Abstract
Heavy metal pollution has emerged as one of the most serious environmental challenges facing the world today. The removal of heavy metals from the effluent is of special environmental concern because of their toxicity and persistence in nature. This study presents the suitability of activated carbon from waste rubber tire as a low-cost adsorbent for multiple adsorption of copper, lead and zinc from wastewater. The adsorbent removed heavy metal ions effectively from solution medium in the order of copper > lead > Zinc. The adsorption process was rapid with all metals reaching equilibrium within 120 min. The optimum pH for Lead was achieved at 5 and 6 for copper and Zinc. The removal of heavy metals was discovered to increase with adsorbent dosage and contact time and reduced with initial concentration. The adsorption of multiple heavy metals was modeled using Freundlich and Langmuir adsorption isotherms to assess the experimental findings. The equilibrium data better fitted to the Langmuir isotherm with regression coefficient (R2) of 0.9831, 0.9992 and 0.9953 for lead, copper and zinc respectively. The maximum adsorption capacities (Qmax) at equilibrium were 9.6805 mg/g, 12.4378 mg/g and 4.9950 mg/g for Lead, Copper and Zinc respectively. The adsorption kinetics indicated that pseudo-second-order kinetic model described well the sorption mechanism for multiple adsorption of heavy metals with R2 of more than 0.99 for all metal ions. An empirical model for predicting and designing of a single batch adsorber for 95 % multiple heavy metal ion removal at any given initial heavy metal ion concentration and effluent volume was further developed using activated carbon from waste rubber tires. Waste rubber tire Activated carbon demonstrated an ability for the treatment of wastewater containing these heavy metals in multimetal solutions.
Keywords: Multiple, Adsorption, Heavy metal, Activated carbon, Waste rubber tires, Adsorption isotherms, Adsorption kinetics
Multiple; Adsorption; Multiple; Heavy Metal; Activated Carbon; Waste rubber tires; Adsorption Isotherms; Adsorption Kinetics.
1. Introduction
Industrial effluents with heavy Metals are a threat to the environment and public health. Heavy metals, unlike organic pollutants, are non-biodegradable and are persistent in the environment making their treatment a challenge [1]. Heavy metals accumulate in living organisms and they have been known to be carcinogenic and cause many disorders in human health [2]. Heavy metals such as Lead, copper, Zinc, Nickel, Cadmium and Chromium are the primary pollutants in Electroplating Effluents [3]. These pollutants are found to be significant contaminants in surface and groundwater [4].
Heavy metals are toxic even at trace level and are currently faced with rigorous measures for maximum allowable limits of their discharge into the environment [5]. Authorities imposing these measures require that treatment methods should be environmentally friendly so as to safeguard public health and the environment [6]. Various conventional methods that have been applied in metal ions removal include; Chemical precipitation, ion exchange, membrane filtration, reverse osmosis, ion exchange, electrodialysis and Adsorption [4, 7, 8]. However, some of these conventional methods have some disadvantages in that they are associated with high costs and less efficiency at low metal concentrations of 1–100 mg/l [9]. Some of the methods also leads to generation of sludge [10], whereby their disposal is an additional burden on the economic feasibility of the treatment. Therefore, these constrains has led to the need of exploring low-cost and efficient adsorbents that are environmentally friendly and remove metal ions to acceptable limits. The potential of adsorbent materials to adsorb metal ions has attracted immense interest in the development of an effective, clean, and low-cost technology for wastewater treatment at metal concentrations as low as 1 mg/L [9]. The most widely recognized materials that have been utilized for low-cost adsorbents production are; sawdust, slime, fly ashes, scrap tires and agricultural by products [11].
Multiple removal of heavy metals ions present in a solution is perhaps the most daunting challenge water purification [12, 13]. A number of research on heavy metal ion adsorption utilizing a variety of adsorbents has been conducted [14]. However, several studies have extensively focused on single metal adsorption from aqueous solution by adsorbent materials [15, 16]. However, very little information is available for multiple adsorption processes [17]. Electroplating wastewater however contain multiple metal ions, which are likely to cause interactive effects depending on the number of metals competing for active sites, levels of initial concentrations, the equilibrium steady state concentration of different metal ions and the mass of the adsorbent [18].
Amongst the few multimetal adsorption processes investigated are the removal of Cd, Cu, Ni and Zn by black gram husk [19]; Cd, Ni, Pb, Cr, Cu, and Zn by Nigerian Bamboo [20]; Ni, Cu and Cr by sunflower plant biomass-based carbon [21]; Pb and Cd by Carpobrotus edulis plant [22]; removal of Pb, Cd, Cu and Ni by Ultrafine Mesoporous Magnetite Nanoparticles [23]; Pb and Cu by Van apple pulp in single- and multi-solute systems [24]; Zn, Ni and Cd by Olive stone [25]; Cu, Ni and Cd by olive stone [26]. No study has yet been carried out on the removal of metal ions by waster rubber tires in multimetal solutions, although several materials have been reported to effectively adsorb them from single metal solutions [27, 28, 29]. Most of the multimetal adsorption studies furthermore were done in shake flasks, which cannot be extrapolated for designing an operational technology without the data obtained from a continuous flow system. Therefore, there is need to study the multiple adsorption of two or more heavy metals and also to quantify the interference of a metal with the adsorption of other.
There have been many batch adsorption studies and experiments to remove contaminants from wastewater [30]. A single batch adsorber is designed to extract rhodium concentrations up to 90% removal efficiency when removing rhodium using gallic acid formaldehyde. For heavy metals' removals from aqueous solutions, where mesoporous metal oxides are used as adsorbents, a two-stage batch adsorber is designed to help remove herbicide [31]. The adsorbent dose is reduced from 78% to 91% compared to a single-batch adsorption system [32]. Batch adsorbers are frequently employed in sorption investigations since they have advantages over continuous flow systems, such as reducing pressure and minimizing mass transfer resistance [32]. It is also necessary to determine the mass of adsorbent and the solution volume required for such adsorption process. These variables are critical and must be carefully studied to completely comprehend the adsorption operation and the design of an adsorber for multiple heavy metal ion adsorption in wastewater. Designing a batch adsorber that can adsorb multi-metals ions such as Lead, Copper and Zinc often found in Electroplating wastewater in the same aqueous solution is imperative.
Waste tires is an alternative source of activated carbon due to its carbon content [33]. Waste tires also contains functional groups like hydroxyl and carboxyl as reported from FT-IR analysis by [27]. In addition, waste rubber tires activated with hydrogen peroxide are particularly effective in various adsorption of heavy metal in wastewater [34]. The adsorbents prepared from waste rubber tires had a good potential for the adsorption of copper from a mono metal systems [28]. Yet not data are available on the use of waste rubber tires for the adsorption of heavy metal ions from multi-metal system. Therefore, the main objective of this study is to evaluate the removal of lead, copper and zinc in a multi-metal solutions using waste rubber tire adsorbents and also to design single batch adsorber for multiple removal of heavy metals.
2. Materials and methods
2.1. Electroplating wastewater and location of study
Electroplating wastewater samples were drawn using a sampler to a depth of 50 cm from the holding tank at the Electroplating company located in Industrial Area, Nairobi, Kenya. The holding tank acts as a meeting point where the effluents are held before they are discharged into municipal sewage.
2.1.1. Characteristics of electroplating wastewater
The wastewater was evaluated for Temperature, pH, Electrical Conductivity, Turbidity, Total Dissolved Solids and heavy metal ions. The pH, temperature and electrical conductivity were measured using HANNA Instruments, H1991300, Romania multiparameter meter and DT-9 digital thermometer. SGZ-B Portable Turbidity Meter was used to measure the turbidity of wastewater. The number of heavy metal ions was assessed using the Flame atomic-absorption spectrophotometer [35]. The various parameters analyzed and the initial concentrations of the heavy metal present in the wastewater are shown in Table 1.
Table 1.
Physico-chemical characteristics of wastewater.
| Parameter | Units | Value | Maximum Permissible limits |
|---|---|---|---|
| Temperature | oC | 23 | 25–30 |
| pH | - | 9.48 | 6.5–8.5 |
| Electrical conductivity | μS | 89.9 | 2000 |
| Turbidity | NTU | 2920.5 | 5 |
| Total Dissolved Solids | mg/l | 44.7 | 1200 |
| Cadmium (Cd) | mg/l | 0.0188 | 0.1 |
| Chromium (VI) | mg/l | 0.0818 | 0.05 |
| Copper (Cu) | mg/l | 138.3767 | 1 |
| Iron (Fe) | mg/l | 4.032 | 10 |
| Nickel (Ni) | mg/l | 0.542 | 1 |
| Lead (Pb) | mg/l | 12.5905 | 0.1 |
| Zinc (Zn) | mg/l | 74.9382 | 5 |
The variables measured did not meet the permissible discharge limits of industrial effluent as per the regulatory body of Kenya [6]. Analytical results for heavy metals in the Electroplating industry manifested high Copper, Lead and Zinc with values of 138.38 mg/L, 12.59 mg/L and 74.94 mg/L, respectively. The water quality Regulation 2006 set limits for copper, lead and zinc in the public sewers discharge as 1 mg/L, 0.1 mg/L and 5 mg/L. Therefore, for this study higher concentration (138 mg/l) was taken for further investigation and the concentration for copper, lead and zinc was taken to be 138 mg/l.
2.2. Adsorbent preparation
Waste rubber tires were at first washed with detergents and sluiced with distilled water to eliminate soil debris and extrinsic and sun-dried for 3 h. Cleaned and dried waste tire was shredded and pyrolyzed at 900 °C without air in Kiln for 3 h. The carbon-based material was then left to cool, grounded and passed through a 1.18 mm sieve to obtained a uniform size of particles. 100 g of the carbonized waste rubber tires was activated by carefully mixing with 150 ml of hydrogen peroxide (50 wt. % in H202) to form a paste; the paste was burnt for 2 h at 500 °C to increase carbon surface area.
2.3. Batch adsorption experiment
The effects of pH, contact time, adsorbent dosage, and initial concentrations were studied in batch experiments. Synthetic wastewater containing the three metals was applied for the experiment. The metal solution was prepared from 1000 mg/l stock solution of the different heavy metals (Lead, Zinc and Copper).
To establish equilibrium, 100 mL of the wastewater sample was poured into a 150 mL flask and 0.8 g of previously made activated carbon was added. The sample bottles were then put in a mechanical shaker and stirred at 250 rpm at room temperature for 120 min. After the agitation time, the contents were centrifuged for 10 min at 1500 rpm and filtered. The remaining metal ions concentration in 5 ml of the filtrates was evaluated using a Flame Atomic Absorption Spectrophotometer (A.A.- 7000 Shimadzu). The wavelength at which copper, lead and zinc were analyzed was 324.8, 283.3 and 213.9 nm respectively. Standard solution of 1000 mg/L of Cu, Pb, and Zn was used to create a standard calibration curve. The calibration curve was determined using serial dilution (0.0, 0.5,1.0, and 2 mg/L).
To determine the percentage of heavy metal removal and WRTA's adsorption capacity (Qe), Eqs. (1) and (2) was utilized respectively as indicated by [22].
| (1) |
| (2) |
Where
Co = initial heavy metal concentration (mg/l),
Ce = equilibrium or final heavy metal concentration in the solution (mg/l),
qe = metal adsorbed at equilibrium (mg/g),
M = Adsorbent mass (g),
V = Solution Volume in (L).
2.4. Adsorption isotherms
2.4.1. Langmuir isotherm
When an adsorbate monolayer saturates the adsorbent, the Langmuir model assumed that equilibrium has been attained [36].
The empirical equation for the linear form of Langmuir adsorption isotherm is illustrated in Eq. (3).
| (3) |
Where;
qe = amount of metals adsorbed at the equilibrium concentration (mg/g),
Ce = Equilibrium concentration of adsorbate in solution (mg/l),
Xm = Langmuir maximum adsorption capacity (mg/g)
KL = Langmuir constant related to a free energy of adsorption (mg/g).
A plot of versus Ce, gives a straight line of slope and intercept .
2.4.2. Freundlich isotherm
The Freundlich model is a simple experimental equation used to illustrate multi-layer adsorption on heterogeneous surfaces under non-ideal adsorption equilibrium [37]. The binding sites available for adsorption are not equivalent.
The linear Equation for Freundlich isotherm is as expressed in Eq. (4)
| (4) |
Where;
qe = is the amount of metals adsorbed at the equilibrium concentration (mg/g),
Ce = is the Equilibrium concentration of adsorbate/solute in solution (mg/l),
Kf = Freundlich constant related with solid adsorption capacity (mg/g),
n = Freundlich constants related to adsorption intensity (mg/g).
A plot of log against log Ce gives a straight line of slope and intercept log Kf, for adsorption data that follow the Freundlich theory.
2.5. Kinetic study
Process design, operation control and adsorption kinetics are essential for any practical application [38]. Adsorption kinetics during wastewater treatment is important as it gives valuable insights into the reaction pathways and mechanism controlling the rate of reaction [39]. Kinetics also describes the metal uptake which in turn controls the residence time of adsorbate at the solid-solution interface.
Adsorption kinetics were investigated to determine the adsorptive absorption of metal ions from wastewater at various time intervals. In this study, the adsorption of heavy metal ions was modeled using pseudo-first order, pseudo-second order kinetic and intraparticle diffusion models. on waste rubber tires adsorbent [40]. When plotted, the linear form of each model indicates if the model is favorable to define the sorption process or not.
The equation for the Pseudo first order model is illustrated Eq. (5) [41].
| (5) |
Pseudo-second order model is described as indicated in Eq. (6) [41].
| (6) |
Where;
qe = amount of metals adsorbed at equilibrium (mg/g)
qt = amount of metals adsorbed at a time, t (mg/g)
K1 = Adsorption rate constant (g/mg min)
K2 = pseudo-second-order reaction rate constant (g/mg min)
| (7) |
Intraparticle diffusion model is given in Eq. (7) [42].Where
Kd is the diffusion rate constants within the adsorbent particle (mg/g min)
A linear plot of Qt versus t 0.5 shows that the adsorption mechanism follows intra particle diffusion and if the intra particle diffusion is the rate controlling step, the line would pass through the origin.
C is the intercept and Kd is obtained from the slope.
2.6. Design of single batch adsorber for adsorption of Cu2+, Pb2+ and Zn2+ ions using WRTA
Adsorption isotherms can be used to design a single batch adsorber to remove heavy metals from wastewater [30, 31, 43]. A single batch adsorber was designed based on the best suited isotherm to scale up laboratory bench-scale experiments for practical applications. The goal of the single-step batch adsorber design is to lower Cu2+, Pb2+, and Zn2+ ions concentrations in effluent volume (L) from initial concentration (Co (mg/L)) to final concentration (C1 (mg/L)). A schematic diagram of the batch adsorber model is shown in Figure 1. This may be utilized to figure out how much adsorbents are required in removing a given percentage of heavy metals from a given volume of effluent.
Figure 1.
Schematic diagram of a Single Batch adsorber process for heavy metals.
M is the amount of adsorbent and the adsorbent solvent load changes from Qo to Qi (mg/g). Qo = 0 at time t = 0, but over a period of time, the mass balance equaled the metal adsorbed from the liquid to the metal adsorbed by the adsorbents solid. The mass balance equation is given as follows:
| (8) |
Where;
Co = initial concentration (mg/l),
Ci = final concentration (mg/l),
M = adsorbent mass in the solution (g).
At equilibrium, C1 and Q1 will be equal to Ce and Qe, respectively. The initial heavy metals adsorbed is Q0 = 0 (mg/g). Q1 (mg/g) is the final heavy metals ion adsorbed. Eq. (8) can therefore be expressed as;
| (9) |
3. Results and discussion
3.1. Adsorbents characteristics
The waste rubber tire activated carbon (WRTA) was characterized to determine chemical composition, elemental composition surface functional groups and surface morphology. The elemental composition was determine using CHNS Elemental Analyzer model AAS iCE 3300 and are presented in Table 2. The adsorbent's surface functional groups were evaluated using a Shimadzu (FTIR-8400) Fourier Transform Infrared Spectrophotometer. The morphological characteristics of the adsorbent were assessed by using a JOEL (JCM-7000) Scanning Electron Microscope. The powder samples of WRTA were covered with a thin layer of gold and an electron acceleration voltage of 10 kv was applied.
Table 2.
Chemical composition of waste rubber tire ash.
| Elemental Constituents | Percentage Composition (%) |
|---|---|
| MgO | 0.008 |
| SiO2 | 65.04 |
| P2O5 | 0.316 |
| S | 15.93 |
| K2O | 0.096 |
| CaO | 2.355 |
| TiO2 | 0.1 |
| Cr | 0.019 |
| Fe | 0.413 |
| Co | 0.011 |
| Ni | 0.001 |
The chemical composition analysis by XRF showed in Table 2 indicates that dormant element expressed as percentage oxide in the unidentified compound making up the carbon matrix is Silicon (expressed as (SiO2) with its percentage content of 65.04 %. Carbon-based materials with high proportion of Silicon are important in terms of surface area and reactive surface functional groups which are significant in the adsorption process [44]. The elemental composition measured by CHNS elemental analyzer of waste rubber tires in terms of carbon, hydrogen, nitrogen and Sulphur were determined (Table 3). The results indicated that the materials contain high amount of carbon and hydrogen and less amount of Sulphur and nitrogen.
Table 3.
Elemental composition of waste rubber tire adsorbent.
| Parameter | Parameter content in percentage (%) |
|---|---|
| Carbon | 90.64 |
| Hydrogen | 7.32 |
| Nitrogen | 0.61 |
| Sulphur | 1.43 |
The interaction among an adsorbate and the active functional groups on the surface of the adsorbent can be analyzed using FTIR spectroscopy. Chemical functional groups like carbonyl, hydroxyl, amine, amide, and others have been identified as potential adsorption sites for binding metallic ions to the adsorbent [30]. The functional groups in both the raw and activated waste rubber adsorbent were identified in the FI-IR spectrum (generated from 4,000 to 500 cm−1). The results from FT-IR test are given in Figure 2 for the raw and activated carbon. The FT-IR analysis revealed that O–H and C=O surface functional groups are liable for adsorption metal ions onto the adsorbents [45]. At band that appeared at 3400 cm−1 is linked to the O–H vibration of the hydroxyl group, which is involved in hydrogen bonding [14]. The carboxyl group stretching vibrations are associated with the occurrence of band at 1596 cm−1 indicating the presence of the C=O bond [46]. The absorption peak of the O–H group transitioned from 3394 cm−1 to 3417 cm−1 for activated samples, which represents the difference between the FTIR spectra of raw adsorbent and activated carbon.
Figure 2.
Fourier Transform Infrared Spectrophotometer spectra for Raw and Activated Carbon.
The SEM micrograph of a raw carbon shown in Figure 3 (a) reveals a slightly porous morphology structure with small pores of various shapes and sizes ranging from 3.025 μm to 20.75 μm as indicated in the diagram. The activated carbon shown in Figure 3 (b) demonstrates an irregular structure with a slightly porous morphology showing that the activation (using hydrogen peroxide) improved the characteristics of the raw carbon with regard to the porosity of the structure. The porosity of the adsorbent was evident on the outer surface that is full of cavities, proving to have high surface area with more or less uniform pore distribution and the size and shape of the pores are the same.
Figure 3.
SEM micrograph of (a) raw carbon (b) activated carbon.
3.2. Effects of pH
The wastewater pH is an essential variable in heavy metals removal from aqueous solution [47]. The effect of wastewater pH on the multiple adsorption of lead, copper and zinc by WRTA is shown in Figure 4. The effects of solution pH were evaluated in the pH range of 2–10. Adsorbent dosage of 1 g was mixed with 100 ml of multi-metal solution and agitated for 90 min. The initial concentration of lead, copper and zinc was 138 mg/Leach.
Figure 4.
Effects of pH on metal ions adsorption using waste rubber tires adsorbent.
The results show that the most favorable pH was 5 for Pb and 6 for Cu and Zn. A percentage removal efficiency of 96.62% for Lead, 93.85% for copper and 89.55% for Zinc was recorded. The percentage of heavy metals removal increases from pH 2 to pH 5 for Lead and from pH 2 to pH 6 for copper and Zinc. Beyond pH 6, the percentage of heavy metal decreased. At low pH, most functional groups protonate and act as positively charged species, competing for active sites with metal ions [28]. The high adsorption efficiency observed between pH 5 and 6 corresponds to an increase in adsorbent surface deprotonation, which results in a reduction in hydrogen ions on the adsorbent surface, creating a more negative charge on the adsorbent surface and favoring adsorption of positively charged sites on the adsorbent [48]. The precipitation of insoluble metal hydroxides is responsible for the decrease in metal removal efficiency at basic pH, which interferes with the active sites and hence the reduction of metal adsorption by restricting the movement of metal ions to the active areas [14, 49]. These findings is compatible with recent studies [50, 51] in their study of removal heavy metals using dairy manure compost and Van apple pulp activated carbon. Kalmykova et al. [39] furthermore stated that the amount of active binding sites in peat increases with pH due to the repelling of negatively charged macromolecules with one another. This concept can also be applied to negatively charged active carbon particles, whose electrokinetic potential increases in the negative direction as solution pH increases, resulting in increased metal ion uptake. Based on the above information the study maintained the pH of the solutions at 6.0 for the rest of the adsorption experiments.
3.3. Effect of adsorbent dosage
The effect of dosage was evaluated at the range of 0.2 g–1.2 g for 100 ml of multi-metal solution for 90 min. The solution's initial pH was set to 6 and the initial concentration was 138 mg/L for all metal ions (Lead, Zinc and Copper). The results are indicated in Figure 5. The results indicate that the optimum dosage was 0.8/100 ml of WRTA. The percentage of metal ions removed increased as the adsorbent dosage increases. This may be attributed to increment in the medium's available active sites [52]. Similar findings were reported by Sabanovic et al. [13], Sdiri and Higashi [53] as they evaluated simultaneous adsorption of heavy metals from water by novel lemon-peel based biomaterial and natural limestones. Beyond the optimum dosage, constant adsorption was observed. This may be attributed to the oversaturation of the adsorbent's attachment sites, which is due to overcrowding of metal ions on the sites [54]. The results revealed the percentage removal efficiency of 96.23%, 94.23 % and 89.34 % for Lead, Copper and Zinc, respectively, at optimum dosage. Thus, 0.8 g/L of the adsorbent dosage was used as operational dosage for the rest of the batch experiments
Figure 5.
Effects of adsorbent dosage on adsorption of metal ions using waste rubber tires adsorbent.
3.4. Effect of contact time on multiple adsorption of heavy metals
The effect of contact duration on the uptake metals ions (Cu2+, Pb 2+ and Zn2+) each with initial concentration of 138 mg/L using the WRT adsorbents is illustrated in Figure 6. The experiment was conducted at room temperature at an interval of 30 min at fixed pH of 6 and adsorbent dosage of 0.8 g.
Figure 6.
Effects of contact time on heavy metals removal using waste rubber tires adsorbent.
Results from the experiment (Figure 6) shows that percentage removal of metal ions increases with increase in contact time. After sometime, there was no significant changes in the percentage removal that was observed, implying that the adsorption sites were saturated due to intense competition among the heavy metals studied. A similar trend was observed by Gupta et al. [55] where the adsorption efficiency of Lead and Nickel by waste rubber tire increased for the first 120 min of contact time after which the adsorption capacity levelled off and Odubiyi et al. [56] in their study of the removal of heavy metals from aqueous solution using activated charcoal produced from cocoa pod husk. The multiple removal efficiency of lead, copper, and zinc was rapid initially and reached equilibrium within 120 min of contact time for all metal ions and then it becomes almost constant at the end of the experiment. The fast adsorption of metal ions at initial stages can be attributed to the initial concentration gradient between the adsorbent in the solution and the availability of unoccupied active sites on the WRTA at the beginning. The gradual increase in adsorption, and thus the attainment of equilibrium adsorption, could be attributed to limited mass transfer of adsorbent molecules from the bulk liquid to the external surface of WRTA [57]. At equilibrium, 98.14 % of the lead ions, 95.53 % of the copper ions, and 89.58 % of the zinc ions were removed from the solution. It can be then concluded that metal-binding rate with adsorbent is more prevalent during early stages and up to the optimum contact time which was discovered to be around 120 min.
3.5. Effects of initial concentration
The influence of multi-metals concentration on its adsorption rate was determined through variation of initial concentration in the solution from 10 to 140 mg/L. The equilibrium concentration of heavy metals was evaluated using an adsorbent weight of 0.8 g and 100 ml of multi-metal solution and the solution pH was initially set to 6. The results from Figure 7 show that the percentage removal of metal ions decreased with increase in the initial metal ion concentration. This is majorly because as the concentration increases, the sorption sites of the adsorbent material become saturated. The number of metal ions available in the solution is less at lower metal ion concentrations when contrasted to the available active sites on the waste rubber tire adsorbent. The available active sites for metal ion adsorption become less at higher metal ion concentrations, and the percentage removal of metal ions is dependent on the initial metal ion concentration. The optimum removal of Pb, Cu, and Zn by WRTA was 99.93%, 98.86%, and 95.27%, respectively. This is consistent with the results obtained by [58].
Figure 7.
Effects of initial concentration on heavy metals removal using waste rubber tires adsorbent.
3.6. Isotherm modelling
The Langmuir and Freundlich models were used to describe the adsorption isotherms, which are commonly used to describe the findings of adsorption on activated carbon in a solution due to their simplicity and applicability. Therefore, the adsorption isotherm data were fitted using these well-known two-parameter models. Figure 8 show Langmuir isotherm for the adsorption of selected heavy metal ions by waste rubber tire activated carbon from multi-metal solution
Figure 8.
Langmuir plot for adsorption of Lead, copper, Zinc unto carbon from waste car tires.
Figure 9 displays the Freundlich isotherm for the adsorption of selected heavy metal ions by waste rubber tire activated carbon from multi-metal solution. The Freundlich isotherm model parameters (Table 4) are derived from the best line of fit's slope, intercept, and correlation coefficient (Figure 9). The data reveal that the simultaneous adsorption of Cu2+, Pb2+ and Zn2+ does not fit well with the Freundlich isotherm model, as R2 for most metals is below 0.99.
Figure 9.
Freundlich plot for adsorption of Lead, copper, Zinc unto carbon from waste car tires.
Table 4.
Constants for the Langmuir and Freundlich isotherm.
| Heavy Metal | Langmuir isotherm |
Freundlich isotherm |
||||
|---|---|---|---|---|---|---|
| Xm (mg/g) |
KL (mg/g) |
R2 | Kf (mg/g) |
1/n (mg/g) |
R2 | |
| Pb | 9.6805 | 0.1137 | 0.9831 | 0.4432 | 2.1149 | 0.9433 |
| Cu | 12.4378 | 0.0233 | 0.9992 | 0.1755 | 1.3743 | 0.9834 |
| Zn | 4.9950 | 0.0248 | 0.9953 | 0.0446 | 1.6162 | 0.9851 |
Table 4 shows the adsorption parameters of the two models studied. The Langmuir model best described the adsorption process for all metal ions evaluated, with R2 values near to unity. Adsorption occurs at the homogeneous sites of the adsorbents, according to the Langmuir isotherm model [36]. This presupposes that the metals are adsorbed onto the adsorbent surface, forming a monolayer [27]. The adsorption was chemisorption, as indicated by the Xm and KL. values in Table 4.
3.7. Adsorption kinetics study
Pseudo-first, pseudo-second-order kinetic and intraparticle diffusion models were used to evaluate the mechanism of metal ion adsorption. Figures 10, 11, and 12 show graphs of kinetic models for multi-metals adsorption of lead, copper and zinc from aqueous solution using activated carbon derived from waste rubber tires.
Figure 10.
The Pseudo First Order Kinetic Model for Cu2+, Pb2+ and Zn2+ ion Adsorption onto WRTA.
Figure 11.
The Pseudo Second Order Kinetic Model for Cu2+, Pb2+ and Zn2+ ion Adsorption onto WRTA.
Figure 12.
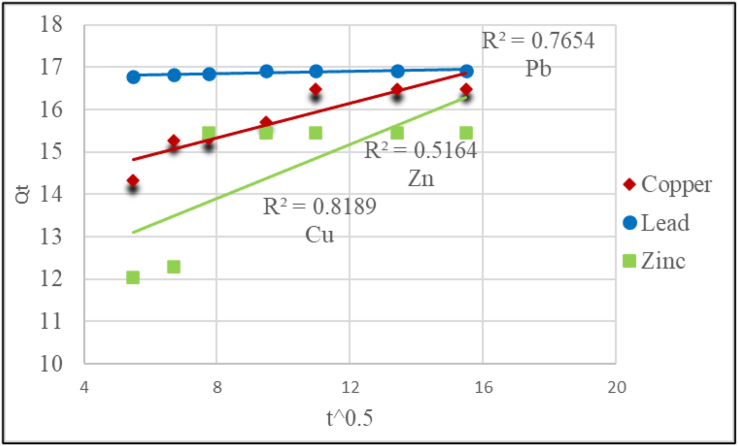
Intraparticle diffusion Model for Cu2+, Pb2+ and Zn2+ ion Adsorption onto WRTA.
In Table 5, the sorption data fit was consistent to pseudo-second-order model, with correlation coefficients R2 close to 1 for the selected metal ions. Furthermore, the Qecal (Calculated) value of the pseudo-second-order model is more comparable to the Qeexp (Qe experimental was calculated from Eq. 2) with a correlation coefficient higher than that of pseudo-first-order. For instance, the calculated Qe of Pb2+ was 16.949 mg/g, which is almost similar to Qeexp value of 16.9533 mg/g, when correlated to Pb2+ of the Pseudo first-order model, which has a calculated Qe of 16.9533 mg/g, which is considerably different from the experimental value of 0.3551 mg/g. The correlation coefficient (R2) for intraparticle diffusion model was less than 0.9 for all metal ions. These findings imply that chemisorption was involved in heavy metals adsorption by activated carbon from waste rubber tires following the pseudo-second-order reaction paradigm. Chemisorption occurs when heavy metals create a chemical bonding with the adsorbent surface and seek out sites that escalate their synchronization number with the surface [59, 60].
Table 5.
Kinetic parameters of the Pseudo first order, Pseudo Second-order and intraparticle diffusion model.
| Heavy Metal ions | Pseudo First Order Kinetics model |
Pseudo Second Order Kinetics model |
Intraparticle diffusion model |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qe(exp) (mg/g) |
Qe(cal) (mg/g) |
K1 (g/mg min) |
R2 | Qe(cal) (mg/g) |
K2 (g/mg min) |
R2 | Qe(cal) (mg/g) |
Kd (g/mg min) |
R2 | |
| Copper | 16.659 | 5.370 | 0.00021 | 0.869 | 16.892 | 0.011 | 0.999 | 16.4789 | 0.2033 | 0.8189 |
| Lead | 16.953 | 0.355 | 0.000193 | 0.988 | 16.949 | 0.204 | 1.000 | 16.9298 | 0.0146 | 0.7654 |
| Zinc | 15.527 | 8.236 | 0.000384 | 0.637 | 16.103 | 0.007 | 0.997 | 15.4526 | 0.3181 | 0.5164 |
3.8. Process parameters from single batch adsorber design
According to the batch adsorption studies and model results, the experimental data were consistent with the Langmuir isotherm model. The Langmuir isotherm equation was used to calculate the mass of waste rubber tire adsorbent needed to remove a given fraction of metal ions from different wastewater volumes. Subsequently, the adsorption isotherm model was substituted for Qe in Eq. (9), and it can be rewritten as in Eq. (10);
| (10) |
Given that the adsorption process involves multiple removal of Cu2+, Pb2+ and Zn2+ ions in the same aqueous solution, the pseudo component approach was used to calculate a constituent balance for every metal ion in solution, allowing Eq. (10) to be rewritten as in Eq. (11);
| (11) |
i = 1,…,nWhere;
Coi = initial concentration of each metal ions simultaneous in solution,
Cei = the final concentration of each metal ions simultaneous adsorbed in solution at equilibrium,
Xm = Langmuir Constant associated with the maximum Adsorption capacity
KL = Langmuir Constant associated with the relative energy of adsorption.
Table 4 shows the Langmuir constants Xm and KL obtained from batch experiments for each metal ion adsorbed.
The limits adopted for this research to develop adsorber for multiple adsorption of heavy metal ions in wastewater were based on the maximum allowable limits of heavy metal ions concentration in wastewater as described by the National Environmental Management Authority (NEMA) in Table 6. This means that 95–99 percent of the metal ions in wastewater must be removed. As a result, the mass of adsorbent required for adsorber design was predicated on a 95 % removal effectiveness.
Table 6.
NEMA guidelines for the concentration of heavy metals ions in wastewater before discharge.
| Heavy Metals | Permissible amount in the wastewater (Mg/l) |
|---|---|
| Copper | 2 |
| Lead | 0.01 |
| Zinc | 3 |
Adsorbent mass (M) required was computed using Eq. (10). An initial metal concentration of 140 mg/L for all the metals (copper, zinc and lead) was applied and the required amount of adsorbent to remove metal ions content by various removal efficiencies (70–99%) and different volumes of wastewater (1,000–10,000 L) was considered for the design. As illustrated in Figure 13, the adsorbent mass (M) needed was plotted versus the volume of wastewater treated.
Figure 13.
Mass of Adsorbent (M) versus effluent volume (V) treated for different percentages of metal ions removal at 140 mg/L initial concentration.
The finding revealed that as the percentage metal ions removal increases, so does the amount of adsorbent require. The figure presents the calculated amount of waste rubber tire adsorbents needed to remove metal ions from aqueous solution with an initial concentration of 140 mg/L for 99%, 95%, 90%, 80%, and 70 % metal ion removal at various volumes (1000–10 000 L) for a single-step batch adsorber, whereby the design process has been established. For all metal ions solution volumes ranging from 1000 to 10,000 L, the mass of WRT adsorbent needed to get rid of 95% of Pb2+, Zn2+, and Cu2+ ions from an aqueous solution of 140 mg/L initial concentration were 0.287, 0.574, 0.861, 1.148,1.435, 1.722, 2.009, 2.296, 2.583, 2.870 kg respectively.
To evaluate the amount of WRT adsorbent (M) required to reduce the multiple ion content of wastewater solutions by 95 % at various volumes (1000 L–10,000 L) and different initial concentrations (ranging from 10 to 140 mg/l), Eq. (10) was applied and the results are plotted in Figure 14.
Figure 14.
Mass of Adsorbent (M) versus initial metal ion concentration for 95 % metal removal at varied volumes of treated solutions (V).
Figure 14 shows the amount of WRT adsorbent is needed to remove multi-metal ions (Pb, Cu, and Zn) from various volumes of wastewater with distinct metal concentrations by 95%. For example, with 10,000 L of wastewater to be treated, the amount of WRT adsorbent required to reduce metal ions contents by 95% increased from 2.4 kg to 2.8 kg as metal ions concentration rose from 10 mg/l to 140 mg/l. Therefore, from Figure 14, the amount of WRT adsorbent required to remove 95 % of metal ions from an aqueous solution can be estimated for any initial metal ions concentration for a fixed volume of effluent. Such a projection can be used to design a single-stage adsorption system.
Therefore, the study came up with an Eq. (11), which can be used to calculate the amount of adsorbent (M) required for multiple adsorption of Pb2+, Cu2+ and Zn2+ ions from electroplating effluent in a single batch adsorber at 95% efficiency. The initial concentrations of the three metal ions are shown in Table 1. The equation was developed by substituting the values of initial concentration of lead, copper and zinc in Table 1 to Eq. (11) to achieve 95% removal efficiency.
| (12) |
Where; V is the volume of effluent to be treated.
The constant 0.000282 was achieved by substituting different parameters of each metal ion in Eq. (10). Thus, in this case, the values of V will vary depending on the volume of effluents to be treated.
4. Conclusions
The study shows that Waste rubber tires is a potent adsorbent for removing multiple metals ions from aqueous solutions. Characterization of waste rubber tire adsorbent revealed that they comprised of carboxyl and hydroxyl functional groups that play a significant role in the removal of heavy metals from wastewater. The adsorption of metal ions onto the WRTA depends on the solution pH, adsorbent dose, contact time and initial metal ion concentration. The maximum adsorption of metal ions by WRTA were recorded at adsorbent dosage (0.8 g), pH 5 (for Pb) and 6 (for Cu and Zn) and contact time (120 min). The adsorption isotherms of Pb, Cu and Zn on WRTA is quite well consistent with the Langmuir model in multi-metal system with R2 of 0.9831, 0.9992 and 0.9953 for lead, copper and zinc respectively. Experimental data fitted well to pseudo-second order kinetic model. This demonstrated that the adsorption of metal ions such as Cu, Pb and Zn ions onto the surface activated waste rubber tires was a monolayer adsorption. The adsorption capacities of heavy metals increase in the order of Cu (12.4378 mg/g) >Pb (9.6805 mg/g)> Zn (4.9950 mg/g). The values of KL were found in the range of 0 and 1 confirming that the adsorption process is favorable in multimetal system.
The kinetic modeling for the design of a single batch adsorber for multiple heavy metal adsorption revealed that the rate-controlling step is a pseudo-second-order kinetic reaction hence chemisorption process. A single-stage batch adsorber was designed for different adsorbent dose to effluent volume ratios using the Langmuir equation. An empirical equation was developed that can then be used for predicting and designing adsorber for the multiple removal of 95% heavy metal ions at any given initial heavy metal ions concentration required for any single-stage adsorption system using waste rubber tire activated carbon.
Declarations
Author contribution statement
Faith Cherono: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Njenga Mburu & Beatrice Kakoi: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by the African Union Commission through Pan African University for Basic Sciences, Technology and Innovation (PAUSTI).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful for the administrative assistance they have received from Pan African University for Basic Sciences, Technology and innovation (PAUSTI).
References
- 1.Edokpayi J.N., Odiyo J.O., Durowoju O.S. Impact of wastewater on surface water quality in developing countries: a case study of South Africa. Water Qual. 2017 [Google Scholar]
- 2.Wojnárovits L., Földváry C.M., Takács E. Radiation-induced grafting of cellulose for adsorption of hazardous water pollutants: a review. Radiat. Phys. Chem. 2010;79(8):848–862. [Google Scholar]
- 3.Mehdipour S., Vatanpour V., Kariminia H. Influence of ion interaction on lead removal by a polyamide nano filtration membrane. 2015;362:84–92. [Google Scholar]
- 4.Fu F., Wang Q. Removal of heavy metal ions from wastewaters : a review. J. Environ. Manag. 2011;92(3):407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 5.UNEP . 1989. United Nations Environmental Programme, Industry and En_vironment Office. Paris. [Google Scholar]
- 6.NEMA K. 2003. Environmental Protection(Standards for Effluents Discharge) Regulations. [Google Scholar]
- 7.Eltaher N., Khan A. Design and preparation of a new and novel nanocomposite with CNTs and its sensor. Integr. Med. Res. 2019;8(2):2238–2246. [Google Scholar]
- 8.Gurgel L.V.A., Júnior O.K., de F. Gil R.P., Gil L.F. Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by cellulose and mercerized cellulose chemically modified with succinic anhydride. Bioresour. Technol. 2008;99(8):3077–3083. doi: 10.1016/j.biortech.2007.05.072. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor A., Viraraghavan T. Heavy metal biosorption sites in Aspergillus Niger. Bioresour. Technol. 1997;61:221–227. doi: 10.1016/s0960-8524(01)00172-9. [DOI] [PubMed] [Google Scholar]
- 10.Akunwa N.K., Muhammad M.N., Akunna J.C. Treatment of metal-contaminated wastewater: a comparison of low-cost biosorbents. J. Environ. Manag. 2014;146:517–523. doi: 10.1016/j.jenvman.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Acevedo B., Barriocanal C. Texture and surface chemistry of activated carbons obtained from tyre wastes. Fuel Process. Technol. 2015;134:275–283. [Google Scholar]
- 12.Nguyen T.C., Loganathan P., Nguyen T.V., Kandasamy J., Naidu R., Vigneswaran S. Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ. Sci. Pollut. Res. 2018;25(21):20430–20438. doi: 10.1007/s11356-017-9610-4. [DOI] [PubMed] [Google Scholar]
- 13.Šabanović E., Memić M., Sulejmanović J., Selović A. Simultaneous adsorption of heavy metals from water by novel lemon-peel based biomaterial. Pol. J. Chem. Technol. 2020;22(1):46–53. [Google Scholar]
- 14.Al-Saadi A.A., Saleh A., Kumar V. Spectroscopic and computational evaluation of cadmium adsorption using activated carbon produced from rubber tires. 2013;188:136–142. [Google Scholar]
- 15.Abdel N.T., Hefny M., Chaghaby G.A.F. Removal of lead from aqueous solution using low cost abundantly available adsorbents. Int. J. Environ. Sci. Tech. 2007;4(1):67–73. [Google Scholar]
- 16.Moura R.C.A., Bertuol D.A., Ferreira C.A., Amado F.D.R. Study of chromium removal by the electrodialysis of tannery and metal-finishing effluents. J. Colloid Interface Sci. 2012 [Google Scholar]
- 17.Luo X., Zhang Z., Zhou P., Liu Y., Ma G., Lei Z. Synergic adsorption of acid blue 80 and heavy metal ions (Cu2+/Ni2+) onto activated carbon and its mechanisms. J. Ind. Eng. Chem. 2015;27:164–174. [Google Scholar]
- 18.Ajmal M., Rao R.A.K., Ahmad R., Ahmad J., Rao L.A.K. Removal and recovery of heavy metals from electroplating wastewater by using Kyanite as an adsorbent. J. Hazard Mater. 2001;87(1–3):127–137. doi: 10.1016/s0304-3894(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 19.Saeed A., Iqbal M., Akhtar M.W. Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk) J. Hazard Mater. 2005;117(1):65–73. doi: 10.1016/j.jhazmat.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Falilat Taiwo A. Sorption characteristics for multiple adsorption of heavy metal ions using activated carbon from Nigerian Bamboo Ademiluyi. Am. J. Chem. Eng. 2016;4(5):105. [Google Scholar]
- 21.Jain M., Garg V.K., Kadirvelu K., Sillanpää M. Adsorption of heavy metals from multi-metal aqueous solution by sunflower plant biomass-based carbons. Int. J. Environ. Sci. Technol. 2016;13(2):493–500. [Google Scholar]
- 22.Chiban M., Soudani A., Sinan F., Persin M. Single, binary and multi-component adsorption of some anions and heavy metals on environmentally friendly Carpobrotus edulis plant. Colloids Surf. B Biointerfaces. 2011;82(2):267–276. doi: 10.1016/j.colsurfb.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Fato F.P., Li D.W., Zhao L.J., Qiu K., Long Y.T. Simultaneous removal of multiple heavy metal ions from river water using ultrafine mesoporous magnetite Nanoparticles. ACS Omega. 2019;4(4):7543–7549. doi: 10.1021/acsomega.9b00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putra W. Biosorption of Cu(II), Pb(II) and Zn(II) ions from aqueous solutions using selected waste materials: adsorption and characterisation studies. J. Encapsulation Adsorpt. Sci. 2014;4(1):25–35. [Google Scholar]
- 25.Alslaibi T.M., Abustan I., Ahmad M.A., Abu Foul A. Comparative studies on the olive stone activated carbon adsorption of Zn2+, Ni2+, and Cd2+ from synthetic wastewater. Desalin. Water Treat. 2015;54(1):166–177. [Google Scholar]
- 26.Bohli T., Ouederni A., Villaescusa I. Simultaneous adsorption behavior of heavy metals onto microporous olive stones activated carbon: analysis of metal interactions. Euro-Mediterranean J. Environ. Integr. 2017;2(1):1–15. [Google Scholar]
- 27.Gupta, Ganjali M.R., Nayak A., Bhushan B., Agarwal S. Enhanced heavy metals removal and recovery by mesoporous adsorbent prepared from waste rubber tire. Chem. Eng. J. 2012;197:330–342. [Google Scholar]
- 28.Mousavi H.Z., Hosseinifar A., Jahed V. Removal of Cu(II) from wastewater by waste tire rubber ash. J. Serb. Chem. Soc. 2010;75(6):845–853. [Google Scholar]
- 29.Mousavi H.Z., Hosseynifar A., Jahed V., Dehghani S.A.M. Removal of lead from aqueous solution using waste tire rubber ash as an adsorbent. Braz. J. Chem. Eng. 2010;27(1):79–87. [Google Scholar]
- 30.Senthil Kumar P., Ramalingam S., Abhinaya R.V., Kirupha S.D., Murugesan A., Sivanesan S. Adsorption of metal ions onto the chemically modified agricultural waste. Clean. 2012;40(2):188–197. [Google Scholar]
- 31.Can M., Bulut E., Örnek A. A batch adsorber design for rhodium adsorption on gallic acid formaldehyde resin. Acta Phys. Pol. A. 2015;127(4):1311–1313. [Google Scholar]
- 32.Pirozzi D., Sannino F. Design of a multi-stage stirred adsorber using mesoporous metal oxides for herbicide removal from wastewaters. J. Environ. Chem. Eng. 2014;2(1):211–219. [Google Scholar]
- 33.Karmacharya M.S., Gupta V.K., Jha V.K. Preparation of activated carbon from waste tire rubber for the active removal of Cr(VI) and Mn(II) ions from aqueous solution. Trans. Indian Ceram. Soc. 2016;75(4):234–241. [Google Scholar]
- 34.Dimpe K.M., Ngila J.C., Nomngongo P.N. Application of waste tyre-based activated carbon for the removal of heavy metals in wastewater. Cogent Eng. 2017;4(1) [Google Scholar]
- 35.Kamau A., Thiong’o G., Kakoi B. Equilibrium studies for removal of cadmium (II) ions removal from water using activated carbon derived from Macadamia Intergrifolia Nutshell waste powder. Int. Res. J. Pure Appl. Chem. 2020;21(23):185–197. [Google Scholar]
- 36.Langmuir I. The adsorption of gases on plane surfaces of glass, mica and PLatinum. J. Am. Chem. Soc. 1918;40(9):1361–1403. [Google Scholar]
- 37.Freundlich H.M. Over the adsorption in solution. J. Phys. Chem. 1906;57:385–471. [Google Scholar]
- 38.Ho Y.S., Ng J.C.Y., McKay G. Kinetics of pollutant sorption by biosorbents: Review. Separ. Purif. Methods. 2000;29(2):189–232. [Google Scholar]
- 39.Kalmykova Y., Strömvall A.M., Steenari B.M. Adsorption of Cd, Cu, Ni, Pb and Zn on Sphagnum peat from solutions with low metal concentrations. J. Hazard Mater. 2008;152(2):885–891. doi: 10.1016/j.jhazmat.2007.07.062. [DOI] [PubMed] [Google Scholar]
- 40.Ademiluyi F.T., David-West E.O. Effect of chemical activation on the adsorption of heavy metals using activated carbons from waste materials. ISRN Chem. Eng. 2012;2012(December 2012):1–5. [Google Scholar]
- 41.Ho Y., McKay G. Pseudo-second order model for sorption processes. Process Chem. 1999;34(6):451–465. [Google Scholar]
- 42.WJ W., JC M. Kinetics of adsorption carbon from solutions. J. Sanit. Eng. Div. Proc. Am. Soc. Civ. Eng. 1963;89:31–60. [Google Scholar]
- 43.Oubagaranadin J.U.K., Murthy Z.V.P. Isotherm modeling and batch adsorber design for the adsorption of Cu(II) on a clay containing montmorillonite. Appl. Clay Sci. 2010;50(3):409–413. [Google Scholar]
- 44.De Gisi S., Lofrano G., Grassi M., Notarnicola M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain. Mater. Technol. 2016;9:10–40. [Google Scholar]
- 45.Czikkely M., Neubauer E., Fekete I., Ymeri P., Fogarassy C. Review of heavy metal adsorption processes by several organic matters from wastewaters. Water (Switzerland) 2018;10(10):1–15. [Google Scholar]
- 46.Sych N.V. Porous structure and surface chemistry of phosphoric acid activated carbon from corncob. Appl. Surf. Sci. 2012;261:75–82. [Google Scholar]
- 47.Kariuki Z., Kiptoo J., Onyancha D. Biosorption studies of lead and copper using rogers mushroom biomass ‘Lepiota hystrix. S. Afr. J. Chem. Eng. 2017;23:62–70. [Google Scholar]
- 48.Lasheen M.R., El-sherif I.Y., El-wakeel S.T. Heavy metals removal from aqueous solution using magnetite Dowex 50WX4 resin nanocomposite. J. Mater. Environ. Sci. 2017;8(Vi):503–511. [Google Scholar]
- 49.Kumar Y.P., King P., Prasad V.S.R.K. Removal of copper from aqueous solution using Ulva fasciata sp.-A marine green algae. J. Hazard Mater. 2006;137(1):367–373. doi: 10.1016/j.jhazmat.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M. Adsorption study of Pb(II), Cu(II) and Zn(II) from simulated acid mine drainage using dairy manure compost. Chem. Eng. J. 2011;172(1):361–368. [Google Scholar]
- 51.Depci T., Kul A.R., Önal Y. Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: study in single- and multi-solute systems. Chem. Eng. J. 2012;200–202:224–236. [Google Scholar]
- 52.Gueye M., Richardson Y., Kafack F.T., Blin J. High efficiency activated carbons from African biomass residues for the removal of chromium(VI) from wastewater. J. Environ. Chem. Eng. 2014;2(1):273–281. [Google Scholar]
- 53.Sdiri A., Higashi T. Simultaneous removal of heavy metals from aqueous solution by natural limestones. Appl. Water Sci. 2013;3(1):29–39. [Google Scholar]
- 54.Malakahmad A., Tan S., Yavari S. Valorization of wasted black tea as a low-cost adsorbent for Nickel and zinc removal from aqueous solution. J. Chem. 2016;2016 [Google Scholar]
- 55.Gupta V., Nayak A., Agarwal S., Tyagi I. Potential of activated carbon from Waste Rubber Tire for the adsorption of phenolics : effect of pre-treatment conditions. J. Colloid Interface Sci. 2014 doi: 10.1016/j.jcis.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 56.Odubiyi O.A., Awoyale A.A., Eloka-Eboka A.C. Wastewater treatment with activated charcoal produced from cocoa pod husk. Int. J. Environ. Bioenergy. 2012;4(3):162–175. [Google Scholar]
- 57.Wu Y., Zhang L., Gao C., Ma J., Ma X., Han R. Adsorption of copper ions and methylene blue on natural wheat straw and modified wheat straw. J. Chem. Eng. Data. 2009;54(12):3229–3234. http://www.scopus.com/inward/record.url?eid=2-s2.0-84886868583&partnerID=40&md5=51b34a4c667cc3a1f3731080ce040ab5 [Online]. Available: [Google Scholar]
- 58.Abdel Salam O.E., Reiad N.A., ElShafei M.M. A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. J. Adv. Res. 2011;2(4):297–303. [Google Scholar]
- 59.Bernard E., Jimoh A., Odigure J.O. 2013. Heavy Metals Removal from Industrial Wastewater by Activated Carbon Prepared from Coconut Shell. July. [Google Scholar]
- 60.Kakoi B., Kaluli J.W., Ndiba P., Thiong G. Removal of lead ( II ) from aqueous solution using natural materials : a kinetic and equilibrium study. J. Sustain. Res. Eng. 2016;3(3):53–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.