Abstract
Objective:
Nocturnal hypertension and non-dipping systolic blood pressure (SBP) are associated with increased cardiovascular disease (CVD) risk. Short and long sleep duration (SSD and LSD) are also associated with increased CVD risk and may be risk factors for nocturnal hypertension and non-dipping SBP. We examined the association between SSD and LSD with sleep BP, nocturnal hypertension, and non-dipping SBP among 647 white and African American Coronary Artery Risk Development in Young Adults (CARDIA) study participants who completed 24-hour ambulatory BP monitoring, wrist actigraphy, and sleep diaries in 2015-2016.
Methods:
The times when participants were asleep and awake were determined from actigraphy complemented by sleep diaries. Nocturnal hypertension was defined as sleep BP ≥120/70 mmHg and non-dipping SBP as mean sleep-to-awake SBP ratio >0.90. Sleep duration was categorized as SSD (<6 hours), normal sleep duration (NSD: 6-8.9 hours), and LSD (≥ 9 hours).
Results:
The prevalence of SSD and LSD were 13.9% and 21.1%, respectively. Compared to participants with NSD, participants with LSD had higher mean sleep SBP (2.1 mmHg, 95% confidence interval [CI] 0.2, 4.1 mmHg) and diastolic BP (1.7 mmHg, 95% CI 0.5, 3.0 mmHg). Participants with LSD had a higher prevalence of nocturnal hypertension (prevalence ratio [PR]: 1.26, 95% CI 1.03-1.54) and non-dipping SBP (PR 1.33, 95% CI 1.03-1.72) compared to participants with NSD. There was no evidence of an association between SSD and sleep SBP or DBP, nocturnal hypertension, or non-dipping SBP.
Conclusions:
These findings suggest that LSD may be associated with nocturnal hypertension and non-dipping SBP.
Keywords: sleep duration, nocturnal hypertension, non-dipping blood pressure
Introduction
Ambulatory blood pressure (BP) monitoring (ABPM) complements BP measured in the clinic setting by measuring BP in the person’s usual environment.1, 2 Typically performed over a 24-hour period, ABPM can characterize several phenotypes including nocturnal hypertension, high BP during sleep, and non-dipping systolic BP ([SBP] i.e., a <10% decline in BP from being awake to asleep).1, 2 In several previous studies, nocturnal hypertension and non-dipping SBP have been associated with an increased prevalence of target organ damage and increased risk for cardiovascular disease (CVD) events and mortality, independent of clinic BP and awake BP as measured by ABPM.3–5
It has been hypothesized that disturbed sleep may be a mechanism underlying nocturnal hypertension and non-dipping BP.6 Both short sleep duration (SSD), defined in some studies7 as sleeping ≤ 6 hours and in other studies as < 6.5 or 7 hours per night8, and long sleep duration (LSD), defined as sleeping ≥ 9 hours per night8, have been associated with higher risk for CVD events and mortality.7–10 Several studies have demonstrated that self-reported SSD and LSD are associated with high clinic BP8, 11–14 , sleep SBP, and non-dipping BP.15 Self-reported habitual sleep duration is subject to recall bias and has previously been shown to have only a modest correlation with actigraphy-assessed sleep duration.16 Few data17–19 are available regarding the association between actigraphy-assessed sleep duration and sleep BP.
We examined the association of actigraphy-assessed SSD and LSD with sleep BP, nocturnal hypertension, and non-dipping SBP in white and African-American adults enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study. As African Americans have a higher prevalence of SSD20–22, nocturnal hypertension and non-dipping SBP compared with whites23, 24, we also evaluated racial differences in the associations of SSD and LSD with sleep BP, nocturnal hypertension, and non-dipping SBP.
Methods
Study Population
CARDIA is a prospective cohort study designed to examine the risk factors for, and the development of, CVD in a population-based sample of 5,115 whites and African Americans. In 1985-1986, adults who were 18 to 30 years of age were recruited at four US centers (Birmingham, AL, Chicago, IL, Minneapolis, MN and Oakland, CA).25 In addition to the baseline exam, participants have completed up to 8 follow-up exams (Supplemental Methods). Participation at the Year 30 exam was 71% of the surviving cohort.
The current cross-sectional analysis was restricted to 831 CARDIA study participants who attended the Year 30 Exam at the Birmingham, AL or Chicago, IL field centers in 2015-2016, self-reported not working at night, and participated in an ancillary study of ABPM following the exam. Of the 786 participants with a complete ABPM recording (defined below), we excluded 25 participants with missing wrist actigraphy data. As daytime naps can alter sleep and 24-hour BP levels18, 26, we also excluded participants (n=114) who reported taking a daytime nap. After these inclusion/exclusion criteria were applied, data from 647 participants were analyzed (Figure 1). The Institutional Review Boards from each field center and the CARDIA Coordinating Center approved the study protocols. All participants provided written informed consent.
Figure 1:
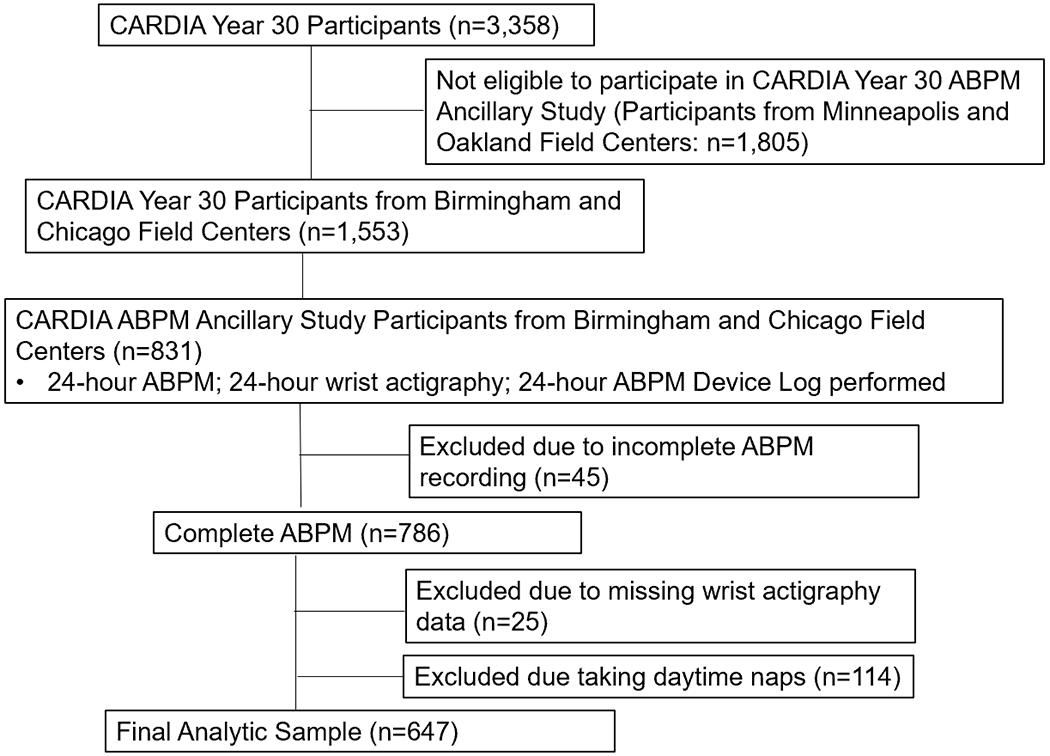
Flow Diagram of the Analytic Sample.
ABPM= Ambulatory blood pressure monitoring
Data Collection
A detailed description of the data collection, methodology, and specimen collection is available in the Supplemental Methods. Data used in the current analysis were collected during the baseline CARDIA Exam and Year 30 Exam as detailed in the Supplemental Methods. Self-administered questionnaires were used to collect information on sociodemographics, selected health behaviors (e.g., smoking status, physical activity, alcohol intake), presence of depressive symptoms (using the 20-item Center for Epidemiologic Studies Depression [CES-D] scale; scores ≥16 indicate high risk for depression27), likelihood for obstructive sleep apnea ([OSA] using the 8-item STOP-BANG questionnaire; scores ≥5 indicate high likelihood of OSA28), habitual sleep duration over the past 30 days using the Pittsburgh Sleep Quality Index (PSQI)29, and antihypertensive medication use. During the Year 30 Exam, trained study staff collected blood and urine samples, which were used to generate estimated glomerular filtration rate (eGFR) and albumin-to-creatinine ratio (ACR), and measured height, weight, and BP. SBP and diastolic BP (DBP) were measured during the Year 30 exam, hereafter referred to as clinic BP, with an Omron HEM 907XL oscillometric device (Omron Healthcare, Inc, Lake Forest, IL) taking three readings using an appropriately sized cuff. There was a 30-second rest period between BP measurements. The second and third BP measurements were averaged and used for this analysis. Clinic hypertension was defined as mean clinic SBP ≥140 mm Hg and/or mean clinic DBP ≥ 90 mm Hg or self-report of current antihypertensive medication use. After the Year 30 Exam, participants were given the opportunity to complete ABPM.
ABPM
ABPM was conducted over a 24-hour period using the SpaceLabs OnTrak 90227 monitor (SpaceLabs Inc., Snoqualmie, WA). Participants were fitted with an appropriately sized cuff on their non-dominant arm and SBP and DBP were measured every 30 minutes. Participants were given a diary to record sleep and awake times including naps and were also fitted with a wrist actigraphy device on their non-dominant arm. The time periods when participants were awake and asleep were determined by actigraphy. Actigraphy-defined sleep and awake time periods were also supplemented using self-reported time periods from participants’ diaries. For the current analysis, a complete ABPM recording was defined as ≥10 awake and ≥5 sleep SBP and DBP measurements.30 Mean awake and sleep SBP and DBP were defined based on the mean of all available readings obtained during the respective actigraphy-defined time periods and 24-hour mean BP was defined as the weighted mean of the awake and sleep BP measurements with weights equal to the proportion of the 24-hour period that each person was awake and asleep.31 Nocturnal hypertension was defined as mean nighttime sleep SBP ≥120 mm Hg and/or mean sleep DBP ≥70 mm Hg.32 BP dipping ratio was calculated as the ratio of mean sleep to mean awake SBP. Non-dipping SBP was defined as mean sleep to mean awake SBP ratio >0.90 (i.e., <10% decrease in mean SBP during sleep).32
Sleep Measures
Participants wore a wrist Actiwatch 2 accelerometer (Philips Respironics, Andover, MA) on their non-dominant wrist throughout the 24-hour ABPM monitoring period. In addition to completing a diary to record sleep and awake times, participants were instructed to press the event marker on the Actiwatch to record when they were going to sleep (“bed time”) and upon waking up the following morning (“awake time”). The Actiwatch was configured to record data continuously, and total activity counts were measured in 15-second epochs. The Actiware software program (version 6.0.9) was used for processing and analysis of sleep and awake periods. A validated algorithm,33 supplemented by sleep diary data and event markers, was used to identify “sleep onset” (when an individual began sleeping after bed time) as well as total minutes of sleep and wakefulness between sleep onset and awake time. Actigraphy-assessed sleep duration was calculated as total minutes of sleep between sleep onset and awake time. Sleep quality during the ABPM period was self-reported and assessed using the question “Did you find the monitor interfered with your normal sleep pattern?” with response options being an integer between 0 (“not at all”) and 10 (“extremely”). A higher score was reflective of worse sleep quality during the ABPM period.
Statistical Analysis
Sleep duration categories were defined using cut-points from prior studies for SSD7 (<6 hours/night), normal sleep duration ([NSD] 6-8.9 hours/night), and LSD (≥ 9 hours/night). Restricted cubic splines with 5 knots were also applied in linear regression models to estimate the association of sleep SBP and DBP, separately, with actigraphy-assessed sleep duration. The cut-points defined using restricted cubic spline analyses were similar to the cut-points defined from prior studies7 for SSD, NSD, and LSD.
Descriptive characteristics for individuals from the Birmingham and Chicago field centers who participated in the ABPM ancillary study compared to individuals from the Birmingham and Chicago field centers who did not participate in the ABPM ancillary study were compared. Participant characteristics and the prevalence of nocturnal hypertension and non-dipping SBP were calculated for the analytic sample, overall and stratified by sleep duration category. We calculated the correlation between self-reported habitual sleep duration and actigraphy-assessed sleep duration as well as the correlation between self-reported sleep duration on 24-hour ABPM sleep diary and actigraphy-assessed sleep duration using Pearson’s correlation coefficients. Linear regression was used to determine the association of actigraphy-assessed SSD and LSD, each versus NSD, with mean sleep SBP and DBP, separately. Poisson regression models with sandwich estimators were used to determine the prevalence ratios (PR) for nocturnal hypertension and separately, non-dipping SBP associated with actigraphy-assessed SSD and LSD, each versus NSD. Five adjusted models were calculated. Model 1 included adjustment for age, sex, race, body mass index (BMI) and field center. Model 2 included the variables in model 1 and history of diabetes, education level, alcohol consumption, smoking status, physical activity, reduced eGFR, ACR ≥30 mg/g, antihypertensive medication use, CES-Depression ≥16, and high likelihood of OSA. Model 3 included adjustment for the variables in model 2 and mean clinic SBP and DBP. Model 4 included the variables in Model 3 and mean awake SBP for the outcomes of mean sleep SBP and mean awake DBP for the outcomes of mean sleep DBP, respectively, awake SBP and DBP for the outcome of nocturnal hypertension, and 24-hour SBP for the outcome of non-dipping BP. Model 5 included the variables in Model 4 and self-reported sleep quality during the ABPM period. Subgroup analyses were conducted by repeating the analyses among African Americans and whites, separately. The tests for interaction between race and sleep duration categories on mean sleep SBP, mean sleep DBP, nocturnal hypertension, and non-dipping SBP were calculated in models including the full sample, main effect terms, and multiplicative interaction terms (e.g., sleep duration category×race). As a sensitivity analysis, we replicated the primary analyses using the 2013 European Society of Hypertension (ESH)34, 35 criteria to define a complete 24-h ABPM recording. These criteria require ≥70% of targeted readings with a minimum of 20 awake and 7 sleep readings. Use of these more stringent criteria excluded an additional 40 participants, reducing the sample size from 647 to 607 for these sensitivity analyses.
The amount of missing data for each variable is provided in Supplemental Table 1. Missing values for covariates were imputed using multivariate chained equations.36 Random forests comprising NTREE=100 decision trees were applied to impute missing values for each variable, separately.37 Ten imputed datasets were generated, analyzed, and their results pooled. P-values <0.05 were considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and R version ≥3.5 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant Characteristics
Supplementary Table 2 provides characteristics of the CARDIA participants from the Birmingham and Chicago Field Centers who participated in versus did not participate in the ABPM ancillary study at the Year 30 exam. Participants who completed the ABPM ancillary study and were included in this analysis were more likely to be female, African American, have a higher BMI, less likely to be physically active, more likely to have diabetes, have prevalent clinic hypertension, be taking antihypertensive medication, and have higher SBP and DBP compared with participants who did not complete the ABPM ancillary study. The mean ± SD age of participants in the current analysis was 54.7 ± 3.7 years; 59.2% were female and 60.9% were African American. The prevalence of nocturnal hypertension and non-dipping SBP was 39.6% and 29.8%, respectively. Mean self-reported habitual sleep duration over the past 30 days was 6.3 ± 1.5 hours. Mean self-reported sleep duration on 24-hour ABPM sleep diary was 7.4 ± 1.7 hours. Mean actigraphy-assessed sleep duration was 7.7 ± 1.7 hours. The correlation between self-reported habitual sleep duration and actigraphy-assessed sleep duration was 0.21 (p value <0.001). The correlation between self-reported sleep duration on 24-hour ABPM sleep diary and actigraphy-assessed sleep duration was 0.76 (p value <0.001).
Figures 2 and 3 depict mean sleep SBP and DBP, respectively, associated with actigraphy-assessed sleep duration and the corresponding cut-points used to define SSD and LSD. Overall, 13.9% and 21.1% of participants had SSD (< 6 hours) and LSD (≥ 9 hours), respectively. Compared to participants with NSD, participants with SSD were more likely to be male, African American, have high risk for depression, and higher mean clinic and 24-h SBP and DBP (Table 1). There were no statistically significant differences in sociodemographic, health, or health behavior characteristics between participants with LSD and NSD. Participants with LSD had higher mean sleep DBP and were more likely to have nocturnal hypertension and non-dipping SBP compared to participants with NSD. There was no evidence of differences in mean awake SBP or DBP between participants with LSD and NSD. The prevalence of clinic hypertension was 41.1% for participants with SSD, 45.0% for participants with NSD, and 52.6% for participants with LSD. Also, 33.3%, 39.1%, and 45.3% of participants with SSD, NSD and LSD were taking antihypertensive medication, respectively.
Figure 2:
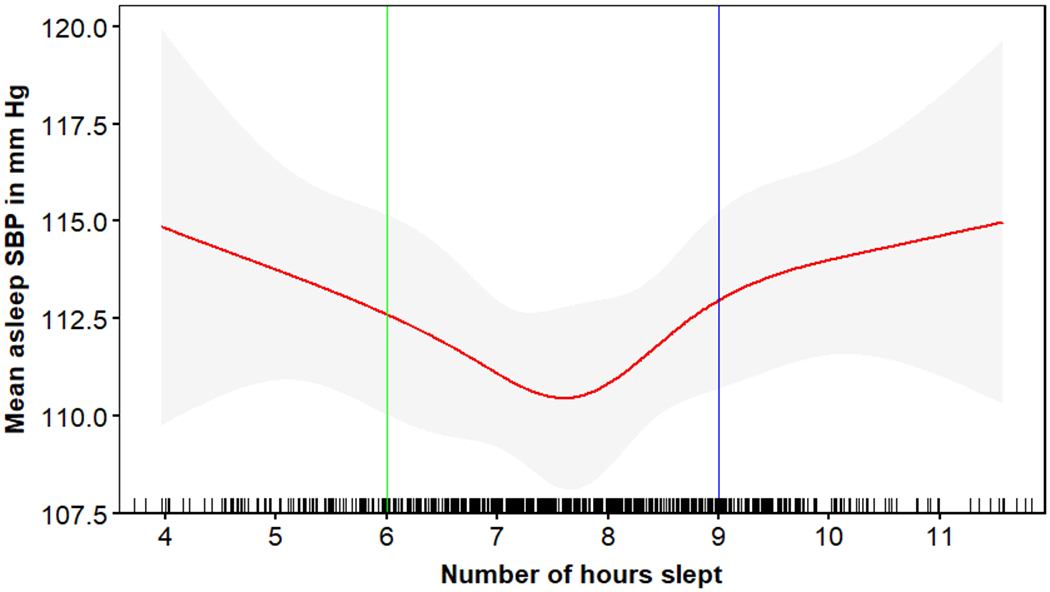
Association* of actigraphy-assessed sleep duration with mean sleep systolic blood pressure.
*The association between sleep duration and mean systolic blood pressure was determined using spline analyses.
Each vertical bar on the x-axis represents a participant with that sleep duration. The red line shows the mean asleep systolic blood pressure associated with actigraphy-assessed sleep duration. Grey regions around the red line represents the 95% confidence interval for the mean sleep systolic blood pressure.
SBP=systolic blood pressure
Figure 3:
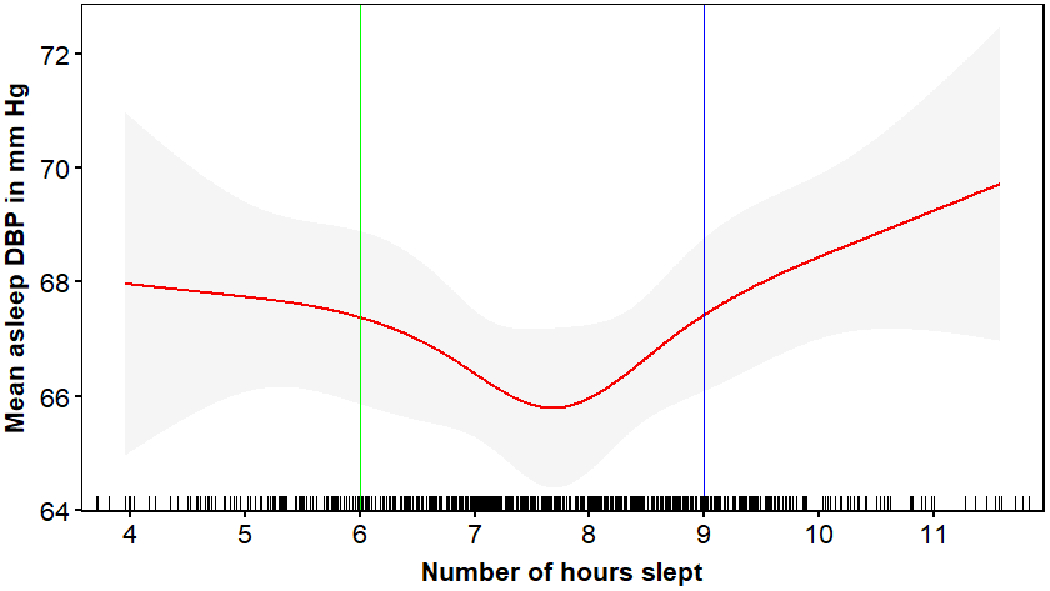
Association* of actigraphy-assessed sleep duration with mean sleep diastolic blood pressure.
*The association between sleep duration and mean diastolic blood pressure was determined using spline analyses.
Each vertical bar on the x-axis represents a participant with that sleep duration. The red line shows the mean asleep diastolic blood pressure associated with actigraphy-assessed sleep duration. Grey regions around the red line represents the 95% confidence interval for the mean sleep diastolic blood pressure.
DBP=diastolic blood pressure
Table 1.
Characteristics of the CARDIA participants included in the analytic sample by actigraphy-assessed sleep duration.
| Short sleep duration (<6 hours) (n=90) |
Normal sleep duration (6-8.9 hours) (n=420) |
Long sleep duration (≥9 hours) (n=137) |
p-value Short vs Normal sleep duration | p-value Long vs Normal sleep duration | |
|---|---|---|---|---|---|
| Age, years | 54.1 (3.8) | 55.0 (3.7) | 54.4 (3.6) | 0.058 | 0.094 |
| Female, % | 46.7 | 60.0 | 66.4 | 0.013 | 0.192 |
| African American, % | 77.8 | 56.9 | 62.8 | <0.001 | 0.210 |
| Education < high school, % | 5.6 | 4.5 | 7.3 | 0.695 | 0.261 |
| aField center-Birmingham | 58.9 | 59.8 | 58.4 | 0.734 | 0.956 |
| Body mass index, kg/m2 | 31.6 (7.1) | 30.9 (6.7) | 31.5 (7.4) | 0.401 | 0.400 |
| bPhysical activity ≥300 EU, % | 23.6 | 35.2 | 33.6 | 0.047 | 0.725 |
| Current smoking, % | 20.0 | 14.3 | 16.9 | 0.168 | 0.459 |
| Alcohol use, % | |||||
| Non-drinker | 50.6 | 52.6 | 52.6 | ||
| Moderate drinker | 39.3 | 34.5 | 36.5 | 0.930 | 0.784 |
| Heavy drinker | 10.1 | 12.9 | 11.0 | ||
| cHigh risk for depression, % | 24.7 | 14.6 | 19.4 | 0.017 | 0.183 |
| dHigh likelihood of OSA, % | 22.7 | 19.2 | 21.3 | 0.443 | 0.584 |
| Diabetes, % | 16.8 | 16.0 | 18.3 | 0.881 | 0.542 |
| eGFR <60 ml/min/m2, % | 2.3 | 3.1 | 6.6 | 0.665 | 0.077 |
| Albumin to creatinine ratio ≥ 30 mg/g, % | 10.1 | 7.1 | 7.3 | 0.336 | 0.951 |
| ePrevalent clinic hypertension, % | 41.1 | 45.0 | 52.6 | 0.510 | 0.111 |
| Antihypertensive medication use, % | 33.3 | 39.1 | 45.3 | 0.319 | 0.195 |
| Mean clinic BP | |||||
| SBP, mm Hg | 126 (21) | 120 (16) | 122 (17) | 0.013 | 0.304 |
| DBP, mm Hg | 77 (14) | 74 (10) | 75 (10) | 0.026 | 0.053 |
| Mean awake BP | |||||
| SBP, mm Hg | 133 (16) | 130 (15) | 130 (17) | 0.098 | 0.664 |
| DBP, mm Hg | 83 (10) | 81 (9) | 81 (9) | 0.057 | 0.350 |
| Mean sleep BP | |||||
| SBP, mm Hg | 115 (16) | 111 (15) | 114 (17) | 0.029 | 0.083 |
| DBP, mm Hg | 69 (11) | 66 (9) | 69 (9) | 0.035 | 0.007 |
| Mean 24-hour BP | |||||
| SBP, mm Hg | 129 (15) | 124 (14) | 123 (16) | 0.003 | 0.819 |
| DBP, mm Hg | 80 (9) | 76 (8) | 76 (8) | 0.001 | 0.954 |
| fBP dipping ratio | 0.13 (0.08) | 0.14 (0.08) | 0.13 (0.08) | 0.215 | 0.033 |
| gNocturnal hypertension | 45.6 | 36.0 | 46.7 | 0.074 | 0.020 |
| hNon-dipping, % | 26.7 | 28.1 | 37.2 | 0.785 | 0.038 |
| Self-reported sleep quality score on ABPM | 3.4 (3.4) | 4.3 (3.0) | 4.1 (3.4) | 0.020 | 0.569 |
| Total sleep time (hours) | 4.5 (0.8) | 6.9 (0.8) | 8.8 (1.4) | <0.001 | <0.001 |
| Wake after sleep onset (hours) | 0.4 (0.3) | 0.6 (0.4) | 0.9 (0.9) | <0.001 | <0.001 |
| Modified sleep efficiency, % | 91.8 | 92.6 | 90.9 | 0.254 | 0.049 |
| Fragmentation index, % | 12.7 | 12.1 | 13.7 | 0.519 | 0.071 |
Data are expressed as percentage or mean (SD) and represent un-imputed results.
The data for the Coronary Artery Risk Development in Young Adults study were collected at Birmingham and Chicago Field Centers.
Physical activity is defined by a score ≥ 300 on the CARDIA physical activity questionnaire.
High risk of depression as determined on the Center for Epidemiologic Studies Depression Scale (Score ≥16).
High likelihood of obstructive sleep apnea as determined on the STOP-BANG questionnaire (Score ≥5).
Prevalent clinic hypertension is defined as a mean clinic systolic blood pressure ≥140 mm Hg or mean clinic diastolic blood pressure ≥90 mm Hg or self-report of current antihypertensive medication use.
Blood pressure dipping ratio was calculated as the ratio of mean sleep to awake systolic blood pressure.
Non-dipping is defined as mean sleep to awake systolic blood pressure ratio >0.90.
Defined as systolic blood pressure ≥120 mm Hg or diastolic blood pressure ≥70 mm Hg.
CES= Center for Epidemiological Studies
eGFR= Estimated glomerular filtration rate
OSA= Obstructive sleep apnea
BP= Blood pressure
SBP= Systolic blood pressure
DBP= Diastolic blood pressure
ABPM= Ambulatory blood pressure monitoring
EU= Exercise units
Associations of SSD and LSD with Sleep BP
There was no evidence of differences in mean sleep SBP between participants with NSD and LSD in models adjusting for sociodemographic, health behavior, and clinical characteristics. (Table 2). After further adjustment for awake SBP and sleep quality on ABPM, mean sleep SBP was 2.1 (95% CI: 0.2, 4.1) mm Hg higher among participants with LSD compared to their counterparts with NSD (p=0.028). Mean sleep DBP was also higher among participants with LSD versus NSD after each level of adjustment. There was no evidence for differences in mean sleep SBP or DBP among participants with SSD compared to their counterparts with NSD. There was no evidence of effect modification between race and sleep duration categories on mean sleep SBP or DBP after multivariable adjustment. Analyses stratified by race are shown in Supplemental Table 2.
Table 2.
Differences in mean sleep blood pressure among CARDIA participants with actigraphy-assessed short and long sleep duration compared to those with normal sleep duration.
| Sleep Duration Categories | P-value | P-interaction by race* | |||||
|---|---|---|---|---|---|---|---|
| Short sleep duration (<6 hours) (n=90) |
Normal sleep duration (6-8.9 hours) (n=420) |
Long sleep duration (≥9 hours) (n=137) |
Short vs Normal sleep duration | Long vs Normal sleep duration | Short sleep duration | Long sleep duration | |
| Mean sleep SBP, mm Hg (SD) | 114.5 (15.6) | 111.1 (15.0) | 113.8 (16.8) | 0.029 | 0.083 | 0.767 | 0.786 |
| Adjusted difference (95% CI) | |||||||
| Model 1 | 2.1 (−1.4, 5.5) |
0 (ref) | 2.6 (−0.4, 5.6) |
0.240 | 0.088 | 0.958 | 0.657 |
| Model 2 | 1.3 (−2.0, 4.6) |
0 (ref) | 2.8 (−0.03, 5.7) |
0.444 | 0.053 | 0.794 | 0.856 |
| Model 3 | −0.3 (−2.9, 2.2) |
0 (ref) | 2.1 (−0.4, 4.5) |
0.796 | 0.103 | 0.165 | 0.675 |
| Model 4 | 0.4 (−1.9, 2.7) |
0 (ref) | 2.1 (0.2, 4.0) |
0.728 | 0.033 | 0.156 | 0.240 |
| Model 5 | 0.6 (−1.7, 2.9) |
0 (ref) | 2.1 (0.2, 4.1) |
0.602 | 0.028 | 0.188 | 0.266 |
| Mean sleep DBP, mm Hg (SD) | 68.2 (10.2) | 66.2 (8.9) | 68.4 (8.9) | 0.035 | 0.007 | 0.433 | 0.243 |
| Adjusted difference | |||||||
| Model 1 | 1.1 (−1.1, 3.3) |
0 (ref) | 2.4 (0.8, 4.0) |
0.327 | 0.004 | 0.544 | 0.253 |
| Model 2 | 0.6 (−1.5, 2.7) |
0 (ref) | 2.5 (0.9, 4.0) |
0.559 | 0.002 | 0.491 | 0.136 |
| Model 3 | 0.1 (−1.7, 1.9) |
0 (ref) | 1.7 (0.3, 3.1) |
0.919 | 0.014 | 0.333 | 0.198 |
| Model 4 | 0.2 (−1.4, 1.8) |
0 (ref) | 1.7 (0.5, 2.9) |
0.828 | 0.005 | 0.252 | 0.578 |
| Model 5 | 0.3 (−1.3, 1.9) |
0 (ref) | 1.7 (0.5, 3.0) |
0.732 | 0.005 | 0.280 | 0.543 |
Model 1 includes adjustment for age, sex, race, body mass index and field center.
Model 2 includes variables in Model 1 and additional adjustment for diabetes, education level, alcohol consumption, smoking status, physical activity, estimated glomerular filtration ratio <60 mL/min per 1.73 m2, albumin to creatinine ratio ≥ 30 mg/g, antihypertensive medication use, CES-Depression score ≥16, and high likelihood of obstructive sleep apnea.
Model 3 includes variables in Model 2 and additional adjustment for mean clinic SBP or DBP for the outcomes of sleep SBP or DBP respectively.
Model 4 includes variables in Model 3 and additional adjustment for mean awake SBP or DBP for the outcomes of sleep SBP or DBP respectively.
Model 5 includes variables in Model 4 and additional adjustment for self-reported sleep quality during ABPM.
The tests for interaction are between race and sleep duration categories on mean sleep SBP and, separately, on mean sleep DBP.
ABPM=ambulatory blood pressure monitoring
CES: Center for Epidemiological Studies
DBP=diastolic blood pressure
SBP=systolic blood pressure
SD= standard deviation
CI= confidence interval
Association of SSD and LSD with Nocturnal Hypertension
The prevalence of nocturnal hypertension among participants with SSD, NSD, and LSD was 45.5%, 35.9%, and 47.1%, respectively (Table 3). The prevalence was not significantly different between SSD and NSD at any level of adjustment. The prevalence of nocturnal hypertension for participants with LSD was greater than for those with NSD (PR 1.26 [95% CI, 1.03-1.54]) after full adjustment. There was no evidence of effect modification by race on the association between sleep duration and nocturnal hypertension.
Table 3.
Prevalence ratios for nocturnal hypertension associated with actigraphy-assessed sleep duration.
| Sleep Duration Categories | P-interaction by race* | ||||
|---|---|---|---|---|---|
| Short sleep duration (<6 hours) (n=90) |
Normal sleep duration (6-8.9 hours) (n=420) |
Long sleep duration (≥9 hours) (n=137) |
Short sleep duration | Long sleep duration | |
| Nocturnal Hypertension, % | 45.5 | 35.9 | 47.1 | 0.542 | 0.717 |
| Prevalence Ratio (95% CI) | |||||
| Model 1 | 1.07 (0.84-1.38) | 1 (Ref) | 1.30 (1.05-1.60) | 0.484 | 0.684 |
| Model 2 | 1.05 (0.82-1.34) | 1 (Ref) | 1.31 (1.06-1.63) | 0.389 | 0.806 |
| Model 3 | 0.94 (0.73-1.20) | 1 (Ref) | 1.28 (1.04-1.57) | 0.763 | 0.786 |
| Model 4 | 0.94 (0.74-1.20) | 1 (Ref) | 1.25 (1.02-1.53) | 0.720 | 0.603 |
| Model 5 | 0.95 (0.74-1.22) | 1 (Ref) | 1.26 (1.03-1.54) | 0.669 | 0.646 |
Model 1 includes adjustment for age, sex, race, body mass index and field center.
Model 2 includes variables in Model 1 and additional adjustment for diabetes, education level, alcohol consumption, smoking status, physical activity, estimated glomerular filtration ratio <60 mL/min per 1.73 m2, albumin to creatinine ratio ≥ 30 mg/g, antihypertensive medication use, CES-Depression score ≥16, and high likelihood of obstructive sleep apnea.
Model 3 includes variables in Model 2 and additional adjustment for mean clinic blood pressure.
Model 4 includes variables in Model 3 and additional adjustment for mean awake blood pressure.
Model 5 includes variables in Model 4 and additional adjustment for self-reported sleep quality on ABPM.
The tests for interaction are between race and sleep duration categories on nocturnal hypertension.
ABPM=ambulatory blood pressure monitoring
CES= Center for Epidemiological Studies
CI= confidence interval
Ref=referent group
The prevalence of nocturnal hypertension was 21.1%, 23.2% and 31.4% for white participants with, SSD, NSD, and LSD, respectively. Among African Americans, the prevalence of nocturnal hypertension was 52.2%, 45.6%, and 56.5% for those with SSD, NSD, and LSD, respectively (Supplemental Table 3).
Association of SSD and LSD with Non-Dipping SBP
The prevalence of non-dipping SBP for participants with SSD, NSD, and LSD was 26.1%, 28.2%, and 37.5%, respectively (Table 4). In a fully-adjusted model, the PRs for having non-dipping SBP for participants with SSD and LSD versus NSD were 0.83 (95% CI, 0.56-1.22) and 1.33 (95% CI, 1.03-1.72), respectively. The prevalence of non-dipping SBP was 36.8%, 15.5%, and 25.5% for white participants with SSD, NSD, and LSD, respectively. The prevalence of non-dipping SBP was 23.2%, 38.0%, and 44.7% for African American participants with SSD, NSD, and LSD, respectively (Supplemental Table 4). After multivariable adjustment and compared to NSD, the PR for non-dipping SBP associated with SSD was 2.15 (95% CI, 1.05-4.39) among whites and 0.67 (95% CI, 0.43-1.04) among African Americans (p-interaction < 0.01).
Table 4.
Prevalence ratios for non-dipping systolic blood pressure associated with actigraphy-assessed sleep duration.
| Sleep Duration Categories | P-interaction by race* | ||||
|---|---|---|---|---|---|
| Short sleep duration (<6 hours) (n=90) |
Normal sleep duration (6-8.9 hours) (n=420) |
Long sleep duration (≥9 hours) (n=137) |
Short sleep duration | Long sleep duration | |
| Non-dipping¥, % | 26.1 | 28.2 | 37.5 | 0.003 | 0.304 |
| Prevalence Ratio (95% CI) | |||||
| Model 1 | 0.86 (0.58-1.27) | 1 (Ref) | 1.30 (1.00-1.68) | 0.008 | 0.250 |
| Model 2 | 0.82 (0.55-1.20) | 1 (Ref) | 1.30 (1.00-1.68) | 0.010 | 0.279 |
| Model 3 | 0.83 (0.56-1.21) | 1 (Ref) | 1.31 (1.01-1.69) | 0.011 | 0.295 |
| Model 4 | 0.82 (0.55-1.20) | 1 (Ref) | 1.31 (1.01-1.69) | 0.008 | 0.307 |
| Model 5 | 0.83 (0.56-1.22) | 1 (Ref) | 1.33 (1.03-1.72) | 0.016 | 0.353 |
Model 1 includes adjustment for age, sex, race, body mass index and field center.
Model 2 includes variables in Model 1 + additional adjustment for diabetes, education level, alcohol consumption, smoking status, physical activity, estimated glomerular filtration ratio <60 mL/min per 1.73 m2, albumin to creatinine ratio ≥ 30 mg/g, antihypertensive medication use, CES-Depression score ≥16, and high likelihood of obstructive sleep apnea.
Model 3 includes variables in Model 2 and additional adjustment for mean clinic systolic blood pressure.
Model 4 includes variables in Model 3 and additional adjustment for mean 24-hour systolic blood pressure.
Model 5 includes variables in Model 4 and additional adjustment for self-reported sleep quality on ABPM.
Non-dipping defined as mean sleep to awake systolic blood pressure ratio > 0.90.
The tests for interaction are between race and sleep duration categories on non-dipping systolic blood pressure.
ABPM=ambulatory blood pressure monitoring
CES= Center for Epidemiological Studies
CI= confidence interval
Ref=referent group
Sensitivity Analyses
Compared to participants with NSD, participants with LSD had higher mean sleep SBP (2.3 mm Hg, 95% CI 0.4, 4.2, p=0.021) and higher mean DBP (1.9 mm Hg, 95% CI 0.7, 3.1, p=0.002) in fully adjusted analyses when analyses were repeated using the ESH criteria to define a complete ABPM period (Supplemental Table 6). Participants with LSD had a higher prevalence of nocturnal hypertension (prevalence ratio [PR]: 1.25, 95% CI 1.01-1.54) and non-dipping SBP (PR 1.35, 95% CI 1.04-1.76) compared to participants with NSD (Supplemental Table 7 and 8 respectively). There was no evidence of an association between SSD and sleep SBP or DBP, nocturnal hypertension, or non-dipping SBP.
Discussion
In this population-based cohort study of white and African American adults, participants with actigraphy-assessed LSD had higher mean sleep BP and a higher prevalence of nocturnal hypertension and non-dipping SBP compared to their counterparts with NSD. In contrast, there was no evidence of an association between SSD and mean sleep BP or nocturnal hypertension. SSD was associated with a higher prevalence of non-dipping SBP among whites. There was no evidence of an association between SSD and non-dipping SBP among African Americans.
In this study, 45.5% of participants with SSD and 47.1% of participants with LSD had nocturnal hypertension. Nocturnal hypertension has been associated with target organ damage and CVD events.3–5 The mechanism(s) underlying these associations remain unknown. Self-reported sleep duration extremes, both a deficit and surfeit, have been associated with an increased risk for CVD events, mortality, and hypertension.8, 10–14
Most studies6, 15, 38 examining the association between sleep duration and out-of-clinic BP in adults have relied on self-reported sleep duration rather than actigraphy-assessed sleep duration. Self-reported sleep duration is subject to recall bias and the computation of mean sleep BP may be affected by errors in self-reported sleep/wake times. Self-reported sleep duration has also been shown to have only a moderate correlation with sleep duration assessed using wrist actigraphy and polysomnography.16
While studies conducted among adolescents17, 18 have demonstrated an association between actigraphy-assessed SSD and higher sleep BP, few studies6, 19 have examined the association of actigraphy-assessed sleep duration and sleep BP in adults. Data from the North Texas Heart Study19 demonstrated that lower actigraphy-assessed sleep duration was associated with higher mean sleep SBP and DBP measured on the same night. However, in that study, mean sleep BP was defined as the average of BP readings that occurred only during a 5-hour fixed-time interval (during the hours of 12:00 AM-5:00 AM) rather than the full actigraphy-defined sleep period. Use of a fixed time interval artificially identifies “sleep” blood pressure based on time versus behavior (i.e. an individual may actually be awake rather than asleep during the fixed time interval). Further, use of fixed time intervals may have led to underestimation of true mean sleep BP especially if BP during this fixed time interval was lower than at other times during the night. In addition, the associations of LSD with sleep BP, nocturnal hypertension, and non-dipping BP were not reported nor were racial differences examined in that study.
In the current study, compared with whites, African-Americans were more likely to have actigraphy-assessed SSD, a finding consistent with prior studies.20–22, 39–41 In one study of 246 African American and white adolescents, shorter sleep duration was associated with higher sleep SBP, sleep DBP, and non-dipping SBP.17 When stratified by race/ethnicity, the association between shorter sleep duration and higher sleep BP was present among white adolescents but not African American adolescents. The current study results do not support the hypothesis that SSD is associated with higher sleep BP levels, nocturnal hypertension, or non-dipping BP among African American adults. Whites with SSD had a nonsignificantly higher prevalence of non-dipping SBP compared to white participants with NSD, a finding that requires confirmation in future studies. In contrast, LSD was associated with higher sleep BP levels, nocturnal hypertension, and non-dipping SBP among whites and African Americans.
Prior studies have demonstrated an association between LSD and cardiometabolic risk factors including diabetes and hypertension among older individuals.42, 43 Also, the association between LSD and ambulatory BP has been described in elderly participants.15 Specifically, Ramos et al.15 demonstrated self-reported LSD (≥ 11 hours/night) to be associated with higher sleep SBP and non-dipping BP compared to a self-reported sleep duration of 6-11 hours/night in a study of 756 participants (mean age 71 ± 9 years). In contrast, in the current study actigraphy-assessed LSD was associated with higher sleep BP levels, nocturnal hypertension, and non-dipping SBP in a cohort of younger individuals (mean age 54.7 ± 3.7 years). The mechanism underlying the association between LSD and BP is unclear, but several hypotheses have been suggested.6, 44 Small studies45, 46 have suggested that higher cortisol levels are associated with higher sleep BP as well as non-dipping BP which may partially explain the association of LSD with high sleep BP levels, nocturnal hypertension, and non-dipping SBP. The association between LSD and sleep BP may also be secondary to chronic medical or psychiatric illnesses, resulting in individuals spending more time in bed and having longer sleep durations.6, 44 Compared to all CARDIA study participants who completed the Year 30 exam, participants in the ABPM ancillary study had more comorbidities. However, we adjusted for several comorbidities, including high risk for depression, that have been associated with both short and long sleep.47, 48
The current study has several strengths. The study was comprised of a well-phenotyped sample of whites and African Americans who completed ABPM following a standardized protocol. We utilized wrist actigraphy to measure sleep duration. In studies with healthy participants, wrist actigraphy has a correlation of 0.9 for the measurement of sleep duration when compared with polysomnography, the gold standard for the assessment of sleep duration.49 Despite these strengths of the study, there are several limitations. While 3,358 participants completed the CARDIA Year 30 Exam, funding was available to conduct ABPM at only two study sites and only a subset of participants completed the ABPM ancillary study. Wrist actigraphy for the assessment of sleep duration was conducted only for 24 hours which may not be representative of “usual” sleep duration averaged over several days or weeks. ABPM was conducted on the same night that sleep duration was assessed. For some individuals, the inflation of the ABPM BP cuff can affect sleep quality.50 However, the significant association between LSD and sleep BP outcomes was not attenuated by statistical adjustment for self-reported sleep quality concurrent with ABPM. Lastly, while participants completed a medication inventory, the use of sleep aides including melatonin and benzodiazepines which are known to lengthen sleep duration was not specifically ascertained. It is possible that individuals with LSD were more likely to use sleep aides.
Conclusions
The prevalence of nocturnal hypertension was high particularly among African American participants with SSD and LSD. Actigraphy-assessed SSD was not associated with nocturnal hypertension or non-dipping SBP. In contrast, actigraphy-assessed LSD was associated with nocturnal hypertension and non-dipping SBP among white and African American adults even after adjusting for factors known to influence nocturnal BP. The current study is consistent with the hypothesis that LSD is associated with an abnormal diurnal pattern on ABPM.
Supplementary Material
Acknowledgements
We thank the CARDIA participants for their time and invaluable contributions to our understanding of cardiovascular health and disease.
Funding Sources
The current study was supported by the American Heart Association grant SFRN 15SFRN2390002 and the Coronary Artery Risk Development in Young Adults Study (CARDIA) which is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI). Dr. Abdalla receives support through 18AMFDP34380732 from the American Heart Association and from the NIH/NHLBI (K23 HL141682-01A1 and R01HL146636-01A1).
Disclosures
Dr. Booth receives salary support for employment at CTI Clinical Trials and Consulting Services, Inc. for work unrelated to the topic of this manuscript, which was undertaken prior to his employment.
References
- 1.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354(22):2368–74. [DOI] [PubMed] [Google Scholar]
- 2.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdalla M, Caughey MC, Tanner RM, Booth JN 3rd, Diaz KM, Anstey DE, Sims M, Ravenell J, Muntner P, Viera AJ, Shimbo D. Associations of Blood Pressure Dipping Patterns With Left Ventricular Mass and Left Ventricular Hypertrophy in Blacks: The Jackson Heart Study. J Am Heart Assoc. 2017;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuspidi C, Facchetti R, Bombelli M, Sala C, Negri F, Grassi G, Mancia G. Nighttime blood pressure and new-onset left ventricular hypertrophy: findings from the Pamela population. Hypertension. 2013;62(1):78–84. [DOI] [PubMed] [Google Scholar]
- 5.Salles GF, Reboldi G, Fagard RH, Cardoso CR, Pierdomenico SD, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, de la Sierra A, Hermida RC, Dolan E, O’Brien E, Roush GC, Investigators A-H. Prognostic Effect of the Nocturnal Blood Pressure Fall in Hypertensive Patients: The Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) Meta-Analysis. Hypertension. 2016;67(4):693–700. [DOI] [PubMed] [Google Scholar]
- 6.Makarem N, Shechter A, Carnethon MR, Mullington JM, Hall MH, Abdalla M. Sleep Duration and Blood Pressure: Recent Advances and Future Directions. Curr Hypertens Rep. 2019;21(5):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep. 2015;38(8):1161–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, Bhatt DL, American Heart Association Obesity BCD, Nutrition Committees of the Council on L, Cardiometabolic H, Council on Cardiovascular Disease in the Y, Council on Clinical C, Stroke C. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation. 2016;134(18):e367–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krittanawong C, Tunhasiriwet A, Wang Z, Zhang H, Farrell AM, Chirapongsathorn S, Sun T, Kitai T, Argulian E. Association between short and long sleep durations and cardiovascular outcomes: a systematic review and meta-analysis. European heart journal Acute cardiovascular care. 2017:2048872617741733. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Bangdiwala SI, Rangarajan S, Lear SA, AlHabib KF, Mohan V, Teo K, Poirier P, Tse LA, Liu Z, Rosengren A, Kumar R, Lopez-Jaramillo P, Yusoff K, Monsef N, Krishnapillai V, Ismail N, Seron P, Dans AL, Kruger L, Yeates K, Leach L, Yusuf R, Orlandini A, Wolyniec M, Bahonar A, Mohan I, Khatib R, Temizhan A, Li W, Yusuf S. Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116 632 people from 21 countries. Eur Heart J. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjorvatn B, Sagen IM, Oyane N, Waage S, Fetveit A, Pallesen S, Ursin R. The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. J Sleep Res. 2007;16(1):66–76. [DOI] [PubMed] [Google Scholar]
- 12.Pretorius S, Stewart S, Carrington MJ, Lamont K, Sliwa K, Crowther NJ. Is There an Association between Sleeping Patterns and Other Environmental Factors with Obesity and Blood Pressure in an Urban African Population? PLoS One. 2015;10(10):e0131081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, Sun Y. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med. 2013;14(4):324–32. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res. 2012;35(10):1012–8. [DOI] [PubMed] [Google Scholar]
- 15.Ramos AR, Jin Z, Rundek T, Russo C, Homma S, Elkind MS, Sacco RL, Di Tullio MR. Relation between long sleep and left ventricular mass (from a multiethnic elderly cohort). Am J Cardiol. 2013;112(4):599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology (Cambridge, Mass). 2008;19(6):838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mezick EJ, Hall M, Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension. 2012;59(3):747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meininger JC, Gallagher MR, Eissa MA, Nguyen TQ, Chan W. Sleep duration and its association with ambulatory blood pressure in a school-based, diverse sample of adolescents. Am J Hypertens. 2014;27(7):948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle CY, Ruiz JM, Taylor DJ, Smyth JW, Flores M, Dietch J, Ahn C, Allison M, Smith TW, Uchino BN. Associations Between Objective Sleep and Ambulatory Blood Pressure in a Community Sample. Psychosom Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covassin N, Greene EL, Singh P, Somers VK. Disparities in Hypertension Among African-Americans: Implications of Insufficient Sleep. Curr Hypertens Rep. 2018;20(7):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carnethon MR, De Chavez PJ, Zee PC, Kim KY, Liu K, Goldberger JJ, Ng J, Knutson KL. Disparities in sleep characteristics by race/ethnicity in a population-based sample: Chicago Area Sleep Study. Sleep Med. 2016;18:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr., Willis M, Yancy CW, American Heart Association Council on E, Prevention, Council on Cardiovascular Disease in the Y, Council on C, Stroke N, Council on Clinical C, Council on Functional G, Translational B, Stroke C. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017;136(21):e393–e423. [DOI] [PubMed] [Google Scholar]
- 23.Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ, Shimbo D. Racial differences in abnormal ambulatory blood pressure monitoring measures: Results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens. 2015;28(5):640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and meta-analysis. Hypertension. 1999;33(5):1099–104. [DOI] [PubMed] [Google Scholar]
- 25.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of clinical epidemiology. 1988;41(11):1105–16. [DOI] [PubMed] [Google Scholar]
- 26.Kallistratos MS, Poulimenos LE, Karamanou A, Kouremenos N, Koukouzeli A, Vrakas S, Chamodraka EF, Tsoukanas K, Martineos A, Manolis AJ. Association of mid-day naps occurrence and duration with bp levels in hypertensive patients. A prospective observational study. Eur Heart J. 2015;36(suppl):1–161.25567812 [Google Scholar]
- 27.Radloff LS. The CES-D Scale:A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 28.Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–21. [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 30.Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Li Y, Dolan E, Tikhonoff V, Seidlerova J, Kuznetsova T, Stolarz K, Bianchi M, Richart T, Casiglia E, Malyutina S, Filipovsky J, Kawecka-Jaszcz K, Nikitin Y, Ohkubo T, Sandoya E, Wang J, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, O’Brien E, Investigators I. The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12(4):255–62. [DOI] [PubMed] [Google Scholar]
- 31.Octavio JA, Contreras J, Amair P, Octavio B, Fabiano D, Moleiro F, Omboni S, Groppelli A, Bilo G, Mancia G, Parati G. Time-weighted vs. conventional quantification of 24-h average systolic and diastolic ambulatory blood pressures. J Hypertens. 2010;28(3):459–64. [DOI] [PubMed] [Google Scholar]
- 32.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Task Force for the Management of Arterial Hypertension of the European Society of H, the European Society of C. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2014;23(1):3–16. [DOI] [PubMed] [Google Scholar]
- 33.Oakley N. Validation with polysomnography of the Sleepwatch sleep/wake scoring algorithm used by the Actiwatch activity monitoring system. Bend: Mini Mitter, Cambridge Neurotechnology. 1997. [Google Scholar]
- 34.O’Brien E, Parati G, Stergiou G. Ambulatory blood pressure measurement: what is the international consensus? Hypertension. 2013;62(6):988–94. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y, European Society of Hypertension Working Group on Blood Pressure M. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731–68. [DOI] [PubMed] [Google Scholar]
- 36.Van Buuren S. Flexible imputation of missing data. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 37.Breiman L. Random Forests. Machine Learning. 2001;45(1):5–32. [Google Scholar]
- 38.Shulman R, Cohen DL, Grandner MA, Gislason T, Pack AI, Kuna ST, Townsend RR, Cohen JB. Sleep duration and 24-hour ambulatory blood pressure in adults not on antihypertensive medications. J Clin Hypertens (Greenwich). 2018;20(12):1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durrence HH, Lichstein KL. The sleep of African Americans: a comparative review. Behav Sleep Med. 2006;4(1):29–44. [DOI] [PubMed] [Google Scholar]
- 40.Hall MH, Matthews KA, Kravitz HM, Gold EB, Buysse DJ, Bromberger JT, Owens JF, Sowers M. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 41.Mezick EJ, Matthews KA, Hall M, Strollo PJ Jr., Buysse DJ, Kamarck TW, Owens JF, Reis SE. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70(4):410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, Rong Y, Jackson CL, Hu FB, Liu L. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–37. [DOI] [PubMed] [Google Scholar]
- 43.Grandner M, Mullington JM, Hashmi SD, Redeker NS, Watson NF, Morgenthaler TI. Sleep Duration and Hypertension: Analysis of > 700,000 Adults by Age and Sex. J Clin Sleep Med. 2018;14(6):1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29(7):881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed N, de la Torre B, Wahlgren NG. Salivary cortisol, a biological marker of stress, is positively associated with 24-hour systolic blood pressure in patients with acute ischaemic stroke. Cerebrovascular diseases (Basel, Switzerland). 2004;18(3):206–13. [DOI] [PubMed] [Google Scholar]
- 46.Holt-Lunstad J, Steffen PR. Diurnal cortisol variation is associated with nocturnal blood pressure dipping. Psychosom Med. 2007;69(4):339–43. [DOI] [PubMed] [Google Scholar]
- 47.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27(1):51–4. [PubMed] [Google Scholar]
- 48.Stickley A, Leinsalu M, DeVylder JE, Inoue Y, Koyanagi A. Sleep problems and depression among 237 023 community-dwelling adults in 46 low- and middle-income countries. Scientific Reports. 2019;9(1):12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tropeano AI, Roudot-Thoraval F, Badoual T, Goldenberg F, Dolbeau G, Gosse P, Macquin-Mavier I. Different effects of ambulatory blood pressure monitoring on subjective and objective sleep quality. Blood Press Monit. 2006;11(6):315–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


