Abstract
Background
Docetaxel (DOC) and abiraterone (ABI) in the upfront setting have separately improved clinical outcomes for metastatic hormone‐sensitive prostate cancer (mHSPC), but there are no studies comparing drug efficacies or the influence of racial disparities.
Materials and Methods
We performed a retrospective multicenter review from Winship Cancer Institute at Emory University and Georgia Cancer Center for Excellence at Grady Memorial Hospital (2014–2020) for patients with mHSPC treated with either upfront DOC or ABI. Outcomes evaluated were overall survival (OS), progression‐free survival (PFS), and prostate‐specific antigen complete response (PSA CR).
Results
A total of 168 patients were included, consisting of 92 (54.8%) Black patients and 76 (45.2%) non‐Black patients (69 White and 7 Asian or Hispanic). Ninety‐four (56%) received DOC and 74 (44%) received ABI. Median follow‐up time was 22.8 months with data last reviewed June 2020. For OS, there was no significant difference between ABI versus DOC and Black versus non‐Black patients. For PFS, DOC was associated with hazard ratio (HR) 1.7 compared with ABI for all patients based on univariate association and HR 2.27 compared with ABI for Black patients on multivariable analysis. For PSA CR, Black patients were less likely to have a CR (odds ratio [OR] = 0.27).
Conclusion
ABI and DOC have similar OS with a trend toward better PFS for ABI in a cohort composed of 54% Black patients. Racial disparities were observed as prolonged PFS for Black patients treated with ABI, more so compared with all patients, and less PSA CR for Black patients. A prospective trial comparing available upfront therapies in a diverse racial population is needed to help guide clinical decision‐making in the era of novel treatment options.
Implications for Practice
Overall survival is similar for abiraterone and docetaxel when used as upfront therapy in metastatic hormone‐sensitive prostate cancer in a cohort composed of 54% Black patients. There is a trend towards improved progression‐free survival for abiraterone in all patients and Black patients. Non‐Black patients were more likely to achieve prostate‐specific antigen (PSA) complete response regardless of upfront therapy.
Keywords: Castration‐sensitive prostate cancer, Racial disparities, Upfront therapy, Abiraterone, Docetaxel
Short abstract
This article presents a retrospective review of 168 patients diagnosed with metastatic hormone‐sensitive prostate cancer who received upfront therapy with either docetaxel (DOC) or abiraterone (ABI), focusing on real‐world clinical outcomes and potential racial disparities.
Introduction
Prostate cancer (PCa) is the most commonly diagnosed malignancy among men in the U.S. based on 2020 data with a 1.04% increase in metastatic cases each year [1, 2, 3, 4]. Although localized disease has a 5‐year survival of 100%, metastatic disease portends a worse prognosis with a 30.2% 5‐year relative survival [1]. In metastatic hormone‐sensitive prostate cancer (mHSPC), clinical outcomes have improved with upfront therapies, such as docetaxel (DOC), abiraterone (ABI), enzalutamide, and apalutamide; however, there are no real‐world studies comparing outcomes among these novel therapeutics [5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. Generally, DOC is used more often for patients with high‐volume disease and good performance status whereas ABI is used in both low‐ and high‐volume disease, worse performance status, and patients who prefer to take pills instead of intravenous chemotherapy [5, 14].
Another unknown within the mHSPC population is the influence of race on clinical outcomes. Black patients consistently have a higher incidence of PCa and mortality from disease compared with all other races (incidence of 175.1 vs. 109.8 per 100,000 and mortality of 36.4 vs 19.1 per 100,000) [5, 15, 16, 17]. Although Black race has been associated with overall greater risk for PCa, recent studies in castration‐resistant prostate cancer (CRPC) have shown better outcomes for Black patients when treated with either DOC or ABI compared with White patients [18, 19, 20, 21, 22, 23]. For the mHSPC patient population, clinical trials leading to approval of DOC and ABI either did not report race or included a predominately White study population with <10% Black participants [6, 10, 11].
We sought to analyze clinical outcomes in a racially diverse population with mHSPC. Our retrospective review consists of 54% Black patients to evaluate clinical outcomes including overall survival (OS), progression‐free survival (PFS), and prostate‐specific antigen complete response (PSA CR) in the real‐world setting to compare the efficacy of upfront DOC and ABI in addition to assessing for racial disparities.
Materials and Methods
Patients
The records of patients with PCa were compiled from pharmacy databases at Winship Cancer Institute of Emory University and Grady Cancer Center for Excellence (2014–2020). Patients treated with either DOC or ABI were identified. Those patients underwent chart review to determine if they received the drug as upfront therapy for mHSPC. Patients were selected for inclusion in our study if they were diagnosed with metastatic hormone‐sensitive prostate cancer, treated with DOC or ABI in the upfront setting, and did not receive any other systemic therapy before DOC or ABI. We did include patients if they received local therapies, such as surgery, radiation, or cryoablation. Institutional review board approval was obtained. Data were collected at baseline, defined as the time before or just after starting upfront therapy, and at 12 weeks after starting the drug. In June 2020, the patient list was reviewed to update data on patient progression or death.
Definitions
Patients were classified as high‐volume disease based on the CHAARTED criteria of visceral metastases or ≥ 4 bone lesions with ≥1 beyond axial skeleton. The number of distant metastases is defined as the number of different anatomical locations, including lymph nodes, bone, liver, lung, and brain.
Clinical outcomes included OS (time from drug initiation to death, transfer to hospice, or lost to follow‐up), PFS (time from drug initiation to biochemical progression, radiographic progression, death, transfer to hospice care, or lost to follow‐up, whichever occurred first), and PSA CR (PSA level ≤ 0.2 ng/mL 12 weeks after treatment with either DOC or ABI). Biochemical progression was based on an increase in PSA on two consecutive measurements with the first measurement noted as time of progression, or if PSA nadir was <4, then the PSA >4 was used as time of progression.
Statistical Analysis
Statistical analysis was conducted using SAS Version 9.4 and SAS macros (SAS Institute, Cary, NC) [24]. The significance level was set at p < .05. Descriptive statistics for each variable were reported. The univariate association of each covariate with treatment drug or PSA CR was assessed using the chi‐square test for categorical covariates and analysis of variance for numerical covariates. The univariate association (UVA) and multivariable analysis (MVA) for OS or PFS was tested by a Cox proportional hazards model with hazard ratio (HR) and its 95% confidence interval (CI) being reported. The UVA and MVA for PSA CR status was performed using a logistic regression model with the odds ratio (OR) and hazard ratio (HR) being reported along with the 95% CI and p value. Variables controlled in the MVA analysis were drug, race, age, Gleason score, disease volume, and Eastern Cooperative Oncology Group (ECOG) performance status. Disease volume was focused on because it encompasses locations and number of metastases. Kaplan‐Meier curves were generated using OS and PFS for the entire cohort and by race group [25]. The effect of upfront treatment in the subgroups was estimated by an MVA model with interaction term between the treatment group and stratified variables.
Results
Patient Characteristics
A total of 168 patients fit our inclusion criteria. Median age of diagnosis was 63.5 years. Ninety‐two patients were Black (54.8%) and 76 patients were non‐Black (45.2%, 69 White and 7 Asian or Hispanic). Median follow‐up time was 22.8 months (95% CI 19.3–25.8 months) for all patients, 22.6 months (95% CI 18.4–27.5 months) for Black patients, and 23 months (95% CI 16.4–27.8 months) for non‐Black patients. For upfront therapy, 94 patients received DOC (55.95%) and 74 received ABI (44.05%). Median follow‐up time for DOC was 29.6 months (95% CI 23.9–35.4 months) and ABI was 15.6 months (95% CI 12.2–19.3 months). The DOC and ABI groups were balanced in regard to race, age at diagnosis, and ECOG performance status. The DOC group was more likely to have high‐volume disease. The ABI group was older (as a continuous variable) and were more likely taking medications for hypertension (Table 1).
Table 1.
Baseline characteristics in DOC and ABI groups
| Variable | DOC (n = 94) | ABI (n = 74) | p value a |
|---|---|---|---|
| Race | |||
| Black | 48 (51.06) | 44 (59.46) | .278 |
| Non‐Black | 46 (48.94) | 27 (36.49) | |
| Age at diagnosis, yr | |||
| <65 | 54 (57.45) | 36 (48.65) | .256 |
| ≥65 | 40 (42.55) | 38 (51.35) | |
| Total Gleason score | |||
| 7 | 6 (6.38) | 17 (22.97) | .008 |
| 8–10 | 64 (68.09) | 42 (56.76) | |
| Unknown | 24 (25.53) | 15 (20.27) | |
| Metastatic disease at initial diagnosis or recurrence | |||
| Initial | 75 (79.79) | 56 (75.68) | .523 |
| After recurrence | 19 (20.21) | 18 (24.32) | |
| ECOG performance status at time of starting treatment b | |||
| 0 | 47 (50) | 29 (39.19) | .077 |
| 1 | 38 (40.43) | 29 (39.19) | |
| 2 | 9 (9.57) | 16 (21.62) | |
| Number of distant metastases c | |||
| 0–1 | 19 (20.21) | 25 (33.78) | .047 |
| 2–4+ | 75 (79.79) | 49 (66.22) | |
| Bone metastases | |||
| No | 15 (15.96) | 17 (22.97) | .250 |
| Yes | 79 (84.04) | 57 (77.03) | |
| Liver metastases | |||
| No | 83 (88.3) | 73 (98.65) | .010 |
| Yes | 11 (11.7) | 1 (1.35) | |
| Brain metastases | |||
| No | 92 (97.87) | 73 (98.65) | .999 |
| Yes | 2 (2.13) | 1 (1.35) | |
| Lung metastases | |||
| No | 74 (78.72) | 65 (87.84) | .121 |
| Yes | 20 (21.28) | 9 (12.16) | |
| Disease volume d | |||
| High | 71 (76.34) | 40 (54.05) | .002 |
| Low | 22 (23.66) | 34 (45.95) | |
| Prior treatment for localized disease e | |||
| No | 63 (67.02) | 44 (59.46) | .312 |
| Yes | 31 (32.98) | 30 (40.54) | |
| Prior treatment: Prostatectomy | |||
| No | 76 (80.85) | 67 (90.54) | .080 |
| Yes | 18 (19.15) | 7 (9.46) | |
| Prior treatment: Radiation | |||
| No | 64 (68.82) | 48 (64.86) | .589 |
| Yes | 29 (31.18) | 26 (35.14) | |
| Aspirin | |||
| No | 65 (69.15) | 51 (68.92) | .974 |
| Yes | 29 (30.85) | 23 (31.08) | |
| Metformin | |||
| No | 83 (88.3) | 67 (90.54) | .641 |
| Yes | 11 (11.7) | 7 (9.46) | |
| Anti‐HTN medications | |||
| No | 46 (48.94) | 21 (28.38) | .007 |
| Yes | 48 (51.06) | 53 (71.62) | |
| Beta blocker | |||
| No | 80 (85.11) | 54 (72.97) | .052 |
| Yes | 14 (14.89) | 20 (27.03) | |
| CCB | |||
| No | 64 (68.09) | 40 (54.05) | .063 |
| Yes | 30 (31.91) | 34 (45.95) | |
| ACEi/ARB | |||
| No | 71 (75.53) | 44 (59.46) | .026 |
| Yes | 23 (24.47) | 30 (40.54) | |
| Age at diagnosis, yr (continuous) | 62.65 | 65.77 | .022 |
Data are presented as n (%).
Bold p values are statistically significant.
The p value is calculated by analysis of variance for numerical covariates and chi‐square test or Fisher's exact for categorical covariates, when appropriate.
Ranging from 0 to 5, with lower scores indicating better functionality.
Number of anatomical locations (lymph nodes = 1, bone = 1, liver = 1, lung = 1, brain = 1).
Disease volume is classified as high‐volume disease based on CHAARTED criteria of visceral metastases or ≥ 4 bone lesions with ≥1 beyond axial skeleton.
Androgen deprivation therapy.
Abbreviations: ABI, abiraterone; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; DOC, docetaxel; ECOG, Eastern Cooperative Oncology Group; EUH,; HTN, hypertension.
Overall Survival
OS in all patients for ABI was 32.2 months with 95.6% survival at 12 months and for DOC was 47.5 months with 92.2% survival at 12 months (Fig. 1A). There was no significant difference in OS between upfront therapies. For Black patients, OS at 12 months was 97.5% for ABI and 89.2% for DOC (Fig. 1B). For non‐Black patients, OS at 12 months was 92.6% for ABI and 95.5% for DOC (Fig. 1C).
Figure 1.
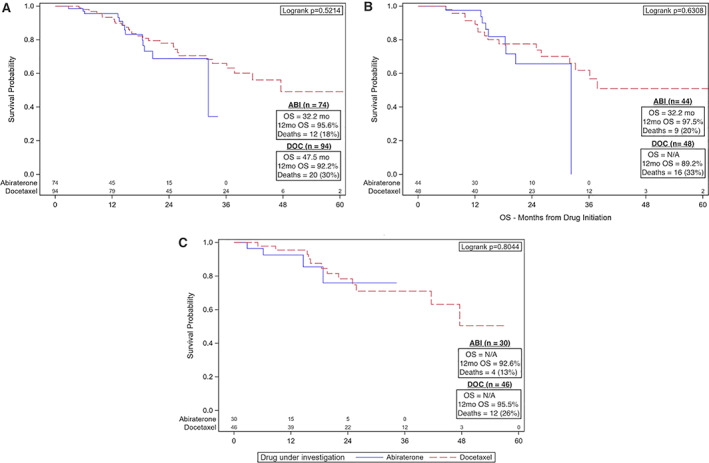
Kaplan‐Meier (KM) plots for OS for patients with metastatic hormone‐sensitive prostate cancer. KM curves were generated using OS both for the entire cohort and by age group. (A): KM plot including all patients in the cohort. The ABI group has OS of 32.2 months and the DOC group has OS of 47.5 months. There was no difference in OS. (B): KM plot for Black patients only showing no difference in OS between Black patients who received ABI and DOC. (C): KM plot for non‐Black patients only also showing no difference in OS based on upfront therapy.
Abbreviations: ABI, abiraterone; DOC, docetaxel; OS, median overall survival; N/A, not applicable; OS, overall survival.
UVA identified shorter OS for ECOG performance status of 2 (HR 2.21, 95% CI 1.01–4.84, p = .048) and liver metastases (HR 3.30, 95% CI 1.46–7.47, p = .004; Table 2; supplemental online Table 1). There was no significant difference in OS between upfront DOC versus ABI or Black versus non‐Black or based on disease volume. Neither MVA nor subgroup analyses identified any significant differences in OS (Table 2; supplemental online Table 4A).
Table 2.
UVA and MVA results for OS, PFS, and PSA CR
| Variable | UVA a | MVA b , c , d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | PFS | PSA CR | OS | PFS | PSA CR | |||||||
| HR (95% CI) | p value | HR (95% CI) | p value | OR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | OR (95% CI) | p value | |
| Drug | ||||||||||||
| DOC | 0.80 (0.40–1.59) | .522 | 1.70 (1.06–2.75) | .029 | 0.47 (0.25–0.89) | .020 | 0.73 (0.34–1.60) | .437 | 1.53 (0.90–2.61) | .119 | 0.46 (0.19–1.13) | .089 |
| ABI | — | — | — | — | — | — | ||||||
| Race | ||||||||||||
| Black | 1.37 (0.73–2.58) | .324 | 1.33 (0.87–2.05) | .190 | 0.22 (0.11–0.42) | <.001 | 1.11 (0.51–2.40) | .794 | 0.90 (0.52–1.57) | .707 | 0.27 (0.11–0.64) | .003 |
| Non‐Black | — | — | — | — | — | — | ||||||
| ECOG performance status | ||||||||||||
| 2 | 2.21 (1.01–4.84) | .048 | 1.78 (0.96–3.28) | .067 | 0.27 (0.10–0.75) | .012 | 1.97 (0.75–5.16) | .167 | 2.23 (1.08–4.60) | .030 | 0.42 (0.11–1.57) | .195 |
| 1 | 0.79 (0.39–1.60) | .507 | 1.31 (0.82–2.10) | .257 | 0.31 (0.16–0.63) | .001 | 0.61 (0.26–1.44) | .257 | 1.44 (0.82–2.53) | .200 | 0.38 (0.15–0.93) | .035 |
| 0 | — | — | — | — | — | — | ||||||
| Disease volume | ||||||||||||
| High | 2.12 (0.88–5.06) | .092 | 2.75 (1.55–4.89) | <.001 | 0.10 (0.05–0.20) | <.001 | 1.72 (0.68–4.35) | .256 | 2.45 (1.32–4.56) | .005 | 0.12 (0.05–0.30) | <.001 |
| Low | — | — | — | — | — | — | ||||||
The full UVA data can be found in the supplemental online materials.
Bold p values are statistically significant.
The univariate association of each covariate with treatment drug was assessed using the chi‐square test for categorical covariates and analysis of variance for numerical covariates. The univariate association of each covariate with OS or PFS was tested by a Cox proportional hazards model with HR and its 95% CI being reported.
Cox proportional hazards model for OS and PFS. The odds ratio and hazard ratio were reported along with 95% CI and p value.
Logistic regression model for PSA CR. The odds ratio and hazard ratio were reported along with 95% CI and p value.
Variables controlled in the MVA analysis were treatment drug, race, age, Gleason score, ECOG performance status, and disease volume.
Abbreviations: —, no data; ABI, abiraterone; CI, confidence interval; DOC, docetaxel; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; MVA, multivariable analysis; OR, odds ratio; PFS, progression‐free survival; PSA CR, prostate‐specific antigen complete response; UVA, univariate association.
Progression‐Free Survival
PFS in all patients for ABI was 26.1 months with 12‐month survival of 71.8%, and that for DOC was 12.9 months with 12‐month survival of 55.1% (Fig. 2A). For Black patients, PFS at 12 months was 72% for ABI and 45.3% for DOC (Fig. 2B). For non‐Black patients, PFS at 12 months was 72.2% for ABI and 65.3% for DOC (Fig. 2C).
Figure 2.
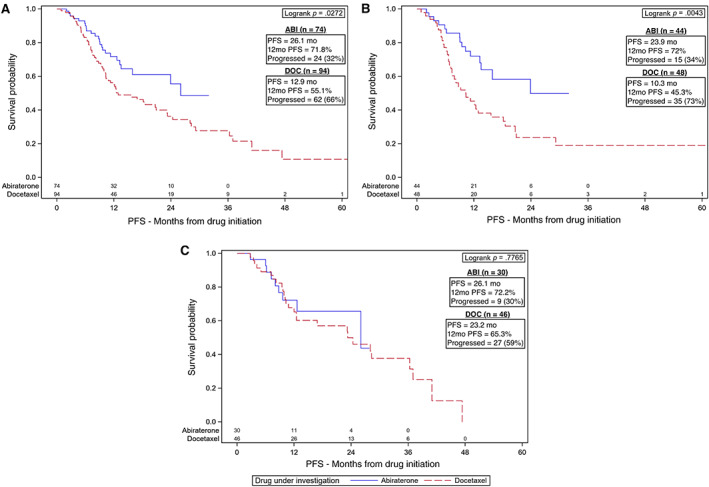
Kaplan‐Meier (KM) plots for PFS for patients with metastatic hormone‐sensitive prostate cancer. KM curves were generated using PFS both for the entire cohort and by age group. (A): KM plot including all patients in the cohort showing improved PFS for those treated with ABI (p = .0272). (B): KM plot for Black patients only showing improved PFS for Black patients treated with ABI (p = .0043). (C): KM plot for non‐Black patients only showing no difference in PFS based on upfront therapies (p = .7765).Abbreviations: ABI, abiraterone; DOC, docetaxel; PFS, progression‐free survival.
Based on our UVA, there was a 70% increased risk of death or progression for patients treated with DOC (HR 1.7, 95% CI 1.06–2.75, p = .029) in all patients with subgroup analyses finding that Black patients receiving DOC were more than two times as likely to progress or die compared with those receiving ABI (HR 2.27, 95% CI 1.16–4.42, p = .016; Table 2; supplemental online Table 4B). This was not seen on MVA. Kaplan‐Meier plots visually illustrate this PFS benefit for ABI compared with DOC in all patients and among Black patients (Fig. 2A, 2B). There were no differences noted for non‐Black patients based on upfront therapy (Fig. 2C).
DOC was also associated with an increased risk for death or progression in patients with high‐volume disease in both the UVA (HR 2.75, 95% CI 1.55–4.89, p < .001) and MVA (HR 2.45, 95% CI 1.32–4.56, p = .005; Table 2; supplemental online Table 2). Similarly, patients with an ECOG performance status of 2 had worse outcomes in the MVA (HR 2.23, 95% CI 1.08–4.60, p = .030; Table 2).
PSA Complete Response
Median PSA at diagnosis was 56.86, ranging from 0.27 to >3,500. PSA CR was achieved in 65 patients (38.69%). Evaluating based on drug, 36 patients received ABI (48.65% of ABI group) and 29 patients received DOC (30.85% of DOC group). Based on race, 21 patients were Black (12.5% of all patients) and 44 were non‐Black (26.19% of all patients; supplemental online Table 3).
Based on MVA findings, PSA CR was more often achieved in non‐Black patients (OR for Black patients = 0.27, 95% CI 0.11–0.64, p = .003), ECOG performance status 0 (OR for ECOG performance status 1 = 0.38, 95% CI 0.15–0.93, p = .035), and low‐volume disease (OR for high‐volume disease = 0.12, 95% CI 0.05–0.30, p < .001; Table 2). The UVA found that ABI was associated with more PSA CR (OR for DOC = 0.47, 95% CI 0.25–0.89, p = .020), but this was not confirmed in MVA. Subgroup analysis did identify that PSA CR was less likely for Black patients treated with DOC (OR 0.20, 95% CI 0.05–0.79, p = .021; Table 2; supplemental online Table 4C).
Discussion
Our study evaluated real‐world outcomes in mHSPC based on upfront therapy (ABI or DOC) and racial disparities in a diverse patient population of 54.8% Black patients and 45.2% non‐Black patients in Atlanta, Georgia. We found similar OS based on upfront therapies (ABI and DOC) and race (Black and non‐Black; Fig. 1; Table 2). There is a trend toward better PFS for ABI, with DOC having an HR of 1.7 (95% CI 1.06–2.75, p = .029) for all patients and 2.27 (95% CI 1.16–4.42, p = .016) for Black patients (Table 2; Fig. 2A, 2B). In non‐Black patients, PFS was similar for ABI and DOC (Table 2; Fig. 2C). PSA CR was more likely in ABI, non‐Black patients, low‐volume disease, and ECOG performance status 0 (Table 2). This is the first study comparing upfront DOC and ABI in mHSPC with approximately half the population being Black, reflecting a more realistic clinical practice.
Our retrospective data illustrate a need for prospective comparisons given similar OS for both drugs and a trend toward better PFS in ABI, suggesting that ABI is a reasonable option in high‐volume disease. DOC was added to mHSPC treatment based on the CHAARTED trial, which demonstrated a 13.6‐month OS benefit with DOC plus androgen deprivation therapy (ADT) compared with ADT alone, which increased to a 17‐month survival benefit in high‐volume disease. The STAMPEDE C arm and STOpCaP meta‐analysis confirmed the improvements in OS and PFS for DOC in high‐volume disease [6, 8, 9, 14]. ABI was added as a treatment option based on the LATITUDE trial reporting a 3‐year survival of 66% for ABI plus ADT compared with 49% in the placebo plus ADT group and a PFS benefit of 33 months for ABI plus ADT compared with 14.8 months for placebo plus ADT; this improvement was confirmed in the STAMPEDE trial arm G [10, 11]. The recent addition of enzalutamide and apalutamide further complicates the choice of upfront therapies without head‐to‐head comparisons or real‐world data to guide treatment decisions. Although at the time of our study there were no available studies to compare upfront therapies in mHSPC, the PEACE‐1 trial was underway to prospectively address outcomes in HSPC treated with ADT alone or in combination with DOC, ADT alone or in combination with ABI, ADT alone or in combination with DOC and radiation, and ADT alone or in combination with DOC and ABI [12, 13, 26].
In mHSPC, there are no data specifically for racial disparities, yet prior studies report that Black patients have a higher incidence of PCa, receive a diagnosis at a younger age, and suffer from more aggressive disease [3, 15, 17, 18, 19, 26, 27]. However, the current understanding of racial disparities in PCa has become increasingly complex, with recent studies in CRPC showing that Black patients may have better clinical outcomes compared with White patients, seen as better OS when treated with DOC, ABI, or enzalutamide [20, 21, 22, 23, 27, 28, 29].
Despite the higher incidence of PCa in Black patients and questions regarding racial disparities, clinical trial populations do not accurately reflect real‐life diversity, with Black patients accounting for only 2.74% of oncology clinical trials. The trials leading to approval for ABI and DOC either did not report race as in STAMPEDE and LATITUDE or had only 9.6% Black participation as in CHAARTED [6, 10, 11, 30]. Additionally, clinical trial patients are on average 6.5 years younger than usual patient populations undergoing treatment [31]. Data from real‐world clinical practice are needed to better understand outcomes in patients who are from different backgrounds, are older, and may have comorbidities.
Our study starts to address the discrepancy in clinical data for Black patients with PCa and specifically mHSPC. We found that Black and non‐Black patients had similar OS and PFS regardless of upfront therapy; however, subgroup analyses illustrate decreased PFS for Black patients treated with DOC compared with ABI (HR 2.27, CI 1.16–4.42, p = .016; Table 2; supplemental online Table 4B). We also found disparities in PSA CR with Black patients being less likely to achieve a CR (OR 0.27, 95% CI 0.11–0.64, p = .003), especially if treated with DOC (OR 0.20, 95% CI 0.05–0.79, p = .021; Table 2; supplemental online Table 4C). Literature review identified one study in mCRPC that also found better PSA responses for Black patients treated with ABI; otherwise, there is minimal information about variations in responses to ABI and DOC based on race [20–22]. A prospective study could help elucidate if these disparities seen in subgroup analyses for PFS and PSA CR translate to differences in OS over time.
Potential explanations for these observed racial disparities include both biological and socioeconomic etiologies. Proposed molecular mechanisms for the increased incidence in PCa have included genetic polymorphisms in the androgen signaling pathway, variations in growth factor expression, differences in microsatellites, and epigenetics [32, 33, 34, 35, 36, 37, 38]. However, a large multiple cohort study of 306,100 patients with localized and metastatic PCa, including 18.1% Black patients, found no significant difference for PCa‐specific outcomes for Black patients in cohorts with equal access to care (i.e., the Department of Veteran's Affaircomplets and National Cancer Institute), suggesting social factors as a primary contributor to racial disparities in PCa [39]. We postulate that the observed improved response to ABI for Black patients in our cohort is multifactorial with potential explanations both biologically and socioeconomically. Other studies have found increased androgen receptor expression and higher levels of circulating androgens in Black patients, which could provide a biological basis for the improved response to androgen synthesis inhibition with ABI [20, 40]. However, identifying potential biological basis is confounded by the social inequalities that Black patients are more likely to face, such as less access to health care, education, social services, and financial support [41, 42, 43]. Our study lacked financial and education data to further differentiate these socioeconomic factors, so we are unable to definitively determine the cause of racial disparities in our patient population. Future studies should include these data points to further distinguish underlying causes of any racial disparities.
There are a few other limitations to our study in addition to the lack of socioeconomic data. As a retrospective review from two hospital centers in Atlanta, Georgia, with a small number of Asian and Hispanic patients, the results might not be generalizable to other settings. The self‐reported nature of race and potential heterogeneity between the two practice sites present challenges to our racial disparity analyses. Additionally, we are unable to control for all possible confounding factors given our population size and expected limitations of retrospective data. The median follow‐up time was 22.8 months, which may not be long enough to fully discern the extent of any differences among our patients’ outcomes. Potentially, the differences seen between ABI and DOC groups could be related to unmeasured differences in baseline populations not accounted for in our analyses, such as the tendency toward DOC for patients who may have poor adherence to daily oral therapies, such as ABI. We did not include other novel oral therapies, such as enzalutamide or apalutamide, owing to the small sample size and short follow‐up for our patients with mHSPC given the recent approval of these therapies. Despite these limitations, we believe the study has several strengths. The inclusion of 54.8% Black patients offers insight into the outcomes of a group that is underrepresented in clinical trials. Our UVAs and MVAs accounted for a large number of clinical and demographic factors. Prospective validation of our results would help clarify differences in outcomes and racial disparities.
Conclusion
Our retrospective multicenter study evaluated clinical outcomes (OS, PFS, and PSA CR) in a population of 54.8% Black patients based on upfront therapies (ABI and DOC) and assessed outcomes for racial disparities. These real‐world data observed similar OS for ABI versus DOC and Black versus non‐Black patients, supporting the current use of ABI and DOC in Black patients with mHSPC despite the underrepresentation of this group in clinical trials. There was a trend toward better PFS for ABI in all patients and Black patients, whereas non‐Black patients had similar PFS for both ABI and DOC. Racial disparities were also observed in PSA CR, with Black patients being less likely to achieve a CR. To our knowledge, this is the first study in mHSPC to evaluate clinical outcomes based on upfront therapy and racial disparities.
Author Contributions
Conception/design: Katherine Emilie Rhoades Smith, Jacqueline Theresa Brown, Limeng Wan, Yuan Liu, Melvin Moore, Omer Kucuk, Bradley Carthon, Bassel Nazha, Mehmet Asim Bilen
Provision of study material or patients: Melvin Moore, Omer Kucuk, Bradley Carthon, Bassel Nazha, Mehmet Asim Bilen, Greta Russler, Lauren Yantorni
Collection and/or assembly of data: Katherine Emilie Rhoades Smith, Jacqueline Theresa Brown
Data analysis and interpretation: Katherine Emilie Rhoades Smith, Jacqueline Theresa Brown, Limeng Wan, Yuan Liu, Mehmet Asim Bilen
Manuscript writing: Katherine Emilie Rhoades Smith, Jacqueline Theresa Brown, Limeng Wan, Yuan Liu, Bassel Nazha, Mehmet Asim Bilen
Final approval of manuscript: Katherine Emilie Rhoades Smith, Jacqueline Theresa Brown, Limeng Wan, Yuan Liu, Greta Russler, Lauren Yantorni, Sarah Caulfield, Jennifer Lafollette, Melvin Moore, Omer Kucuk, Bradley Carthon, Bassel Nazha, Mehmet Asim Bilen
Disclosures
Bassel Nazha: Exelixis (C/A); Mehmet Asim Bilen: Exelixis, Bayer, Bristol‐Myers Squibb, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, Sanofi (C/A), Xencor, Bayer, Bristol‐Myers Squibb, Genentech/Roche, Seattle Genetics, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Genome & Company, AAA, Peloton Therapeutics, Pfizer (RF [to institution, for work performed outside of the current study]). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table 1 Complete UVA for OS.
Supplementary Table 2. Complete UVA for PFS.
Supplementary Table 3. Complete UVA for PSA CR.
Supplementary Table 4. Cox Proportional Hazard Models Comparing Clinical Outcomes Based on Race and Upfront Therapy. A) OS, B) PFS, D) PSA CR.
Acknowledgments
Part of the data presented in this manuscript was presented at the 2020 American Society of Clinical Oncology Genitourinary Cancers Symposium. This work was supported by the National Institutes of Health/National Cancer Institute and the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute of Emory University under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
Editor's Note: See the related commentary, “Nature versus Nurture: Investigating Racial Disparity in Advanced Prostate Cancer,” by Nishita Tripathi, Neeraj Agarwal, and Abhishek Tripathi, on page 904 of this issue.
References
- 1. National Cancer Institute . Cancer Stat Facts: Common Cancer Sites. Available at https://seer.cancer.gov/statfacts/html/common.html. Accessed July 6, 2020.
- 2. Dalela D, Sun M, Diaz M et al. Contemporary trends in the incidence of metastatic prostate cancer among US men: Results from nationwide analyses. Eur Urol Focus 2019;5:77–90. [DOI] [PubMed] [Google Scholar]
- 3. Kelly SP, Anderson WF, Rosenberg PS et al. Past, current, and future incidence rates and burden of metastatic prostate cancer in the United States. Eur Urol Focus 2018;4:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiner AB, Matulewicz RS, Eggener SE et al. Increasing incidence of metastatic prostate cancer in the United States (2004‐2013). Prostate Cancer Prostatic Dis 2016;19:395–397. [DOI] [PubMed] [Google Scholar]
- 5. Hahn AW, Higano CS, Taplin M‐E et al. Metastatic castration sensitive prostate cancer: Optimizing patient selection and treatment. Am Soc Clin Oncol Educ Book 2018;38:363–371. [DOI] [PubMed] [Google Scholar]
- 6. Sweeney CJ, Chen Y‐H, Carducci MA et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer. N Engl J Med 2015;373:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gravis G, Boher JM, Joly F et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: Impact of metastatic burden and long‐term survival analysis of the randomized phase 3 GETUG‐AFU15 trial. Eur Urol 2016;70:256–262. [DOI] [PubMed] [Google Scholar]
- 8. James ND, Sydes MR, Clarke NW et al. Addition of docetaxel, zoledronic acid, or both to first‐line long‐term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vale CL, Burdett S, Rydzewska LHM et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone‐sensitive prostate cancer: A systematic review and meta‐analyses of aggregate data. Lancet Oncol 2016;17:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fizazi K, Tran NP, Fein L et al. Abiraterone plus prednisone in metastatic, castration‐sensitive prostate cancer. N Engl J Med 2017;377:352–360. [DOI] [PubMed] [Google Scholar]
- 11. James ND, de Bono JS, Spears MR et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017;377:338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armstrong AJ, Szmulewitz RZ, Petrylak DP et al. ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone‐sensitive prostate cancer. J Clin Oncol 2019;37:2974–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith MR, Saad F, Chowdhury S et al. Apalutamide treatment and metastasis‐free survival in prostate cancer. N Engl J Med 2018;378:1408–1418. [DOI] [PubMed] [Google Scholar]
- 14. Sweeney C, Chen Y, Liu G et al. Long term efficacy and QOL data of chemohormonal therapy (C‐HT) in low and high volume hormone naïve metastatic prostate cancer (PRCA): E3805 CHAARTED trial. Ann Oncol 2016;27:243–265. [Google Scholar]
- 15. National Cancer Institute . Cancer State Facts: Cancer Disparities. Available at https://seer.cancer.gov/statfacts/html/disparities.html. Accessed July 6, 2020.
- 16. Howlader N, Noone AM, Krapcho M et al. Cancer Statistics Review SEER, 1975. ‐2017, National Cancer Institute. Available at https://seer.cancer.gov/csr/1975_2017/. Published April 2020. [Google Scholar]
- 17. Kelly SP, Rosenburg PS, Anderson WF et al. Trends in the incidence of fatal prostate cancer in the United States by race. Eur Urol 2017;71:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nettey OS, Walker AJ, Keeter MK et al. Self‐reported Black race predicts significant prostate cancer independent of clinical setting and clinical and socioeconomic risk factors. Urol Oncol 2018;36:501.e1–501.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gaines AR, Turner EL, Moorman PG et al. The association between race and prostate cancer risk on initial biopsy in an equal access, multiethnic cohort. Cancer Causes Control 2014;25:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramalingam S, Humeniuk MS, Hu R et al. Prostate‐specific antigen response in black and white patients treated with abiraterone acetate for metastatic castrate‐resistant prostate cancer. Urol Oncol 2017;35:418–424. [DOI] [PubMed] [Google Scholar]
- 21. Bitting RL, Goodman M, George DJ. Racial disparity in response to prostate cancer systemic therapies. Curr Oncol Rep 2020;22:96. [DOI] [PubMed] [Google Scholar]
- 22. George GJ, Heath EI, Sartor AO et al. Abi Race: A prospective, multicenter study of black (B) and white (W) patients (pts) with metastatic castrate resistant prostate cancer (mCRPC) treated with abiraterone acetate and prednisone (AAP). J Clin Oncol 2018;36(suppl 18):LBA5009. [Google Scholar]
- 23. Halabi S, Dutta S, Tangen CM et al. Overall survival of black and white men with metastatic castration‐resistant prostate cancer treated with docetaxel. J Clin Oncol 2019;37:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Nickleach D, Zhang C et al. Carrying out streamlined routine data analyses with reports for observational studies: Introduction to a series of generic SAS® macros. F1000Res 2018;7:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal 1999;30:253–270. [Google Scholar]
- 26. Clinical Trials.gov . A Phase III Study for Patient with Metastatic Hormone‐Naïve Prostate Cancer (PEACE1). Available at https://clinicaltrials.gov/ct2/show/NCT01957436. Published April 22, 2020. Accessed July 6, 2020.
- 27. Smith ZL, Eggener SE, Murphy AB. African‐American prostate cancer disparities. Curr Urol Rep 2017;18:81. [DOI] [PubMed] [Google Scholar]
- 28. Bigler SA, Pound CR, Zhou X. A retrospective study on pathologic features and racial disparities in prostate cancer. Prostate Cancer 2011;2011:239640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McNamara MA, George DJ, Ramaswamy K et al. Overall survival by race in chemotherapy‐naïve metastatic castration‐resistant prostate cancer (mCRPC) patients treated with abiraterone acetate or enzalutamide. J Clin Oncol 2019;37(suppl 7S):212a [Google Scholar]
- 30. Woodcock J, Whyte J, Henderson MB. 2015. ‐2016 Global Participation in Clinical Trials Report. U.S. Food and Drug Administration. Available at https://www.fda.gov/media/106725/download.
- 31. Ludmir EB, Mainwaring W, Lin TA et al. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol 2019;5:1769–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh SK, Lillard JW, Singh R. Molecular basis for prostate cancer racial disparities. Front Biosci (Landmark Ed) 2017;22:428–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaston KE, Kim D, Singh S et al. Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J Urol 2003;170:990–993. [DOI] [PubMed] [Google Scholar]
- 34. Irvine RA, Yu MC, Ross RK et al. The CAG and GGC microsatellites of the androgen receptor gene are in linkage disequilibrium in men with prostate cancer. Cancer Res 1995;55:1937–1940. [PubMed] [Google Scholar]
- 35. Koga Y, Son H, Chalmers ZR et al. Genomic profiling of prostate cancers from men with African and European ancestry. Clin Cancer Res 2020;26:4651–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He Y, Mi J, Olson A et al. Androgen receptor with short polyglutamine tract preferably enhances Wnt/β‐catenin‐mediated prostatic tumorigenesis. Oncogene 2020;39:3276–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shui IM, Mucci LA, Kraft P et al. Vitamin D‐related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: A prospective nested case‐control study. J Natl Cancer Inst 2012;104:690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Devaney JM, Wang S, Furbert‐Harris P et al. Genome‐wide differentially methylated genes in prostate cancer tissues from African‐American and Caucasian men. Epigenetics 2015;10:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dess RT, Hartman HE, Mahal BA et al. Association of Black race with prostate cancer–specific and other‐cause mortality. JAMA Oncol 2019;5:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang BD, Yang Q, Ceniccola K et al. Androgen receptor‐target genes in African American prostate cancer disparities. Prostate Cancer 2013;2013:763569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Major JM, Oliver NM, Doubeni CA et al. Socioeconomic status, health care density, and risk of prostate cancer among African‐American and Caucasian men in a large prospective study. Cancer Causes Control 2012;23:1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coughlin SS. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int 2020;8:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borno H, George DJ, Schnipper LE et al. All men are created equal: Addressing disparities in prostate cancer care. Am Soc Clin Oncol Educ Book 2019;39:302–308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table 1 Complete UVA for OS.
Supplementary Table 2. Complete UVA for PFS.
Supplementary Table 3. Complete UVA for PSA CR.
Supplementary Table 4. Cox Proportional Hazard Models Comparing Clinical Outcomes Based on Race and Upfront Therapy. A) OS, B) PFS, D) PSA CR.


