Abstract
Background
The suitability criteria for accelerated partial breast irradiation (APBI) from the American Brachytherapy Society (ABS), American Society for Radiation Oncology (ASTRO), and The Groupe Européende Curiethérapie European SocieTy for Radiotherapy & Oncology (GEC‐ESTRO) have significant differences.
Materials and Methods
This is a single institution retrospective review of 946 consecutive patients with invasive breast cancer who underwent lumpectomy and APBI intracavitary brachytherapy from 2003 to 2018. Overall survival (OS), breast cancer‐specific survival (BCSS), relapse‐free survival (RFS), and ipsilateral breast tumor recurrence (IBTR) were estimated with Kaplan‐Meier method.
Results
Median follow‐up time was 60.2 months. Median age was 68 years (46–94 years). The majority of patients had estrogen receptor (ER)–positive disease (94%). There were 821 (87%) cases of invasive ductal carcinoma and 68 cases (7%) of invasive lobular carcinoma (ILC). The 5‐year OS, BCSS, RFS, and IBTR were 93%, 99%, 90%, and 1.5%, respectively. Upon univariate analysis, ILC (hazard ratio [HR], 4.6; p = .008) and lack of nodal evaluation (HR, 6.9; p = .01) were risk factors for IBTR. The 10‐year IBTR was 2.5% for IDC and 14% for ILC. While the ABS and ASTRO criteria could not predict IBTR, the GEC‐ESTRO intermediate risk group was associated with inferior IBTR (p = .04) when compared to both low risk and high risk groups. None of the suitability criteria was able to predict RFS.
Conclusion
These results show that APBI is an effective treatment for patients with invasive breast cancer. Expansion of the current eligibility criteria should be considered, although prospective validation is needed. Caution is required when considering APBI for patients with ILC.
Implications for Practice
In a large retrospective review of 946 patients with early breast cancer treated with partial mastectomy and accelerated partial breast irradiation (APBI) intracavitary brachytherapy, this study demonstrates durable local control. Patients deemed unsuitable or high risk by the American Brachytherapy Society, American Society for Radiation Oncology, and European Society for Radiotherapy and Oncology guidelines were not at increased risk for ipsilateral breast tumor recurrence (IBTR), suggesting that expansion of the current criteria should be considered. Importantly, however, these results demonstrate that caution should be taken when considering APBI for patients with invasive lobular carcinoma, as these patients had relatively high risk for IBTR (10‐year IBTR, 14%).
Keywords: Breast cancer, Accelerated partial breast irradiation, Brachytherapy, Radiation, Breast conserving therapy
Short abstract
In this retrospective review of 946 patients, the authors investigate the effectiveness of accelerated partial breast irradiation for select patients with invasive breast cancer and determined that patients deemed unsuitable for this treatment by criteria established by the American Brachytherapy Society or the American Society for Radiation Oncology were not at increased risk for ipsilateral breast tumor recurrence
Introduction
Breast conservation therapy with partial mastectomy and whole breast irradiation (WBI) provides many early‐stage patients with outcomes similar to radical mastectomy [1, 2]. However, in select cases the entire breast may not require radiation, but rather only the tumor cavity and surrounding breast tissue at highest risk for recurrence [3]. This has led to the advent of accelerated partial breast irradiation (APBI), a targeted approach that reduces the volume of irradiated breast tissue with the potential to minimize toxicity without increasing the risk of ipsilateral breast tumor recurrence (IBTR). APBI can be delivered via different techniques, including multicatheter interstitial brachytherapy, balloon‐based brachytherapy, and external beam radiotherapy (EBRT) [4, 5]. There is a strong body of evidence that supports the use of APBI over WBI in select populations, as it has been demonstrated to have less acute toxicity, better cosmetic outcomes, and similar quality of life [6, 7, 8, 9]. The long‐term results of RTOG 0413 and the Florence Trial are promising, with favorable toxicity profiles and a low 10‐year cumulative incidence of IBTR (3.7%–4.6%) after APBI [10, 11].
Given the targeted approach to therapy, optimal patient selection is crucial for APBI to ensure a low risk of IBTR. Three prominent governing bodies, the American Brachytherapy Society (ABS), the American Society for Radiation Oncology (ASTRO), and Groupe Européen de Curiethérapie and the European SocieTy for Radiotherapy Oncology (GEC‐ESTRO), have published guidelines delineating the appropriate patient population who will benefit from APBI treatment (Table 1) [12, 13, 14]. Although all three guidelines use similar patient and tumor features to identify a subpopulation at low risk for IBTR, there remain important differences. Invasive lobular carcinoma (ILC) subtype, for example, is an intermediate risk factor per GEC‐ESTRO guidelines and a cautionary factor per ASTRO guidelines, but is not taken into consideration per ABS guidelines. Similarly, whereas the ABS and GEC‐ESTRO guidelines do not include estrogen receptor (ER) status, ASTRO deems ER‐negative patients cautionary for APBI.
Table 1.
APBI suitability criteria for patients with invasive breast cancer
| Risk Factor | ABS, Suitable | ASTRO | GEC‐ESTRO | ||||
|---|---|---|---|---|---|---|---|
| Suitable | Cautionary | Unsuitable | Low risk | Intermediate risk | High risk | ||
| Age, yr | ≥45 | ≥50 | 40–49 a | <40 | >50 | 40–50 | ≤40 |
| Size, cm | ≤3 | ≤2 | 2.1–3 | >3 | ≤3 | ≤3 | >3 |
| Margins | ≥2 mm | ≥2 mm | <2 mm | Positive | ≥2 mm | <2 mm | Positive |
| Histology | ILC | ILC | |||||
| Hormone status | ER‐positive | ER‐negative | |||||
| LVSI | Not present | Not present | Limited/Focal | Not present | Not present | Present | |
| EIC | – | ≤3 cm | >3 cm | Not present | Not present | Present | |
| Focality | Clinically unifocal, ≤2 cm total | Clinically unifocal, total size 2.1–3 cm | Multifocal, >3 cm | Unifocal | Multifocal, ≤2 cm | Multifocal, >2 cm | |
| Centricity | Unicentric | Multicentric | Unicentric | Multicentric | |||
| Nodal status | pN0 | pN0 | ≥ pN1 | pN0 | pN1mi, pN1a b | ≥ pN2a, pNx | |
If all other criteria for suitable are met. Age ≥ 50 years if any of the cautionary factors are present.
Confirmed by axillary lymph node dissection.
Abbreviations: ABS, American Brachytherapy Society; ASTRO, American Society for Radiation Oncology; EIC, extensive intraductal component; ER, estrogen receptor; GEC‐ESTRO, The Groupe Européende Curiethérapie ‐ European SocieTy for Radiotherapy & Oncology; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LVSI, lymphovascular space invasion.
Given the importance of careful patient selection for APBI and the significant discrepancies within the suitability guidelines, the purpose of this study is to assess these guidelines using a single institutional experience with APBI intracavitary brachytherapy in treating invasive breast cancer.
Materials and Methods
Study Design
This is a retrospective review of 946 patients with invasive breast cancer treated with partial mastectomy followed by APBI intracavitary brachytherapy high dose rate (HDR) from 2003 to 2018. This study was approved by the institutional review board. Women with pathologic confirmation of invasive breast cancer and clinically node‐negative, clinically unicentric, and clinically unifocal disease met the inclusion criteria. Of note, the vast majority of cases lacked pathologic details of the extent of extensive intraductal component (EIC) and lymphovascular space invasion (LVSI); the presence of either feature was considered to be a cautionary or high‐risk factor by ASTRO and GEC‐ESTRO criteria, respectively (Table 1). In addition, cases without surgical lymph node evaluation were considered to be high risk by GEC‐ESTRO criteria but were not classified by ASTRO criteria.
Treatment Details
Patients underwent partial mastectomy and placement of a cavity evaluation device (CED). Once final pathology was determined, the partial breast intracavitary applicator was placed. The intracavitary brachytherapy devices used included single‐lumen and multi‐lumen Mammosite (Hologic Inc., Marlborough, MA), Contura (Hologic Inc., Marlborough, MA), and SAVI (Cianna Medical, Aliso Viejo, CA.). Each patient was treated with Ir‐192 HDR unit with a prescription of 340 cGy per fraction to 1 cm from the applicator surface for 10 fractions occurring twice per day, with each fraction separated by at least 6 hours each day.
Statistical Analysis
Clinical features were summarized using descriptive statistics. Primary outcomes, including overall survival (OS), breast cancer‐specific survival (BCSS), IBTR, and relapse‐free survival (RFS), were measured from the time of APBI and estimated with the Kaplan‐Meier method, with the log‐rank test to test differences in IBTR and RFS by suitability criteria. BCSS accounted only for breast cancer–related deaths, whereas OS accounted for death of any cause. Recurrence in any quadrant of the treated breast was considered an event for IBTR. Failure in the treated breast, contralateral breast, regional lymph nodes, distant regions, or death from any cause were considered events when calculating RFS. The Cox proportional‐hazards model was used for univariate analysis of IBTR and RFS. Two‐tailed p values <.05 were considered to indicate statistical significance. Statistical analyses were performed using JMP 15 (SAS Institute Inc., Cary, NC).
Results
Patient, Tumor, and Treatment Characteristics
The median age at the time of APBI was 68 years (Table 2). The majority of cases were ER‐positive (94%), progesterone receptor (PR)–positive (83%), and HER2‐receptor negative (88%). There were 821 patients (87%) with invasive ductal carcinoma and 68 patients (7%) with ILC. A minority of patients (12%) were prescribed adjuvant chemotherapy. The majority (80%) of patients were prescribed endocrine therapy, and of those with adequate follow up (n = 393), 153 patients (38.9%) had confirmed adherence to endocrine therapy for 5 years.
Table 2.
Patient, tumor, and treatment characteristics (n = 946)
| Characteristic | Entire cohort (n = 946), n (%) | Cases of IBTR (n = 18), n (% with IBTR) |
|---|---|---|
| Age at treatment, median (range) | 68 (46–94) | |
| ≥50 years | 932 (98.5) | 16 (1.7) |
| <50 years | 14 (1.5) | 2 (7.4) |
| Menopausal status | ||
| Premenopausal | 10 (1.1) | 0 (0) |
| Postmenopausal | 936 (98.9) | 18 (1.9) |
| Laterality | ||
| Right | 450 (47.6) | 6 (1.3) |
| Left | 496 (52.4) | 12 (2.4) |
| Histology | ||
| IDC | 821 (86.8) | 12 (1.5) |
| ILC | 68 (7.2) | 4 (5.9) |
| Other | 57 (6.0) | 2 (3.5) |
| Tumor size, median (range) cm | 1.1 (0.07–3.5) | |
| ≤3cm | 942 (99.7) | 18 (1.9) |
| >3cm | 3 (0.3) | 0 (0) |
| Nodal Stage | ||
| pNx | 15 (1.6) | 2 (13.3) |
| pN0 | 927 (97.9) | 16 (1.7) |
| pN1 | 3 (0.3) | 0 (0) |
| pN2 | 1 (0.1) | 0 (0) |
| Grade | ||
| 1 | 372 (39.3) | 3 (0.8) |
| 2 | 424 (44.8) | 10 (2.4) |
| 3 | 148 (15.6) | 5 (3.4) |
| Unknown | 2 (0.2) | 0 (0) |
| Multifocal | 13 (1.4) | 0 (0) |
| LVSI | 43 (4.6) | 1 (2.3) |
| EIC | 128 (15.9) | 0 (0) |
| Hormone status | ||
| ER+ | 887 (93.8) | 17 (1.9) |
| ER− | 59 (6.2) | 1 (1.7) |
| PR+ | 783 (82.8) | 13 (1.7) |
| PR− | 163 (17.2) | 5 (3.1) |
| Receptor subtype | ||
| HR+/HER2− | 797 (84.3) | 14 (1.8) |
| HR+/HER2+ | 40 (4.2) | 3 (7.5) |
| HR−/HER2+ | 9 (1.0) | 0 (0) |
| HR−/HER2− | 39 (4.1) | 1 (2.6) |
| Unknown | 61 (6.5) | 0 (0) |
| Margin status | ||
| ≥2 mm | 752 (79.5) | 12 (1.6) |
| <2 mm | 71 (7.5) | 3 (4.2) |
| Negative, NOS | 37 (3.9) | 0 (0) |
| Positive | 52 (5.5) | 1 (1.9) |
| Unknown | 34 (3.6) | 2 (5.9) |
| APBI device | ||
| SAVI | 207 (21.9) | 0 (0) |
| Mammosite | 153 (16.2) | 8 (5.2) |
| Mammosite ML | 275 (29.1) | 1 (3.6) |
| Contura | 210 (22.2) | 2 (1.0) |
| Unknown | 6 (0.6) | 3 (50.0) |
| Chemotherapy | 110 (11.6) | 6 (5.5) |
| Endocrine therapy | ||
| Patients prescribed endocrine therapy | 753 (79.6) | 14 (1.9) |
| Confirmed adherence for 5 years | 153 (38.9) | 2 (1.3) |
Abbreviations: APBI, accelerated partial breast irradiation; EIC, extensive intraductal component; ER, estrogen receptor; HR, hormone receptor; IBTR, ipsilateral breast tumor recurrence; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LVSI, lymphovascular space invasion; PR, progesterone receptor; ML, multilumen; NOS, not otherwise specified.
There were 852, 862, and 882 patients with adequate clinicopathologic details for suitability determination by the ABS, ASTRO, and GEC‐ESTRO guidelines, respectively (Table 3). By the ABS criteria, 693 (81%) patients were deemed suitable. By the ASTRO criteria, 465 (54%), 339 (39%), and 58 (7%) were deemed suitable, cautionary, and unsuitable, respectively. By the GEC‐ESTRO criteria, 524 (59%), 136 (15%), and 222 (25%) were deemed low risk, intermediate risk, and high risk, respectively.
Table 3.
Outcomes by suitability criteria
| Guideline | n (%) | 5‐year IBTR, % | p value | 5‐year RFS, % | p value |
|---|---|---|---|---|---|
| ABS (n = 852) | .185 | .155 | |||
| Suitable | 693 (81.3) | 1.3 | 96.8 | ||
| Unsuitable | 159 (18.7) | 2.8 | 91.0 | ||
| ASTRO (n = 862) | .693 | .591 | |||
| Suitable | 465 (53.9) | 1.3 | 90.8 | ||
| Cautionary | 339 (39.3) | 1.8 | 88.7 | ||
| Unsuitable | 58 (6.7) | 2.0 | 91.5 | ||
| GEC‐ESTRO (n = 882) | .038 | .537 | |||
| Low risk | 524 (59.4) | 0.9 | 90.4 | ||
| Intermediate risk | 136 (15.4) | 4.6 | 87.4 | ||
| High risk | 222 (25.2) | 1.1 | 90.9 |
Abbreviations: ABS, American Brachytherapy Society; ASTRO, American Society for Radiation Oncology; GEC‐ESTRO, European Society for Radiotherapy & Oncology; IBTR, ipsilateral breast tumor recurrence; RFS, relapse‐free survival.
Clinical Outcomes
The median follow up from the end of APBI was 60.2 months (interquartile range: 36.2–94.2 months). For the entire cohort, the 10‐year OS (Fig. 1A), BCSS (Fig. 1B), RFS (Fig. 1C), and IBTR (Fig. 1D) were 82.7%, 97.5%, 79.0%, and 3.6%, respectively. A single patient underwent mastectomy after developing IBTR.
Figure 1.
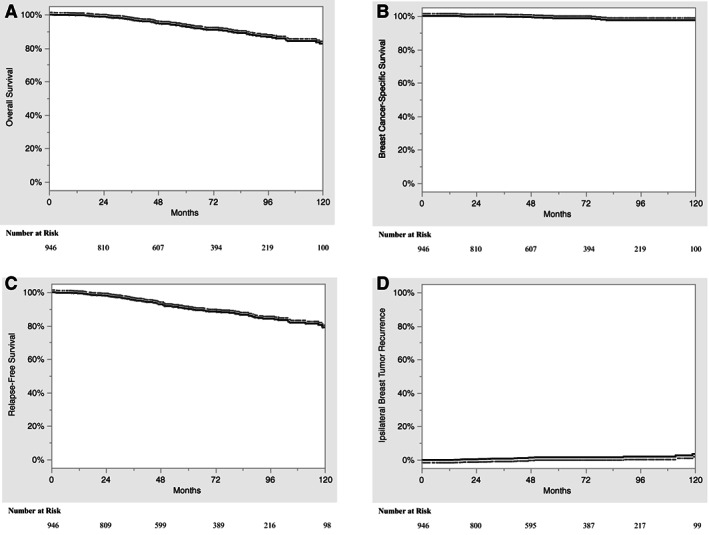
Kaplan‐Meier curve depicting the overall survival (A), breast cancer–specific survival (B), relapse‐free survival (C), and ipsilateral breast tumor recurrence (D) for the entire cohort.
The ABS, ASTRO, and GEC‐ESTRO suitability criteria failed to significantly predict RFS (Table 3). The ABS and ASTRO criteria were also unable to predict IBTR. Cases deemed intermediate risk by the GEC‐ESTRO criteria had a significantly higher risk of IBTR (10‐year IBTR 9.2%; p = .038).
Upon univariate analysis, invasive lobular carcinoma (hazard ratio [HR], 4.6; p = .008) and the absence of nodal evaluation (HR, 6.9; p = .011) were associated with higher rates of IBTR (Table 4). The 5‐ and 10‐year IBTR for IDC were 1.3% and 2.5%, respectively (Fig. 2). The 5‐ and 10‐year IBTR for ILC were 3.5% and 14%, respectively. Of the 68 cases of ILC, 54 (78%) had magnetic resonance imaging (MRI) prior to surgery, and all 4 cases of ILC that developed IBTR had MRI prior to surgery. Close margin status (<2 mm) was the only significant risk factor for poor RFS (HR, 1.9; p = .023).
Table 4.
Univariate analysis for ipsilateral breast tumor recurrence and relapse‐free survival
| Characteristic | IBTR | RFS | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | ||||
| ≥50 years (reference) | 1.00 | 1.00 | ||
| <50 years | .999 | .999 | ||
| Size | ||||
| ≤3 cm (reference) | 1.00 | 1.00 | ||
| >3 cm | .999 | .999 | ||
| Margin | ||||
| ≥2 mm (reference) | 1.00 | 1.00 | ||
| <2 mm | 2.09 (0.58–7.46) | .258 | 1.91 (1.10–3.32) | .023 |
| Positive | 1.42 (0.18–11.0) | .738 | 0.81 (0.30–2.22) | .688 |
| Histology | ||||
| IDC (reference) | 1.00 | 1.00 | ||
| ILC | 4.62 (1.49–14.4) | .008 | 1.51 (0.79–2.91) | .212 |
| Other | 2.70 (0.60–12.1) | .194 | 0.69 (0.25–1.87) | .461 |
| ER status | ||||
| Positive (reference) | 1.00 | 1.00 | ||
| Negative | 0.71 (0.09–5.38) | .743 | 1.08 (0.55–2.14) | .824 |
| Grade | ||||
| 1 (reference) | 1.00 | 1.00 | ||
| 2 | 2.94 (0.81–10.7) | .101 | 1.26 (0.83–1.92) | .278 |
| 3 | 3.86 (0.92–16.2) | .065 | 1.30 (0.76–2.24) | .340 |
| LVSI | ||||
| Absent (reference) | 1.00 | 1.00 | ||
| Present | 0.89 (0.12–6.73) | .907 | 0.80 (0.33–1.97) | .632 |
| EIC | ||||
| Absent (reference) | 1.00 | 1.00 | ||
| Present | .999 | 0.98 (0.49–1.98) | .959 | |
| Focality | ||||
| Unifocal (reference) | 1.00 | 1.00 | ||
| Multifocal | .999 | .999 | ||
| Nodal status | ||||
| pN0 (reference) | 1.00 | 1.00 | ||
| pN1 | .999 | .999 | ||
| pNx | 6.85 (1.56–30.0) | .011 | 1.45 (0.46–4.57) | .525 |
Abbreviations: CI, confidence interval; EIC, extensive intraductal carcinoma; ER, estrogen receptor; HR, hazard ratio; IBTR, ipsilateral breast tumor recurrence; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LVSI, lymphovascular space invasion; RFS, relapse‐free survival.
Figure 2.
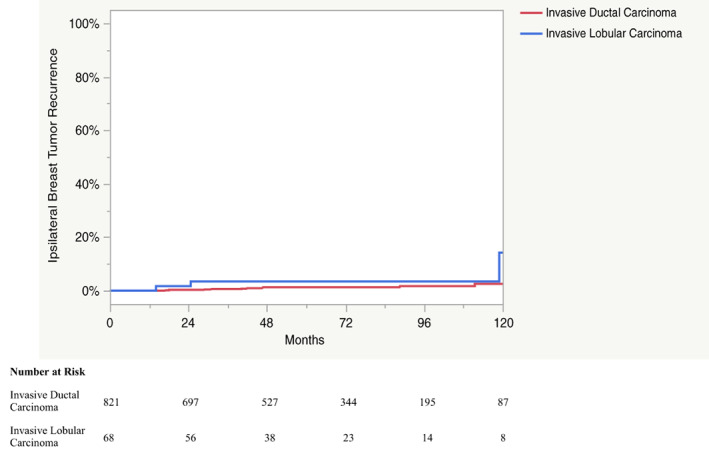
Kaplan Meier curve depicting the ipsilateral breast tumor recurrence for patients with invasive ductal carcinoma and for patients with invasive lobular carcinoma.
Toxicity
There were 211 (22%) cases of postoperative seroma, 25 (3%) cases of postoperative infection, and 128 (14%) cases complicated by fat necrosis. There were higher rates of seroma with multilumen Mammosite (37%) and Contura (28%) devices when compared with other APBI devices (p < .001). There were higher rates of fat necrosis with multilumen Mammosite (29%) and single lumen Mammosite (25%) when compared with other APBI devices (p = .04). The rates of infection did not differ by APBI device (p = .835).
Discussion
APBI provides a promising alternative for breast conservation therapy, with multiple clinical trials demonstrating decreased toxicity, improved cosmesis, and comparable local control to WBI [6, 7, 8, 9]. However, the inclusion criteria for these trials were highly variable, and significant heterogeneity exists in patient selection criteria recommended by ASTRO, GEC‐ESTRO, and ABS (Table 1). The present study is an attempt to add to the existing literature in determining the most appropriate guidelines for APBI patient selection.
We demonstrate effective and durable local control after APBI with 10‐year IBTR of 3.6% (Fig. 1D), similar to the long‐term results of recent clinical trials (10‐year IBTR, 3.7%–5.9%) [10, 11, 15]. The Budapest phase III trial, which randomized patients to WBI and multicatheter interstitial brachytherapy APBI, demonstrated no significant difference in 10‐year local recurrence (5.9% vs. 5.1%), with APBI associated with more favorable cosmetic results [15]. The Florence trial, a phase III trial comparing WBI with intensity‐modulated radiation therapy (IMRT) APBI, also found no significant difference in IBTR between the treatment arms (10‐year IBTR, 3.7% vs. 2.5%) [11]. The Rapid trial, a phase III trial comparing WBI with external beam APBI delivered twice per day, found APBI to be noninferior to WBI in preventing IBTR [6]. The RTOG 0413, a similar phase III trial that included both EBRT and brachytherapy APBI, found that APBI did not meet criteria for equivalence with WBI [10]. However, the difference in IBTR at 10 years was less than 1% (4.6% vs. 3.9%). Taken together, these results indicate that APBI via multiple modalities can provide effective local control, but the heterogeneity of inclusion criteria used by each trial underscores the importance of careful patient selection.
Patient selection for APBI is an evolving field, with regular updates made to the consensus guidelines as recently as 2017 [13]. However, prior studies have been unable to demonstrate the ability for these guidelines to significantly predict IBTR [11, 16, 17, 18, 19, 20, 21]. Budrukkar et al. investigated the utility of the current guidelines in a retrospective study of 240 women treated with multicatheter interstitial brachytherapy APBI [20]. Although local control did not differ for the risk groups of ABS, ASTRO, or GEC‐ESTRO, they did find that these guidelines significantly predicted rates of OS and disease‐free survival. Conversely, the present study demonstrates that none of the guidelines were able to predict RFS (Table 3). Although the risk groups of ABS and ASTRO did not differ in IBTR, the GEC‐ESTRO intermediate‐risk group had a higher risk of IBTR (5‐year IBTR, 4.6%) compared with the low‐risk (5‐year IBTR, 0.9%) and high‐risk groups (5‐year IBTR, 1.1%; Table 3). A significantly higher proportion of patients less than 50 years of age (14%) and ILC cases (46%) within the GEC‐ESTRO intermediate‐risk group likely contributed to the higher rates of IBTR in this subgroup. In contrast, the ASTRO cautionary group had significantly fewer cases of younger patients (5%) and ILC (19%).
Prior studies have identified ER‐negativity [16, 17, 22, 23, 24, 25], tumor size [10, 22], young age [23, 26], positive margins [27], high grade [21, 27], LVSI [21, 25], and EIC [25] as significant risk factors for IBTR after APBI. The present study found that only ILC and the absence of surgical lymph node evaluation were associated with IBTR on univariate analysis (Table 4). However, it should be noted that there were few cases of ER‐negativity, positive margins, young age, or high grade in the present study, which likely limited the analysis (Table 2).
The suitability of patients with ILC for APBI is controversial. Historically, patients with ILC were considered high risk for local recurrence after APBI because of higher rates of multifocal disease and positive resection margins [28]. For this reason, MRI prior to surgery for ILC cases can help to exclude high‐risk patients. However, it should be noted that all four patients with ILC who developed IBTR underwent preoperative MRI. Whereas some studies have shown a higher risk of IBTR for ILC [25, 29], other studies have failed to identify ILC as a significant predictor of IBTR after APBI [26, 30, 31]. The prospective experience with ILC is limited, as the histology accounts for less than 10% of the study population in select trials [10, 11], whereas others have excluded ILC cases altogether [6, 8, 15]. Although the current ASTRO and GEC‐ESTRO guidelines consider ILC to be a cautionary and intermediate risk factor, respectively, the ABS guidelines consider these patients suitable for APBI (Table 1) [12, 13, 14]. The present study demonstrates that ILC cases are at an increased risk for IBTR, with a 10‐year IBTR of 14% (Table 4; Fig. 2), supporting the notion that these patients should remain intermediate risk for APBI. However, further prospective studies with larger numbers of ILC cases are required to clarify the suitability of these patients for APBI.
This study has significant limitations, including its nonrandomized, retrospective nature, which creates the opportunity for selection bias, as well as a relatively limited follow up. There were a limited number of patients within certain categories of the consensus criteria, such as node‐positive disease, that limited the evaluation of the consensus guidelines. Another important limitation was the lack of LVSI and EIC pathologic data that are required for accurate classification of certain cases by the ASTRO and GEC‐ESTRO selection criteria. Additionally, our study does not report on cosmetic outcomes, an important potential benefit of APBI over WBI.
Conclusion
Overall, our data demonstrate the efficacy of APBI even in patients deemed unsuitable by ABS, ASTRO, or GEC‐ESTRO criteria. This study contributes to the growing body of evidence indicating that expansion of these guidelines may be warranted; however, prospective trials are required to validate these findings. Our data indicate that caution is required when considering APBI for patients with ILC.
Author Contributions
Conception/design: Matthew Mills, Ronica H. Nanda, Sunny Raiker, Jessica Jastrzebski, Jason P. Wilson, Taghrid A. Altoos, Kathleen G. Allen, Peter W. Blumencranz, Roberto Diaz
Provision of study material or patients: Ronica H. Nanda, Sunny Raiker, Jessica Jastrzebski, Taghrid A. Altoos, Kathleen G. Allen, Peter W. Blumencranz, Roberto Diaz
Collection and/or assembly of data: Matthew Mills, Nicholas W. Russo, Matthew Fahey, Lisa L. Stout
Data analysis and interpretation: Matthew Mills, Nicholas W. Russo, Roberto Diaz
Manuscript writing: Matthew Mills, Nicholas W. Russo, Roberto Diaz
Final approval of manuscript: Matthew Mills, Nicholas W. Russo, Matthew Fahey, Ronica H. Nanda, Sunny Raiker, Jessica Jastrzebski, Lisa L. Stout, Jason P. Wilson, Taghrid A. Altoos, Kathleen G. Allen, Peter W. Blumencranz, Roberto Diaz
Disclosures
Jason Wilson: Lumicell (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This work was supported by the Morton Plant Mease Foundation.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Fisher B, Anderson S, Bryant J et al. Twenty‐year follow‐up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233–1241. [DOI] [PubMed] [Google Scholar]
- 2. Veronesi U, Cascinelli N, Mariani L et al. Twenty‐year follow‐up of a randomized study comparing breast‐conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 3. Smith TE, Lee D, Turner BC et al. True recurrence vs. New primary ipsilateral breast tumor relapse: An analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys 2000;48:1281–1289. [DOI] [PubMed] [Google Scholar]
- 4. Bennion NR, Baine M, Granatowicz A et al. Accelerated partial breast radiotherapy: A review of the literature and future directions. Gland Surg 2018;7:596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cox JA, Swanson TA. Current modalities of accelerated partial breast irradiation. Nat Rev Clin Oncol 2013;10:344–356. [DOI] [PubMed] [Google Scholar]
- 6. Whelan TJ, Julian JA, Berrang TS et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node‐negative breast cancer (rapid): A randomised controlled trial. Lancet 2019;394:2165–2172. [DOI] [PubMed] [Google Scholar]
- 7. Livi L, Meattini I, Marrazzo L et al. Accelerated partial breast irradiation using intensity‐modulated radiotherapy versus whole breast irradiation: 5‐year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 2015;51:451–463. [DOI] [PubMed] [Google Scholar]
- 8. Franceschini D, Loi M, Chiola I et al. Preliminary results of a randomized study on postmenopausal women with early stage breast cancer: Adjuvant hypofractionated whole breast irradiation versus accelerated partial breast irradiation (HYPAB trial). Clin Breast Cancer 2021;21:231–238. [DOI] [PubMed] [Google Scholar]
- 9. Schafer R, Strnad V, Polgar C et al. Quality‐of‐life results for accelerated partial breast irradiation with interstitial brachytherapy versus whole‐breast irradiation in early breast cancer after breast‐conserving surgery (GEC‐ESTRO): 5‐year results of a randomised, phase 3 trial. Lancet Oncol 2018;19:834‐844. [DOI] [PubMed] [Google Scholar]
- 10. Vicini FA, Cecchini RS, White JR et al. Long‐term primary results of accelerated partial breast irradiation after breast‐conserving surgery for early‐stage breast cancer: A randomised, phase 3, equivalence trial. Lancet 2019;394:2155–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meattini I, Marrazzo L, Saieva C et al. Accelerated partial‐breast irradiation compared with whole‐breast irradiation for early breast cancer: Long‐term results of the randomized phase III APBI‐IMRT‐Florence trial. J Clin Oncol 2020;38:4175–4183. [DOI] [PubMed] [Google Scholar]
- 12. Shah C, Vicini F, Shaitelman SF et al. The American Brachytherapy Society consensus statement for accelerated partial‐breast irradiation. Brachytherapy 2018;17:154–170. [DOI] [PubMed] [Google Scholar]
- 13. Correa C, Harris EE, Leonardi MC et al. Accelerated partial breast irradiation: Executive summary for the update of an ASTRO evidence‐based consensus statement. Pract Radiat Oncol 2017;7:73–79. [DOI] [PubMed] [Google Scholar]
- 14. Polgar C, Van Limbergen E, Potter R et al. Patient selection for accelerated partial‐breast irradiation (APBI) after breast‐conserving surgery: Recommendations of the Groupe Europeen de Curietherapie‐European Society for Therapeutic Radiology and Oncology (GEC‐ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol 2010;94:264–273. [DOI] [PubMed] [Google Scholar]
- 15. Polgar C, Fodor J, Major T et al. Breast‐conserving therapy with partial or whole breast irradiation: Ten‐year results of the Budapest randomized trial. Radiother Oncol 2013;108:197–202. [DOI] [PubMed] [Google Scholar]
- 16. Christoudias MK, Collett AE, Stull TS et al. Are the American Society for Radiation Oncology guidelines accurate predictors of recurrence in early stage breast cancer patients treated with balloon‐based brachytherapy? Int J Surg Oncol 2013;2013:829050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beitsch P, Vicini F, Keisch M et al. Five‐year outcome of patients classified in the “unsuitable” category using the American Society of Therapeutic Radiology and Oncology (ASTRO) consensus panel guidelines for the application of accelerated partial breast irradiation: An analysis of patients treated on the American Society of Breast Surgeons MammoSite(r) registry trial. Ann Surg Oncol 2010;17(suppl 3):219–225. [DOI] [PubMed] [Google Scholar]
- 18. Jawad MS, Shah C, Wilkinson JB et al. Seven‐year outcomes following accelerated partial breast irradiation stratified by ASTRO consensus groupings. Am J Clin Oncol 2017;40:483–489. [DOI] [PubMed] [Google Scholar]
- 19. Wadasadawala T, Mondal M, Paul SN et al. Should molecular subtype be recommended as one of the selection criteria for accelerated partial breast irradiation? Preliminary results from an Asian cohort. J Contemp Brachytherapy 2018;10:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Budrukkar A, Telkhade T, Wadasadawala T et al. A comparison of long‐term clinical outcomes of accelerated partial breast irradiation using interstitial brachytherapy as per GEC‐ESTRO, ASTRO, updated ASTRO, and ABS guidelines. Brachytherapy 2020;19:337–347. [DOI] [PubMed] [Google Scholar]
- 21. Gabani P, Cyr AE, Zoberi JE et al. Long‐term outcomes of APBI via multicatheter interstitial HDR brachytherapy: Results of a prospective single‐institutional registry. Brachytherapy 2018;17:171–180. [DOI] [PubMed] [Google Scholar]
- 22. Shah C, Badiyan S, Ben Wilkinson J et al. Treatment efficacy with accelerated partial breast irradiation (APBI): Final analysis of the American Society of Breast Surgeons MammoSite((r)) breast brachytherapy registry trial. Ann Surg Oncol 2013;20:3279–3285. [DOI] [PubMed] [Google Scholar]
- 23. Wilkinson JB, Shah C, Amin M et al. Outcomes according to breast cancer subtype in patients treated with accelerated partial breast irradiation. Clin Breast Cancer 2017;17:55–60. [DOI] [PubMed] [Google Scholar]
- 24. Shah C, Wilkinson JB, Lyden M et al. Predictors of local recurrence following accelerated partial breast irradiation: A pooled analysis. Int J Radiat Oncol Biol Phys 2012;82:e825–830. [DOI] [PubMed] [Google Scholar]
- 25. Cannon DM, McHaffie DR, Patel RR et al. Locoregional recurrence following accelerated partial breast irradiation for early‐stage invasive breast cancer: Significance of estrogen receptor status and other pathological variables. Ann Surg Oncol 2013;20:3446–3452. [DOI] [PubMed] [Google Scholar]
- 26. Ott OJ, Hildebrandt G, Potter R et al. Accelerated partial breast irradiation with interstitial implants: Risk factors associated with increased local recurrence. Int J Radiat Oncol Biol Phys 2011;80:1458–1463. [DOI] [PubMed] [Google Scholar]
- 27. Anderson BM, Kamrava M, Wang PC et al. Locoregional recurrence by molecular subtype after multicatheter interstitial accelerated partial breast irradiation: Results from the pooled registry of multicatheter interstitial sites research group. Brachytherapy 2016;15:788–795. [DOI] [PubMed] [Google Scholar]
- 28. Biglia N, Maggiorotto F, Liberale V et al. Clinical‐pathologic features, long term‐outcome and surgical treatment in a large series of patients with invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC). Eur J Surg Oncol 2013;39:455–460. [DOI] [PubMed] [Google Scholar]
- 29. Ribeiro GG, Magee B, Swindell R et al. The Christie Hospital breast conservation trial: An update at 8 years from inception. Clin Oncol (R Coll Radiol) 1993;5:278–283. [DOI] [PubMed] [Google Scholar]
- 30. Shah C, Wilkinson JB, Shaitelman S et al. Clinical outcomes using accelerated partial breast irradiation in patients with invasive lobular carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e547–551. [DOI] [PubMed] [Google Scholar]
- 31. Shaikh AY, LaCombe MA, Du H et al. Accelerated partial breast irradiation using once‐daily fractionation: Analysis of 312 cases with four years median follow‐up. Radiat Oncol 2012;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]


