Abstract
Prevalence of neurodevelopmental disorders (NDDs) with social deficits is conspicuously rising, particularly in boys. Flame retardants (FRs) have long been associated with increased risk, and prior work by us and others in multiple species has shown that developmental exposure to the common FR mixture Firemaster 550 (FM 550) sex-specifically alters socioemotional behaviors including anxiety and pair bond formation. In rats, FRs have also been shown to impair aspects of osmoregulation. Because vasopressin (AVP) plays a role in both socioemotional behavior and osmotic balance we hypothesized that AVP and its related nonapeptide oxytocin (OT) would be vulnerable to developmental FM 550 exposure. We used the prairie vole (Microtus ochrogaste) to test this because it is spontaneously prosocial. Using siblings of prairie voles used in a prior study that assessed behavioral deficits resulting from developmental FM 550 exposure across 3 doses, here we tested the hypothesis that FM 550 sex-specifically alters AVP and OT neuronal populations in critical nuclei, such as the paraventricular nucleus (PVN), that coordinate those behaviors, as well as related dopaminergic (determined by tyrosine hydroxylase (TH) immunolabeling) populations. Exposed females had fewer AVP neurons in the anterior PVN and more A13 TH neurons in the zona incerta than controls. By contrast, in FM 550 males, A13 TH neuron numbers in the zona incerta were decreased but only in 1 dose group. These results expand on previous work showing evidence of endocrine disruption of OT/AVP pathways, including to subpopulations of PVN AVP neurons that coordinate osmoregulatory functions in the periphery.
Keywords: Social, anxiety, neurodevelopment, neural, endocrine disruptors, endocrine disrupting chemicals, oxytocin, vasopressin, dopamine
Chemical flame retardants (FRs), particularly halogenated FRs, have long been associated with numerous adverse outcomes with endocrine underpinnings including higher risk of neurodevelopmental disorders (NDDs). While most work to date has focused on thyroid disruption (1, 2), converging evidence from us and others suggest oxytocin (OT) and arginine vasopressin (AVP) signaling pathways are potential targets of disruption by some FRs (3). Here we used the common FR mixture Firemaster 550 (FM 550) to test the hypothesis that developmental exposure would sex-specifically alter OT, AVP and related neuronal populations.
Both OT and AVP are primarily synthesized by magnocellular and parvocellular neurons in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus (4). Subpopulations of OT and AVP magnocellular neurons release OT and AVP directly into the bloodstream via the posterior pituitary gland, where they coordinate peripheral functions such as uterine contraction and osmoregulation respectively (5). Centrally, OT and AVP play critical roles in coordinating socioemotional behaviors but generally OT is anxiolytic and prosocial, while AVP is anxiogenic and heightens depressive-like behaviors (3, 6). Interactions with the mesolimbic dopamine system promote prosocial behavior by mediating reward (7-9). Influence by central dopamine also modulates cognitive and behavioral processes essential to pair bonding including olfaction, learning, memory, and social memory (10-12).
Significantly, exposure to FRs has been linked to both osmoregulatory and behavioral deficits, strongly implicating OT/AVP pathways as a target of toxicity. Compelling evidence in male rats suggests that some FRs disrupt osmotic balance (13) as well as PVN and SON AVP levels in response to prolonged hyperosmotic challenge (14). In humans, developmental FR exposure has been linked to behavioral and cognitive impairments, including heightened risk of autism spectrum disorders (ASDs), attention deficit and hyperactivity disorder (ADHD), and other neurodevelopmental disorders (NDDs) (15-18). Prevalence of NDDs is conspicuously rising, particularly in boys, provoking some to declare it a silent pandemic (17, 19). Epidemiological and experimental evidence has linked elevated risk of NDDs with prenatal exposure to air pollution, pesticides, FRs, and some endocrine disrupting chemicals (EDCs), but definitive evidence linking any chemical exposure to specific NDDs remains sparse (18, 20-23). Thus, there is a pressing need to understand the mechanisms by which sexually dimorphic neuroendocrine pathways underlying prosocial behaviors may be vulnerable to chemical exposures (17, 24, 25).
There are many types of FRs, the most intensively studied of which are the polybrominated diphenyl ethers (PBDEs), which were phased out of most uses in the mid-2000s due to evidence of thyroid disruption and developmental neurotoxicity (16, 26). Consequently, use of alternative FRs rapidly increased, including alternative brominated FRs (BFRs) and a newer FR class: organophosphate esters (OPFRs) (27-30). We focused on FM 550 because it contains both BFRs and OPFRs, and we and others have shown developmental exposure sex-specifically alters socioemotional behaviors (31) in rodents (32-34), and possibly humans (35).
FM 550 comprises 2 brominated compounds, 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and bis(2-ethylhexyl) 2,3,4,5- tetrabromophthalate (BEH-TEBP), previously abbreviated TBPH), and the OPFR triphenyl phosphate (TPHP, previously abbreviated TPP) and a suite of ITPs (29, 36). Human exposure is typically via ingestion and inhalation of contaminated house dust and, because of their frequent hand to mouth behavior and time on the floor, young children consistently have higher body burdens than adults (37-39). FM 550 components are regularly detected in human tissues including blood, hair, breast milk, fingernails, and urine (40-44). Some OPFRs are also used as plasticizers, in personal care products, and for other purposes so human exposure is ubiquitous and dates back decades (27, 45). Human blood levels are generally in the low ng/mL range (27) but exposure can be much higher for carpet installers, nail salon workers, fire fighters, and other FR-intensive occupations (46). In animal models, FM 550 and its individual components have been shown to induce abnormal behavioral phenotypes including elevated anxiety, hyperactivity, impaired cognitive function, and social deficits, but it remains unclear how exposure might induce social deficits such as impaired attachment (31-33, 47, 48). Nothing is known about how FM 550 exposure might impact peripheral action of OT or AVP.
The present study builds on our prior work showing perinatal FM 550 exposure at environmentally relevant doses (500, 1000, or 2000 µg/day) impacted a range of socioemotional behaviors in adult prairie voles (Microtus ochrogaster) (31). As a developmental neurotoxicology model, the prairie vole is uniquely valuable because it is a socially monogamous and biparental species that spontaneously displays prosocial traits such as affiliation and pair bonding (8, 49). The bulk of the work establishing that OT, AVP, and the sexually dimorphic pathways by which they interact with the mesolimbic dopamine system coordinate human-relevant prosocial traits including pair bonding, paternal care, and social recognition was conducted in voles (8, 50-55). The translational utility of the vole model for ASDs has already been established (56), and we have used it for other EDC studies (57, 58). Sex-specific effects of developmental FM 550 exposure on pair bonding were observed, with females displaying a heightened partner preference and lack of partner preference observed in males. In both sexes, developmental FM 550 exposure impaired sociality, but social and generalized anxiety were greater and dose responsive in females (31). The experiments herein used their siblings, who were identically exposed but did not undergo behavioral testing.
The number of dopaminergic neurons in the zona incerta (ZI), which lies just caudal to the PVN, along with the ventral tegmental area (VTA), and the medial division of the bed nucleus of the stria terminalis (BNST) were also quantified because they play a role in locomotor activity, social stress, and motivation (59-63). Dopaminergic neuron numbers, identified by tyrosine hydroxylase (TH)-immunoreactivity (ir), are known to be sexually dimorphic in the BNST with male prairie voles having more (11). The A13 dopaminergic (DAergic) neuron population, located within the ZI, are thought to contribute to motor control (64), specifically defensive motor behaviors (65, 66). The VTA was of interest because exposed sibling voles displayed disrupted pair formation, and the mesolimbic dopamine system facilitates pair bonding by modulating reward-seeking behaviors (67).
OT and AVP neurons were quantified such that we could distinguish potential effects in different subregions of the PVN, which was critical for understanding how disruption might impact the central and peripheral functions of these nonapeptides. The data contribute to our fundamental understanding of how the developing brain is vulnerable to EDCs and other anthropogenic chemicals.
Materials and Methods
The ARRIVE (Animal Research: Reporting of In Vivo Experiments) Guidelines Checklist for Reporting Animal Research was used in the construction of this manuscript with all elements met (68). The ARRIVE guidelines were developed in consultation with the scientific community as part of an NC3Rs (National Centre for the Replacement Refinement and Reduction of Animals in Research) initiative to improve the standard of reporting of research using animals.
Animals
All tissues were obtained from sibling prairie voles of animals used in a prior, published study describing the impacts of developmental FM 550 exposure on socioemotional behavior (31). Animal care, maintenance, and experimental protocols met the standards of the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for the Care and use of Laboratory Animals” and were approved by the North Carolina State University (NCSU) Institutional Animal Care and Use Committee. A supervising veterinarian approved and monitored all procedures throughout the duration of the project. Prairie voles (Microtus ochrogaster) were obtained from founders generously gifted by Bruce S. Cushing at the University of Texas El Paso in 2017 and bred in house in a manner that maintains genetic diversity in humidity- and temperature-controlled rooms at 22°C and 30% average humidity, each with 12 hour:12 hour light:dark cycles (lights on at 6 am EST) in the Assessment and Accreditation of Laboratory Animal Care approved Biological Resource Facility at NCSU. Food (Lab Diet 5326 high-fiber rabbit diet) and water were provided ad libitum. As in our prior studies (32, 69) and in accordance with recommended practices for EDC research (70), all animals were housed in conditions specifically designed to minimize unintended EDC exposure including use of glass water bottles with metal sippers, woodchip bedding, and thoroughly washed polysulfone caging. The diet is not a low-phytoestrogen diet but phytoestrogens are required to maximize health and fertility of this herbivorous species (71).
Dose Preparation
As we have done previously (31, 32, 72), the sesame oil–based dosing solutions were prepared and coded by the Stapleton laboratory at Duke University and transferred to the Patisaul laboratory at NCSU. A commercial mixture of FM 550 was obtained from Great Lakes Chemical (West Lafayette, IN, USA) (30) and each dosing solution (0, 500, 1000, and 2000 μg/20 μL vehicle) was prepared by weighing the appropriate amount of FM 550 and diluting it in sesame oil (Sigma) with stirring for 6 h, and then stored in amber bottles at 4°C until use. Dams were not dosed by individual weight, but rather the average colony weight of 50 g during pregnancy, producing exposures of approximately 10 mg/kg body weight (BW), 20 mg/kg BW, and 40 mg/kg BW. This approach was chosen to minimize handling stress but does mean the per BW exposure varies across pregnancy and lactation as the dam’s weight changes. The global serotonin agonist, 5MT, was used as a positive control for chemical disruption of social behavior because prior work in rats and voles showed perinatal exposure alters social behavior and dysregulation of the OT and AVP systems (73-75). 5MT was obtained from Aldrich chemistry (lot number BCBT9524, 97% pure, 286583-1G, St Louis, MO) and dosing solution was prepared by dissolving in 100% ethanol to give the final concentration of 50 μg 5MT/20 μL and approximately 1 mg/kg BW. Despite prior studies reporting tonic saline as the vehicle, we found 5MT to be insoluble in saline regardless of concentration.
Exposure
Animals used herein were siblings of voles used in our previously published behavioral study (31), and underwent the same exposure paradigm. Pairs from the breeding colony were selected and the dams randomly assigned to an exposure group: FM 550 (0, 500, 1000, 2000 μg) or 5MT (50 μg). All pairs had reared prior litters and were experienced parents. Because voles breed continuously and typically become pregnant with 48 hours after birth, exposure to the dam via subcutaneous (SC) injection began the day after parturition of a litter. Designated as gestational day (GD) 0 for the exposed, gestating litter, daily SC injections to the dam continued through the day before birth of the experimental offspring. No dosing occurred on the day of birth. Offspring were then dosed directly by SC injection from the day after parturition, postnatal day (PND) 1, until weaning, PND 21. Offspring exposed to 5MT started to develop skin irritation at the injection sites, thus 5MT exposure ceased on PND 7. Dosing occurred daily beginning at 2:00 pm EST. Although oral exposure is considered more human relevant, SC injection was chosen because, at the time, the pharmacokinetics of FM 550 in prairie voles was unknown. To assess internal levels following dosing, a separate set of sexually naïve male (n = 4) and female (n = 5) adult prairie voles were exposed to 2000 µg of FM 550 for 5 days via SC injection and their blood collected 4 hours after injection. Serum levels of EH-TBB were between 15 and 25 ng/mL, and TPHP and several of the ITP isomers ranged from 0.2 to 20 ng/mL. Although BEH-TEBP accounts for ~14% of FM 550, it was not detected in serum, which is likely due to its rapid body clearance (76). While fetal levels are unknown, they are likely far lower than dam serum levels.
After weaning, offspring were housed by same sex littermates or conspecifics at 2 or 3 per cage. Final litter numbers were 11 control, 11 500 µg FM 550, 9 1000 µg FM 550, 12 2000 µg FM 550, and 10 5MT. Up to 2 male (M) and female (F) pups per litter (there was only 1 instance where 3 males were selected from a 500-µg litter) were randomly selected for neuroanatomical evaluations, with their siblings reserved for the socioemotional behavioral testing study that has already been published (31). Although litter is the preferred statistical unit for toxicological studies, the individual pups were used here for logistical reasons (this species produces small litters and must remain pair bonded, which limits breeding options) and because the colony is wild-derived and thus considerably more genetically diverse than typical laboratory rodent strains. Because our breeding colony is small, in some cases pairs produced multiple experimental litters. Most of those pairs produced a control litter and then 1 of the FM 550 litters. The remaining produced a lower than higher dose of FM 550 pups (so a 500-µg dam would then produce a 1000- or 2000-µg litter). The number of litters represented by offspring sex in each group is control (10 F, 11 M), 500 µg FM 550 (10 F, 11 M), 1000 µg FM 550 (8 F, 9 M), 2000 µg FM 550 (7 F, 12 M), 5MT (7 F, 10 M). The number of pups represented by offspring sex in each group is control (12 F, 15 M), 500 µg FM 550 (13 F, 12 M), 1000 µg FM 550 (11 F, 12 M), 2000 µg FM 550 (8 F, 13 M), 5MT (8 F, 15 M).
Tissue Collection
Animals were euthanized by transcardial perfusion and the brains removed, cryoprotected, and flash frozen as previously described (58) on PNDs 77-85. Brains were cut coronally until the caudal portion of the ventromedial nucleus was reached and then cut sagittally. We did not have to control for female estrous cycle when animals were sacrificed because prairie voles are spontaneous ovulators. Brains were sectioned at 40 µm on a frozen sliding microtome. For each region of interest (ROI) a 1:4 series of sections for each individual was collected and processed for immunohistochemistry. The sagittal sections were primarily used for another study, but remaining VTA sections were used herein. All other ROIs were selected from the coronal sections. All antibodies are validated by the Antibody Registry (https://antibodyregistry.org/) and are listed in Table 1.
Table 1.
Antibodies used
| Peptide/Protein target | Name of antibody | Catalog number, manufacturer, lot number, RRID | Species raised in monoclonal or polyclonal | Dilution used |
|---|---|---|---|---|
| OT | Anti-oxytocin, clone 4G11 | MAB5296; Chemicon; 1979939; AB_2157626 | Mouse monoclonal | 1:10 000 |
| AVP | Anti-AVP antibody | 20069; Immnostar; 1004001; AB_572219 | Rabbit polyclonal | 1:5000 |
| TH | Anti-tyrosine hydroxylase antibody | AB152; Millipore, 1994651; AB_390204 | Rabbit polyclonal | 1:4000 |
OT and AVP Labeling in the PVN
For each animal, 7 consecutive PVN sections were immunolabeled for OT and AVP using routine procedures described previously (58, 77). Sections were washed in cold potassium phosphate buffer solution (KPBS) and preincubated for 24 hours in 0.02 M KPBS, 0.3% Triton X-100, and 2% normal donkey serum at 4°C. Sections were then incubated in a primary antibody cocktail consisting of 1:10 000 monoclonal mouse anti-OT (MAB5296; Millipore) and 1:5000 polyclonal rabbit anti-AVP (20069; Immunostar) for 72 hours on a shaker at 4°C. Sections were washed then incubated in a cocktail of Alexa Fluor 555 donkey antimouse (Invitrogen; A21206) and Alexa Fluor 488 donkey antirabbit (Invitrogen; A31570) secondary antibodies (both at 1:200) for 90 minutes. After a final series of washes, sections were mounted on Fisher Superfrost Plus glass slides (catalog number 12-550-15), coverslipped with a glycerol mountant, and stored at −20°C.
TH Labeling in the PVN, pBNST, and VTA
For each individual, 4 caudal PVN, 3 pBNST, and 6 VTA, sections were immunolabeled for TH and counterstained with Hoescht as described previously (58, 77). Sections were washed then preincubated for 24 hours in 0.02 M KPBS, 0.3% Triton X-100, and 2% normal donkey serum at 4°C. Sections were then incubated with the polyclonal rabbit anti-TH (catalog number AB152; Millipore) primary antibody at 1:4000 for 72 hours on a shaker at 4°C. After a series of washes they were then incubated with the Alexa Fluor 488 donkey antirabbit secondary antibody at 1:200 for 120 minutes, washed, and counterstained with Hoechst (catalog number H3569; Invitrogen Life Technologies) for 45 seconds. After a final round of washes in cold KPBS, sections were mounted on Fisher super frost plus glass slides, coverslipped with a glycerol mountant, and stored at −20°C.
Region of Interest Identification and Quantification
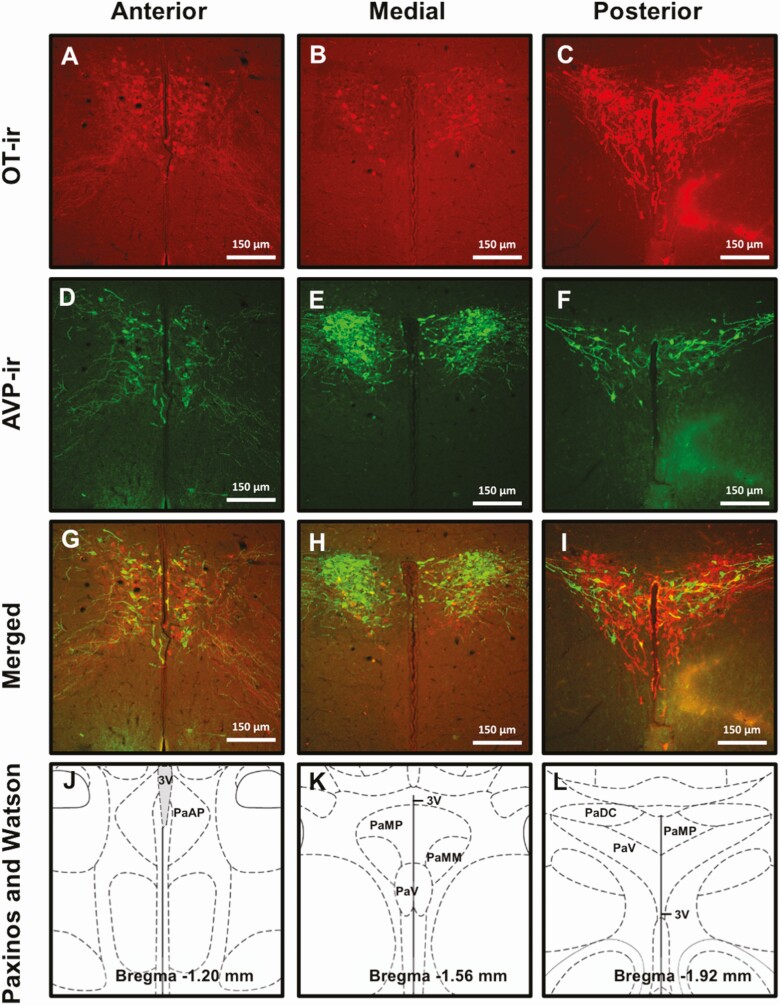
As done previously by our laboratory and others using prairie voles, we used the Paxinos and Watson rat brain atlas to identify the ROIs and surrounding, defining landmarks (58, 78). As we and others have previously done for prairie voles (58, 79), for quantification of OT and AVP labeling, PVN sections were subcategorized into anterior, medial, and posterior subregions (Fig. 1). The 2 anterior sections corresponded with Bregma –1.08 to –1.32 mm, the 3 medial sections corresponded to Bregma –1.44 to –1.80 mm, and the single posterior section corresponded to Bregma –1.92 mm. Two sections corresponding to Bregma –1.08 to –1.20 mm were selected for SON quantification. A single section was selected for quantification of TH-ir in the region containing A13 dopamine neurons (Bregma –2.28 mm), the STMAM and STAML region of the BNST (Bregma –0.48 mm), and the VTA (lateral 0.90 mm). All sections were imaged using a fluorescent Leica DM5500 Q scope and OT-ir, AVP-ir, and TH-ir neurons were counted unilaterally using the cell counter plug-in for Image J. In brain subregions with more than 1 section, the number of neurons was averaged for each animal.
Figure 1.
Representative images of each PVN region of interest (ROI) immunolabeled for OT (A-C), AVP (D-F), and both (G-I). Corresponding illustrations adapted from the Paxinos and Watson rat brain atlas depicting the landmarks and features that identify each subregion: anterior (Bregma –1.08 to –1.32 mm), medial (Bregma –1.44 to –1.80 mm), and posterior (Bregma –1.92 mm) (J-L). Scale bars, 1 μm. PaAP, paraventricular nucleus of the hypothalamus anterior parvicellular; PaDC, paraventricular nucleus of the hypothalamus dorsal cap; PaMP, paraventricular nucleus of the hypothalamus medial parvicellular; PaMM, paraventricular hypothalamic nucleus, medial magnocellular part; PaV, paraventricular nucleus ventral portion; 3V, third ventricle.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 8.4.2 (La Jolla, CA, USA). A ROUT outliers test (Q = 1%) was used to identify and remove statistical outliers. Only 1 outlier was identified and removed from the 1000 µg (n = 9) and 2000 µg (n = 10) FM 550 males for the AVP-ir neurons within the anterior PVN. The statistical results were the same with or without the outliers included thus removal did not affect the conclusions. For all analyses, statistical significance was defined as α ≤ .05. The statistical approach was specifically designed to test planned comparisons and minimize false positives. Unexposed males and females were compared using a student’s 1-tailed t-test to check for known and hypothesized sex differences in brain regions known or suspected to be sexually dimorphic (ex. TH-ir neurons in the BNST). Confirmation of known sex differences was considered assurance that the study was sufficiently powered to detect biologically meaningful differences. In brain regions not known to be sexually dimorphic, control males and females were compared using a 2-tailed t-test to test for potential sex differences. Only if there was a sex difference within the controls were follow up t-tests then used to probe if sex differences remained within an exposure group. Average cell numbers per region were evaluated within sex by 1-way analysis of variance (ANOVA) to test for a main effect of FM 550 exposure and followed up with a Fisher least significant difference post hoc test for multiple comparisons when significant. The 5MT group was considered separately from the FM 550 groups because it was used as a positive control for neural effects, thus inclusion with the FM 550 groups would bias the ANOVA. A 2-tailed t-test was used to compare 5MT and unexposed controls of the same sex. For each outcome, as recommended by multiple behavioral neuroscience groups including the American Psychological Association in the 5th edition of their manual effect size was calculated. ANOVA effect size was determined by Eta squared (η2). Effects are defined as small at 0.01, medium at 0.06, and large at 0.14. The t-test effect size was calculated by Cohen’s d, which is defined as small at 0.2, medium at 0.5, and large at 0.81. If the ANOVA was significant or the effect size was medium or large, a follow-up linear trend test was used to assess potential dose responsivity for the FM 550 groups. For each endpoint, post hoc tests (Fisher’s least significant difference) were also performed when effect sizes were medium or large to gain more information about potentially meaningful effects for future and further study.
Results
Oxytocin
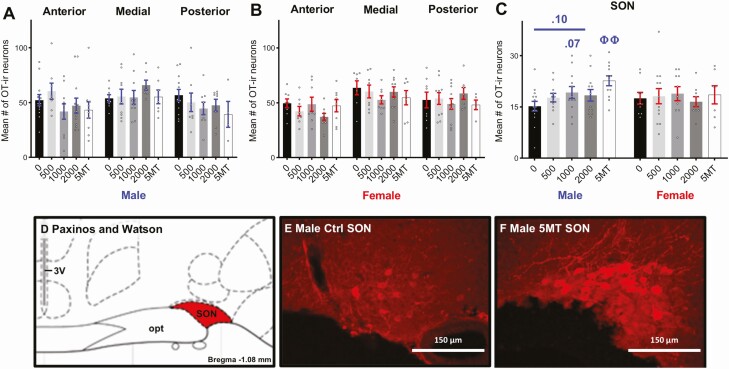
No baseline sex differences in OT-ir neuron numbers were detected in any subregion of the PVN or the SON (Fig. 2A-2C). Exposure to FM 550 and 5MT had no significant effects on OT-ir neuron numbers in any subregion of the PVN for either sex (Fig. 2A and 2B). No significant differences in OT-ir neurons numbers in the SON were found in FM 550 or 5MT females (Fig. 2C). Although not statistically significant, FM 550 exposure was suggested to have an increasing effect on SON OT-ir neurons in males (Fig. 2C, F3,45 = 1.35, P = .27, η 2 = 0.08) with a medium effect size. Follow-up analyses suggested that 1000 µg FM 550 males had more OT-ir neurons than controls (P = .07, d = 0.71) and there was a dose-dependent increase in OT-ir neurons in male SONs (Fig. 2C, F1,45 = 2.87, P = .10), although neither finding reached statistical significance. Males exposed to 5MT had significantly more OT-ir neurons in the SON than controls (Fig. 2C, 2E, and 2F, P ≤ .001, d = 1.43).
Figure 2.
No significant effects of FM 550 exposure on OT-ir neurons in the PVN (A-B) or SON (C) were observed in either sex. Males exposed to 5MT had significantly more OT-ir neurons in the SON than control males (C). Illustration adapted from the Paxinos and Watson rat brain atlas depicting the landmarks used to identify the SON (Bregma –1.08 to –1.20 mm) (D). Representative images depicting increased OT-ir neuron numbers in the 5MT male SON (F) compared with control males (E). Scale bars, 150 μm. 3V, third ventricle, SON supraoptic nucleus, opt, optic tract. Bar graphs depict mean ± SEM; individual animals are depicted by circles. Significant differences due to exposure of 5MT denoted by ΦΦ (P ≤ .01).
Vasopressin
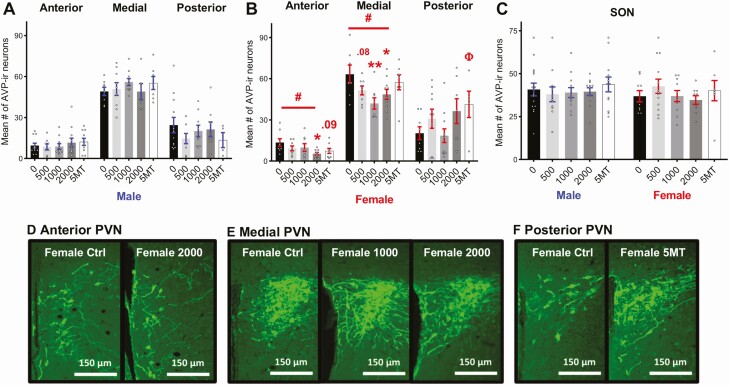
No baseline sex differences in AVP-ir neuron number were detected in any PVN subregion or the SON (Fig. 3A-3C). Effects of FM 550 on AVP-ir neuron numbers in the PVN were sex specific, and significant findings were only observed in females (Fig. 3B). A main effect of FM 550 exposure on female AVP-ir neurons in the anterior PVN was suggested by a large effect size (Fig. 3B, F3,29 = 2.38, P = .09, η 2 = 0.20) but did not quite reach statistical significance. Post hoc testing found a dose-dependent decrease in anterior PVN AVP-ir neurons in FM 550 females (Fig. 3B, F1,29 = 6.12, P ≤ .02) with females in the highest dose group having significantly fewer AVP-ir neurons (Fig. 3B, P ≤ .01, d = 1.40) than controls. A significant effect of FM 550 exposure on female AVP-ir neurons was detected in the medial PVN (Fig. 3B, F3,29 = 3.72, P ≤ .02, η 2 = 0.28) with a dose-dependent decrease (F1,29 = 6.21, P ≤ .02). Fewer AVP-ir neurons in were found in the medial PVN of 1000 µg (Fig. 2B, P ≤ .002, d = 1.44) and 2000 µg (P ≤ .02, d = 1.03) FM 550 females than in controls. Females exposed to 500 µg of FM 550 also had fewer AVP-ir neurons in the medial PVN, although not statistically significant (Fig. 3B, P = .08, d = 0.86). No significant effects of FM 550 exposure were observed in the posterior PVN of either sex.
Figure 3.
No significant effects of FM 550 or 5MT exposure were observed on AVP-ir neuron numbers in male PVN (A) and SON (C). Dose dependent decreases in AVP-ir neuron numbers were observed in the anterior and medial PVN in FM 550 females (B). 5MT females had more AVP-ir neurons in the posterior PVN than control females (B). No effect of FM 550 or 5MT exposure was observed in female SON. Representative images depicting decreased AVP-ir neuron numbers in anterior (A) and medial (B) PVN of FM 550 females and increased AVP-ir neuron numbers in 5MT females (F) when compared to control females. Scale bars, 150 μm. Bar graphs depict mean ± SEM; individual animals are depicted by circles. Significant differences due to FM 550 exposure denoted by * and Φ for 5MT. #Dose–response effect within sex (*, #, and Φ P ≤ .05 and ⁎⁎P ≤ .01).
Sex-specific effects of 5MT exposure on AVP-ir neurons in the PVN were also only observed in females (Fig. 3A and 3B). The large effect size suggested 5MT females had fewer AVP-ir neurons in the anterior PVN (Fig. 3B, P = .09, d = 0.91) than controls. No significant differences were detected in the medial PVN but 5MT females had more AVP-ir neurons in the posterior PVN than controls (Fig. 3B, P ≤ .05, d = 0.91). No significant effects of FM 550 or 5MT exposure on AVP-ir neurons in the SON were detected for either sex (Fig. 3C).
Tyrosine Hydroxylase
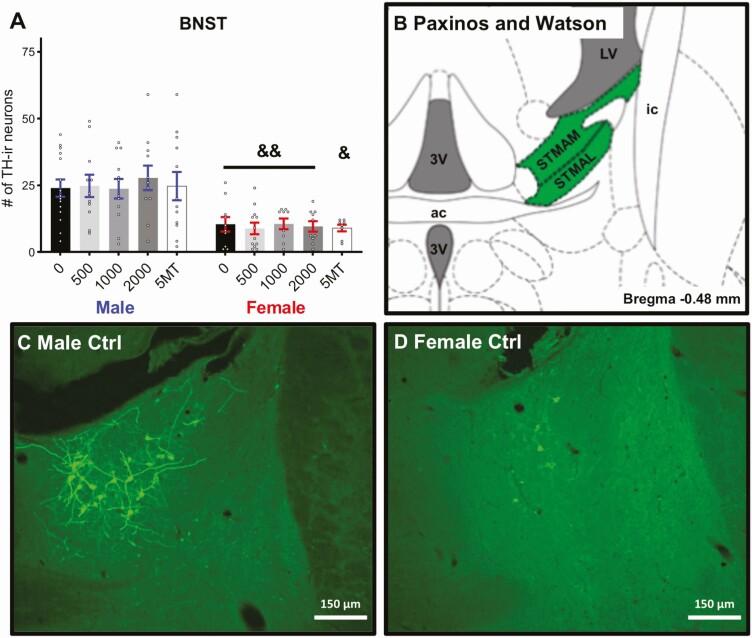
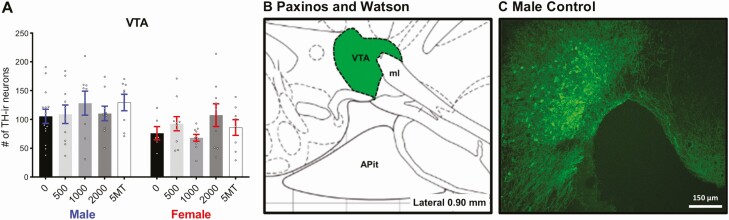
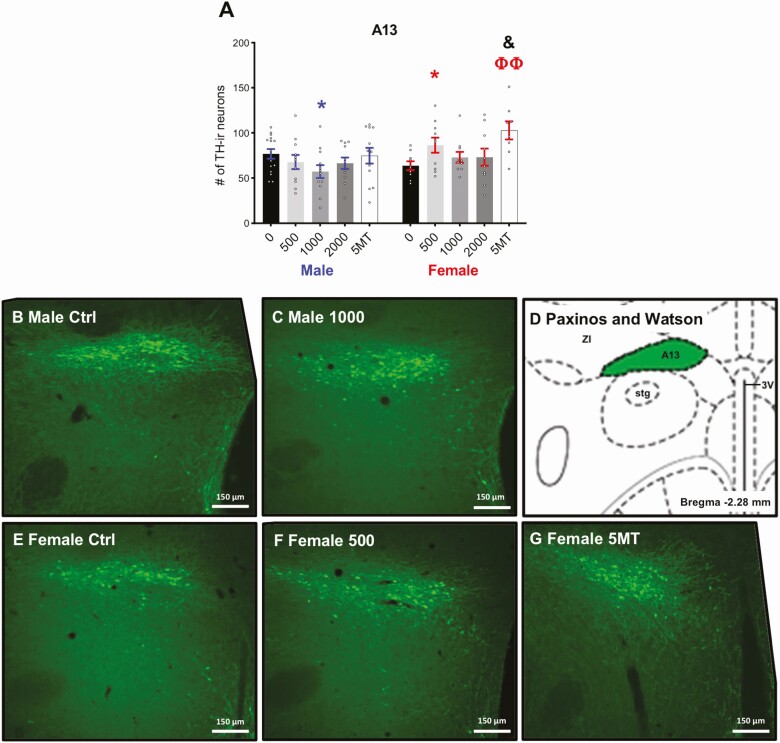
As expected, there was a significant sex difference in the number of TH-ir neurons in the anteromedial and anterolateral part of the medial division of the BNST (Fig. 4A and 4B, P ≤ .003, d = 1.44), with control males having more than control females. No sex difference was detected in the VTA between control males and females (Fig. 5A, P = .17, d = 0.70). There were no significant effects of FM 550 or 5MT exposure on TH-ir neuron numbers in the medial BNST or VTA for either sex. The sex difference in the number of TH-ir neurons of the BNST were maintained in all exposure groups. A baseline sex difference in TH-ir neurons in the A13 region just posterior to the PVN was suggested by a medium effect size (Fig. 6A, P = .11, d = 0.70) with control males having more than control females. An effect of FM 550 exposure in females was suggested by a medium effect size (Fig. 6A, F3, 35 = 1.52, P = .23, η 2 = 0.12). Post hoc testing found 500 µg FM 550 females had significantly more TH-ir neurons (Fig. 6A, P ≤ .04, d = 1.05) than control females. Within males, an overall effect of FM 550 exposure on TH-ir neurons in the A13 region was also only suggestive by a medium effect size (Fig. 6A, F = 1.61, P = .20, η 2 = 0.10) with 1000 µg FM 550 males having significantly fewer TH-ir neurons than controls (Fig. 6A, P ≤ .03, d = 0.87). Medium effect sizes within the 500 µg (P = .12, d = 0.74) and 1000 µg (P = .12, d = 0.70) FM 550 groups implied a reversal of the baseline sex difference, with exposed females having more TH-ir neurons in the A13 region than exposed males. Females exposed to 5MT had significantly more TH-ir neurons in the A13 region than control females (Fig. 6A, P ≤ .002, d = 1.78) and no significant differences were observed in 5MT males. Within the 5MT dose group, exposed females had significantly more TH-ir neurons than exposed males (Fig. 6A, P ≤ .01, d = 0.95).
Figure 4.
Males had significantly more TH-ir neurons in the BNST than females (A) and no significant effects of FM 550 or 5MT were observed in either sex. Bar graphs depict mean ± SEM; individual animals are depicted by circles. An illustration adapted from the Paxinos and Watson rat brain atlas depicting the landmarks used to identify the BNST (Bregma –0.48 mm) (B). Representative images depicting more AVP-ir neuron numbers in males (A) than females (D). Scale bars, 150 μm. Bar graphs depict mean ± SEM; individual animals are depicted by circles. Significant difference between sexes within exposure indicated by & (&&P ≤ .01). 3V, 3rd ventricle; ac, anterior commissure; STMAM, bed nucleus of the stria terminalis, medial division, anteromedial part; STMAL, bed nucleus of the stria terminalis, medial division, anterolateral part; LVe, lateral vestibular nucleus; ic, internal capsule.
Figure 5.
No significant effects of FM 550 or 5MT exposure were observed on TH-ir neuron numbers in VTA (A). An illustration adapted from the Paxinos and Watson rat brain atlas depicting the landmarks used to identify the VTA (Lateral 0.90 mm) (B). Representative image of TH-ir neurons in the VTA (C). Scale bars, 150 μm. Bar graphs depict mean ± SEM; individual animals are depicted by circles.
Figure 6.
Females exposed to 500 μg of FM 550 and 5MT had significantly more TH-ir A13 neurons than control females (A). There were fewer TH-ir neurons in 1000-µg males than in control males. Representative images depicting decreased TH-ir neuron numbers in the 1000-µg males (C) compared with control males (B) and increased TH-ir neuron numbers in the 500 µg FM 550 (F) and 5MT females (G) when compared with control females (E). A corresponding illustration adapted from the Paxinos and Watson rat brain atlas depicting the landmarks used to identify the BNST (Bregma –2.28 mm) (D). Bar graphs depict mean ± SEM; individual animals are depicted by circles. Significant differences due to FM 550 exposure denoted by * and Φ for 5MT. Significant difference between sexes within exposure indicated by & (* and & P ≤ .05; ΦΦ P ≤ .01). ZI, zona incerta; A13, A13 dopamine cells; Stg, sigmoid hypothalamic nucleus; 3V, 3rd ventricle.
Discussion
In concordance with our behavioral findings in sibling prairie voles (31), effects on neuron numbers examined were sex specific with greater and dose–responsive effects in females (summarized in Table 2). FM 550–exposed females had fewer AVP-ir neurons in the anterior and medial PVN. Furthermore 500 µg FM 550 females had fewer A13 TH-ir neurons within the ZI than controls. By contrast, in FM 550 males, A13 TH-ir neuron numbers in the ZI were decreased but only in the 1000-µg dose group. No significant impacts on OT-ir neuron numbers were detected in either sex. Although these results provide limited mechanistic insight regarding how FM 550 exposure induces social behavior deficits, the location of the disrupted population of AVP neurons suggests FM 550 may impact AVP control of peripheral functions.
Table 2.
Summary of significant neuroanatomical effects
| Sex | Male | Female | ||
|---|---|---|---|---|
| Endpoint | FM 550 | 5MT | FM 550 | 5MT |
| OT | — | ↑ OT-ir neurons in SON (5MT) | — | — |
| AVP | — | — | ↓ AVP-ir neurons in medial PVN (1000 µg) ↓ AVP-ir neurons in anterior/medial PVN (2000 µg) |
↑ AVP-ir neurons in posterior PVN (5MT) |
| TH | ↓ TH-ir A13 neurons in ZI (1000ug) | — | ↑ TH-ir A13 neurons in ZI (500 µg) | ↑ TH-ir A13 neurons in ZI (5MT) |
The most robust effect observed was a dose-dependent decrease in AVP-ir neurons in the anterior and medial PVN of exposed females. Notably, this is an area where AVP-ir neurons are most dense and numerous. In prairie voles, most OT/AVP neurons within these PVN regions have been characterized as magnocellular neurohypophysial neurons (79) with terminal projections in the posterior lobe of the pituitary where AVP is released into circulation to regulate osmolality. Thus, the data suggest possible dysregulation of AVP-mediated peripheral actions including antidiuretic and vasoconstrictive functions. Prior work using other FRs support this hypothesis. For example, exposure to a PBDE FR mixture (DE-71), significantly decreased somatodendritic AVP release from rat SON punches in response to dehydration (80), and developmental DE-71 exposure impaired osmoregulatory and cardiovascular responses to hyperosmotic stress in rats (13). Similarly, adult rats perinatally exposed to a mixture of PBDEs or PCBs failed to upregulate magnocellular AVP production in either the PVN or SON in response to prolonged hyperosmotic challenge (14). Organohalogens with other adverse effects on neurodevelopment, the PBDEs and PCBs are structurally similar with the former being brominated and the latter chlorinated. The PBDE and PCB data suggest that the brominated compounds in the FM 550 mixture may be responsible for the effects on AVP neuron numbers reported herein. Given that these brominated compounds are used for other applications and human exposure is widespread, further work should explore the potential for FM 550 and its components to alter peripheral vasopressinergic systems.
Effects of FM 550 exposure on TH-ir neurons were sex specific and dose specific, with effects only observed in the A13 DA neurons in the ZI. TH-ir neuron numbers were significantly increased in females exposed to 500 µg while males exposed to 1000 µg had fewer than their corresponding controls. The A13 DA neurons within this region have been shown to have descending DAergic projections to the mesencephalic locomotor region, which is an important area for initiating and modulating locomotion (64). In rats, they have also been shown to project to the medial preoptic area (MPOA), where they are thought to influence maternal and other social behaviors (81). Although we have previously reported a sex difference in prairie vole TH-ir neuron numbers in this region (58), with males having more DA neurons than females, reports of this sex difference in the literature are inconsistent (82). Here, only a suggestive sex-difference was observed in unexposed controls with males having more TH-ir neurons than females. This may result from use of only a single brain section to quantify A13 TH-ir neuron numbers, while our prior study included multiple sections that extended more rostrally (58). Similarly, although not statistically significant, FM 550 appears capable of reversing the sex difference. This effect would be consistent to what we have previously seen with bisphenol A where male voles exposed to 50 µg and 50 mg of bisphenol A had significantly less DA neurons in this region than females, reversing the sex difference seen in control animals (58).
Although we found no significant effects on VTA TH-ir neurons this was not entirely unexpected. Dopamine activity, rather than neuron number, is more likely to be environmentally vulnerable. A notable limitation is that because most available sagittal sections were used for a different study, limited sections remained for the present study, thus only a single section was used. This likely contributes to the large variance in neuron counts. Given the effects on the A13 DA neurons in the ZI reported herein, and that exposed sibling voles displayed disrupted pair formation, future work should further interrogate other regions of the mesolimbic dopamine system including the prefrontal cortex and the nucleus accumbens (NAcc), which facilitates pair bonding by modulating reward seeking behaviors (67).
Our findings support the hypothesis that the mesolimbic dopamine system may be a potential target for FM 550 exposure and are consistent with previous reports of FR disruption of DA circuits (83, 84). For example, the brominated FR hexabromocyclododecane (HBCD) targets the mesohippocampal dopamine circuit in male mice (85) which could potentially account for memory impairments associated with BFR exposure in humans (86-88). Dopamine uptake into synaptic vesicles has been shown to be inhibited by HBCD, tetrabromobisphenol A (TBBPA), pentaBDE (85), and PCBs (89). FM 550 has not been tested for this potential effect. In male prairie voles, amphetamine-induced inhibition of the formation of mating-induced partner preference is accompanied by altered mesocorticolimbic signaling with altered DA receptor expression in the NAcc (90).
Although PVN OT neuron numbers were unaltered in exposed females and no significant effects on OT/AVP neuron numbers were detected in exposed males, disruption within these pathways cannot be ruled out. Comparative studies across vole species with different degrees of social monogamy have found only small differences in the distribution of OT, AVP, and DA neurons compared with the location and densities of their respective receptors particularly in the NAcc, ventral pallidum, and lateral septum (LS) (91-94). Thus, quantification of OT and AVP receptor density is an obviously needed next step. Furthermore, other extrahypothalamic AVP neuron populations have been shown to regulate social behavior and cognitive functions, such as those in the BNST, suprachiasmatic nucleus, and medial nucleus of the amygdala (95, 96). AVP levels in these brain regions are known to be sexually dimorphic with males having more AVP expressing neurons in the BNST and amygdala, and more terminal projections to the LS than females. (97-100). The serotonin agonist, 5MT, was used as a positive control for effect because a range of socioemotional behaviors have been found to be impacted following developmental exposure in rats (101-104) and prairie voles (73). Here we found increased numbers of OT-ir neurons in the SON of 5MT males, AVP-ir neurons in the posterior PVN of 5MT females, and A13 TH-ir neurons in the ZI of 5MT females. Critically, a prior study using this same dose but a longer exposure window, found decreased numbers of OT-ir and AVP-ir neurons in the PVN following perinatal exposure in male prairie voles (73). We did not find any effects on OT-ir neurons in the PVN; however, 5MT females had fewer AVP-ir neurons in the anterior PVN but this outcome did not reach statistical significance. Surprisingly, we found no significant effects in the PVN of 5MT males, and thus did not replicate the previously reported finding. This is likely due to the shortened postnatal exposure period, which ceased on PND 7 and use of a different vehicle, ethanol, after discovering that 5MT does not fully dissolve in tonic saline. However, our results build and expand on the previous study by including females and providing additional evidence that developmental exposure to a 5-HT agonist disrupts OT and AVP systems outside of the PVN.
Conclusions
The results herein expand upon previous work by us and others in multiple species showing evidence of disrupted neurodevelopment and behavior following developmental exposure to FM 550 (33, 105-107), or its component classes (27, 34, 108-113). Exposure to FM 550 compounds, particularly the OPFRs, is widespread because they are also used as plasticizers in a wide variety of consumer products, including polyvinyl chloride products, and personal care products such as nail polish (45); thus developmental exposure is a major human health concern. This study also reinforces the utility of the prairie vole as a uniquely appropriate animal model to explore chemical exposure effects on neural circuits of relevance to prosocial behaviors not displayed by laboratory rats or mice. That the results suggest FM 550 may impact peripheral actions of AVP is compelling because it is consistent with effects produced by organohalogens structurally similar to the BFR components. Finally, additional follow-up studies will probe potential disruption of OT, AVP, and DA receptor expression levels in areas of relevance to prosocial behavior, and their interface with the mesolimbic dopamine pathway.
Acknowledgments
Financial Support: AR160055 to H.B.P., P30ES025128 to NCSU, R01ES016099 to H.M.S.
Glossary
Abbreviations
- ANOVA
analysis of variance
- ASD
autism spectrum disorder
- AVP
vasopressin
- BEH-TEBP
bis(2-ethylhexyl) 2,3,4,5- tetrabromophthalate
- BFR
brominated flame retardant
- BNST
bed nucleus of the stria terminalis
- BW
body weight
- DA, dopamine; EDC
endocrine disrupting chemical
- FR
flame retardant
- EH-TBB
2-ethylhexyl-2,3,4,5-tetrabromobenzoate
- GD
gestational day
- KPBS
potassium phosphate buffer solution
- ir
immunoreactivity
- NAcc
nucleus accumbens; MPOA, medial preoptic area
- NDD
neurodevelopmental disorder
- OPFR
organophosphate ester flame retardant
- OT
oxytocin
- PBDE
polybrominated diphenyl ether
- PND
postnatal day
- PVN
paraventricular nucleus
- ROI
region of interest
- SC
subcutaneous
- SON
supraoptic nucleus
- TH
tyrosine hydroxylase
- TPHP
triphenyl phosphate
- VTA
ventral tegmental area
- ZI
zona incerta
Additional Information
Disclosures: The authors have nothing to declare.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Dufour P, Charlier C. Brominated flame retardant: environmental and exposed individuals’ health impact. Ann Biol Clin (Paris). 2017;75(2):146-157. [DOI] [PubMed] [Google Scholar]
- 2. Xiong P, Yan X, Zhu Q, et al. A review of environmental occurrence, fate, and toxicity of novel brominated flame retardants. Environ Sci Technol. 2019;53(23):13551-13569. [DOI] [PubMed] [Google Scholar]
- 3. Patisaul HB. Endocrine disruption of vasopressin systems and related behaviors. Front Endocrinol (Lausanne). 2017;8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25(3-4):150-176. [DOI] [PubMed] [Google Scholar]
- 5. Baribeau DA, Anagnostou E. Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits. Front Neurosci. 2015;9:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649-659. [DOI] [PubMed] [Google Scholar]
- 7. Lee NS, Beery AK. The role of dopamine signaling in prairie vole peer relationships. Horm Behav. 2021;127:104876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walum H, Young LJ. The neural mechanisms and circuitry of the pair bond. Nat Rev Neurosci. 2018;19(11):643-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lieberwirth C, Wang Z. The neurobiology of pair bond formation, bond disruption, and social buffering. Curr Opin Neurobiol. 2016;40:8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El-Ghundi M, O’Dowd BF, George SR. Insights into the role of dopamine receptor systems in learning and memory. Rev Neurosci. 2007;18(1):37-66. [DOI] [PubMed] [Google Scholar]
- 11. Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol. 2011;32(1):53-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández M, Mollinedo-Gajate I, Peñagarikano O. Neural circuits for social cognition: implications for autism. Neuroscience. 2018;370:148-162. [DOI] [PubMed] [Google Scholar]
- 13. Shah A, Coburn CG, Watson-Siriboe A, et al. Altered cardiovascular reactivity and osmoregulation during hyperosmotic stress in adult rats developmentally exposed to polybrominated diphenyl ethers (PBDEs). Toxicol Appl Pharmacol. 2011;256(2):103-113. [DOI] [PubMed] [Google Scholar]
- 14. Mucio-Ramírez S, Sánchez-Islas E, Sánchez-Jaramillo E, et al. Perinatal exposure to organohalogen pollutants decreases vasopressin content and its mRNA expression in magnocellular neuroendocrine cells activated by osmotic stress in adult rats. Toxicol Appl Pharmacol. 2017;329:173-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landrigan PJ, Lambertini L, Birnbaum LS. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect. 2012;120(7):a258-a260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Messer A. Mini-review: polybrominated diphenyl ether (PBDE) flame retardants as potential autism risk factors. Physiol Behav. 2010;100(3):245-249. [DOI] [PubMed] [Google Scholar]
- 17. Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vuong AM, Yolton K, Cecil KM, Braun JM, Lanphear BP, Chen A. Flame retardants and neurodevelopment: An updated review of epidemiological literature. Curr Epidemiol Rep. 2020;7(4):220-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics. 2011;127(6):1034-1042. [DOI] [PubMed] [Google Scholar]
- 20. Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Curr Probl Pediatr Adolesc Health Care. 2014;44(10):277-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moosa A, Shu H, Sarachana T, Hu VW. Are endocrine disrupting compounds environmental risk factors for autism spectrum disorder? Horm Behav. 2018;101:13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chun H, Leung C, Wen SW, McDonald J, Shin HH. Maternal exposure to air pollution and risk of autism in children: A systematic review and meta-analysis. Environ Pollut. 2020;256:113307. [DOI] [PubMed] [Google Scholar]
- 23. Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: Current findings and future directions. Horm Behav. 2018;101:94-104. [DOI] [PubMed] [Google Scholar]
- 24. Rebuli ME, Patisaul HB. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. J Steroid Biochem Mol Biol J Steroid Biochem. 2015;160:148-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sealey LA, Hughes BW, Sriskanda AN, et al. Environmental factors in the development of autism spectrum disorders. Environ Int. 2016;88:288-298. [DOI] [PubMed] [Google Scholar]
- 26. Dorman DC, Chiu W, Hales BF, et al. Polybrominated diphenyl ether (PBDE) neurotoxicity: a systematic review and meta-analysis of animal evidence. J Toxicol Environ Health B Crit Rev. 2018;21(4):269-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blum A, Behl M, Birnbaum L, et al. Organophosphate ester flame retardants: are they a regrettable substitution for polybrominated diphenyl ethers? Environ Sci Technol Lett. 2019;6(11):638-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stapleton HM, Sharma S, Getzinger G, et al. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46(24):13432-13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119-1153. [DOI] [PubMed] [Google Scholar]
- 30. Stapleton HM, Allen JG, Kelly SM, et al. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol. 2008;42(18):6910-6916. [DOI] [PubMed] [Google Scholar]
- 31. Gillera SEA, Marinello WP, Horman BM, et al. Sex-specific effects of perinatal FireMaster® 550 (FM 550) exposure on socioemotional behavior in prairie voles. Neurotoxicol Teratol. 2020;79:106840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patisaul HB, Roberts SC, Mabrey N, et al. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27(2):124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baldwin KR, Phillips AL, Horman B, et al. Sex specific placental accumulation and behavioral effects of developmental Firemaster 550 exposure in Wistar rats. Sci Rep. 2017;7(1):7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wiersielis KR, Adams S, Yasrebi A, Conde K, Roepke TA. Maternal exposure to organophosphate flame retardants alters locomotor and anxiety-like behavior in male and female adult offspring. Horm Behav. 2020;122:104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castorina R, Bradman A, Stapleton HM, et al. Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere. 2017;189:574-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phillips AL, Hammel SC, Konstantinov A, Stapleton HM. Characterization of individual isopropylated and tert-butylated triarylphosphate (ITP and TBPP) isomers in several commercial flame retardant mixtures and house dust standard reference material SRM 2585. Environ Sci Technol. 2017;51(22):13443-13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim UJ, Wang Y, Li W, Kannan K. Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the United States. Environ Int. 2019;125:342-349. [DOI] [PubMed] [Google Scholar]
- 38. Ospina M, Jayatilaka NK, Wong LY, Restrepo P, Calafat AM. Exposure to organophosphate flame retardant chemicals in the U.S. general population: Data from the 2013-2014 National Health and Nutrition Examination Survey. Environ Int. 2018;110:32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gibson EA, Stapleton HM, Calero L, et al. Differential exposure to organophosphate flame retardants in mother-child pairs. Chemosphere. 2019;219:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoffman K, Fang M, Horman B, et al. Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster® 550. Environ Health Perspect. 2014;122(9):963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeng Y, Pan W, Ding N, et al. Brominated flame retardants in home dust and its contribution to brominated flame retardants bioaccumulation in children hair. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2020;55(13):1528-1533. [DOI] [PubMed] [Google Scholar]
- 42. Chupeau Z, Bonvallot N, Mercier F, Le Bot B, Chevrier C, Glorennec P. Organophosphorus flame retardants: a global review of indoor contamination and human exposure in Europe and epidemiological evidence. Int J Environ Res Public Health. 2020;17(18):6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hu L, Luo D, Wang L, et al. Levels and profiles of persistent organic pollutants in breast milk in China and their potential health risks to breastfed infants: A review. Sci Total Environ. 2021;753:142028. [DOI] [PubMed] [Google Scholar]
- 44. Hammel SC, Zhang S, Lorenzo AM, Eichner B, Stapleton HM, Hoffman K. Young infants’ exposure to organophosphate esters: Breast milk as a potential source of exposure. Environ Int. 2020;143:106009. [DOI] [PubMed] [Google Scholar]
- 45. Mendelsohn E, Hagopian A, Hoffman K, et al. Nail polish as a source of exposure to triphenyl phosphate. Environ Int. 2016;86:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Estill CF, Slone J, Mayer A, Chen IC, La Guardia MJ. Worker exposure to flame retardants in manufacturing, construction and service industries. Environ Int. 2020;135:105349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Witchey SK, Al Samara L, Horman BM, Stapleton HM, Patisaul HB. Perinatal exposure to FireMaster® 550 (FM550), brominated or organophosphate flame retardants produces sex and compound specific effects on adult Wistar rat socioemotional behavior. Horm Behav. 2020;126:104853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dishaw LV, Macaulay LJ, Roberts SC, Stapleton HM. Exposures, mechanisms, and impacts of endocrine-active flame retardants. Curr Opin Pharmacol. 2014;19:125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnson ZV, Young LJ. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev. 2017;76(Pt A):87-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McGraw LA, Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 2010;33(2):103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Young LJ. The Neural Basis of Pair Bonding in a Monogamous Species: A Model for Understanding the Biological Basis of Human Behavior. In: National Research Council (US) Panel for the Workshop on the Biodemography of Fertility and Family Behavior. National Academies Press (US); 2003:4. [Google Scholar]
- 52. Aragona BJ, Liu Y, Yu YJ, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9(1):133-139. [DOI] [PubMed] [Google Scholar]
- 53. Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109(4):782-789. [DOI] [PubMed] [Google Scholar]
- 54. Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545-548. [DOI] [PubMed] [Google Scholar]
- 55. Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400(6746):766-768. [DOI] [PubMed] [Google Scholar]
- 56. Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm Behav. 2012;61(3):340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rebuli ME, Gibson P, Rhodes CL, Cushing BS, Patisaul HB. Sex differences in microglial colonization and vulnerabilities to endocrine disruption in the social brain. Gen Comp Endocrinol. 2016;238:39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sullivan AW, Beach EC, Stetzik LA, et al. A novel model for neuroendocrine toxicology: neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster). Endocrinology. 2014;155(10):3867-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471(4):396-433. [DOI] [PubMed] [Google Scholar]
- 60. Northcutt KV, Wang Z, Lonstein JS. Sex and species differences in tyrosine hydroxylase-synthesizing cells of the rodent olfactory extended amygdala. J Comp Neurol. 2007;500(1):103-115. [DOI] [PubMed] [Google Scholar]
- 61. Northcutt KV, Lonstein JS. Neuroanatomical projections of the species-specific tyrosine hydroxylase-immunoreactive cells of the male prairie vole bed nucleus of the stria terminalis and medial amygdala. Brain Behav Evol. 2011;77(3):176-192. [DOI] [PubMed] [Google Scholar]
- 62. Been LE, Petrulis A. Chemosensory and hormone information are relayed directly between the medial amygdala, posterior bed nucleus of the stria terminalis, and medial preoptic area in male Syrian hamsters. Horm Behav. 2011;59(4):536-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. J Neuroendocrinol. 1994;6(4):433-442. [DOI] [PubMed] [Google Scholar]
- 64. Sharma S, Kim LH, Mayr KA, Elliott DA, Whelan PJ. Parallel descending dopaminergic connectivity of A13 cells to the brainstem locomotor centers. Sci Rep. 2018;8(1):7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Messanvi F, Eggens-Meijer E, Roozendaal B, van der Want JJ. A discrete dopaminergic projection from the incertohypothalamic A13 cell group to the dorsolateral periaqueductal gray in rat. Front Neuroanat. 2013;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fougère M, Flaive A, Frigon A, Ryczko D. Descending dopaminergic control of brainstem locomotor circuits. Curr Opin Physiol. 2019;8:30-35. [Google Scholar]
- 67. Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Curr Opin Behav Sci. 2015;3:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother. 2010;1(2):94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Patisaul HB, Todd KL, Mickens JA, Adewale HB. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology. 2009;30(3):350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li AA, Baum MJ, McIntosh LJ, Day M, Liu F, Gray LE Jr. Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology. 2008;29(3):504-519. [DOI] [PubMed] [Google Scholar]
- 71. National Research Council (U.S.). Subcommittee on Laboratory Animal Nutrition. Nutrient Requirements of Laboratory Animals. 4th ed. National Academy of Sciences. [Google Scholar]
- 72. Baldwin KR, Horman B, Phillips AL, et al. EDC IMPACT: molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain. Endocr Connect. 2018;7:305-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Martin MM, Liu Y, Wang Z. Developmental exposure to a serotonin agonist produces subsequent behavioral and neurochemical changes in the adult male prairie vole. Physiol Behav. 2012;105(2):529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McNamara IM, Borella AW, Bialowas LA, Whitaker-Azmitia PM. Further studies in the developmental hyperserotonemia model (DHS) of autism: social, behavioral and peptide changes. Brain Res. 2008;1189:203-214. [DOI] [PubMed] [Google Scholar]
- 75. Edwards KA, Madden AMK, Zup SL. Serotonin receptor regulation as a potential mechanism for sexually dimorphic oxytocin dysregulation in a model of Autism. Brain Res. 2018;1701:85-92. [DOI] [PubMed] [Google Scholar]
- 76. Knudsen GA, Sanders JM, Birnbaum LS. Disposition of the emerging brominated flame retardant, bis(2-ethylhexyl) tetrabromophthalate, in female Sprague Dawley rats: effects of dose, route and repeated administration. Xenobiotica. 2017;47(3):245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Adewale HB, Todd KL, Mickens JA, Patisaul HB. The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2011;32(1):38-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502(6):1109-1122. [DOI] [PubMed] [Google Scholar]
- 79. Ross HE, Cole CD, Smith Y, et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Coburn CG, Currás-Collazo MC, Kodavanti PRS. Polybrominated diphenyl ethers and ortho-substituted polychlorinated biphenyls as neuroendocrine disruptors of vasopressin release: effects during physiological activation in vitro and structure–Activity relationships. Toxicol Sci. 2007;98(1):178-186. [DOI] [PubMed] [Google Scholar]
- 81. Miller SM, Lonstein JS. Dopaminergic projections to the medial preoptic area of postpartum rats. Neuroscience. 2009;159(4):1384-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lansing SW, Lonstein JS. Tyrosine hydroxylase-synthesizing cells in the hypothalamus of prairie voles (Microtus ochrogaster): sex differences in the anteroventral periventricular preoptic area and effects of adult gonadectomy or neonatal gonadal hormones. J Neurobiol. 2006;66(3):197-204. [DOI] [PubMed] [Google Scholar]
- 83. Genskow KR, Bradner JM, Hossain MM, Richardson JR, Caudle WM. Selective damage to dopaminergic transporters following exposure to the brominated flame retardant, HBCDD. Neurotoxicol Teratol. 2015;52(Pt B):162-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Oliveri AN, Ortiz E, Levin ED. Developmental exposure to an organophosphate flame retardant alters later behavioral responses to dopamine antagonism in zebrafish larvae. Neurotoxicol Teratol. 2018;67:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pham-Lake C, Aronoff EB, Camp CR, Vester A, Peters SJ, Caudle WM. Impairment in the mesohippocampal dopamine circuit following exposure to the brominated flame retardant, HBCDD. Environ Toxicol Pharmacol. 2017;50:167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chevrier C, Warembourg C, Le Maner-Idrissi G, et al. Childhood exposure to polybrominated diphenyl ethers and neurodevelopment at six years of age. Neurotoxicology. 2016;54:81-88. [DOI] [PubMed] [Google Scholar]
- 87. Cowell WJ, Lederman SA, Sjödin A, et al. Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3-7 years. Neurotoxicol Teratol. 2015;52(Pt B):143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen A, Yolton K, Rauch SA, et al. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: the HOME study. Environ Health Perspect. 2014;122(8):856-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159(1-2):11-21. [DOI] [PubMed] [Google Scholar]
- 90. Liu Y, Aragona BJ, Young KA, et al. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc Natl Acad Sci U S A. 2010;107(3):1217-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366(4):726-737. [DOI] [PubMed] [Google Scholar]
- 93. Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89(13):5981-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14(9):5381-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25(9):1565-1574. [DOI] [PubMed] [Google Scholar]
- 96. Ring RH. The central vasopressinergic system: examining the opportunities for psychiatric drug development. Curr Pharm Des. 2005;11(2):205-225. [DOI] [PubMed] [Google Scholar]
- 97. De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273(2):307-317. [DOI] [PubMed] [Google Scholar]
- 98. De Vries GJ, Best W, Sluiter AA. The influence of androgens on the development of a sex difference in the vasopressinergic innervation of the rat lateral septum. Brain Res. 1983;284(2-3):377-380. [DOI] [PubMed] [Google Scholar]
- 99. Vries GJ. Sex differences in neurotransmitter systems. J Neuroendocrinol. 1990;2(1):1-13. [DOI] [PubMed] [Google Scholar]
- 100. Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav Neurosci. 2001;115(4):910-919. [DOI] [PubMed] [Google Scholar]
- 101. Whitaker-Azmitia PM, Shemer AV, Caruso J, Molino L, Azmitia EC. Role of high affinity serotonin receptors in neuronal growth. Ann N Y Acad Sci. 1990;600:315-330. [DOI] [PubMed] [Google Scholar]
- 102. Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23(1):75-83. [DOI] [PubMed] [Google Scholar]
- 103. Kahne D, Tudorica A, Borella A, et al. Behavioral and magnetic resonance spectroscopic studies in the rat hyperserotonemic model of autism. Physiol Behav. 2002;75(3):403-410. [DOI] [PubMed] [Google Scholar]
- 104. Shemer AV, Azmitia EC, Whitaker-Azmitia PM. Dose-related effects of prenatal 5-methoxytryptamine (5-MT) on development of serotonin terminal density and behavior. Brain Res Dev Brain Res. 1991;59(1):59-63. [DOI] [PubMed] [Google Scholar]
- 105. Rock KD, St Armour G, Horman B, et al. Effects of prenatal exposure to a mixture of organophosphate flame retardants on placental gene expression and serotonergic innervation in the fetal rat brain. Toxicol Sci. 2020;176(1):203-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bailey JM, Levin ED. Neurotoxicity of FireMaster 550® in zebrafish (Danio rerio): Chronic developmental and acute adolescent exposures. Neurotoxicol Teratol. 2015;52(Pt B):210-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rock KD, Horman B, Phillips AL, et al. EDC IMPACT: Molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain. Endocr Connect. 2018;7(2):305-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Adams S, Wiersielis K, Yasrebi A, et al. Sex- and age-dependent effects of maternal organophosphate flame-retardant exposure on neonatal hypothalamic and hepatic gene expression. Reprod Toxicol. 2020;94:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Krumm EA, Patel VJ, Tillery TS, et al. Organophosphate flame-retardants alter adult mouse homeostasis and gene expression in a sex-dependent manner potentially through interactions with ERα. Toxicol Sci. 2018;162(1):212-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Slotkin TA, Skavicus S, Stapleton HM, Seidler FJ. Brominated and organophosphate flame retardants target different neurodevelopmental stages, characterized with embryonic neural stem cells and neuronotypic PC12 cells. Toxicology. 2017;390:32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhang S, Ireland D, Sipes NS, Behl M, Collins ES. Screening for neurotoxic potential of 15 flame retardants using freshwater planarians. Neurotoxicol Teratol. 2019;73:54-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Behl M, Rice JR, Smith MV, et al. Editor’s Highlight: Comparative Toxicity of Organophosphate Flame Retardants and Polybrominated Diphenyl Ethers to Caenorhabditis elegans. Toxicol Sci. 2016;154(2):241-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Behl M, Hsieh JH, Shafer TJ, et al. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol Teratol. 2015;52(Pt B):181-193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.