Abstract
We describe the isolation of Photorhabdus (Xenorhabdus) luminescens from four Australian patients: two with multiple skin lesions, one with bacteremia only, and one with disseminated infection. One of the patients had multiple skin lesions following the bite of a spider, while the lesions in the other patient were possibly associated with a spider bite. The source of infection for the remaining two patients is unknown. As a member of the family Enterobacteriaceae, P. luminescens is unusual in that it fails to reduce nitrate and ferments only glucose and mannose. It gives negative reactions for lysine decarboxylase, arginine dihydrolase, and ornithine decarboxylase (Moeller). The species is motile, utilizes citrate, hydrolyzes urea, and usually produces a unique type of annular hemolysis on sheep blood agar plates incubated at 25°C. A weak bioluminescence is the defining characteristic. P. luminescens is an insect pathogen and is symbiotically associated with entomopathogenic nematodes. Its isolation from human clinical specimens has been reported previously from the United States. Restriction fragment length polymorphism-PCR analysis of the 16S rRNA gene demonstrated a high level of similarity among the Australian clinical strains and significant differences between the Australian clinical strains and the U.S. clinical strains. However, numerical analyses of the data suggest that the two groups of clinical strains are more similar to each other than they are to the symbiotic strains found in nematodes. This is the first report of the isolation of P. luminescens from infected humans in Australia and the second report of the isolation of this species from infected humans worldwide.
The first isolations of Photorhabdus (Xenorhabdus) luminescens from human clinical specimens were reported by the Centers for Disease Control (CDC) in the United States in 1989 (8). The identification of five strains of this species was described, and the first of those strains had been submitted to CDC for identification in 1977. A sixth U.S. isolate was reported in an addendum to that 1989 publication. Since then, no further isolations of P. luminescens from human clinical specimens have been reported.
P. luminescens and species of the related genus, Xenorhabdus, are usually associated with nematodes and the biological control of insect pests, not human infections. These bacteria occur in the gut of juvenile nematodes as symbionts. The nematodes invade the larvae of susceptible insects and penetrate to the hemocoel, where they release their symbiotic bacteria. The bacteria proliferate, kill the insect larvae, and promote nematode reproduction by providing nutrients from the actions of degradative enzymes on the insect cadaver and by producing antibiotics that inhibit the growth of other microorganisms (2, 9). A striking feature of P. luminescens (and Xenorhabdus spp.) is phase variation, which affects a large number of membrane-bound, intra- and extracellular proteins and secondary metabolites (3, 9). Phase I variants are involved in the symbiotic relationship with entomopathogenic nematodes and are isolated from the nonfeeding infective stage nematodes and the body cavities of insects killed by these nematodes. No role in symbiosis has yet been determined for phase II, which is associated only with entomopathogenic nematodes under laboratory conditions. It is the potential for biological control of insect pests that drives most of the scientific research on these bacteria. P. luminescens was originally classified within the family Enterobacteriaceae as a species of the genus Xenorhabdus (18). However, on the basis of the results of DNA relatedness and phenotypic studies, it was subsequently transferred to a new genus, Photorhabdus, still in the same family (5). More extensive DNA relatedness and 16S ribosomal DNA (rDNA) studies with the genus Photorhabdus showed evidence of three DNA relatedness groups, one of which consisted only of the U.S. clinical strains (3, 17). As indicated by its specific epithet, P. luminescens is bioluminescent, a characteristic that is unique not only in the family Enterobacteriaceae but also among terrestrial bacteria generally. As a member of this family, it is also unusual in its inability to reduce nitrate and in the very limited number of carbohydrates that it ferments (8).
We report on the isolation and identification of P. luminescens from four infected Australian patients, one in 1994 and three in 1998. Three of the patients lived in the state of Victoria, two in Melbourne and one in rural Victoria, and the fourth patient came from northern New South Wales.
CASE REPORTS
Case report 1: strain 1.
An 11-year-old girl first presented in early February 1994 with a painful lump on the back of her right thigh. Apart from mild asthma, she had an unremarkable medical history, had never traveled outside Australia, and had no pets. Treatment with cortisone cream was prescribed, and the cortisone cream was used for a few days, but without benefit. In mid-February, the girl re-presented with an additional lesion, this time on the inner left thigh. Oral flucloxacillin was prescribed. However, when she returned on 25 February, the lesion on her left thigh had increased greatly in size to a diameter of about 15 cm. The lesion was lanced and a large amount of pus was released. Treatment with flucloxacillin was continued for another 4 days, but, although the lesion on the right thigh appeared to be resolving, that on the left thigh was not healing. She was referred to a children's hospital in Melbourne.
At the time of her presentation at the hospital on 9 March, the girl had three lesions: a small, palpable lump with red-purple discoloration on her right thigh; a painful lesion of about 2.5 cm diameter, also with red-purple discoloration, on her left thigh; and a painful lesion about the size of a pea on her left chest. No inguinal lymphadenopathy was detected. A swab of the left thigh lesion yielded Staphylococcus aureus and a non-lactose-fermenting, gram-negative, rod-shaped bacterium. The latter was not investigated further at this stage. Treatment with amoxicillin-clavulanic acid was commenced but resulted in only slight improvement.
Eight days later, she was admitted to hospital with fever and chills and the still unresolved lesions. Cultures of swabs of the surface of the left thigh lesion collected on 18 March and on 21 March grew non-lactose-fermenting, gram-negative rods that resembled the previous isolate but in pure culture. Antimicrobial susceptibility testing was performed with this isolate by the agar dilution method (23). It was found to be susceptible to amoxicillin-clavulanic acid, gentamicin, nalidixic acid, and trimethoprim and showed intermediate susceptibility to cephalothin and resistance to amoxicillin. The isolate was referred to the Microbiological Diagnostic Unit (MDU) at the University of Melbourne, where it was identified as P. luminescens. Therapy with flucloxacillin (administered intravenously) was continued for 1 week from the day of admission. Four days after admission, a 3-day course of treatment with intravenous gentamicin was added. Surface swabs of the lesions on the left thigh and left chest and pus from the left thigh collected after this therapy (1 week after admission) showed no growth on culture. However, P. luminescens and Escherichia coli were cultured from a tissue biopsy specimen taken from the left thigh lesion at this time. Histopathologic examination of the tissue, which consisted of small skin fragments and underlying soft tissue up to 1 cm in diameter, showed hyperkeratosis of the squamous epithelium and infiltration of the adjacent dermis by many chronic inflammatory cells as well as occasional giant cells. Tuberculoid granulomas were not seen. These features were reported as being consistent with chronic inflammation. Oral amoxicillin-clavulanic acid therapy replaced the intravenous therapy for the remaining 4 days of the patient's stay in hospital. She was discharged on 27 March.
On 2 May, some 3 months after she originally sought medical help, the girl was readmitted to hospital as a day surgical patient for debridement of a recurrent, nonhealing ulcer on her left thigh. On this occasion, culture of the surface of the ulcer and of the tissue biopsy specimen yielded S. aureus but not P. luminescens. A diagnosis of inflammatory ulcer was made on the basis of the histopathologic examination of this tissue. The patient was subsequently lost to follow-up. Her medical records at the hospital include a reference to her father's concern that his daughter's initial wound may have been due to the bite of a spider.
Case report 2: strain 2.
On 10 January 1998, a 90-year-old man presented with cough and fever. He had no known underlying medical condition, so apart from his advanced age, he had no specific medical reason to be immunocompromised. Blood was collected and cultured. A gram-negative, rod-shaped bacterium grew on the second day of incubation. Antimicrobial susceptibility testing by the National Committee for Clinical Laboratory Standards disk diffusion method (23) indicated that the isolate was susceptible to cefotaxime and gentamicin and resistant to ampicillin and cephalothin. The isolate was identified at MDU as P. luminescens. The man became afebrile after 2 days of treatment with ceftriaxone. He has since remained healthy.
Case report 3: strain 3.
On 18 January 1998, a 50-year-old greenkeeper at a golf course in a country town in Victoria drove his ride-on lawn mower into the web of a golden orb spider. He then noticed the large (presumably female) spider on the upper side of his left forearm. As he instinctively brushed the spider off his arm, he felt a bite. The next day, the greenkeeper experienced swelling in the left arm and aching of the right arm, as well as blurring of vision, loss of balance, and uncontrollable shaking. He was admitted to a regional hospital and was treated with cefotaxime and metronidazole. Blood cultures were not performed. Even though he remained febrile, he was discharged the day after admission and returned to work the following day.
Later that same day, he presented to a local medical clinic with an erythematous lump of increasing size on his left arm. The lesion was lanced and pus was drained. He was readmitted to the regional hospital. The gram-negative rods grown in pure culture from swabs taken at this stage were referred to MDU and were identified as P. luminescens. The patient remained in hospital for several days, where he was treated with cefotaxime and metronidazole.
On 5 February, he transferred to a private hospital with an ulcerative abscess at the site of the spider bite, from which P. luminescens was again isolated. Blood samples for culture collected at this stage were negative. He was commenced on a regimen of cefotaxime, gentamicin, and metronidazole, to which imipenem (500 mg three times daily) was added 1 week later. Antimicrobial treatment continued until 23 February. Despite the treatment, he had three lesions when he presented for surgical debridement on 24 February: one on the upper left arm, one on the lower left arm, and another on the back of the upper left leg. Swab specimens from all three sites yielded P. luminescens. Further debridement was undertaken on 19 March, this time with skin grafting. Once again, and now 2 months after the initial spider bite, cultures were positive for P. luminescens. Antimicrobial therapy, which consisted of gentamicin (80 mg three times daily) and ciprofloxacin (500 mg twice daily), was instituted. Cultures of swabs collected 5 days later grew S. aureus alone. Altogether, MDU received four isolates of P. luminescens from this patient, including one from a fine-needle aspirate of a “closed” abscess (not at the spider-bite site) and one from granulomatous tissue.
Case report 4: strain 4.
A 55-year-old cattle farmer from northern New South Wales was admitted to hospital in February 1998 with fever, malaise, and multiple painful skin lesions. One week previously, he had developed painful subcutaneous nodules over the right tibial plateau and below the umbilicus. Diffuse, erythematous, indurated, subcutaneous swellings then appeared over his left lateral thigh, left lateral malleolus, the dorsa of both feet, and his proximal upper limbs. A total of eight distinct cutaneous lesions were observed. The patient also had a slight cough and was sweaty and febrile with a temperature of 39°C. A chest radiograph showed a right perihilar infiltrate, but the initial set of blood cultures was negative.
Hematologic findings and investigations of immune function revealed no abnormality. The latter included laboratory assessment of immunoglobulin levels, lymphocyte subsets, neutrophil function, and human immunodeficiency virus antibody status. The erythrocyte sedimentation rate was 90 mm/h, and hepatic transaminase levels were approximately twice the normal levels. Histopathologic examination of the cutaneous lesion from his left thigh showed acute dermal abscess formation in association with septic vasculitis. Thrombosis and a neutrophilic infiltrate were present in dermal vessels. Gram, Brown-Hopp (12), and Warthin-Starry silver staining procedures demonstrated the presence of small, gram-negative, rod-shaped bacteria within the inflammatory infiltrate. A gram-negative, rod-shaped bacterium, later identified as P. luminescens, was isolated in pure culture from the skin biopsy specimen. Antimicrobial susceptibility testing by the National Committee for Clinical Laboratory Standards disk diffusion method (23) indicated that the isolate was susceptible to gentamicin. Despite initial therapy with intravenous gentamicin (240 mg daily), the patient remained febrile with drenching sweats which continued over the next 3 days. In addition, cultures of blood and sputum, which were collected while the patient was being treated with gentamicin, subsequently yielded P. luminescens. Antibiotic therapy was changed to intravenous ceftazidime therapy (2 g every 8 h), which resulted in resolution of the fever within 24 h. This therapy was continued for 6 days, after which the patient was treated with oral ciprofloxacin (750 mg twice daily) as an outpatient.
In the following weeks, the patient improved, apart from persistent bilateral foot pain. A technetium skeletal survey revealed features suggestive of osteomyelitis in the tarsal bones of both feet. Treatment with ciprofloxacin was continued for 3 months, by which time all symptoms had resolved. The patient had previously been well and had never traveled outside Australia. As a farmer, he was exposed to cattle and horses and had recently been digging holes for fence posts. He did not recall any recent insect or spider bite.
MATERIALS AND METHODS
Phenotypic characterization.
On receipt of the isolates for identification at MDU, subcultures were made to plates of Columbia agar (CM331; Oxoid, Basingstoke, United Kingdom) containing 5% horse blood (HBA) that were incubated aerobically and anaerobically and to plates of nutrient agar (Oxoid nutrient broth no. 2 CM067 with 0.3% yeast extract LP21 and 1% agar LP11) and MacConkey agar (Oxoid CM507) that were incubated aerobically. All aerobic cultures were incubated at temperatures of 28 and 35°C. After incubation at 35°C for 24 h, Gram-stained smears of the colonies were examined and oxidase and catalase activities were recorded (11). Motility was determined by the hanging-drop method. Conventional tests were used to determine the biochemical reactions of the isolates (7, 11, 13), except that the indicator for detection of acid production from carbohydrates was bromcresol purple, not the Andrade indicator. Columbia agar (Oxoid) containing 5% sheep blood (SBA) and incubation at 25°C were used to investigate hemolysis (3, 8).
Storage of isolates.
Because a temperature of 4°C may be lethal for many symbiotic strains of P. luminescens, isolates are stored short term at 10 to 12°C for up to 2 weeks or, less reliably, at −20°C for a few months. Isolates of P. luminescens are stored long term in 20% glycerol at −80°C or in liquid nitrogen or by lyophilization in skim milk. Strain 1 from patient 1, which was obtained in 1994, lost viability while being stored in the Protect bead storage system (Technical Service Consultants, Heywood, United Kingdom) at −20°C.
Antimicrobial susceptibility testing.
The antimicrobial susceptibilities of strains 2, 3, and 4 from patients 2, 3, and 4, respectively, were determined by the disk diffusion method of the National Committee for Clinical Laboratory Standards (23). Strain 1 from patient 1 was not included because it had lost viability on storage. The following antimicrobial agents were tested: amoxicillin-clavulanic acid, ampicillin, ceftazidime, ceftriaxone, cephalothin, chloramphenicol, ciprofloxacin, gentamicin, imipenem, kanamycin, nalidixic acid, penicillin, tetracycline, ticarcillin, ticarcillin-clavulanic acid, tobramycin, trimethoprim, and trimethoprim-sulfamethoxazole.
Bioluminescence.
The emission of light was initially investigated by observing cultures on agar plates in a darkroom (under conditions of total darkness) for up to 20 min. The light produced by aqueous suspensions of strain 2 and the four isolates of strain 3 was then measured by placing suspensions of these strains in the well of a tray and positioning the well directly above an opening to a photomultiplier tube connected to a microphotometer for readings of light output. A distilled water blank and a suspension of E. coli were used as negative controls. Extraneous light was excluded by the use of reflective foil to cover the tray and by enclosing the tray and photomultiplier tube in a light-tight box (21). The bioluminescence of strains 2, 3, and 4 was also measured in a scintillation counter (LKB WALLAC, Rackbeta, Sweden), as were those of the five U.S. clinical isolates provided by CDC and designated 3265.86 (ATCC 43950 [American Type Culture Collection, Manassas, Va.]), 2617.87 (ATCC 43951), 1216.79 (ATCC 43948), 3105.77 (ATCC 43949), and 2407.88 (ATCC 43952). Distilled water and a suspension of E. coli were used as controls. For each strain, a 1.5-cm circle of bacterial growth from a 24-h culture was suspended in 5 ml of sterile water, the suspension was transferred to a vial containing 20 ml of scintillation fluid, and the emissions were counted for 1 min with the window fully open.
Pathogenicity for insect larvae.
Viable strains 2, 3, and 4 were injected into the hemocoels of greater wax moth larvae (Galleria mellonella). The tip of an insect pin (size 0) was touched onto a colony and was used to pierce the cuticle of each insect. Each strain was injected into 10 larvae. Two control groups were also injected: one with a sterile pin and the other with a pin touched onto a colony of E. coli JM109. The insect larvae were incubated on dry filter paper at 28°C for 3 days, at which time the numbers of dead larvae were recorded. The presence of P. luminescens in the body contents of the dead larvae was tested by suspending samples in water and measuring bioluminescence in a Beckman LS 2800 scintillation counter (tritium setting).
Bacterial strains used in restriction fragment length polymorphism (RFLP)-PCR studies.
Thirteen strains of P. luminescens were analyzed. In addition to the three viable and one non-viable Australian clinical strains described above, the five U.S. clinical isolates from CDC were used. Four symbiotic strains of P. luminescens from the culture collection of the Division of Entomology, Commonwealth Scientific and Industrial Research Organization, chosen as representatives of the DNA relatedness groups identified by Akhurst et al. (3), were also included. They were Hb/1 (ATCC 29999T; symbiont of Heterorhabditis bacteriophaga Brecon), C1/1 (symbiont of H. bacteriophaga NC1), Meg/1 (symbiont of Heterorhabditis megidis Ohio), and D1/1 (symbiont of Heterorhabditis indicus D1).
Sample preparation and DNA extraction.
Each viable strain of P. luminescens to be tested was inoculated onto tryptic soy agar (Acumedia, Baltimore, Md.) containing 5% sheep blood and was incubated at 30°C for 48 h. A reference culture of Proteus vulgaris ATCC 49132 was grown on nutrient agar (Acumedia) at 35°C for 24 h. DNA was extracted from suspensions of these bacteria and the nonviable Australian strain 1 with the IsoQuick nucleic acid extraction kit (Orca Research, Bothell, Wash.) according to the manufacturer's instructions. Each DNA sample was diluted 20-fold in pure water to be used as a template for PCR.
Oligonucleotide primers.
One DNA segment containing a trimmed portion of the 16S rRNA gene (approximately 1.4 kb) was amplified from each strain. The two oligonucleotide sequences used as primers were 5′-GGT GAG TAA TGT CTG GGG AT-3′ (sense) (GenBank Nucleotide Sequence Database accession no. X82248) and 5′-AAG GAG GTG ATC CAG CCG CA-3′ (antisense) (19). Synthesized and purified oligonucleotides were obtained from Gibco BRL (Life Technologies, Grand Island, N.Y.).
Amplification of the 16S rDNA segments.
The template DNA was amplified in a volume of 50 μl which contained 10 pM primers, 0.2 mM (each) deoxynucleoside triphosphates (Pharmacia Biotech, Uppsala, Sweden), 1.5 mM MgCl2, and 2.5 U of Taq polymerase (Gibco BRL). Negative controls contained all components for the PCR except the template DNA. The mixtures were incubated in a DNA thermal cycler (Perkin-Elmer Corp., Norwalk, Conn.) for a 35-cycle amplification series. After initial denaturation of the reaction mixture at 94°C for 3 min, each cycle included denaturation at 94°C for 1 min, annealing at 56°C for 1 min, and extension at 72°C for 2 min. The final extension was carried out at 72°C for 5 min. The reaction products were separated by electrophoresis on a 1.5% agarose gel containing ethidium bromide (0.5 μg per ml), visualized under UV light, and photographed with a Polaroid DS-34 camera of fixed focal length with a 667 film and a deep yellow (no. 15) filter.
Purification of PCR products.
PCR products were purified with the JET quick PCR Purification Spin Kit 50 (Astral Scientific, Sydney, Australia) according to the manufacturer's instructions. DNA was eluted in 50 μl of sterile water.
RFLP analysis of 16S rDNA PCR products.
PCR products were cleaved with restriction endonucleases AluI, CfoI, HaeIII, HinfI, and MspI (Gibco BRL) according to the manufacturer's instructions and were separated by electrophoresis on a 2% agarose gel containing 0.5 μg of ethidium bromide per ml in 1× Tris-borate-EDTA buffer (0.089 M; pH 8.3) at 80 V. The gels were visualized and photographed as described above.
Cluster analysis.
The RFLP-PCR data were examined by cluster analysis to assess relatedness. Levels of similarity were calculated by use of the Jaccard coefficient (16). Several clustering algorithms were applied to test the reliability of the results. These were unweighted group mean average, weighted group mean average, furthest neighbor, nearest neighbor, Lance and Williams (L&W) flexible, increment in sum of squares (ISS) flexible, and ISS variable.
RESULTS
Phenotypic characteristics of isolates.
All isolates grew equally well on HBA at 28 and 35°C, giving colonies up to 2 mm in diameter after 24 h and up to 4 mm in diameter after 48 h. Cultures typically contained some colonies that were only about half the diameter of that of the majority of colonies. Except for strain 2, the edges of the colonies showed a tendency to swarm. However, nonswarming strain 2 was the only one to produce a yellow pigment. Pigment production by this strain was enhanced by incubation at the lower temperature of 28°C and was more obvious on blood-free media. All isolates produced smaller colonies on both nutrient agar and MacConkey agar, e.g., 1 to 1.5 mm after 24 h and 2 to 2.5 mm after 48 h at both 28 and 35°C. Gram-stained smears of the colonies showed gram-negative, rod-shaped bacteria with some bipolar staining and vacuolation and occasional long filamentous forms. All isolates were facultative, oxidase negative, strongly catalase positive, fermentative, and motile. The biochemical characteristics of seven isolates of the four clinical strains (i.e., four isolates of strain 3 plus one each of strains 1, 2, and 4) are given in Table 1. On the basis of growth characteristics and colonial morphology, staining reaction and microscopic morphology, and the biochemical reactions, the isolates were identified as P. luminescens. In addition, strains 1, 2, and 4 produced a thin line of annular hemolysis on SBA incubated at 25°C at a distance of 6 to 12 mm from the colony edge. However, this distinctive type of hemolysis was not observed for any of the four isolates of strain 3.
TABLE 1.
Conventional biochemical reactions of the four clinical strains of P. luminescens isolated from Australian patients
| Testa | Cumulative % positive at the following times:
|
||
|---|---|---|---|
| 24 h | 48 h | 7 days | |
| Indole production | NTb | 0 | 0 |
| Methyl red reaction | NT | 0 | 0 |
| Voges-Proskauer reaction | NT | 0 | 0 |
| Citrate utilization (Simmons) | 50 | 50 | 100 |
| Citrate utilization (Christensen) | 100 | 100 | 100 |
| H2S production (TSIc) | 0 | 0 | 0 |
| Urea hydrolysis | 25 | 100 | 100 |
| Phenylalanine deaminase reaction | 0 | 0 | 0 |
| Lysine decarboxylase (Moeller) reaction | 0 | 0 | 0 |
| Arginine dihydrolase (Moeller) reaction | 0 | 0 | 0 |
| Ornithine decarboxylase (Moeller) reaction | 0 | 0 | 0 |
| Gelatin hydrolysis (28°C) | 25 | 100 | 100 |
| Acid production fromd: | |||
| d-Glucose | 100 | 100 | 100 |
| d-Mannose | 100 | 100 | 100 |
| Gas from glucose | 0 | 0 | 0 |
| Esculin hydrolysis | 0 | 0 | 0 |
| Tartrate (Jordan) reaction | NT | 25 | 25 |
| Acetate utilization | 0 | 0 | 0 |
| DNase (25°C) reaction | NT | 0 | 0 |
| Nitrate reduction | 0 | 0 | 0 |
| Tyrosine clearing | 0 | 0 | 100 |
The incubation temperature was 35°C unless otherwise specified.
NT, not tested at 24 h.
TSI, triple sugar iron agar.
Acid not produced from adonitol, arabinose, cellobiose, glycerol, inositol, lactose, maltose, mannitol, melibiose, raffinose, rhamnose, salicin, sorbitol, sucrose, trehalose, or xylose.
Antimicrobial susceptibility.
Strains 2, 3, and 4 were resistant to ampicillin, cephalothin, and penicillin but were susceptible to all the other antimicrobial agents tested (Table 2).
TABLE 2.
Antimicrobial susceptibilities of clinical strains 2, 3, and 4 of P. luminescens isolated from Australian patients
| Strain no. | Zone size (mm) fora:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMC | AMP | CAZ | CRO | CEF | CHL | CIP | GEN | IPM | KAN | NAL | PEN | TET | TIC | TIM | TOB | TMP | SXT | |
| 2 | 20 | <6 | 31 | 31 | <6 | 28 | 32 | 23 | 25 | 28 | 33 | <6 | 28 | 32 | 32 | 22 | 24 | 28 |
| 3 | 20 | <6 | 31 | 31 | <6 | 28 | 32 | 24 | 25 | 26 | 32 | <6 | 27 | 32 | 32 | 22 | 23 | 28 |
| 4 | 19 | <6 | 31 | 31 | <6 | 28 | 32 | 21 | 24 | 26 | 32 | <6 | 26 | 32 | 32 | 22 | 24 | 28 |
| Mean | 19.6 | 31 | 31 | 28 | 32 | 22.7 | 24.7 | 26.7 | 32.3 | 27 | 32 | 32 | 22 | 23.7 | 28 | |||
| SD | 0.6 | 0 | 0 | 0 | 0 | 1.5 | 0.6 | 1.6 | 0.6 | 1 | 0 | 0 | 0 | 0.6 | 0 | |||
| CDC meanb | 10 | 0 | 39 | 26 | 32 | 36 | 6 | 33 | ||||||||||
| % Susceptible | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 100 | 100 |
AMC, amoxicillin-clavulanic acid (20/10 μg); AMP, ampicillin (10 μg); CAZ, ceftazidime (30 μg); CRO, ceftriaxone (30 μg); CEF, cephalothin (30 μg); CHL, chloramphenicol (30 μg); CIP, ciprofloxacin (5 μg); GEN, gentamicin (10 μg); IPM, imipenem (10 μg); KAN, kanamycin (30 μg); NAL, nalidixic acid (30 μg); PEN, penicillin (10 U); TET, tetracycline (30 μg); TIC, ticarcillin (75 μg); TIM, ticarcillin-clavulanic acid (75/10 μg); TOB, tobramycin (10 μg); TMP, trimethoprim (5 μg); SXT, trimethoprim-sulfamethoxazole (1.25/23.75 μg).
Previously published zone sizes for U.S. clinical strains of P. luminescens.
Bioluminescence.
Weak luminescence was observed in the darkroom for all strains but for only one of the four isolates of strain 3. However, the four isolates of strain 3 gave (positive) readings similar to that obtained for light emission from strain 2, as registered on the microphotometer connected to the photomultiplier detection system. The scintillation counts for each strain were as follows: strain 2, 240 kcpm; strain 3, 660 kcpm; strain 4, 1,200 kcpm. Counts for the U.S. clinical strains of P. luminescens were as follows: strain 3265.86, 410 kcpm; strain 2617.87, 140 kcpm; strain 1216.79, 2,600 kcpm; strain 3105.77, 740 kcpm; strain 2407.88, 200 kcpm. E. coli gave a count of 38 cpm, and distilled water gave a count of 32 cpm.
Pathogenicity for insect larvae.
At 3 days, all larvae injected with strains 2, 3, and 4 were dead, whereas none of those that were injected with E. coli or that were sham injected was dead. Luminescence was detected in all the dead larvae.
PCR amplification of 16S rDNA and RFLP analysis.
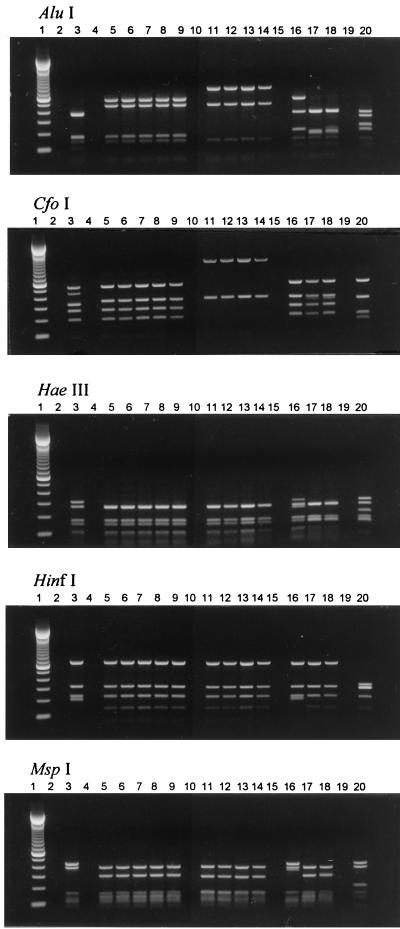
DNA segments of approximately 1,400 bp were amplified from each of the 13 strains of P. luminescens by using the primers described above. The four Australian clinical strains displayed similar restriction patterns with each of the five endonucleases (AluI, CfoI, HaeIII, HinfI, and MspI). The restriction patterns of the five U.S. clinical strains were also similar to one another but displayed differences from the patterns of the Australian clinical strains with restriction enzymes AluI and CfoI (Fig. 1).
FIG. 1.
Gel electrophoresis of restriction endonuclease digests of 16S rDNAs of P. luminescens strains. Lanes: 1, 100-bp molecular size marker; 2, negative control; 3, Hb/1 (ATCC 29999T) symbiotic strain; 4, negative control; 5 through 9, U.S. clinical strains 3265.86, 2617.87, 1216.79, 3105.77, and 2407.88, respectively; 10, negative control; 11 through 14, Australian clinical strains 4, 2, 3, and 1, respectively; 15, negative control; 16 through 18, symbiotic strains D1/1, C1/1, and Meg/1, respectively; 19, negative control; 20, P. vulgaris ATCC 49132. Restriction patterns for the Australian clinical strains are similar to one another, as are those for the U.S. clinical strains, but differences between the two groups are evident with restriction enzymes AluI and CfoI.
Cluster analysis.
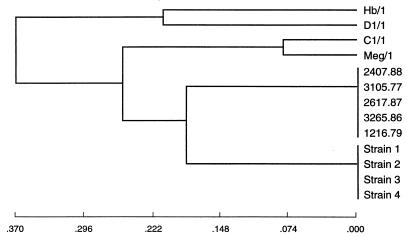
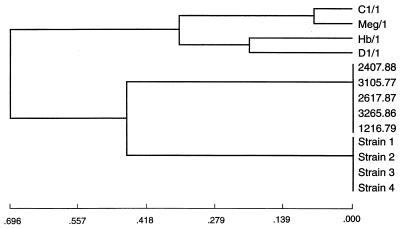
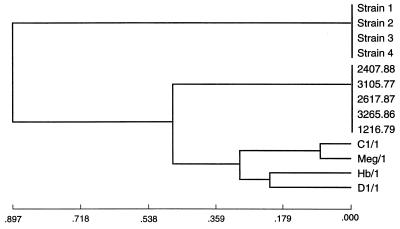
In four of the analyses (by the unweighted group mean average, weighted group mean average, L&W flexible, and furthest neighbor methods), the Australian clinical strains clustered with the U.S. clinical strains before joining any of the symbiotic strains. By both of the group mean average analyses, Australian clinical strains clustered with U.S. clinical strains and then joined symbiotic strains C1/1 and Meg/1 before joining symbiotic strains Hb/1 and D1/1 (Fig. 2). By L&W flexible and furthest neighbor analyses, Australian clinical strains clustered with U.S. clinical strains and then joined the clustered symbiotic strains (Fig. 3). The nearest neighbor analysis showed the simultaneous clustering of the Australian, U.S., and C1/1–Meg/1 clusters. In both ISS analyses, the U.S. clinical strains joined the symbiotic strains before clustering with the Australian clinical strains (Fig. 4).
FIG. 2.
Dendrogram derived from unweighted group mean average analysis of RFLP-PCR analysis data for 16S rDNAs of P. luminescens strains showing clustering of the Australian clinical strains (strains 1 through 4) with the U.S. clinical strains (strains 2407.88, 3105.77, 2617.87, 3265.86, and 1216.79) before they joined symbiotic strains C1/1 and Meg/1 and then symbiotic strains Hb/1 and D1/1.
FIG. 3.
Dendrogram derived from L&W flexible analysis of the RFLP-PCR analysis data for 16S rDNAs of P. luminescens strains showing clustering of the Australian clinical strains with the U.S. clinical strains before they joined the clustered symbiotic strains.
FIG. 4.
Dendrogram derived by ISS variable analysis of RFLP-PCR analysis data for 16S rDNAs of P. luminescens strains showing clustering of the U.S. clinical strains with the symbiotic strains before they joined the Australian clinical strains.
DISCUSSION
Key positive characteristics for P. luminescens are motility, citrate utilization, urea hydrolysis, and the fermentation of glucose and mannose. Key negative characteristics are the inability to reduce nitrate, negative decarboxylase and dihydrolase reactions, and the lack of fermentation of a wide range of carbohydrates. Such negative reactions are unusual, but not unknown, among members of the family Enterobacteriaceae. However, the combination of the key positive and the unusual negative characteristics in an isolate leads to its recognition as P. luminescens. The identity can then be confirmed by demonstrating the property of bioluminescence, which is the definitive characteristic of this species. However, testing for light production in an instrument, such as a scintillation counter, is generally more sensitive and reliable than observation of weak luminescence in a darkroom with dark-adapted eyes. Another distinctive characteristic is the production of annular hemolysis on SBA incubated at 25°C. In this type of hemolysis, which was shown by three of the four strains, a thin line of complete hemolysis forms at a distance of about 10 mm around the edge of the colonies (3, 8). Pigmentation is not so characteristic. Whereas four of the five U.S. clinical strains of P. luminescens reported previously did produce a yellow pigment (8), only one of the four strains being reported on here was pigmented.
P. luminescens has some similarities to nonpigmented strains of Chromobacterium violaceum. One of the strains studied by Farmer et al. (8) had initially been reported to be “most similar to Chromobacterium violaceum; arginine negative.” An arginine-negative, nonpigmented strain of C. violaceum was also considered as a possible identification for one of the strains in our series by the laboratory that originally investigated the isolate. Nonpigmented strains of C. violaceum may give biochemical profiles similar to those of P. luminescens because of their variability in certain biochemical characteristics, such as oxidase reaction, citrate utilization, urea hydrolysis, and sucrose fermentation. However, C. violaceum is 97% positive for nitrate reduction and 100% positive by the Moeller arginine dihydrolase reaction (20), whereas P. luminescens is 100% negative for each of these tests. In addition, nonpigmented strains of C. violaceum are frequently indole positive, whereas P. luminescens is indole negative.
The parallels between the clinical presentations of patients infected with the Australian strains of P. luminescens and those previously reported for humans from the United States infected with this species are obvious (8). In both series, P. luminescens resulted in disseminated infection and was isolated from multiple sites for patients with cutaneous lesions and from blood cultures for older individuals. Overall, cutaneous lesions were described in three of the four Australian patients and in five of the six U.S. patients. The widespread distribution of these lesions in individual patients and their histopathologic appearance suggest hematogenous dissemination of infection. Bacteremia was documented in 4 of the 10 patients, although it is interesting that for one of the Australian patients, P. luminescens was isolated only after repeated culture of blood. Two of the U.S. patients were immunocompromised by diabetes, and with the exception of one of these, all were middle-aged to elderly. In contrast, although three of the four Australian patients were middle-aged to elderly, all had previously been healthy with no evidence of immunosuppression, and the first Australian case of P. luminescens infection occurred in a previously healthy, 11-year-old girl. The clinical picture reported here suggests that this species can be highly pathogenic for humans, so it seems surprising that more cases have not been described. All four infected Australian patients presented during the Southern Hemisphere summer months of January and February.
It is difficult to make specific recommendations about antimicrobial therapy, given the small number of cases. Gentamicin therapy appears to have been associated with a poor early clinical response in several patients, despite the susceptibility of the isolates to aminoglycosides. In one of the Australian patients, bacteremia was documented 3 days after the start of treatment with intravenous gentamicin. In another Australian patient, P. luminescens was isolated from a biopsy specimen of a skin lesion taken 3 days after the start of gentamicin treatment. In one of the previously reported U.S. patients, a purulent collection of material from which P. luminescens had been isolated persisted on the lower limb of a patient after 1 week of treatment with intravenous gentamicin and oxacillin (8). All patients appear to have ultimately been cured with various combinations of β-lactam antibiotics (except penicillin, ampicillin, and cephalothin) and ciprofloxacin. However, the time taken to achieve cure was often prolonged.
Of particular interest is the association of multiple skin lesions with the bite of a spider. This was reported for one of the U.S. patients and for one, and possibly two, of the Australian patients. In these cases, the course of infection was characterized by the development of metastatic, purulent lesions over a period of 2 or 3 months, despite treatment with antibiotics over this period. The spider that was implicated in one of the Australian patients was a large golden orb-weaving spider (Nephila sp.). From the geographic location and known species distribution, it was probably Nephila edulis (M. A. Elgar, Department of Zoology, University of Melbourne [6]). The size indicates that it was female since male Nephila spp. are significantly smaller and may be less than 1/10 the size of females (6).
Necrotizing arachnidism is a syndrome associated with spider bites. The syndrome involves skin blistering, ulceration, and necrosis and is sometimes associated with severe, ongoing pain and extensive tissue destruction (10, 14). However, the lesions are nonpurulent, and although satellite lesions may develop, metastatic lesions in other parts of the body have not been observed (K. D. Winkel, Australian Venom Research Unit, University of Melbourne [22]). The white-tailed spider (Lampona sp.) is most frequently implicated as the cause of this syndrome in Australia (10, 14), whereas in the United States, a related but distinct syndrome, known as loxoscelism, is caused by the brown recluse spider, Loxosceles reclusa (22). Nephila spp. have not been associated with these syndromes (22). Recent evidence is against the involvement of Mycobacterium ulcerans in necrotizing arachnidism, and no other microbial agent has been implicated (4).
Phase variation has been demonstrated for all P. luminescens and Xenorhabdus spp. isolated from entomopathogenic nematodes, but its significance is not understood. Phase I plays a significant role in the symbiosis by providing nutrients and antimicrobial compounds and is the only phase of the bacteria demonstrated to be associated with entomopathogenic nematodes collected from nature (1, 2). However, the role of phase II, which is found to be associated only with entomopathogenic nematodes under laboratory conditions, is uncertain. Although their more efficient nutrient uptake and higher levels of respiratory activity suggest that phase II variants are better adapted than phase I variants to a free-living existence, they have not been isolated as free-living bacteria from the aquatic or terrestrial environments (15). Interestingly, all of the clinical strains isolated in Australia and the United States were phase II, at least at the time that the phenotypic characterizations were conducted. As the role and ecology of phase II P. luminescens are not understood, the phase status of clinical strains at the time of initial isolation may provide some useful clues about the function of phase variation in this species.
RFLP-PCR analysis of the 16S rRNA gene clearly demonstrates a high level of similarity among the Australian clinical strains and significant differences between the Australian and the U.S. clinical strains. Numerical analyses of the RFLP-PCR analysis data suggest that the two groups of clinical isolates may be more similar to each other than they are to the symbiotic strains. However, the production of four different relationship patterns by the seven clustering algorithms does not allow one to draw the conclusion that the clinical strains are completely distinct from the symbiotic strains. Further analysis, such as sequencing of the 16S rRNA genes and/or DNA relatedness studies, will be required to better define the relationship of the Australian clinical strains to other strains of P. luminescens.
ACKNOWLEDGMENTS
We thank Geoffrey Hogg, Teresa Lazzaro, Micheleine Uhe, and Janet Strachan; Robert Baird and Haydn Smith; and Malcolm Eaton, Robin Martin, and Lance Meng for clinical information on patients 1, 2, and 3 respectively. We also thank Bruce Livett of the Russell Grimwade School of Biochemistry and Molecular Biology at The University of Melbourne for assistance in the detection of the luminescence of strains 2 and 3 and J. J. Farmer III of CDC Atlanta, Ga., who confirmed the compatibility of the biochemical profile of strain 2 with identification as P. luminescens by comparison with the strain profiles of the species in the database of the CDC computer program Strain Matcher.
REFERENCES
- 1.Akhurst R J. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J Gen Microbiol. 1980;121:303–309. [Google Scholar]
- 2.Akhurst R J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982;128:3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- 3.Akhurst R J, Mourant R G, Baud L, Boemare N E. Phenotypic and DNA relatedness between nematode symbionts and clinical strains of the genus Photorhabdus (Enterobacteriaceae) Int J Syst Bacteriol. 1996;46:1034–1041. doi: 10.1099/00207713-46-4-1034. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson R K, Farrell D J, Leis A P. Evidence against the involvement of Mycobacterium ulcerans in most cases of necrotic arachnidism. Pathology. 1995;27:53–57. doi: 10.1080/00313029500169462. [DOI] [PubMed] [Google Scholar]
- 5.Boemare N E, Akhurst R J, Mourant R G. DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int J Syst Bacteriol. 1993;43:249–255. [Google Scholar]
- 6.Elgar, M. A. Personal communication.
- 7.Farmer J J, III, Asbury M A, Hickman F W, Brenner D J the Enterobacteriaceae Study Group. Enterobacter sakazakii: a new species of “Enterobacteriaceae” isolated from clinical specimens. Int J Syst Bacteriol. 1980;30:569–584. [Google Scholar]
- 8.Farmer J J, III, Jorgensen J H, Grimont P A D, Akhurst R J, Poinar G O, Jr, Ageron E, Pierce G V, Smith J A, Carter G P, Wilson K L, Hickman-Brenner F W. Xenorhabdus luminescens (DNA hybridization group 5) from human clinical specimens. J Clin Microbiol. 1989;27:1594–1600. doi: 10.1128/jcm.27.7.1594-1600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forst S, Dowds B, Boemare N, Stackebrandt E. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu Rev Microbiol. 1997;51:47–72. doi: 10.1146/annurev.micro.51.1.47. [DOI] [PubMed] [Google Scholar]
- 10.Gray M. A significant illness that was produced by the white-tailed spider, Lampona cylindrata. Med J Aust. 1989;151:114–116. doi: 10.5694/j.1326-5377.1989.tb101182.x. [DOI] [PubMed] [Google Scholar]
- 11.Hendrickson D A, Krenz M M. Reagents and stains. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 1289–1314. [Google Scholar]
- 12.Luna L G. Histopathologic methods and color atlas of special stains and tissue artifacts. Gaithersburg, Md: American Histolabs Inc; 1992. pp. 194–195. [Google Scholar]
- 13.Nash P, Krenz M M. Culture media. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 1226–1288. [Google Scholar]
- 14.Skinner M W, Butler C S. Necrotising arachnidism treated with hyperbaric oxygen. Med J Aust. 1995;162:372–373. doi: 10.5694/j.1326-5377.1995.tb139942.x. [DOI] [PubMed] [Google Scholar]
- 15.Smigielski A J, Akhurst R J, Boemare N E. Phase variation in Xenorhabdus nematophilus and Photorhabdus luminescens: differences in respiratory activity and membrane energization. Appl Environ Microbiol. 1994;60:120–125. doi: 10.1128/aem.60.1.120-125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sneath P H A, Sokal R R. Numerical taxonomy: the principles and practice of numerical classification. W. H. San Francisco, Calif: Freeman & Co.; 1973. [Google Scholar]
- 17.Szállás E, Koch C, Fodor A, Burghardt J, Buss O, Szentirmai A, Nealson K H, Stackebrandt E. Phylogenetic evidence for the taxonomic heterogeneity of Photorhabdus luminescens. Int J Syst Bacteriol. 1997;47:402–407. doi: 10.1099/00207713-47-2-402. [DOI] [PubMed] [Google Scholar]
- 18.Thomas G M, Poinar G O., Jr Xenorhabdus gen. nov., a genus of entomopathogenic, nematophilic bacteria of the family Enterobacteriaceae. Int J Syst Bacteriol. 1979;29:352–360. [Google Scholar]
- 19.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weyant R S, Moss C W, Weaver R E, Hollis D G, Jordan J G, Cook E C, Daneshvar M I. Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1996. [Google Scholar]
- 21.White T D, Bourke J E, Livett B G. Direct and continuous detection of ATP secretion from primary monolayer cultures of bovine adrenal chromaffin cells. J Neurochem. 1987;49:1266–1273. doi: 10.1111/j.1471-4159.1987.tb10019.x. [DOI] [PubMed] [Google Scholar]
- 22.Winkel, K. D. Personal communication.
- 23.Woods G L, Washington J A. Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 1327–1341. [Google Scholar]