythm disturbance, heart failure, or dyspnea, and (2) who did not have more than mild neurologic or gastrointestinal (GI) symptoms (excluding erectile dysfunction, constipation, and carpal tunnel syndrome). Patients with the predominantly neurologic phenotype were those (1) with walking disability of any severity, other neurologic symptoms of any severity, or GI symptoms (early satiety, nausea, vomiting, unintentional weight loss, diarrhea, constipation, or fecal incontinence) of any severity, and (2) who did not have abnormal ECG due to rhythm disturbance, heart failure, or dyspnea. Patients with the mixed phenotype were all remaining symptomatic patients who did not meet the criteria for either cardiac or neurologic phenotype.
Several clinical characteristics were assessed at enrollment. Neurologic impairment was measured by a derived Neuropathy Impairment Score in the Lower Limbs (NIS-LL; ranging from 0 [normal] to 88 [total impairment]) [11]. The NIS-LL scale includes reflex, motor, and sensory subscales measuring key physiological responses. The Karnofsky Performance Status Scale was used to quantify the patient’s ability to perform normal daily life activities and their need for assistance (ranging from 0 [dead] to 100 [normal; no complaints]). Quality of life was assessed using the (1) EQ-5D-3L, a self-administered health status instrument by which respondents rated their current health state on five dimensions (mobility, self-care, usual activities, pain or discomfort, and anxiety or depression), with each dimension having three levels of function (1 = no problems, 2 = some problems, and 3 = extreme problems) [12], and (2) Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (Norfolk QoL-DN), a patient-reported questionnaire that returns a total quality of life score ranging from −4 to 136, with higher scores reflecting poorer quality of life [11, 13]. Measures of cardiac disease were left ventricular (LV) septal thickness (mm) and LV ejection fraction (%).
Statistical Analysis
Data are presented as mean (standard deviation [SD]) and percentages unless stated otherwise.
Results
Overall Population
There were 379 patients enrolled in the six THAOS sites in Spain at the time of data cutoff. Most patients had the Val30Met mutation (69.1%) or ATTRwt amyloidosis (15.6%) (Table 1). Almost half of the patients (43.5%) were included from the two Val30Met endemic foci in Spain (Majorca and Huelva). There was a greater proportion of men in the whole cohort (58.0% male), which was more pronounced among patients with ATTRwt amyloidosis (84.7% male). The median age at onset of earliest ATTR amyloidosis was 51.4 years (10th percentile 27.5, 90th percentile 74.4), with a mean duration of disease of 9.7 years (SD 8.4) (Table 2). Finally, 91.0% of the overall population was Caucasian.
Table 1.
Genotype distribution: overall population and symptomatic patients
| Overall population N = 379, n (%) | Symptomatic patients N = 240, n (%) | |
|---|---|---|
| Val30Met (p.Val50Met) | 262 (69.1) | 146 (60.8) |
| Wild-type | 59 (15.6) | 57 (23.8) |
| Val122Ile (p.Val142Ile) | 14 (3.7) | 10 (4.2) |
| delVal122 (p.delVal142) | 14 (3.7) | 7 (2.9) |
| Glu89Lys (p.Glu109Lys) | 12 (3.2) | 9 (3.8) |
| Ala45Thr (p.Ala65Thr) | 3 (0.8) | 2 (0.8) |
| Glu89Gln (p.Glu109Gln) | 3 (0.8) | 1 (0.4) |
| Ile107Met (p.Ile127Met) | 3 (0.8) | 2 (0.8) |
| Ser77Tyr (p.Ser97Tyr) | 3 (0.8) | 1 (0.4) |
| Val71Ala (p.Val91Ala) | 2 (0.5) | 1 (0.4) |
| Asp18Asn (p.Asp38Asn) | 1 (0.3) | 1 (0.4) |
| Asp38Ala (p.Asp58Ala) | 1 (0.3) | 1 (0.4) |
| Glu89Gly (p.Glu109Gly) | 1 (0.3) | 1 (0.4) |
| Thr60Ala (p.Thr80Ala) | 1 (0.3) | 1 (0.4) |
Data cutoff: January 6, 2020
Table 2.
Demographic and baseline characteristics: overall population and symptomatic patients
| Overall population (N = 379) | Symptomatic patients (N = 240) | |||
|---|---|---|---|---|
| Cardiac phenotypea (N = 33) | Neurologic phenotypeb (N = 121) | Mixed phenotypec (N = 86) | ||
| Age at signed, informed consent, years | ||||
| n | 379 | 33 | 121 | 86 |
| Mean (SD) | 56.1 (17.8) | 71.4 (13.3) | 55.3 (14.3) | 65.6 (18.0) |
| Median (10th percentile, 90th percentile) | 55.4 (34.2, 80.0) | 72.8 (55.4, 85.8) | 54.9 (37.2, 74.8) | 69.6 (40.2, 87.2) |
| Age at onset of earliest ATTR amyloidosis symptoms, years | ||||
| n | 277 | 33 | 121 | 86 |
| Mean (SD) | 51.0 (17.6) | 63.8 (17.1) | 46.7 (15.8) | 53.2 (18.5) |
| Median (10th percentile, 90th percentile) | 51.4 (27.5, 74.4) | 68.5 (42.4, 82.5) | 47.7 (25.6, 69.0) | 55.1 (27.1, 77.0) |
| Duration of disease at time of consent, years | ||||
| n | 260 | 33 | 121 | 86 |
| Mean (SD) | 9.7 (8.4) | 7.5 (6.4) | 8.6 (7.5) | 12.4 (9.3) |
| Median (10th percentile, 90th percentile) | 7.9 (0.9, 19.9) | 5.2 (1.8, 14.8) | 7.2 (0.7, 17.2) | 10.1 (2.1, 25.3) |
| Time from symptom onset to diagnosis, yearsd | ||||
| n | 187 | 28 | 97 | 62 |
| Mean (SD) | 5.0 (7.5) | 4.0 (5.4) | 3.3 (7.0) | 8.1 (8.1) |
| Median (10th percentile, 90th percentile) | 2.7 (0.0, 17.0) | 2.5 (0.0, 9.3) | 1.3 (–1.4, 13.9) | 5.5 (0.3, 18.3) |
Data cutoff: January 6, 2020
Overall population includes asymptomatic and symptomatic patients. Patients were grouped into a predominant clinical phenotype based on clinical presentation at enrollment
ATTR amyloidosis transthyretin amyloidosis, ECG electrocardiogram, GI gastrointestinal, SD standard deviation
aPatients with the cardiac phenotype are those (1) with abnormal ECG due to rhythm disturbance, heart failure, or dyspnea, and (2) who do not have more than mild neurologic or GI symptoms (excluding erectile dysfunction, constipation, and carpal tunnel syndrome)
bPatients with the neurologic phenotype are those (1) with walking disability of any severity, other neurologic symptoms of any severity, or GI symptoms (early satiety, nausea, vomiting, unintentional weight loss, diarrhea, constipation, or fecal incontinence) of any severity, and (2) who do not have abnormal ECG due to rhythm disturbance, heart failure, or dyspnea
cPatients with the mixed phenotype are all remaining symptomatic patients who do not meet the criteria for either cardiac or neurologic phenotype
dNegative percentile reflects that date of diagnosis is earlier than date of symptom onset
Of all 379 patients, 240 (63.3%) were symptomatic. Of those, 150 (62.5%) were male and 146 (60.8%) carried the Val30Met mutation. Half of the symptomatic patients (50.4%) had a neurologic phenotype (Table 2). Patients with a cardiac phenotype had a higher mean age at symptom onset than those with a mixed or neurologic phenotype (63.8 years [SD 17.1] vs. 53.2 years [SD 18.5] vs. 46.7 years [SD 15.8], respectively), while patients with a mixed phenotype had the longest delay from symptom onset to diagnosis (8.1 years [SD 8.1]) (Table 2).
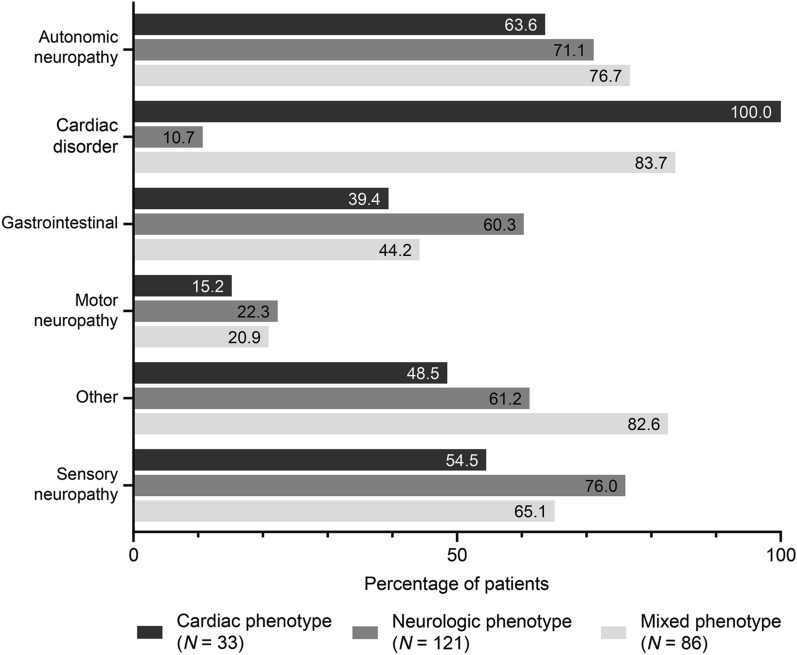
Patients reported a range of ATTR amyloidosis signs and symptoms at enrollment, with autonomic neuropathy and sensory neuropathy common in all phenotypes (Fig. 1). Clinical characteristics at enrollment for symptomatic patients were consistent with phenotype, with Norfolk QoL-DN total quality of life score appearing to be poorest in patients with a mixed phenotype (Table 3).
Fig. 1.
Signs and symptoms at enrollment by predominant phenotype. Data cutoff: January 6, 2020. Cardiac phenotype, n = 33; neurologic phenotype, n = 121; mixed phenotype, n = 86. “Autonomic neuropathy” includes dizziness, dry eye, dyshidrosis, palpitations, recurrent urinary tract infections, urinary incontinence, urinary retention, vomiting, constipation, diarrhea, early satiety, fecal incontinence, nausea, erectile dysfunction. “Cardiac disorder” includes coronary artery disease, dyspnea, heart failure, other cardiovascular disease, rhythm disturbance, syncope, myocardial infarction. “Gastrointestinal” includes constipation, diarrhea, early satiety, fecal incontinence, nausea, unintentional weight loss, vomiting. “Motor neuropathy” includes muscle weakness, walking disability. “Other” includes adrenal insufficiency, cerebrovascular accident/stroke, cognitive decline, depression, dialysis, fractures, glaucoma, hyperlipidemia, inflammatory arthritis, inflammatory bowel disease, osteoarthritis, osteoporosis, other endocrine/metabolic disease, other eye disease, other gastrointestinal disease, other genitourinary/reproductive disease, other musculoskeletal disease, other neurologic diagnosis, other psychiatric diagnosis, other respiratory disease, pneumonia, renal impairment, thyroid dysfunction, visual impairment and vitrectomy, transplant, kidney stones, diabetes mellitus, asthma, chronic obstructive pulmonary disease, hepatitis, peptic ulcer disease, chronic demyelinating inflammatory polyneuropathy, carpal tunnel syndrome, seizures, schizophrenia. “Sensory neuropathy” includes balance abnormality, neuropathic arthropathy or pain/paresthesia, numbness, temperature/pain insensitivity, tingling. Categories are not mutually exclusive
Table 3.
Clinical characteristics at enrollment, by predominant phenotype: symptomatic patients (N = 240)
| Cardiac phenotypea (N = 33) | Neurologic phenotypeb (N = 121) | Mixed phenotypec (N = 86) | |
|---|---|---|---|
| Derived NIS-LL score | |||
| n | 16 | 53 | 51 |
| Mean (SD) | 8.0 (14.1) | 10.6 (20.2) | 7.0 (12.3) |
| Median (10th percentile, 90th percentile) | 2.5 (0.0, 39.0) | 0.0 (0.0, 53.0) | 0.0 (0.0, 20.0) |
| Reflex score | |||
| n | 19 | 84 | 69 |
| Mean (SD) | 1.3 (2.7) | 1.7 (3.1) | 0.8 (2.3) |
| Median (10th percentile, 90th percentile) | 0.0 (0.0, 7.0) | 0.0 (0.0, 8.0) | 0.0 (0.0, 2.0) |
| Motor score | |||
| n | 20 | 65 | 63 |
| Mean (SD) | 1.9 (5.5) | 5.8 (14.6) | 0.9 (3.7) |
| Median (10th percentile, 90th percentile) | 0.0 (0.0, 9.0) | 0.0 (0.0, 26.0) | 0.0 (0.0, 2.0) |
| Sensory score | |||
| n | 14 | 66 | 55 |
| Mean (SD) | 5.7 (10.4) | 7.7 (17.9) | 6.6 (14.7) |
| Median (10th percentile, 90th percentile) | 0.0 (0.0, 26.0) | 0.0 (0.0, 28.0) | 0.0 (0.0, 32.0) |
| KPS score, % | |||
| n | 27 | 67 | 62 |
| Mean (SD) | 81.5 (12.9) | 86.9 (13.8) | 77.9 (17.1) |
| Median (10th percentile, 90th percentile) | 80.0 (60.0, 100.0) | 90.0 (70.0, 100.0) | 80.0 (50.0, 100.0) |
| LV septum, mm | |||
| n | 16 | 49 | 70 |
| Mean (SD) | 17.1 (3.0) | 13.2 (4.1) | 15.5 (4.7) |
| Median (10th percentile, 90th percentile) | 17.5 (12.0, 21.0) | 12.0 (8.0, 19.0) | 16.0 (10.0, 21.5) |
| LV ejection fraction, % | |||
| n | 15 | 48 | 68 |
| Mean (SD) | 55.3 (11.5) | 60.9 (7.2) | 55.9 (12.1) |
| Median (10th percentile, 90th percentile) | 60.0 (36.0, 65.0) | 60.0 (54.0, 70.0) | 57.0 (38.0, 69.0) |
| EQ-5D health state | |||
| n | 20 | 43 | 68 |
| Mean (SD) | 71.4 (18.1) | 72.7 (15.6) | 67.5 (19.1) |
| Median (10th percentile, 90th percentile) | 70.0 (45.0, 99.0) | 80.0 (50.0, 90.0) | 70.0 (40.0, 90.0) |
| EQ-5D index, derived value | |||
| n | 20 | 43 | 68 |
| Mean (SD) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) |
| Median (10th percentile, 90th percentile) | 0.8 (0.6, 1.0) | 0.8 (0.5, 1.0) | 0.8 (0.4, 1.0) |
| Norfolk QoL-DN: total quality of life score | |||
| n | 19 | 48 | 59 |
| Mean (SD) | 26.6 (25.5) | 24.2 (26.8) | 31.5 (33.1) |
| Median (10th percentile, 90th percentile) | 31.0 (–1.0, 58.0) | 13.5 (0.0, 67.0) | 20.0 (0.0, 89.0) |
Data cutoff: January 6, 2020
Patients were grouped into a predominant clinical phenotype based on clinical presentation at enrollment
ECG electrocardiogram, EQ-5D EuroQol quality-of-life questionnaire, GI gastrointestinal, KPS Karnofsky Performance Status, LV left ventricular, NIS-LL Neuropathy Impairment Score in the Lower Limbs, Norfolk QoL-DN Norfolk Quality of Life-Diabetic Neuropathy questionnaire, SD standard deviation
aPatients with the cardiac phenotype are those (1) with abnormal ECG due to rhythm disturbance, heart failure, or dyspnea, and (2) who do not have more than mild neurologic or GI symptoms (excluding erectile dysfunction, constipation, and carpal tunnel syndrome)
bPatients with the neurologic phenotype are those (1) with walking disability of any severity, other neurologic symptoms of any severity, or GI symptoms (early satiety, nausea, vomiting, unintentional weight loss, diarrhea, constipation, or fecal incontinence) of any severity, and (2) who do not have abnormal ECG due to rhythm disturbance, heart failure, or dyspnea
cPatients with the mixed phenotype are all remaining symptomatic patients who do not meet the criteria for either cardiac or neurologic phenotype
The overall phenotype distribution for symptomatic patients (n = 240) was neurologic (50.4%), mixed (35.8%), and cardiac (13.8%). Most patients with the Val30Met variant had a neurologic phenotype (67.8%), but there were many with a mixed (21.2%) or cardiac (11.0%) phenotype. Notably, most patients with ATTRwt amyloidosis had a mixed phenotype (64.9%), with just over one-third presenting with a cardiac (22.8%) or neurologic (12.3%) phenotype (Table 4).
Table 4.
ATTR amyloidosis phenotype distribution by genotype: symptomatic patients (N = 240)
| Genotype | Overall, N | Cardiaca | Neurologicb | Mixedc | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Val30Met (p.Val50Met) | 146 | 16 | 11.0 | 99 | 67.8 | 31 | 21.2 |
| Wild-type | 57 | 13 | 22.8 | 7 | 12.3 | 37 | 64.9 |
| Val122Ile (p.Val142Ile) | 10 | 2 | 20.0 | 4 | 40.0 | 4 | 40.0 |
| Glu89Lys (p.Glu109Lys) | 9 | 0 | 0.0 | 4 | 44.4 | 5 | 55.6 |
| delVal122 (p.delVal142) | 7 | 1 | 14.3 | 3 | 42.9 | 3 | 42.9 |
| Ala45Thr (p.Ala65Thr) | 2 | 0 | 0.0 | 0 | 0.0 | 2 | 100.0 |
| Ile107Met (p.Ile127Met) | 2 | 0 | 0.0 | 2 | 100.0 | 0 | 0.0 |
| Asp18Asn (p.Asp38Asn) | 1 | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 |
| Asp38Ala (p.Asp58Ala) | 1 | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 |
| Glu89Gln (p.Glu109Gln) | 1 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Glu89Gly (p.Glu109Gly) | 1 | 0 | 0.0 | 1 | 100.0 | 0 | 0.0 |
| Ser77Tyr (p.Ser97Tyr) | 1 | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 |
| Thr60Ala (p.Thr80Ala) | 1 | 0 | 0.0 | 1 | 100.0 | 0 | 0.0 |
| Val71Ala (p.Val91Ala) | 1 | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 |
Data cutoff: January 6, 2020
ATTR amyloidosis transthyretin amyloidosis, ECG electrocardiogram, GI gastrointestinal
aPatients with the cardiac phenotype are those (1) with abnormal ECG due to rhythm disturbance, heart failure, or dyspnea, and (2) who do not have more than mild neurologic or GI symptoms (excluding erectile dysfunction, constipation, and carpal tunnel syndrome)
bPatients with the neurologic phenotype are those (1) with walking disability of any severity, other neurologic symptoms of any severity, or GI symptoms (early satiety, nausea, vomiting, unintentional weight loss, diarrhea, constipation, or fecal incontinence) of any severity, and (2) who do not have abnormal ECG due to rhythm disturbance, heart failure, or dyspnea
cPatients with the mixed phenotype are all remaining symptomatic patients who do not meet the criteria for either cardiac or neurologic phenotype
Discussion
In this first descriptive analysis of the ATTR amyloidosis population in Spain we found, as expected, that Val30Met is, by far, the most frequent variant. Although Spain has two endemic foci of patients with ATTR amyloidosis with the Val30Met variant, we found that more than half of the patients with ATTR amyloidosis enrolled in THAOS in Spain were included from non-endemic sites. This shows the wide dispersion of the disease across the country, and the need for clinical suspicion in non-endemic areas. Moreover, our results highlight the multisystemic nature and phenotypic heterogeneity associated with ATTR amyloidosis in Spain and the importance of a comprehensive multidisciplinary approach for all patients with ATTR amyloidosis [14].
The neurologic phenotype was the predominant clinical picture of patients with ATTR amyloidosis in Spain, followed by the mixed phenotype. The neurologic phenotype is also predominant in other countries with endemic foci of patients with the Val30Met variant, including Portugal, Sweden, Japan, and Brazil [4, 15, 16]. The cardiac phenotype is more commonly seen in other European countries such as Germany, Denmark, and Italy, where there is a greater distribution of genotypes [4], and in the United States, where a large proportion of patients have ATTRwt amyloidosis or the Val122Ile (p.Val142Ile) mutation [17]. More recent studies suggest that a mixed phenotype is relatively common [4, 18].
Over 30% of patients with the Val30Met variant experienced cardiac symptoms, with or without neurologic symptoms. Cardiac involvement has previously been reported to be present in similar proportions (30–36%) among patients with Val30Met [4, 15, 16, 19] and, as expected, is more frequent among those patients with late-onset disease [4, 15]. This reinforces the need for a multidisciplinary approach in endemic areas, independent of the most frequent phenotype of the area.
More remarkable is the fact that 11.0% of patients with Val30Met had a cardiac phenotype, which has been shown in other analyses [15, 20]. Isolated cardiac involvement in patients with Val30Met is rare.
Phenotypic variations are observed between populations of patients with the Val30Met mutation. As previously published [8], the ATTR amyloidosis Val30Met population in Spain presents with a “middle-age” disease onset. Clinically, this finding is reflected in the high proportion of patients showing autonomic neuropathy (typical of early disease onset) and sensorimotor neuropathy or mixed phenotype (typical of late disease onset) [3]. In Portugal, onset is early (around 30 years), with high penetrance, and the disease is characterized by length-dependent small-fiber sensory-motor polyneuropathy and severe autonomic dysfunction [2, 21]. Conversely, the age of onset is later (after 50 years) and penetrance much lower in Sweden, and neuropathy tends to affect all fibers, with mild autonomic symptoms [2, 22]. Onset can be early or late in Japan, with corresponding differences in the clinical presentation [23]. Genetic factors aside from the TTR mutation could play a role in the phenotypic variation observed between populations. Prior reports have identified several genetic factors that may influence the age of onset such as C1Q polymorphisms [24, 25], single-nucleotide haplotype polymorphisms in the TTR gene [26, 27], mitochondrial haplogroup polymorphisms [28], RBP4 and AR gene variation [29], and mitochondrial DNA copy number [30]. Environmental factors may also play a role.
Diagnostic delay was pronounced among patients with the cardiac and mixed phenotypes. On the other hand, diagnostic delay among patients with the neurologic phenotype was limited. This reflects the high suspicion index and targeted follow-up of asymptomatic carriers of TTR mutations around Val30Met foci [31].
Diagnostic delay in transthyretin amyloid cardiomyopathy is frequent [32, 33] and has been reported to be more than 1 year after first cardiac evaluation. However, if diagnostic delay is defined as the time between symptom onset and diagnosis, this time would be greater, as seen in our analysis. Similar diagnostic delay was observed in a previous THAOS analysis [19].
One remarkable finding is the diagnostic delay among patients of mixed phenotype, which could be explained by the high proportion of patients with ATTRwt amyloidosis in this subgroup (64.9%). Although neurologic manifestations are increasingly being recognized in ATTRwt amyloidosis [34], the high proportion of mixed and neurologic phenotypes among patients with ATTRwt amyloidosis was surprising. We speculate that autonomic symptoms, carpal tunnel syndrome, or lumbar spinal stenosis in some patients could have been attributed to neurologic involvement in the patients registered as mixed phenotype; however, in the literature, this phenotype is characterized by sensorimotor neuropathy, which has been considered rare in ATTRwt amyloidosis [34]. Previously, symptomatic neuropathy was reported in up to 12% of cases but with limited description of associated neuropathy [35]. Recently, a single referral center review showed a prevalence of 30.5% for neuropathic symptoms among 163 patients with ATTRwt amyloidosis, with no evidence of alternative factors that cause these neuropathic symptoms [34], suggesting that neuropathic symptoms may be more common in ATTRwt amyloidosis than previously thought. However, not all patients in the current analysis had a neurologic evaluation, and most of our results are based on analysis of a small number of patients, so a multifactorial cause could not be ruled out, especially given that risk factors for neuropathy are very common in the ATTRwt amyloidosis population [16].
Our study has some limitations: (1) patients in Spain included in THAOS are being followed in referral centers; thus the cohort may be not representative of all patients with ATTR amyloidosis in Spain, especially in terms of diagnostic delay; (2) patients with ATTRv amyloidosis in Spain are probably underrepresented in this analysis, as just six hospitals participated in this THAOS analysis, though the analysis did include the two endemic foci of Val30Met in Spain (Huelva and Majorca); and (3) ATTRwt amyloidosis is likely also underrepresented, as underdiagnosis is frequent [36], and also because the THAOS registry was initially focused on ATTRv amyloidosis. There are also limitations inherent to a large global registry with the different specialties of enrolling centers potentially focusing on different clinical assessments and not all assessments available for all patients.
The main strength of the study is that in Spain there is no centralized patient registry, so THAOS represents the first national epidemiological approach to ATTR amyloidosis. More efforts are needed to increase our understanding of regional characteristics of patients with ATTR amyloidosis in order to help optimize clinical assessment and disease management.
Conclusions
ATTR amyloidosis in Spain is typically a disease that occurs in middle age, associated mainly with the Val30Met variant, and presents with neurologic or mixed phenotype. Cardiac manifestations among patients in this cohort with the Val30Met variant and neurologic complaints among those with ATTRwt amyloidosis were not uncommon, which highlights the importance of a multidisciplinary evaluation of patients with ATTR amyloidosis.
Acknowledgements
We thank Dr. Jan Kiszko for his contributions to earlier versions of this work. We thank all THAOS patients and investigators for their important contributions to this study.
Funding
The THAOS registry and this analysis were sponsored by Pfizer. Pfizer also provided the funding for the journal’s Rapid Service Fee.
Medical writing/Editorial assistance
Manuscript formatting support was provided by Emily Balevich, PhD, of Engage Scientific Solutions, funded by Pfizer; no contribution was made to editorial content.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
All authors provided substantial contributions to the design, acquisition, analysis, or interpretation of data for this analysis, and participated in revising the manuscript critically for important intellectual content.
Prior Presentation
A prior version of the analysis in this manuscript was presented at the XVII International Symposium of Amyloidosis Online Event, September 14–18, 2020.
Disclosure
Juan González-Moreno reports speaker fees from Pfizer, Alnylam, and Akcea. Inés Losada-López reports investigator fees paid to her institution for clinical trials promoted by Pfizer and Alnylam; speaker fees, travel, and accommodation remuneration from Pfizer and Alnylam; and investigation-financial support from Akcea. Eugenia Cisneros-Barroso declares that she has no competing interests. Pablo Garcia-Pavia reports speaker fees from Pfizer, Eidos, Alnylam, and Akcea; consulting fees from Pfizer, Eidos, Neurimmune, Alnylam, Prothena, and Akcea; and research/educational support to his institution from Pfizer, Eidos, and Alnylam. José González-Costello reports attending advisory boards for Pfizer and Alnylam. Francisco Muñoz-Beamud reports speaker and consultant fees from Pfizer, Alnylam, and Akcea. Josep Maria Campistol declares that he has no competing interests. Roberto Fernandez-Torron declares that he has no competing interests. Doug Chapman and Leslie Amass are full-time employees of Pfizer and hold stock options with Pfizer.
Compliance with Ethics Guidelines
All study sites received ethical or institutional review board approval prior to patient enrollment, and each patient provided written, informed consent. The study followed the Good Pharmacoepidemiology Practice guidelines and the principles of the Declaration of Helsinki.
Data Availability
Pfizer provides secure access to anonymized patient-level data to qualified researchers in response to scientifically valid research proposals. Further details can be found at https://www.pfizer.com/science/clinical-trials/trial-data-and-results.
References
- 1.Ando Y, Coelho T, Berk JL, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8:31. doi: 10.1186/1750-1172-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parman Y, Adams D, Obici L, et al. Sixty years of transthyretin familial amyloid polyneuropathy (TTR-FAP) in Europe: where are we now? A European network approach to defining the epidemiology and management patterns for TTR-FAP. Curr Opin Neurol. 2016;29(Suppl 1):S3–13. doi: 10.1097/WCO.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conceição I, Coelho T, Rapezzi C, et al. Assessment of patients with hereditary transthyretin amyloidosis - understanding the impact of management and disease progression. Amyloid. 2019;26:103–111. doi: 10.1080/13506129.2019.1627312. [DOI] [PubMed] [Google Scholar]
- 4.Damy T, Kristen AV, Suhr OB, et al. Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS) Eur Heart J. 2019 doi: 10.1093/eurheartj/ehz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conceição I, De Carvalho M. Clinical variability in type I familial amyloid polyneuropathy (Val30Met): comparison between late- and early-onset cases in Portugal. Muscle Nerve. 2007;35:116–118. doi: 10.1002/mus.20644. [DOI] [PubMed] [Google Scholar]
- 6.Hörnsten R, Pennlert J, Wiklund U, Lindqvist P, Jensen SM, Suhr OB. Heart complications in familial transthyretin amyloidosis: impact of age and gender. Amyloid. 2010;17:63–68. doi: 10.3109/13506129.2010.483114. [DOI] [PubMed] [Google Scholar]
- 7.Suhr OB, Lundgren E, Westermark P. One mutation, two distinct disease variants: unravelling the impact of transthyretin amyloid fibril composition. J Intern Med. 2017;281:337–347. doi: 10.1111/joim.12585. [DOI] [PubMed] [Google Scholar]
- 8.Cisneros-Barroso E, González-Moreno J, Rodríguez A, et al. Anticipation on age at onset in kindreds with hereditary ATTRV30M amyloidosis from the Majorcan cluster. Amyloid. 2020;27:254–258. doi: 10.1080/13506129.2020.1789580. [DOI] [PubMed] [Google Scholar]
- 9.Munar-Qués M. Polineuropatía amiloidótica familiar 2003 [Familial amyloid polyneuropathy 2003] Med Clin (Barc) 2003;121:100–101. doi: 10.1016/S0025-7753(03)73869-X. [DOI] [PubMed] [Google Scholar]
- 10.Planté-Bordeneuve V, Suhr OB, Maurer MS, White B, Grogan DR, Coelho T. The Transthyretin Amyloidosis Outcomes Survey (THAOS) registry: design and methodology. Curr Med Res Opin. 2013;29:77–84. doi: 10.1185/03007995.2012.754349. [DOI] [PubMed] [Google Scholar]
- 11.Coelho T, Vinik A, Vinik EJ, Tripp T, Packman J, Grogan DR. Clinical measures in transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2017;55:323–332. doi: 10.1002/mus.25257. [DOI] [PubMed] [Google Scholar]
- 12.EuroQol: EQ-5D, Helping the World Make Better Health Decisions™. 2020. https://euroqol.org/. Accessed 19 Nov 2020.
- 13.Vinik EJ, Vinik AI, Paulson JF, et al. Norfolk QOL-DN: validation of a patient reported outcome measure in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2014;19:104–114. doi: 10.1111/jns5.12059. [DOI] [PubMed] [Google Scholar]
- 14.Losada I, González-Moreno J, Rodriguez A, et al. Multidisciplinary approach in the management of hATTR. Eur J Clin Invest. 2020;50:e13296. [DOI] [PubMed]
- 15.Sekijima Y, Mundayat R, Ishii T, Ando Y. The current status of the Transthyretin Amyloidosis Outcomes Survey (THAOS) in Japan. Amyloid. 2019;26:61–62. doi: 10.1080/13506129.2019.1583182. [DOI] [PubMed] [Google Scholar]
- 16.Cruz MW, Pinto MV, Pinto LF, et al. Baseline disease characteristics in Brazilian patients enrolled in Transthyretin Amyloidosis Outcome Survey (THAOS) Arq Neuropsiquiatr. 2019;77:96–100. doi: 10.1590/0004-282x20180156. [DOI] [PubMed] [Google Scholar]
- 17.Maurer MS, Hanna M, Grogan M, et al. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey) J Am Coll Cardiol. 2016;68:161–172. doi: 10.1016/j.jacc.2016.03.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentile L, Tournev I, Amass L, Chapman D, Mazzeo A, et al. Phenotypic differences of Glu89Gln genotype in ATTR amyloidosis from endemic loci: update from THAOS. Cardiol Ther. 2021 doi: 10.1007/s40119-021-00226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coelho T, Maurer MS, Suhr OB. THAOS—the transthyretin amyloidosis outcomes survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29:63–76. doi: 10.1185/03007995.2012.754348. [DOI] [PubMed] [Google Scholar]
- 20.Ripoll-Vera T, Alvarez J, Buades J, et al. Cardiac involvement in a large cohort of patients with Val30Met transthyretin amyloidosis from Majorca focus. Amyloid. 2019;26:15–16. doi: 10.1080/13506129.2019.1582482. [DOI] [PubMed] [Google Scholar]
- 21.Adams D, Ando Y, Beirao JM, et al. Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy. J Neurol. 2021;268:2109–2122. doi: 10.1007/s00415-019-09688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellman U, Alarcon F, Lundgren HE, Suhr OB, Bonaiti-Pellie C, Plante-Bordeneuve V. Heterogeneity of penetrance in familial amyloid polyneuropathy, ATTR Val30Met, in the Swedish population. Amyloid. 2008;15:181–186. doi: 10.1080/13506120802193720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobue G, Koike H, Misu K, et al. Clinicopathologic and genetic features of early- and late-onset FAP type I (FAP ATTR Val30Met) in Japan. Amyloid. 2003;10(Suppl 1):32–38. doi: 10.1080/13506129.2003.12088566. [DOI] [PubMed] [Google Scholar]
- 24.Dias A, Santos D, Coelho T, et al. C1QA and C1QC modify age-at-onset in familial amyloid polyneuropathy patients. Ann Clin Transl Neurol. 2019;6:748–754. doi: 10.1002/acn3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreou S, Panayiotou E, Michailidou K, et al. Epidemiology of ATTRV30M neuropathy in Cyprus and the modifier effect of complement C1q on the age of disease onset. Amyloid. 2018;25:220–226. doi: 10.1080/13506129.2018.1534731. [DOI] [PubMed] [Google Scholar]
- 26.Soares ML, Coelho T, Sousa A, et al. Haplotypes and DNA sequence variation within and surrounding the transthyretin gene: genotype-phenotype correlations in familial amyloid polyneuropathy (V30M) in Portugal and Sweden. Eur J Hum Genet. 2004;12:225–237. doi: 10.1038/sj.ejhg.5201095. [DOI] [PubMed] [Google Scholar]
- 27.Alves-Ferreira M, Coelho T, Santos D, et al. A trans-acting factor may modify age at onset in familial amyloid polyneuropathy ATTRV30M in Portugal. Mol Neurobiol. 2018;55:3676–3683. doi: 10.1007/s12035-017-0593-4. [DOI] [PubMed] [Google Scholar]
- 28.Olsson M, Hellman U, Plante-Bordeneuve V, Jonasson J, Lang K, Suhr OB. Mitochondrial haplogroup is associated with the phenotype of familial amyloidosis with polyneuropathy in Swedish and French patients. Clin Genet. 2009;75:163–168. doi: 10.1111/j.1399-0004.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 29.Santos D, Coelho T, Alves-Ferreira M, et al. Variants in RBP4 and AR genes modulate age at onset in familial amyloid polyneuropathy (FAP ATTRV30M) Eur J Hum Genet. 2016;24:756–760. doi: 10.1038/ejhg.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos D, Santos MJ, Alves-Ferreira M, et al. mtDNA copy number associated with age of onset in familial amyloid polyneuropathy. J Neurol Neurosurg Psychiatry. 2018;89:300–304. doi: 10.1136/jnnp-2017-316657. [DOI] [PubMed] [Google Scholar]
- 31.Conceição I, Damy T, Romero M, et al. Early diagnosis of ATTR amyloidosis through targeted follow-up of identified carriers of TTR gene mutations. Amyloid. 2019;26:3–9. doi: 10.1080/13506129.2018.1556156. [DOI] [PubMed] [Google Scholar]
- 32.Dang D, Fournier P, Cariou E, et al. Gateway and journey of patients with cardiac amyloidosis. ESC Heart Fail. 2020;7:2418–2430. doi: 10.1002/ehf2.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ladefoged B, Dybro A, Povlsen JA, Vase H, Clemmensen TS, Poulsen SH. Diagnostic delay in wild type transthyretin cardiac amyloidosis—a clinical challenge. Int J Cardiol. 2020;304:138–143. doi: 10.1016/j.ijcard.2019.12.063. [DOI] [PubMed] [Google Scholar]
- 34.Wajnsztajn Yungher F, Kim A, Boehme A, et al. Peripheral neuropathy symptoms in wild type transthyretin amyloidosis. J Peripher Nerv Syst. 2020;25:265–272. doi: 10.1111/jns.12403. [DOI] [PubMed] [Google Scholar]
- 35.Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165:1425–1429. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- 36.González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Pfizer provides secure access to anonymized patient-level data to qualified researchers in response to scientifically valid research proposals. Further details can be found at https://www.pfizer.com/science/clinical-trials/trial-data-and-results.