Abstract
Introduction
In a pivotal study, apomorphine sublingual film (APL; KYNMOBI®) was an effective and generally well-tolerated on-demand treatment of “OFF” episodes in patients with Parkinson’s disease (PD), approved across the dose range of 10–30 mg. Pharmacokinetics and comparative bioavailability of APL and two subcutaneous (SC) apomorphine formulations (SC-APO [APOKYN®] and SC-APO-GO [APO-go® PEN]) were evaluated in a randomized, three-way crossover, open-label study (NCT03292016).
Methods
Patients with PD and “OFF” episodes received an open-label randomized sequence of single doses of SC-APO and SC-APO-GO at the currently prescribed dose (2/3/4/5 mg) and APL doses with similar plasma exposure (15/20/25/30 mg) with ≥ 1-day washout between formulations. Plasma pharmacokinetics of apomorphine and apomorphine sulfate (major inactive metabolite) were measured 0–6 h postdose.
Results
Median time to maximum plasma concentration (tmax) of apomorphine was 0.63–0.75 h for APL and 0.25–0.38 h for SC-APO and SC-APO-GO. Geometric mean maximum plasma concentration (Cmax) of apomorphine was 4.31–11.2 ng/ml across APL doses and was generally lower compared with SC apomorphine formulations within dose groups. Area under the concentration-time curve from time 0 to infinity (AUC∞) was similar across apomorphine formulations within most dose groups. Relative bioavailability of APL was ~ 17% of SC apomorphine by AUC∞; SC-APO and SC-APO-GO had similar bioavailability (98% and 83% by AUC∞ and Cmax, respectively). Apomorphine sulfate exposure was ~ three-fold higher for APL versus SC-APO and SC-APO-GO by AUC∞ and Cmax.
Conclusion
In patients with PD and “OFF” episodes, APL demonstrated lower Cmax and relative bioavailability but similar exposures (AUCs) versus SC apomorphine within the approved dose range.
Trial Registration
ClinicalTrials.gov, NCT03292016.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-021-00251-6.
Keywords: APL-130277, APL, Apomorphine sublingual film, Bioavailability, Exposure, “OFF” episodes, Parkinson’s disease, Pharmacokinetics, Subcutaneous apomorphine
Key Summary Points
| Why carry out this study? |
| Parkinson’s disease (PD) is a neurodegenerative disorder for which oral carbidopa/levodopa (CD/LD) is the mainstay of treatment; however, many patients develop episodes of suboptimal response to medication after years of CD/LD treatment, resulting in the reappearance or worsening of their symptoms (“OFF” episodes). |
| Administration of apomorphine is an accepted approach for the treatment of “OFF” episodes associated with PD, with several formulations approved for administration, including subcutaneous (SC) injection (SC-APO), a multiple-dose pen injector (SC-APO-GO), and a minipump/syringe driver. Apomorphine sublingual film was recently approved for this indication with a recommended dose range of 10–30 mg. |
| Considering the lack of studies comparing the pharmacokinetics and bioavailability of apomorphine sublingual film, SC-APO, and SC-APO-GO, the objective of this study was to evaluate these parameters in patients with PD and “OFF” episodes across the effective dose range for these treatments. |
| What was learned from the study? |
| Apomorphine sublingual film is absorbed quickly, achieves a peak concentration (Cmax) that is generally lower, has similar systemic exposure as described by the area under the concentration-time curve (AUC), and has lower relative bioavailability compared with subcutaneous apomorphine at comparable dose levels across the approved dose range of 10–30 mg. |
| The similar overall exposure of apomorphine sublingual film compared with subcutaneous apomorphine, without a rapid early rise in drug concentration, may contribute to a more favorable safety profile. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14394227.
Introduction
Parkinson’s disease (PD) is a chronic, progressive neurodegenerative disorder [1] that affects nearly 1 million people in North America and 6.1 million globally [2, 3]. The mainstay of treatment for PD is oral carbidopa/levodopa (CD/LD) to replenish striatal dopamine levels [4]; however, 70% of patients develop motor fluctuations after 9 years of CD/LD treatment [5] that are characterized by episodes of suboptimal response to medication, resulting in the reappearance or worsening of their symptoms (“OFF” episodes) [1]. Different types of “OFF” episodes can occur, including upon awakening (morning “OFF” or morning akinesia), at the end of a CD/LD dose (wearing “OFF”), with delay in onset of effect from a CD/LD dose (delayed “ON”), with failure of a CD/LD dose (suboptimal “ON,” dose failure), and unexpectedly, without an apparent dose relationship (unpredictable “OFF”) [1, 6].
Apomorphine is a non-ergoline dopamine agonist approved as a subcutaneous (SC) injection at doses of 2–6 mg for the acute intermittent treatment of “OFF” episodes [7, 8]. Subcutaneous apomorphine (APOKYN® [herein referred to as SC-APO]) is administered by a multiple-dose pen injector in the US [8] and Europe (APO-go® PEN [herein referred to as SC-APO-GO]) or as a minipump/syringe driver in Europe (APO-go® PFS) [7, 9]; additional formulations of SC apomorphine have been approved in other regions. Apomorphine SC injection was developed to avoid first-pass metabolism as apomorphine is almost completely metabolized when delivered orally (< 4% of the total dose is bioavailable) [10]. In a phase 2 study of 16 patients with PD treated with SC-APO, pharmacokinetic (PK) parameters measured in eight patients at doses of 2, 3, 5, and 6 mg were associated with a time to maximum plasma concentration (tmax) of 0.367–0.383 h, maximum plasma concentration (Cmax) of 7.5–22.6 ng/ml, terminal elimination phase half-life (t1/2) of 0.520–0.793 h, and area under the concentration-time curve (AUC) from 0 to 2 h of 5.770–22.04 µg/l·h [11]. To date, clinical utilization of SC apomorphine may be limited because of difficulty with device assembly and use, injection site reactions, and other adverse effects [12, 13].
To overcome limitations associated with SC apomorphine such as challenging injection pen assembly, psychological resistance to the idea of injection, and injection site reactions [14–16], apomorphine sublingual film (APL; KYNMOBI®) was developed to deliver apomorphine through systemic absorption from the oral mucosa, thereby bypassing extensive first-pass metabolism associated with oral administration of the compound [10, 12]. Apomorphine sublingual film consists of a thin bilayer film designed to ensure drug stability, rapid drug diffusion, and bioavailability [12]. The first layer contains apomorphine, and the second layer contains a buffer designed to neutralize acid generation after drug absorption [12].
The efficacy and safety of apomorphine sublingual film were evaluated in a phase 3, randomized, double-blind, placebo-controlled trial of levodopa-responsive patients with PD and “OFF” episodes [17]. Apomorphine sublingual film at doses of 10–35 mg (n = 109; dosed as 10 mg [18%], 15 mg [27%], 20 mg [21%], 25 mg [19%], 30 mg [8%], and 35 mg [6%; administered as one 20-mg film followed by one 15-mg film]) significantly improved motor function 30 min postdose at week 12 as assessed by the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III score compared with placebo (− 11.1 vs. – 3.5, respectively; least squares mean difference, − 7.6; p = 0.0002) [17, 18]. Separation from placebo was seen as early as 15 min and persisted up to the 90-min time point. The self-rated FULL “ON” response rate within 30 min postdose at week 12 was significantly higher for apomorphine sublingual film compared with placebo (35% vs. 16%, respectively; p = 0.043) [17]. The most common adverse reactions (incidence ≥ 10%) in patients treated with apomorphine sublingual film, and with an incidence greater than placebo, were nausea, oral/pharyngeal soft tissue swelling, oral/pharyngeal soft tissue pain and paresthesia, dizziness, and somnolence [18]. Apomorphine sublingual film is approved for the acute, intermittent treatment of “OFF” episodes in patients with PD across a dose range of 10–30 mg [18] in the US and Canada.
In a previous phase 1, crossover, bioavailability study that evaluated 15 mg of apomorphine sublingual film versus 2 mg of SC-APO-GO, respectively, apomorphine sublingual film showed a longer tmax (0.85 vs. 0.38 h), longer t1/2 (1.75 vs. 0.90 h), lower Cmax (4.95 vs. 6.15 ng/ml), and higher AUC from time 0 to the last measurable plasma concentration (AUClast; 9.79 vs. 7.61 h·ng/ml) and AUC from time 0 extrapolated to infinity (AUC∞; 10.4 vs. 7.87 h·ng/ml), indicating that a 15-mg dose of apomorphine sublingual film was approximately equivalent to a 2-mg dose of SC-APO-GO [19]. Estimated bioavailability of apomorphine sublingual film relative to SC-APO-GO was approximately 11% and 19% for Cmax and AUC∞, respectively (unpublished). No studies comparing the PK and bioavailability of apomorphine sublingual film [18], SC-APO [8], and SC-APO-GO [9] at higher dosages have been reported to date.
The objective of this current study was to evaluate the PK and comparative bioavailability of a single dose of apomorphine sublingual film compared with SC-APO and SC-APO-GO in patients with PD and “OFF” episodes across the effective dose range for these treatments.
Methods
This was a randomized, three-way crossover, open-label study (ClinicalTrials.gov: NCT03292016) conducted at three sites in the US. The study was designed, conducted, and monitored in accordance with the World Medical Association Declaration of Helsinki (1989) and International Council for Harmonisation guidelines. The study protocol and patient informed consent form were approved by an institutional review board (Copernicus Group, IRB, reference number INC1-17-218 [Cary, NC, USA]).
Patients
Eligible patients were males or females ≥ 18 years of age with a clinical diagnosis of idiopathic PD, consistent with UK Brain Bank Criteria and modified Hoehn and Yahr scale stage ≤ 3 in the “ON” state. Patients had a clinically meaningful response to oral CD/LD with well-defined “OFF” episodes, as determined by the investigator. Patients were required to be receiving SC-APO at ≤ 5 mg per dose for ≥ 4 weeks and stable doses of CD/LD (immediate or sustained release) administered ≥ 4 times daily or extended-release CD/LD three times daily for ≥ 4 weeks. Adjunctive PD medications must have been maintained at a stable dose for ≥ 4 weeks except for monoamine oxidase-B inhibitors, which had to be maintained at a stable dose for ≥ 8 weeks. Patients must have had well-defined “OFF” episodes in the morning and must have been willing to delay morning doses on the three study dosing days.
Patients were excluded if they had atypical or secondary parkinsonism; major psychiatric disorders; previous treatment with continuous SC apomorphine infusion or CD/LD infusion; contraindications or hypersensitivity to SC apomorphine; received selective 5-HT3 antagonists, dopamine antagonists (excluding quetiapine and clozapine), or dopamine-depleting agents within the past 30 days; or mouth cankers or sores within the past 30 days.
Study Design
During the study period, patients received three treatments with a minimum 1-day washout interval between each visit. Patients were randomized to an open-label sequence of single doses of SC-APO (APOKYN®; US WorldMeds, LLC, Louisville, KY, USA [8]) at their current prescribed dose, SC-APO-GO (APO-go® PEN; Britannia Pharmaceuticals Ltd, Berkshire, UK [9]) at the same dose as SC-APO, and apomorphine sublingual film (KYNMOBI®; Sunovion Pharmaceuticals Inc., Marlborough, MA, USA) [18] at doses believed to achieve similar plasma concentrations. The doses of apomorphine sublingual film for this study (15, 20, 25, and 30 mg) were chosen to evaluate the PK and comparative bioavailability of a range of proposed doses of SC-APO and SC-APO-GO and were based on results from an open-label, proof-of-concept, phase 2 study [20] and a phase 1 bioavailability study [19]. The open-label, proof-of-concept study demonstrated that patients with PD and “OFF” episodes who received apomorphine sublingual film at doses of 10–30 mg achieved a clinically confirmed FULL "ON" with all responders turning FULLY "ON" between 15 and 30 min after administration (mean "ON" duration of 50 min) [20]. The bioavailability study demonstrated that a 15-mg dose of apomorphine sublingual film is approximately equivalent to a 2-mg dose of SC-APO [19]. In the present study, the dosing algorithm included four dose levels of apomorphine sublingual film, SC-APO, and SC-APO-GO (Table 1), and patients were randomized to one of six possible treatment sequences (Table S1 in the electronic supplementary material [ESM]).
Table 1.
Dosing algorithma
| APL | SC-APO | SC-APO-GO |
|---|---|---|
| 15 mg | 2 mg | 2 mg |
| 20 mg | 3 mg | 3 mg |
| 25 mg | 4 mg | 4 mg |
| 30 mg | 5 mg | 5 mg |
aThe SC-APO dose was based on a patient’s current prescribed dose; the SC-APO-GO dose was given at the same dose as SC-APO; the APL dose was given at doses with similar plasma exposure based on results from an open-label, proof-of-concept, phase 2 study [20] and a phase 1 bioavailability study [16]
APL apomorphine sublingual film, SC-APO subcutaneous apomorphine injection, SC-APO-GO subcutaneous apomorphine prefilled injection pen
Patients were required to not administer SC apomorphine for ≥ 1 day before each study period. Patients arrived in the clinic on each study day after their prescribed morning dose of PD medication but before taking their second dose of medication. After confirmation by the investigator that the patient was “OFF,” study drug was administered according to the randomized treatment sequence. Other PD medications were withheld until 60 min after study drug dosing.
Assessments
The primary objective was to characterize the PK profile of apomorphine and its inactive metabolites, apomorphine sulfate and norapomorphine [21, 22]. Assessments of apomorphine PK parameters included Cmax, tmax, AUC from time 0 to 24 h (AUC0–24), AUClast, AUC∞, and t1/2. Apomorphine metabolite PK parameters included Cmax, tmax, AUClast, AUC∞, and metabolite-to-parent ratios of Cmax, AUClast, and AUC∞.
Comparative bioavailability among the three study drugs was also evaluated using dose-normalized Cmax, AUC0–24, AUClast, and AUC∞. Blood was collected for PK analyses at time point 0 (right before dosing) and at 0.25, 0.5, 0.75, 1, 1.5, 3, and 6 h postdose.
Safety and tolerability were assessed via evaluation of clinical laboratory tests, 12-lead electrocardiograms, physical examinations, vital signs, and treatment-emergent adverse events (TEAEs).
Statistical Analysis
With a sample size of 12 patients, a two-sided 90% confidence interval for the difference in paired PK parameter means on the log scale would have had an interval that extended no more than 0.221 units from the observed difference with 90% coverage probability. After an unplanned interim analysis, it was determined that sufficient data were obtained from eight patients to meet the primary objective of the study; therefore, additional patients were not recruited.
The PK population included all patients who received ≥ 1 dose of study drug and had ≥ 1 quantifiable PK concentration. The safety population included all patients who received ≥ 1 dose of study drug.
Continuous variables were summarized using the number of observations; arithmetic mean; standard deviation (SD) of the arithmetic mean, geometric mean; and percent coefficient of variation of the geometric mean, median, and range.
PK parameters were derived using noncompartmental methods employing Phoenix WinNonlin version 6.3 (Certara, Princeton, NJ, USA). PK analysis was conducted using actual time elapsed from dosing concentration-time data for apomorphine, apomorphine sulfate, and norapomorphine; PK profiles had to have at least four quantifiable postdose concentrations to be included.
To analyze comparative bioavailability of the three study drugs, a mixed-effects model with fixed effects for treatment and period, with patients nested within sequence as a random effect, was used to analyze the natural log–transformed dose-normalized Cmax, AUC0–24, AUClast, and AUC∞. For each treatment comparison, a point estimate and 90% confidence interval were provided for the geometric mean ratio upon back-transformation.
Results
Patient Disposition and Clinical Characteristics
Eight patients were screened at three clinical sites in the US and randomized into the three-way crossover study. At enrollment, three patients received 3 mg of SC-APO, two patients received 4 mg of SC-APO, and three patients received 5 mg of SC-APO (and corresponding doses of SC-APO-GO and apomorphine sublingual film); no patients were enrolled at the lowest dose level of 2 mg of SC-APO (Table S2 in the ESM). Of the eight patients randomized, seven completed the study in their randomized treatment sequence and one withdrew from the study due to a TEAE of transient dyskinesia on day 3 of period 2 after administration of 5 mg of SC-APO-GO.
Patients had a mean (SD) time since PD diagnosis of 11.3 (5.6) years and mean (SD) age of 67.3 (8.7) years; 75.0% were male; and all were White (Table 2). The mean (SD) time since the onset of motor fluctuations was 5.9 (3.8) years. “OFF” episodes included: wearing “OFF” (87.5%), delayed “ON” (87.5%), morning akinesia (75.0%), sudden “OFF” (75.0%), and dose failure (50.0%). The mean (SD) number of “OFF” episodes per day was 4.0 (1.8), and the mean (SD) duration of “OFF” episodes was 50.6 (13.7) min. All patients were receiving levodopa-containing agents, and 87.5% were also receiving dopamine agonists; no patients were receiving catechol-O-methyltransferase inhibitors (Table 2).
Table 2.
Patient demographics and baseline clinical characteristics
| Overall (N = 8) | |
|---|---|
| Age, years | 67.3 (8.7) |
| Male | 6 (75.0) |
| White race | 8 (100.0) |
| Weight, kg | 77.4 (15.9) |
| BMI, kg/m2 | 25.9 (2.8) |
| Time since PD diagnosis, years | 11.3 (5.6) |
| Time since motor fluctuations started, years | 5.9 (3.8) |
| Modified Hoehn and Yahr score when “ON” | 2.4 (0.4) |
| Number of “OFF” episodes per day | 4.0 (1.8) |
| Type of “OFF” episodes experienced | |
| Wearing “OFF” | 7 (87.5) |
| Delayed “ON” | 7 (87.5) |
| Morning akinesia | 6 (75.0) |
| Sudden “OFF” | 6 (75.0) |
| Dose failure | 4 (50.0) |
| Concomitant PD medications | |
| Levodopa-containing agents | 8 (100.0) |
| Dopamine agonists | 7 (87.5) |
| Monoamine oxidase-B inhibitors | 3 (37.5) |
| Amantadine | 1 (12.5) |
| Rivastigmine | 1 (12.5) |
Data are n (%) or mean (SD)
BMI body mass index, PD Parkinson’s disease, SD standard deviation
Plasma Apomorphine PK Evaluation (Summary Findings)
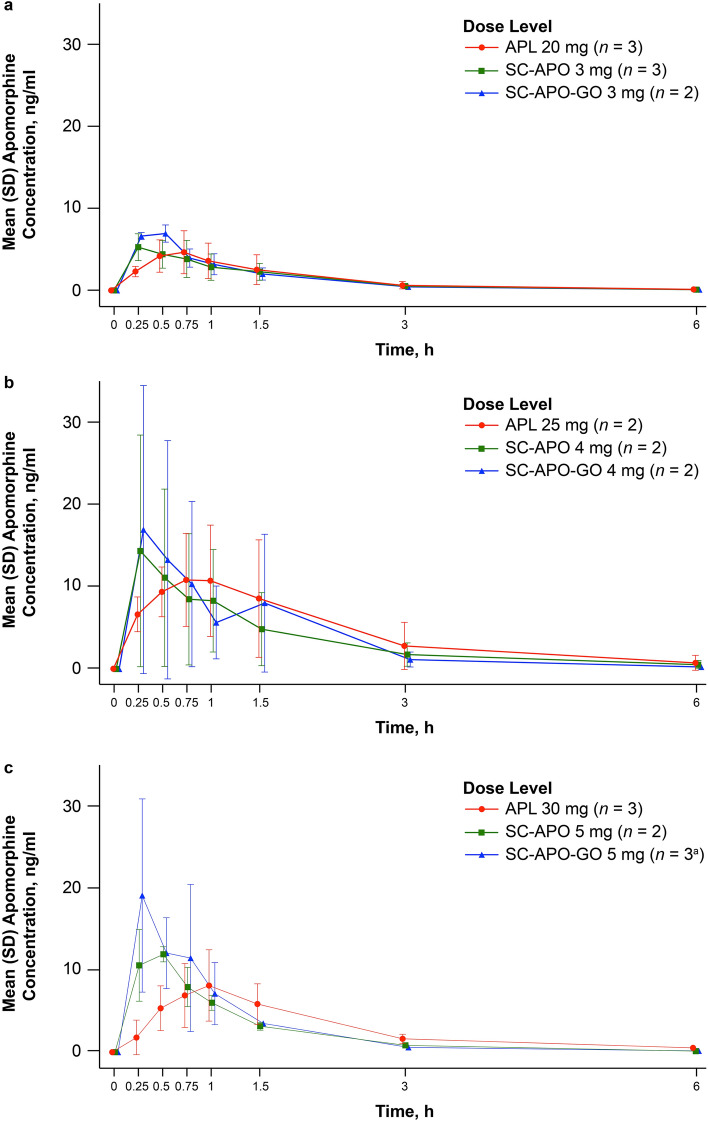
Seven of eight patients had quantifiable concentrations of plasma apomorphine by 0.25 h after drug administration, and concentrations remained quantifiable through the entire 6-h sampling window. In one patient treated with SC-APO, drug administration apparently failed (either due to user error or device malfunctioning), and no apomorphine concentration was detectable postdose; on concentration-time plots, Cmax of apomorphine generally occurred by 0.25–0.5 h with SC-APO and SC-APO-GO and by 0.5–1.0 h with apomorphine sublingual film (Fig. 1). Mean concentrations of apomorphine were similar across all three formulations beginning at about 0.75–1.0 h postdose.
Fig. 1.
Plasma apomorphine concentration time data for a APL 20 mg, b APL 25 mg, and c APL 30 mg and corresponding doses of SC-APO and SC-APO-GO. APL apomorphine sublingual film, SC-APO subcutaneous apomorphine injection, SC-APO-GO subcutaneous apomorphine prefilled injection pen, SD standard deviation. a Data were missing for one patient at the 6-h time point
Median tmax values occurred later across the 20-, 25-, and 30-mg doses of apomorphine sublingual film compared with the 3-, 4-, and 5-mg doses of SC-APO and SC-APO-GO (0.63–0.75 h vs. 0.25–0.38 h, respectively; Table 3). For apomorphine sublingual film, geometric mean t1/2 ranged from 1.09 to 1.21 h, whereas the mean t1/2 was generally shorter (0.75–1.17 h) for the SC apomorphine formulations (Table 3).
Table 3.
Summary of apomorphine plasma PK parameters
| PK parameter | APL 20 mg/SC-APO 3 mg/SC-APO-GO 3 mga | APL 25 mg/SC-APO 4 mg/SC-APO-GO 4 mg | APL 30 mg/SC-APO 5 mg/SC-APO-GO 5 mgb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| APL (n = 3) | SC-APO (n = 2) | SC-APO-GO (n = 2) | APL (n = 2) | SC-APO (n = 2) | SC-APO-GO (n = 2) | APL (n = 3) | SC-APO (n = 2) | SC-APO-GO (n = 3) | |
| Cmax, ng/ml | 4.31 (53.2) | 6.01 (9.7) | 6.95 (13.8) | 11.2 (48.1) | 10.3 (182.8) | 11.5 (216.0) | 7.72 (60.3) | 12.4 (13.7) | 16.8 (85.7) |
| tmax, h | 0.75 (0.52–0.75) | 0.38 (0.25–0.50) | 0.38 (0.25–0.50) | 0.63 (0.25–1.02) | 0.25 (0.25–0.25) | 0.26 (0.25–0.27) | 0.75 (0.50–1.00) | 0.38 (0.25–0.50) | 0.25 (0.25–0.50) |
| AUC0–24, h·ng/ml | 7.23 (52.4) | 7.35 (33.3) | 7.99 (32.2) | 22.8 (99.5) | 16.0 (140.5) | 15.8 (169.6) | 15.4 (44.3) | 14.3 (4.0) | 16.7 (41.0) |
| AUClast, h·ng/ml | 7.03 (53.5) | 7.23 (33.6) | 7.91 (31.5) | 21.9 (92.7) | 15.3 (141.2) | 15.4 (174.0) | 14.4 (47.4) | 14.1 (3.4) | 16.5 (42.1) |
| AUC∞, h·ng/ml | 7.23 (52.4) | 7.35 (33.3) | 7.99 (32.2) | 22.8 (99.5) | 16.0 (140.6) | 15.8 (169.6) | 15.4 (44.3) | 14.3 (4.0) | 16.7 (41.0) |
| t1/2, h | 1.11 (15.6) | 0.98 (9.9) | 0.87 (12.6) | 1.09 (34.3) | 1.17 (20.6) | 1.02 (19.7) | 1.21 (20.5) | 0.89 (18.7) | 0.75 (35.5) |
Data are geometric mean (geometric CV%) except for tmax, which is reported as median (range)
Geometric mean indicates exp (mean of log e transformed data); geometric CV% indicates (sqrt [exp (variance for log e transformed data) − 1])·100
APL apomorphine sublingual film, AUC area under the concentration-time curve, AUC0–24 AUC from time 0 to 24 h, AUC∞ AUC from time 0 extrapolated to infinity, AUClast AUC from time 0 to the last measurable plasma concentration, Cmax maximum observed plasma concentration, CV, coefficient of variation, PK pharmacokinetics, SC-APO subcutaneous apomorphine injection, SC-APO-GO subcutaneous apomorphine prefilled injection pen, t1/ 2 terminal elimination phase half-life, tmax time to maximum concentration
aPK parameters for SC-APO and SC-APO-GO for one patient were excluded because of incomplete dose administration and uncharacteristically low concentration levels
bOne patient did not complete the full three-way crossover
For all formulations, geometric mean apomorphine plasma concentrations were lower overall in the lowest dose group (Fig. 1a) compared with the higher dose groups (Fig. 1b, c). Geometric mean Cmax values of apomorphine sublingual film ranged from 4.31 to 11.2 ng/ml across doses and were generally lower than Cmax levels of SC-APO (6.01–12.4 ng/ml) and SC-APO-GO (6.95–16.8 ng/ml) within each dose group (Table 3). Exposure levels as described by observed and extrapolated estimation (AUC0–24, AUClast, and AUC∞) were similar across the three apomorphine formulations within each dose group (Table 3).
Plasma Apomorphine PK Evaluation (Apomorphine Sublingual Film vs. SC-APO and SC-APO-GO)
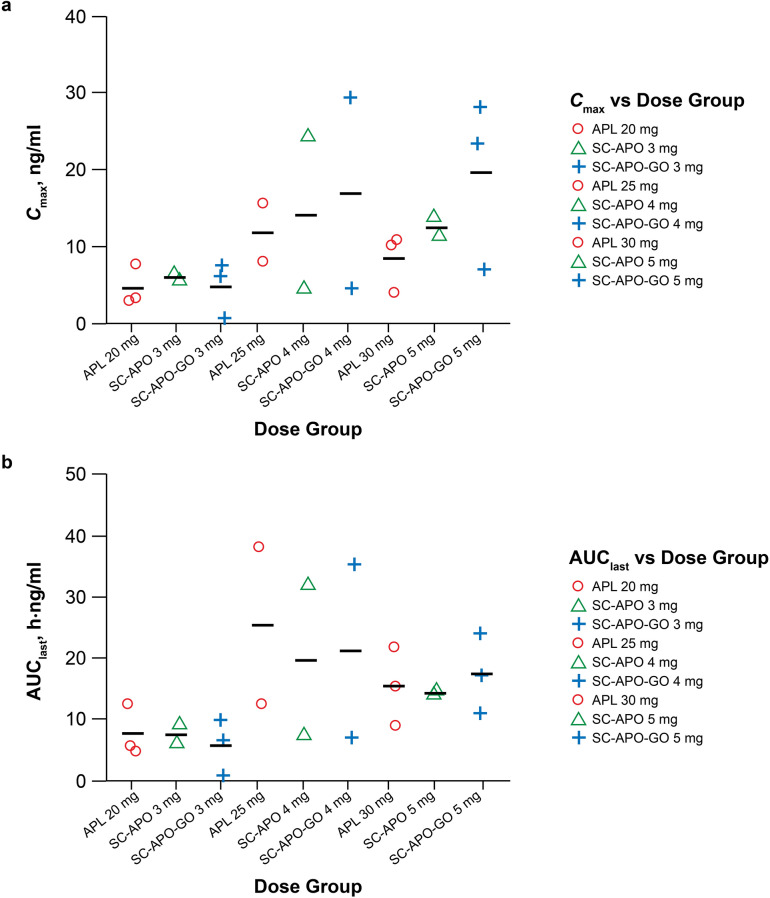
Within each of the three dose levels of apomorphine (i.e., 20 mg sublingual/3 mg SC, 25 mg sublingual/4 mg SC, and 30 mg sublingual/5 mg SC), Cmax of apomorphine occurred later after sublingual administration (Fig. 1), with tmax values occurring roughly 15 min later than after SC-APO and SC-APO-GO administration (Table 3). Geometric mean Cmax values were approximately 30–70% lower in the 20- and 30-mg apomorphine sublingual film dose groups compared with the 3- and 5-mg doses of SC-APO and SC-APO-GO, whereas the Cmax value for 25 mg of apomorphine sublingual film was approximately 3% lower to 8% higher versus 4 mg of SC-APO and SC-APO-GO. Geometric mean apomorphine AUC values (AUC0–24, AUClast, and AUC∞) were similar for 20- and 30-mg doses of apomorphine sublingual film and for 3- and 5-mg doses of SC-APO and SC-APO-GO, whereas 25 mg of apomorphine sublingual film led to AUC values that were approximately 40% higher compared with 4 mg of SC-APO and SC-APO-GO. Interpatient variability in observed Cmax and AUClast values was greatest in the 25-mg apomorphine sublingual film and the 4-mg SC-APO and SC-APO-GO dose groups (Fig. 2a, b). Dose-normalized PK parameters (Cmax, AUC0–24, AUClast, and AUC∞) for apomorphine sublingual film were approximately 10–20% of those observed after SC-APO and SC-APO-GO administration (Table 4).
Fig. 2.
Plot of individual apomorphine a Cmax and b AUClast values vs. dose of apomorphine sublingual film, SC-APO, and SC-APO-GO. Symbols denote the individual values and the line denotes the mean. APL apomorphine sublingual film, AUClast area under the concentration-time curve from time 0 to the last measurable plasma concentration, Cmax maximum plasma concentration, SC-APO subcutaneous apomorphine injection, SC-APO-GO subcutaneous apomorphine prefilled injection pen
Table 4.
Dose-normalized PK parameters for apomorphine by treatment group
| PK parameter | APL (n = 8) | SC-APO (n = 6) | SC-APO-GO (n = 7) |
|---|---|---|---|
| Cmax/dose, ng/ml/mg | 0.28 (57.5) | 2.34 (60.8) | 2.89 (80.9) |
| AUC0–24/dose, h·ng/ml/mg | 0.52 (66.4) | 3.03 (57.9) | 3.28 (61.4) |
| AUClast/dose, h·ng/ml/mg | 0.50 (66.0) | 2.97 (57.5) | 3.23 (62.2) |
| AUC∞/dose, h·ng/ml/mg | 0.52 (66.4) | 3.03 (58.0) | 3.28 (61.4) |
Data are geometric mean (geometric CV%)
Geometric CV% indicates (sqrt [exp (variance for log e transformed data) − 1])·100
APL apomorphine sublingual film, AUC area under the concentration-time curve, AUC0–24 AUC from time 0 to 24 h, AUC∞ AUC from time 0 extrapolated to infinity, AUClast AUC from time 0 to the last measurable plasma concentration, Cmax maximum observed plasma concentration, CV coefficient of variation, PK pharmacokinetics, SC-APO subcutaneous apomorphine injection, SC-APO-GO subcutaneous apomorphine prefilled injection pen
When AUClast values of apomorphine for apomorphine sublingual film were plotted against SC-APO, a clear positive linear relationship was observed with a slope of approximately 0.9 and 0.8 between apomorphine AUClast values for apomorphine sublingual film versus SC-APO, with and without substitution for missing SC-APO data from SC-APO-GO, respectively, suggesting that apomorphine sublingual film and SC-APO exposures are similar (Fig. 3a, b). Geometric mean t1/2 values were approximately 1 h after both apomorphine sublingual film and SC-APO and SC-APO-GO across dose levels (Table 3).
Fig. 3.
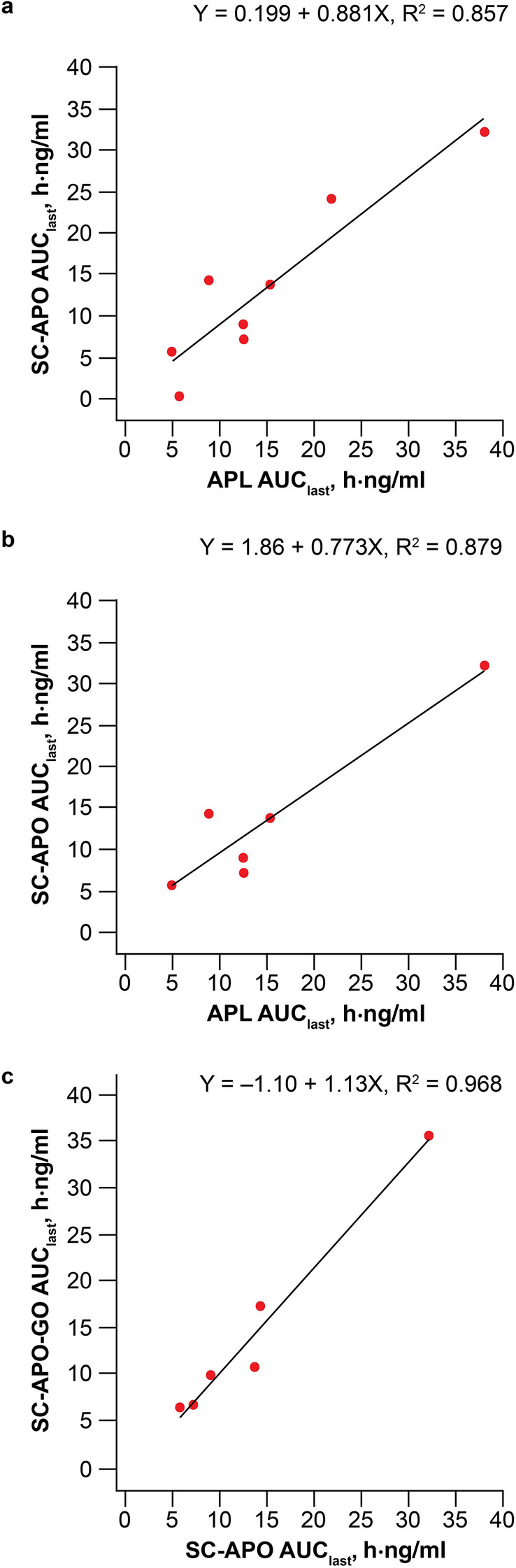
Plot of apomorphine AUClast for a SC-APO vs. APL with substitution of missing SC-APO data for SC-APO-GO, b SC-APO vs. APL without substitution of missing SC-APO data for SC-APO-GO, and c SC-APO-GO vs. SC-APO. The black line denotes the linear regression line and the equation represents the slope. APL apomorphine sublingual film, AUClast area under the concentration-time curve from time 0 to the last measurable plasma concentration, SC-APO subcutaneous apomorphine injection, SC-APO-GO subcutaneous apomorphine prefilled injection pen
Plasma Apomorphine PK Evaluation (SC-APO vs. SC-APO-GO)
For SC-APO-GO versus SC-APO, exposures (AUC and Cmax) across all three dose levels were nearly identical (Table 3). Cmax was observed within 30 min of administration for both SC formulations (Fig. 1). Geometric mean Cmax values were higher for SC-APO-GO in each dose group, but values were within 35% of each other (Table 3). Geometric mean AUC values were similar for the 3-mg and 4-mg dose levels but were about 20% higher for SC-APO-GO for the 5-mg dose. Dose-normalized apomorphine PK parameters were comparable for SC-APO-GO versus SC-APO (Table 4). When AUClast values of apomorphine for SC-APO-GO were plotted against SC-APO, a positive linear relationship was observed with a slope of approximately 1 (Fig. 3c). Geometric mean t1/2 was approximately 1 h for both treatments across dose levels (Table 3).
Relative Bioavailability of Apomorphine Sublingual Film, SC-APO, and SC-APO-GO
The relative bioavailability of apomorphine exposure after sublingual administration was approximately 17% for AUC∞ and approximately 10% for Cmax compared with SC-APO and SC-APO-GO (Table 5). The two SC formulations of apomorphine were nearly identical to each other, with relative bioavailability of approximately 98% for AUC∞ and approximately 83% for Cmax, indicating similar PK for SC-APO and SC-APO-GO.
Table 5.
Comparative bioavailability of apomorphine sublingual film and subcutaneous apomorphine formulations
| PK parameter | APL (n = 8) | SC-APO (n = 6) | SC-APO-GO (n = 7) |
|---|---|---|---|
| Cmax/dose, ng/ml/mg | |||
| LS meana (90% CI) | 0.28 (0.19–0.41) | 2.29 (1.46–3.59) | 2.74 (1.82–4.13) |
| GMRb (90% CI) vs. SC-APO | 12.3 (7.5–20.3) | – | – |
| GMR (90% CI) vs. SC-APO-GO | 10.3 (6.5–16.3) | 83.4 (50.5–137.6) | – |
| AUC0–24/dose, h·ng/ml/mg | |||
| LS mean (90% CI) | 0.52 (0.36–0.76) | 2.97 (2.01–4.39) | 3.04 (2.08–4.45) |
| GMR (90% CI) vs. SC-APO | 17.6 (13.7–22.5) | – | – |
| GMR (90% CI) vs. SC-APO-GO | 17.2 (13.7–21.6) | 97.8 (76.6–124.8) | – |
| AUClast/dose, h·ng/ml/mg | |||
| LS mean (90% CI) | 0.50 (0.34–0.73) | 2.91 (1.97–4.30) | 3.00 (2.05–4.39) |
| GMR (90% CI) vs. SC-APO | 17.2 (13.1–22.5) | – | – |
| GMR (90% CI) vs. SC-APO-GO | 16.7 (13.0–21.3) | 96.9 (74.2–126.4) | – |
| AUC∞/dose, h·ng/ml/mg | |||
| LS mean (90% CI) | 0.52 (0.36–0.76) | 2.97 (2.02–4.39) | 3.04 (2.08–4.45) |
| GMR (90% CI) vs. SC-APO | 17.6 (13.7–22.5) | – | – |
| GMR (90% CI) vs. SC-APO-GO | 17.2 (13.7–21.6) | 97.8 (76.6–124.8) | – |
APL apomorphine sublingual film, AUC area under the concentration-time curve, AUC0–24 AUC from time 0 to 24 h, AUC∞ AUC from time 0 extrapolated to infinity, AUClast AUC from time 0 to the last measurable plasma concentration, CI confidence interval, Cmax maximum observed plasma concentration, GMR geometric mean ratio, LS least squares, PK pharmacokinetics, SC-APO subcutaneous apomorphine injection, SC-APO-GO subcutaneous apomorphine prefilled injection pen
aLS mean and 90% CI for LS mean have been exponentiated
bEstimates of the mean and CI limits for the natural log values were exponentiated and multiplied by 100
PK Evaluation of Apomorphine Metabolites
Cmax of the apomorphine sulfate metabolite occurred between 1 and 3 h postdose across the three study drugs (Fig. S1 in the ESM). Across all apomorphine formulations, apomorphine sulfate exposure was much greater than that of the parent, apomorphine, based on geometric mean metabolite-to-parent ratios of Cmax (apomorphine sublingual film: 32- to 62-fold higher; SC-APO and SC-APO-GO: 7- to 15-fold higher) and AUCs (apomorphine sublingual film: 46- to 98-fold higher; SC-APO and SC-APO-GO: 16- to 37-fold higher; Table S3 in the ESM). Metabolite-to-parent ratios based on Cmax or AUCs were approximately three- to four-fold higher with apomorphine sublingual film than with SC apomorphine at all dose levels (Table S3 in the ESM). No quantifiable concentrations of norapomorphine were detected in the analyses.
Safety
Overall, across all treatment periods, the most common TEAEs that occurred in the study were nausea (n = 3; 38%), dyskinesia, somnolence, injection site bruising, fall, rib fracture, splenic rupture, and hyperhidrosis (n = 1; 13% for each). Safety findings were similar across the three apomorphine formulations. No unexpected or oral adverse reactions were reported with apomorphine sublingual film in this single-dose study of patients on a stable dose of SC apomorphine. Vesicular eruption was noted on exam with a corresponding injection site bruising TEAE in one (13%) patient receiving SC-APO-GO at 2 h postdose; there were no other postdose abnormal findings on examination of the injection site following administration of SC-APO or SC-APO-GO. Overall, changes in mean vital sign values (systolic blood pressure and diastolic blood pressure, pulse rate, respiration rate, body temperature, and body weight) were minimal and not clinically meaningful. Change from period baseline for supine and standing systolic blood pressure was greater for SC-APO and SC-APO-GO compared with apomorphine sublingual film at 15 and 45 min postdose (Table S4 in the ESM).
Discussion
Subcutaneous apomorphine (SC-APO and SC-APO-GO) and apomorphine sublingual film are approved treatments for “OFF” episodes associated with PD [8, 9, 18]. Apomorphine sublingual film was developed in part to provide a more convenient, easy-to-use formulation that bypasses extensive first-pass metabolism associated with oral administration of apomorphine [10, 12]. In a pivotal phase 3 trial, apomorphine sublingual film was shown to be an effective and generally safe and well-tolerated treatment for multiple “OFF” episodes, including morning akinesia, wearing “OFF,” delayed “ON,” dose failure, and sudden “OFF” [17]. To date, there have been no previous studies comparing the PK and bioavailability of these apomorphine formulations.
In this crossover study of patients with PD and “OFF” episodes treated with SC-APO, PK comparisons showed that Cmax occurred quickly (< 1 h) after administration of all three apomorphine formulations, but Cmax occurred 15 min later after apomorphine sublingual film administration, likely a result of differences in the drug dissolution and absorption processes. In addition, Cmax was approximately 30–70% lower in the 20- and 30-mg apomorphine sublingual film dose groups than with SC-APO and SC-APO-GO, and compared with both SC apomorphine formulations, the relative bioavailability of apomorphine sublingual film was approximately 17% based on AUC∞. Despite these differences, the overall systemic exposure to apomorphine was similar between the three formulations based on AUC levels (AUC0–24, AUClast, and AUC∞) within dose groups; t1/2 values were also similar. The consistency of these findings across dose groups provides evidence that apomorphine sublingual film is bioavailable, and the extent of apomorphine exposure after sublingual administration at all dose levels in this study was approximately equal to the corresponding SC apomorphine dose levels within each dose group evaluated (i.e., 20 mg to 3 mg, 25 mg to 4 mg, and 30 mg to 5 mg). Overall, the two SC apomorphine formulations showed a similar rate and comparable extent of exposure. Concentrations peaked at 30 min, and Cmax values and AUCs were higher for SC-APO-GO versus SC-APO but were within a range of 20–35% of each other. The relative bioavailability of the two SC formulations was ~ 98% based on AUC∞, indicating that they have indistinguishable PK.
The observed PK profile and relative bioavailability of apomorphine sublingual film and SC-APO and SC-APO-GO compare well with observations from the phase 1, crossover, bioavailability study where the tmax of apomorphine sublingual film was longer, Cmax was lower, and the relative bioavailability based on Cmax and AUC∞ were 11% and 19%, respectively [19]. One difference between the two studies was the 25% higher AUC with apomorphine sublingual film versus SC-APO in the phase 1 study, which mirrored the current study’s findings observed in the middle dose group (25 mg of apomorphine sublingual film and 4 mg of SC-APO and SC-APO-GO), but not the lowest and highest dose groups where AUCs were similar between sublingual and SC apomorphine formulations. The basis for this difference is unclear but may reflect interpatient variability in absorption, which was greatest for the 25-mg dose in the current study due to one patient within this dose group with higher exposures after all three treatments.
Although sufficient data were obtained to meet the primary objective of characterizing the PK profile of apomorphine and its inactive metabolites, we were not able to formally evaluate dose proportionality in this study because of the overall small number of patients evaluated at each dose level. However, the available evidence suggests that apomorphine sublingual film shows PK characteristics that are less than dose proportional across the approved therapeutic dose range, most likely attributable to interpatient differences in absorption, which may be more pronounced in patients with PD (i.e., swallowing the dose before absorption, reduced contact time of the film under the tongue) [23]. Additional PK studies of apomorphine sublingual film may provide further clarification on dose proportionality.
The metabolism of apomorphine occurs via sulfation, glucuronidation, and N-demethylation, resulting in norapomorphine, apomorphine sulfate, and apomorphine glucuronide [21]. Apomorphine sulfate is a major inactive metabolite in plasma that represents approximately 63% of exposure by AUC; therefore, analysis of the PK profile of apomorphine sulfate was of interest [21]. Apomorphine sulfate Cmax occurred later than apomorphine Cmax; however, concentrations were similar across all three treatments. The exposure ratio of apomorphine sulfate (inactive metabolite) to apomorphine (active parent) was higher with apomorphine sublingual film than with either SC apomorphine formulation where the levels were comparable. This may be due in part to the large sublingual dose needed to achieve comparable exposure levels to the SC formulation (e.g., 15-mg apomorphine sublingual film dose vs. 2-mg SC-APO dose) assuming that a large fraction of the dose (up to 80%) may be swallowed and undergoes first-pass metabolism to produce more inactive metabolite.
Clinical Relevance
Compared with the SC apomorphine formulations, Cmax was lower, tmax was longer, and overall systemic apomorphine exposure (AUCs) was similar with apomorphine sublingual film. These PK properties may contribute to a more favorable safety profile for apomorphine sublingual film (i.e., lower incidence of class effects [nausea, vomiting, somnolence, etc.]), as similar overall exposure is achieved without a rapid early rise in drug concentration. In the double-blind maintenance phase of the pivotal trial of apomorphine sublingual film, nausea, somnolence, and vomiting occurred in 28%, 13%, and 7% of patients, respectively, whereas in the pivotal study of SC-APO, nausea, or vomiting occurred in 30% of patients and drowsiness or somnolence occurred in 35% of patients [8, 18]. Moreover, the overall similarity in apomorphine exposures across formulations within a dose level, combined with evidence of interpatient variability in absorption, supports the use of apomorphine sublingual film as a treatment of “OFF” episodes across the approved therapeutic dose range of 10–30 mg.
Although not specifically measured in this subgroup of patients, clinical and patient-reported findings from studies of apomorphine sublingual film help contextualize the PK findings of this report. In a post hoc analysis of the pivotal phase 3 trial, patients receiving apomorphine sublingual film experienced a ~ two-fold higher magnitude of motor response at 15 min postdose versus patients treated with CD/LD, the gold standard of therapy for PD [24]. In the pivotal trial, 37% of patients receiving apomorphine sublingual film noted improvement in their condition compared with 20% receiving placebo, as measured by the Patient Global Impression of Improvement scale; patients also reported improvement in their quality of life compared with placebo as measured by European Quality of Life–5 Dimensions instrument [17]. Additionally, an online survey of patients with PD and “OFF” episodes who were asked about their preferences for hypothetical “OFF” episode on-demand treatments suggested that patients rated a dissolvable sublingual film as the easiest potential route of administration (vs. inhaled or injected medication) and preferred administration via dissolvable sublingual film versus the alternative routes of administration, despite the potential for mouth/lip sores [25]. Taken together, the comparable PK parameters of apomorphine formulations presented herein, along with demonstration of a rapid onset of action for apomorphine sublingual film that resulted in patient-reported improvements in disease outcomes, tied to previous patient preference findings, suggest that apomorphine sublingual film may be a valuable option to patients with PD as a treatment for their “OFF” episodes.
Study Limitations
This study was conducted in a small number of patients. However, interim analyses suggested that the data were sufficiently consistent to meet the primary objective of the study. Nonetheless, larger samples at each dose level may provide additional evidence for interpatient differences in absorption related to disease state and further explain differences in dose levels required to achieve efficacy among patients with PD. Exposure-response modeling based on data from > 600 patients with PD enrolled across several apomorphine sublingual film studies demonstrated substantial interpatient variability in both plasma levels of apomorphine and changes in the MDS-UPDRS Part III scores, suggesting that a plasma concentration to achieve FULL “ON” will vary by patient [26]. In other clinical trials and in real-world use, at initiation of treatment, apomorphine sublingual film is titrated to the maximum efficacious and tolerated dose to provide a clinical benefit for the individual patient, thus overcoming some of the interpatient variability concerns observed in this small, single-dose study [17, 18, 24].
Because the design of the current study was based on each patient’s current prescribed dose of SC-APO, some commonly used dosages of apomorphine sublingual film (< 20 mg and > 30 mg) were not evaluated, although results from an earlier phase 1 bioavailability study comparing the observed PK profile and relative bioavailability of 15 mg of apomorphine sublingual film to 2 mg of SC-APO-GO were generally consistent with those reported herein [19].
Conclusions
In patients with PD and “OFF” episodes, apomorphine sublingual film demonstrated lower Cmax, similar exposures (AUCs), and lower relative bioavailability compared with SC apomorphine and is a practical treatment across the approved dose range.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
The study and the journal's Rapid Service Fee was supported by funding from Sunovion Pharmaceuticals Inc. (Marlborough, Massachusetts).
Medical Writing and Editorial Assistance
Medical writing and editorial assistance were provided by Robert Schupp, PharmD, CMPP, of The Lockwood Group (Stamford, Connecticut) and Lisa Baker, PhD, CMPP, on behalf of The Lockwood Group, and were supported by funding from Sunovion Pharmaceuticals Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author's Contributions
FA, Y-YC, and BN contributed to the study concept and design. SJB and PB conducted the statistical analysis. All authors contributed to data acquisition, analysis, or interpretation, drafting and critical review of the manuscript, and approval of the final version for submission.
List of Investigators
The authors would like to thank Aaron Ellenbogen for his contribution as a coinvestigator to this study.
Prior Presentation
These data have been presented in whole or in part at the 5th European Academy of Neurology Congress; June 29–July 2, 2019; Oslo, Norway.
Disclosures
Stuart H. Isaacson has received honoraria for continuing medical education, consultancy services, research grants, or promotional speaking from AbbVie, Acadia, Acorda, Adamas, Addex, Allergan, Amarantus, Axovant, Benevolent, Biogen, Britannia, Cerecor, Eli Lilly, Enterin, GE Healthcare, Global Kinetics, Impax, Intec Pharma, Ipsen, Jazz, Kyowa, Lundbeck, Michael J. Fox Foundation, Neurocrine, NeuroDerm, Parkinson Study Group, Pharma Two B, Roche, Sanofi, Sunovion Pharmaceuticals Inc., Teva, Theravance, UCB, US WorldMeds, and Zambon. Ramon Gil has received honoraria for speaker participation or grant/research support from Abbott, AbbVie, Acadia, Adamas, Auspex, Civitas, Cynapsus, Eli Lilly, Impax, Ipsen, Kyowa Kirin Pharmaceutical Development, Lundbeck, Mediti, Neurocrine, Neuroderm, Pharma Two B, Sunovion Pharmaceuticals Inc., and US WorldMeds. Scott J. Brantley is an employee of Nuventra, Inc., and has received consultancy fees from Sunovion Pharmaceuticals Inc. Felix Agbo, Yu-Yuan Chiu, and Bradford Navia are employees of Sunovion Pharmaceuticals Inc. Parul Bhargava is a former employee of Sunovion Pharmaceuticals Inc.
Compliance with Ethics Guidelines
The study was designed, conducted, and monitored in accordance with the World Medical Association Declaration of Helsinki (1989) and International Council for Harmonisation guidelines. The study protocol and patient informed consent form were approved by an institutional review board (Copernicus Group, IRB, reference number INC1-17-218 [Cary, NC, USA]). Written informed consent was obtained prior to patients’ participation in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available after a research proposal is submitted online (http://clinicalstudydatarequest.com) and receives approval from the Independent Review Panel and after a data-sharing agreement is in place. Access will be provided for an initial period of 12 months, but an extension can be granted, when justified, for up to an additional 12 months.
References
- 1.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009) Neurology. 2009;72(21 Suppl 4):S1–136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- 2.GBD Parkinson's Disease Collaborators Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, et al. Prevalence of Parkinson's disease across North America. NPJ Parkinson's Dis. 2018;4:21. doi: 10.1038/s41531-018-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olanow CW, Stocchi F. Levodopa: a new look at an old friend. Mov Disord. 2018;33(6):859–866. doi: 10.1002/mds.27216. [DOI] [PubMed] [Google Scholar]
- 5.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16(3):448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 6.Chou KL, Stacy M, Simuni T, Miyasaki J, Oertel WH, Sethi K, et al. The spectrum of "off" in Parkinson's disease: what have we learned over 40 years? Parkinsonism Relat Disord. 2018;51:9–16. doi: 10.1016/j.parkreldis.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 7.APO-go PFS 5 mg/ml Solution for Infusion in Pre-filled Syringe. Summary of Product Characteristics. Berkshire: Britannia Pharmaceuticals Ltd.; 2018.
- 8.APOKYN® (apomorphine hydrochloride injection) [Prescribing information]. Louisville: US WorldMeds, LLC; 2020.
- 9.APO-go Pen 10 mg/ml Solution for Injection. Summary of Product Characteristics. Berkshire: Britannia Pharmaceuticals Ltd.; 2018.
- 10.Gancher ST, Nutt JG, Woodward WR. Absorption of apomorphine by various routes in parkinsonism. Mov Disord. 1991;6(3):212–216. doi: 10.1002/mds.870060304. [DOI] [PubMed] [Google Scholar]
- 11.Nomoto M, Kubo S, Nagai M, Yamada T, Tamaoka A, Tsuboi Y, et al. A randomized controlled trial of subcutaneous apomorphine for Parkinson disease: a repeat dose and pharmacokinetic study. Clin Neuropharmacol. 2015;38(6):241–247. doi: 10.1097/WNF.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 12.Bilbault T, Taylor S, Walker R, Grundy SL, Pappert EJ, Agro A. Buccal mucosal irritation studies of sublingual apomorphine film (APL-130277) in Syrian golden hamsters. Ther Deliv. 2016;7(9):611–618. doi: 10.4155/tde-2016-0043. [DOI] [PubMed] [Google Scholar]
- 13.Trenkwalder C, Chaudhuri KR, Garcia Ruiz PJ, LeWitt P, Katzenschlager R, Sixel-Doring F, et al. Expert Consensus Group report on the use of apomorphine in the treatment of Parkinson's disease–clinical practice recommendations. Parkinsonism Relat Disord. 2015;21(9):1023–1030. doi: 10.1016/j.parkreldis.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Bhidayasiri R, Chaudhuri KR, LeWitt P, Martin A, Boonpang K, van Laar T. Effective delivery of apomorphine in the management of Parkinson disease: practical considerations for clinicians and Parkinson nurses. Clin Neuropharmacol. 2015;38(3):89–103. doi: 10.1097/WNF.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 15.Bowron A. Practical considerations in the use of apomorphine injectable. Neurology. 2004;62(6 Suppl 4):S32–6. [DOI] [PubMed]
- 16.Manson AJ, Turner K, Lees AJ. Apomorphine monotherapy in the treatment of refractory motor complications of Parkinson's disease: long-term follow-up study of 64 patients. Mov Disord. 2002;17(6):1235–1241. doi: 10.1002/mds.10281. [DOI] [PubMed] [Google Scholar]
- 17.Olanow CW, Factor SA, Espay AJ, Hauser RA, Shill HA, Isaacson S, et al. Apomorphine sublingual film for off episodes in Parkinson's disease: a randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurol. 2020;19(2):135–144. doi: 10.1016/S1474-4422(19)30396-5. [DOI] [PubMed] [Google Scholar]
- 18.KYNMOBI® (apomorphine hydrochloride) sublingual film [Prescribing information]. Marlborough: Sunovion Pharmaceuticals Inc.; 2020.
- 19.Chen YL, Shi L, Agbo F, Yong SH, Tan PS, Ngounou Wetie AG. LC-MS/MS simultaneous quantification of apomorphine and its major metabolites in human plasma: application to clinical comparative bioavailability evaluation for the apomorphine sublingual film and a subcutaneous product. J Pharm Biomed Anal. 2020;190:113493. doi: 10.1016/j.jpba.2020.113493. [DOI] [PubMed] [Google Scholar]
- 20.Hauser RA, Olanow CW, Dzyngel B, Bilbault T, Shill H, Isaacson S, et al. Sublingual apomorphine (APL-130277) for the acute conversion of OFF to ON in Parkinson's disease. Mov Disord. 2016;31(9):1366–1372. doi: 10.1002/mds.26697. [DOI] [PubMed] [Google Scholar]
- 21.Argiolas A, Hedlund H. The pharmacology and clinical pharmacokinetics of apomorphine SL. BJU Int. 2001;88(Suppl 3):18–21. doi: 10.1046/j.1464-4096.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- 22.Campbell A, Kula NS, Jeppsson B, Baldessarini RJ. Oral bioavailability of apomorphine in the rat with a portacaval venous anastomosis. Eur J Pharmacol. 1980;67(1):139–142. doi: 10.1016/0014-2999(80)90022-9. [DOI] [PubMed] [Google Scholar]
- 23.Agbo F, Crass RL, Chiu Y-Y, Chapel S, Galluppi G, Blum D, et al. Population pharmacokinetic model of apomorphine sublingual film or subcutaneous apomorphine in healthy subjects and patients with Parkinson’s disease. Clin Transl Sci. 2021. Epub ahead of print 1 Mar 2021. 10.1111/cts.13008. [DOI] [PMC free article] [PubMed]
- 24.Hui JS, Fox SH, Neeson W, Bhargava P, Pappert E, Blum D, et al. Open-label titration of apomorphine sublingual film in patients with Parkinson's disease and "OFF" episodes. Parkinsonism Relat Disord. 2020;79:110–116. doi: 10.1016/j.parkreldis.2020.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Thach A, Sutphin J, Coulter J, Mansfield C. Patient experiences and preferences for specific on-demand treatments for Parkinson's Disease-related “OFF” episodes. Houston: Academy of Managed Care Pharmacy; 2020. p. S40. [Google Scholar]
- 26.Agbo F, Chiu Y-Y, Chapel S, Navia B. Exposure-response efficacy model of apomorphine sublingual film for the treatment of “OFF” episodes in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2020;79(Suppl 1):E85–E86. doi: 10.1016/j.parkreldis.2020.06.311. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available after a research proposal is submitted online (http://clinicalstudydatarequest.com) and receives approval from the Independent Review Panel and after a data-sharing agreement is in place. Access will be provided for an initial period of 12 months, but an extension can be granted, when justified, for up to an additional 12 months.