Abstract
Diffuse axonal injury (DAI) is one of the most severe types of primary traumatic brain injury. In recent years, MR imaging has been gaining popularity as an adjunctive imaging method in patients with DAI. In this case report, we describe MRI findings of an 11-year-old male patient diagnosed with DAI and discuss the role of different sequences in the evaluation of DAI.
Keywords: Diffuse axonal injury, Magnetic resonance imaging, Diffusion weighted imaging, Susceptibility weighted imaging, Diffusion tensor imaging
Introduction
Diffuse axonal injury (DAI) is one of the most severe types of primary traumatic brain injury and a major cause of unconsciousness and persistent vegetative state after severe head trauma. The most common etiology of diffuse axonal injury involves high-speed motor vehicle accidents [1]. The most common mechanism involves a sudden rotational accelerating and decelerating motion that leads to the shearing of the brain's long connecting nerve fibers. This causes macroscopic and microscopic damage to the axons at the grey-white matter junction. Cortex and white-matter have different densities and therefore rotate at different speeds during a closed head injury, leading to misaligned axons or stretched axons (rarely sheared). The stretching of axons causes depolarization, metabolic alterations, cellular swelling, cytotoxic oedema, and apoptosis [2].
Histopathological grading of DAI into grades 1 to 3 was first proposed by Adams and associates in 1989. In grade 1 there is histological evidence of axonal injury in the white matter of the cerebral hemispheres, the corpus callosum, the brain stem and, less commonly, the cerebellum. In grade 2 there is also a focal lesion in the corpus callosum. In grade 3 there is in addition a focal lesion in the dorsolateral quadrant or quadrants of the rostra1 brain stem [3].
Computed tomography (CT) findings in DAI are typically limited to microhemorrhages in the white matter or traumatic edema of the brain which can be subtle and make it difficult to be diagnosed. MRI is the modality of choice for assessing suspected DAI even in patients with normal CT brain, especially those with unexplained neurologic deficit after severe head trauma.
In recent years, MRI is increasingly being used in the diagnosis and prognosis of DAI. T2 GRE and SWI are MRI sequences that are particularly sensitive in detecting hemorrhagic lesions [4,5]. FLAIR and DWI have been proven to be sensitive in detecting non-hemorrhagic lesions seen with DAI [6,7]. DTI can detect decreased fractional anisotropy (FA) which implies neuronal disruption. 3-D tractography can reveal interruption of the white matter fibers [8,9].
Case report
An 11–year old boy was admitted to the emergency department after a high-speed vehicle accident. He was 5 feet 1 inch (5′ 1″) and 99 lbs. On arrival to the emergency department, the patient had a Glasgow Coma Scale (GCS) score of 9, right-sided hemiparesis, fractured right clavicle, femur and the following initial vital signs: blood pressure 100/60 mm Hg, pulse rate 86 beats/min, respiratory rate 20 breaths/min, body temperature 37°C, oxygen saturation 98% on room air.
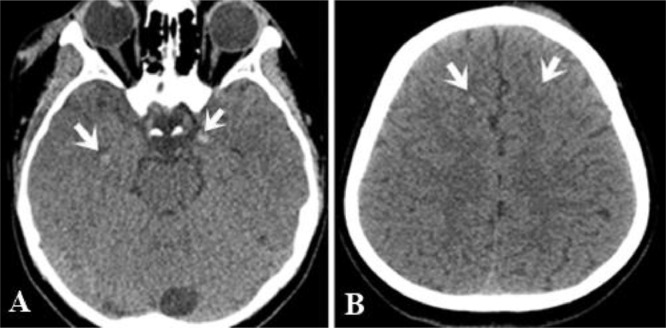
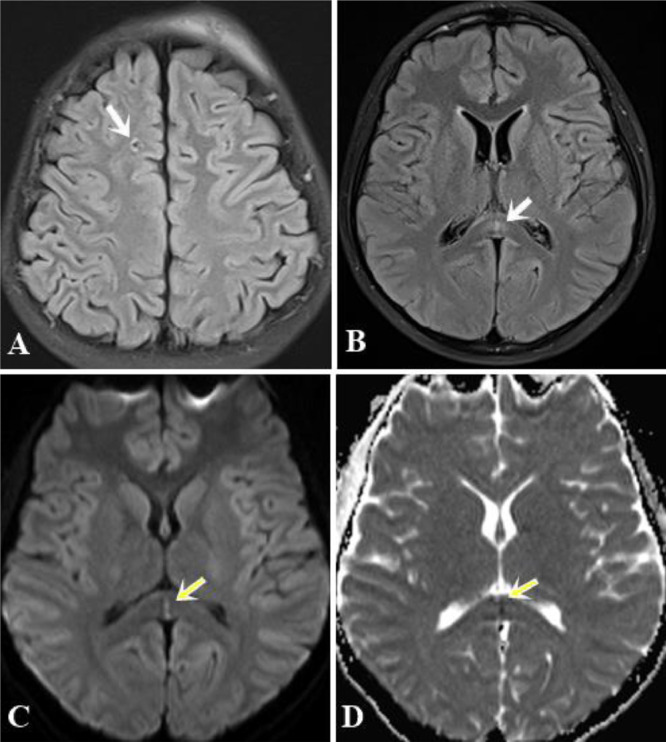
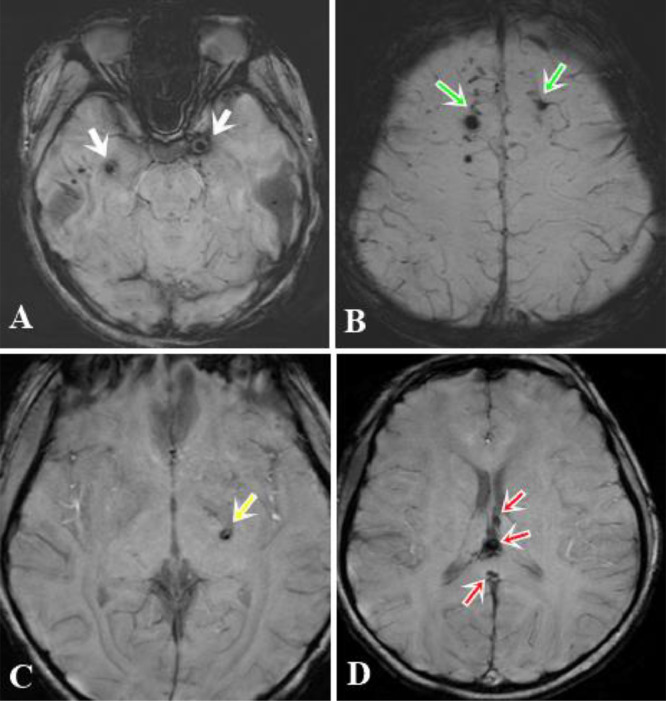
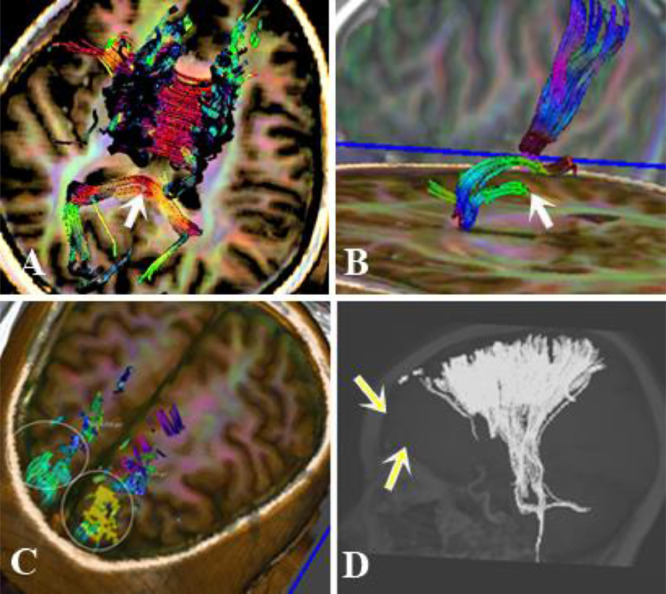
A non-contrast computed tomography scan was performed and revealed some punctate hyperdense foci at the grey–white matter junction of bilateral frontal and temporal lobes (Fig 1). DAI was suspected and the patient underwent a head MRI scan for further evaluation. FLAIR depicted some hyperintense punctate foci involving subcortical white matter of bilateral frontal and temporal lobes, posterior limb of left internal capsule and splenium of corpus callosum (Fig. 2-A,B). DWI showed restricted diffusion within corpus callosum (Fig. 2C-D). SWI minimum intensity projection (mIP) showed some hypointense foci at the grey-white matter junction of bilateral frontal and temporal lobes, fornix commissure, left crus of the fornix, splenium of corpus callosum, which indicated their hemorrhagic nature (Fig. 3). Diffusion tractography showed decreased fractional anisotropy at the grey-white matter junction of bilateral frontal lobes and corpus callosum, decreased number of subcortical frontal white matter fibers, several disrupted white matter fibers in the posteroinferior aspect of the splenium, commissure and left crus of fornix (Fig. 4).
Fig. 1.
Multiple punctate hyperdense foci (microhemorrhage) at the gray-white matter junction of bilateral temporal (white arrow, A) and frontal lobes (white arrows, B)
Fig. 2.
(A) and (B). Axial FLAIR images showed some punctate hyperintense foci in the subcortical white matter of the right frontal lobe (arrow) and the splenium of the corpus callosum (white arrows). (C) and (D). DWI and ADC maps indicated restricted diffusion within the splenium of the corpus callosum (yellow arrows) (Color version of figure is available online)
Fig. 3.
SWI illustrated multiple hypointense foci of hemorrhagic lesions at the grey-white matter junction of bilateral temporal lobes (white arrows, A), bilateral frontal lobes (green arrows, B), posterior limb of the left internal capsule (yellow arrow, C), the fornix commissure, and the splenium of the corpus callosum (red arrows, D) (Color version of figure is available online)
Fig. 4.
Diffusion Tensor Imaging with 3D-Fiber Tractography revealed disrupted white matter fibers in the posteroinferior aspect of the splenium (arrow, A), left crus of the fornix (arrow, B), and subcortical frontal tracts (circle C and arrow D)
Discussion
Despite having an incidence of only 3.5%, DAI is one of the most severe types of primary traumatic brain injury and takes a toll on patients [10]. In clinical practice, a diagnosis of DAI is considered in patients with traumatic brain injury having low GCS score, especially those with unexplained neurologic deficit. The clinical presentation of patients with DAI relates to the severity of axonal damage. In this case, the patient had a GCS score of nine and right-sided hemiparesis, head CT scan detected several petechial hemorrhages at the grey–white matter junction of bilateral frontal and temporal lobes without significant mass effect, midline shift or cerebral edema. Clinical assessment and CT-scan findings suggested the diagnosis of DAI.
DAI typically presents with hemorrhagic and non-hemorrhagic lesions. CT is the imaging technique of choice in the setting of acute head trauma. CT findings in DAI are subtle and typically limited to microhemorrhages at the grey-white matter junction, corpus callosum, especially splenium, brainstem, especially dorsolateral midbrain and upper pons. According to recent studies, CT has low sensitivity for detecting DAI [11].
MRI with different sequences has been considered superior to CT in detecting hemorrhagic and non-hemorrhagic DAI lesions.
Some studies reported that SWI is the most sensitive sequence to reveal hemorrhagic lesions in patients with DAI due to its sensitivity to paramagnetic blood products [4,5,7]. Patients with these lesions usually have a poor prognosis [12]. In this case, SWI also depicted a few more hemorrhagic lesions than CT scan and FLAIR image do.
DWI is valuable in detecting additional shearing injuries not visible on T2/FLAIR or T2 GRE sequences [7] and plays important role in prognosis. There is a correlation between clinical severity and lesions visible on DWI. The regions demonstrating restricted diffusion on DWI are assumed to have suffered irreversible injuries. Schaefer et al. concluded that the volume of alterations on DWI has a stronger correlation with clinical outcome and Glasgow coma scale than on FLAIR [6]. Depending on signal characteristics on DWI and apparent diffusion coefficient (ADC) maps, lesions are classified into three categories: type 1, DWI- and ADC-hyperintense most likely representing lesions with vasogenic edema; type 2, DWI-hyperintense, ADC-hypointense indicating cytotoxic edema; type 3, central hemorrhagic lesion surrounded by an area of increased diffusion [6]. Our patient had type 2 cytotoxic edema at the splenium and type 3 lesions at the subcortical frontal white matter.
DTI is an advanced technique in neuroradiology. On DTI, areas of decreased fractional anisotropy (FA) imply neuronal disintegration. In this case, fractional anisotropy decreased at the grey-white matter junction of bilateral frontal lobes and the corpus callosum. 3D-Fiber Tractography revealed disrupted white matter fibers at these regions (Fig. 4). Sugiyama reported that disruptions of white matter fibers in the corpus callosum and the fornix were found in patients with impaired intelligence, and attention deficit and memory disorders that prevent physical activities [9]. Using 3-D tractography, Le et al. described disrupted white matter fibers in the posteroinferior aspect of the splenium in correlation with the patient's left hemialexia, a functional deficit resulting from the disconnection of the right visual cortex from the language centers of the dominant left hemisphere [8].
Conclusion
Diffuse axonal injury is a rare but serious primary traumatic brain injury and takes a toll on afflicted patients. MRI with different specific sequences for different purposes: T2GRE or SWI for microhemorrhages; T2/FLAIR and DWI for non-hemorrhagic lesions [6,7], DWI to determine additional shearing injuries not visible on T2/FLAIR or T2GRE sequences; DTI analyzing water motion to evaluate the integrity of white matter tracts; 3-D tractography which reveals disruptions of white matter fibers – provide useful information in the diagnosis and prognosis of diffuse axonal injury, in addition to CT scanning.
Footnotes
Acknowledgements: We would like to thank the patient and his family for the permission to publish this report.
Competing interests: There are no conflicts of interest to declare
Patient consent: The protocol was reviewed and approved by the Human Research Ethics Committee of the University of Medicine and Pharmacy at Ho Chi Minh City. The study was performed in accordance with the Declaration of Helsinki.
References
- 1.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann neurol. 1982;12(6):557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 3.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15(1):49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 4.Ashwal S, Babikian T, Gardner-Nichols J, Freier M-C, Tong KA, Holshouser BA. Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. Arch physic med rehabilitat. 2006;87(12):50–58. doi: 10.1016/j.apmr.2006.07.275. [DOI] [PubMed] [Google Scholar]
- 5.Tao J-J, Zhang W-J, Wang D, Jiang C-J, Wang H, Li W. Susceptibility weighted imaging in the evaluation of hemorrhagic diffuse axonal injury. Neural Regen Res. 2015;10(11):1879–1881. doi: 10.4103/1673-5374.170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hergan K, Schaefer PW, Sorensen AG, Gonzalez RG, Huisman TA. Diffusion-weighted MRI in diffuse axonal injury of the brain. Euro radiol. 2002;12(10):2536–2541. doi: 10.1007/s00330-002-1333-2. [DOI] [PubMed] [Google Scholar]
- 7.Huisman TA, Sorensen AG, Hergan K, Gonzalez RG, Schaefer PW. Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J comput assist tomograph. 2003;27(1):5–11. doi: 10.1097/00004728-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Le TH, Mukherjee P, Henry RG, Berman JI, Ware M, Manley GT. Diffusion tensor imaging with three-dimensional fiber tractography of traumatic axonal shearing injury: an imaging correlate for the posterior callosal "disconnection" syndrome: case report. Neurosurgery. 2005;56(1):189. [PubMed] [Google Scholar]
- 9.Sugiyama K, Kondo T, Higano S, Endo M, Watanabe H, Shindo K. Diffusion tensor imaging fiber tractography for evaluating diffuse axonal injury. Brain inj. 2007;21(4):413–419. doi: 10.1080/02699050701311042. [DOI] [PubMed] [Google Scholar]
- 10.Jeong HW, Choi SW, Youm JY, Lim JW, Kwon HJ, Song SH. Mortality and epidemiology in 256 cases of pediatric traumatic brain injury: korean neuro-trauma data bank system (KNTDBS) 2010-2014. J Korean Neurosurg Soc. 2017;60(6):710–716. doi: 10.3340/jkns.2016.1010.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis PC. Head trauma. AJNR. Am J neuroradiol. 2007;28(8):1619–1621. [PMC free article] [PubMed] [Google Scholar]
- 12.Paterakis K, Karantanas AH, Komnos A, Volikas Z. Outcome of patients with diffuse axonal injury: the significance and prognostic value of MRI in the acute phase. J trauma. 2000;49(6):1071–1075. doi: 10.1097/00005373-200012000-00016. [DOI] [PubMed] [Google Scholar]