Abstract
Introduction
This study aimed to explore the precipitating factors and evaluate the impact of different stenosis types on treatment outcomes in patients with idiopathic intracranial hypertension (IIH) and venous sinus stenosis (VSS).
Methods
We recruited patients with IIH who presented with VSS, either intrinsic or extrinsic. We observed the clinical and laboratory findings, and we then compared the outcomes of stenting and medical treatment in different stenosis types.
Results
Among 145 patients with IIH and VSS, 59 were of the intrinsic type and 86 were of the extrinsic type. Patients in the intrinsic group were older (42 vs. 34 years old, P < 0.001) and presented with higher pre-op gradient pressure (15 mmHg vs. 12 mmHg, P < 0.001). There was no significant difference between groups regarding other precipitating factors (P > 0.05). Stenting was significantly associated with complete resolution of the headache and impaired vision both in intrinsic (adjusted OR 0.017, 95% CI 0.001–0.35, P = 0.011; adjusted OR 0.056, 95% CI 0.004–0.697, P = 0.025, respectively) and extrinsic types of stenosis (adjusted OR 0.072, 95% CI 0.015–0.343, P = 0.001; adjusted OR 0.241, 95% CI 0.062–0.931, P = 0.039, respectively). Meanwhile, stenting was significantly associated with improvement of the papilledema in extrinsic-type stenosis compared with medical treatment (adjusted OR 0.017, 95% CI 0.002–0.135, P < 0.001).
Conclusion
Stenting may provide substantial clinical improvement in patients with IIH regardless of intrinsic or extrinsic stenosis type in our patient population, as noted in other series.
Trial Registration
Clinical trial registration number ChiCTR-ONN-17010421.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-021-00281-0.
Keywords: Idiopathic intracranial hypertension, Venous sinus stenosis, Intrinsic, Extrinsic, Treatment, Outcomes
Key Summary Points
| The precipitating factors of idiopathic intracranial hypertension might be similar in both intrinsic and extrinsic types of stenosis. |
| Stenting treatment results in better improvement of the clinical symptoms than medical treatment regardless of different stenosis types. |
| Pre-operative pressure gradient seems to be an essential factor in determining treatment outcome. |
| Early stenting treatment is recommended for patients with idiopathic intracranial hypertension who are refractory to medical therapy. |
Introduction
Idiopathic intracranial hypertension (IIH) is raised intracranial pressure (ICP) due to an unknown cause [1, 2]. It occurs at reproductive age and mainly affects overweight women. Its incidence and prevalence are increasing, as is obesity worldwide. Headache is the most common symptom of IIH [2–4], and visual impairment is the significant morbidity for IIH related to papilledema [5]. An entire understanding of the etiology of IIH remains elusive as the underlying pathogenesis mechanisms of IIH are not fully established [2–7]. It is still questionable whether elevated ICP is directly caused by IIH or by secondary causative factors. Later discoveries demonstrated that structural alterations, including stenosis, in the cerebral venous sinuses could be a vital contributor to the pathophysiology of IIH [8]. A recent study by Dinkin and Oliveira [7] has summarized the foundation theory of IIH, which depicts the venous outflow obstruction and elevated cerebrospinal fluid (CSF) pressure in a positive feedback loop. This study describes the anatomic features of venous sinus stenosis, including the dominant characteristics of venous sinus stenosis, degree of stenosis, and types of stenosis (intrinsic or extrinsic), which involved the cyclic pathophysiologic mechanisms of IIH. However, there is still a lack of clinical evidence for further understanding of the underlying mechanisms of IIH because of limited studies and their low numbers of patients.
Stenting treatment has a safety profile and may provide significant clinical improvement in patients with IIH refractory to medical therapy compared to other treatment modalities such as optic nerve fenestration and CSF diversion [9]. Despite the promising results from stenting treatment, there remain patients who were unresponsive to the stenting treatment. These results imply that the pathophysiologic mechanisms of IIH may play an essential role in patient selection for IIH treatment. Here, we explored and evaluated 145 patients with confirmed IIH and the clinical characteristics, radiological, laboratory findings, and different treatment modalities. We then further analyzed the impact of different types of stenosis on stenting and medical treatment.
Methods
Study Design and Participants
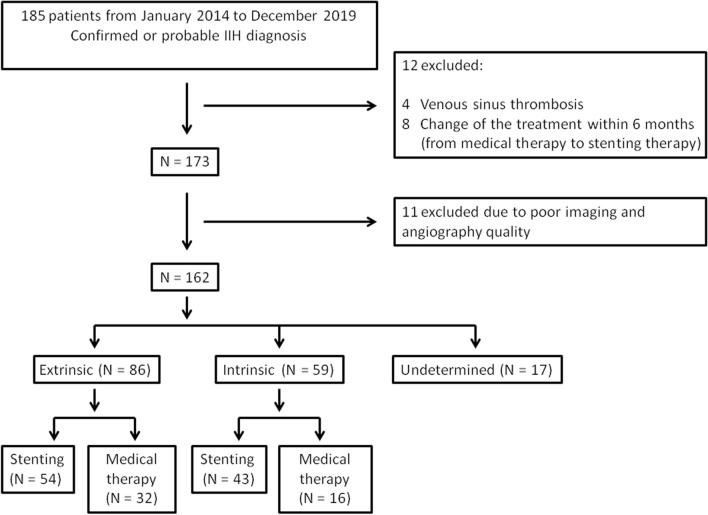
This registry study (clinical trial registration number ChiCTR-ONN-17010421) included patients with IIH treated at Beijing Tiantan Hospital from January 2014 to December 2019 (Fig. 1). All patients fulfilled the following criteria [1,2]: (1) papilledema; (2) normal neurological examination except for cranial nerve abnormalities; (3) neuroimaging revealed normal brain parenchyma without hydrocephalus, mass, or any structural lesion and no evidence of meningeal enhancement on magnetic resonance imaging (MRI) or computed tomography (CT); (4) normal CSF composition; (5) elevated CSF opening pressure (greater than 250 mmH2O in an adequately performed lumbar puncture). In addition, a diagnosis of IIH can also be made even in the absence of papilledema if criteria no. 2 and 3 are satisfied with unilateral or bilateral abducens nerve palsy. A diagnosis of IIH can also be made if the following neuroimaging features are present: (1) empty sella; (2) flattening of the posterior aspect of the globe; (3) dilation of the peri-optic subarachnoid space with or without a tortuous optic nerve; (4) transverse venous sinus stenosis. Our exclusion criteria were (1) concomitant venous sinus thrombosis; (2) change of the treatment modalities within 6 months; (3) poor angiography quality to identify the type of stenosis lesion. The Institutional Review Board of Beijing Tiantan Hospital (KY2016-039-02) approved this study. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. We obtained informed consent from all patients or relatives before the study.
Fig. 1.
Flow chart of recruited patients in the present study
Data Collection
We recorded the patients’ demographic and clinical data, including age, sex, body mass index (BMI), onset to treatment time, and CSF opening pressure. We also recorded the assessment of medical records including patients with existing signs, symptoms, and comorbidities. The blood serum at admission was collected, and a series of blood markers were analyzed.
Image Interpretation
The pre-treatment neurovascular images were interpreted from CT, MRI (T1WI, T2WI), and digital subtraction angiography (DSA) examination. The lesion characteristics were evaluated, including the dominance sites of the stenosis (unilateral (right/left) or codominance), stenosis location (transverse sinus or sigmoid sinus or transverse sinus to sigmoid sinus); furthermore, the stenosis length and stenosis rate were measured according to previous studies. We then classified the lateral sinus stenosis into intrinsic or extrinsic on the basis of DSA and MRI (Fig. 2). The presence of intraluminal lesions, such as subarachnoid granulation (a rounded endosinusal image) and septations, was considered the intrinsic type. In comparison, long sinus stenosis without an endoluminal image caused by compression of the venous sinus from brain parenchyma was considered the extrinsic type [7–10].
Fig. 3.
Bar graphs showing changes in symptoms between groups in intrinsic-type stenosis
Fig. 4.
Bar graphs showing changes in symptoms between groups in extrinsic-type stenosis
Fig. 2.
a Axial postcontrast MRI venography (MRV) demonstrating intrinsic stenosis (white arrow). b Contrast-enhanced 3D-MRV image shows intrinsic stenosis from arachnoid granulations (white arrow). c Pre- and d post-stenting of intrinsic stenosis. e Axial postcontrast MRV demonstrating extrinsic stenosis (white arrow). f Contrast-enhanced 3D-MRV image shows typical long smooth narrowing extrinsic stenosis (white arrow). g Pre- and h post-stenting of extrinsic stenosis
Venography, Manometry, and Treatment Strategies
The management flow chart of diagnosis and treatment of IIH in our institution is shown in Supplementary Fig. 1. Venography and manometry were performed under conscious sedation via right femoral venous puncture. Access into the superior sagittal sinus was usually straightforward with a Rebar-27 microcatheter (Medtronic, Minneapolis, Minnesota, USA) over a microwire. A contrast agent was injected through the microcatheter with a venographic assessment of the superior sagittal sinus and transverse and sigmoid sinuses by attaching a pressure transducer to the microcatheter and taking a zero-reference point at the midaxillary line [11, 12].
The treatment decision was a consensus decision between the neurointerventionalist, neurologist, neuroradiologist, and ophthalmologist. Patients were either treated with stenting or medical therapy. We performed stent placement when the pressure gradient stenosis was at least 8 mmHg [8, 12, 13]. However, some of the patients refused to have stenting treatment even though their pressure gradient value was greater than 8 mmHg because of the fear of the surgical risk or they declined to take life-long anticoagulation medication after stenting treatment. We grouped these patients into the medical therapy group; they received maximal medical therapy, including a weight loss program with a low-calorie diet (at most 425 kcal/day), use of acetazolamide (0.25–1.5 g/day) [2, 4], short-term mannitol (bolus of 0.25–1 g/kg body weight) [14] or repeated lumbar puncture to reduce ICP (20 ml per lumbar puncture) [15], and taking analgesics for headache. Patients with severe papilledema had dexamethasone at 4 mg every 6 h in the local hospital.
Outcome Measures
The main symptoms measured at follow-up were headache, neck pain, tinnitus, visual impairment, and sixth nerve palsy. Ophthalmologic examinations were assessed on presentation and during follow-up. Evaluations included visual acuity and funduscopic examination. Papilledema severity was graded according to the Frisen scale. At the 1-, 3-, and 6-month follow-up periods, the clinical outcomes were defined as asymptomatic, improved, and unchanged (or worse) [16]. Asymptomatic was defined as the complete resolution of the symptoms or other focal objective neurological symptoms. Improved was defined as residual symptoms or other focal objective neurological symptoms that did not require further intervention. Unchanged (or worse) was defined as no change or even deterioration in the symptoms mentioned above.
Statistical Analysis
We described the patient’s baseline characteristics, laboratory findings, and lesion outcome characteristics using medians (25th and 75th percentiles) for continuous variables. Frequencies or proportions represent the categorical variables. We compare the frequencies or proportions between groups using the Pearson chi-square test or Fisher’s exact test. To compare the differences of means or medians for continuous variables, we used the independent-samples t test or the nonparametric test (Mann–Whitney U test). We performed univariate logistic regression analysis adjusted for potential confounders to evaluate the association of different treatment modalities with outcomes in different stenosis groups. The potential confounders were the variables of baseline and lesion characteristics with significant differences between stenting and medical therapy (P < 0.05) in different stenosis types (Supplementary Tables 1 and 2). P < 0.05 was considered statistically significant. We used SPSS 23.0 software (IBM, Armonk, NY, USA) to conduct the statistical analysis.
Results
Baseline Characteristics of Intrinsic and Extrinsic Venous Sinus Stenosis
Of a total of 185 patients, we excluded 23 patients from the analysis because of venous sinus thrombosis (4 patients), change of the treatment from medical treatment to stenting within 6 months (8 patients), and poor imaging and angiography quality (11 patients). Of the remaining 162 patients, we could not determine the stenosis in 17 patients. In these patients, angiographic and imaging examination showed local stenosis without focal filling defect, which did not conform to typical intrinsic or extrinsic type imaging characteristics described previously. Finally, 145 patients with IIH and venous sinus stenosis with definite intrinsic and extrinsic stenosis types were analyzed.
A total of 59 patients (40.7%) were of the intrinsic stenosis type, while 86 patients (59.3%) were of the extrinsic stenosis type. As shown in Table 1, patients in the intrinsic group were older than the extrinsic group (42 vs. 34 years old, P < 0.001). Those with the extrinsic stenosis type had longer stenosis length with a median of 26.4 (IQ 20.8–34.9) vs. 16.4 (IQ 11–24.1) (P < 0.001). We noted higher pre-op pressure gradient (15 mmHg vs. 12 mmHg, P < 0.001), and lower post-op pressure gradient (1.5 mmHg vs. 2 mmHg, P < 0.001) in the intrinsic group.
Table 1.
Baseline and lesion characteristics of the IIH in different stenosis types
| Variables | Total | Intrinsic (N = 59) | Extrinsic (N = 86) | P value |
|---|---|---|---|---|
| Age | 37 (29–44) | 42 (38–48) | 34 (27–40.3) | < 0.001 |
| Female | 115 (79.3) | 47 (79.7) | 68 (79.1) | 0.931 |
| Male | 30 (20.7) | 12 (20.3) | 18 (20.9) | |
| SBP (mmHg, IQR) | 129 (119.5–139.5) | 132 (120–145) | 127.5 (118.5–136) | 0.229 |
| DBP (mmHg, IQR) | 85 (78–93) | 86 (78–96) | 83 (79–92.3) | 0.394 |
| Onset to treatment time (months, IQR) | 4 (1.5–10.5) | 6 (1.7–12) | 3 (1.5–9) | 0.409 |
| Symptoms and signs | ||||
| Headache | 116 (80) | 45 (76.3) | 71 (82.6) | 0.352 |
| Neck pain | 33 (22.8) | 10 (16.9) | 23 (26.7) | 0.167 |
| Tinnitus | 33 (22.8) | 14 (23.7) | 19 (22.1) | 0.817 |
| Impaired vision | 114 (78.6) | 45 (76.3) | 69 (80.2) | 0.568 |
| Papilledema | 115 (79.3) | 47 (79.7) | 68 (79.1) | 0.931 |
| Sixth nerve palsy | 25 (17.2) | 8 (13.6) | 17 (19.8) | 0.331 |
| Obesity and metabolism disorder | ||||
| BMI | 26.2 (24–29.6) | 25.7 (22.6–28.9) | 26.9 (24.4–30.1) | 0.384 |
| Hypertension | 44 (30.3) | 21 (35.6) | 23 (26.7) | 0.255 |
| Diabetes mellitus | 3 (2.1) | 2 (3.4) | 1 (1.2) | 0.74 |
| Hyperlipidemia | 12 (8.3) | 6 (10.2) | 6 (7) | 0.493 |
| Thyroid disorder | 2 (1.4) | 2 (3.4) | 0 | 0.164 |
| Female-related factors | ||||
| Anemia | 8 (5.5) | 2 (3.4) | 6 (7) | 0.576 |
| Menstrual disorder | 8 (5.5) | 2 (3.4) | 6 (7) | 0.576 |
| Uterine myoma | 6 (4.1) | 4 (6.8) | 2 (2.3) | 0.369 |
| Polycystic ovary syndrome | 0 | 0 | 0 | |
| Abortion history | 7 (4.8) | 2 (3.4) | 5 (5.8) | 0.784 |
| Contraceptive drug | 7 (4.8) | 3 (5.1) | 4 (4.7) | 1 |
| Pregnancy | 0 | 0 | 0 | |
| Immunity and inflammatory related factors | ||||
| Allergic history | 14 (9.7) | 4 (6.8) | 10 (11.6) | 0.493 |
| Rheumatic history | 6 (4.1) | 3 (5.1) | 3 (3.5) | 0.96 |
| Common cold | 25 (17.2) | 7 (11.9) | 18 (20.9) | 0.156 |
| Others history | ||||
| Diuretics use | 85 (58.6) | 37 (62.7) | 48 (55.8) | 0.407 |
| Other head and neck disorders | 13 (9) | 5 (8.5) | 8 (9.3) | 0.864 |
| Steroid therapy | 33 (22.8) | 15 (25.4) | 18 (20.9) | 0.526 |
| Anticoagulation therapy | 38 (26.2) | 19 (32.2) | 19 (22.1) | 0.174 |
| CSF pressure | ||||
| 200–249 mmH2O | 15 (10.3) | 9 (15.3) | 6 (7) | 0.237 |
| 250–329 mmH2O | 48 (33.1) | 17 (28.8) | 31 (36) | |
| ≥ 330 mmH2O | 82 (56.6) | 33 (55.9) | 49 (57) | |
| Transverse sinus dominance | ||||
| Unilateral dominance | 111 (76.6) | 49 (83.1) | 62 (72.1) | 0.126 |
| Codominance | 34 (23.4) | 10 (16.9) | 24 (27.9) | |
| Stenosis location | ||||
| Transverse sinus | 46 (31.7) | 17 (28.8) | 29 (33.7) | 0.736 |
| Sigmoid sinus | 6 (4.1) | 2 (3.4) | 4 (4.7) | |
| Transverse-sigmoid sinus | 93 (64.1) | 40 (67.8) | 53 (61.6) | |
| Stenosis length, median (mm, IQR) | 22 (15.7–31.3) | 16.4 (11–24.1) | 26.4 (20.8–34.9) | < 0.001 |
| Stenosis rate, median (%, IQR) | 76.1 (68.6–82.4) | 75.9 (67.4–81.9) | 76.1 (68.6–82.8) | 0.572 |
| Pre-op pressure gradient (mmHg) | 13.5 (10–19) | 15 (12–19) | 12 (9–18.1) | < 0.001 |
| Post-op pressure gradient (mmHg) | 2 (1–3.5) | 1.5 (0–3.5) | 2 (1–3.5) | < 0.001 |
| Treatment | ||||
| Stenting | 97 (66.9) | 43 (72.9) | 54 (62.8) | 0.205 |
| Medical therapy | 48 (33.1) | 16 (27.1) | 32 (37.2) | |
SBP systolic blood pressure, DBP diastolic blood pressure, BMI body mass index, CSF cerebrospinal fluid, IQR interquartile range
There is no significant difference between the groups regarding symptoms and signs, metabolism disorder (BMI, hypertension, diabetes mellitus, hyperlipidemia, thyroid disorder), female-related comorbidities (menstrual disorder, uterine myoma, abortion history, and contraceptive medication), and immunity and related inflammatory comorbidities (allergic history, rheumatic history and recently suffered from common cold), as well as diuretic, other head and neck disorders, anticoagulation therapy, steroid therapy (P > 0.05 for all). Similarly, we did not identify any significant differences between the groups in terms of the level of BP, CSF pressure, stenosis location, and stenosis rate (P > 0.05 for all, Table 1). Although triglycerides level was higher in the extrinsic group, the level was within the normal range. There is no significant difference between groups regarding other serum markers examined (P > 0.05 for all, Table 2).
Table 2.
Blood serum markers in different stenosis types
| Variables | Total | Intrinsic | Extrinsic | P value |
|---|---|---|---|---|
| Metabolism disorder | ||||
| FBG (mmol/L) | 4.6 (4.3–4.9) | 4.6 (4.4–5.2) | 4.5 (4.2–4.9) | 0.605 |
| Total cholesterol (mmol/L) | 4.4 (3.8–5) | 4.4 (3.9–5.2) | 4.3 (3.8–4.9) | 0.123 |
| Triglycerides (mmol/L) | 1.6 (1–2.2) | 1.5 (0.9–2.4) | 1.6 (1.1–2.1) | 0.008 |
| LDL (mmol/L) | 2.7 (2.2–3.2) | 2.7 (2.2–3.3) | 2.7 (2.3–2) | 0.424 |
| HDL (mmol/L) | 1.1 (1–1.3) | 1.1 (1–1.3) | 1.1 (1–1.3) | 0.282 |
| ApoA1 (g/L) | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) | 0.703 |
| ApoB (g/L) | 0.9 (0.7–1.1) | 0.9 (0.7–1.2) | 0.9 (0.7–1) | 0.073 |
| TSH (µIU/mL) | 1.6 (1.1–2.4) | 1.3 (1–1.9) | 1.8 (1.2–2.8) | 0.176 |
| FT3 (pmol/L) | 4.3 (3.8–4.7) | 4.4 (3.8–4.9) | 4.3 (3.8–4.7) | 0.313 |
| FT4 (pmol/L) | 12.3 (11–13.3) | 12.4 (10.9–13.3) | 12.1 (11.2–13.4) | 0.933 |
| Anemia | ||||
| Hemoglobin (g/L) | 126 (110–141.5) | 129 (114–141) | 125 (107.8–142) | 0.147 |
| Immunity-related factors | ||||
| Autoimmune profile | 14 (12.2) | 7 (15.9) | 7 (9.9) | 0.335 |
| ASO (IU/mL) | 76.2 (36.1–121.8) | 76.2 (40–111) | 77.8 (34.9–129.8) | 0.868 |
| RF (IU/mL) | 10.6 (9.5–11.3) | 10.6 (9.5–11.4) | 10.6 (9.5–10.9) | 0.468 |
| C3 complement (g/L) | 1.2 (1–1.3) | 1.2 (1–1.3) | 1.2 (1–1.4) | 0.652 |
| C4 complement (g/L) | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 0.252 |
| Coagulation factors | ||||
| Platelet (109/L) | 261 (212.5–311.5) | 247 (208–302) | 275 (225.8–321) | 0.078 |
| Prothrombin time (s) | 10.9 (10.3–11.5) | 10.6 (10.3–11.3) | 11 (10.4–11.8) | 0.757 |
| APTT (s) | 28.5 (25.6–31.2) | 29.1 (26.5–31.1) | 28.1 (25.4–31.2) | 0.168 |
| INR | 1 (0.9–1) | 1 (0.9–1) | 1 (0.9–1.1) | 0.43 |
| Homocysteine (µmol/L) | 10.4 (8–13.5) | 10.6 (8.2–12.8) | 10.2 (7.5–14) | 0.074 |
| D-dimer (µm/mL) | 0.5 (0.4–0.7) | 0.5 (0.4–0.7) | 0.5 (0.4–0.8) | 0.704 |
| Inflammatory marker | ||||
| WBC (109/L) | 6.3 (5.1–7.4) | 6.1 (4.8–7.3) | 6.3 (5.3–7.6) | 0.142 |
| Neutrophil (%) | 60.1 (56.7–65.4) | 59.8 (54.6–65.6) | 60.2 (57.2–64.8) | 0.725 |
| Lymphocyte (%) | 30.6 (25.8–35.4) | 30.7 (24.7–36.9) | 30.5 (26.2–33.8) | 0.857 |
| ESR (mm/H) | 12 (6–18) | 13 (6–22) | 11 (7–18) | 0.977 |
| CRP (mg/L) | 1.1 (0.4–3.1) | 0.9 (0.5–22) | 1.2 (0.4–3.8) | 0.562 |
| SCRP (mg/L) | 0.9 (0.3–2.8) | 0.8 (0.3–2.2) | 1.1 (0.2–3.2) | 0.688 |
| Cerebrospinal fluid | ||||
| CSF total cell count (/µL) | 3 (1–102.5) | 3 (1–101.8) | 4 (1–103) | 0.25 |
| CSF WBC (/µL) | 2 (1–3) | 2 (1–3) | 2 (1–4) | 0.074 |
| CSF protein (mg/mL) | 24.9 (19–34.6) | 22.2 (18.3–36) | 25.5 (19–33.2) | 0.364 |
| CSF polynucleus (%) | 0 (0–25) | 0 (0–0) | 0 (0–33.1) | 0.583 |
| CSF mononucleus (%) | 0 (0–50) | 0 (0–0) | 0 (0–66.9) | 0.194 |
FBG fasting blood glucose, LDL low density lipoprotein, HDL high density lipoprotein, ApoA1 apolipoprotein A1, ApoB apolipoprotein B, TSH thyroid stimulating hormone, FT3 free triiodothyronine 3, FT4 free triiodothyronine 4, ASO antistreptolysin O, RF rheumatoid factor, APTT activated partial thromboplastin time, INR international normalized ratio, WBC white blood cells, ESR erythrocyte sedimentation rate, CRP C-reactive protein, SCRP sensitive C-reactive protein, CSF cerebrospinal fluid
Stenting Versus Medical Treatment Baseline Characteristics in Different Stenosis Type
As shown in Supplementary Tables 1 and 2, in the intrinsic stenosis group, 43 patients received stenting treatment, while 16 patients received medical treatment. Patients who received stenting treatment had higher DBP (88 mmHg vs. 79.5 mmHg, P = 0.002), more presented with impaired vision and papilledema on admission (83.7% vs. 56.2%, 90.7% vs. 50%, P = 0.027 and P = 0.002, respectively). In the extrinsic stenosis group, papilledema was identified more in the stenting group (87% vs. 65.6%, P = 0.018). Diuretics use was found to be higher in the medical treatment group (71.9% vs. 46.3%, P = 0.021). The stenosis length was longer in the medical treatment group (30.3 mm vs. 23.7 mm, P = 0.008), while the stenosis rate was higher in the stenting group (79.2% vs. 71.6%, P = 0.013). We did not notice any significant difference between the stenting and medical treatment groups regarding the other baseline characteristics in these patients.
Outcome Analysis
As shown in Supplementary Table 3, both medical and stenting-treated patients had improvements in papilledema at 1-, 3-, and 6-month follow-ups. However, improvements were more rapid among the stented patients in extrinsic stenosis than the intrinsic stenosis group (P < 0.05). Most of the patients had a visual examination at the 3-month follow-up. We detected a slight improvement of the visual acuity in patients who received stenting treatment. However, there was no significant difference between the stenting and medical treatment groups of the intrinsic and extrinsic groups (P > 0.05).
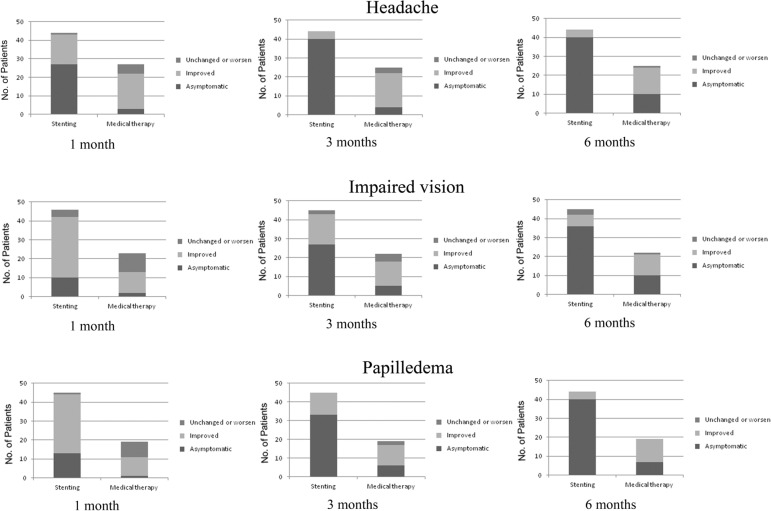
The outcomes at follow-up are shown in Tables 3, 4, Supplementary Table 4, Figs. 3 and 4. At the 1-month follow-up, there was significant improvement of the major symptoms, such as headache and impaired vision, in patients who received stenting treatment (P < 0.05) both in intrinsic- and extrinsic-type stenosis. Consistently, patients who had stenting also showed improvement in minor symptoms, including neck pain and tinnitus (P < 0.05). The symptoms also improved at the 3- and 6-month follow-ups in patients who had stenting treatment.
Table 3.
Outcomes at follow-up grouped by stenting and medical therapy in intrinsic stenosis types
| Stenting | Medical therapy | Adjusted OR (95% CI) | P value | |
|---|---|---|---|---|
| Headache | ||||
| Asymptomatic at 1 month | 16 (51.6) | 0 | ||
| Asymptomatic at 3 months | 24 (77.4) | 1 (7.1) | 0.04 (0.003–0.568) | 0.017 |
| Asymptomatic at 6 months | 26 (86.7) | 3 (23.1) | 0.017 (0.001–0.39) | 0.011 |
| Impaired vision | ||||
| Asymptomatic at 1 month | 6 (16.7) | 0 | ||
| Asymptomatic at 3 months | 18 (50) | 0 | ||
| Asymptomatic at 6 months | 25 (71.4) | 1 (12.5) | 0.056 (0.004–0.697) | 0.025 |
| Papilledema | ||||
| Asymptomatic at 1 month | 10 (25.6) | 0 | ||
| Asymptomatic at 3 months | 26 (66.7) | 2 (28.6) | 3.356 (0.448–25.133) | 0.239 |
| Asymptomatic at 6 months | 32 (86.5) | 3 (42.9) | 8.116 (0.952–69.21) | 0.056 |
Adjusted OR for diastolic blood pressure, impaired vision, papilledema, and CSF pressure
Table 4.
Outcomes at follow-up grouped by stenting and medical therapy in extrinsic stenosis types
| Stenting | Medical therapy | Adjusted OR (95% CI) | P value | |
|---|---|---|---|---|
| Headache | ||||
| Asymptomatic at 1 month | 27 (61.4) | 3 (11.1) | 0.145 (0.033–0.643) | 0.011 |
| Asymptomatic at 3 months | 40 (90.9) | 4 (16) | 0.024 (0.004–0.141) | < 0.001 |
| Asymptomatic at 6 months | 40 (90.9) | 10 (40) | 0.072 (0.015–0.343 | 0.001 |
| Impaired vision | ||||
| Asymptomatic at 1 month | 10 (21.7) | 2 (8.7) | 0.333 (0.041–2.693) | 0.302 |
| Asymptomatic at 3 months | 27 (60) | 5 (22.7) | 0.135 (0.03–0.609) | 0.009 |
| Asymptomatic at 6 months | 36 (80) | 10 (45.5) | 0.241 (0.062–0.931) | 0.039 |
| Papilledema | ||||
| Asymptomatic at 1 month | 13 (28.9) | 1 (5.3) | 0.067 (0.006–0.769) | 0.030 |
| Asymptomatic at 3 months | 33 (73.3) | 6 (31.6) | 0.082 (0.018–0.365) | 0.001 |
| Asymptomatic at 6 months | 40 (90.9) | 7 (36.8) | 0.017 (0.002–0.135) | < 0.001 |
Adjusted OR for papilledema, stenosis length, stenosis rate, and pre-op pressure gradient
In the intrinsic stenosis type, univariate logistic regression analysis showed a correlation with the complete resolution of headache at 3- and 6-month follow-ups (OR 0.04, 95% CI 0.003–0.568, P = 0.017; OR 0.017, 95% CI 0.001–0.39, P = 0.011, respectively) and resolution of the impaired vision at 6-month follow-up (OR 0.056, 95% CI 0.004–0.697, P = 0.025) even after adjustment for some potential confounders. Similarly for the extrinsic stenosis type, there was a correlation of the stenting treatment with complete resolution of headache (OR 0.024, 95% CI 0.004–0.141, P < 0.001; OR 0.072, 95% CI 0.015–0.343, P = 0.001, respectively) and resolution of the impaired vision at 6-month follow-up (OR 0.135, 95% CI 0.03–0.607, P = 0.009; OR 0.241, 95% CI 0.062–0.931, P = 0.039, respectively) were also seen at 3- and 6-month follow-ups even after adjustment for some potential confounders.
Discussion
The present study did not find a significant difference regarding the precipitating factors inducing IIH in both the intrinsic and extrinsic types of VSS. This issue remains uncertain and needs further study for verification. Stenting substantially improved the clinical symptoms in those of the intrinsic and extrinsic types. Pre-operative pressure gradient seems to be an essential factor in determining the treatment outcome. We recommended early stenting treatment for patients with IIH who are refractory to medical treatment.
To date, there are two morphological types of venous stenosis, intrinsic and extrinsic. The intrinsic type has relatively fixed intraluminal structures characterized by focal filling defects. These intraluminal structures include fenestrations, arachnoid granulations, organized thrombus, or fibrous septae [17–21]. The extrinsic type is a smooth, gradually narrowing tapered stenosis due to increased intracranial pressure [17, 18]. The theoretical underpinnings of IIH have been well described by Dinkin and Oliveira [7]. In normal conditions, the arachnoid villi regulate the passive drainage of CSF. Enlarged arachnoid granulations can potentially obstruct the sinus lumen as well as organized chronic thrombus. There were various inducing factors for these changes, including metabolism, endocrine disorders, inflammatory change, and female-related comorbidities [2, 7, 22–27]. There is still controversy about the precipitating factors causing the initial elevation in ICP leading to secondary venous hypertension. The current study did not find any significant difference between intrinsic and extrinsic stenosis regarding the related inducing factors, including blood serum markers, which suggests that influencing factors may precipitate the ICP elevation regardless of different anatomic features of VSS.
A recent study by Lenck et al. [21] reported that the age of symptom and age at stenting differed in different types of stenosis. Our finding also demonstrated older age in patients with intrinsic-type stenosis. However, there was no statistical significance regarding the duration of symptoms, BMI, pre-treatment CSF opening pressure, symptoms and signs, or pre- or post-operative pressure gradient over the stenosis. We identified slightly higher pre-operative gradient pressure in intrinsic-type stenosis than extrinsic type (15 mmHg vs. 12 mmHg). However, the current study did not observe that higher gradient pressure causes more significant elevation of the CSF pressure and exacerbates the symptoms in intrinsic stenosis type, which might be due to the prominent use of diuretics in this group that might blur the actual impact of the different stenosis types on the degree of severity of the IIH. Nevertheless, we observed lower post-op gradient pressure in this group, suggesting a trend towards a more significant reduction in those who had higher pre-operative gradient pressure.
Pre-operative pressure gradient seems to be an essential factor in determining the treatment outcome. Despite significant heterogeneity of outcomes in previous studies, a recent meta-analysis showed that venous sinus stenting provides a comparable efficacy and safety profile over other treatments such as optic nerve fenestration and CSF diversion [9]. Stenting improves venous outflow obstruction, whether due to the anatomically fixed intraluminal structures in intrinsic stenosis [28, 29] or by external compression of the venous sinus [11, 30], and is initially effective in both types of stenosis [18, 21, 31]. Our results also show that stenting treatment initially provides substantial clinical improvement compared with medical treatment for patients with IIH and VSS, regardless of the stenosis type.
It is noteworthy to understand better the underlying mechanism of substantial clinical improvement from the stenting treatment in different stenosis types. As was described previously, the anatomically fixed intraluminal structures in intrinsic stenosis leading to venous sinus flow obstruction may further alter the flow dynamics. Thus, the stenting is expected to alter the impaired hemodynamic flow from the obstruction of the intraluminal structure. In contrast, stenting of the extrinsic stenosis aims to recover the passive venous drainage, which is disturbed by external compression of the venous sinus due to an increase in ICP. Although the present study still could not explain the “chicken or egg problem,” it seems that reconstruction of the disturbed venous drainage by stenting is the central resolution for treating patients with IIH and VSS regardless of different stenosis types. Furthermore, this might also explain why stenting is efficient in both stenosis types.
In addition, pressure gradient seems to be an essential factor in determining the treatment outcome as we observed a significant improvement of the symptoms and signs from stenting in extrinsic-type stenosis. We noted that the pre-operative gradient pressure was higher in the stenting group than in the medical therapy group. Meanwhile, there is no significant difference in the pre-operative pressure gradient between stenting and medical treatment groups in intrinsic type of stenosis. Previous studies have reported that those with favorable outcomes had a higher mean pre-stent trans-stenotic pressure gradient [7, 32]. Our results confirmed this finding. Pre-operative pressure gradient seems to have an essential role in determining the outcomes; although the cutoff value for a favorable outcome is not yet clear, further study is still needed in the future to evaluate the extent and impact of the pressure gradient on the treatment outcomes.
Previous studies have reported improved visual function after stenting, although objective assessments are rarely performed in those studies [11, 33, 34]. We also noted improved visual acuity in patients who had stenting treatment during follow-up, although relatively insignificant. This result might be attributed to the uneven baseline visual acuity in both stenting and medical treatment groups. Patients with stenting treatment presented lower visual acuity (0.8 vs. 0.9) both in intrinsic and extrinsic types. Another possible reason might be that most patients seek treatment after 3 months or longer, meaning that the optic nerve might have been partially damaged, leading to irreversible visual function. Nonetheless, this result also highlights the importance of early treatment of IIH.
The strength of our study lies in the large number of patients. To the best of our knowledge, the current study is the largest among all relevant studies conducted. However, our study has several limitations. First, the uneven sample distribution of the stenosis type might cause a biased result. Second, patients in the stenting treatment group had more severe symptoms; this could lead to a selection bias, reducing the effect of stenting treatment. Third, the outcome measure for headache, neck pain, and tinnitus is too subjective; this also could lead to a biased result. Fourth, incomplete post-treatment evaluation might also reduce the objectivity of the result in different treatment modalities. Fifth, our study was limited to a single center and Chinese patients; thus, this result cannot be generalized to the global population. In addition, despite our finding that demonstrated the benefit of stenting treatment over medical therapy, randomized controlled trials comparing the stenting and medical therapy are warranted for validation. However, this study may provide further information regarding the clinical characteristics and treatment outcomes in different types of stenosis.
Conclusions
The precipitating factors of IIH might be similar in both intrinsic and extrinsic types of stenosis. Stenting treatment provides better improvement of the clinical symptoms over medical treatment regardless of different stenosis types. However, further randomized controlled trials are needed for further confirmation of the results.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study
Funding
This work was supported by the Beijing Municipal Administration of Hospitals Incubating Program, Grant number [PX2017009]; China Postdoctoral Science Foundation, Grant number [2020-YJ-008]. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
DPM and ZRM planned the study; R, XCH, and HCY analysed the data, interpreted the findings and wrote the manuscript; ZYW, XT, XQL and SRW contributed to data collection and analyses; HCY and LL provided critical comments/ revisions of the manuscript. DPM and ZRM are responsible for the overall content.
Disclosures
Raynald, Xiaochuan Huo, Hongchao Yang, Zhengyang Wang, Xu Tong, Xiaoqing Li, Lian Liu, Shuran Wang, Zhongrong Miao, and Dapeng Mo declared that they have no conflict of interests.
Compliance with Ethics Guidelines
This registry study were registered with clinical trial registration number ChiCTR-ONN-17010421. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The study protocol, informed consent forms, and other study-related documents were reviewed and approved by the Institutional Review Board of Beijing Tiantan Hospital (KY2016-039-02). Written consent to participate in the studies was obtained from all patients.
Data Availability
Anonymous data that support the findings of this study are available on reasonable request from the corresponding author.
Footnotes
Dapeng Mo and Zhongrong Miao are the corresponding authors in this work.
Contributor Information
Zhongrong Miao, Email: doctorzhongrongm@126.com.
Dapeng Mo, Email: bjttmodp@163.com.
References
- 1.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159–1165. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 2.Mollan SP, Ali F, Hassan-Smith G, Botfield H, Friedman DI, Sinclair AJ. Evolving evidence in adult idiopathic intracranial hypertension: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2016;87(9):982–992. doi: 10.1136/jnnp-2015-311302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulla Y, Markey KA, Woolley RL, Patel S, Mollan SP, Sinclair AJ. Headache determines quality of life in idiopathic intracranial hypertension. J Headache Pain. 2015;16:521. doi: 10.1186/s10194-015-0521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89(10):1088–1100. doi: 10.1136/jnnp-2017-317440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol. 2016;15(1):78–91. doi: 10.1016/S1474-4422(15)00298-7. [DOI] [PubMed] [Google Scholar]
- 6.Radhakrishnan K, Thacker AK, Bohlaga NH, Maloo JC, Gerryo SE. Epidemiology of idiopathic intracranial hypertension: a prospective and case–control study. J Neurol Sci. 1993;116(1):18–28. doi: 10.1016/0022-510X(93)90084-C. [DOI] [PubMed] [Google Scholar]
- 7.Dinkin M, Oliveira C. Men are from mars, idiopathic intracranial hypertension is from venous: the role of venous sinus stenosis and stenting in idiopathic intracranial hypertension. Semin Neurol. 2019;39(6):692–703. doi: 10.1055/s-0039-3399506. [DOI] [PubMed] [Google Scholar]
- 8.King JO, Mitchell PJ, Thomson KR, Tress BM. Cerebral venography and manometry in idiopathic intracranial hypertension. Neurology. 1995;45(12):2224–2228. doi: 10.1212/WNL.45.12.2224. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson P, Brinjikji W, Radovanovic I, et al. Venous sinus stenting for idiopathic intracranial hypertension: a systematic review and meta-analysis. J Neurointerv Surg. 2019;11(4):380–385. doi: 10.1136/neurintsurg-2018-014172. [DOI] [PubMed] [Google Scholar]
- 10.Sundararajan SH, Ramos AD, Kishore V, et al. Dural venous sinus stenosis: why distinguishing intrinsic-versus-extrinsic stenosis matters. AJNR Am J Neuroradiol. 2021;42(2):288–296. doi: 10.3174/ajnr.A6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinkin MJ, Patsalides A. Venous sinus stenting in idiopathic intracranial hypertension: results of a prospective trial. J Neuroophthalmol. 2017;37(2):113–121. doi: 10.1097/WNO.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 12.Bussiere M, Falero R, Nicolle D, Proulx A, Patel V, Pelz D. Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2010;31(4):645–650. doi: 10.3174/ajnr.A1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982;45(1):13–18. doi: 10.1136/jnnp.45.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangel-Castilla L, Gopinath S, Robertson CS. Management of intracranial hypertension. Neurol Clin. 2008;26(2):521–541. doi: 10.1016/j.ncl.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shenouda S, Al-Farawi K, Dolan J, Flesher SL. Idiopathic intracranial hypertension as a presenting sign of adrenal insufficiency. SAGE Open Med Case Rep. 2018;6:2050313X17753787. doi: 10.1177/2050313X17753787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JN, Cousins C, Owler BK, Sarkies N, Pickard JD. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry. 2003;74(12):1662–1666. doi: 10.1136/jnnp.74.12.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60(9):1418–1424. doi: 10.1212/01.WNL.0000066683.34093.E2. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol. 2011;32(8):1408–1414. doi: 10.3174/ajnr.A2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick MW, Bartels HG, Rodriguez A, Johnson JE, Janjua RM. Anatomical variations of the transverse–sigmoid sinus junction: implications for endovascular treatment of idiopathic intracranial hypertension. Anat Rec (Hoboken) 2016;299(8):1037–1042. doi: 10.1002/ar.23370. [DOI] [PubMed] [Google Scholar]
- 20.Strydom MA, Briers N, Bosman MC, Steyn S. The anatomical basis of venographic filling defects of the transverse sinus. Clin Anat. 2010;23(2):153–159. doi: 10.1002/ca.20911. [DOI] [PubMed] [Google Scholar]
- 21.Lenck S, Vallee F, Labeyrie MA, et al. Stenting of the lateral sinus in idiopathic intracranial hypertension according to the type of stenosis. Neurosurgery. 2017;80(3):393–400. doi: 10.1227/NEU.0000000000001261. [DOI] [PubMed] [Google Scholar]
- 22.Sinclair AJ, Ball AK, Burdon MA, et al. Exploring the pathogenesis of IIH: an inflammatory perspective. J Neuroimmunol. 2008;201–202:212–220. doi: 10.1016/j.jneuroim.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Klein A, Stern N, Osher E, Kliper E, Kesler A. Hyperandrogenism is associated with earlier age of onset of idiopathic intracranial hypertension in women. Curr Eye Res. 2013;38(9):972–976. doi: 10.3109/02713683.2013.799214. [DOI] [PubMed] [Google Scholar]
- 24.Glueck CJ, Aregawi D, Goldenberg N, Golnik KC, Sieve L, Wang P. Idiopathic intracranial hypertension, polycystic-ovary syndrome, and thrombophilia. J Lab Clin Med. 2005;145(2):72–82. doi: 10.1016/j.lab.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Glueck CJ, Iyengar S, Goldenberg N, Smith LS, Wang P. Idiopathic intracranial hypertension: associations with coagulation disorders and polycystic-ovary syndrome. J Lab Clin Med. 2003;142(1):35–45. doi: 10.1016/S0022-2143(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 26.Sundholm A, Burkill S, Waldenlind E, Bahmanyar S, Nilsson Remahl AIM. Infectious and inflammatory disorders might increase the risk of developing idiopathic intracranial hypertension: a national case–control study. Cephalalgia. 2020;40(10):1084–1094. doi: 10.1177/0333102420928079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finsterer J, Kuntscher D, Brunner S, Krugluger W. Pseudotumor cerebri from sinus venous thrombosis, associated with polycystic ovary syndrome and hereditary hypercoagulability. Gynecol Endocrinol. 2007;23(3):179–182. doi: 10.1080/09513590701237290. [DOI] [PubMed] [Google Scholar]
- 28.Fields JD, Javedani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg. 2013;5(1):62–68. doi: 10.1136/neurintsurg-2011-010156. [DOI] [PubMed] [Google Scholar]
- 29.Ducruet AF, Crowley RW, McDougall CG, Albuquerque FC. Long-term patency of venous sinus stents for idiopathic intracranial hypertension. J Neurointerv Surg. 2014;6(3):238–242. doi: 10.1136/neurintsurg-2013-010691. [DOI] [PubMed] [Google Scholar]
- 30.Kumpe DA, Seinfeld J, Huang X, et al. Dural sinus stenting for idiopathic intracranial hypertension: factors associated with hemodynamic failure and management with extended stenting. J Neurointerv Surg. 2017;9(9):867–874. doi: 10.1136/neurintsurg-2016-012810. [DOI] [PubMed] [Google Scholar]
- 31.Asif H, Craven CL, Siddiqui AH, et al. Idiopathic intracranial hypertension: 120-day clinical, radiological, and manometric outcomes after stent insertion into the dural venous sinus. J Neurosurg. 2018;129(3):723–731. doi: 10.3171/2017.4.JNS162871. [DOI] [PubMed] [Google Scholar]
- 32.McDougall CM, Ban VS, Beecher J, Pride L, Welch BG. Fifty shades of gradients: does the pressure gradient in venous sinus stenting for idiopathic intracranial hypertension matter? A systematic review. J Neurosurg. 2018;130(3):999–1005. doi: 10.3171/2017.8.JNS17459. [DOI] [PubMed] [Google Scholar]
- 33.Elder BD, Goodwin CR, Kosztowski TA, et al. Venous sinus stenting is a valuable treatment for fulminant idiopathic intracranial hypertension. J Clin Neurosci. 2015;22(4):685–689. doi: 10.1016/j.jocn.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Young CC, Morton RP, Ghodke BV, Levitt MR. Retrograde 3D rotational venography (3DRV) for venous sinus stent placement in idiopathic intracranial hypertension. J Neurointerv Surg. 2018;10(8):777–779. doi: 10.1136/neurintsurg-2017-013533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymous data that support the findings of this study are available on reasonable request from the corresponding author.