Abstract
Background:
Several states opted to expand Medicaid under the Affordable Care Act (ACA), which offers insurance coverage to low-income individuals up to 138% of the federal poverty level. This expansion of Medicaid to a medically vulnerable population can potentially reduce cancer outcome disparities, especially in screening-amenable cancers. The study objective was to estimate the effect of Medicaid expansion on the proportion of adults from low-income communities with screening-amenable cancers who present with metastatic disease.
Methods:
Using state cancer registry data linked with block group-level income data, 12,760 individuals aged 30–64 years who were diagnosed with incident invasive breast (female), cervical, colorectal, or lung cancer in 2011 – 2016, and were uninsured or had Medicaid insurance at diagnosis were identified. This sample was probability weighted based on income to reflect potential Medicaid eligibility under the ACA’s Medicaid expansion. Then, a multivariable logistic model was fitted to examine the independent association between the exposure (pre-expansion, years 2011–2013 versus post-expansion, years 2014–2016) and the outcome (metastatic versus non-metastatic disease at diagnosis).
Results:
After adjusting for potential confounders, individuals diagnosed post-expansion had 15% lower odds of having metastatic disease compared to pre-expansion (Adjusted Odds Ratio: 0.85, 95% Confidence Interval: 0.77 – 0.93). As a control, a separate analysis that focused on individuals with private insurance from high-income communities found nonsignificant pre/post-expansion changes in the outcome (Adjusted Odds Ratio: 1.02, 95% Confidence Interval: 0.96 – 1.09).
Conclusions:
Medicaid expansion is associated with a narrowing of a critical cancer outcome disparity in adults from low-income communities.
Keywords: Patient Protection and Affordable Care Act, Medicaid, breast neoplasms, uterine cervical neoplasms, colorectal neoplasms, lung neoplasms, cancer staging
Introduction
Under the Affordable Care Act (ACA), Medicaid expansion was broadly implemented in 2014.1 The law simplified Medicaid eligibility criteria to a strictly income-based means test (≤138% of the federal poverty level2). As a result, states that opted to expand Medicaid have seen substantially increased enrollment,3 with the majority of new enrollees reporting being previously uninsured (voluntary private-public substitution with Medicaid has been rare4). Within the context of cancer care, researchers have documented numerous positive associations between Medicaid expansion and health care utilization and screening rates.5,6
To evaluate whether increased access to healthcare services and preventive care have translated to meaningful improvements in cancer control, researchers have begun to investigate the association between Medicaid expansion and stage-at-diagnosis. Initial studies have shown shifts toward early-stage diagnosis using models that investigate the most common cancers individually and combined.7–9 In general, these increases have been modest (varying from 0.4 to 3 percent) and of varying statistical significance. These findings may be partially explained by the limited study follow-up periods, which did not extend past 2014.7–9
In order to more fully understand the impact that Medicaid expansion has had on stage-at-diagnosis in screening-amenable cancers10–13 (breast, cervical, colorectal, and lung) among adults living in low-income communities (“low-income individuals”), we assessed the probability of having metastatic disease at diagnosis in the three years preceding (2011 – 2013) and following (2014 – 2016) Medicaid expansion. The study focuses on Ohio, a state that was part of the first wave of expansion in 2014,1 making evaluating Medicaid expansion’s impact on stage-at-diagnosis over a longer time period possible. Additionally, the burden of cancer in Ohio, both in incidence and mortality, is higher than the national average.14,15 For example, the mortality rates per 100,000 in Ohio are 23.0 (versus 21.2 nationally) for breast cancer, 2.4 (versus 2.3 nationally) for cervical cancer, 17.5 (versus 15.9 nationally) for colorectal cancer, and 55.2 (versus 47.2 nationally) for lung cancer.16–19 Therefore, the present study provides an opportunity to evaluate the potential ability of Medicaid expansion to mitigate cancer disparities, especially those associated with low-income populations.14 We hypothesized that among those who are potentially eligible for Medicaid under the ACA’s expansion, the probability of having metastatic disease at diagnosis decreased post-expansion (years 2014 – 2016) compared to the pre-expansion (years 2011 – 2013) period.
Materials and Methods
The study data was from NEO-CASE (Northeast Ohio Cancer Risk Assessment and Surveillance Engine), a cancer-focused multilevel data infrastructure previously developed by the authors. From NEO-CASE, we extracted a flat file that contained individual cancer cases (original source: OCISS, the Ohio Cancer Incidence Surveillance System), linked to census block group-level income data (original source: ACS, the American Community Survey of the US Census) using the patient’s geocoded address at diagnosis.
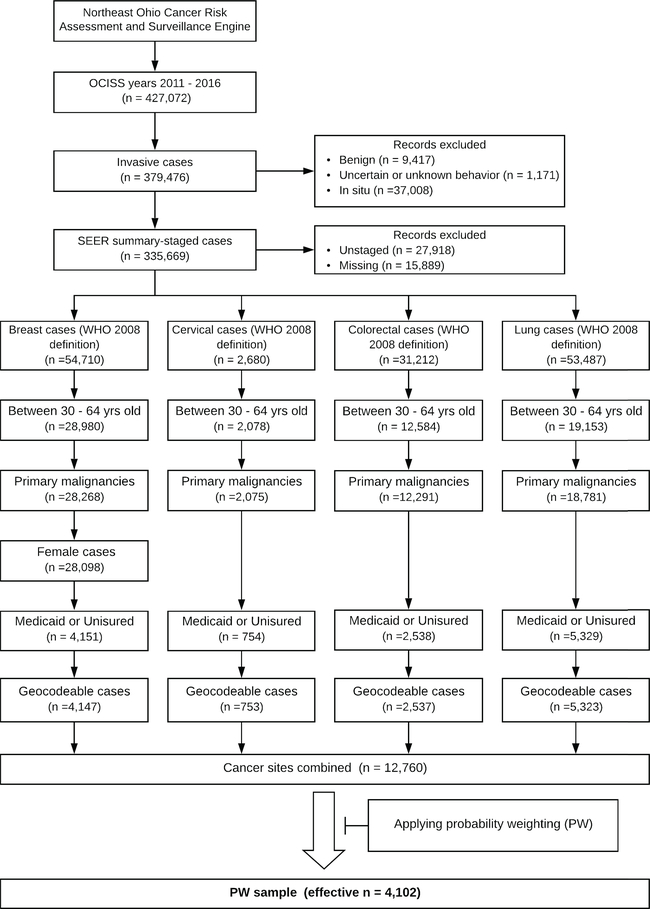
The study population included non-elderly adults from Ohio who were diagnosed with incident invasive breast (female), cervical, colorectal, or lung cancer in 2011 – 2016 and were uninsured or had Medicaid insurance at diagnosis (n = 12,772). Non-elderly adults in this study were defined as those between 30–64 years of age, to exclude adolescent and young adult cancer patients (commonly defined as those between 15 – 29 years of age20). Individuals with unstaged or unknown stage cancers and those who could not be geocoded were excluded. Within a given cancer site, if individuals had multiple tumor records, only the earliest record was kept (the presumed primary malignancy) to exclude records representing synchronous, metachronous, and recurrent tumors, leading to a sample of 12,760.
The OCISS, like most cancer registries,21 does not capture individual-level income, so studies generally approximate individual-level income using community-level income (for example, using zip-code or census tract-level data).7,8 In this study, we approximated individual-level income using income aPFPL (as percent of the federal poverty level) from the patient’s block group of residence (ACS table C17002). This income data, specifically the count of individuals in a given block group with incomes aPFPL ≤138, was used to probability weight the sample. Because income aPFPL ≤138 does not coincide with the pre-tabulated income categories of ACS table C17002 (Under 50, 50 to 99, 100 to 124, 125 to 149, 150 to 184, 185 to 199, or 200 and over), the count of individuals between income aPFPL 125 to 138 was linearly interpolated using an approach described elsewhere.22 This interpolation procedure assumes a flat distribution in the 125 to 149 income aPFPL category, an assumption that is supported by other census income data (see Appendix for further detail).
The sample was probability weighted to account for the fact that those living in low-income communities have the highest likelihood of being eligible for Medicaid under expansion, while at the same time, those living in more affluent communities may also be eligible (although the likelihood decreases as community-level income increases). For example, the probability weight for individuals from a block group where 950 of the 1000 residents had incomes aPFPL ≤138 would be 0.95. Since these individuals live in a community where the vast majority of residents have low incomes (income aPFPL is ≤138), the likelihood of these individuals being Medicaid-eligible is very high. This is why their probability weight is close to the maximum value of 1. Conversely, the probability weight for individuals from a block group where 50 of the 1000 residents had incomes aPFPL ≤138 would be 0.05. Since these individuals live in a community where the vast majority of residents have incomes aPFPL >138, the likelihood of these individuals being Medicaid-eligible is very low. This is why their probability weight is close to the minimum value of 0. Thus, probability weighting effectively reduces sample size by diminishing the influence of more wealthy individuals who would likely be ineligible for Medicaid under expansion. The main analyses were conducted on this Probability Weighted sample (“PW sample,” effective n = 4,102). The probability weights in this study are not to be confused with the sample or design weights in survey analyses, which redistribute the sample size to achieve representative estimates. A flow diagram showing exclusions is provided in Figure 1, and further details on the probability weighting are described in the Appendix.
Figure 1.
Flow diagram of study population
This study was approved by the institutional review boards of the Ohio Department of Health and the Case Western Reserve University. All data management and analysis procedures were conducted in R.
Data Sources
OCISS:
The OCISS is Ohio’s Population-Based cancer registry. By law, all providers or hospitals providing cancer services must report incident cases within six months of diagnosis, with few exceptions.23 The OCISS is a NAACCR- (North American Association of Central Cancer Registries) certified registry and is 98% complete across the 785 NAACR-required data variables.21,24
The American Community Survey of the US Census:
We used pre-tabulated block group-level income data from the 2016 5-year American Community Survey (ACS). The 5-year ACS data provide statistically reliable estimates for the computed measures for small geographies nationwide, including block groups.25 Block groups are geographic units that nest within census tracts, containing between 600–3,000 people.26 There are over 9,200 block groups in Ohio.
Exposure and Study Variables
Exposure:
The exposure was whether patients were diagnosed “pre-expansion” (years 2011 – 2013), or “post-expansion” (years 2014 – 2016).
Outcome Variable:
The study outcome was having metastatic disease at diagnosis. The study outcome was derived from the SEER (Surveillance, Epidemiology, and End Results) summary stage variable in the OCISS, which is coded as “localized only,” “regional by direct extension only,” “regional lymph nodes only,” “regional by both direction extension and lymph node involvement,” and “distant site(s)/node(s) involved.” The last SEER summary stage was coded as “metastatic disease” for the study outcome, and the remaining SEER summary stages were coded as “non-metastatic disease.”
Covariates:
The covariates derived from the OCISS were cancer site (breast, cervical, colorectal, or lung), age at diagnosis (30–39, 40–49, 50–59, or 60–64), race (White, Black or All Others), ethnicity (non-Hispanic, Hispanic, or unknown), and marital status (married/partnered vs all others).
The covariate derived from the ACS was patient income aPFPL (less than 100, 100 and over), approximated using the median income aPFPL of the patient’s block group of residence. This covariate adjusts for the effect of income within the low-income population that is potentially eligible for Medicaid under expansion. The patient income covariate is not to be confused with the probability weighting procedure described previously (which serves to isolate the population that is potentially eligible for Medicaid under expansion).
Analysis
The aim of our study was to examine the change in the odds that low-income, potentially Medicaid-eligible individuals were diagnosed with metastatic disease in screening-amenable cancers as a result of Medicaid expansion. We modeled this change using a multivariate logistic regression model, applied to the PW sample, and adjusting for the covariates described above.
We also conducted several subgroup analyses (“SGA”) and sensitivity analyses (“SA”). This included four subgroup analyses (modeling breast - SGA1, cervical - SGA2, colorectal - SGA3, and lung - SGA4 cancers separately). Sex, in addition to the covariates described previously, was included in the models for SGA3 and SGA4. In SA1, using the PW sample, we modelled the exposure as discrete years. In SA2, as a control to the main analysis, we modelled the same exposure and outcome in adults from higher-income communities (“high-income individuals”) who had private insurance at diagnosis. These individuals were likely to be relatively unaffected by Medicaid expansion, allowing for the examination of possible temporal trends in stage-at-presentation. In SA2, the sample was weighted by 1 minus the probability weight (“1-PW sample”) to isolate the high-income individuals.
Results
Sample characteristics
Table 1 presents the characteristics of the non-PW (n = 12,760) and PW (effective n = 4,101) samples across the covariates included in our model. Overall, the PW sample included more individuals who were Black or had incomes aPFPL <100 compared to the non-PW sample. Post-expansion, individuals tended to be slightly older in both the non-PW (p <0.01) and PW (p = 0.02) samples compared to the pre-expansion period. Additionally, there were slightly fewer persons with unknown ethnicity in both the non-PW (p <0.01) and the PW (p = 0.01) samples post-expansion. Otherwise, the non-PW and PW samples were similar between the exposure groups across the other covariates.
Table 1.
Descriptive statistics for the non-Probability Weighted (non-PW) and Probability Weighted (PW) sample
| non-PW sample (probability weight not applied to observations) |
PW sample (probability weight applied to observations) |
||||||
|---|---|---|---|---|---|---|---|
| Pre-expansion | Post-expansion | p | Pre-expansion | Post-expansion | p | ||
| n | 6088 | 6672 | 1968 | 2134 | |||
| Cancer Site | 0.53 | 0.42 | |||||
| Breast | 2017 (33.1) | 2130 (31.9) | 622 (31.6) | 640 (30.0) | |||
| Cervical | 359 (5.9) | 394 (5.9) | 117 (6.0) | 134 (6.3) | |||
| Colorectal | 1195 (19.6) | 1342 (20.1) | 385 (19.6) | 427 (20.0) | |||
| Lung | 2517 (41.3) | 2806 (42.1) | 844 (42.9) | 932 (43.7) | |||
| Sex | 0.58 | 0.47 | |||||
| Female | 4147 (68.1) | 4513 (67.6) | 1334 (67.8) | 1432 (67.1) | |||
| Male | 1941 (31.9) | 2159 (32.4) | 633 (32.2) | 702 (32.9) | |||
| Age at Diagnosis | <0.01 | <0.01 | |||||
| 30 – 39 | 413 (6.8) | 459 (6.9) | 125 (6.3) | 148 (6.9) | |||
| 40 – 49 | 1335 (21.9) | 1313 (19.7) | 424 (21.6) | 405 (19.0) | |||
| 50 – 59 | 2914 (47.9) | 3197 (47.9) | 965 (49.0) | 1034 (48.4) | |||
| 60 – 64 | 1426 (23.4) | 1703 (25.5) | 454 (23.1) | 548 (25.7) | |||
| Race | 0.31 | 0.09 | |||||
| White (%) | 4662 (76.6) | 5104 (76.5) | 1351 (68.7) | 1456 (68.2) | |||
| Black (%) | 1281 (21.0) | 1434 (21.5) | 575 (29.2) | 645 (30.2) | |||
| All Others (%) | 145 (2.4) | 134 (2.0) | 42 (2.1) | 33 (1.6) | |||
| Ethnicity | <0.01 | 0.01 | |||||
| Non - Hispanic (%) | 5883 (96.6) | 6497 (97.4) | 1895 (96.3) | 2073 (97.2) | |||
| Hispanic | 88 (1.4) | 103 (1.5) | 37 (1.9) | 39 (1.8) | |||
| Unknown (%) | 117 (1.9) | 72 (1.1) | 36 (1.8) | 22 (1.0) | |||
| Marital Status | 0.27 | 0.78 | |||||
| Married/Partnered (%) | 1874 (30.8) | 1993 (29.9) | 513 (26.1) | 552 (25.8) | |||
| All Others (%) | 4214 (69.2) | 4679 (70.1) | 1454 (73.9) | 1582 (74.2) | |||
| Income as percent of federal poverty level | 0.52 | ||||||
| Less than 100 (%) | 538 (8.8) | 567 (8.5) | 379 (19.2) | 403 (18.9) | |||
| 100 or more (%) | 5550 (91.2) | 6105 (91.5) | 1589 (80.8) | 1731 (81.1) | |||
| Probability weights | 0.34 | - | |||||
| Mean (SD) | 0.32 (0.20) | 0.32 (0.20) | - | - | |||
Changes to the percentage uninsured and in the crude odds of having metastatic disease
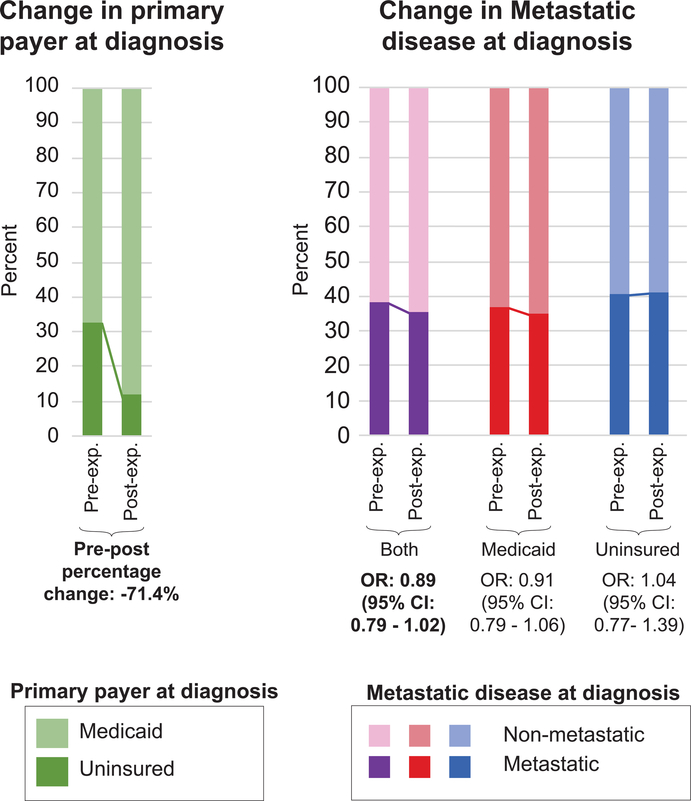
Figure 2 presents the pre/post-expansion changes in the PW sample in primary payer at diagnosis (Medicaid, uninsured) and the unadjusted (crude) odds of having metastatic disease, stratified by primary payer at diagnosis (Medicaid, uninsured, both). There was a 71.4% decrease in the proportion of individuals who were uninsured at diagnosis post-expansion. Examining the crude odds of having metastatic disease at diagnosis, there appears to be a shift toward non-metastatic disease post-expansion overall, but a similar shift was not apparent for those who reported being uninsured at diagnosis.
Figure 2.
Changes in primary payer at diagnosis and crude odds of having metastatic disease in the Probability Weighted (PW) sample, pre-expansion and post-expansion
Effect of Medicaid expansion on stage-at-diagnosis
Table 2 presents the effect size estimates and the corresponding 95% confidence intervals of the exposure and covariates of the logistic regression model. The results from this model showed that for breast, cervical, colorectal, and lung cancers, after adjusting for the potential confounders, individuals diagnosed post-expansion had 15% lower odds of having metastatic disease compared to pre-expansion (Adjusted Odds Ratio [AOR]: 0.85, 95% Confidence Interval [CI]: 0.77 – 0.93).
Table 2.
Changes to the odds of being diagnosed with metastatic disease after Medicaid expansion, adjusting for covariates
| Odds Ratio | 95% confidence interval | p | |
|---|---|---|---|
| Exposure | |||
| Pre-expansion | 1.00 | reference | |
| Post-expansion | 0.85 | 0.77 – 0.93 | <0.01 |
| Covariates | |||
| Cancer Site | |||
| Breast | 1.00 | reference | |
| Cervical | 1.45 | 1.11 – 1.89 | <0.01 |
| Colorectal | 3.63 | 3.11 – 4.23 | <0.01 |
| Lung | 11.81 | 10.32 – 13.51 | <0.01 |
| Age at Diagnosis | 0.34 | ||
| 30 – 39 | 1.00 | reference | |
| 40 – 49 | 1.09 | 0.86 – 1.38 | 0.47 |
| 50 – 59 | 1.13 | 0.90 – 1.42 | 0.28 |
| 60 – 64 | 1.12 | 0.88 – 1.42 | 0.36 |
| Race | |||
| White | 1.00 | reference | |
| Black | 0.98 | 0.88 – 1.11 | 0.78 |
| All Others | 1.17 | 0.80 – 1.73 | 0.42 |
| Ethnicity | |||
| Non - Hispanic | 1.00 | reference | |
| Hispanic | 0.70 | 0.46 – 1.07 | 0.10 |
| Unknown | 0.31 | 0.18 – 0.54 | <0.01 |
| Marital Status | |||
| Married/Partnered | 1.00 | reference | |
| All Others | 1.08 | 0.97 – 1.20 | 0.18 |
| Income as percent of federal poverty level | |||
| Less than 100 (%) | 1.00 | reference | |
| 100 or more (%) | 1.02 | 0.88– 1.19 | 0.77 |
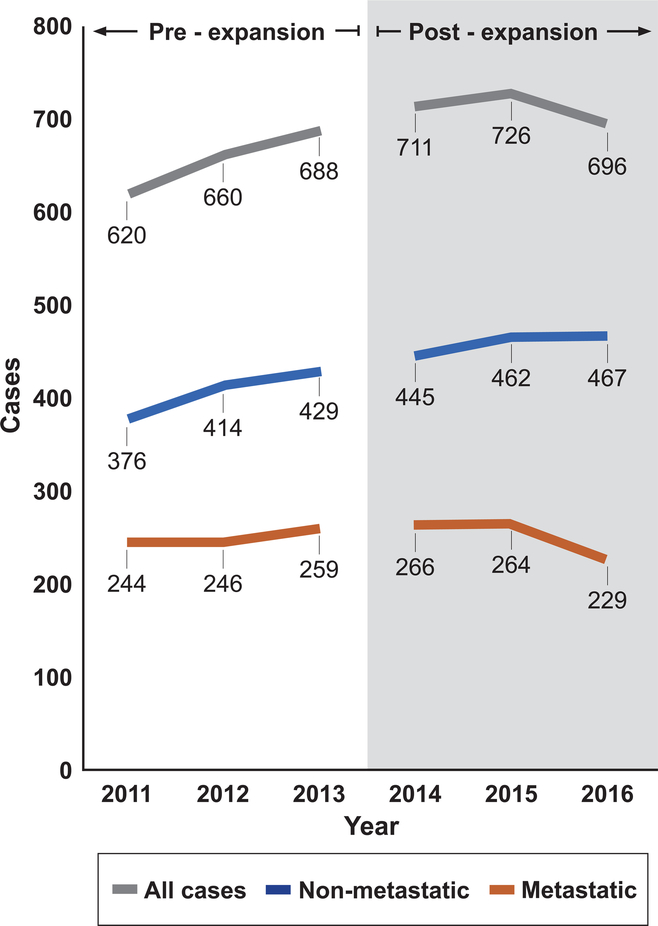
To explore the extent to which declining odds of metastatic disease were driven by a decrease in the number of metastatic cases in the numerator versus an increase in non-metastatic cases in the denominator, we examined the counts of each type of case in the PW sample year-to-year. These numbers (Figure 3) suggest an upward trend in total cases during the pre-expansion years driven by increases in both non-metastatic and metastatic cases. In the post-expansion period, non-metastatic cases continued to climb, while metastatic cases decreased. As a post-hoc analysis, we applied a one-sided Cochran-Armitage test for trend to these case counts. In 2011–2013, the Cochran-Armitage test did not detect a decreasing trend in the proportion of metastatic cases (p = 0.27), but in 2014–2016, the test suggested a decreasing trend (p = .04).
Figure 3.
Metastatic, non-metastatic, and total cases in the Probability Weighted (PW) sample during the pre-expansion and post-expansion periods
Subgroup and Sensitivity analyses
The four subgroup analyses (SGA1–4) in which we modeled each cancer site separately (Tables A2–A5 of the Appendix) showed that post-expansion, there were 15% (AOR: 0.85, 95% CI: 0.67 – 1.07), 37% (AOR: 0.63, 95% CI: 0.40 – 1.03), 3% (AOR: 0.97, 95% CI: 0.80 – 1.18), and 18% (AOR: 0.82, 95% CI: 0.72 – 0.94) decreases in the odds of having metastatic disease compared to pre-expansion for breast, cervical, colorectal, and lung cancer, respectively.
When we modeled the exposure as discrete years (SA1) with 2011 as the reference, we found non-statistically significant shifts toward non-metastatic disease in 2012 (AOR: 0.91, 95% CI: 0.76 – 1.08) and 2013 (AOR: 0.96, 95% CI: 0.82 – 1.14). In the post-expansion years, there were consistent, larger shifts toward metastatic disease. In 2014 the shift was non-significant (AOR: 0.88, 95% CI: 0.75 – 1.04), but in 2015 and 2016, the odds of metastatic disease decreased significantly by 17% (AOR: 0.83, 95% CI: 0.70 – 0.98) and 28% (AOR: 0.72, 95% CI: 0.61 – 0.86), respectively, compared to 2011. Table A1 of the Appendix summarizes these findings.
SA2 serves as a control to our main analysis. In SA2, we modelled the changes in our main outcome in the population who reported having private insurance at the time diagnosis (n = 35,274). Weighting the observations by 1 minus the probability weight led to an effective sample size of n = 28,658 (1-PW sample), representing high-income, privately insured adults. The 1-PW sample was comprised of individuals who were less likely to have lung cancer (but more likely to have breast cancer), and were less likely to be male, Black, Hispanic, and unmarried/unpartnered compared to the main PW sample (see Table A6 of the Appendix). The logistic regression model applied to the 1-PW sample showed that there were minimal changes in the odds of being diagnosed with metastatic disease in post-expansion compared to pre-expansion (AOR: 1.02, 95% CI: 0.96 – 1.09)(see Table A7 of the Appendix).
Discussion
Medicaid expansion under the Affordable Care Act decreased the number of uninsured in the low-income population, and there have been several other population health benefits, including improving access to care, utilization patterns, and self-reported health outcomes.6,27–29 Our results suggest that Medicaid expansion may have also improved outcomes in screening-amenable cancers. In the population of low-income, potentially Medicaid-eligible adults in Ohio (a state that was part of the first round of expansions in 2014), the odds of being diagnosed with metastatic breast, cervical, colorectal, and lung cancers decreased in the post-expansion period. Since stage-at-diagnosis is perhaps the most important predictor of survival in these cancers, our findings suggest that Medicaid expansion has likely had a meaningful clinical and public health impact in the context of cancer care since breast, cervical, colorectal, and lung cancers collectively contribute to 33% and 50% of all cancer deaths in men and women, respectively.30
Lower income and insurance status have traditionally been associated with more advanced stage-at-diagnosis.31–34 In an ad-hoc analysis of the unadjusted case counts in the OCISS, we found that 24% of all breast, cervical, colorectal, and lung cancer cases were metastatic at diagnosis in 2011–2013. The figures for those with private insurance, Medicaid insurance, and no insurance were 18%, 33%, and 37%, respectively. The stage-at-diagnosis disparities between those with private insurance and those with Medicaid or no insurance have historically been persistent, as the rates of metastatic disease among these three groups have been relatively consistent going back 15 years.
Thus, the impact that Medicaid expansion has had over a relatively short time frame on stage-at-diagnosis in the low-income population is noteworthy. Our study estimates that individuals have a 15% lower odds of being diagnosed with metastatic cancer post-expansion, an effect size that is substantially larger than earlier estimates.7,8 Previous studies likely underestimated the effect of Medicaid expansion given their short follow-up periods (1 year or less, post-expansion7,8). Our study covers the three years following the implementation of Medicaid expansion, which includes both the ramp-up and steady-state periods with respect to Medicaid enrollment under expansion. Indeed, in SA1, we found that the likelihood of being diagnosed with metastatic disease decreased in each progressive year after 2014, corresponding to Medicaid enrollment patterns, which did not stabilize until early to mid 2015 in Ohio.35
There may be several mechanistic explanations for how Medicaid expansion has improved stage-at-diagnosis in these cancers. First, and perhaps most importantly, Medicaid expansion provided coverage for many low-income individuals who previously did not have insurance,36 improving access to care in this medically vulnerable population. Second, Medicaid expansion may have specifically increased access and uptake of preventive health services,6 such as cancer screenings.5 In this population, screening access likely improved the most in colorectal and lung cancer since analogs to the Breast and Cervical Cancer Early Detection Program (a federal-state partnership program that provides low-income women with free guideline-consistent screening for breast and cervical cancer) do not exist for these cancer sites. Recent estimates show that overall guideline-concordant screening rates are 71.5%, 83%, 62.4%, and 4.4% in breast, cervical, colorectal, and lung cancer, respectively.37,38 Finally, Medicaid expansion may have altered the timing of enrollment into Medicaid relative to cancer diagnosis. Since Medicaid expansion greatly simplified the eligibility criteria to a strictly income-based means test, a greater number of individuals may have enrolled in Medicaid in advance of their eventual cancer diagnosis (“pre-diagnosis” enrollees) rather than around the time of their diagnosis or because of their cancer diagnosis (“peri/post-diagnosis” enrollees). Previous studies have shown that pre-diagnosis enrollees have more favorable cancer survival outcomes compared to peri/post-diagnosis enrollees, perhaps reflecting the better access to care for pre-diagnosis enrollees.39,40 Additionally, pre-diagnosis enrollees may be more likely to present for care in the face of early cancer symptoms.
The observed reduction in the odds of metastatic disease in this study could be driven by an increase in the number of non-metastatic cases (perhaps as a result of improved access to care), a decrease in the number of metastatic cases (perhaps as a result of improved uptake of preventive services), or a combination of the two. Any of these scenarios would be noteworthy, but a decrease in the number of metastatic cases would have the most proximate impact in reducing the total cancer morbidity and mortality burden. Figure 3 provides tentative evidence that a reduction in the number of metastatic cases post-expansion at least partially contributed to the relative reduction in metastatic disease, a trend that is particularly pronounced in the final study year. As additional years of data become available, this trend should be examined further.
Given year-to-year stochastic variations in incidence, cancer statistics are often reported across multiple aggregated years. Thus, an a priori decision was made to model the exposure dichotomously in the main analysis. Nonetheless, we performed a sensitivity analysis (SA1) to model the exposure as discrete years. The results in SA1 generally paralleled the main analysis, but there were some year-to-year variations in the pre-expansion period worth noting. Compared to 2011, we observed 9% and 4% lower odds in 2012 and 2013, respectively, of metastatic disease. Larger, monotonic trends (congruent with Medicaid enrollment patterns, as mentioned previously) were detected in the post-expansion years (12%, 17%, and 28% decreases in the odds for years 2014, 2015, and 2016, respectively). If the pre-expansion shifts toward non-metastatic disease in SA1 represent an early phase of a larger secular trend, the main model may overestimate the impact of Medicaid expansion. To explore this possibility further, we conducted a post-hoc, one-sided Cochran-Armitage test for trend that only detected a decreasing trend in the proportion of metastatic cases in the post-expansion period. While we cannot fully rule out a pre-expansion secular trend toward non-metastatic disease in our study population, the results of these tests for trend are consistent with the evidence from our control population (high-income, privately insured adults), which is modelled in SA2. In SA2, there was no effect of ACA on metastatic disease (AOR 1.02 for post-expansion compared to pre-expansion, 95% Confidence Interval: 0.96 – 1.09).
The subgroup analyses suggest that Medicaid expansion may have had differential impacts across the four sites. The results from SGA1–4 show that while all sites saw shifts toward non-metastatic disease, the largest improvements were seen in cervical (AOR: 0.63, 95% CI: 0.40 – 1.03) and lung cancer (AOR: 0.82: 95% CI: 0.72 – 0.94). The shifts for breast and colorectal cancer were non-significant. This pattern of cervical and lung cancer seeing the largest improvements has also been documented in a previous study,8 while another study found non-significant changes in adjusted, site-specific models.7 It may become possible to clarify the site-specific relationships between Medicaid expansion and stage-at-diagnosis as additional years of data become available.
The main limitation of our study is that we examined cases in one Medicaid expansion state, so the findings may not generalize to all Medicaid expansion states. Additionally, we could not control for factors not captured in the OCISS, like comorbidities. These limitations are tempered by several strengths. First, we used the OCISS, a cancer registry that provided nearly full coverage of the breast, cervical, colorectal, and lung cases in our study area. Second, we used data covering three post-expansion years, allowing for a longer follow-up time compared to previous studies and potentially enabling us to capture a more accurate estimate of the effect of Medicaid expansion on stage-at-diagnosis. Finally, our study used the fully identified OCISS data, which included patient addresses at diagnosis. This allowed us to more precisely estimate patient income using census data compared to other studies, potentially allowing us to more accurately define the low-income Medicaid-eligible population.
Other studies have approximated patient income using median family income data in dollars from the patients’ zip codes or census tracts of residence.8 In contrast, this analysis utilized income aPFPL data (a more relevant income measure in the context of Medicaid studies since it accounts for family size) for block groups (a geographic area that is smaller than both zip codes and census tracts). Furthermore, income is usually incorporated in models as a stratifying variable7,8 (applying cutoffs to different income levels), which may underestimate the population of low-income, potentially Medicaid-eligible individuals. This is because individuals living in more affluent communities (and are thus assigned a higher estimated patient-level income) are completely excluded from the lower-income strata. Rather than stratifying, we applied weights to the observations equal to the probability of income aPFPL ≤138. The probability weighting approach may potentially identify the Medicaid-eligible population more accurately (by not completely excluding higher-income individuals who are potentially eligible for Medicaid), and by the same token, it can increase power since no observations are explicitly excluded. Our probability weighting approach has not yet been validated, and future studies doing so would be valuable given its potential applicability to other cancer prevention and control studies.
Thus, the approaches utilized in this analysis have the potential to reduce confounding related to income approximation compared to other studies by using a small geographic unit of analysis to approximate individual-level (census block groups), the most policy relevant measure of income (family income as a percent of the federal poverty level), and probabilistically isolating the potentially Medicaid expansion-eligible population (see Appendix for further comparisons the income approximation method used in this analysis versus previous studies).
Conclusions
We found a significant reduction in the odds that low-income Ohio residents would be diagnosed with metastatic screening-amenable cancer following the implementation of Medicaid expansion. Given that this study has a longer follow-up time compared to previous studies, the effect estimates may provide a more comprehensive assessment of the impact Medicaid expansion has had on an important clinical outcome. During this time of rapid health system evolution, many states that have not expanded Medicaid are reconsidering their positions. At the same time, there are ongoing attempts to dismantle Medicaid expansion through legislative and executive actions at the federal level, and through state-level Medicaid reforms instituting Medicaid work requirements or enrollment caps. To varying degrees, these maneuvers could have the effect of limiting Medicaid expansion. State-level waiver requests that allow for work requirements and enrollment caps have already been approved by the Centers for Medicare and Medicaid Services.41–43 Policymakers should consider the broad consequences of both implementing Medicaid expansion or limiting Medicaid expansion, including the impact such changes might have on cancer outcomes.
Supplementary Material
Acknowledgments
Cancer incidence data used in these analyses were obtained from the Ohio Cancer Incidence Surveillance System (OCISS), Ohio Department of Health (ODH), a cancer registry partially supported by the National Program of Cancer Registries at the Centers for Disease Control and Prevention (CDC). Use of these data does not imply that ODH or CDC agrees or disagrees with the analyses, interpretations or conclusions in this report (or publication / presentation)
Funding Statement
This study was supported by the NIGMS (GM007250), Clinical and Translational Science Collaborative of Cleveland (NCATS 1TL1TR002549; NCATS UL1TR000439), Pharmaceutical Research and Manufacturers of America Foundation (PDHO18), Prevention Research Center for Healthy Neighborhoods at CWRU (cooperative agreement 1U48DP005013 from the CDC), Case Comprehensive Cancer Center (NCI P30 CA043703), CWRU Center for Reducing Health Disparities, CWRU Center for Community Health Integration, and the University Hospitals Cleveland Medical Center Department of Family Medicine and Community Health. The findings and conclusions of this study are those of the authors and do not necessarily represent the official positions of the funders.
Conflict of Interest Statement
Uriel Kim and Abby Statler have no conflicts of interests to disclose. Siran Koroukian is supported in part by a grant from Celgene Corporation. Johnie Rose is co-founder and Chief Medical Advisor of VINYA Intelligence, Inc., a healthcare artificial intelligence firm developing remote patient monitoring solutions; none of the firm’s work relates directly to the content of the manuscript.
References
- 1.The Henry J. Kaiser Family Foundation. Status of State Action on the Medicaid Expansion Decision. https://www.kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed August 22, 2019.
- 2.Koh HK, Sebelius KG. Promoting Prevention through the Affordable Care Act. N Engl J Med. 2010;363(14):1296–1299. doi: 10.1056/NEJMp1008560 [DOI] [PubMed] [Google Scholar]
- 3.Miller S, Wherry LR. Health and Access to Care during the First 2 Years of the ACA Medicaid Expansions. N Engl J Med. 2017;376(10):947–956. doi: 10.1056/NEJMsa1612890 [DOI] [PubMed] [Google Scholar]
- 4.Seiber E, Sahr T. 2015. Update on Public-Private Substitution among Adults in Ohio Medicaid. 2016:0–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedewa SA, Yabroff KR, Smith RA, Goding Sauer A, Han X, Jemal A. Changes in Breast and Colorectal Cancer Screening After Medicaid Expansion Under the Affordable Care Act. Am J Prev Med. 2019. doi: 10.1016/j.amepre.2019.02.015 [DOI] [PubMed] [Google Scholar]
- 6.Sommers BD, Blendon RJ, Orav EJ, Epstein AM. Changes in Utilization and Health Among Low-Income Adults After Medicaid Expansion or Expanded Private Insurance. JAMA Intern Med. 2016;176(10):1501–1509. doi: 10.1001/jamainternmed.2016.4419 [DOI] [PubMed] [Google Scholar]
- 7.Han X, Yabroff KR, Ward E, Brawley OW, Jemal A. Comparison of Insurance Status and Diagnosis Stage Among Patients With Newly Diagnosed Cancer Before vs After Implementation of the Patient Protection and Affordable Care Act. JAMA Oncol. 2018;4(12):1713–1720. doi: 10.1001/jamaoncol.2018.3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jemal A, Lin CC, Davidoff AJ, Han X. Changes in Insurance Coverage and Stage at Diagnosis Among Nonelderly Patients With Cancer After the Affordable Care Act. J Clin Oncol. 2017;35(35):3906–3915. doi: 10.1200/JCO.2017.73.7817 [DOI] [PubMed] [Google Scholar]
- 9.Soni A, Simon K, Cawley J, Sabik L. Effect of Medicaid Expansions of 2014 on Overall and Early-Stage Cancer Diagnoses. Am J Public Health. 2018;108(2):216–218. doi: 10.2105/AJPH.2017.304166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Final Recommendation Statement: Breast Cancer: Screening - US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Accessed August 13, 2019.
- 11.Final Update Summary: Cervical Cancer: Screening - US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Accessed August 13, 2019.
- 12.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for Colorectal Cancer. JAMA. 2016;315(23):2564. doi: 10.1001/jama.2016.5989 [DOI] [PubMed] [Google Scholar]
- 13.Final Recommendation Statement: Lung Cancer: Screening - US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lung-cancer-screening. Accessed August 13, 2019.
- 14.Ohio Department of Health, The Ohio State University. Ohio Cancer Atlas 2019.; 2019. https://odh.ohio.gov/wps/portal/gov/odh/know-our-programs/ohio-cancer-incidence-surveillance-system. Accessed August 22, 2019. [Google Scholar]
- 15.SEER*Explorer, Version April 15, 2019. https://seer.cancer.gov/explorer/index.html. Accessed August 13, 2019. [Google Scholar]
- 16.Ohio Department of Health. Breast Cancer in Ohio, 2010–2014. https://odh.ohio.gov/. Accessed October 10, 2019.
- 17.Ohio Department of Health. Cervical Cancer in Ohio, 2011–2015. https://odh.ohio.gov/. Accessed October 10, 2019.
- 18.Ohio Department of Health. Colon & Rectum Cancer in Ohio, 2007 – 2011. https://odh.ohio.gov/. Accessed October 10, 2019.
- 19.Ohio Department of Health. Lung & Bronchus Cancer in Ohio, 2008 – 2012. https://odh.ohio.gov/. Accessed October 10, 2019.
- 20.Barr RD, Holowaty EJ, Birch JM. Classification schemes for tumors diagnosed in adolescents and young adults. Cancer. 2006;106(7):1425–1430. doi: 10.1002/cncr.21773 [DOI] [PubMed] [Google Scholar]
- 21.Hofferkamp J, Havener LA. Standards for Cancer Registries Volume II.; 2007. http://www.naaccr.org. Accessed August 8, 2019.
- 22.California State Data Center. Re-Calculating Medians and Their Margin of Errors for Aggregated ACS Data (from the January 2011 Network News).; 2011. http://www.dof.ca.gov/Forecasting/Demographics/Census_Data_Center_Network/documents/How_to_Recalculate_a_Median.pdf. Accessed August 8, 2019.
- 23.Ohio Cancer Incidence Surveillance System (OCISS). https://odh.ohio.gov/wps/portal/gov/odh/know-our-programs/ohio-cancer-incidence-surveillance-system/welcome-to/. Accessed August 8, 2019.
- 24.OCISS. OCISS July 2016 Quarterly Newsletter.; 2016. https://odh.ohio.gov/wps/wcm/connect/gov/cfa8c0e4-766b-424c-b7f9-c0df3bdd4a79/OCISS+Quarterly+Newsletter+July+2016.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=ROOTWORKSPACE.Z18_M1HGGIK0N0JO00QO9DDDDM3000-cfa8c0e4-766b-424c-b7f9-c0df3bdd4a79-mD4Tw2f.
- 25.US Census Bureau. American Community Survey Information Guide. https://www.census.gov/content/dam/Census/programs-surveys/acs/about/ACS_Information_Guide.pdf. Accessed August 8, 2019.
- 26.US Census Bureau. Glossary. https://www.census.gov/programs-surveys/geography/about/glossary.html#par_textimage_13. Accessed August 8, 2019.
- 27.Klein EY, Levin S, Toerper MF, et al. The Effect of Medicaid Expansion on Utilization in Maryland Emergency Departments. Ann Emerg Med. 2017;70(5):607–614.e1. doi: 10.1016/J.ANNEMERGMED.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 28.Huguet N, Valenzuela S, Marino M, et al. Following Uninsured Patients Through Medicaid Expansion: Ambulatory Care Use and Diagnosed Conditions. Ann Fam Med. 2019;17(4):336–344. doi: 10.1370/afm.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonisse L, Garfield R, Rudowitz R, Artiga S. The Effects of Medicaid Expansion under the ACA: Updated Findings from a Literature Review. doi: 10.1111/jrh.12234/full [DOI] [Google Scholar]
- 30.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 31.Lantz PM, Mujahid M, Schwartz K, et al. The Influence of Race, Ethnicity, and Individual Socioeconomic Factors on Breast Cancer Stage at Diagnosis. Am J Public Health. 2006;96(12):2173–2178. doi: 10.2105/AJPH.2005.072132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–231. doi: 10.1016/S1470-2045(08)70032-9 [DOI] [PubMed] [Google Scholar]
- 33.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–435. doi: 10.1007/s10552-008-9256-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarazi WW, Bradley CJ, Bear HD, Harless DW, Sabik LM. Impact of Medicaid disenrollment in Tennessee on breast cancer stage at diagnosis and treatment. Cancer. 2017;123(17):3312–3319. doi: 10.1002/cncr.30771 [DOI] [PubMed] [Google Scholar]
- 35.Total Monthly Medicaid and CHIP Enrollment | The Henry J. Kaiser Family Foundation. https://www.kff.org/health-reform/state-indicator/total-monthly-medicaid-and-chip-enrollment/?activeTab=graph¤tTimeframe=0&startTimeframe=64&selectedDistributions=total-monthly-medicaidchip-enrollment&selectedRows=%257B%2522states%2522:%257B%2522ohio%2522:%257B%257. Accessed August 13, 2019. [Google Scholar]
- 36.Berchick ER, Hood E, Barnett JC. Health Insurance Coverage in the United States: 2017 Current Population Reports.; 2018. https://www.census.gov/content/dam/Census/library/publications/2018/demo/p60-264.pdf. Accessed August 13, 2019.
- 37.White A, Thompson TD, White MC, et al. Cancer Screening Test Use - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. doi: 10.15585/mmwr.mm6608a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards TB, Doria-Rose VP, Soman A, et al. Lung Cancer Screening Inconsistent With U.S. Preventive Services Task Force Recommendations. Am J Prev Med. 2019;56(1):66–73. doi: 10.1016/j.amepre.2018.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koroukian SM, Bakaki PM, Raghavan D. Survival disparities by Medicaid status. Cancer. 2012;118(17):4271–4279. doi: 10.1002/cncr.27380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koroukian SM, Bakaki PM, Htoo PT, et al. The Breast and Cervical Cancer Early Detection Program, Medicaid, and breast cancer outcomes among Ohio’s underserved women. Cancer. 2017;123(16):3097–3106. doi: 10.1002/cncr.30720 [DOI] [PubMed] [Google Scholar]
- 41.Trump Administration Approves Medicaid Work Requirements in Utah - The New York Times. https://www.nytimes.com/2019/03/29/us/politics/medicaid-trump-utah.html. Accessed August 14, 2019. [Google Scholar]
- 42.Trump Greenlights Major Medicaid Changes | The Pew Charitable Trusts. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2019/03/26/trump-greenlights-major-medicaid-changes. Accessed August 14, 2019. [Google Scholar]
- 43.Christensen A. S.B.96 Medicaid Expansion Adjustments. https://le.utah.gov/~2019/bills/sbillenr/SB0096.pdf. Accessed August 14, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.