Abstract
Context
The Igls criteria were developed to provide a consensus definition for outcomes of β-cell replacement therapy in the treatment of diabetes during a January 2017 workshop sponsored by the International Pancreas & Islet Transplant Association (IPITA) and the European Pancreas & Islet Transplant Association. In July 2019, a symposium at the 17th IPITA World Congress was held to examine the Igls criteria after 2 years in clinical practice, including validation against continuous glucose monitoring (CGM)-derived glucose targets, and to propose future refinements that would allow for comparison of outcomes with artificial pancreas system approaches.
Evidence acquisition
Utilization of the criteria in various clinical and research settings was illustrated by population as well as individual outcome data of 4 islet and/or pancreas transplant centers. Validation against CGM metrics was conducted in 55 islet transplant recipients followed-up to 10 years from a fifth center.
Evidence synthesis
The Igls criteria provided meaningful clinical assessment on an individual patient and treatment group level, allowing for comparison both within and between different β-cell replacement modalities. Important limitations include the need to account for changes in insulin requirements and C-peptide levels relative to baseline. In islet transplant recipients, CGM glucose time in range improved with each category of increasing β-cell graft function.
Conclusions
Future Igls 2.0 criteria should consider absolute rather than relative levels of insulin use and C-peptide as qualifiers with treatment success based on glucose assessment using CGM metrics on par with assessment of glycated hemoglobin and severe hypoglycemia events.
Keywords: pancreas transplantation, islet transplantation, type 1 diabetes, β-cell replacement, continuous glucose monitoring
The aim of β-cell replacement therapy is to achieve near-normal glycemic control in the absence of clinically significant hypoglycemia for patients with diabetes and β-cell failure experiencing severe hypoglycemia, hypoglycemia unawareness, and/or marked glycemic lability, and for patients with diabetes already committed to immunosuppression in support of another organ transplant. Current options for β-cell replacement include whole pancreas (1) or isolated islet transplantation (2), both of which can restore endogenous insulin secretion and improve glycemic control and stability, ameliorate clinically significant hypoglycemia, and reduce diabetes-related complications (3). As an alternative to restoration of endogenous insulin secretion, the artificial pancreas (AP) uses continuous glucose monitoring (CGM) to automate exogenous insulin delivery (4). Despite varying uses and options for β-cell replacement therapy, there had been a lack of clear and standardized definitions for graft function and clinical success, as well as poor alignment of glycemic control metrics used to evaluate AP systems impeding comparison of outcomes with cellular and technological approaches to therapy (5). To that end, in January 2017, the International Pancreas and Islet Transplant Association (IPITA) and the European Pancreas and Islet Transplant Association held a 2-day workshop in Igls, Austria, to develop a standardized definition for functional and clinical outcomes of β-cell replacement therapy, now known as the Igls criteria (6, 7).
The Igls criteria define β-cell graft function as optimal, good, marginal, or failure, based on glycated hemoglobin (HbA1c); severe hypoglycemia events (SHEs); insulin requirements; and C-peptide levels (Table 1). A SHE is defined as an event associated with loss of consciousness or requiring third-party assistance for recovery (8). Optimal graft function requires near-normal glycemic control defined by HbA1c ≤6.5% (48 mmol/mol), absence of SHE, insulin independence (including absence of other antihyperglycemic therapy), and a C-peptide increase over pretransplant measurement. Good β-cell graft function requires on-target glycemic control defined by HbA1c <7.0% (53 mmol/mol), absence of SHE, a reduction in insulin requirements of more than 50% compared with pretransplant (or use of noninsulin antihyperglycemic therapy), and a C-peptide increase over pretransplant measurement. Marginal graft function is defined by either HbA1c ≥7.0% (53 mmol/mol), occurrence of any SHE, or a reduction in insulin requirements of less than 50% in the presence of a C-peptide increase from pretransplant. When C-peptide measures less than 0.5 ng/mL (0.17 nmol/L), or lower than the patient’s baseline before transplantation, the graft is considered to have functionally failed (6, 7). Optimal and good function are considered clinically successful outcomes, whereas marginal and failure are not.
Table 1.
Igls definition of functional and clinical outcomes for β-cell replacement therapy (6, 7) (joint publication)
| β-cell graft functional status | HbA1c, % (mmol/mol)a | Severe hypoglycemia, events per y | Insulin requirements, U·kg-1·d-1 | C-peptide | Treatment success |
|---|---|---|---|---|---|
| Optimal | ≤6.5(48) | None | None | >Baselineb | Yes |
| Good | <7.0(53) | None | <50% baselinec | >Baselineb | Yes |
| Marginal | Baseline | <Baselined | ≥50% baseline | >Baselineb | Noe |
| Failure | Baseline | Baselinef | Baseline | Baselineg | No |
Baseline, pretransplant assessment (not applicable to total pancreatectomy with islet autotransplantation patients).
a Mean glucose should be used to provide an estimate of the glycated hemoglobin, termed the glucose management indicator, in the setting of disordered red blood cell life span.
b Should also be > 0.5 ng/mL (>0.17 nmol/L) fasting or stimulated.
c Should also be < 0.5 U·kg-1·d-1; might include the use of noninsulin antihyperglycemic agents.
d Should severe hypoglycemia occur following treatment, then continued benefit may require assessment of hypoglycemia awareness, exposure to serious hypoglycemia (<54 mg/dL [3.0 mmol/L]), and/or glycemic variability/lability with demonstration of improvement from baseline.
e Clinically, benefits of maintaining and monitoring β-cell graft function may outweigh risks of maintaining immunosuppression.
f If severe hypoglycemia was not present before β-cell replacement therapy, then a return to baseline measures of glycemic control used as the indication for treatment (6, 7) may be consistent with β-cell graft failure.
g May not be reliable in uremic patients and/or in those patients with evidence of C-peptide production before β-cell replacement therapy.
In July 2019, a daylong symposium was held as part of the 17th IPITA World Congress in Lyon, France, to examine implementation of the Igls criteria after 2 years of use in clinical practice. The aims included evaluating the utility and limitations of the current criteria in assessing β-cell graft function, identifying possible areas for improvement, and proposing further refinements to the original criteria. Five experienced transplant centers illustrated of the usefulness of the Igls criteria in various clinical and research settings, and the symposium included discussion of limitations and recommendations for paving the way toward future implementation of the Igls criteria to compare outcomes of β-cell replacement therapies with AP system approaches to diabetes management.
Methods
Utilization of the Igls criteria
To illustrate the various uses of the Igls criteria, patient data from 4 transplant centers were examined. Usefulness in a clinical setting on a population level was demonstrated by data from center A. All consecutive data on patients that had completed at least 1 year of follow-up after either an islet or solitary pancreas transplant in this center were included. In addition, all patients were included who received a simultaneous pancreas-kidney (SPK) transplant in the year 2014 to provide at least 4 years of follow-up. Igls criteria were assessed at 6 months and 1, 2, and 4 years posttransplantation and are presented as a percentage of the population for each of the three β-cell replacement therapy groups (ie, islet transplantation, solitary pancreas transplantation, and SPK transplantation).
Usefulness of the criteria in a clinical setting on an individual level was illustrated by centers B and C using data from islet and pancreas transplant recipients who had completed at least 2 years of follow-up. For center B, patients were assessed at 6 months and 1 and 2 years posttransplantation, longitudinally describing individual patients’ graft function according to the Igls criteria using all functional categories. For center C, individual patients’ graft function was longitudinally delineated using the dichotomous Igls criteria definition of treatment success (optimal or good β-cell graft function) and treatment failure (marginal or failed β-cell graft function).
Usefulness of the criteria in a research setting was illustrated by data of center D, describing consecutive islet transplant recipients included in a research study of a novel immunosuppressive approach that avoided calcineurin inhibitors as previously reported (9), followed now over a 10-year period.
Comparison with CGM metrics
To address whether CGM metrics should be included as part of functional criteria that would better align glycemic control metrics with the AP field, validation of the Igls criteria against standard CGM metrics of glycemic control was provided using data from another transplant center, experienced with CGM in islet transplant recipients.
All CGM data collected during annual posttransplant follow-up in a cohort of patients with type 1 diabetes (T1D) before and after islet transplantation in center E were analyzed (10). CGM metrics were assessed using a blinded system (Medtronic MiniMed, Northridge, CA) for 3 to 5 consecutive days during usual daily life activities and diet as previously described (11). The percentages of glucose time in range (TIR) 70 to 180 mg/dL (3.9-10 mmol/L) and time below range (TBR) <70 mg/dL (3.9 mmol/L) were categorized according to the Igls criteria as optimal, good, marginal, and failure based on 146, 36, 90, and 30 patient assessments, respectively, and evaluated using 1-way ANOVA.
Results
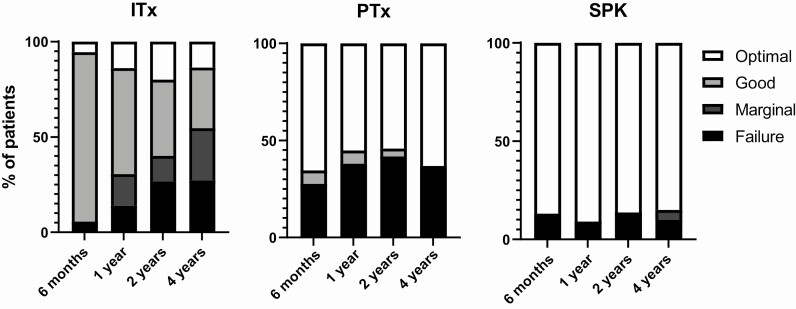
The Igls criteria provide the ability to present and compare data on multiple clinically important levels. On a population level, the Igls criteria are useful to cross-sectionally present and compare functional outcomes of different β-cell replacement modalities (Fig. 1). Using the Igls criteria, functional outcomes of 36 islet transplant recipients (30 islet after kidney [IAK] (12), 4 islet alone [ITA], 2 islet-after-lung (13)), 29 solitary pancreas transplant recipients (26 pancreas after kidney, 3 pancreas transplant alone [PTA]), and 23 SPK recipients from center A were evaluated at 6 months and 1, 2, and 4 years posttransplantation. Good and marginal β-cell graft function is experienced most often with islet transplantation, and optimal and failure with solitary pancreas transplantation, such that treatment success (optimal or good) is experienced by ~60% of recipients over the first 2 years, with more durable function in the pancreas than islet group at 4 years. The highest rate of treatment success is seen with SPK.
Figure 1.
Igls criteria in a clinical setting on a population level. Illustration of the Igls criteria utility for cross-sectional comparison between β-cell replacement modalities, illustrated by consecutive data from center A at 0.5, 1, 2, and 4 years posttransplantation. Igls criteria functional categories are presented as a percentage of each population for islet transplantation (ITx; n = 36), solitary pancreas transplantation (PTx; n = 29), and SPK (n = 23), respectively. Describing the natural course posttransplantation according to current clinical practice, this includes 17 islet transplant recipients that received a subsequent islet infusion by the 2-year assessment, and 1 pancreas transplant recipient with a failed graft at 1 and 2 years receiving a subsequent whole pancreas transplant with optimal graft function at 4 years. SPK, simultaneous pancreas-kidney transplantation.
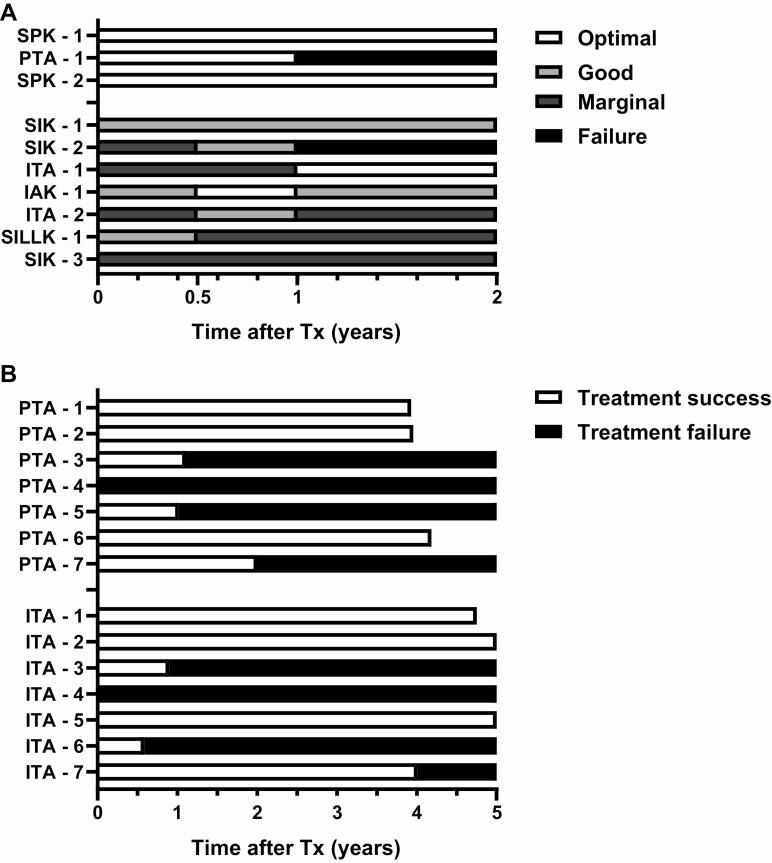
The Igls criteria can also be used for individual longitudinal description of β-cell graft function over time. Graft function in individual patients following islet transplantation (1 IAK (14), 2 ITA, 3 simultaneous islet-kidney, 1 simultaneous islet-liver-lung-kidney (15)), 1 solitary pancreas transplantation (PTA), and 8 SPK was assessed at 6 months and 1 and 2 years posttransplantation by center B (Fig. 2A). Islet transplant recipients more often experienced good and marginal functional outcomes with high fluctuation between functional categories, whereas pancreas transplant recipients showed either optimal function or graft failure. Describing β-cell graft function using the binary Igls criteria outcome measure of treatment success (optimal or good) vs treatment failure (marginal or failed) in 7 individuals following ITA and 7 following PTA from center C (Fig. 2B) shows that achieving treatment success is less fluctuant and follows similar patterns in ITA compared with PTA recipients.
Figure 2.
Igls criteria in a clinical setting on an individual level. Illustration of the Igls criteria utility for individual longitudinal description of β-cell graft function over time for individual patients after ITA, IAK, simultaneous islet-kidney (SIK), simultaneous islet-liver-lung-kidney (SILLK), PTA, and SPK. (A) Illustrated by data of patients from center B followed-up at 0.5, 1, and 2 years posttransplantation, using all functional categories of the Igls criteria. (B) Illustrated by data of patients from center C, using the binary Igls criteria outcomes of treatment success (optimal or good β-cell graft function) vs treatment failure (marginal or failed β-cell graft function). IAK, islet after kidney; ITA, islet alone; PTA, pancreas transplant alone; SPK, simultaneous pancreas-kidney.
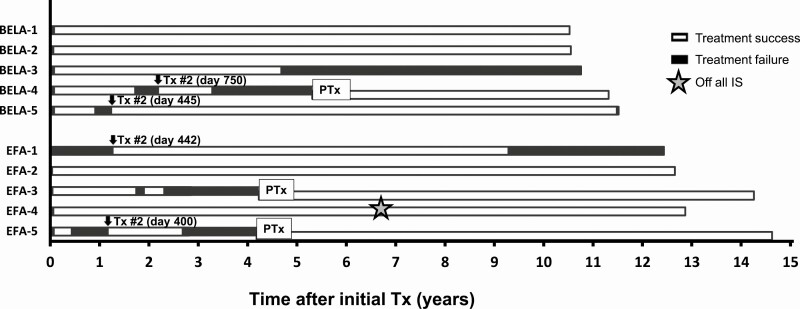
Apart from clinical settings, the Igls criteria can also be used in a research setting to describe and compare β-cell graft function. Graft function according to the Igls criteria was structurally assessed in 10 consecutive ITA recipients from center D that received islet transplantation under protocols evaluating belatacept or efalizumab (Fig. 3). A switch in graft function from treatment success to treatment failure according to the Igls criteria always predated the clinical decision to perform a supplemental islet infusion (16, 17) or subsequent pancreas transplant (18).
Figure 3.
Igls criteria in a research setting. Illustration of the Igls criteria utility for individual longitudinal description of β-cell graft function over time in a research setting, illustrated by 10 structurally and consecutively followed patients that received islet transplantation under protocols investigating belatacept (BELA) or efalizumab (EFA) from center D. Both islet and pancreas transplants were applied in these patients. The binary Igls criteria outcomes of treatment success (optimal or good β-cell graft function) vs treatment failure (marginal or failed β-cell graft function) were used. IS, immunosuppression.
CGM data were collected in a cohort of 55 patients with T1D before and after ITA (n = 39) or IAK (n = 16) in center E, providing >302 patient-years based on individual follow-up periods of 1 to 10 years (10). After islet transplantation, median (interquartile range) TIR was incrementally improved at 100% (95-100; optimal function), 90% (78-97; good function), 75% (64-89; marginal function) and 58% (44-73; failure) compared with 54% (44-71) pretransplant (P < 0.0001, Fig. 4A). Similarly, TBR was 0% (0-1; optimal function), 0% (0-5; good function), 2% (0-7; marginal function) and 7% (3-13; failure), compared with 9% (3-15) pretransplant (P < 0.0001, Fig. 4B).
Figure 4.
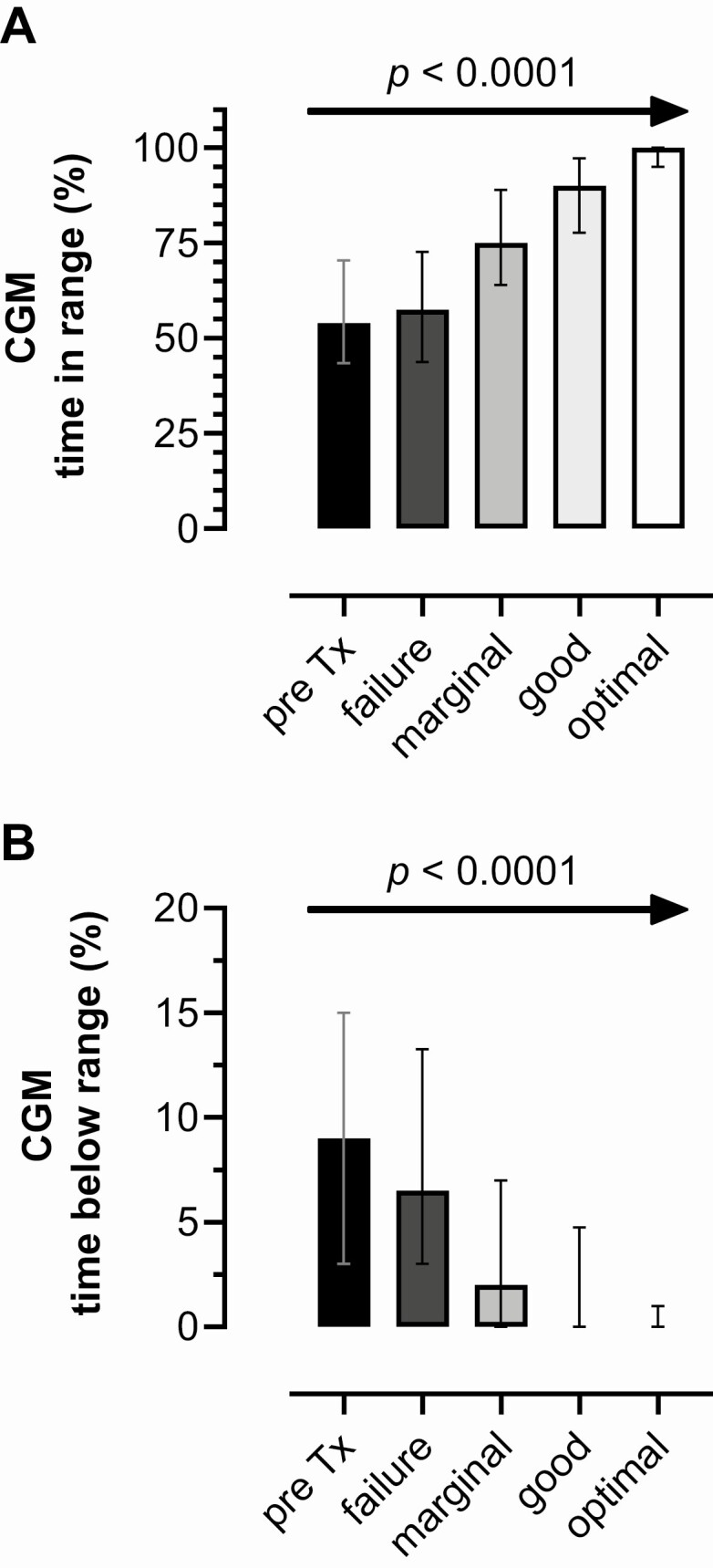
Igls criteria and continuous glucose monitoring metrics of glycemic control. Continuous glucose monitoring (CGM) metrics of glycemic control categorized according to the Igls criteria for scoring β-cell graft function as optimal, good, marginal, or failure, using data from a cohort of islet transplant recipients (n = 55) followed-up to 10 years from center E. CGM parameters incrementally improved with each consecutive category of Igls classification following islet transplantation (P < 0.0001 for both, 1-way ANOVA test for linear trend). Values are represented as median (interquartile range). (A) Glucose time-in-range (TIR, %) 70-180 mg/dL (3.9-10 mmol/L) for each of the functional categories of the Igls criteria. (B) Glucose time-below-range (TBR, %) <70 mg/dL (<3.9 mmol/L) for each of the functional categories of the Igls criteria.
Discussion
The Igls criteria represent an important step forward in the process of standardizing the assessment of outcomes for β-cell replacement therapy, allowing for individual patient monitoring and the comparison of outcomes by different treatment approaches (ie, islet and pancreas transplantation). Illustrated by outcome data of experienced transplant centers, the criteria have shown versatility to capture information on different levels in a clinical (at both a treatment group and at an individual patient level) as well as in a research setting.
Existing registries for pancreas (International Pancreas Transplant Registry [IPTR]) and islet (Collaborative Islet Transplant Registry [CITR]) transplantation have used different definitions for functional graft outcomes. IPTR previously defined pancreas graft failure or success by whether insulin was used or not, irrespective of glucose regulation. Recently, this definition has been revised to insulin requirements ≥0.5 units/kg per day (19), which remains limited as an outcome in the absence of glucose criteria. In addition to insulin requirements, CITR requires reporting of measures for glucose regulation (HbA1c, fasting glucose, severe hypoglycemia events) and C-peptide levels, with primary outcomes defined for insulin independence, HbA1c ≤6.5% (48 mmol/mol), absence of SHE, and C-peptide ≥0.3 ng/mL (0.10 nmol/L) (20). Similar metrics are being collected by CITR for a registry of patients undergoing total pancreatectomy with islet autotransplantation (21). Thus, CITR is positioned to implement assessment by the Igls criteria across both allogeneic and autologous islet transplantation, and IPTR could expand its data reporting requirements for pancreas transplant recipients. By combining measures of glucose regulation and β-cell graft function, the Igls criteria allow for treatment success of whole pancreas, isolated islet, or future stem cell-derived islet transplantation with ongoing insulin use, provided goals for glycemic control and elimination of severe hypoglycemia are met, and clinically significant endogenous insulin secretion (C-peptide) has been restored.
Limitations of the Igls criteria
The basis of β-cell graft functional categories on the achievement of HbA1c targets, absence of SHE, reduction in insulin requirements, and restoration of clinically significant C-peptide production is currently limited by the requirement for baseline measures before transplantation. In addition, although the thresholds used for defining a successful graft outcome are unavoidably arbitrary, the rationale for glycemic control metrics (ie, HbA1c and severe hypoglycemia events) is stronger than that for those reflecting graft function to secrete insulin (ie, insulin use and C-peptide levels).
The requirement for good β-cell graft function of a 50% reduction in insulin use (which should also be <0.5 units/kg per day) is based on expert opinion (22). Insulin requirements are, however, highly variable and depend on factors that not only vary day to day but are also independent of β-cell graft secretory capacity, such as dietary habits, physical activity, insulin sensitivity, kidney function, and the use of noninsulin antihyperglycemic agents. Patient requirements for glucocorticoid therapy, particularly the maintenance of supraphysiologic dosing in combined islet and lung transplants for individuals with β-cell failure because of cystic fibrosis (13, 15), may result in higher insulin requirements from steroid-induced insulin resistance despite all other criteria being optimal/good. Thus, when insulin requirements are the only component leading to classification of marginal β-cell graft function with glycemic control targets being met, it may be difficult to conclude that the treatment is not clinically successful.
For patients with chronic pancreatitis undergoing total pancreatectomy with islet autotransplantation, the assessment for a reduction of insulin requirements or an increase in C-peptide levels relative to baseline prior to intervention (prepancreatectomy) is not possible. Thus, good β-cell graft function that is required to meet criteria for treatment success depends on the presence of insulin requirements <0.5 units/kg per day and C-peptide levels that are >0.5 ng/mL (0.17 nmol/L) fasting or stimulated. In the absence of a stimulated C-peptide, a recent validation study of the Igls criteria in autologous islet recipients substituted a fasting C-peptide ≥0.2 ng/mL (0.07 nmol/L) that was highly predictive of a stimulated C-peptide >0.5 ng/mL (0.17 nmol/L) when both measures were available for analysis (23). Measurement of C-peptide provides an estimate of the contribution of engrafted islets to glycemic control, enabling determination of whether improvements in HbA1c are due to changes in insulin dosing or to effective secretory function of the β-cell graft.
Incorporation of CGM metrics
At the time of the IPITA/European Pancreas and Islet Transplant Association Opinion Leaders Workshop in 2017, consensus targets for CGM-derived metrics of glycemic control had not been established. Since then, the use of CGM has increasingly expanded in clinical practice. The use of CGM metrics such as TIR may identify changes in glycemia sooner than a change in HbA1c, allow simultaneous assessment of hypoglycemia from TBR, and would allow for more direct comparison of β-cell replacement with AP system outcomes (24, 25). In addition, glucose variability has gained increasing importance as both a therapeutic target and an outcome measure in diabetes clinical trials (26), including of islet transplantation (27), where improvement in glucose variability may be related to improvements in measures of neuropathy (28).
The Igls criteria were well-correlated to CGM parameters in the allogeneic islet transplant recipients reported here, with similar findings recently demonstrated in a smaller cohort of autologous islet transplant recipients (23). These results support an approach that applies CGM metrics to the assessment of β-cell graft function, thus further enabling comparisons of results with AP system technology. As even a marginal β-cell graft function is enough to increase TIR and decrease TBR, these results further support that marginal function could still provide benefit to an individual patient by reducing the risk for experiencing future SHE (29-31).
The increasing use of CGM has led to the recent publication of an international consensus for TIR targets, which may soon be adopted as a surrogate for HbA1c (25). In the international consensus, two situations were distinguished: for adults with T1D or type 2 diabetes, TIR should be greater than 70%, TBR less than 4%, and time above range less than 25%. For older or high-risk patients, avoidance of hypoglycemia is prioritized such that the goal is first aimed at limiting TBR to less than 1%, and decreasing the requirement of TIR to greater than 50% with time above range less than 50% (25). Although such a compromise in glycemic control is appropriate when hypoglycemia is a significant risk, the objective of β-cell replacement therapies to eliminate hypoglycemia should allow for the achievement of TIR >70% to 80% even for high-risk individuals such as those with hypoglycemia unawareness or having already undergone kidney transplantation. These TIR targets are based on validation against HbA1c, whereby TIR >50% relates to HbA1c <8.0%, TIR > 60% to HbA1c <7.5%, TIR > 70% to HbA1c <7.0%, and a TIR > 80% to HbA1c ≤6.5% (32).
In the results from center E, and as previously reported by the same group (10, 11), those with a failed islet transplant spent only 58% TIR but with 7% TBR, and so clearly struggled with achieving even the less stringent CGM criteria for high-risk patients with T1D. Those with marginal β-cell graft function spent 75% TIR with only 2% TBR, and so are most often achieving adult standards for glycemic control. Those with good or optimal β-cell graft function spent 90 and 100% TIR, respectively, with no TBR, clearly meeting stringent glycemic control targets. Thus, there is close agreement of the Igls criteria for defining β-cell graft function with increasing time spent in the target glucose range and decreasing time spent with exposure to hypoglycemia. This relationship of CGM time spent both within and below the normal glucose range with the CGM-independent metrics used in Igls 1.0 should enable the adoption of CGM metrics as the most accurate approach to compare both cellular therapies and technological approaches to glycemic control.
For high-risk individuals being considered for and receiving β-cell replacement therapy, it is particularly important to also examine time spent with serious, clinically significant hypoglycemia <54 mg/dL (3.0 mmol/L) (33) and glucose variability that more strongly relate to risk for experiencing SHE (34). Moreover, because β-cell replacement therapy targets near-normal glycemic control (even for high-risk patients), <4% TBR is acceptable as long as time spent <54 mg/dL (3.0 mmol/L) is negligible (<1%) because this amount of CGM measured hypoglycemia is present in healthy, nondiabetic individuals (35).
Looking forward: paving the way for Igls 2.0
In summary, the Igls criteria are considered a great improvement for standardized classification of graft function and treatment success for current β-cell replacement therapies, including both isolated islet and whole pancreas transplantation. Temporal assessment is important and should be included any time a clinical change in β-cell graft function is suspected and at the time of any additional β-cell transplant. Limitations include the absence of CGM metrics that preclude direct comparison of outcomes to AP systems. In addition, insulin requirements were found to be very dependent on confounding factors such as diet, exercise, and glucocorticoids rather than β-cell graft function, and the requirement for obtaining a stimulated C-peptide >0.5 ng/mL (>0.17 nmol/L) to document β-cell graft function in cases in which the fasting level fell below this threshold was felt too cumbersome. Additionally, the dichotomous outcome definition of treatment success and treatment failure was thought to be insensitive to the clinical benefits associated with a marginal β-cell graft function. Together with insulin requirements, C-peptide levels also cannot be used for comparison of cellular to technologic treatment approaches to glycemic control.
Future steps forward to improve upon the current criteria should incorporate CGM metrics to ensure comparison between β-cell replacement therapies and new developments in AP systems technology. We suggest that a new Igls 2.0 form composite criteria in which clinical outcome based on glucose regulation is separated from β-cell graft function, with the latter considered only for further qualification of β-cell replacement modalities (Table 2). Clinical outcome would encompass glycemic control and hypoglycemia and be sufficient for defining treatment success, and only the assessment of β-cell graft function would further require the addition of C-peptide and insulin use criteria. Reflecting the potential of a marginal β-cell function providing clinical benefit, this subdivision also would ensure the possibility for scoring treatment success, even with marginal β-cell graft function. Glycemic control and hypoglycemia could be assessed with or without CGM. Glucose regulation in patients with CGM could be assessed through %TIR and %TBR, whereas in those without CGM through HbA1c and the occurrence of SHE.
Table 2.
Proposed Igls criteria 2.0
| Treatment outcome | Glycemic control | Hypoglycemia | Treatment success | ||
|---|---|---|---|---|---|
| HbA1c, % (mmol/mol)a | CGM, % time-in-range | Severe hypoglycemia, events per y | CGM, % time < 54 mg/dl (3.0 mmol/L) | ||
| Optimal | ≤6.5 (48) | ≥80 | None | 0 | Yes |
| Good | <7.0 (53) | ≥70 | None | <1 | Yes |
| Marginal | ≤Baseline | >Baseline | <Baselineb | <Baseline | Noc |
| Failure | ~Baseline | ~Baseline | ~Baselined | ~Baseline | No |
| β-cell graft functione | C-peptide, ng/mL (nmol/L) f | Insulin use or noninsulin antihyperglycemic therapy | |||
| Optimal | Any | None | |||
| Good | >0.5 (0.17) stimulated ≥0.2 (0.07) fasting |
Any | |||
| Marginal | 0.3-0.5 (0.10-0.17) stimulated 0.1-<0.2 (0.04-<0.07) fasting |
Any | |||
| Failure | <0.3 (0.10) stimulated <0.1 (0.04) fasting |
Any |
Baseline, pretransplant assessment (not applicable to total pancreatectomy with islet autotransplantation patients).
Abbreviations: CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin.
a Mean glucose should be used to provide an estimate of the HbA1c, termed the glucose management indicator, in the setting of disordered red blood cell life span.
b Should severe hypoglycemia occur following treatment, then continued benefit may require assessment of hypoglycemia awareness, exposure to serious hypoglycemia (<54 mg/dL [3.0 mmol/L]), and/or glycemic variability/lability with demonstration of improvement from baseline.
c Clinically, benefits of maintaining and monitoring β-cell graft function may outweigh risks of maintaining immunosuppression.
d If severe hypoglycemia was not present before β-cell replacement therapy, then a return to baseline measures of glycemic control used as the indication for treatment (6, 7) may be consistent with β-cell graft failure.
e Categorization of β-cell graft function must first meet treatment outcome based on measures of glucose regulation.
f May not be reliable in uremic patients and/or in those patients with evidence of C-peptide production before β-cell replacement therapy.
Because insulin requirements are extremely dependent on individual lifestyle-related factors (36), and are not useful for comparison to AP systems, it was suggested to remove percent reductions for defining β-cell graft function in a future Igls 2.0 criteria. Furthermore, although a threshold for insulin requirements < 0.5 units/kg body weight per day was felt by some to represent a reasonable expectation of a clinically successful β-cell graft with good function (consistent with the IPTR) (19), others felt that only insulin independence should be required for defining optimal β-cell graft function. By removing the amount of insulin that may be required to optimize glycemic control, these revised criteria for insulin use would also allow for more direct application of the Igls criteria to patients undergoing total pancreatectomy with islet autotransplantation.
The treatment goal for C-peptide level as a functional measure of β-cell graft insulin secretion should still meet the stimulated threshold >0.5 ng/mL (0.17 nmol/L) established by the Diabetes Control and Complications Trial as associated with reduced risk for experiencing severe hypoglycemia events as well as for the development and progression of microvascular complications (37). This threshold is also associated with improved glycemic control and avoidance of hypoglycemia following islet transplantation for T1D (38), where it is usually related with a fasting C-peptide of at least 0.2 ng/mL (0.07 nmol/L) (23). C-peptide below this threshold, but at least 0.3 ng/mL (0.10 nmol/L) stimulated (39) (as reported in CITR) (20) or 0.1 ng/mL (0.03 nmol/L) fasted, could be compatible with a marginal β-cell graft. Although lower levels of residual C-peptide detectable by high-sensitivity assays have been associated with a reduced risk of hypoglycemia in T1D (39, 40), in the phase 3 Clinical Islet Transplantation Consortium trial involving individuals with T1D complicated by hypoglycemia unawareness, only those transplant recipients who lost islet graft function defined by a stimulated C-peptide <0.3 ng/mL (0.10 nmol/L) experienced a recurrence of severe hypoglycemia (41), and so should be considered failed.
It is still not known whether the Igls criteria may predict outcomes in β-cell replacement therapy, nor whether the Igls criteria may guide physicians in clinical decision making (eg, whether a shift from optimal to good function should prompt closer metabolic monitoring or immunological surveillance). Finally, given the heavy psychological burden of T1D affecting disease management (42), recent clinical trials of diabetes treatments increasingly include patient-reported outcomes (PROs), which have been recognized as a clinically meaningful outcome in T1D (24). Future updates to the criteria should also take into account PROs, including health-related quality of life, diabetes distress, fear of hypoglycemia, and patient satisfaction with their current treatment (43). It is important to note that the herewith-proposed Igls 2.0 criteria are only preliminary. We propose that experts and practitioners in the field reconvene for another workshop to generate consensus of the incorporation of CGM metrics as proposed here, as well as considering the addition of PROs that could be applied to comparative effectiveness evaluation of both β-cell replacement and AP system approaches to diabetes treatment.
Acknowledgments
The authors thank Suzanne Landis of the International Pancreas and Islet Transplant Association, a section of the Transplantation Society, for assistance with organization and filming of the symposium.
Funding: M.R.R. is supported in part by U.S. Public Health Services research grant R01 DK091331.
Glossary
Abbreviations
- AP
artificial pancreas
- CGM
continuous glucose monitoring
- CITR
Collaborative Islet Transplant Registry
- HbA1c
glycated hemoglobin
- IAK
islet after kidney
- IPITA
International Pancreas & Islet Transplant Association
- IPTR
International Pancreas Transplant Registry
- ITA
islet transplant alone
- PRO
patient-reported outcome
- PTA
pancreas transplant alone
- SHE
severe hypoglycemia event
- SPK
simultaneous pancreas-kidney
- TBR
time below range
- T1D
type 1 diabetes
- TIR
time in range.
Additional Information
Disclosures: M.R.R. has been a consultant to Semma Therapeutics and Sernova Corporation, and has received research grant support from Xeris Pharmaceuticals. The remaining authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Larsen JL. Pancreas transplantation: indications and consequences. Endocr Rev. 2004;25(6):919-946. [DOI] [PubMed] [Google Scholar]
- 2. Rickels MR, Robertson RP. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr Rev. 2019;40(2):631-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vantyghem MC, de Koning EJP, Pattou F, Rickels MR. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet. 2019;394(10205):1274-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beck RW, Bergenstal RM, Laffel LM, Pickup JC. Advances in technology for management of type 1 diabetes. Lancet. 2019;394(10205):1265-1273. [DOI] [PubMed] [Google Scholar]
- 5. Bartlett ST, Markmann JF, Johnson P, et al. . Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation. 2016;100(Suppl 2):S1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rickels MR, Stock PG, de Koning EJP, et al. . Defining outcomes for beta-cell replacement therapy in the treatment of diabetes: a consensus report on the Igls criteria from the IPITA/EPITA opinion leaders workshop. Transplantation. 2018;102(9):1479-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rickels MR, Stock PG, de Koning EJP, et al. . Defining outcomes for beta-cell replacement therapy in the treatment of diabetes: a consensus report on the Igls criteria from the IPITA/EPITA opinion leaders workshop. Transpl Int. 2018;31(4):343-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seaquist ER, Anderson J, Childs B, et al. . Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab. 2013;98(5):1845-1859. [DOI] [PubMed] [Google Scholar]
- 9. Posselt AM, Szot GL, Frassetto LA, et al. . Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation. 2010;90(12):1595-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vantyghem MC, Chetboun M, Gmyr V, et al. . Ten-year outcome of islet alone or Islet after kidney transplantation in type 1 diabetes: a prospective parallel-arm cohort study. Diabetes Care. 2019;42(11):2042-2049. [DOI] [PubMed] [Google Scholar]
- 11. Vantyghem MC, Raverdy V, Balavoine AS, et al. . Continuous glucose monitoring after Islet transplantation in type 1 diabetes: an excellent graft function (beta-score greater than 7) is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (beta-score greater than 3). J Clin Endocrinol Metab. 2012;97(11):E2078-E2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nijhoff MF, Engelse MA, Dubbeld J, et al. . Glycemic stability through Islet-after-kidney transplantation using an alemtuzumab-based induction regimen and long-term triple-maintenance immunosuppression. Am J Transplant. 2016;16(1):246-253. [DOI] [PubMed] [Google Scholar]
- 13. Spijker HS, Wolffenbuttel BH, van der Bij W, Engelse MA, Rabelink TJ, de Koning EJ. Islet-after-lung transplantation in a patient with cystic fibrosis-related diabetes. Diabetes Care. 2014;37(7):e159-160. [DOI] [PubMed] [Google Scholar]
- 14. Lablanche S, Vantyghem MC, Kessler L, et al. . Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(7):527-537. [DOI] [PubMed] [Google Scholar]
- 15. Kessler L, Bakopoulou S, Kessler R, et al. . Combined pancreatic islet-lung transplantation: a novel approach to the treatment of end-stage cystic fibrosis. Am J Transplant. 2010;10(7):1707-1712. [DOI] [PubMed] [Google Scholar]
- 16. Faradji RN, Tharavanij T, Messinger S, et al. . Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;86(12):1658-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koh A, Imes S, Kin T, et al. . Supplemental islet infusions restore insulin independence after graft dysfunction in islet transplant recipients. Transplantation. 2010;89(3):361-365. [DOI] [PubMed] [Google Scholar]
- 18. Wisel SA, Gardner JM, Roll GR, et al. . Pancreas-after-islet transplantation in nonuremic type 1 diabetes: a strategy for restoring durable insulin independence. Am J Transplant. 2017;17(9):2444-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gruessner AC, Gruessner RW. Pancreas transplantation of US and non-US cases from 2005 to 2014 as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2016;13(1):35-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barton FB, Rickels MR, Alejandro R, et al. . Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35(7):1436-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bellin MD, Gelrud A, Arreaza-Rubin G, et al. . Total pancreatectomy with islet autotransplantation: summary of an NIDDK workshop. Ann Surg. 2015;261(1):21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davies MJ, D’Alessio DA, Fradkin J, et al. . Management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McEachron KR, Yang Y, Hodges JS, et al. . Performance of modified Igls criteria to evaluate islet autograft function after total pancreatectomy with islet autotransplantation - a retrospective study. Transpl Int. 2021; 34(1):87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agiostratidou G, Anhalt H, Ball D, et al. . Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, the Leona M. and Harry B. Helmsley charitable trust, the Pediatric Endocrine Society, and the T1D exchange. Diabetes Care. 2017;40(12):1622-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Battelino T, Danne T, Bergenstal RM, et al. . Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilmot EG, Choudhary P, Leelarathna L, Baxter M. Glycaemic variability: the under-recognized therapeutic target in type 1 diabetes care. Diabetes Obes Metab. 2019;21(12):2599-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jalbert M, Zheng F, Wojtusciszyn A, et al. . Glycemic variability indices can be used to diagnose islet transplantation success in type 1 diabetic patients. Acta Diabetol. 2019; 57(3):335-345. [DOI] [PubMed] [Google Scholar]
- 28. Vantyghem MC, Quintin D, Caiazzo R, et al. . Improvement of electrophysiological neuropathy after islet transplantation for type 1 diabetes: a 5-year prospective study. Diabetes Care. 2014;37(6):e141-142. [DOI] [PubMed] [Google Scholar]
- 29. Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50(12):2553-2561. [DOI] [PubMed] [Google Scholar]
- 30. Henriksen MM, Andersen HU, Thorsteinsson B, Pedersen-Bjergaard U. Hypoglycemic exposure and risk of asymptomatic hypoglycemia in type 1 diabetes assessed by continuous glucose monitoring. J Clin Endocrinol Metab. 2018;103(6):2329-2335. [DOI] [PubMed] [Google Scholar]
- 31. Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C. The association of biochemical hypoglycemia with the subsequent risk of a severe hypoglycemic event: analysis of the DCCT data set. Diabetes Technol Ther. 2019;21(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beck RW, Bergenstal RM, Cheng P, et al. . The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. International Hypoglycaemia Study G. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40(1):155-157. [DOI] [PubMed] [Google Scholar]
- 34. Senior PA, Bellin MD, Alejandro R, et al. . Consistency of quantitative scores of hypoglycemia severity and glycemic lability and comparison with continuous glucose monitoring system measures in long-standing type 1 diabetes. Diabetes Technol Ther. 2015;17(4):235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shah VN, DuBose SN, Li Z, et al. . Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pilacinski S, Zozulinska-Ziolkiewicz DA. Influence of lifestyle on the course of type 1 diabetes mellitus. Arch Med Sci. 2014;10(1):124-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26(3):832-836. [DOI] [PubMed] [Google Scholar]
- 38. Brooks AM, Oram R, Home P, Steen N, Shaw JAM. Demonstration of an intrinsic relationship between endogenous C-peptide concentration and determinants of glycemic control in type 1 diabetes following islet transplantation. Diabetes Care. 2015;38(1):105-112. [DOI] [PubMed] [Google Scholar]
- 39. Lachin JM, McGee P, Palmer JP, Group DER . Impact of C-peptide preservation on metabolic and clinical outcomes in the diabetes control and complications trial. Diabetes. 2014;63(2):739-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jeyam A, Colhoun H, McGurnaghan S, et al. . Clinical impact of residual C-peptide secretion in type 1 diabetes on glycemia and microvascular complications. Diabetes Care. 2021;44(2):390-398. [DOI] [PubMed] [Google Scholar]
- 41. Hering BJ, Clarke WR, Bridges ND, et al. . Phase 3 trial of transplantation of human Islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Duinkerken E, Snoek FJ, de Wit M. The cognitive and psychological effects of living with type 1 diabetes: a narrative review. Diabet Med. 2020;37(4):555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Foster ED, Bridges ND, Feurer ID, et al. . Improved health-related quality of life in a phase 3 islet transplantation trial in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2018;41(5):1001-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.