Abstract
Background
Grade 3 gastroenteropancreatic neuroendocrine neoplasms (G3 GEPNENs) are often aggressive, and the optimal treatment is unclear for this subgroup of neuroendocrine neoplasms (NENs). Temozolomide (TEM)‐based regimens have been increasingly used to treat grade 1–2 NENs, but their efficacy in G3 NENs remains undetermined. We aimed to assess the clinical efficacy of TEM‐containing regimens in advanced grade 3 GEPNENs.
Materials and Methods
A multicenter retrospective review (2008–2018) of patients with metastatic/unresectable G3 GEPNENs who received a TEM‐containing regimen was undertaken within a North American partnership to pool data. The primary endpoint was time to treatment failure (TTF). Radiologic response was extracted from local reports.
Results
One hundred and thirty patients in six high‐volume NEN centers were included (median age 55, 64% male, 18% functional, 67% pancreatic NEN). Forty‐nine percent were well‐differentiated, 35% poorly differentiated, and 15% unknown based on local pathology reports. The regimen used was capecitabine and temozolomide (CAPTEM) in 92% and TEM alone in 8%. Radiological response by local assessment was seen in 36% of patients. Median TTF was 3.6 months and median overall survival (OS) 19.2 months. Six percent of patients required discontinuation of therapy due to adverse events. TTF was longer in first‐line treatment (7.8 months vs. 2.9 months; hazard ratio, 1.62; 95% confidence interval, 1.11–2.36; p = .015) and in patients with pancreatic NENs (panNENs) compared with gastrointestinal NENs (5.8 months vs 1.8 months; p = .04). The overall response rate was higher in the first‐line setting (51% vs 29%; p = .02) and in panNEN (41% vs 23%; p = .04).
Conclusion
This is the largest TEM treatment series in G3 NEN, involving collaboration of several major North American NEN centers as a partnership. Thirty‐six percent of patients showed some degree of radiographic response, and treatment was generally well tolerated, although the median duration of response was short. Response rates and time to treatment failure were superior in the first‐line setting. CAPTEM should be considered a viable treatment option in this setting. Further randomized trials are warranted.
Implications for Practice
Neuroendocrine neoplasms (NENs) are heterogeneous, and optimal treatment for aggressive grade 3 (G3) NENs remains undetermined. The capecitabine and temozolomide (CAPTEM) regimen has been used in low‐grade pancreas NENs but there are few data for its safety and efficacy in the G3 setting. This article reports on the efficacy of temozolomide‐containing regimens, particularly CAPTEM, in management of G3 NENs. The good tolerance and response rate show that CAPTEM should be considered a viable regimen in treatment of G3 NENs pending confirmatory prospective studies.
Keywords: Neuroendocrine tumors, Chemotherapy, Temozolomide, Capecitabine and temozolomide, Outcomes
Short abstract
There has been increasing interest in the combination of capecitabine and temozolomide (CAPTEM) for the treatment of gastroenteropancreatic neuroendocrine neoplasm (GEPNEN). This review reports a large retrospective analysis to report the efficacy and tolerability of temozolomide (TEM)‐containing regimens, particularly CAPTEM, in patients with grade 3 GEPNENs.
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of tumors originating from cells in the diffuse neuroendocrine system [1]. They are located throughout the body, being most commonly found in the gastrointestinal (GI) tract (stomach, small and large bowel, pancreas) and the lungs. Anatomically, gastrointestinal NENs can be divided into those from foregut, midgut and hindgut origin [2].
Systemic therapy is the mainstay of treatment for nonoperable and metastatic NENs. Treatment choice for these advanced NENs is often driven by the grade of the tumor. Gastroenteropancreatic neuroendocrine neoplasms (GEPNENs) are generally graded by the World Health Organization (WHO) 2017 (pancreas) and 2019 (GI) classification systems, relying on the mitotic count and Ki‐67 index to divide tumors into three grades. Grade 1 tumors generally display indolent behavior, whereas grade 3 tumors predict a poor prognosis despite aggressive treatment [3, 4, 5]. There are multiple proven treatment options for grade 1–2 tumors such as somatostatin analogs (octreotide, lanreotide), targeted agents such as everolimus and sunitinib [6, 7, 8], and peptide receptor radionuclide therapy [9]. However, the optimal treatment for G3 NENs remains unknown.
Grade 3 GEPNENs remain a challenging group of tumors to manage despite recent advances in understanding their biology. These aggressive neoplasms are often treated with cytotoxic chemotherapy, but there are no randomized data to support this practice. Platinum‐containing chemotherapy is often used as first‐line treatment, but there are few proven options in the second‐line setting. The new WHO classifications subdivided G3 GEPNENs into G3 well‐differentiated neuroendocrine tumors (WDNENs) and G3 poorly differentiated neuroendocrine carcinomas (PDNECs), and recent data have shown poorer outcomes with PDNECs [10, 11, 12]. However, there are few data to date suggesting that WDNENs and PDNECs have differential response rates to systemic therapies. Therefore, differentiation status cannot currently be considered as a strong predictive biomarker to guide therapy choices for G3 NENs. In addition, as multiple histological features are considered in determining differentiation status, some challenging cases may display some features of both WDNENs and PDNECs, making this binary classification difficult to apply in all cases. It remains unclear whether G3 WDNENs should be treated with targeted agents such as everolimus and sunitinib, chemotherapy, somatostatin analogs, peptide receptor radionuclide therapy (PRRT), or other modalities [13, 14]. Without clear evidence to demonstrate the efficacy of these regimens, there is the real risk that patients with G3 GEPNENs miss out on efficacious treatment in an aggressive disease, resulting in poorer outcomes.
There has been increasing recent interest regarding the combination of capecitabine and temozolomide (CAPTEM) in the treatment of GEPNENs. Capecitabine is a prodrug of 5‐fluorouracil, a thymidylate synthase inhibitor. Temozolomide is an alkylating agent that is also used in glioblastoma multiforme [15]. The CAPTEM combination showed promising results in multiple retrospective studies of patients with pancreatic neuroendocrine tumors [16, 17, 18, 19]. A recent prospective randomized trial has shown that CAPTEM is superior to temozolomide monotherapy in G1–2 pancreatic NEN [20]. Median progression‐free survival in the CAPTEM arm of this study was 22.7 months versus 14.4 months in the temozolomide (TEM) arm (hazard ratio [HR] 0.58; p = .023), and response rates were 33.3% and 27.8%, respectively. Although there is thought to be clinical utility from CAPTEM in the NEN treatment community, there are more limited data on its efficacy in extrapancreatic NENs and grade 3 NENs. These data have been hard to obtain because of the uncommon nature of NENs and particularly G3 NENs, as well as the changing pathological landscape. Recognizing that any attempt at better understanding the efficacy of this promising treatment combination would require collaboration among the neuroendocrine tumour (NET) community, we performed a large retrospective analysis to report the efficacy and tolerability of TEM‐containing regimens, particularly CAPTEM, in patients with grade 3 GEPNENs.
Materials and Methods
This was a multicenter retrospective review conducted at six high‐volume NEN centers in North America, collaborating as a consortium. A common data collection form was used by each participating institution.
Inclusion and Exclusion Criteria
Patients were identified from institutional databases and chart review. Patients were eligible for inclusion if they had a histologically confirmed diagnosis of unresectable/metastatic neuroendocrine neoplasm with gastrointestinal or pancreatic origin, classified as grade 3 by the WHO 2017 or WHO 2019 criteria, respectively, as defined by a Ki‐67 index of >20% and/or a mitotic count greater than 20 per 10 high power fields [4, 5]. Tissue samples were not subject to review at time of data abstraction. Patients with tumors of unknown primary, clinically suspected to be from the gastrointestinal tract or pancreas, were eligible. Included patients needed to have been prescribed temozolomide‐containing therapy from January 2008 to December 2018 at the participating center for at least one cycle and be aged 18 or over at the time of commencing such therapy. In keeping with the real‐world nature of this study, no minimum dose of TEM was required for inclusion. Previous lines of therapy were allowed. Patients were excluded if they had a diagnosis of Merkel cell carcinoma or mixed neuroendocrine non‐neuroendocrine neoplasm.
Study Endpoints
The primary endpoint of the study was time to treatment failure (TTF), defined as the time from initiating TEM treatment to cessation of such treatment or death, censored at the date of last follow‐up. Secondary endpoints included response rate (defined as a composite of complete and partial responses extracted from local reports), overall survival (OS; the time from initiating treatment to death, censored at date of last follow‐up), and the incidence of dose reductions/discontinuations due to adverse events.
Statistical Analysis
Patient demographics were presented descriptively. Data were analyzed on an intention‐to‐treat basis. The median TTF and OS were calculated and presented using the Kaplan‐Meier method. Subgroup analyses were performed based on regimen (CAPTEM vs. TEM alone vs other), differentiation status (well‐differentiated G3 [WD] vs. poorly differentiated G3[PD]), and primary tumor site (pancreatic NEN vs. other GI NEN). Statistical analyses were performed using Microsoft Excel (Microsoft, Redmond, WA) and Graphpad Prism (version 8.3.1; Graphpad Software, San Diego, CA) to conduct χ2 and log‐rank tests as appropriate.
Ethical Approval
This study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board, reference number 257‐2017, as well as the relevant regulatory authorities at each participating site.
Results
Demographics
One hundred and thirty patients were identified from six participating centers (Table 1). Approximately two thirds of the patients were male, and the median age at TEM initiation was 56 (range, 20–95). As expected, the majority of included patients had a pancreatic primary (67%). Approximately half of patients had well‐differentiated tumors, and 67% of patients had tumors with a Ki‐67 index <55%. The median Ki‐67 index was 40% (range, 20%–95%). Of the patients who underwent functional imaging (n = 69; 43 with OctreoScan (Covidien, Hazelwood, Missouri, U.S.A.), 21 with 68Gallium‐based positron emission tomography (PET), 5 with both), 81% had uptake in at least one site of disease. Most of the patients were treated with CAPTEM (92%) rather than TEM monotherapy (8%); no other TEM‐containing regimens were used. The median follow‐up time from the start of TEM therapy was 16.8 months.
Table 1.
Baseline characteristics (n = 130)
| Patient characteristics | n (%) |
|---|---|
| Age, median (range), yr | 55 (20–95) |
| Gender | |
| Male | 83 (64) |
| Female | 47 (36) |
| Functional tumor | |
| Yes | 23 (18) |
| No | 107 (82) |
| Primary tumor site | |
| Pancreas | 87 (67) |
| Colorectal | 9 (7) |
| Small bowel | 6 (5) |
| Other GI | 12 (9) |
| Unknown, thought to be of GI origin | 16 (12) |
| Ki‐67 index | |
| <55% | 87 (67) |
| ≥55% | 29 (22) |
| Unknown | 14 (11) |
| Differentiation | |
| Well‐differentiated | 64 (49) |
| Poorly differentiated | 46 (35) |
| Unknown | 20 (15) |
| Number of prior systemic therapies | |
| 0 | 37 (28) |
| 1 | 58 (45) |
| 2+ | 35 (27) |
| Prior therapies used | |
| Platinum‐based chemotherapy | 58 (45) |
| Other chemotherapy | 11 (8) |
| Everolimus or sunitinib | 11 (8) |
| Somatostatin analogs | 35 (27) |
| Initial regimen used | |
| CAPTEM | 120 (92) |
| TEM | 10 (8) |
Abbreviations: CAPTEM, capecitabine and temozolomide; GI, gastrointestinal; TEM, temozolomide.
Dose Intensity and Dose Reductions
Dose reductions occurred in 14/103 patients on whom these data were available (14%)—9 from cytopenias (2 with leukopenia/neutropenia, 4 with thrombocytopenia, 3 with pancytopenia), 4 from general fatigue/nausea, and 1 from diarrhea. Thirteen patients (10%) were hospitalized during treatment (pain, 3; fatigue, 3; fever, 2; cardiac vasospasm, dehydration, gastrointestinal bleed, hypercalcemia, diarrhea, 1 each). There were no recorded deaths due to treatment toxicity. Treatment was predominantly given every 4 weeks, with 14 days of capecitabine treatment and 5 days of temozolomide treatment concurrent with the capecitabine. Full information on therapy dose was available on 48 patients (37%). The median starting dose of capecitabine was 1,500 mg b.i.d. (interquartile range [IQR], 1,250–1,500 mg), and the median starting dose of temozolomide was 200 mg/m2 daily (IQR, 200–200 mg/m2). The median duration of treatment was 3.6 months.
The reason for discontinuation of CAPTEM therapy was disease progression in 78 (60%), toxicity in 8 (6%), patient decision for treatment discontinuation in 9 (7%), other in 7 (5%), and unknown in 22 (17%). At time of data analysis, CAPTEM therapy was ongoing in six patients (5%). Cited reasons for discontinuation due to toxicity included fatigue, cardiac vasospasm, and hand‐foot syndrome.
Treatment Efficacy
The median time to treatment failure in the overall cohort was 3.6 months (Table 2). TTF was longer in patients treated with CAPTEM compared with TEM monotherapy, although this was not statistically significant (4.6 months vs. 1.2 months; p = .09). TTF was longer in patients on first‐line treatment compared with later‐line treatment (7.8 months vs. 2.9 months; HR, 1.62; 95% CI, 1.11–2.36; p = .015; Fig. 1) and in patients with a pancreatic NEN (panNEN) compared with a GI NEN (5.8 months vs. 1.8 months; p = .04). TTF did not differ significantly by Ki‐67 index (Ki‐67 < 55%, 6.1 months vs. Ki‐67 > 55%, 2.3 months; p = .28), nor by differentiation status (WD, 5.7 months vs. PD, 2.0 months; p = .33).
Table 2.
Treatment efficacy outcomes stratified by differentiation status
| Efficacy measure | All patients | WD G3 NETs | G3 NECs |
|---|---|---|---|
| TTF | 3.6 months | 5.7 months | 2.0 months (vs. G3 NETs: p = .33) |
| OS | 19.2 months | 31.7 months | 13.1 months (vs. G3 NETs: p = .01) |
| RR | 36% | 41% | 26% (vs. G3 NETs: p = .63) |
Abbreviations: G3, grade 3; NEC, neuroendocrine carcinoma; NET, neuroendocrine tumour; OS, overall survival; RR, response rate; TTF, time to treatment failure; WD, well‐differentiated.
Figure 1.
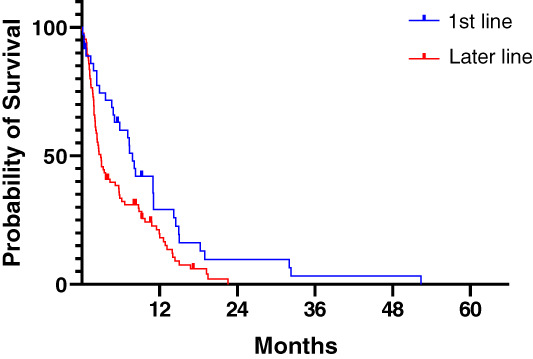
Time to treatment failure (TTF) by line of therapy. The median TTF was 7.8 months for patients treated in the first‐line setting compared with 2.9 months in later lines (p = .015, log‐rank test).
The response rate reported by local radiological reports was 36%. The best response achieved was complete response in 1 patient (1%), partial response in 45 (35%), stable disease in 26 (20%), progressive disease in 38 (29%), and unknown in 20 (15%). The response rate was higher in the first‐line setting compared with later‐line settings (51% vs. 29%; χ2 test p = .02), and in patients with panNEN compared with those with GI NEN (41% vs. 23%; χ2 test p = .04). Response rates were non‐significantly higher in well‐differentiated NENs compared with poorly differentiated NECs (41% vs. 26%; p = .63), and in those with Ki‐67 index <55% compared with those with Ki‐67 index >55% (39% vs. 14%; p = .14). The response rate was 37% (44/120) in patients receiving CAPTEM compared with 20% (2/10) in patients receiving temozolomide monotherapy (p = .29).
Overall Survival
The median overall survival for this cohort was 19.2 months. OS was longer in patients treated in the first‐line setting compared with later‐line settings (41.2 months vs. 15.2 months; HR, 0.51; 95% CI, 0.32–0.82; p = .015) and longer in patients with well‐differentiated NEN than in those with PDNEC (31.7 months vs. 13.1 months; p = .01; Fig 2). OS was not significantly different by Ki‐67 index, primary site, or regimen choice.
Figure 2.
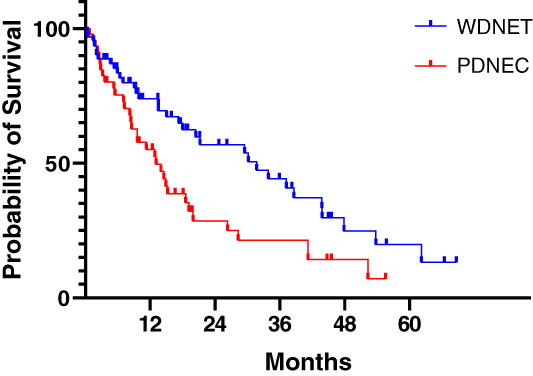
Overall survival (OS) by differentiation status. The median OS was 31.7 months for WDNET versus 13.1 months for PDNEC (hazard ratio, 0.55; 95% confidence interval, 0.33–0.92; p = .01, log‐rank test).
Abbreviations: PDNEC, poorly differentiated neuroendocrine carcinoma; WDNET, well‐differentiated neuroendocrine tumor.
These results are comparable to those recently published from the NORDIC database [12].
Discussion
Neuroendocrine neoplasms are heterogeneous diseases. The current project was motivated by an increasing use of CAPTEM as a regimen for treating G1–2 pancreatic NENs in clinical practice, combined with a lack of efficacy data regarding CAPTEM in patients with G3 disease. Some options used in this setting include platinum/etoposide regimens extrapolated from small cell lung cancer, platinum doublet regimens extrapolated from colorectal data [21, 22], and second‐line therapies used in small cell lung cancer such as topotecan and the combination of cyclophosphamide, doxorubicin, and vincristine [23]. All of these are relatively toxic with modest response rates.
This is the largest series investigating the outcomes of TEM‐based therapies in G3 GEPNENs, involving several major North American NEN centers. We enrolled 130 patients, showing a response rate of 36% and median TTF of 3.6 months. These outcomes are comparable to that achieved with (most commonly) first‐line platinum doublet therapy in a large cohort of patients with G3 NEN (31%) [3]. This is especially encouraging as 72% of patients in the current series were receiving second‐ or later‐line CAPTEM treatment, which would be anticipated to decrease the above measures of efficacy compared with that seen in the first‐line setting. We acknowledge the potential confounding effect of patients with panNEN, who made up 67% of the current cohort but only 25% of the study cited above [3]. Some significant series of patients with NENs treated with CAPTEM have been published, but they have each reported on fewer numbers of patients with G3 disease [18, 24, 25, 26, 27, 28, 29, 30]. Although the data regarding CAPTEM in grade 1–2 well‐differentiated GEPNENs have shown significant promise, grade 3 NENs are a clinically and genetically distinct group with a much worse prognosis. Therefore, our paper demonstrates a significant effect from CAPTEM for the first time in a large cohort of patients with poor‐prognosis disease. Although the response rate in our series was high, the median time to treatment failure was only 3.6 months. This is comparable to prior data regarding the efficacy of platinum doublet chemotherapy in the NORDIC NEC study [3].
We observed better treatment outcomes with CAPTEM in patients with pancreatic NEN compared with those with GI NEN. This is not surprising given the results from E2211 [20]. It potentially reflects a greater sensitivity of panNENs to TEM‐based regimens, stemming from genetic differences between NENs of different primary sites elucidated in recent studies [31]. We also showed that patients with well‐differentiated NET (WDNET) had better overall survival compared with those with PDNEC, confirming the poor prognosis with PDNEC previously shown in pancreatic NENs [10].
The current manuscript describes the largest series by far regarding the efficacy of CAPTEM in G3 NENs. It provides support to the use of CAPTEM in these patients who have limited other treatment options. This regimen should be considered a viable option with acceptable toxicity in this NEN subgroup while awaiting confirmation from prospective trials. Although we acknowledge the retrospective design of this study, this enabled the collaboration of several high‐volume sites in an uncommon tumor. We analyzed a combined cohort of G3 PDNECs and WDNETs, which have acknowledged differences in genetic mutations and clinical outcome (as shown in the subgroup analyses); future studies may confirm a difference in efficacy by NEN differentiation status. Further analysis may show the prognostic and predictive value of 18F‐fluorodeoxyglucose (FDG) PET in this setting [32, 33]. The lack of a standardized imaging protocol means that time to treatment failure was used as a primary endpoint as opposed to progression‐free survival. We did not analyze the MGMT methylation status of NENs in this clinically focused paper; we note that current studies show conflicting evidence as to the predictive power of O‐6‐Methylguanine‐DNA Methyltransferase (MGMT) in predicting TEM efficacy [16, 34]. Finally, the response rate was assessed by review of local radiological reports rather than RECIST; this may overestimate the response rate compared with measurement by RECIST version 1.1 criteria. Work is underway to formally review cross‐sectional imaging collected in this study.
Future Research
This study suggests several avenues of future research. A prospective trial (EA2142, NCT02595424) is currently underway randomizing patients with advanced G3 NEN to cisplatin/etoposide and CAPTEM in the first‐line setting. These results are eagerly awaited and will hopefully confirm the significant activity of CAPTEM shown in our manuscript. Given the conflicting evidence to date, future studies investigating predictors of TEM efficacy, including MGMT methylation status, are warranted [16]. In addition, there has been some conjecture as to a differential response to CAPTEM and platinum doublet regimens according to differentiation status. Specifically, some experts think that poorly differentiated NECs may respond better to cisplatin/etoposide, whereas WDNETS may respond better to CAPTEM [35]. Although the response rate was numerically higher in patients with Ki‐67 < 55% and in WDNENs in our cohort, the difference was not statistically significant. A recent trial has investigated the combination of PRRT with CAPTEM, showing an increase in response rate from combination therapy [36]. This combination might be one way to incorporate CAPTEM into treatment regimens for patients with G3 NENs given that significant numbers of these patients still have significant avidity on somatostatin receptor PET (e.g., 68Ga‐DOTATATE PET) [36]. The ongoing NETTER‐2 trial (NCT03972488) may shed light on the effectiveness of PRRT in patients with G3 NENs and a Ki‐67 index of less than 55%. Future studies may also shed more light on the interplay of differentiation status and Ki‐67 index and its effect on CAPTEM efficacy in G3 NENs.
Conclusion
TEM‐based chemotherapy appears to be an effective therapy in G3 GEPNEN and to be well tolerated, with a response rate of 36%. Responses were more common in the first‐line setting and in patients with pancreatic NEN. TEM‐based regimens should be considered viable treatment options in the setting of G3 WDNEN, particularly earlier in the treatment course, and further research is warranted to confirm the above findings.
Author Contributions
Conception/design: David L. Chan, Emily K. Bergsland, Jennifer A. Chan, Thorvardur R. Halfdanarson, Pamela L. Kunz, Diane L. Reidy, Simron Singh
Provision of study material or patients: David L. Chan, Emily K. Bergsland, Jennifer A. Chan, Rujuta Gadgil, Thorvardur R. Halfdanarson, Kathleen Hornbacker, Virginia Kelly, Pamela L. Kunz, Patrick W. McGarrah, Nitya P. Raj, Diane L. Reidy, Alia Thawer, Julia Whitman, Linda Wu, Christoph Becker, Simron Singh
Collection and/or assembly of data: David L. Chan, Emily K. Bergsland, Jennifer A. Chan, Rujuta Gadgil, Thorvardur R. Halfdanarson, Kathleen Hornbacker, Virginia Kelly, Pamela L. Kunz, Patrick W. McGarrah, Nitya P. Raj, Diane L. Reidy, Alia Thawer, Julia Whitman, Linda Wu, Christoph Becker, Simron Singh
Data analysis and interpretation: David L. Chan, Emily K. Bergsland, Jennifer A. Chan, Rujuta Gadgil, Thorvardur R. Halfdanarson, Kathleen Hornbacker, Virginia Kelly, Pamela L. Kunz, Patrick W. McGarrah, Nitya P. Raj, Diane L. Reidy, Alia Thawer, Julia Whitman, Linda Wu, Christoph Becker, Simron Singh
Manuscript writing: David L. Chan, Emily K. Bergsland, Jennifer A. Chan, Thorvardur R. Halfdanarson, Pamela L. Kunz, Patrick W. McGarrah, Nitya P. Raj, Diane L. Reidy, Simron Singh
Final approval of manuscript: David L. Chan, Emily K. Bergsland, Jennifer A. Chan, Rujuta Gadgil, Thorvardur R. Halfdanarson, Kathleen Hornbacker, Virginia Kelly, Pamela L. Kunz, Patrick W. McGarrah, Nitya P. Raj, Diane L. Reidy, Alia Thawer, Julia Whitman, Linda Wu, Christoph Becker, Simron Singh
Disclosures
David L. Chan: Ipsen, Novartis (H), Novartis (RF); Emily K. Bergsland: UpToDate (H), Hutchison MediPharma (C/A), Merck, Novartis (RF); Jennifer A. Chan: Merck (OI), Ipsen, Novartis, Lexicon (C/A), Novartis, Sanofi, Eli Lilly & Co. (RF); Thorvardur R. Halfdanarson: Thermo Fisher Scientific, Novartis, Advanced Accelerator Applications, Curium, Terumo, ITM (C/A), Basilea, Agios, Novartis, Advanced Accelerator Applications, Turnstone Biologics, Genentech (RF); Pamela L. Kunz: Ipsen, Lexicon, Novartis (C/A); Nitya P. Raj: Novartis, Xencor (RF); Diane L. Reidy: Ipsen, Novartis (C/A); Simron Singh: Novartis, Ipsen (C/A), EMD Serono (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Modlin IM, Oberg K, Chung DC et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61–72. [DOI] [PubMed] [Google Scholar]
- 2. Raphael MJ, Chan DL, Law C et al. Principles of diagnosis and management of neuroendocrine tumours. CMAJ 2017;189:E398–E404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sorbye H, Welin S, Langer SW et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol 2013;24:152–160. [DOI] [PubMed] [Google Scholar]
- 4. Lloyd RV, Osamura RY, Klöppel G et al. eds. WHO Classification of Tumours of Endocrine Organs. 4th ed. Lyon: International Agency for Research on Cancer, 2017. [Google Scholar]
- 5. WHO Classification of Tumours Editorial Board , ed. WHO Classification of Tumours of the Digestive System. Vol 1. 5th ed. Lyon: International Agency for Research on Cancer, 2019. [Google Scholar]
- 6. Caplin ME, Pavel M, Cwikla JB et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224–233. [DOI] [PubMed] [Google Scholar]
- 7. Raymond E, Dahan L, Raoul JL et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501–513. [DOI] [PubMed] [Google Scholar]
- 8. Yao JC, Fazio N, Singh S et al. Everolimus for the treatment of advanced, non‐functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT‐4): A randomised, placebo‐controlled, phase 3 study. Lancet 2016;387:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strosberg J, El‐Haddad G, Wolin E et al. Phase 3 trial of 177 Lu‐Dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basturk O, Yang Z, Tang LH et al. The high‐grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 2015;39:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heetfeld M, Chougnet CN, Olsen IH et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2015;22:657–664. [DOI] [PubMed] [Google Scholar]
- 12. Elvebakken H, Perren A, Scoazec JY et al. A consensus developed morphological re‐evaluation of 196 high‐grade gastroenteropancreatic neuroendocrine neoplasms and its clinical correlations. Neuroendocrinology 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13. Garcia‐Carbonero R, Sorbye H, Baudin E et al. ENETS consensus guidelines for high‐grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016;103:186–194. [DOI] [PubMed] [Google Scholar]
- 14. Strosberg JR, Coppola D, Klimstra DS et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high‐grade) extrapulmonary neuroendocrine carcinomas. Pancreas 2010;39:799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perry JR, Laperriere N, O'Callaghan CJ et al. Short‐course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med 2017;376:1027–1037. [DOI] [PubMed] [Google Scholar]
- 16. Cives M, Ghayouri M, Morse B et al. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer 2016;23:759–767. [DOI] [PubMed] [Google Scholar]
- 17. Fine RL, Gulati AP, Krantz BA et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well‐differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol 2013;71:663–670. [DOI] [PubMed] [Google Scholar]
- 18. Peixoto RD, Noonan KL, Pavlovich P et al. Outcomes of patients treated with capecitabine and temozolamide for advanced pancreatic neuroendocrine tumors (PNETs) and non‐PNETs. J Gastrointest Oncol 2014;5:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strosberg JR, Fine RL, Choi J et al. First‐line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kunz PL, Catalano PJ, Nimeiri H et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG‐ACRIN Cancer Research Group (E2211). J Clin Oncol 2018;36(suppl 15):4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hadoux J, Malka D, Planchard D et al. Post‐first‐line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer 2015;22:289–298. [DOI] [PubMed] [Google Scholar]
- 22. Hentic O, Hammel P, Couvelard A et al. FOLFIRI regimen: An effective second‐line chemotherapy after failure of etoposide‐platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr Relat Cancer 2012;19:751–757. [DOI] [PubMed] [Google Scholar]
- 23. von Pawel J, Schiller JH, Shepherd FA et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small‐cell lung cancer. J Clin Oncol 1999;17:658–667. [DOI] [PubMed] [Google Scholar]
- 24. Sahu A, Jefford M, Lai‐Kwon J et al. CAPTEM in metastatic well‐differentiated intermediate to high grade neuroendocrine tumors: A single centre experience. J Oncol 2019;2019:9032753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas K, Voros BA, Meadows‐Taylor M et al. Outcomes of capecitabine and temozolomide (CAPTEM) in advanced neuroendocrine neoplasms (NENs). Cancers (Basel) 2020;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crespo G, Lopez C, Jimenez‐Fonseca P et al. Capecitabine‐temozolomide in G3 neuroendocrine neoplasms. Paper presented at: 13th Annual European Neuroendocrine Tumor Society conference; March 9–11, 2016; Barcelona, Spain. Available at https://www.enets.org/abstracts.1405.html. Accessed October 7, 2020.
- 27. Chatzellis E, Angelousi A, Daskalakis K et al. Activity and safety of standard and prolonged capecitabine/temozolomide administration in patients with advanced neuroendocrine neoplasms. Neuroendocrinology 2019;109:333–345. [DOI] [PubMed] [Google Scholar]
- 28. Wang W, Zhang Y, Peng Y et al. A Ki‐67 index to predict treatment response to the capecitabine temozolomide (CAPTEM) regimen in neuroendocrine neoplasms: A retrospective multicenter study. Neuroendocrinology 2021;111:752–763. [DOI] [PubMed] [Google Scholar]
- 29. Apostolidis L, Dal Buono A, Merola E et al. Multicenter analysis of treatment outcomes for well differentiated grade 3 neuroendocrine tumors (NET G3). J Clin Oncol 2020;38(suppl 15):4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu AJ, Ueberroth BE, McGarrah PW et al. Treatment outcomes of well‐differentiated high‐grade neuroendocrine tumors. The Oncologist 2021;26:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mafficini A, Scarpa A. Genetics and epigenetics of gastroenteropancreatic neuroendocrine neoplasms. Endocr Rev 2019;40:506–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Binderup T, Knigge U, Loft A et al. 18F‐fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res 2010;16:978–985. [DOI] [PubMed] [Google Scholar]
- 33. Chan DL, Bernard E, Schembri G et al. High metabolic tumour volume on FDG PET predicts poor survival from neuroendocrine neoplasms. Neuroendocrinology 2020;110:950–958. [DOI] [PubMed] [Google Scholar]
- 34. Cros J, Hentic O, Rebours V et al. MGMT expression predicts response to temozolomide in pancreatic neuroendocrine tumors. Endocr Relat Cancer 2016;23:625–633. [DOI] [PubMed] [Google Scholar]
- 35. Espinosa‐Olarte P, La Salvia A, Riesco‐Martinez MC et al. Chemotherapy in NEN: Still has a role? Rev Endocr Metab Disord 2021. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pavlakis N, Ransom DT, Wyld D et al. Australasian Gastrointestinal Trials Group (AGITG) CONTROL NET study: Phase II study evaluating the activity of 177Lu‐Octreotate peptide receptor radionuclide therapy (LuTate PRRT) and capecitabine, temozolomide (CAPTEM)—First results for pancreas and updated midgut neuroendocrine tumors (pNETS, mNETS). J Clin Oncol 2020;38(suppl 15):4608. [Google Scholar]


