Abstract
This cohort study investigates the association between low levels of ERBB2 expression and progression-free survival among patients with HR+/ERBB2− metastatic breast cancer treated with CDK4/6 inhibitors.
Introduction
Among the major breakthroughs in the treatment of hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/ERBB2− [formerly HER2−]) metastatic breast cancer (MBC), the introduction of 3 cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors into the treatment arsenal has been associated with significant gains in progression-free survival (PFS) and overall survival of patients in first-line and second-line settings.1 However, phenotypic and genetic analysis has yet to identify robust predictive markers associated with the efficacy of these treatments. A potential marker candidate could be associated with the newly proposed subgroup with the nomenclature of ERBB2-low (formerly HER2-low), defined as tumors with an ERBB2 immunohistochemistry (IHC) score of 1+ or 2+ with negative in situ hybridization assay. Approximately 50% of all breast cancers fall under such definition. Studies have suggested bidirectional crosstalk between ERBB2 and HR pathways as a potential mechanism of hormonal resistance and poor outcomes.2 However, an association between ERBB2-low status and treatment outcomes of CDK4/6 inhibitors among patients with MBC was not established. We investigated the association between low levels of ERBB2 expression and clinical outcomes among patients with HR+/ERBB2− MBC treated with CDK4/6 inhibitors.
Methods
This cohort study was approved by the Kowloon Central/Kowloon East Research Ethics Committee, Hospital Authority, Hong Kong, which approved a waiver of informed consent because the data were deidentified. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
We identified consecutive patients with HR+/ERBB2− MBC who received CDK4/6 inhibitors with letrozole or fulvestrant from March 2017 to June 2020 from a single institutional cancer registry in Hong Kong. ERBB2-low expression was defined as an IHC score of 1+ or 2+ with a negative in situ hybridization. PFS was defined as the time from the initiation of CDK4/6 inhibitor to the date of radiological or clinical progression or death. The association between ERBB2 expression levels and PFS was evaluated using log-rank test and multivariable Cox regression modeling. Covariates included the line of treatment, progesterone receptor status, and disease extent. An α level of 2-sided P ≤ .05 denoted statistical significance. Statistical analyses were performed using SPSS statistical software version 23.0 (IBM). Data were analyzed from July 2020 through January 2021.
Results
There were 106 women with MBC eligible for analysis. The median (range) age at treatment was 58.0 (23.0-91.4) years. Most patients received palbociclib (90 patients [84.9%]), while the rest received ribociclib (16 patients [15.1%]). CDK4/6 inhibitor was used as the first-line treatment in 54 patients (50.9%). Most patients had tumors that were of ductal histology (88 patients [83.0%]), had a high estrogen receptor H score (ie, H ≥ 200; 76 patients [71.7%]), and had progesterone receptor positive status (81 patients [76.4%]). There were 24 patients [22.6%] with bone-only disease (Table).
Table. Demographic and Clinical Characteristics.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Total (N = 106) | ERBB2-low (n = 82)a | ERBB2 IHC score 0 (n = 24)a | |
| Age, y | |||
| Median (range) | 58.0 (23.0-91.4) | 58.6 (23.0-91.4) | 57.0 (35.7-73.6) |
| <65 | 81 (76.4) | 62 (75.6) | 19 (79.2) |
| ≥65 | 25 (23.6) | 20 (24.4) | 5 (20.8) |
| Disease status | |||
| De novo | 47 (44.3) | 40 (48.8) | 10 (41.7) |
| Relapse | 59 (55.7) | 42 (51.2) | 14 (58.3) |
| Disease site | |||
| Bone only | 24 (22.6) | 19 (23.1) | 5 (20.8) |
| Visceral | 59 (55.7) | 46 (56.1) | 12 (50.0) |
| Line of treatment | |||
| First line | 54 (50.9) | 39 (47.6) | 15 (62.5) |
| Second or third line | 27 (25.5) | 21 (25.6) | 6 (25.0) |
| Estrogen receptor (H score)b | |||
| ≥200 | 76 (71.7) | 60 (73.2) | 15 (62.5) |
| <200 | 27 (25.5) | 18 (22.0) | 9 (37.5) |
| Progesterone receptor status | |||
| Positive | 81 (76.4) | 63 (76.8) | 18 (75.0) |
| Negative | 25 (23.6) | 19 (23.2) | 6 (25.0) |
| CDK4/6 inhibitor | |||
| Ribociclib | 16 (15.1) | 14 (17.1) | 2 (8.3) |
| Palbociclib | 90 (84.9) | 68 (82.9) | 22 (91.7) |
Abbreviations: CDK, cyclin dependent kinase; IHC, immunohistochemistry.
Formerly HER2.
Data on estrogen receptor H score were unavailable for 3 patients.
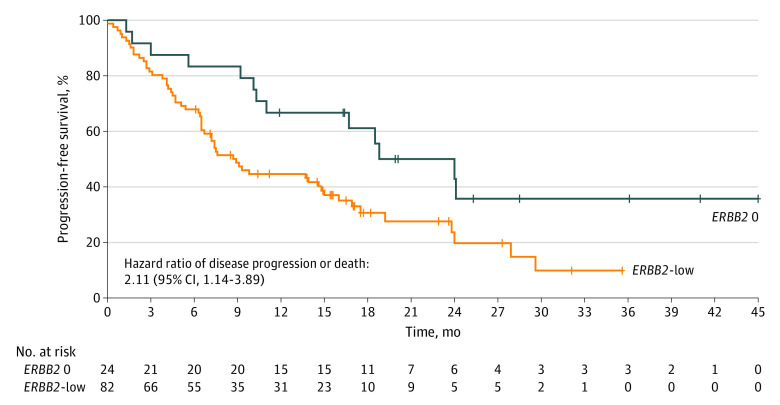
There were 82 patients (77.3%) considered ERBB2-low expressing, which was associated with a shorter median PFS compared with 24 patients with ERBB2 IHC score of 0 (8.9 months; 95% CI, 6.49-11.30 months vs 18.8 months; 95% CI, 9.44-28.16 months; P = .01) (Figure). In multivariable analysis, ERBB2-low expression remained associated with an inferior PFS (hazard ratio [HR], 1.96; 95% CI, 1.03-3.75; P = .04) after adjusting for line of treatment (HR for first line vs beyond first line, 0.30; 95% CI, 0.18-0.53; P < .001), progesterone receptor status (HR for positive vs negative, 1.48; 95% CI, 0.62-3.50; P = .38), and disease extent (HR for bone-only disease vs extraosseous disease, 0.50; 95% CI, 0.26-0.97; P = .04).
Figure. Kaplan-Meier Survival Analyses With Log-Rank Test for Progression-Free Survival.
ERBB2 0 indicates an ERBB2 immunohistochemistry score of 0; ERBB2-low, an ERBB2 immunohistochemistry score of 1+ or 2+ with negative in situ hybridization.
Discussion
Among patients with HR+/ERBB2− MBC treated with CDK4/6 inhibitors, we observed that ERBB2-low expression was associated with an inferior PFS. This may serve as a potential marker candidate associated with CDK4/6 inhibitor efficacy. An intrinsic subtypes genomic analysis of the MONALEESA studies3 found that, overall, the ERBB2-enriched (formerly HER2-enriched) subtype was associated with 2.3-fold increased risk of disease progression compared with the luminal A subtype. Our results provide phenotypical evidence suggesting the inferior efficacy of CDK4/6 inhibitors in the ERBB2-low expression subgroup. Some limitations of this study include its retrospective nature with limited sample size, the lack of patients receiving abemaciclib, and the lack of data on PIK3CA mutation status. Given that novel anti-ERBB2 antibody-drug conjugates, such as trastuzumab deruxtecan, demonstrated clinical efficacy in ERBB2-low–expressing MBC,4 coupled with emerging evidence supporting the combination use of CDK4/6 inhibitors with anti-ERBB2 agents,5 this subgroup may be of high clinical relevance and warrant prospective evaluations in future trials.
References
- 1.Schettini F, Giudici F, Giuliano M, et al. Overall survival of CDK4/6-inhibitor-based treatments in clinically relevant subgroups of metastatic breast cancer: systematic review and meta-analysis. J Natl Cancer Inst. 2020;112(11):1089-1097. doi: 10.1093/jnci/djaa071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano M, Trivedi MV, Schiff R. Bidirectional crosstalk between the estrogen receptor and human epidermal growth factor receptor 2 signaling pathways in breast cancer: molecular basis and clinical implications. Breast Care (Basel). 2013;8(4):256-262. doi: 10.1159/000354253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prat A, Chaudhury A, Solovieff N, et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol. 2021;39(13):1458-1467. doi: 10.1200/JCO.20.02977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modi S, Park H, Murthy RK, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887-1896. doi: 10.1200/JCO.19.02318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolaney SM, Wardley AM, Zambelli S, et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21(6):763-775. doi: 10.1016/S1470-2045(20)30112-1 [DOI] [PubMed] [Google Scholar]