Abstract
Background
We investigated the radiodensity of epicardial (EAT), subcutaneous (SAT), and visceral adipose tissue (VAT) before and after treatment with anthracyclines in a population of breast cancer (BC) patients, and in controls not treated with anthracyclines, to detect a potential role of EAT density as a biomarker of changes related to chemotherapy cardiotoxicity.
Methods
We reviewed BC patients treated with anthracyclines who underwent CT before (CT-t0) and after (CT-t1) chemotherapy, and age- and sex-matched controls who underwent two CT examinations at comparable intervals. On non-contrast scans, EAT was segmented contouring the pericardium and thresholding between -190 and -30 Hounsfield units (HU), and SAT and VAT were segmented with two 15-mm diameter regions of interest thresholded between -195 and -45 HU.
Results
Thirty-two female patients and 32 controls were included. There were no differences in age (p = 0.439) and follow-up duration (p = 0.162) between patients and controls. Between CT-t0 and CT-t1, EAT density decreased in BC patients (-66 HU, interquartile range [IQR] -71 to -63 HU, to -71 HU, IQR -75 to -66 HU, p = 0.003), while it did not vary in controls (p = 0.955). SAT density increased from CT-t0 to CT-t1 in BC patients (-107 HU, IQR -111 to -105 HU, to -105 HU, IQR -110 to -100 HU, p = 0.014), whereas it did not change in controls (p = 0.477). VAT density did not vary in either BC patients (p = 0.911) or controls (p = 0.627).
Conclusions
EAT density appears to be influenced by anthracycline treatment for BC, well known for its cardiotoxicity, shifting towards lower values indicative of a less active metabolism.
Keywords: Anthracyclines; Cardiotoxicity; Breast neoplasms; Biomarkers; Tomography, X-ray computed
Key points
We retrospectively assessed epicardial adipose tissue (EAT) CT density in breast cancer patients.
EAT CT density decreased after anthracyclines treatment.
Lower EAT density after chemotherapy may be an indicator of lower cardiac metabolism.
Introduction
Breast cancer (BC) is the most common malignancy in women, with estimates indicating that women in the European Union have a 1 in 11 chance of developing BC before 74 years of age [1]. While 5-year survival rates have risen to about 90% due to improvements in treatment and screening effectiveness, the incidence of BC is rising yet again [2]. Thus, a growing body of BC survivors is facing new health complications related to BC and its treatment, the most worrisome being cardiovascular disease, a leading cause of mortality in this population [3]. Indeed, many BC treatment options yield significant cardiotoxicity: anthracyclines and radiation therapy have long been linked to a dose-related development of myocardial fibrosis, whereas trastuzumab and immune checkpoint inhibitors have been associated to stochastic cardiotoxic effects [4]. Nonetheless, anthracyclines still represent a cornerstone of BC treatment, especially in patients with advanced disease or triple negative neoplasms [5]. In addition to the well-known toxic effects on cardiomyocytes, anthracyclines are metabolised by adipose tissue and may produce shifts in adipocytes activity in different adipose tissues [6].
Epicardial adipose tissue (EAT) is a layer of beige adipose tissue located between the epicardium and visceral pericardium, which enacts both mechanical and biochemical functions to support the myocardium [7]. In particular, EAT provides a source of nutrition to the heart through the regulation of the local metabolism of free fatty acids and adipokines [8]. Therefore, dysregulations of EAT functions, macroscopically marked by an increase in EAT volume, have been related to an increased cardiovascular risk [9]. More recently, EAT density, measured via computed tomography (CT) in Hounsfield units (HU), has been proposed as a cardiovascular risk biomarker, displaying correlations with the onset of coronary artery disease and atrial fibrillation, as well as cardiac involvement in severe novel coronavirus diseases [10–12].
Given the crucial role of EAT in the development of cardiovascular disease, and the effects of anthracycline treatment on both adipose tissue and myocardium, we might hypothesise that variations in EAT density could be related to the cardiotoxic effects of anthracyclines. Thus, the purpose of our study was to assess the CT density of EAT before and after treatment with anthracyclines in a population of BC patients, and in controls who underwent CT examinations at comparable time intervals, to detect a potential role of EAT in changes related to chemotherapy cardiotoxicity. Pre- and post-treatment densities of subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) were also assessed in the two populations, considering the potential confounding effect of treatment on systemic adipose tissue.
Materials and methods
Ethics committee
The local Ethics Committee (Ethics Committee of IRCCS Ospedale San Raffaele) approved this retrospective study (protocol code “CardioRetro”, number 122/int/2017; approved on September 14, 2017, and amended on February 10, 2021), and informed consent was waived due to the retrospective nature of the study.
Study population
We retrospectively reviewed BC patients treated with an anthracycline-based chemotherapy regimen, and who underwent at least two chest CT examinations for disease staging or re-staging at our institution between March 2012 and June 2020, one acquired before the start of chemotherapy (CT-t0), and the other following the end of treatment (CT-t1). As CT-t1, we considered the examination at the longest available follow-up, at least 3 months after the end of chemotherapy, to avoid potential confounding from myocardial oedema due to chemotherapy myocardial toxicity. Each CT examination had to include one unenhanced scan. Patients who underwent radiation therapy to the left breast between CT-t0 and CT-t1 were excluded, because of potential confounders [13]. Moreover, we excluded all CT scans which presented artefacts that would hinder the segmentation of EAT, SAT, or VAT. Our patient population was already included in a study appraising the role of myocardial extracellular volume in anthracycline cardiotoxicity [14]. For each patient, we retrieved demographical, BC, staging, and treatment data.
As a control population, we selected age-matched women who had undergone chest CT examinations including at least one unenhanced scan for any indication except those pathologies yielding known or potential myocardial injury (such as pulmonary embolism or mediastinal lymphomas), at time intervals comparable to those in-between CT-t0 and CT-t1 in our population of patients, and who did not undergo chemotherapy with anthracyclines, radiation therapy, or any other cardiotoxic treatment.
Image acquisition
Patients were studied using a 64-row CT scan (Somatom Definition, Siemens Medical Solution, Erlangen, Germany) with 120 kVp, tube current ranging from 100 to 200 mAs depending on automatic exposure control system (CARE Dose 4D, Siemens Medical Solution, Erlangen, Germany), rotation speed 0.5 s, pitch 1, B30f medium smooth for kernel reconstruction technique and abdominal CT window, or a 16-row CT scan (Emotion 16, Siemens Medical Solution, Erlangen, Germany) with 130 kVp, tube current ranging from 100 to 200 mAs depending on automatic exposure control system (CARE Dose 4D, Siemens Medical Solution, Erlangen, Germany), rotation speed 0.5 s, pitch 1, B30f medium smooth for kernel reconstruction technique. All patients underwent CT protocols based on their clinical status and indications, and all CT protocols included an unenhanced scan which was acquired with the above-mentioned parameters and was then used for adipose tissue segmentation.
Image analysis
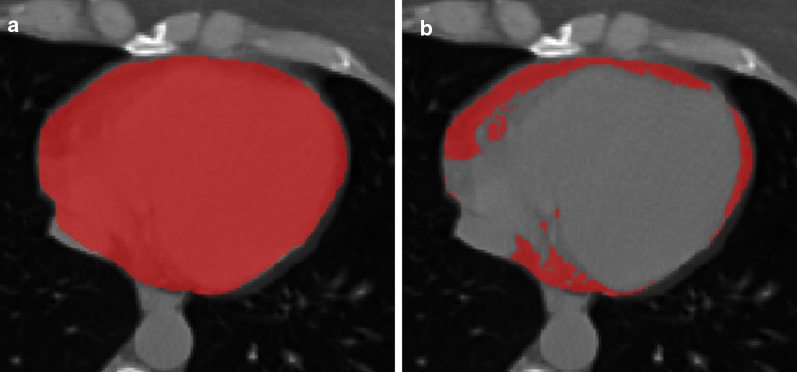
One reader, a medical student with 2 years of experience in cardiovascular CT performed image segmentation with the supervision of a more experienced reader (C.B.M.) with 5 years of experience in cardiovascular CT. Image segmentation was performed using ITK-SNAP Version 3.8.0 (www.itksnap.org [15]). For EAT assessment, the whole pericardium was contoured and the region of interest (ROI) generated from this segmentation was then thresholded between -190 and -30 HU, as already described [16]. An example of EAT segmentation is presented in Fig. 1. Afterwards, to appraise the reproducibility of EAT density, a third reader (MDMGO) with 3 years of experience in cardiovascular CT performed all EAT segmentations, following the same procedure.
Fig. 1.
Epicardial adipose tissue segmentation. a contouring of the pericardium; b subsequent thresholding between -190 and -30 Hounsfield units
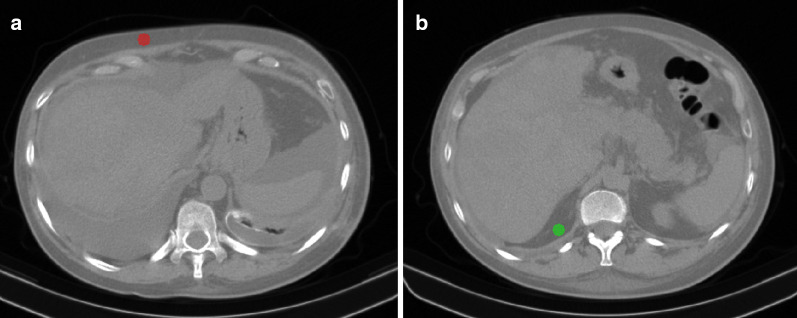
For the segmentation of SAT and VAT, the first reader placed two ROIs with a diameter of 15 mm in the context of abdominal SAT and VAT (Fig. 2). Such ROIs were then thresholded between -45 and -195 HU [17].
Fig. 2.
Segmentation of: a subcutaneous adipose tissue and (b) visceral adipose tissue by placement of round regions of interest with a 15 mm diameter
Statistical analysis
Data distributions were assessed both visually and with the aid of the Shapiro–Wilk test. Normally distributed variables were reported as mean and standard deviation, whereas non-normally distributed variables were reported as median and interquartile range (IQR). Correlations were assessed with Pearson r or Spearman ρ according to data distribution normality, and resulting correlation coefficients were reported as proposed by Evans [18]. Differences between variables were assessed with t tests or Wilcoxon and Mann-Whitney U, according to data distribution normality. Reproducibility was appraised with Bland–Altman analysis and reported as bias and coefficient of repeatability. Statistical analyses were performed using Python 3.7.6, and p values below 0.05 were considered as statistical significant [19].
Results
Study population
Overall, 33 patients referring to our institution for BC treatment met the inclusion criteria for this study. However, one patient who received radiation therapy to the left breast was excluded, leading to a final study population of 32 patients having undergone an anthracycline-based chemotherapy regimen. All patients were females, and median age at CT-t0 was 55 years (IQR 48−68 years). All patients had a histologic demonstration of BC: 29/32 patients (91%) had infiltrating ductal carcinoma, 2/32 (6%) had a poorly differentiated carcinoma, and 1/32 (3%) had a neuroendocrine carcinoma. All patients had stage II or higher BC (10/32 (31%) stage II, 13/32 (41%) stage III, and 9/32 (28%) stage IV) and had undergone anthracycline-based chemotherapy with dosages adjusted to body surface area according to clinical guidelines: 26 patients received epirubicin with a total dose of 360 mg/m2, 4 patients received mitoxantrone with a total dose of 40 mg/m2, and 2 patients received 240 mg/m2 of adriamycin. Median left ventricular ejection fraction before chemotherapy was 66% (IQR 60−68%), and median time between CT-t0 and CT-t1 was 31 months (IQR 20−53 months). No patient treated with anthracyclines showed overt cardiotoxicity, with left ventricular ejection fraction remaining within normal range and displaying no significant decreases.
The 32 matched controls were referred to CT for colon cancer (16/32, 50%), liver disease (11/32, 34%), or evaluation of pulmonary lesions (5/32, 16%). Controls had a median age comparable to that of BC patients (55 years, IQR 48−67 years, p = 0.439) and had undergone the two CT examinations in comparable time frames (27 months, IQR 13−43 months, p = 0.162). Demographic data of patients and controls are reported in Table 1.
Table 1.
Demographical and adipose tissue data for breast cancer (BC) patients undergoing treatment with anthracyclines and controls
| Breast cancer patients | Controls | p | |||
|---|---|---|---|---|---|
| N | 32 | 32 | - | ||
| Females (n, %) | 32 (100) | 32 (100) | - | ||
| Age (years) | 55 (48 to 68) | 55 (48 to 67) | 0.439 | ||
| Pathology (n, %) | Cancer stage | CT indication | |||
| II | 10 (31) | Colon cancer | 16 (50) | - | |
| III | 13 (41) | Liver disease | 11 (34) | - | |
| IV | 9 (28) | Pulmonary lesions | 5 (16) | - | |
| CT-t0 EAT density (HU) | -66 (-71 to -63) | -64 (-74 to -60) | 0.439 | ||
| CT-t0 SAT density (HU) | -107 (-111 to -105) | -104 (-109 to -101) | 0.030 | ||
| CT-t0 VAT density (HU) | -107 (-112 to -102) | -106 (-113 to -97) | 0.501 | ||
| Interval CT-t0–CT-t1 (months) | 31 (20 to 53) | 27 (13 to 43) | 0.162 |
CT computed tomography, CT-t0 baseline CT, CT-t1 follow-up CT, EAT epicardial adipose tissue, SAT subcutaneous adipose tissue, VAT visceral adipose tissue, HU Hounsfield units. Quantitative data are reported as median and interquartile range
Concerning the kVp of CT acquisitions in the BC cohort, 27/32 patients (84%) were acquired at 130 kVp and 5/32 (16%) at 120 kVp at CT-t0, without significant differences from CT-t1 when 28/32 patients (88%) were acquired at 130 kVp and 4/32 (12%) at 120 kVp (p = 0.999). Among controls, at CT-t0 16/32 patients (50%) were acquired at 130 kVp and 16/32 (50%) at 120 kVp, whereas at CT-t1 2/32 patients (7%) were acquired at 130 kVp and 30/32 (93%) at 120 kVp (p = 0.002).
Epicardial adipose tissue
At CT-t0, patients receiving anthracyclines showed a median EAT density similar to that of controls (-66 HU, IQR -71 to -63 HU versus -64 HU, IQR -74 to -60 HU, p = 0.439). Between CT-t0 and CT-t1, EAT density decreased in these patients reaching a median value of -71 HU (IQR -75 to -66 HU, p = 0.003), while it did not vary significantly in controls, with a median CT-t1 density of -67 HU (IQR -71 to -62 HU, p = 0.955). The median difference in EAT density between CT-t0 and CT-t1 in BC patients receiving anthracyclines was -2 HU (IQR -5 to 0 HU). Such difference in EAT density did not display significant correlations with anthracycline dose (ρ = 0.004, p = 0.982), BC histology (ρ = 0.042, p = 0.818), pre-treatment ejection fraction (ρ = 0.144, p = 0.513), or patients’ age (ρ = 0.180, p = 0.324). EAT density distributions at CT-t0 and CT-t1 in patients receiving anthracyclines and controls are displayed in Fig. 3.
Fig. 3.
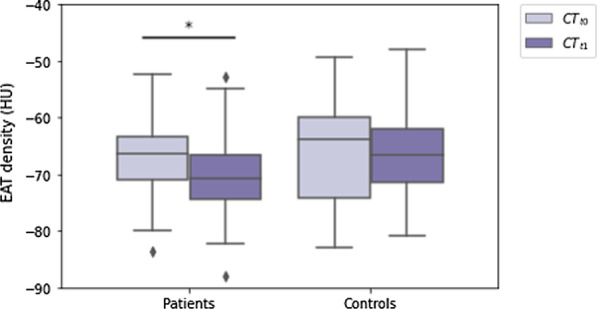
Boxplots depicting the distributions of epicardial adipose tissue density at the first (CT-t0) and second (CT-t1) computed tomography examination in patients with breast cancer who underwent treatment with anthracyclines, and in controls with no breast cancer who did not undergo cardiotoxic treatment. * denotes statistical significance
At Bland–Altman analysis, EAT density showed a bias of 2 HU, with a coefficient of repeatability of 10 HU. The Bland–Altman plot for EAT density reproducibility is displayed in Fig. 4.
Fig. 4.
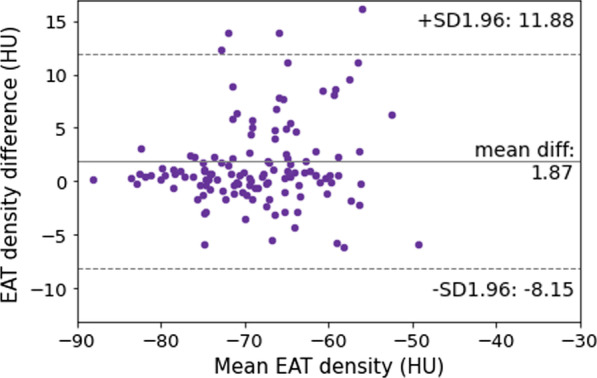
Bland–Altman plot for inter-reader reproducibility of epicardial adipose tissue (EAT) density
Subcutaneous and visceral adipose tissue
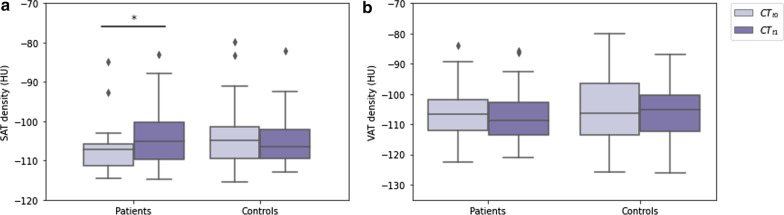
At CT-t0, SAT density was lower in patients receiving anthracyclines than in controls (-107 HU, IQR -111 to -105 HU versus -104 HU, IQR -109 to -101 HU, p = 0.030). SAT density increased from CT-t0 to CT-t1 in these patients, reaching a median of -105 HU (IQR -110 to -100 HU, p = 0.014), whereas it did not change significantly in controls, with a median at CT-t1 of -106 HU (IQR -109 to -102 HU, p = 0.477). SAT density distributions at CT-t0 and CT-t1 in BC patients receiving anthracyclines and controls are displayed in Fig. 5.
Fig. 5.
Boxplots depicting: a the distributions of subcutaneous adipose tissue and (b) visceral adipose tissue density at the first (CT-t0) and second (CT-t1) computed tomography examination in patients with breast cancer who underwent treatment with anthracyclines, and in controls with no breast cancer who did not undergo cardiotoxic treatment. * denotes statistical significance
At CT-t0, VAT density did not display significant differences between patients receiving anthracyclines and controls (-107 HU, IQR -112 to -102 HU versus -106 HU, IQR -113 to -97 HU, p = 0.501), and it did not change in either group with CT-t1 density values of -109 HU (IQR -114 to -103 HU, p = 0.911) in patients receiving anthracyclines and -105 HU, (IQR -112 to -100 HU, p = 0.627) in controls. VAT density distributions at CT-t0 and CT-t1 in patients receiving anthracyclines and controls are displayed in Fig. 5.
Discussion
Given the potential cardiotoxicity related to treatment with anthracycline-based regimens, in this work we assessed pre- and post-treatment CT EAT density in BC patients, observing a significant decrease in EAT density which was not evident in controls, who were not treated with anthracyclines.
The median values of EAT density on unenhanced scans at CT-t0 for patients (-66 HU) and controls (-64 HU) observed in our study are slightly higher than those reported by previous studies, as that by Goeller et al. [11] who described a mean EAT density of -76.2 HU in asymptomatic patients undergoing cardiac CT for the assessment of coronary artery disease. This difference could be due to the different demographics: the average age of our patients (55 years) was lower than that of patients in the referenced work (60 years). Indeed, a study by Nerlekar et al. [16] outlined how EAT density decreases with age, with a mean decrease of 10 HU over about 4 years. Concerning SAT and VAT density, the median values observed in our patients and controls (-107 HU and -104 HU, respectively, for SAT, and -107 HU and -106 HU, respectively, for VAT) are relatively close to those reported by previous works [20, 21]. The lower SAT density at CT-t0 observed in our patients group compared to controls might be an indicator of the fact that BC patients may present with a less active, more lipidic adipose tissue, which could be linked to a higher risk of both BC cardiometabolic disease [22].
The decrease in EAT density observed in patients after chemotherapy with anthracyclines can be explained by two main hypotheses. First, as EAT plays a pivotal role in sustaining cardiac metabolism, myocyte necrosis and consequent cardiac fibrosis associated with anthracycline treatment could lead to a decrease in the metabolic activity of the myocardium, which could in turn lead to a decrease in EAT metabolism and consequential adipose tissue whitening [23]. Alternatively, the decrease in EAT density could be due to the lower metabolic requirements of the myocardium of cancer patients undergoing chemotherapy, who most often become less active due to disease- and treatment-related side effects [24]. Nevertheless, our control group was composed by patients with colon cancer, who undergo chemotherapy as well, albeit with considerably less cardiotoxic regimens, and who might too therefore prove less active [25]. Conversely, the concomitant increase in SAT density detected in patients undergoing anthracycline-based chemotherapy regimens might be due to an overall loss of adipose tissue observed in patients with advanced cancer, as all our patients presented with stage II or higher BC, which would actually lead to a loss of adipocytes lipids with a consequent increase in CT radiodensity [26]. Moreover, anthracyclines may be directly metabolised by subcutaneous adipocytes, which might sequester chemotherapeutic agents and thus lead to lower treatment efficacy in obese subjects, and the accumulation of anthracyclines metabolites along with the metabolic changes they provoke might produce a higher CT density [27]. The difference in kVp used in controls between CT-t0 and CT-t1 with lower values at CT-t1 further enhances the lack of decrease in EAT density, as lower kVp acquisition values have been correlated with lower adipose tissue density at CT [28].
The lack of correlations between the decrease in EAT density and anthracycline dosage might be explained by the fact that while anthracycline-related cardiotoxicity is indeed dose-related, not all patients treated with anthracyclines respond by developing cardiotoxicity with consequent myocardial necrosis and fibrosis, thus leading to a high interpersonal variability [29].
While our population was already involved in a previous work assessing the role of myocardial extracellular volume as a biomarker of anthracycline cardiotoxicity [14], such analyses require the presence of both unenhanced and contrast-enhanced CT scans including the heart and might therefore prove less widely feasible than the sole assessment of EAT density on unenhanced scans, hence providing an edge to the use of EAT density in this context.
Our study presents some limitations. First, all CT scans included in this study were non-electrocardiographically gated, and therefore, the movement of the heart might have led to motion artefacts and inaccurate voxel density values for EAT. Nevertheless, all segmentations of the pericardium were thresholded between -190 and -30 HU, thus only included voxels containing adipose tissue, and the median EAT density values in our patients and controls were only slightly higher than those reported by previous works, in line with trends reported in the literature [11]. Additionally, inter-reader reproducibility for EAT density proved high, with a small bias (2 HU) compared to EAT density magnitude. Second, we did not assess SAT and VAT density over one whole slice, but rather from single, round ROIs. Nevertheless, such ROIs were carefully placed in regions containing SAT and VAT, respectively, and subsequently thresholded, excluding all but adipose tissue according to voxel density. Third, our patient population is small and only includes patients with BC, which is not the only malignancy treated with anthracyclines [30]. However, the main aim of our work was an initial appraise potential effects on EAT, SAT, and VAT of anthracycline treatment, and further studies are warranted to better clarify the modifications in adipose tissue density caused by anthracyclines, and their relative associations with cardiotoxicity or other side effects. Lastly, due to the retrospective nature of this work we could not retrieve data concerning the metabolic status of patients, such as blood test results or morphometric information, at uniform times and intervals, and thus, we could not analyse the relationship between EAT density and such variables. Thus, further prospective studies are warranted to clarify these issues.
In conclusion, our results showed that variations in EAT CT density may reflect local changes secondary to anthracycline treatment. Thus, considering that unenhanced, non-gated CT scans are often part of the clinical workflow of BC patients who receive an anthracycline-based chemotherapy regimen, the evaluation of EAT density could be implemented in such setting to review its potential role as a biomarker of cardiotoxicity.
Acknowledgements
We would like to thank Doctor Federica Boselli for her precious support in data collection.
Abbreviations
- BC
Breast cancer
- CT
Computed tomography
- CT-t0
Pre-chemotherapy CT scan
- CT-t1
Post-chemotherapy CT scan
- EAT
Epicardial adipose tissue
- HU
Hounsfield units
- IQR
Interquartile range
- ROI
Region of interest
- SAT
Subcutaneous adipose tissue
- VAT
Visceral adipose tissue
Authors' contributions
FSe and FSa were scientific guarantors; CBM and FSe contributed to study conceptualisation; CBM, MDMGO, MZ, EDB, and AGL were involved in data collection; CBM, DC, MZ, FSe, and FSa contributed to data analysis; and CBM, SS, MDMGO, DC, MZ, PS, FSe, and FSa were involved in manuscript writing and revision. All authors read and approved the final manuscript.
Funding
This study was partially supported by Ricerca Corrente funding from Italian Ministry of Health to IRCCS Policlinico San Donato.
Availability of data and materials
The dataset used during the current study is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The local Ethics Committee (Ethics Committee of IRCCS Ospedale San Raffaele) approved this retrospective study (protocol code “CardioRetro”, number 122/int/2017; approved on September 14, 2017, and amended on February 10, 2021), and informed consent was waived due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
Prof. Francesco Sardanelli has received research grants from and is member of speakers’ bureau and of advisory group for General Electric, Bayer, and Bracco. Simone Schiaffino has received travel support from Bracco Imaging and is a member of the speakers’ bureau for General Electric Healthcare. Moreno Zanardo is member of the Insights into Imaging Editorial Board. He has not taken part in the review or selection process of this article. All other authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.European Cancer Information System (ECIS) (2019) Breast cancer burden in EU-27. https://ecis.jrc.ec.europa.eu/pdf/Breast_cancer_factsheet-Oct_2020.pdf. Accessed 23 Aug 2021
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Coughlin SS, Ayyala D, Majeed B, Cortes L, Kapuku G (2020) Cardiovascular Disease among Breast Cancer Survivors. Cardiovasc Disord Med 2(1):10.31487/j.cdm.2020.01.01 [DOI] [PMC free article] [PubMed]
- 4.Yeh ETH, Ewer MS, Moslehi J, et al. Mechanisms and clinical course of cardiovascular toxicity of cancer treatment I. Oncol Semin Oncol. 2019;46:397–402. doi: 10.1053/j.seminoncol.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Narui K, Ishikawa T, Shimizu D, et al. Anthracycline could be essential for triple-negative breast cancer: a randomised phase II study by the Kanagawa Breast Oncology Group (KBOG) 1101. Breast. 2019;47:1–9. doi: 10.1016/j.breast.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Cespedes Feliciano EM, Chen WY, Lee V, et al. Body composition, adherence to anthracycline and taxane-based chemotherapy, and survival after nonmetastatic breast cancer. JAMA Oncol. 2020;6:264. doi: 10.1001/jamaoncol.2019.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raggi P. Epicardial adipose tissue and progression of coronary artery calcium. JACC Cardiovasc Imaging. 2014;7:917–919. doi: 10.1016/j.jcmg.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Deng G, Long Y, Yu Y-RR, Li M-RR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK–eNOS Pathway. Int J Obes. 2010;34:165–171. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- 9.Yerramasu A, Dey D, Venuraju S, et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220:223–230. doi: 10.1016/j.atherosclerosis.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 10.Klein C, Brunereau J, Lacroix D, et al. Left atrial epicardial adipose tissue radiodensity is associated with electrophysiological properties of atrial myocardium in patients with atrial fibrillation. Eur Radiol. 2019;29:3027–3035. doi: 10.1007/s00330-018-5793-4. [DOI] [PubMed] [Google Scholar]
- 11.Goeller M, Achenbach S, Marwan M, et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. 2018;12:67–73. doi: 10.1016/j.jcct.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iacobellis G, Secchi F, Capitanio G, et al. Epicardial Fat Inflammation in Severe COVID-19. Obesity. 2020;28:2260–2262. doi: 10.1002/oby.23019. [DOI] [PubMed] [Google Scholar]
- 13.Jacob S, Pathak A, Franck D, et al. Early detection and prediction of cardiotoxicity after radiation therapy for breast cancer: the BACCARAT prospective cohort study. Radiat Oncol. 2016;11:54. doi: 10.1186/s13014-016-0627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monti CB, Zanardo M, Bosetti T, et al. Assessment of myocardial extracellular volume on body computed tomography in breast cancer patients treated with anthracyclines. Quant Imaging Med Surg. 2020;10:934–944. doi: 10.21037/qims.2020.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Nerlekar N, Thakur U, Lin A, et al. The Natural history of Epicardial Adipose Tissue Volume and Attenuation: A long-term prospective cohort follow-up study. Sci Rep. 2020;10:7109. doi: 10.1038/s41598-020-63135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therkelsen KE, Pedley A, Rosenquist KJ, et al. Adipose tissue attenuation as a marker of adipose tissue quality: Associations with six-year changes in body weight. Obesity. 2016;24:499–505. doi: 10.1002/oby.21337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans J. Straightforward statistics for behavioral sciences. Pacific Grove, California: Brooks/Cole Publishing; 1996. [Google Scholar]
- 19.Di Leo G, Sardanelli F (2020) Statistical significance: p value, 0.05 threshold, and applications to radiomics—reasons for a conservative approach. Eur Radiol Exp 4:18 [DOI] [PMC free article] [PubMed]
- 20.Rosenquist KJ, Pedley A, Massaro JM, et al. Visceral and Subcutaneous Fat Quality and Cardiometabolic Risk. JACC Cardiovasc Imaging. 2013;6:762–771. doi: 10.1016/j.jcmg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvey NJ, Pedley A, Rosenquist KJ, et al. Association of Fat Density With Subclinical Atherosclerosis. J Am Heart Assoc. 2014;3(4):e000788. doi: 10.1161/JAHA.114.000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk. JAMA Oncol. 2015;1:611. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonopoulos AS, Antoniades C. The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles. J Physiol. 2017;595:3907–3917. doi: 10.1113/JP273049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero SAD, Brown JC, Bauml JM, et al. Barriers to physical activity: a study of academic and community cancer survivors with pain. J Cancer Surviv. 2018;12:744–752. doi: 10.1007/s11764-018-0711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker K, Erckenbrecht JF, Häussinger D, Fueling T. Cardiotoxicity of the antiprolif erative compound fluorouracil. Drugs. 1999;57(4):475–484. doi: 10.2165/00003495-199957040-00003. [DOI] [PubMed] [Google Scholar]
- 26.Baba S, Jacene HA, Engles JM, Honda H, Wahl RL. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med. 2010;51:246–250. doi: 10.2967/jnumed.109.068775. [DOI] [PubMed] [Google Scholar]
- 27.Mittelman SD, Orgel E. Adipocyte metabolism of the chemotherapy daunorubicin. Oncoscience. 2018;5:146–147. doi: 10.18632/oncoscience.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma R, Ties D, van Assen M, et al. Towards reference values of pericoronary adipose tissue attenuation: impact of coronary artery and tube voltage in coronary computed tomography angiography. Eur Radiol. 2020;30:6838–6846. doi: 10.1007/s00330-020-07069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig LA, Ekert PG, Conyers R, Elliott DA. Genetic determinants of anthracycline cardiotoxicity – ready for the clinic? Br J Clin Pharmacol. 2017;83:1141–1142. doi: 10.1111/bcp.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaqub F. Mechanism of action of anthracycline drugs. Lancet Oncol. 2013;14:e296. doi: 10.1016/S1470-2045(13)70118-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used during the current study is available from the corresponding author on reasonable request.