Abstract
Background
Implantable continuous-flow left ventricular assist device (LVAD) improve renal function in advanced heart failure. However, the long-term effects of LVAD on renal function have not been investigated thoroughly. We aimed to assess long-term renal function in patients with LVAD support and to identify predictors for late deterioration in renal function (LDRF).
Methods
One hundred patients underwent LVAD implantation as a bridge to transplant at the University of Tokyo Hospital between May 2011 and December 2018. We assessed renal function at intervals (preoperative, 1, 6, 12, 18, 24 and 30 months after LVAD implantation). We divided patients into two groups: “with LDRF,” whose renal function at 30 months had decreased by >25% compared with preoperatively (n = 14), and “without LDRF” (n = 55).
Results
Renal function improved at 1 month, returned to preoperative levels at 6 months, and remained there up to 30 months after LVAD implantation. However, renal function impairment became evident in patients with LDRF 18 months after LVAD implantation. A ratio of right atrial pressure/pulmonary artery wedge pressure > 0.57 and left ventricular dimension diastole ≤ 67 mm were preoperative independent risk factors for LDRF. In addition, the incidence of perioperative acute kidney injury, ventricular arrhythmia, aortic insufficiency, and late right ventricular failure was significantly higher in patients with LDRF.
Conclusion
LDRF after LVAD implantation corresponded to several risk factors, including a small left ventricle and LVAD-related complications, such as right ventricular failure.
Keywords: Left ventricular assist devices, Renal function, Right ventricular failure
1. Introduction
Left ventricular assist device (LVAD) implantation has become the standard therapy for end-stage congestive heart failure [1], [2], [3]. Patients with severe heart failure experience dysfunction of other organs because of low cardiac output and venous congestion. An LVAD can provide sufficient blood flow to improve end-organ function. Studies have reported that the estimated glomerular filtration rate (eGFR) increased significantly up to 1 month after LVAD implantation [4]. However, data on the effects of long-term LVAD support on end-organ function are limited. Yoshioka et al. reported that the renal function improvement in most patients returns to baseline after a prolonged LVAD support period [5]. In Japan, donor hearts are in short supply, which increases the length of LVAD support time until heart transplantation. Therefore, a focus on long-term renal function is important for the management of LVAD implantation. In this study, we assessed long-term renal function in patients on LVAD support and investigated the predictors of long-term deterioration in renal function [6].
2. Methods
2.1. Patients
This retrospective study was conducted by extracting data from the medical records of 100 consecutive Japanese patients who received an implantable LVAD as a bridge to transplant (BTT) at the University of Tokyo Hospital between May 2011 and December 2018, with the LVAD remaining in place for at least 1 year (Fig. 1). Forty-four patients received a HeartMateⅡ (HMII; Abbott Laboratories, Chicago, IL, USA), 24 received an EVAHEART (Sun Medical, Nagano, Japan), and 21 received the Jarvik 2000 (Jarvik Heart, New York, NY, USA). Ten were given a DuraHeart (Terumo Heart, Ann Arbor, MI, USA), and one had an HVAD (Medtronic, Minneapolis, MN, USA). Patients who converted from extracorporeal LVAD, central extracorporeal membrane oxygenation, or Impella were excluded. The study protocol conformed to the tenets of the Declaration of Helsinki and was reviewed and approved by the University of Tokyo Institutional Review Board (approval number: 2650).
Fig. 1.
Flow chart of patients who met inclusion criteria for study population. LVAD; left ventricular assist device, LDRF; late deterioration in renal function.
2.2. Follow-up
All patients received hemodynamic ramp tests for LVAD speed optimization 1 month after LVAD implantation. A monthly follow-up examination was performed after initial discharge. To assess renal function, we collected the preoperative eGFR and the rates at 1, 6, 12, 18, 24, 30 months after LVAD implantation. Preoperative eGFR was taken from just before LVAD implantation and those was defined as baseline eGFR. When a patient received treatment, such as an inotropic agent or hemodialysis, the prior eGFR was obtained after LVAD implantation. The eGFR was calculated using the following Japanese equation: eGFR = 194 × (serum creatinine [mg/dL])−1.094 × (age [years])−0.287 × (0.739, if female) [7]. In patients aged under 18 years, the eGFR was calculated using the Japanese Children equation [8]. We then analyzed the changes in the eGFR after LVAD implantation using 100 patients. We defined late deterioration in renal function (LDRF) as a decrease from the preoperative eGFR of>25% and we assess the characteristics of LDRF using patients who could be followed until appropriate durations. To assess the predictors of LDRF, we collected the following information: age at LVAD implantation; sex; body mass index; Brinkman index; diabetes; preoperative profile—whether registered with the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). We recorded preoperative hemodynamic status using right heart catheterization (RHC). According to echocardiographic parameters, preoperative left ventricular dimension diastolic (LVDD) and the grade of tricuspid regurgitation were checked. Preoperative laboratory data including albumin, blood urea nitrogen (BUN), total bilirubin, b-type natriuretic peptide and proteinuria were measured. We also collected perioperative incidence of acute kidney injury (AKI) and laboratory data, hemodynamic status and treatment such as β blocker or Renin-angiotensin aldosterone system inhibitor (RAASi) at 1 month after LVAD implantation. Adverse events recorded after discharge from hospital following the index LVAD implantation up to 30 months included AKI, pump thrombosis, aortic valve insufficiency (AI), drive-line infection, stroke, ventricular arrhythmia (VA), right ventricular failure (RVF), and all-cause mortality. In order to demonstrate the effect of preoperative renal dysfunction on the postoperative clinical course, we also assessed long-term renal function after categorizing patients by with or without preoperative eGFR below 60 mL/min/1.73 m2.
Perioperative and post-discharge AKI was defined by using KDIGO criteria [9]. Suspected pump thrombosis was defined as a clinical diagnosis of pump-related malfunction and hemolysis as reported by the previous publications [10]. Moderate or severe aortic regurgitation was counted as an event of AI. RVF was defined and classified according to the INTERMACS RVF criteria. In this study, we defined late RVF as when patients met the moderate or severe INTERMACS RVF criteria after discharge following the index LVAD implant. This included the use of inotropes, intravenous or inhaled pulmonary vasodilators for a duration > 7 days, or the requirement of a right ventricular (RV) assist device implantation [11]. When an electrocardiogram, a pacemaker, an implantable cardioverter-defibrillator, or cardiac resynchronization therapy recorded VA, we counted it as a VA event.
2.3. Statistical analyses
Data are presented as the mean ± standard deviation or median (interquartile range). Statistical analyses were performed with JMP software, version 14.2 (SAS Institute, Cary, NC, USA). Student’s t-test or the Mann–Whitney Utest was used for continuous variables, and Fisher’s exact test was used for categorical variables. Univariate analyses of the patients’ characteristics were performed, and the odds ratio and 95% confidence interval (95% CI) were computed for the prediction of LDRF. The optimal cutoff point for the prediction of LDRF was determined using a receiver operating characteristic curve. Multivariate analysis for the prediction of LDRF was performed using variables with P value < 0.05 in univariate analyses. The change from the preoperative eGFR at each point after LVAD implantation was tested using a paired t-test or Wilcoxon signed-rank tests. The Bonferroni method was used to assess the significance of multiple comparisons. To assess the eGFR over time, the mean eGFR at each period was examined graphically for all patients and stratified according to baseline renal function. A value of P < 0.05 was considered statistically significant in all analyses.
3. Results
3.1. Change in renal function after LVAD implantation
We initially assessed the change in the eGFR after LVAD implantation. The duration of LVAD support was 39.6 ± 14.4 months (3.3 ± 1.2 years). The percentage of males was 78.2%, and the average age at LVAD implantation was 41.3 ± 12.7 years. The study subjects included those with dilated cardiomyopathy (DCM; N = 73), dilated-phase hypertrophic cardiomyopathy (dHCM; N = 9), drug-induced cardiomyopathy (N = 3), restrictive cardiomyopathy (RCM; N = 3), arrhythmogenic right ventricular cardiomyopathy (ARVC; N = 2), ischemic cardiomyopathy (ICM; N = 6), and others (N = 4).
The eGFR 1 month after LVAD implantation was significantly higher than the preoperative eGFR (P < 0.0001). By contrast, the eGFR had almost returned to the preoperative level 6 months after LVAD implantation, and the eGFR were comparable as compared with preoperative eGFR after that time (Fig. 2). A subgroup analysis of the longitudinal change in the eGFR according to the preoperative eGFR revealed that renal function in patients with a lower preoperative eGFR was lower at all points than those with a higher preoperative eGFR. However, the eGFR in patients with a preoperative eGFR < 60 mL/min/1.73 m2 remained higher than the preoperative level even 30 months after LVAD implantation in the same patient group. The number of patients, who had eGFR above 60 mL/min/1.73 m2 before LVAD implantation and eGFR below 60 mL/min/1.73 m2 after LVAD implantation, were 2 (1 month after LVAD implantation), 4 (6 months), 6 (12 months), 6 (18 months),7 (24 months) and 9 (30 months) patients, respectively.
Fig. 2.
Change in the estimated glomerular filtration rate (eGFR) in all patients and the results of multiple comparisons by Bonferroni method. Solid line = all patients; dotted line = preoperative eGFR ≥ 60 mL/min/1.73 m2; dashed line = preoperative eGFR < 60 mL/min/1.73 m2. LVAD; left ventricular assist device.
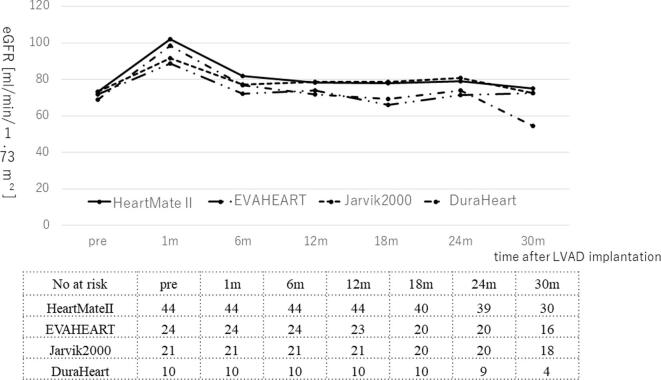
Next, we investigated the incidence of eGFR < 60 mL/min/1.73 m2 at each time and the change of eGFR in different LVAD. The incidence of eGFR < 60 mL/min/1.73 m2 was 11%, 21%, 24%, 25.3%, 27%, and 31.8% respectively, from 38% preoperatively (Fig. 3). There was no difference for change in renal function among each LVAD type (Supplementary Fig. 1).
Fig. 3.
Change in the number at risk and the percentage of patients with eGFR < 60 mL/min/1.73 m2 at each time point. LVAD; left ventricular assist device, eGFR; estimated glomerular filtration rate.
3.2. Long term deterioration in renal function after LVAD implantation
Next, we explored the characteristics of LDRF after LVAD implantation. We set 30 months as the timing of the long term, which was used for evaluation of LDRF. In this analysis, patients who underwent heart transplantation (n = 13), LVAD removal (n = 2), or died (n = 4) or who lacked follow-up data (n = 12) within 30 months of LVAD implantation were excluded from the consecutive cases (n = 100).
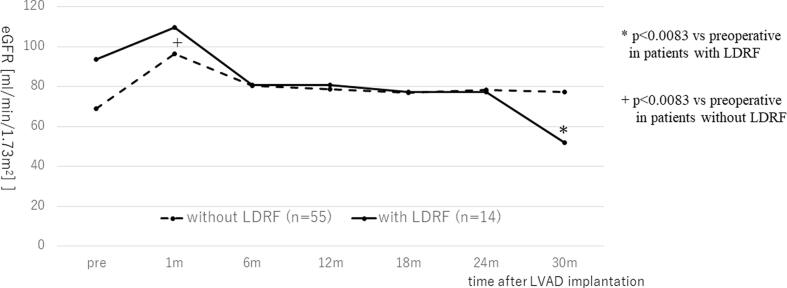
Subsequently, we divided patients into two groups, with or without a 25% decline in the eGFR at 30 months after LVAD implantation: patients with LDRF (N = 14) and without LDRF (N = 55). In patients without LDRF, their eGFR were maintained significantly higher than preoperative eGFR. On the other hand, in patients with LDRF, there was significantly decline in the eGFR 18 months after LVAD implantation (P = 0.034 at 18 months, P = 0.0067 at 24 months, and P < 00001 at 30 months (whose significance was verified after Bonferroni correction)) (Fig. 4).
Fig. 4.
Change in the eGFR in patients with LDRF (n = 14) and without LDRF (n = 55) and the results of multiple comparisons by Bonferroni method. Solid line = patients with LDRF; dotted line = patients without LDRF. LVAD; left ventricular assist device, eGFR; estimated glomerular filtration rate, LDRF; late deterioration in renal function.
Regarding risk factors for LDRF (Table 1), the patient’s background, including sex, age, body mass index, Brinkman index, or INTERMACS scoring, did not differ between groups. The pulmonary artery wedge pressure (PAWP) determined during preoperative RHC was significantly lower in patients with LDRF (16.8 ± 8.6 vs 22.8 ± 8.2 mmHg, P = 0.018). There were significant differences between the patients with and without LDRF in the ratio of patients with right atrial pressure (RAP)/PAWP and right ventricular stroke work index (RVSWI), which have been demonstrated previously as indicating risk for RVF [12] (RAP/PCWP: 0.78 ± 0.65 vs 0.41 ± 0.25, P = 0.0012; RVSWI: 4.4 ± 2.7 vs 7.6 ± 3.4 g·m/m2 /beat, P = 0.0019). Further, the preoperative eGFR was significantly higher in patients with LDRF (93.7 ± 38.6 vs 68.9 ± 28.4 mL/min/1.73 m2, P = 0.011). The LVDD in patients with LDRF was significantly smaller than in those without LDRF (62.2 ± 12.2 vs 73.9 ± 15.3 mm, P = 0.0093). Univariate analysis showed the preoperative eGFR, LVDD, PAWP, RAP/PAWP and RVSWI were significantly different between LDRF and non-LDRF (eGFR: Odds ratio (95% CI) = 45.9 (1.7–1234.6), P = 0.016, PAWP: OR = 0.039 (0.0024–0.65), P = 0.016, RAP/PAWP: OR = 62.8 (2.5–1584.7), P = 0.0044, RVSWI: OR = 62.8 (6.3e-6-0.11), P = 0.0006). We used RAP/PAWP as a risk for RVF in further analysis of risk factor for LDRF among RAP/PCWP, RVSWI and PAWP because it correlated with RVSWI or PAWP closely (RAP/PAWP vs PAWP; P = 0.0006, RAP/PAWP vs RVSWI; P < 0.0001). The cutoff values of preoperative LVDD and RAP/PAWP for the prediction of LDRF, determined by receiver operating characteristic curve analysis, were 67.0 mm and 0.57, respectively (area under the curve; LVDD = 0.73, RAP/PAWP = 0.65). Multivariate analysis confirmed that LVDD ≤ 67 mm and RAP/PAWP > 0.57 were independent risk factors of LDRF (Table 2).
Table 1.
Patient characteristics at preoperative point in patients with and without late deterioration in renal dysfunction and regression analysis for the prediction of late deterioration in renal dysfunction.
| with LDRF (n = 14) | without LDRF (n = 55) | p value | odds ratio | 95% CI | p value | |
|---|---|---|---|---|---|---|
| Sex (male, %) | 92.8 | 72.7 | 0.080 | 0.20 | 0.025–1.70 | 0.079 |
| Age (years) | 40.5 ± 11.6 | 39.9 ± 13.2 | 0.87 | 1.2 | 0.14–10.32 | 0.87 |
| BMI | 20.4 ± 2.3 | 20.3 ± 2.9 | 0.91 | 1.2 | 0.069–19.88 | 0.91 |
| Brinkman index | 48 [0–385] | 0 [0–360] | 0.75 | 0.8 | 0.071–10.65 | 0.91 |
| Diabetes mellitus (%) | 7.1 | 14.5 | 0.43 | 0.45 | 0.052–3.95 | 0.43 |
| DCM (%) | 50 | 76.4 | 0.061 | 0.31 | 0.092–1.05 | 0.061 |
| INTERMACS 2 (%) | 21.4 | 20.0 | 0.91 | |||
| 3 (%) | 57.2 | 56.4 | 0.96 | |||
| 4 (%) | 21.4 | 23.6 | 0.86 | |||
| Preoperative IABP support, (%) | 21.4 | 20.0 | 0.91 | 1.1 | 0.26–4.59 | 0.91 |
| β blocker (%) | 85.7 | 94.5 | 0.29 | 0.35 | 0.052–2.31 | 0.29 |
| RAASi (%) | 35.7 | 56.4 | 0.16 | 0.43 | 0.13–1.45 | 0.17 |
| Albumin (mg/dL) | 3.4 ± 0.5 | 3.6 ± 0.51 | 0.17 | 0.2 | 0.02–1.99 | 0.17 |
| BUN (ng/dL) | 16.3 ± 5.1 | 19.0 ± 7.9 | 0.23 | 0.08 | 0.001–4.74 | 0.19 |
| eGFR (mL/min/1.73 m2) | 93.7 ± 38.6 | 68.9 ± 28.4 | 0.011* | 45.9 | 1.7–1234.6 | 0.016* |
| Total bilirubin (mg/dL) | 1.2 ± 0.7 | 1.3 ± 0.7 | 0.76 | 0.65 | 0.04–10.43 | 0.75 |
| BNP (pg/mL) | 700.8 [276.7–1127.7] | 657.6 [443.3–1155.4] | 0.71 | 1.2 | 0.1–13.3 | 0.89 |
| proteinuria (%) | 28.6 | 16.4 | 0.32 | 2.0 | 0.52–7.98 | 0.30 |
| LVDD (mm) | 62.2 ± 12.1 | 73.9 ± 15.2 | 0.0093* | 0.004 | 4.63e-5-0.29 | 0.0054* |
| TR > moderate (%) | 50 | 23.6 | 0.061 | 3.2 | 0.95–10.92 | 0.059 |
| RAP (mmHg) | 10.1 ± 5.8 | 8.8 ± 4.2 | 0.36 | 2.9 | 0.29–28.76 | 0.36 |
| PAWP (mmHg) | 16.8 ± 8.6 | 22.8 ± 8.2 | 0.018* | 0.039 | 0.0024–0.65 | 0.016* |
| Cardiac Index (L/min/m2) | 2.0 ± 0.6 | 2.0 ± 0.4 | 0.89 | 1.3 | 0.048–33.95 | 0.88 |
| RAP/PAWP | 0.78 ± 0.65 | 0.41 ± 0.25 | 0.0012* | 62.8 | 2.5–1584.7 | 0.0044* |
| RVSWI (g·m/m2 /beat) | 4.4 ± 2.7 | 7.6 ± 3.4 | 0.0019* | 0.0008 | 6.3e-6-0.11 | 0.0006* |
| PAPi | 2.2 ± 1.6 | 3.1 ± 2.5 | 0.19 | 0.005 | 2.8e-6-10.1 | 0.11 |
*: Significantly different between with and without LDRF
LDRF; late deterioration in renal dysfunction, CI; confidence interval, BMI: body mass index, DCM: dilated cardiomyopathy, IABP: intra-aortic balloon pumping, RAASi; renin-angiotensin-aldosterone system inhibitor, AI; aortic insufficiently, eGFR; estimated glomerular filtration rate, BUN; blood urea nitrogen, BNP; brain natriuretic peptide, LVDD; left ventricular dimension diastolic, TR; tricuspid valve regurgitation, RAP; right atrial pressure, PAWP; pulmonary artery wedge pressure, RVSWI; right ventricular stroke work index, PAPi; pulmonary artery pulsatility index
Table 2.
Multivariate regression analysis of preoperative status for prediction of late deterioration in renal dysfunction.
| odds ratio | 95% CI | p value | |
|---|---|---|---|
| eGFR ≧ 60 mL/min/1.73 m2 | 1.70 | 0.38–7.54 | 0.48 |
| LVDD ≦ 67 mm | 5.49 | 1.24–24.26 | 0.025* |
| RAP / PAWP > 0.57 | 6.64 | 1.49–29.62 | 0.013* |
*: Significantly different between with and without late renal dysfunction
CI; confidence interval, eGFR; estimated glomerular filtration rate, LVDD; left ventricular dimension diastolic, RAP; right atrial pressure, PAWP; pulmonary artery wedge pressure
3.3. Association between LDRF and postoperative factors
During the perioperative period, although the incidence of perioperative AKI (57.1% vs 16.4%, P = 0.0028) was significantly higher in patients with LDRF, there was no significant difference between the two groups in RHC and laboratory examination, including the eGFR 1 month after LVAD implantation in univariate analysis (Table 3). The occurrence of complications such as AI, VA and late RVF was significantly higher in patients with LDRF (AI: 57.1% vs 18.9%, P = 0.0061; VA: 57.1% vs 21.8%, P = 0.012; late RVF: 35.7% vs 0%, P < 0.0001). Above all, late RVF events were most strongly associated with LDRF. No patient was depended on hemodialysis.
Table 3.
Perioperative parameters and clinical events in patients with and without late deterioration in renal function and univariate regression analysis for prediction of late deterioration in renal function.
| with LDRF (n = 14) | without LDRF (n = 55) | p value | Odds ratio | 95% CI | p value | |
|---|---|---|---|---|---|---|
| LVAD implantation - discharge | ||||||
| perioperative AKI (%) | 57.1 | 16.4 | 0.0028* | 6.8 | 1.90–24.43 | 0.0032* |
| Alb (mg/dL)(1) | 3.1 ± 0.5 | 3.2 ± 0.4 | 0.23 | 0.12 | 0.0035–3.89 | 0.22 |
| eGFR (mL/min/1.73 m2) (1) | 108.7 ± 45.8 | 96.4 ± 27.5 | 0.20 | 5.9 | 0.38–93.11 | 0.20 |
| T-bil (mg/dL)(1) | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.29 | 4.4 | 0.28–69.48 | 0.30 |
| BNP (pg/mL)(1) | 310.4 [ 177.9–732.0] | 236.9 [143.2–395.9] | 0.23 | 11.3 | 0.76–168.65 | 0.079 |
| RAP (mmHg)(1) | 9.4 ± 4.4 | 7.8 ± 3.9 | 0.20 | 4.9 | 0.43–55.79 | 0.20 |
| PAWP (mmHg)(1) | 10.1 ± 5.7 | 8.4 ± 4.9 | 0.27 | 3.8 | 0.34–43.75 | 0.28 |
| Cardiac Index (L/min/m2)(1) | 2.5 ± 0.8 | 2.5 ± 0.5 | 0.94 | 0.8 | 0.025–31.36 | 0.94 |
| RVSWI (g·m/m2/beat)(1) | 3.6 ± 3.4 | 4.0 ± 1.8 | 0.61 | 0.4 | 0.0082–16.3 | 0.60 |
| PAPi(1) | 2.1 ± 1.4 | 2.1 ± 1.5 | 0.86 | 0.7 | 0.013–35.97 | 0.86 |
| Aortic valve opening (%)(1) | 50.0 | 34.5 | 0.29 | 1.9 | 0.58–6.2 | 0.29 |
| Events from discharge to 30 months after LVAD implantation | ||||||
| AKI (%)(2) | 35.7 | 23.6 | 0.37 | 1.8 | 0.51–6.31 | 0.36 |
| Pump thrombosis (%)(2) | 14.3 | 12.7 | 0.88 | 1.1 | 0.21–6.22 | 0.88 |
| AI (%)(2) | 57.1 | 18.9 | 0.0061* | 5.7 | 1.62–20.26 | 0.0061* |
| DLI (%)(2) | 28.6 | 43.6 | 0.30 | 0.52 | 0.14–1.85 | 0.31 |
| Stroke (%)(2) | 7.1 | 23.6 | 0.13 | 0.25 | 0.030–2.08 | 0.13 |
| VA (%)(2) | 57.1 | 21.8 | 0.012* | 4.8 | 1.39–16.46 | 0.013* |
| late RVF (%)(2) | 35.7 | 0 | <0.0001* | 6.7 × 107 | n/a | <0.0001* |
| RAASi at 30 months (%) | 42.8 | 60 | 0.25 | 0.5 | 0.15–1.64 | 0.25 |
*: Significantly different between with and without LDRF
(1): result at 1 month after LVAD implantation
(2): the incidence of each adverse event after discharge from hospital following the index LVAD implantation up to 30 months
(3): the degree of AI could not be estimated in two patients due to poor study.
LVAD; left ventricular assist device, LDRF; late deterioration in renal function, CI: confidence interval, AKI; acute kidney injury, Alb; albumin, T-bil; total-bilirubin, BNP; brain natriuretic peptide, RAP; right atrial pressure, PAWP; pulmonary artery wedge pressure, RVSWI; right ventricular stroke work index, PAPi; pulmonary artery pulsatility index, RAASi; renin-angiotensin-aldosterone system inhibitor, AI; aortic insufficiently, DLI; driveline infection, VA; ventricular arrhythmia, RVF; right ventricular failure
4. Discussion
In this study, we analyzed the long-term renal function changes in patients with LVAD implanted as BTT. There were three notable findings in this study: (1) the eGFR was increased significantly at 1 month, returned to preoperative levels at 6 months, and then generally remained unchanged until 30 months after LVAD implantation; (2) high ratio of RAP/PAWP and small LVDD were risk factors of LDRF 30 months after LVAD implantation; and (3) LDRF under long-term LVAD support was related to perioperative AKI, late RVF, AI, and VA, particularly late RVF.
Several studies have assessed renal function under LVAD support for up to 1 year; [5], [13] however, there are not many studies on long-term renal function.
In this study, renal function generally remained unchanged until 30 months after LVAD implantation. Yoshioka et al. reported that the initial improvement in renal function is largely transient, and 78% of patients showed a gradual decline in eGFR [5]. Advanced age or heart failure with an ischemic cause were risk factors of LDRF. As reasons for that, they reported that in addition to insufficient blood flow, impaired renal function in elderly patients or those with ICM might also be caused by a reduced latent renal functional reserve (RFR). RFR is defined as the capacity of the kidney to increase eGFR in response to certain physiological or pathological stimuli or conditions [5], [14]. Hasin T et al. reported that early gains in renal function after LVAD implantation are not sustained in many patients and longer intraoperative bypass time and lower albumin 3 months after LVAD are associated with decline in eGFR at 12 months after LVAD [15]. Compared with these studies, most of our patients were young with a nonischemic cause of heart failure, which might be derived from common characteristics of candidate of heart transplantation in Japan [16], and they could maintain good nutritional status 1 month after LVAD implantation. As a result, renal function generally remained unchanged during LVAD support. On the other hand, several studies have reported that patients with a preoperative eGFR below 60 mL/min/1.73 m2 improved more in long-term LVAD support than those with a preoperative eGFR above that level [17], which corresponded to the results in our current study.
Concerning determinant factors for LDRF, Ross DW et al. reported that non-pulsatile flow LVAD, hemolysis, and RVF were risk factors of late kidney injury under LVAD [18]. On the other hand, analysis of the INTERMACS data showed that a gradual late decline in the eGFR was observed with both pulsatile and continuous-flow LVAD [19]. In this study, all patients had a non-pulsatile, continuous-flow LVAD, so we could not compare with pulsatile LVAD. We could, however, assess the long-term renal function by whether the LVAD was a centrifugal or an axial pump type. There were 21 patients with a centrifugal pump and 48 with an axial pump. The result was that there were no significant between-group differences in long-term renal function (percentage of LDRF; centrifugal type: 19.1% vs axial type: 20.8%, P = 0.87). Mehra MR et al. reported that although centrifugal pumps have a lower rate of reoperation for pump malfunction than axial pumps, there were no significant differences in renal function within 6 months of LVAD implantation, which was similar with our result [20]. We also assessed the impact of the presence of aortic valve opening on long-term renal function. There were 26 patients with aortic valve opening and 43 patients without aortic valve opening at 1 month after LVAD implantation. There were no significant differences between patients with and without aortic valve opening in long-term renal function (percentage of LDRF; 26.9% vs 16.3%, P = 0.29) but as this study was small, an additional larger-scale study is necessary for the confirmation of these results.
Late RVF is another important factor for worsening renal function [9], [21]. High ratio of RAP/PAWP, low RVSWI and small LVDD, known risk factors of late RVF, were associated with LDRF in this study. Late RVF can be secondary to causes such as VA, progression of tricuspid regurgitation, interventricular septal shift, and pulmonary hypertension. Although RVF can develop at any time during LVAD support, Rich et al. reported that most patients developed late RVF and required the initiation of inotropes>1 year after implantation [22]. After LVAD implantation, cardiac output increases, which increases RV wall stress [23], [24]. In addition, the chronic leftward shift of the LV septum may contribute to the gradual development and worsening of tricuspid regurgitation, leading to late RVF development [25], [26]. RV dysfunction will progress gradually under LVAD. In patients with late RVF, renal congestion, low renal perfusion (partly resulting from the adjustment of the LVAD pump speed), or the use of high-dose diuretic agents might significantly decrease the eGFR [27]. Indeed, five patients in this study developed late RVF after long-term LVAD support (average time to developing RVF, 26.4 ± 4.8 months), and their renal function declined (eGFR at 30 months after LVAD implantation in patients with late RVF, 35.5 ± 15.6 mL/min/1.73 m2).
Small LV was also associated with low cardiac output [28] or VAD thrombosis. [29] VAD thrombosis does not only develop low cardiac output with insufficient renal blood flow, but also induce hemolysis. Hemolysis, the breakdown of red blood cells and release of free hemoglobin, can induce acute tubular necrosis, leading to the decrease of renal function [30]. In this study, although the incidence of the VAD thrombosis or hemolysis was not different between two groups, the level of lactate dehydrogenase in patients with LVDD ≤ 67 mm at 30 months after LVAD implantation was significantly higher compared with LVDD > 67 mm (LVDD ≤ 67 mm vs LVDD > 67 mm: 481.3 ± 270.4 vs 360.6 ± 181.7 U/L, P = 0.023), so VAD thrombosis or hemolysis derived from small LV might have some impacts on the development of LDRF.
Interestingly, perioperative AKI, and post-discharge VA and AI were also related to developing LDRF. Perioperative AKI occurs frequently after LVAD implantation and has a detrimental effect on survival [22]. Our study determined that although preoperative renal function in patients with perioperative AKI was lower than in those without, the difference in the eGFR between patients with and without perioperative AKI diminished gradually over the 30 months after LVAD implantation (Supplementary Fig. 2.). In contrast, patients with LDRF had a high probability of developing perioperative AKI. Patients with perioperative AKI and patients with LDRF might be different, however, have some common characteristics, such as baseline RAP/PAWP. However, Wettersten N, reported younger age and lower eGFR were associated with improvements in eGFR at later months [31]. Thus, various factors might be intertwined to improvement and exacerbation for renal function in each time after LVAD implantation.
Postimplant VA is common after LVAD implantation and is associated with increased hospitalization, RVF, and increased mortality [32]. In this study, the incidence of VA was significantly associated with renal dysfunction; however, the impact of VAs on renal function is unknown, although there are several possibilities. Bedi et al. reported that VAs could contribute to significant RV dysfunction and diminished pump preload [32]. Antiarrhythmic drugs might also have a negative inotropic effect on the right heart [33]. Galand et al. reported that patients who developed late VAs were significantly more likely to die from a cardiovascular cause, such as RVF, LVAD thrombosis, or electrical storm [34].
Postoperative AI is also frequent after continuous-flow LVAD implantation, and the underlying mechanisms are probably multifactorial [35]. AI diminishes cardiac output, induces RVF, and contributes to renal dysfunction.
In this study, we defined LDRF as a decrease in the eGFR by>25% following several studies which investigated about worsening renal function in heart failure [36], [37], [38], [39], and we found a good correlation between LDRF and clinical events after LVAD implantation. The determining factor for the deterioration of renal function was changed if the criteria of LDRF would be changed. When we used other criteria such as an eGFR decline > 30% or eGFR below 60 mL/min/1.73 m2 at 30 months, the result did not change in the former criteria and the latter criteria led to the results which was heavily affected by preoperative renal function (data not shown). Indeed, the fine correlation between clinical events and LDRF in this study was demonstrated so that the clinical relevance of the definition of LDRF in this study was considered to be comparatively high. We believe that further studies, which include the eGFR of another population, are required.
Our study had some limitations. First, this was a retrospective study in a single center. The number of patients with LDRF was low, thereby limiting the statistical power. Second, we could not exclude the impact of medications that affected renal function. For instance, some patients required long-term antimicrobial medications for LVAD-related infections. Third, we defined eGFR just before LVAD implantation as baseline eGFR, which might be affected by the condition of advanced heart failure. Forth, renal function was assessed by the eGFR based on serum creatinine, and other factors, such as changes in weight or physical constitution, were unknown. The data of albuminuria was not available, therefore the assessment of chronic kidney disease using KDIGO guideline could not be performed. Fifth, when analyzing long-term changes in renal function, we only used the data of patients who continued 1 year after LVAD implantation and survived at least 30 months under LVAD support. Therefore, patients who underwent heart transplantation, LVAD removal, died within 30 months or <30 months under LVAD were excluded from the consecutive cases, which might lead to some selection bias. Eleven patients died within 30 months of LVAD implantation from stroke, infection, and RVF, and the interaction between survival rate and renal function was not obvious in this study.
In conclusion, renal function improved after LVAD implantation; however, there were some cases of LDRF in long-term follow-up under LVAD implantation. The risk factors for LDRF were a high RAP/PAWP ratio, and small LVDD. In addition, the presence of LDRF corresponded to LVAD-related complications including RVF.
CRediT authorship contribution statement
Chie Bujo: Conceptualization, Methodology, Data curation, Validation, Writing – original draft, Writing – review & editing. Eisuke Amiya: Conceptualization, Methodology, Validation, Data curation, Writing – review & editing. Masaru Hatano: Supervision. Junichi Ishida: . Masaki Tsuji: Data curation. Nobutaka Kakuda: Data curation. Koichi Narita: Data curation. Akihito Saito: Data curation. Hiroki Yagi: Data curation. Masahiko Ando: . Shogo Shimada: . Mitsutoshi Kimura: . Osamu Kinoshita: . Minoru Ono: Supervision. Issei Komuro: Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [EA, MH, and MT belong to the Department of Therapeutic Strategy for Heart Failure, Graduate School of Medicine, University of Tokyo, which is endowed by NIPRO-Corp, Terumo-Corp., Senko-Medical-Instrument-Mfg., Century-Medical-Inc., ONO-pharmaceutical-Co.,Ltd. Medtronic-JAPAN Co.,Ltd, Nippon-Shinyaku Co.,Ltd, Mochida Pharmaceutical Co., Boehringer Ingelheim Pharmaceuticals Inc, Abiomed-Inc, AQuA-Inc, Fukuda-Denshi Co., Ltd, and Sun-Medical-Technology-Research Corp. The other authors have no conflicts of interest to disclose. There are no patents, products in development, or marketed products to declare.].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100907.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
References
- 1.Rose E.A., Gelijns A.C., Moskowitz A.J., Heitjan D.F., Stevenson L.W., Dembitsky W., Long J.W., Ascheim D.D., Tierney A.R., Levitan R.G., Watson J.T., Ronan N.S., Shapiro P.A., Lazar R.M., Miller L.W., Gupta L., Frazier O.H., Desvigne-Nickens P., Oz M.C., Poirier V.L., Meier P. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin J.K., Pagani F.D., Kormos R.L., Stevenson L.W., Blume E.D., Myers S.L., Miller M.A., Baldwin J.T., Young J.B., Naftel D.C. Eighth annual INTERMACS report. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36(10):1080–1086. doi: 10.1016/j.healun.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Haddad M., Hendry P.J., Masters R.G., Mesana T., Haddad H., Davies R.A., Mussivand T.V., Struthers C., Keon W.J. Ventricular assist devices as a bridge to cardiac transplantation: the Ottawa experience. Artif Organs. 2004;28(2):136–141. doi: 10.1111/j.1525-1594.2003.47331.x. [DOI] [PubMed] [Google Scholar]
- 4.Hasin T., Topilsky Y., Schirger J.A., Li Z., Zhao Y., Boilson B.A., Clavell A.L., Rodeheffer R.J., Frantz R.P., Edwards B.S., Pereira N.L., Joyce L., Daly R., Park S.J., Kushwaha S.S. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol. 2012;59(1):26–36. doi: 10.1016/j.jacc.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Yoshioka D., Takayama H., Colombo P.C., Yuzefpolskaya M., Garan A.R., Topkara V.K., Han J., Kurlansky P., Naka Y., Takeda K. Changes in end-organ function in patients with prolonged continuous-flow left ventricular assist device support. Ann Thorac Surg. 2017;103(3):717–724. doi: 10.1016/j.athoracsur.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima N., Ono M., Saiki Y., Sawa Y., Nunoda S., Isobe M. Registry report on heart transplantation in Japan (June 2016) Circ J. 2017;81(3):298–303. doi: 10.1253/circj.CJ-16-0976. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., Yamagata K., Tomino Y., Yokoyama H., Hishida A. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 8.Uemura O., Nagai T., Ishikura K., Ito S., Hataya H., Gotoh Y., Fujita N., Akioka Y., Kaneko T., Honda M. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol. 2014;18(4):626–633. doi: 10.1007/s10157-013-0856-y. [DOI] [PubMed] [Google Scholar]
- 9.Davison SN, Levin A, Moss AH, Jha V, Brown EA, Brennan F, Murtagh FE, Naicker S, Germain MJ, O’Donoghue DJ, Morton RL, Obrador GT, Kidney Disease: Improving Global Outcomes. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int 2015;88:447-459. [DOI] [PubMed]
- 10.Starling R.C., Moazami N., Silvestry S.C., Ewald G., Rogers J.G., Milano C.A., Rame J.E., Acker M.A., Blackstone E.H., Ehrlinger J., Thuita L., Mountis M.M., Soltesz E.G., Lytle B.W., Smedira N.G. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 11.Lampert B.C., Teuteberg J.J. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant. 2015;34(9):1123–1130. doi: 10.1016/j.healun.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Kormos R.L., Teuteberg J.J., Pagani F.D., Russell S.D., John R., Miller L.W., Massey T., Milano C.A., Moazami N., Sundareswaran K.S., Farrar D.J., HeartMate I.I. Clinical Investigators. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–1324. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Sandner S.E., Zimpfer D., Zrunek P., Rajek A., Schima H., Dunkler D., Grimm M., Wolner E., Wieselthaler G.M. Renal function and outcome after continuous flow left ventricular assist device implantation. Ann Thorac Surg. 2009;87(4):1072–1078. doi: 10.1016/j.athoracsur.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A., Mucino M.J., Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract. 2014;127(1-4):94–100. doi: 10.1159/000363721. [DOI] [PubMed] [Google Scholar]
- 15.Hasin T., Grupper A., Dilon J.J., Maleszewski J.J., Li Z., Topilsky Y., Frantz R.P., Edwards B.S., Pereira N.L., Maltais S., Stulak J.M., Joyce L., Dalay R., Park S.J., Kushwaha S.S. Early Gains in Renal Function Following Implantation of HeartMate Ⅱ Left Ventricular Assist Devices May Not Persist to One Year. ASAIO J. Jul/Aug. 2017;63:401–407. doi: 10.1097/MAT.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 16.Nakatani T., Fukushima N., Ono M., Saiki Y., Matsuda H., Nunoda S., Sawa Y., Isobe M. The registry Report of Heart Transplantation in Japan (1999–2014) Circ J. 2016;80(1):44–50. doi: 10.1253/circj.CJ-15-0975. [DOI] [PubMed] [Google Scholar]
- 17.Daimee UA, Wang M, Papernov A, Sherazi S, McNitt S, Vidula H, Chen L, Alexis JD, Kutyifa V. Renal function changes following left ventricular assist device implantation. Am J Cardiol 2017;120:2213-2220.17. [DOI] [PubMed]
- 18.Ross D.W., Stevens G.R., Wanchoo R., Majure D.T., Jauhar S., Fernandez H.A., Merzkani M., Jhaveri K.D. Left ventricular assist devices and the Kidney. Clin J Am Soc Nephrol. 2018;13(2):348–355. doi: 10.2215/CJN.04670417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brisco M.A., Kimmel S.E., Coca S.G., Putt M.E., Jessup M., Tang W.W.H., Parikh C.R., Testani J.M. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail. 2014;7(1):68–75. doi: 10.1161/CIRCHEARTFAILURE.113.000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC, Colombo PC, Walsh MN, Milano CA, Patel CB, Jorde UP, Pagani FD, Aaronson KD, Dean DA, McCants K, Itoh A, Ewald GA, Horstmanshof D, Long JW, Salerno C, MOMENTUM 3 Investigators. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 2017;376:440-450. [DOI] [PubMed]
- 21.Kato T.S., Farr M., Schulze P.C., Maurer M., Shahzad K., Iwata S., Homma S., Jorde U., Takayama H., Naka Y., Gillam L., Mancini D. Usefulness of two-dimensional echocardiographic parameters of the left side of the heart to predict right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2012;109(2):246–251. doi: 10.1016/j.amjcard.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Rich J.D., Gosev I., Patel C.B., Joseph S., Katz J.N., Eckman P.M., Lee S., Sundareswaran K., Kilic A., Bethea B., Soleimani B., Lima B., Uriel N., Kiernan M. Evolving Mechanical Support Research Group. The incidence, risk factors, and outcomes associated with late right-sided heart failure in patients supported with an axial-flow left ventricular assist device. J Heart Lung Transplant. 2017;36(1):50–58. doi: 10.1016/j.healun.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Barbone A., Holmes J.W., Heerdt P.M., The’ A.H.S., Naka Y., Joshi N., Daines M., Marks A.R., Oz M.C., Burkhoff D. Comparison of right and left ventricular responses to left ventricular assist device support in patients with severe heart failure: a primary role of mechanical unloading underlying reverse remodeling. Circulation. 2001;104(6):670–675. doi: 10.1161/hc3101.093903. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad T., Wang T., O’Brien E.C., Samsky M.D., Pura J.A., Lokhnygina Y., Rogers J.G., Hernandez A.F., Craig D., Bowles D.E., Milano C.A., Shah S.H., Januzzi J.L., Felker G.M., Patel C.B. Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation, and renal injury. JACC Heart Fail. 2015;3:30–39. doi: 10.1016/j.jchf.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Romano M.A., Cowger J., Aaronson K.D., Pagani F.D. Diagnosis and management of right-sided heart failure in subjects supported with left ventricular assist devices. Curr Treat Options Cardiovasc Med. 2010;12(5):420–430. doi: 10.1007/s11936-010-0091-8. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi K., Homma S., Han J., Takayama H., Colombo P.C., Yuzefpolskaya M., Garan A.R., Farr M.A., Kurlansky P., Di Tullio M.R., Naka Y., Takeda K. Usefulness of tricuspid annular diameter to predict late right sided heart failure in patients with left ventricular assist device. Am J Cardiol. 2018;122(1):115–120. doi: 10.1016/j.amjcard.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Gambardella I., Gaudino M., Ronco C., Lau C., Ivascu N., Girardi L.N. Congestive kidney failure in cardiac surgery: the relationship between central venous pressure and acute kidney injury. Interact Cardiovasc Thorac Surg. 2016;23(5):800–805. doi: 10.1093/icvts/ivw229. [DOI] [PubMed] [Google Scholar]
- 28.Imamura T., Kinugawa K., Kato N., Muraoka H., Fujino T., Inaba T., Maki H., Kinoshita O., Hatano M., Kyo S., Ono M. Late-onset right ventricular failure in patients with preoperative small left ventricle after implantation of continuous flow left ventricular assist device. Circ J. 2014;78(3):625–633. doi: 10.1253/circj.cj-13-1201. [DOI] [PubMed] [Google Scholar]
- 29.Chivukula V.K., Beckman J.A., Prosco A.R., Lin S., Dardas T.F., Cheng R.K., Farris S.D., Smith J.W., Mokadam N.A., Mahr C., Aliseda A. Small Left Ventricular Size Is an Independent Risk Factor for Ventricular Assist Device Thrombosis. ASAIO J. 2019;65:152–159. doi: 10.1097/MAT.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues J., Alam A., Podymow T., Bernard C., Giannetti N. Secondary hemosiderosis on kidney biopsy in a patient with a left ventricular assist device. Am J Med Sci. 2014;347(2):172–173. doi: 10.1097/MAJ.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 31.Wettersten N., Estrella M., Brambatti M., Horiuchi Y., Adler E., Pretorius V., Murray P.T., Shlipak M., Ix J.H. Kidney Function Following Left Ventricular Assist Device Implantation: An Observational Cohort Study. Kidney Med. 2021;3(3):378–385.e1. doi: 10.1016/j.xkme.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedi M., Kormos R., Winowich S., McNamara D.M., Mathier M.A., Murali S. Ventricular arrhythmias during left ventricular assist device support. Am J Cardiol. 2007;99(8):1151–1153. doi: 10.1016/j.amjcard.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 33.Faber T.S., Zehender M. Antiarrhythmic therapy in patients with heart failure. Ther Umsch. 2000;57:324–332. doi: 10.1024/0040-5930.57.5.324. [DOI] [PubMed] [Google Scholar]
- 34.Galand V., Flécher E., Auffret V., Boulé S., Vincentelli A., Dambrin C., Mondoly P., Sacher F., Nubret K., Kindo M., Cardi T., Gaudard P., Rouvière P., Michel M., Gourraud J.B., Defaye P., Chavanon O., Verdonk C., Ghodbane W., Pelcé E., Gariboldi V., Pozzi M., Obadia J.F., Litzler P.Y., Anselme F., Babatasi G., Belin A., Garnier F., Bielefeld M., Hamon D., Radu C., Pierre B., Bourguignon T., Eschalier R., D’Ostrevy N., Bories M.C., Marijon E., Vanhuyse F., Blangy H., Verhoye J.P., Leclercq C., Martins R.P., ASSIST-ICD Investigators Predictors and clinical impact of late ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. JACC Clin Electrophysiol. 2018;4:1166–1175. doi: 10.1016/j.jacep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Bouabdallaoui N., El-Hamamsy I., Pham M., Giraldeau G., Parent M.-C., Carrier M., Rouleau J.L., Ducharme A. Aortic regurgitation in patients with a left ventricular assist device: A contemporary review. J Heart Lung Transplant. 2018;37(11):1289–1297. doi: 10.1016/j.healun.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Vardeny O., Wu D.H., Desai A., Rossignol P., Zannad F., Pitt B., Solomon S.D., Investigators R.A.L.E.S. Influence of baseline and worsening renal function on efficacy of spironolactone in patients With severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study) J Am Coll Cardiol. 2012;60:2082–2089. doi: 10.1016/j.jacc.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 37.Rossignol P., Cleland J.G.F., Bhandari S., Tala S., Gustafsson F., Fay R., Lamiral Z., Dobre D., Pitt B., Zannad F. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the Eplerenone Post – Acute Myocardial Infarction Heart Failure Efficacy and Survival Study. Circulation. 2012;125(2):271–279. doi: 10.1161/CIRCULATIONAHA.111.028282. [DOI] [PubMed] [Google Scholar]
- 38.Damman K., Solomon S.D., Pfeffer M.A., Swedberg K., Yusuf S., Young J.B., Rouleau J.L., Granger C.B., McMurray J.J.V. Worsening renal function and outcome in heart failure patients with reduced and preserved ejection fraction and the impact of angiotensin receptor blocker treatment: data from the CHARM – study programme. Eur J Heart Fail. 2016;18(12):1508–1517. doi: 10.1002/ejhf.609. [DOI] [PubMed] [Google Scholar]
- 39.Mullens W., Damman K., Testani J.M., Martens P., Mueller C., Lassus J., Tang W.H.W., Skouri H., Verbrugge F.H., Orso F., Hill L., Ural D., Lainscak M., Rossignol P., Metra M., Mebazaa A., Seferovic P., Ruschitzka F., Coats A. Evaluation of kidney function throughout the heart failure trajectory – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:584–603. doi: 10.1002/ejhf.1697. [DOI] [PubMed] [Google Scholar]