Abstract
Scientific research into developing new antimicrobials from plants continues to be an interesting area for many scientists. This is because the resistance of microorganisms to anti-infective agents has affected a wide range of conditions, some of which are life-threatening. This study aimed to investigate the antimicrobial properties of Cnestis ferruginea (CF). Powdered roots of Cnestis ferruginea were extracted with petroleum ether (CFP), ethyl acetate (CFE) and methanol (CFM). The antimicrobial and microbial resistance modifying activity profiles of the extracts were studied against Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 4853, Staphylococcus aureus ATCC 25923, clinical strains of Methicillin-Resistant Staphylococcus aureus, Streptococcus pyogenes, Klebsiella pneumonia, Staphylococcus epidermidis, Proteus mirabilis and Candida albicans. CFP and CFE showed no activity against the test organisms. CFM had mean zones of growth inhibition in the range of 11.0 ± 0.5 to 22.17 ± 0.24 mm against the test organisms. The MIC of CFM was within the range of 0.31 and 5.0 mg/mL, with MBC/MFC range of 2.5–20.0 mg/mL. The time-kill kinetics studies showed CFM is a static agent. At sub-inhibitory concentrations, CFM was able to increase the susceptibility of the test organisms to standard antibiotics from the range of 1–8 folds. CFM reduced the formation of biofilms from 100% to 56.59%, 62.33%, 65.89% and 71.88% against K. pneumonia, S. aureus, E. coli and P. aeruginosa, respectively. The findings of this study show that C. ferruginea possesses antimicrobial activity and therefore gives credence to its folkloric use.
Keywords: Cnestis ferruginea, Biofilm, Resistance modifying activity, Time-kill kinetics, Antimicrobial
Graphical abstract
Cnestis ferruginea, Biofilm, Resistance modifying activity, Time-kill kinetics, Antimicrobial.
1. Introduction
Man has exploited different parts of plants in managing and preventing various ailments before the advent of chemotherapeutic assays [1]. History has it that, our ancestors discovered the healing prowess of medicinal plants through trial and error. The use of medicinal plants for therapy therefore is based on the verifiable findings from over a hundred years ago [2]. Majority of plant-based drugs came into existence through the isolation of the pharmacologically active agents [3]. These researches have led to the better management of some infections and ailments through the novelty of isolated compounds which have been developed into various medications such as strychnine, aspirin and taxol [4]. Again, it is worth mentioning that diseases that arise from microbial infections often cause disabilities, mortality and adverse socio-economic effects on people [5]. Therefore, many types of research are still ongoing as the quest for new and effective antibiotics continues to be of interest to scientists [6].
Statistically, about 75–80% of the population in many developing countries still uses Herbal medicine. Also, the last few years have experienced a major rise in the use of medicinal plants for therapy in developed countries [4]. Their use in primary health care is often based on its easy availability and because they are of better cultural acceptability with fewer side effects [7]. They are often cheap or obtained free of any charges [4]. Above all, plants are biodegradable, easy to handle and generally safe for humans and their surroundings [8].
Over several years of antibiotic existence, the misuse and inappropriate dosing have led to the microorganisms, becoming resistant to the once effective agents. This adaptation by the microorganisms has been achieved by several mechanisms of resistance [9]. The resistance of microorganisms to existing antibiotics has increased in recent times and has necessitated an increase in scientific researches into finding new antibiotics and modifying the existing antibiotics [10].
Cnestis ferruginea Vahl ex DC. is a widely distributed perennial shrub which is found across the savannah region of tropical West Africa. It has a typical height of about 3.0–3.6 m. It has pinnate leaves and reddish-brown fruits. The plant flowers between January to March [11]. The roots of C. ferruginea is used as a laxative, aphrodisiac, remedy to ovarian disorders, abortion complications, treatment for some skin infections, sore throat, migraines and sinusitis [12]. It is used in combination with other plants in a decoction in the management of gonorrhoea, joint and waist pains, arthritis, rheumatism, stroke and syphilis by herbalists in Eastern Nigeria [13]. Biological and pharmacological activities of the plant include anti-inflammatory and anti-nociceptive properties [14], antioxidant [15], hypoglycaemic [16], aphrodisiac [17], antimicrobial [18] and laxative activities [19]. This study, therefore, seeks to explore the antimicrobial properties of Cnestis ferruginea.
2. Materials and methods
2.1. Plant collection
Collection of the roots of C. ferruginea was conducted on the campus of the University of Cape Coast, Ghana (Ghana post location: CC-140-7474, William Amo road) in the Central Region of Ghana between October and November, 2018. Herbarium specimen (KNUST/HM1/2018/R005) was kept in the Department of Pharmacognosy, Faculty of Pharmacy and Pharmaceutical Sciences, KNUST, Kumasi.
2.2. Preparation of extracts
The roots of C. ferruginea were washed with water and the bark, scraped using a knife and dried between the temperatures of 28–35 °C for two weeks. They were then cut out into pieces and powdered using a mechanical grinder. Extraction took successive turns with petroleum ether, ethyl acetate and methanol. Three hundred (300) grams of the powdered root bark were weighed and 3 L of petroleum ether (Sigma- Aldrich, London, UK) was added. This mixture was swirled and left to stand at room temperature (25 °C) with intermittent shaking for 72 h. The mixture was then filtered through Whatman filter papers (number 1) (Sigma- Aldrich, Michigan, USA). The filtrate was evaporated to dryness using a rotary evaporator (Rotavapor BUCHI R-200 with heating bath B-490, Buchi, Konstanz, Germany) at 40 °C under reduced pressure. The extract obtained was labelled and kept in a desiccator at 28 °C. The residue was re-weighed and taken through the same extraction procedure using ethyl acetate (Sigma- Aldrich, London, UK) as the next solvent for extraction. The residue from the ethyl acetate extraction was further extracted with methanol (Sigma- Aldrich, London, UK) after drying. The extracts obtained were named CFP (Cnestis ferruginea petroleum ether extract), CFE (Cnestis ferruginea ethyl acetate extract) and CFM (Cnestis ferruginea methanol extract). The percentage yield of extracts was then calculated.
2.3. Determination of antimicrobial activity
2.3.1. Test organisms
Pure cultures of Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 4853, Staphylococcus aureus ATCC 25923, clinical strains of Methicillin-Resistant Staphylococcus aureus, Streptococcus pyogenes, Klebsiella pneumonia, Staphylococcus epidermidis, Proteus mirabilis and Candida albicans were obtained from the Microbiology Section, Department of Pharmaceutics, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana, and kept on 20 mL nutrient agar (Oxoid Ltd, Basingstoke, UK) and sabouraud agar (Oxoid Ltd, Basingstoke, UK) slants and stored at -4 °C. Before use, the organisms were sub-cultured aseptically on freshly prepared nutrient agar and incubated for 24 h. The test organisms were standardized to a 0.5 M McFarland for use in each experiment. The identity of each test organism was confirmed using appropriate selective media, morphological features and biochemical tests.
2.4. Determination of microbial susceptibility of CFP, CFE and CFM
The antimicrobial activities of the plant extracts (CFP, CFE and CFM) were determined using the agar well diffusion method. Nutrient agar and Sabouraud agar (Oxoid Limited, United Kingdom) were used. Molten sterile agar (20 mL) was aseptically inoculated with 0.1 mL of a 24 h suspension of the test organisms containing approximately 106 CFU/mL and rolled in the palm to mix thoroughly. The seeded agar was then aseptically transferred into sterile Petri dishes. After the agar was set, cork borer No. 5 (diameter 10 mm) was flamed and used to bore four (4) wells equidistant from each other. The wells were labelled and filled with 0.2 mL of 4 different concentrations (20, 10, 5 and 2.5 mg/mL) of the plant extract. The diameter zones of growth inhibition (the area around the well where no visible microbial growth was observed) were measured using a millimetre rule after 24 h of incubation at 37 °C for bacteria and 72 h at 28 °C for fungus. Ciprofloxacin in concentrations of 4, 2, 1 and 0.5 μg/mL, and ketoconazole in concentrations of 64, 32, 16 and 8 μg/mL were used as standard drugs. Tween 80 (Carl Roth GmbH, Karlsruhe, Germany) was tested to rule out any antimicrobial activity or contamination [20]. The above procedure was carried out in three replicates.
2.5. Determination of minimum inhibitory concentrations (MICs) of CFM
The minimum inhibitory concentrations of CFP, CFE and CFM were determined using the broth dilution method [21,22]. Ninety-six (96)-well micro-titre plates (Bio-Tek Instruments GmbH, Germany) were each filled with 100 μL of sterile double strength nutrient broth (Oxoid Limited, United Kingdom). A concentration of 50 mg/mL of CFM was prepared with sterile water. The same concentration was prepared for CFP and CFE with sterile water and Tween 80. Aliquots from the stock solutions ranging between 10 to 80 μL were added to the wells to achieve a concentration range of 0.04–20 mg/mL. Appropriate volumes of water, as well as an inoculum size of 20 μL (1.0 × 106 CFU/mL) of test organisms, were added to appropriately labelled wells to make the final volume of 200 μL each. The plates were then incubated at 37 °C for 24 h. Similarly, stock concentrations were prepared for ketoconazole, ciprofloxacin, amoxicillin, clarithromycin and tetracycline. Aliquots between 10 and 80 μL were added to their respective wells with appropriate volumes of water where necessary to achieve concentrations in the ranges of 64 to 0.50 μg/mL for ketoconazole, 4 to 0.01 μg/mL for ciprofloxacin, and 80 to 0.04 mg/mL for amoxicillin, tetracycline and clarithromycin. After incubation, 20 μL (1.25 mg/mL) of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was added to each well and incubated again at 37 °C for 30 min. The appearance of a purple colour after incubation indicated growth in the wells. The MIC was determined as the lowest concentration of the extract that inhibited growth [20]. The experiment was carried out in three replicates.
2.6. Determination of minimum bactericidal and fungicidal concentrations of CFM
The procedure for determining MICs was used in this study. However, after incubation, aliquots of 100 μL were pipetted out of the various wells and inoculated into freshly prepared 1 mL Mueller- Hinton broth. They were labelled and incubated at 37 °C for another 24 h. The minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) of the extracts were determined as the least concentration of the extract which produced no purple colour after the addition of 20 μL of MTT followed by incubation at 37 °C for 30 min. The experiment was carried out in three replicates [20].
2.7. Microbial resistance modifying activity of CFM
The micro-dilution method was used [22]. The MICs of the various antibiotics were noted and re-determined against the test organisms in the presence of sub-inhibitory concentrations of CFM. Micro-titre plates of ninety-six wells were filled with 100 μL of sterile double strength nutrient broth each. Aliquots of between 10 and 80 μL of the reference drugs were added to their respective wells with appropriate volumes of water where necessary to achieve a range of concentrations of 0.5–64 μg/mL for ketoconazole; 0.01–4 μg/mL for ciprofloxacin, 0.04–80 mg/mL for amoxicillin, tetracycline and clarithromycin. CFM was added to appropriate wells to produce sub-inhibitory concentrations against the various organisms. Twenty microlitres (20 μL) of suspension containing 1.0 × 106 CFU/mL of the test organisms were finally added to the appropriate wells. The plates were then incubated for 24 h at 37 °C, after which 20 μL of MTT was added to the wells and MIC re-determined as the lowest concentration at which no growth was observed (no purple colour observed after 30 min of incubation at 37 °C).
2.8. Time-kill kinetic studies of CFM
The MIC, twice the MIC and four times the MICs of CFM against the test organisms were prepared and added to 5 mL sterile double strength nutrient broth separately with appropriate volumes of sterile water to produce 9 mL each. This was followed by the addition of an inoculum size of 1 mL (1.0 × 106 CFU/mL) of the various organisms to their appropriate test tubes (Labchem Ltd, Dublin, UK). The tubes were incubated at 37 °C and 0.1 mL of the medium was taken at time intervals of 0, 2, 4, 6, 12 and 24 h and inoculated aseptically into 20 mL sterile nutrient agar and incubated at 37∘ C for 24 h. A control test was performed for the organisms without CFM or reference antibiotics. The colony-forming unit (CFU) of the organisms was determined and the procedure was performed in triplicate (three independent experiments). A graph of the log CFU/mL was plotted against time [20, 23].
2.9. Determination of the effect of CFM on some biofilm-producing organisms
The effect of the methanol extract of C. ferruginea (CFM) on biofilm production by P. aeruginosa, K. pneumoniae, E. coli and S. aureus were determined using a modified method [24]. The test organisms were cultivated in a 96-well microtitre plate in the presence and absence of the extract and the extent of biofilm production was determined by measuring the optical density at 490 nm using a multi-mode micro-titre plate reader (Bio-Tek Instruments GmbH, Germany) after the formed biofilm was stained with 0.1% w/v crystal violet dissolved in ethanol-acetic acid (3:7 ratio) (Sigma- Aldrich, London, UK). The above procedure was carried out in triplicates.
2.10. Statistical analysis
Data were analyzed with Graph Pad Prism Version 8.0 for Windows (Graph Pad Software Inc, San Diego, CA, USA). One-way ANOVA followed by Dunnett's post hoc test was employed in the analysis of data for the time-kill studies and the biofilm inhibition assay.
3. Results
3.1. Susceptibility testing using the agar well diffusion method
The agar well diffusion method was used in this assay as it is simple and reproducible [25]. CFP and CFE showed no activity against all the test organisms. CFM exhibited antimicrobial activity against the test organisms (Table 1). The most susceptible organism to CFM was K. pneumoniae with mean diameter zones of inhibition ranging from 14.33 ± 0.47 to 22.17 ± 0.24 mm. Mean diameter zones of inhibition of CFM against E. coli ranged from 11.17 ± 0.24 to 15.0 ± 0.41 mm, 12.67 ± 0.47 to 19.5 ± 0.41 mm for S. pyogenes, 11.67 ± 0.58 to 13.83 ± 0.29 mm for P. mirabilis, 13.5 ± 0.50 to 18.17 ± 0.76 mm for S. epidermidis, 12.67 ± 0.47 to 17.83 ± 0.24 mm for S. aureus and 11.00 ± 0.5 to 15.83 ± 0.29 mm for MRSA. The most resistant organism to CFM was P. aeruginosa with mean diameter zones of inhibition in the range of 12.33 ± 0.47 to 13.5 ± 0.41 mm. CFM was active against C. albicans with mean diameter zones of inhibition in the range of 14.00 ± 0.00 to 18.17 ± 0.29 mm.
Table 1.
Mean zones of growth inhibition of CFM against test organisms.
| Agent | Conc. | Mean zones of growth inhibition (mm) ± SEM of CFM |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PA | EC | PM | SP | SA | KP | CA | MRSA | SE | ||
| CFM (mg/mL) | 20 | 13.5 ± 0.41 | 15.0 ± 0.41 | 13.83 ± 0.29 | 19.5 ± 0.41 | 17.8 ± 0.24 | 22.1 ± 0.24 | 18.17 ± 0.29 | 15.83 ± 0.29 | 18.17 ± 0.76 |
| 10 | 12.3 ± 0.47 | 11.17 ± 0.24 | 11.67 ± 0.58 | 14.0 ± 0.41 | 16.3 ± 0.47 | 19.0 ± 0.00 | 17.00 ± 0.00 | 14.00 ± 0.00 | 16.67 ± 0.58 | |
| 5 | NA | NA | NA | 12.6 ± 0.47 | 16.0 ± 0.00 | 17.5 ± 0.41 | 15.00 ± 0.00 | 12.67 ± 0.58 | 14.67 ± 0.58 | |
| 2.5 | NA | NA | NA | NA | 12.6 ± 0.47 | 14.3 ± 0.47 | 14.00 ± 0.00 | 11.00 ± 0.5 | 13.5 ± 0.50 | |
| Cipro (μg/mL) | 4 | 21.6 ±0.47 | 16.20 ± 0.37 | 18.00 ± 0.47 | 17.3 ± 0.29 | 30.3 ± 0.57 | 30.5 ± 0.67 | ND | 17.67 ± 0.26 | 18.67 ± 0.29 |
| 2 | 16.6 ±0.47 | 13.83 ± 0.41 | 14.67 ± 0.41 | 14.17 ± 0.39 | 31.5 ± 0.47 | 25.5 ± 0.31 | ND | 17.0 ± 0.41 | 17.33 ± 0.43 | |
| 1 | 14.0 ±0.41 | 11.24 ± 0.28 | 14.50 ± 0.53 | NA | 25.6 ± 0.24 | 22.1 ± 0.67 | ND | 14.83 ± 0.26 | 15.17 ± 0.50 | |
| 0.5 | 11.3 ± 0.42 | NA | 12.67 ± 0.47 | NA | 23.2 ± 0.41 | 18.5 ± 0.41 | ND | NA | 12.83 ± 0.47 | |
| Keto (μg/mL) | 64 | ND | ND | ND | ND | ND | ND | NA | ND | ND |
| 32 | ND | ND | ND | ND | ND | ND | NA | ND | ND | |
| 16 | ND | ND | ND | ND | ND | ND | 14.67 ± 0.47 | ND | ND | |
| 8 | ND | ND | ND | ND | ND | ND | 11.50 ± 0.24 | ND | ND | |
NA (no activity), ND (not determined), PA (P. aeruginosa), EC (E. coli), SP (S. pyogenes), SA (S. aureus), KP (K. pneumoniae), CA (Candida albicans), MRSA (Methicillin-Resistant S. aureus), PM (P. mirabilis), SE (S. epidermidis), Values expressed as mean ± SEM, n (3), the diameter of well (10 mm), Conc. (Concentration), CFM (methanol extract of C. ferruginea), Cipro (Ciprofloxacin), Keto (Ketoconazole).
3.2. Minimum inhibitory concentrations of CFM, CFE and CFP, and minimum bactericidal/fungicidal (MBC/MFC) concentrations of CFM
MICs for CFP and CFE were greater than 20 mg/mL, thus they were not further investigated. The MIC of CFM against P. aeruginosa, P. mirabilis and E. coli was 5 mg/mL and is being reported as the highest MIC value for CFM. MIC of CFM against S. epidermidis, K. pneumonia, S. aureus, MRSA and C. albicans were 2.5, 0.31, 0.63, 2.5 and 0.63 mg/mL, respectively (Table 2). The MBCs of CFM ranged from 2.5 to 20 mg/mL whereas the MFC was 10 mg/mL.
Table 2.
MICs of CFM, CFE and CFP, and minimum bactericidal/fungicidal (MBC/MFC) concentrations of CFM.
| Test organisms | CFM |
CFE |
CFP |
Reference drugs |
||
|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC/MFC (mg/mL) | (mg/mL) | Cipro (μg/mL) | Keto (μg/mL) | ||
| P. aeruginosa | 5 | 10 | - | - | 1 | ND |
| E. coli | 5 | 20 | - | - | 1 | ND |
| P. mirabilis | 5 | 10 | - | - | 1 | ND |
| S. epidermidis | 2.5 | 10 | - | - | 0.25 | ND |
| S. pyogenes | 1.25 | 2.5 | - | - | 1 | ND |
| S. aureus | 0.63 | 5 | - | - | 0.13 | ND |
| K. pneumoniae | 0.31 | 2.5 | - | - | 0.25 | ND |
| MRSA | 2.5 | 10 | - | - | 1 | ND |
| C. albicans | 0.63 | 10.0 | - | - | ND | 32 |
CFM (Cnestis ferruginea methanol extract), CFE (Cnestis ferruginea ethyl acetate extract), CPE (Cnestis ferruginea petroleum ether extract), Cipro (Ciprofloxacin), Keto (Ketoconazole), ND (not determined), - (No activity)/>20 mg/mL.
3.3. Microbial resistance modifying activity of CFM
The MICs of 4 standard antibiotics; amoxicillin, ciprofloxacin, clarithromycin, tetracycline and one antifungal agent, ketoconazole were determined (Table 3). The most susceptible organism to amoxicillin was K. pneumoniae (MIC at 0.31 mg/mL) while the most resistant was P. mirabilis (MIC at 40 mg/mL). The most susceptible organism to ciprofloxacin was S. aureus (MIC at 0.13 μg/mL). The highest MIC at 1 μg/mL was exhibited by P. aeruginosa, P. mirabilis, E. coli, MRSA and S. pyogenes. K. pneumoniae was the most susceptible organism to clarithromycin whereas P. mirabilis and E. coli showed the most resistance to clarithromycin. S. epidermidis was the most susceptible to tetracycline while P. aeruginosa showed the most resistance to tetracycline. The minimum inhibitory concentration of ketoconazole against C. albicans was 32 μg/mL.
Table 3.
MIC of test antibiotics alone and in the presence of sub-inhibitory concentrations of CFM.
| Test Organisms | Antibiotic alone |
In the presence of sub-inhibitory concentrations of CFM |
The ratio of MIC of antibiotics alone to MIC of antibiotic in the presence of CFM |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cipro |
Amoxi |
Clari |
Tetra |
Cipro |
Amoxi |
Clari |
Tetra |
Cipro | Amoxi | Clari | Tetra | |
| (μg/mL) | (mg/mL) | (μg/mL) | (mg/mL) | |||||||||
| P. aeruginosa | 1 | 5 | 5 | 10 | 0.5 | 2.5 | 5 | 2.5 | 2 | 2 | 1 | 4 |
| P. mirabilis | 1 | 40 | 40 | 5 | 0.5 | 10 | 5 | 5 | 2 | 4 | 8 | 1 |
| E. coli | 1 | 5 | 40 | 5 | 0.5 | 2.5 | 5 | 1.25 | 2 | 2 | 8 | 4 |
| S. epidermidis | 0.25 | 0.16 | 0.08 | 0.08 | 0.06 | <0.04 | 0.04 | <0.04 | 4 | >2 | 2 | >2 |
| S. aureus | 0.13 | 0.63 | 0.16 | 0.66 | 0.13 | 0.31 | 0.08 | 0.16 | 1 | 2 | 2 | 4 |
| K. pneumoniae | 0.25 | 0.31 | 0.08 | 1.25 | 0.13 | 0.08 | 0.04 | <0.04 | 2 | 4 | 2 | >2 |
| MRSA | 1 | 5 | 0.31 | 1.25 | 0.5 | 2.5 | 0.31 | 1.25 | 2 | 2 | 1 | 1 |
| S. pyogenes | 1 | 10 | 1.25 | 5 | 0.5 | 5 | 0.12 | 2.50 | 2 | 2 | 8 | 2 |
ND (not determined), Cipro (Ciprofloxacin), Amoxi (Amoxicillin), Clari (Clarithromycin), Tetra (Tetracycline), MRSA (Methicillin-Resistant Staphylococcus aureus).
The MICs of the various reference drugs were re-tested against the microorganisms after addition of sub-inhibitory concentrations of CFM. This was to investigate the modifying effect of CFM on the reference antibiotics (Table 3). CFM was able to influence the initial MICs of the standard drugs and this was indicated by the number of folds (Table 3) that it either potentiated or otherwise. The most susceptible organism after sub-inhibitory concentrations of CFM was added to ciprofloxacin was S. epidermidis with MIC of 0.06 μg/mL while P. aeruginosa, P. mirabilis, E. coli, MRSA and S. pyogenes were the most resistant with MICs of 0.5 μg/mL each. S. epidermidis was the most susceptible to amoxicillin with MIC less than 0.04 mg/mL. The most resistant organism was P. mirabilis with MIC of 10 mg/mL. For tetracycline, K. pneumoniae and S. epidermidis were the most susceptible with MIC less than 0.04 mg/mL while P. mirabilis was the most resistant with MIC of 5 mg/mL. For clarithromycin, P. aeruginosa, P. mirabilis and E. coli were the most resistant with MIC of 5 mg/mL while S. epidermidis was the most susceptibility with MIC of 0.04 mg/mL.
3.4. Time-kill kinetic studies of CFM
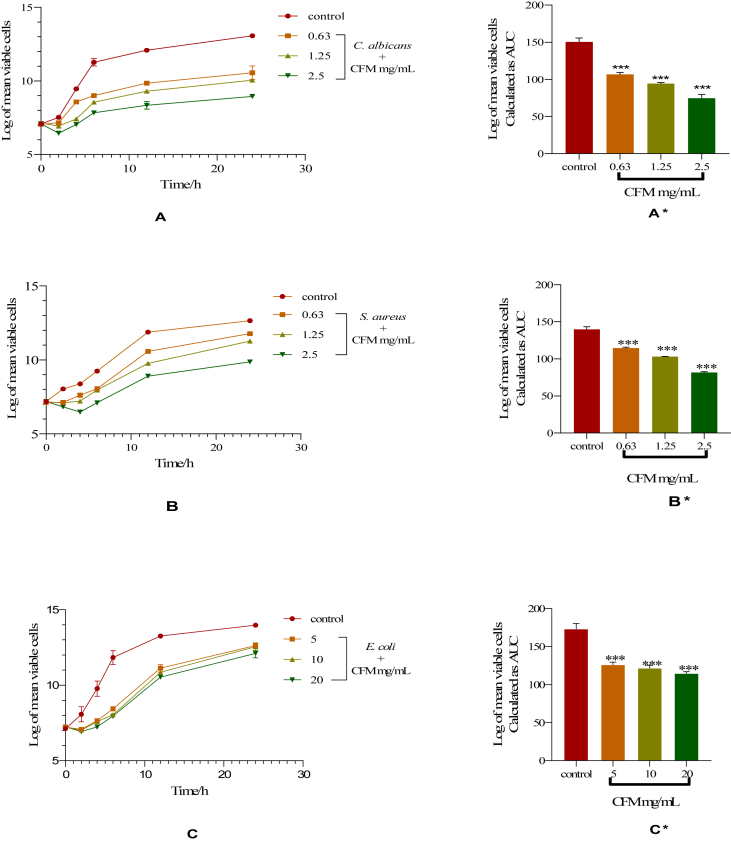
The graphs (Figures 1, 2, and 3) show the time-kill kinetics study which indicates whether the test antimicrobial agent is static or cidal. It also shows the rate at which an agent inhibits or kill microbes [23]. The time-kill kinetics of CFM against C. albicans showed a gradual increase in the number of organisms at 0.63 mg/mL from 0 to 24 h. The organisms in the presence of 1.25 and 2.5 mg/mL of CFM showed a decrease in the number of viable cells between the first 2 h followed by a gradual increase in the number of viable cells (A). For S. aureus, there was a reduction in the number of viable cells over the first 2 h at 0.63 and 1.25 mg/mL and a gradual increase in the number of organisms in both concentrations thereafter until the 24th h. At 2.5 mg/mL, CFM revealed a decrease in the number of viable cells over the first 4 h followed by a rise in the number of viable cells (B). The time-kill kinetics of CFM against E. coli showed a concentration-dependent decrease in the number of viable cells over the first 2 h after which there was a gradual rise in the number of organisms until the 24th h (C). The area under the curve (AUC) representing the activity of CFM against C. albicans, S. aureus and E. coli compared to the control showed that the number of organisms at the various concentrations reduced significantly (∗∗∗p < 0.001) (A∗, B∗, and C∗, respectively) (Figure 1).
Figure 1.
Time-kill kinetics of CFM against C. albicans, S. aureus and E. coli. A, B and C: Time-kill kinetics curve of C. albicans, S. aureus and E. coli, respectively. A∗, B∗, and C∗: AUCs of time-kill kinetics of C. albicans, S. aureus and E. coli, respectively. Comparison of AUCs to control indicates significance levels of ∗∗∗p < 0.001 (One-way ANOVA followed by Dunnett's post hoc test).
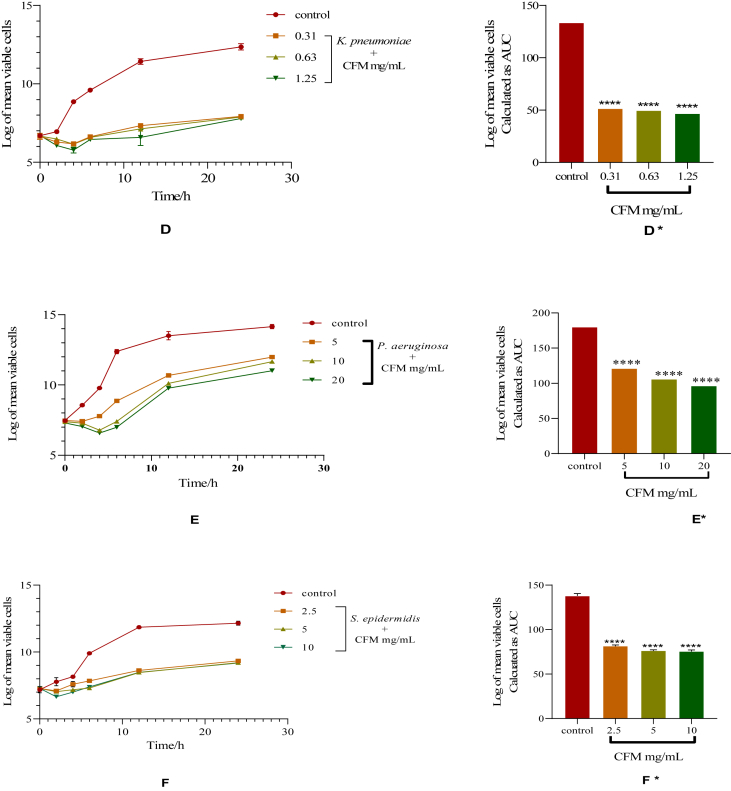
Figure 2.
Time-kill kinetics of CFM against K. pneumoniae, P. aeruginosa and S. epidermis. D, E and F: Time-kill kinetics curve of K. pneumonia, P. aeruginosa and S. epidermis, respectively. D∗, E∗, and F∗: AUCs of time-kill kinetics of K. pneumonia, P. aeruginosa and S. epidermis, respectively. Comparison of AUCs to control indicates significance levels of ∗∗∗∗p < 0.0001 (One-way ANOVA followed by Dunnett's post hoc test).
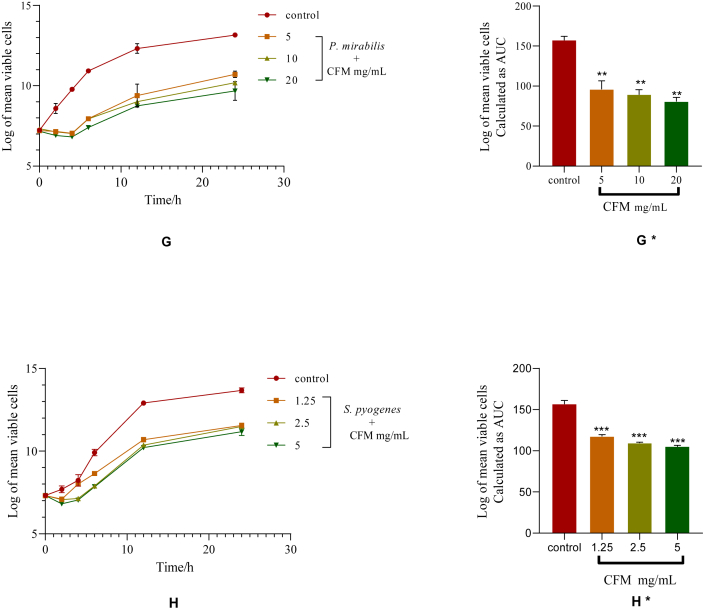
Figure 3.
Time-kill kinetics of CFM against P. mirabilis and S. pyogenes. G and H: Time-kill kinetics curve of P. mirabilis and S. pyogenes, respectively. G∗ and H∗: AUCs of time-kill kinetics of P. mirabilis and S. pyogenes, respectively. Comparison of AUCs to control indicates significance levels of ∗∗∗p < 0.001 (One-way ANOVA followed by Dunnett's post hoc test).
There was a concentration-dependent reduction in the number of viable cells over the first 4 h followed by a gradual rise in the number of organisms until the 24th h in the time-kill kinetics of CFM against K. pneumoniae (D). The time-kill kinetics of CFM against P. aeruginosa revealed a decrease in the number of viable cells over the first 2 h at 5 mg/mL. There was a gradual increase in the number of organisms thereafter until the 24th h. CFM at 10 and 20 mg/mL reduced the number of viable cells over the first 4 h followed by a gradual rise in the number of viable cells (E). The time-kill kinetics of CFM against S. epidermidis showed a concentration-dependent decrease in the number of viable cells over the first 2 h after which there was a gradual rise in the number of organisms until the 24th h (F). The AUC of CFM against K. pneumonia, P. aeruginosa and S. epidermidis when compared to the control showed that the number of organisms reduced significantly at the various concentrations (p < 0.0001) (D∗, E∗, and F∗, respectively) (Figure 2).
There was a concentration-dependent reduction in the number of viable cells over the first 4 h followed by a gradual rise in the number of organisms until the 24th h in the time-kill kinetics of CFM against P. mirabilis (G). The time-kill kinetics of CFM against S. pyogenes showed a concentration-dependent decrease in the number of viable cells over the first 2 h after which there was a gradual rise in the number of organisms until the 24th h (H). The AUC of CFM against P. mirabilis and S. pyogenes, when compared to the control, showed that the number of organisms reduced significantly at the various concentrations (p < 0.0001) (G∗ and H∗, respectively) (Figure 3).
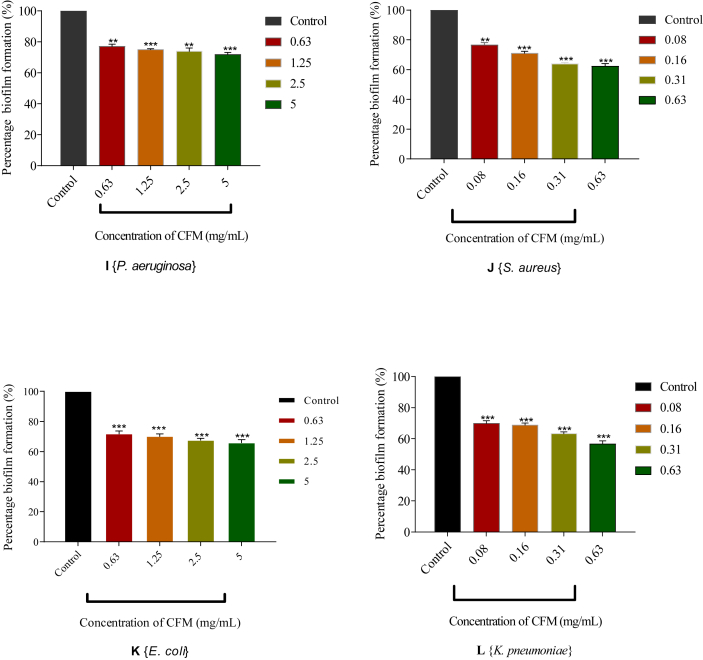
3.5. The effect of CFM on biofilm production by some selected test organisms
CFM was tested for its ability to inhibit the formation of biofilms in four organisms namely; P. aeruginosa, S. aureus, E. coli and K. pneumoniae. These selected organisms are usually implicated in wounds and easily form biofilms [26, 27]. The results indicated a concentration-dependent inhibition to the formation of biofilms in all the test organisms. CFM at its highest concentration (5 mg/mL) was able to inhibit 28.12% (∗∗∗p < 0.001) of biofilms from being formed by P. aeruginosa. The least inhibition was observed at a concentration of 0.63 mg/mL with a percentage inhibition of 22.98% (∗∗∗p < 0.001) (I). CFM at 5 and 0.63 mg/mL inhibited the formation of biofilms in E. coli by 34.11% and 28.19%, respectively (∗∗∗p < 0.001) (K). In S. aureus (J) and K. pneumonia (L), there was 36.66 and 43.41% (∗∗∗p < 0.001) of biofilm inhibition at 0.63 mg/mL when compared to the control. At 0.08 mg/mL of CFM, S. aureus (J) and K. pneumonia (L) inhibited the formation of biofilms by 23.47 and 30.10%, respectively (∗∗∗p < 0.001) (Figure 4).
Figure 4.
Influence of CFM on biofilm formation of selected organisms. I, J, K and L: Percentage biofilm formation of CFM against P. aeruginosa, S. aureus, E. coli and K. pneumoniae, respectively. Comparison to the control indicates significance levels of ∗∗∗p < 0.001 (Unpaired t-test with Welch's correction).
4. Discussion
Cnestis ferruginea is a common plant in Ghana popularly called “Apↄↄsee or Apↄwose” which means “teeth bleacher” by the Akans due to its common usage of cleaning the teeth with the fruits of the plant [28, 29]. Cnestis ferruginea has antibacterial properties [30]. Roots and fruits extracts of C. ferruginea have been reported to exhibit very good antifungal activity against Aspergillus niger and three other filamentous dermatophytes [31]. The fruits of C. ferruginea had activity against some bacteria usually implicated in orofacial infections [29].
The MICs of CFM against the test organisms (Table 2) indicates that C. feruginea contained broad-spectrum antimicrobial agents as it was effective against both Gram-positive and Gram-negative organisms. It also had antifungal properties. This confirmed the traditional usage of the roots of the plant in the treatment of skin infections and its broad-spectrum antimicrobial properties [18, 32]. CFP and CFE had no activity against all the test organisms in the agar well diffusion assay, as well as the broth dilution assay. This occurrence may be as a result of the types or contents of secondary metabolites present in the extracts [32].
Different studies have identified some compounds from the leaves and fruits of C. feruginea. An isoflavone glycoside: afrormosin-7-O-β -D-galactoside (a polar compound), which exhibited antimicrobial activity against S. aureus, E. coli and C. albicans has been isolated from the fruit of C. feruginea [33]. Benzene-1, 4-diol and caffeic acid methyl ester (polar compounds) have also been isolated from the leaves which are believed to confer its antimicrobial activities [34]. Amentoflavone from the roots of the plants has been isolated and investigated for its anti-inflammatory and analgesic properties [14]. The isolated compounds were all polar, thus giving a probability that the antimicrobial compounds present in the roots may also be polar. However, the observed activity in the roots may be as a result of an entirely different compound(s) that are present. Based on the promising antimicrobial activity of CFM compared to CFP and CFE, CFM was subsequently used for the remainder of the studies.
Time-kill kinetics studies were conducted to confirm whether CFM is static or cidal. Determination of cidal or static potential by time-kill kinetics is often desirable. This is because it shows the rate at which an agent can inhibit or kill microbes and the time range in which this inhibition or killing occurs [20, 23]. Observation of 3 log-cycles reductions of the initial number of viable cells, usually over the first 6 h of incubation is indicative of a cidal agent whereas a reduction of less than 3 log-cycle of the initial number of viable cells is indicative of a static agent [23, 35]. CFM reduced the initial number of organisms by less than 3 log-cycles in all test organisms (Figures 1, 2, and 3). However, there was regrowth of the organisms after the reductions observed, making CFM a static agent. This agrees with the findings that some plants do possess static agents [20].
Since bacteria may genetically develop resistance to antibacterial agents after some time [36], it is important to protect these antibacterial agents. This makes resistance modifying activities of microorganisms a significant area in research. Such researches have led to the successful production of combined drugs such as amoxicillin and clavulanic acid combination (Augmentin) [37]. Plant compounds are useful in resistance modifying and as such crude extract screening for such activities is a stepping stones in the identification of such compounds [38]. Sub-inhibitory concentrations of CFM added to amoxicillin, clarithromycin, ciprofloxacin and tetracycline showed that CFM was able to increase the susceptibility of most of the test organisms to reference antibiotics (Table 3). At sub-inhibitory concentrations, some plant-based antibacterial agents increase the antimicrobial activities of existing antibiotics by acting in synergy with the antibiotics [39]. For instance, at sub-MIC (5 mg/mL), the aqueous crude of khat (Catha edulis) when added to tetracycline showed a 2 to 4-folds potentiation against resistant strains of Streptococcus sanguis, Streptococcus oralis and Fusobacterium nucleatum [40].
Many bacteria confine themselves in colonies which are enveloped in a matrix composed of extracellular polymeric substances called biofilms. These biofilms are very difficult to eradicate [41, 42]. CFM was able to inhibit biofilm formation in the selected test organisms. This could be attributed to the presence of secondary metabolites such as terpenoids [43]. The biofilm inhibition property of CFM agrees with reports that some plant extracts do possess antibiofilm activity [42, 43, 44]. For instance, it is reported that plant extracts of Mentha piperita L exhibited antibiofilm activity against S. aureus and E. coli [45].
5. Conclusion
The methanol root extract of C. ferruginea (CFM) showed a broad-spectrum antimicrobial activity. The extract had static activity against the test organisms and exhibited concentration-dependent biofilm inhibition activity. Sub-inhibitory concentrations of CFM modified the activities of some selected antibiotics.
Data availability
The datasets used and/or analyzed during the current study are included within the article. Further clarification can be obtained from the Corresponding author.
Declarations
Author contribution statement
Akosua Dufie Ankomah: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yaw Duah Boakye: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Theresa Appiah Agana: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Francis Adu & Christian Agyare: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank the Technicians at the Microbiology Section, Department of Pharmaceutics, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana for their support.
References
- 1.Chah K.F., Eze C.A., Emuelosi C.E., Esimone C.O. Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J. Ethnopharmacol. 2006;104:164–167. doi: 10.1016/j.jep.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 2.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Aspect. Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Ramawat K.G., Dass S., Mathur M. Herb. Drugs Ethnomedicine to Mod. Med. 2008. The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants; pp. 7–32. [Google Scholar]
- 4.Agyare C., Koffuor G.A., Boamah V.E., Adu F., Mensah K.B., Adu-Amoah L. Antimicrobial and anti-inflammatory activities of Pterygota macrocarpa and Cola gigantea (Sterculiaceae), evidence-based complement. Altern. Med. 2012;2012:1–9. doi: 10.1155/2012/902394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.W.H.O . World Heal. Organ.; 2010. First Report on Neglected Tropical Diseases: Working to Overcome the Global Impact of Neglected Tropical Diseases; pp. 1–184.https://www.who.int/neglected_diseases/resources/9789241564090/en/ Available at. [Google Scholar]
- 6.Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahomoodally M.F. Traditional medicines in Africa: an appraisal of ten potent African medicinal plants, Evidence-Based Complement. Altern. Med. 2013;2013:14. doi: 10.1155/2013/617459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akharaiyi F.C., Ilori R.M., Adesida J.A. Antibacterial effect of Terminalia catappa on some selected pathogenic bacteria. Int. J. Pharm. Biomed. Res. 2011;2:64–67. [Google Scholar]
- 9.Oluwatuyi M., Kaatz G.W., Gibbons S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry. 2004;65:3249–3254. doi: 10.1016/j.phytochem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Bin Zaman S., Hussain M.A., Nye R., Mehta V., Mamun K.T., Hossain N. A Review on antibiotic resistance: alarm bells are ringing. Cureus. 2017;9:1–9. doi: 10.7759/cureus.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atere T.G., Ajao A.T. Toxicological implications of crude alkaloidal fraction from Cnestis ferruginea D . C root on liver function indices of male Wistar rats. Int. J. Biomed. Heal. Sci. 2009;5:145–156. [Google Scholar]
- 12.Ha A. Therapeutic potentials of Cnestis ferruginea: a review. J. Pharmacogn. Phytochem. 2017;6:1397–1401. [Google Scholar]
- 13.Okwu D.E., Iroabuchi F. Phytochemical composition and biological activities of Uvaria chamae and Clerodendoron splendens. E-Journal Chem. 2009;6:553–560. [Google Scholar]
- 14.Ishola I.O., Akindele A.J., Adeyemi O.O. Analgesic and anti-inflammatory activities of Cnestis ferruginea Vahl ex DC (Connaraceae) methanolic root extract. J. Ethnopharmacol. 2011;135:55–62. doi: 10.1016/j.jep.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Ita B. Antioxidant activity of Cnestis ferruginea and Uvaria chamae seed extracts. Br. J. Pharmaceut. Res. 2017;16:1–8. [Google Scholar]
- 16.Adisa R.A., Khan A.A., Oladosu I., Ajaz A., Choudhary M.I., Olorunsogo O., Ur Rahman A. Purification and characterization of phenolic compounds from the leaves of Cnestis ferruginea (De Candolle): investigation of antioxidant property. Res. J. Phytochem. 2011;5:177–189. [Google Scholar]
- 17.Toyin Y.M., Olaide N.Q. Effects of aqueous extract of Cnestis ferruginea (Vahl ex De Cantolle) root on paroxetine-induced sexual dysfunction in male rats. Asian Pacific J. Reprod. 2012;1:111–116. [Google Scholar]
- 18.Acheampong A., Okyem S., Akoto B.A.K., Clement Osei. Antioxidant, antimicrobial and FTIR analysis of methanol root extract of Cnestis ferruginea and ethanol root extract of Citrus limon. J. Pharmacogn. Phytochem. 2018;7:2938–2946. [Google Scholar]
- 19.Yakubu M.T., Adams D.M., Akanji M.A., Oladiji A.T. Laxative activity of aqueous root extract of Cnestis ferruginea (VAHL EX DC) in loperamide-induced constipated rats. Niger. J. Gastroenterol. Hepatol. 2011;3:21–29. [Google Scholar]
- 20.Boakye Y.D., Agyare C., Hensel A. Anti-infective properties and time-kill kinetics of Phyllanthus muellerianus and its major constituent, geraniin. Med. Chem. (Los. Angeles). 2016;6:95–104. [Google Scholar]
- 21.Salie F., Eagles P.F.K., Leng H.M.J. Preliminary antimicrobial screening of four South African Asteraceae species. J. Ethnopharmacol. 1996;52:27–33. doi: 10.1016/0378-8741(96)01381-5. [DOI] [PubMed] [Google Scholar]
- 22.Adu F., Sam G.H., Agyare C., Apenteng J.A., Boamah V.E., Mintah D.N. Influence of methanol fruit and leaf extracts of Myristica fragrans (Myristicaceae) on the activity of some antibiotics. Afr. J. Microbiol. Res. 2014;8:1982–1986. [Google Scholar]
- 23.Petersen P.J., Jones C.H., Bradford P.A. In vitro antibacterial activities of tigecycline and comparative agents by time-kill kinetic studies in fresh Mueller-Hinton broth. Diagn. Microbiol. Infect. Dis. 2007;59:347–349. doi: 10.1016/j.diagmicrobio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Niu C., Gilbert E.S. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl. Environ. Microbiol. 2004;70:6951–6956. doi: 10.1128/AEM.70.12.6951-6956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiegand I., Hilpert K., Hancock R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 26.Omar A., Wright J., Schultz G., Burrell R., Nadworny P. Microbial biofilms and chronic wounds. Microorganisms. 2017;5:9. doi: 10.3390/microorganisms5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Percival S.L., Hill K.E., Williams D.W., Hooper S.J., Thomas D.W., Costerton J.W. 2012. A Review of the Scientific Evidence for Biofilms in Wounds, Wound Repair and Regeneration. [DOI] [PubMed] [Google Scholar]
- 28.Addo-Fordjour P., Anning A.K., Belford E.J.D., Akonnor D. Diversity and conservation of medicinal plants in the Bomaa community of the Brong Ahafo region, Ghana. J. Med. Plants Res. 2013;2:226–233. [Google Scholar]
- 29.Ndukwe K.C., Okeke I.N., Lamikanra A., Adesina S.K., Aboderin O. Antibacterial activity of aqueous extracts of selected chewing sticks. J. Contemp. Dent. Pract. 2005;6:86–94. [PubMed] [Google Scholar]
- 30.Boakye Yiadom K., Konning G.H. Incidence of antibacterial activity in the connaraceae. Planta Med. 1975;28:397–400. doi: 10.1055/s-0028-1097878. [DOI] [PubMed] [Google Scholar]
- 31.le Grand A., Wondergem P.A., Verpoorte R., Pousset J.L. Anti-infectious phytotherapies of the tree-savannah of Senegal (West-Africa) II. Antimicrobial activity of 33 species. J. Ethnopharmacol. 1988;22:25–31. doi: 10.1016/0378-8741(88)90227-9. [DOI] [PubMed] [Google Scholar]
- 32.Maganha E.G., da C. Halmenschlager R., Rosa R.M., Henriques J.A.P., de P. Ramos A.L.L., Saffi J. Pharmacological evidences for the extracts and secondary metabolites from plants of the genus Hibiscus. Food Chem. 2010;118:1–10. [Google Scholar]
- 33.Parvez M., Rahman A. A novel antimicrobial isoflavone galactoside from Cnestis ferruginea (connaraceae) Front. Microbiol. 1992;14:221–223. [Google Scholar]
- 34.Kouakou K., Panda S.K., Yang M.R., Lu J.G., Jiang Z.H., Van Puyvelde L., Luyten W. Isolation of antimicrobial compounds from Cnestis ferruginea vahl ex. DC (Connaraceae) leaves through bioassay-guided fractionation. Front. Microbiol. 2019;10:705. doi: 10.3389/fmicb.2019.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keepers T.R., Gomez M., Celeri C., Nichols W.W., Krause K.M. Bactericidal activity, absence of serum effect, and time-kill kinetics of ceftazidime-avibactam against β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014;58:5297–5305. doi: 10.1128/AAC.02894-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nascimento G.G.F., Locatelli J., Freitas P.C., Silva G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000;31:247–256. [Google Scholar]
- 37.Cole M., Reading C. Clavulanic acid : beta-lactamase-inhibiting beta-lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 1977;11:852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibanda T., Okoh A.I. The challenges of overcoming antibiotic resistance: plant extracts as potential sources of antimicrobial and resistance modifying agents. Afr. J. Biotechnol. 2007;6:2886–2896. [Google Scholar]
- 39.Kamatou G.P.P., Van Zyl R.L., Van Vuuren S.F., Viljoen A.M., Figueiredo A.C., Barroso J.G., Pedro L.G., Tilney P.M. Chemical composition, leaf trichome types and biological activities of the essential oils of four related Salvia species indigenous to Southern Africa. J. Essent. Oil Res. 2006;18:72–79. [Google Scholar]
- 40.Al-hebshi N., Al-haroni M., Skaug N. In vitro antimicrobial and resistance-modifying activities of aqueous crude khat extracts against oral microorganisms. Arch. Oral Biol. 2006;51:183–188. doi: 10.1016/j.archoralbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 41.De Kievit T.R., Iglewski B.H. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 43.Raut J.S., Shinde R.B., Chauhan N.M., Mohan Karuppayil S. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling. 2013;29:87–96. doi: 10.1080/08927014.2012.749398. [DOI] [PubMed] [Google Scholar]
- 44.Upadhyay A., Upadhyaya I., Kollanoor-Johny A., Venkitanarayanan K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013;36:79–89. doi: 10.1016/j.fm.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Bazargani M.M., Rohloff J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Contr. 2016;61:156–164. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are included within the article. Further clarification can be obtained from the Corresponding author.
Data included in article/supplementary material/referenced in article.