Abstract
Background
Germline genetic testing is universally recommended for patients with pancreatic cancer, but testing remains infrequent. In May 2018, we implemented a systematic patient intake workflow featuring an in‐clinic genetic testing station (GTS) at the University of California San Francisco (UCSF) to expedite genetic counseling and facilitate sample collection. We sought to determine the impact of this innovation on rates of genetic counseling and testing.
Methods
Medical records, patient intake records, and genetic test reports were retrospectively reviewed for new patients with pancreatic cancer eligible for germline testing at UCSF from May 2018 to May 2019. Primary outcomes included the rate of offered genetic counseling and confirmed germline testing. Data were compared for periods before and after GTS implementation. Associations between demographic characteristics and testing rates were assessed.
Results
Genetic counseling/testing was offered to 209 (94%) of 223 eligible patients, and 158 (71%) completed testing (135 at UCSF, 23 elsewhere). Compared with a traditional referral‐based genetic counseling model, confirmed testing increased from 19% to 71%, patient attrition between referral and genetics appointment decreased from 36% to 3%, and rate of pathogenic variant detection increased from 20% to 33%. Patients who were younger, identified as non‐Hispanic White, and spoke English as a primary language were more likely to complete testing.
Conclusions
Implementation of a systematic patient intake workflow and in‐clinic GTS resulted in the highest reported real‐world rate of germline testing for patients with pancreatic cancer. Health care disparities were identified and will guide future innovation. This report provides a model for other centers to create a similar testing infrastructure.
Implications for Practice
This study demonstrates that a systematic patient intake workflow and associated in‐clinic genetic testing station improve delivery of genetic counseling and completion of germline testing for patients with pancreatic cancer. This study achieved, to the authors’ knowledge, the highest real‐world rate of confirmed genetic testing in this patient population. This article describes this innovation in detail to guide replication at other medical centers and facilitate guideline‐concordant care for patients with pancreatic cancer. This infrastructure can also be applied to other cancers for which germline testing is recommended.
Keywords: Pancreatic neoplasms, Genetic testing, Precision medicine, Guideline adherence, Quality improvement
Short abstract
Germline genetic testing is universally recommended for patients with pancreatic cancer, but testing remains infrequent. This article reports a patient intake workflow that was implemented to expedite genetic counseling and facilitate sample collection. The authors describe the impact of this innovation on rates of genetic counseling and testing.
Introduction
Over 60,000 patients are diagnosed with pancreatic ductal adenocarcinoma (PDAC) in the U.S. annually [1]. Although screening, diagnostic, and therapeutic advances have recently reduced the overall national burden and mortality of cancer, PDAC remains an exception: incidence and mortality rates are increasing, and PDAC is projected to be the second‐leading cause of U.S. cancer‐related deaths from 2030 [2] to 2040 [3] and beyond. The development of screening programs and biomarker‐driven therapies has established the importance of universal germline genetic testing to improve PDAC outcomes. However, implementing this testing for all patients with PDAC remains an ongoing challenge [4, 5].
Hereditary predisposition accounts for up to 10% of PDAC cases [6]. Identification of deleterious germline mutations through multigene genetic testing can inform treatments for the proband and screening for family members. Patients with pathogenic mutations in BRCA1/BRCA2 are candidates for maintenance therapy with the poly(ADP‐ribose) polymerase (PARP) inhibitor olaparib [7]; DNA mismatch repair–deficient tumors harboring mutations in MLH1, MSH2, MSH6, or PMS2 can be treated with immunotherapy [8]; and alterations in DNA homologous recombination repair (HRR)–related genes may confer susceptibility to platinum‐based chemotherapy [9, 10]. Other findings may inform clinical trial candidacy. Even if test results are not therapeutically actionable for the proband, they can prompt cascade testing for family members and surveillance of mutation carriers with the goal of detecting cancers at early stages [11, 12].
Population‐based studies have shown that pathogenic germline mutations are detected at high rates in patients regardless of whether they exhibit conventional features of hereditary cancer syndromes based on age, race/ethnicity, or family history [13, 14, 15, 16]. In light of these data, in 2018 the National Comprehensive Cancer Network [17] and the American Society of Clinical Oncology [18] expanded their recommendations for germline testing to all patients with PDAC. Adopting these recommendations required innovations in genetic counseling/testing workflow to accommodate increased patient volume [19].
In a traditional genetic testing model, providers refer patients at risk of a hereditary cancer syndrome to a genetic counselor (GC) or medical geneticist for discussion and consideration of germline testing. This process relies on subjective provider‐specific assessment of risk and/or familiarity with guidelines for universal testing, and it is prone to both bias and patient attrition. For example, at our medical center from 2015 to 2017, 32% of patients with PDAC were referred for genetic counseling and only 19% completed germline testing [20]. These data highlighted the need for practice‐specific initiatives to improve patient education and testing rates.
To address this need at our medical center, we designed and implemented a new intake workflow, featuring an embedded in‐clinic genetic testing station (GTS) staffed by trained genetic counseling assistants (GCA), for patients referred to our Gastrointestinal (GI) Medical Oncology clinic. The primary objectives of our new testing infrastructure were to (a) systematically engage all patients to discuss germline testing, (b) assess whether patients had already completed counseling/testing at another medical center, (c) offer standardized and accessible educational materials, (d) coordinate genetic testing sample collection with the initial medical oncology visit, (e) increase rates of germline testing while minimizing requisite expansion of genetic counseling and clinic resources, and (f) gather data to drive further improvements in testing practices. Herein, we report our experience from the first year of GTS operation and evaluate achievement of these objectives.
Materials and Methods
Study Population
This study included patients newly referred to University of California San Francisco (UCSF) GI Medical Oncology with a diagnosis of PDAC during the first year of GTS operation. There were no demographic or disease‐specific exclusion criteria, but patients in an external integrated health system who were authorized only for a second‐opinion medical oncology visit at UCSF were not eligible for testing at our site and were thus excluded. GTS efficacy was assessed by comparing the study population with a separate population of UCSF patients with PDAC over a 3‐year period prior to GTS implementation; this population has been previously described [20]. This study was approved by the UCSF Human Research Protection Program Institutional Review Board (10‐02541).
Traditional Testing Model
Prior to GTS implementation, patients with PDAC were referred to the UCSF Clinical Genetics program based on providers’ suspicion of a hereditary cancer syndrome. Patients were evaluated by a GC, and, if deemed appropriate, germline genetic testing was ordered via a variety of testing panels ranging in size from 2 to 479 genes. Panels were selected based on clinical data including family history, patient preference, and insurance coverage. Patients then met with a GC again to review results and, if indicated, discuss cascade testing of family members.
Genetic Testing Station Workflow
Figure 1 diagrams the GTS workflow. In our clinic, referred patients are first contacted by a PDAC Nurse Navigator (NN) who completes an intake process, confirms the appropriateness of the referral for GI Medical Oncology, and then alerts a New Patient Coordinator (NPC) to schedule an initial visit. In the GTS workflow, the NN also introduces the concept of universally recommended germline testing (supplemental online Appendix) and determines if the patient has already completed counseling/testing at another medical center. If the patient has not received counseling/testing and agrees to a GTS visit, the NN alerts the NPC to place a GTS referral, schedule a GTS kiosk appointment on or near the same day as the initial medical oncology visit, and begin the process of insurance authorization for a follow‐up visit with a GC.
Figure 1.
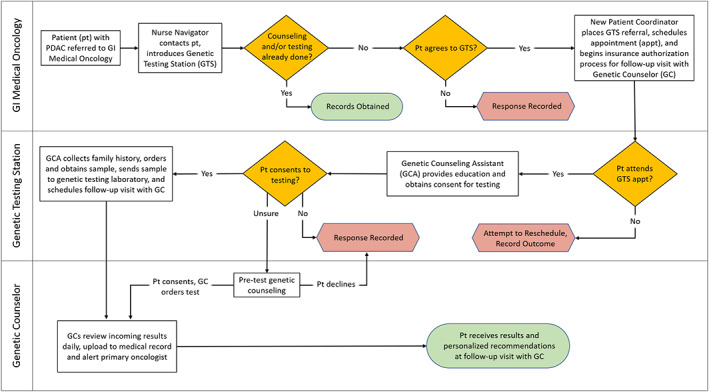
Operational workflow for genetic testing station referral and germline testing of patients with pancreatic ductal adenocarcinoma at the University of California San Francisco. Abbreviations: GC, genetic counselor; GCA, genetic counseling assistant; GI, gastrointestinal; GTS, genetic testing station; pt, patient.
The GTS kiosk is staffed by GCAs under the supervision of a GC. Once the patient arrives at the kiosk, they receive video‐based education about hereditary cancer and genetic testing (available at https://kintalk.org/videos/), provide consent for testing and tracking results for research purposes, report personal and family medical history, provide a saliva sample for testing, and schedule a follow‐up visit with a GC. Patients have the opportunity to receive additional pretest counseling by scheduling a visit with a GC prior to providing a sample. If patients decline GTS referral or germline testing, GTS staff record their rationale for refusal.
During the GTS kiosk appointment, GCAs also review cost information with patients. For the duration of the study, germline testing was supported via philanthropic funds at no cost to the patients. The GTS kiosk appointment is a no‐fee visit, and GC visits are billed to patients’ insurance.
Testing is performed with a custom Invitae panel including sequencing and/or copy number analysis of 133 genes (supplemental online Appendix). The reporting laboratory categorizes test results for each gene as pathogenic/likely pathogenic (grouped as “pathogenic” for this study), variant of unknown significance (VUS), or negative (wild type). Results are reviewed by GCs daily, uploaded to the medical record, and immediately made available to treating oncologists. The GC then reviews the results with patients at a follow‐up visit. If a patient has died prior to the follow‐up visit, results are disclosed to a prespecified designee.
Data Collection and Analysis
Demographic and disease‐specific data were abstracted via retrospective chart reviews. Patient reports of counseling/testing at other centers were obtained from GTS records. Germline test results for patients tested at UCSF were recorded from original reports.
If patients did not undergo testing, the reason for attrition was determined from GTS records and retrospective chart reviews: patients either (a) were not offered germline testing, (b) were not referred to the GTS, (c) did not attend the GTS appointment, (d) declined testing after GTS and/or GC education, or (e) did not provide an additional sample after the initial test failed. Associations between demographic characteristics and testing completion were assessed to identify populations at higher risk of attrition.
We evaluated the impact of the GTS on patient engagement and testing completion by comparing clinical efficacy metrics during time periods prior to GTS implementation (January 2015 to October 2017) and after GTS implementation (May 2018 to May 2019). Proportions of Clinical Genetics/GTS referrals, referral appointment attendance, testing completion, and pathogenic variant detection were compared. In addition, a key feature of the GTS workflow is the ability to systematically track completion of genetic counseling/testing at other medical centers prior to establishing care at UCSF. To quantify this benefit, we compared the overall proportion of UCSF patients who were confirmed to have completed testing at any site between the two time periods.
All associations were evaluated via two‐tailed chi‐square test for categorical variables and t test for continuous variables. Values of p < .05 were considered significant. Data were processed and analyzed in Stata version 16.1 (College Station, TX).
Results
During the 1‐year study period, 223 eligible patients with PDAC were seen at UCSF. Patients were an average of 64.6 years old at the time of their initial UCSF appointment, 52% were male, 66% identified as non‐Hispanic White race/ethnicity, and 90% spoke English as their preferred language (Table 1).
Table 1.
Demographic characteristics for patients with pancreatic ductal adenocarcinoma eligible for genetic testing at University of California San Francisco during the first year of GTS operation
| Characteristic | Eligible population (n = 223) | Completed testing (n = 158) | Missed testing (n = 65) | p value a |
|---|---|---|---|---|
| Age at appointment, yr, mean ± SD | 64.6 ± 12.2 | 63.0 ± 11.8 | 68.5 ± 12.2 | .002 |
| Sex, n (%) | ||||
| Male | 116 (52) | 84 (53) | 32 (49) | .59 |
| Female | 107 (48) | 74 (47) | 33 (51) | |
| Race/ethnicity, n (%) | ||||
| Non‐Hispanic White | 147 (66) | 111 (70) | 36 (55) | .03 b |
| Asian | 43 (19) | 25 (16) | 18 (28) | |
| Hispanic/Latino | 12 (5) | 9 (6) | 3 (5) | |
| Black/African American | 9 (4) | 7 (4) | 2 (3) | |
| Multiethnic/other | 5 (2) | 3 (2) | 2 (3) | |
| Unknown/declined | 7 (3) | 3 (2) | 4 (6) | |
| Preferred language, n (%) | ||||
| English | 200 (90) | 149 (94) | 51 (78) | <.001 c |
| Non‐English | 23 (10) | 9 (6) | 14 (22) | |
| Spanish | 7 (3) | 2 (1) | 5 (8) | |
| Mandarin | 5 (2) | 1 (1) | 4 (6) | |
| Russian | 3 (1) | 1 (1) | 2 (3) | |
| Cantonese | 3 (1) | 2 (1) | 1 (2) | |
| Korean | 2 (1) | 1 (1) | 1 (2) | |
| Punjabi | 1 (1) | 1 (1) | 0 | |
| Tagalog | 1 (1) | 1 (1) | 0 | |
| Vietnamese | 1 (1) | 0 | 1 (2) |
Two‐tailed chi‐square (categorical variables) or t test (continuous variable).
Comparing proportions of non‐Hispanic White versus the collective other groups.
Comparing proportions of English versus non‐English preferred language.
GTS Workflow
Figure 2 shows a comprehensive record of all eligible patients with PDAC at UCSF in the first year of GTS operation. Germline testing and GTS referral were discussed with 209 of 223 (94%) patients before or during their initial medical oncology visit. Twenty‐three (10%) patients reported completion of genetic counseling/testing at another medical center, and 147 (66%) were referred to the GTS. In total, 158 (71%) eligible patients with PDAC completed counseling/testing (135 at UCSF and 23 at other centers). For the 65 patients who did not, the majority of attrition was patient‐driven: 24 patients declined GTS referral, and 13 missed initial GC or GTS appointments. However, the GTS was not discussed with 14 patients, referrals were omitted without clearly documented rationale for 10 patients, and testing was unsuccessful for four patients. Patients who completed testing were more likely to be younger (mean age ± SD: 63.0 ± 11.8 vs. 68.5 ± 12.2; p = .002), to be non‐Hispanic White (70% vs. 55%, p = .03), and to speak English as their preferred language (94% vs. 78%, p = <.001).
Figure 2.
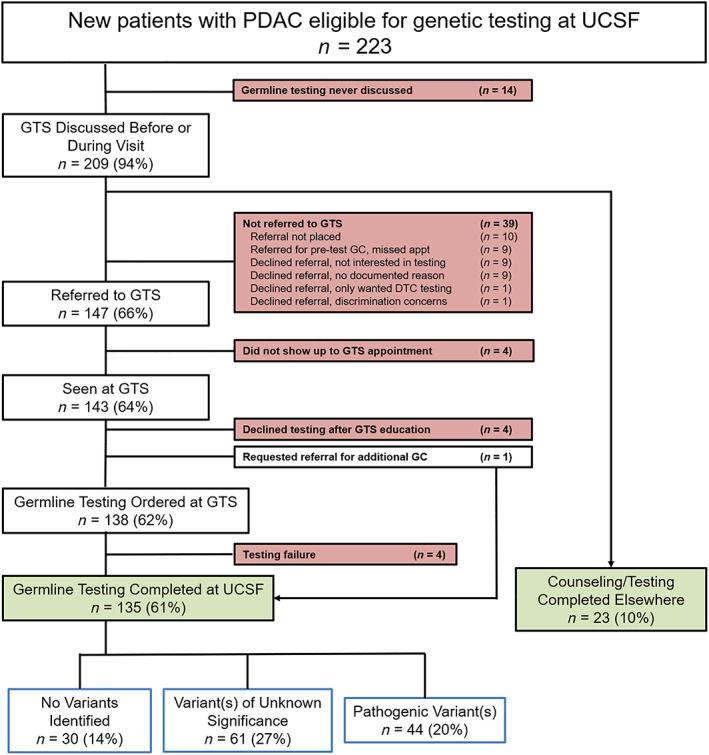
Genetic testing station referrals and germline testing for patients with PDAC during the first year of GTS operation. Abbreviations: DTC, direct‐to‐consumer; GC, genetic counselor; GTS, genetic testing station; PDAC, pancreatic ductal adenocarcinoma; UCSF, University of California San Francisco.
Implementation of the GTS statistically significantly improved all clinical efficacy metrics (Table 2). Prior to GTS implementation, 32% of patients with PDAC seen at UCSF were referred to Clinical Genetics; after GTS implementation, 66% were referred to the GTS (p < .001). Of the 32% of patients referred to Clinical Genetics in the traditional model, 64% attended their appointment and 60% completed testing. Of the 66% of patients referred to the GTS, 97% attended their appointment and 92% completed testing (p < .001). Overall, germline testing at any medical center was confirmed for 19% of patients seen prior to implementation of the GTS versus 71% of patients after implementation (p < .001).
Table 2.
Comparison of UCSF Clinical Genetics engagement and germline testing for patients with PDAC before and after implementation of GTS
| Clinical efficacy metric | Before GTS | After GTS | p value a |
|---|---|---|---|
| Total patients with PDAC seen at UCSF | n = 432 | n = 223 | |
| Referred to Clinical Genetics/GTS | 137/432 (32%) | 147/223 (66%) | <.001 |
| Referral appointment attended | 88/137 (64%) | 143/147 (97%) | <.001 |
| Testing completed at UCSF | 82/137 (60%) | 135/147 (92%) | <.001 |
| Pathogenic variant detected | 16/82 (20%) | 44/135 (33%) | .04 |
| Overall germline testing confirmed | 82/432 (19%) | 158/223 (71%) | <.001 |
Abbreviations: PDAC, pancreatic ductal adenocarcinoma; GTS, genetic testing station; UCSF, University of California San Francisco.
Two‐tailed chi‐square.
Germline Test Results
Compared with the traditional testing model, the rate of pathogenic variant detection was higher after GTS implementation (33% vs. 20%, p = .04). Among patients who underwent testing via the GTS, 49 pathogenic variants were discovered in 44 patients, and at least one VUS was detected in another 61 patients (Table 3). Of the pathogenic variants, 15 (31%) were in HRR‐related genes (ATM (n = 3), BRCA2 (n = 3), CHEK2 (n = 3), PALB2 (n = 2), BRCA1 (n = 1), BRIP1 (n = 1), FANCC (n = 1), FANCL (n = 1)), and one (2%) was in a mismatch repair–related gene: PMS2. Three patients with BRCA1/BRCA2 mutations and one with a PALB2 mutation received targeted therapy with a PARP inhibitor, and the patient with a PMS2 mutation was treated with immunotherapy.
Table 3.
Demographic and family history data for University of California San Francisco patients with pancreatic ductal adenocarcinoma and pathogenic germline variants
| Pt | Gene | Pathogenic variant | Age | Sex | Race/ ethnicity | Other cancer in patient (age if known) | Cancer in first‐degree relative (age if known) | Cancer in second‐degree relative (age if known) | VUS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | APC | c.3920T > A (p.Ile1307Lys) | 55 | Male | NHW | — | Pancreatic (64), colon (70) | Colon, breast (36), stomach | CFTR, TSC1 |
| 2 | ATM | c.7705_7706delGA (p.Asp2569*) | 51 | Male | Hispanic/ Latino | — | — | — | BLM, CTRC, DICER1, GALNT12 |
| 3 | ATM | c.7886_7890delTATTA (p.Ile2629Serfs*25) | 71 | Male | NHW | Basal cell carcinoma (46) | Breast (60s), lung | — | ERCC2 |
| 4 | ATM | c.1524del (p.Gly509Glufs*3) | 80 | Female | NHW | — | Breast, colon | — | |
| 4 | CHEK2 | c.470T > C (p.Ile157Thr) (low penetrance) | |||||||
| 5 | BRCA1 | c.5266dup (p.Gln1756Profs*74) | 47 | Female | NHW | Melanoma (42), melanoma (45) | Pancreatic (85), leukemia (74), ocular melanoma (77) | Breast (22) | CEP57 |
| 6 | BRCA2 | c.5946del (p.Ser1982Argfs*22) | 70 | Female | NHW | — | Brain (85), stomach (66) | — | |
| 7 | BRCA2 | c.3109C > T (p.Gln1037*) | 71 | Male | Asian | — | Bladder, ovarian (54), lymphoma (70) | — | BRCA2, BAP1, FANCM, KIT, MRE11A |
| 8 | BRCA2 | c.3165_3168delTCAA (p.Asn1055Lysfs*4) | 86 | Female | Asian | — | — | PSU (85) | FANCD2, FANCF, GALNT12, POLE |
| 8 | FANCC | c.389_390delAA (p.Glu130Glyfs*21) | |||||||
| 8 | MSH3 | c.708C > G (p.Tyr236*) | |||||||
| 9 | BRIP1 | Partial deletion (exon 7) | 76 | Male | Asian | — | Breast, ovarian, lung | — | AXIN2, BLM, BRCA2, RAD51C |
| 10 | CFTR | c.274G > T (p.Glu92*) | 72 | Female | NHW | — | Uterine (69), stomach | — | BAP1, FANCM |
| 10 | CFTR | c.1210‐34TG [11]T [5] (intronic) | |||||||
| 11 | CFTR | c.1210‐34TG [11]T [5] (intronic) | 77 | Male | NHW | — | Breast (82), lung, prostate (60), colon (65) | — | APC |
| 11 | RECQL4 | c.3433C > T (p.Gln1145*) | |||||||
| 12 | CFTR | c.489 + 3A > G (intronic) | 65 | Female | NHW | — | Oral (70) | — | CTC1 |
| 13 | CFTR | c.1521_1523delCTT (p.Phe508del) (ΔF508) | 54 | Male | NHW | — | — | — | |
| 14 | CFTR | c.1521_1523delCTT (p.Phe508del) (ΔF508) | 54 | Male | NHW | — | Lung (70s), pancreatitis (75) | Skin, skin | BRIP1 |
| 15 | CFTR | c.1521_1523delCTT (p.Phe508del) (ΔF508) | 69 | Female | NHW | — | Melanoma (27), oral, leukemia (6) | PSU (25) | FANCD2 |
| 16 | CFTR | c.1521_1523delCTT (p.Phe508del) (ΔF508) | 62 | Female | NHW | — | Adopted— unknown | Adopted— unknown | CFTR |
| 17 | CFTR | c.1210‐34TG [11]T [5] (intronic) | 57 | Female | Unknown | — | — | PSU | |
| 18 | CFTR | c.1210‐34TG [11]T [5] (intronic) | 60 | Male | NHW | — | Nasal | PSU | FANCD2, RET |
| 19 | CFTR | c.1210‐34TG [11]T [5] (intronic) | 63 | Male | NHW | — | Breast (40) | Stomach (40) | |
| 20 | CFTR | c.1210‐34TG [11]T [5] (intronic) | 67 | Female | NHW | — | Leukemia (80) | — | TSC2 |
| 21 | CFTR | c.1210‐34TG [11]T [5] (intronic) | 69 | Male | Black/African American | — | — | — | AXIN2, CEBPA, POL, POT1, RPS26 |
| 22 | CFTR | c.1210‐34TG [11]T [5] (intronic) | 71 | Female | NHW | — | Uterine, lymphoma (50) | — | POLE |
| 23 | CFTR | c.1210‐34TG [11]T [5] (intronic) | 79 | Male | NHW | — | — | — | BLM, PTCH1 |
| 24 | CFTR | c.1210‐34TG [11]T [5] (intronic) | 80 | Male | Black/African American | — | Prostate | — | |
| 25 | CFTR | c.1210‐34TG [11]T [5] (intronic) | 69 | Male | NHW | — | Breast (54) | Colon (70), PSU (65) | |
| 26 | CFTR | c.1210‐34TG [12]T [5] (intronic) | 59 | Male | NHW | — | Breast, PSU | — | |
| 27 | CFTR | c.1210‐34TG [12]T [5] (Intronic) | 72 | Female | Asian | — | Lymphoma (83) | — | |
| 28 | CFTR | c.1210‐34TG [12]T [5] (intronic) | 78 | Female | NHW | — | — | Breast (50) | NTHL1, RECQL4 |
| 29 | CFTR | c.1210‐34TG [12]T [5] (intronic) | 75 | Male | NHW | — | PSU | — | NTHL1 |
| 30 | CFTR | c.1210‐34TG [12]T [5] (intronic) | 59 | Female | Asian | — | Pancreatic (54) | — | APC, BAP1, POLE, RAD50, SDHA |
| 31 | CFTR | c.1210‐34TG [12]T [5] (intronic) | 54 | Female | Asian | — | — | — | DIS3L2 |
| 32 | CFTR | c.2249C > T (p.Pro750Leu) | 76 | Female | NHW | Thyroid (45), melanoma, melanoma | Melanoma, melanoma (37) | — | |
| 33 | CHEK2 | c.470T > C (p.Ile157Thr) (low penetrance) | 38 | Female | NHW | — | — | Breast (80s), prostate, PSU | BLM, FANCM, PTCH1 |
| 34 | CHEK2 | c.470T > C (p.Ile157Thr) (low penetrance) | 69 | Female | NHW | — | — | Breast (60s), skin | KIF1B, PIK3CA |
| 35 | CTC1 | c.724_727del (p.Lys242Leufs*41) | 56 | Female | Other | — | Colon (66), prostate (60) | — | PDGFRA, RAD51D |
| 36 | CTRC | c.760C > T (p.Arg254Trp) (increased risk allele) | 59 | Female | NHW | — | Esophageal (80) | Liver (40s), stomach | APC, RINT1 |
| 37 | FANCL | c.36C > A (p.Cys12*) | 56 | Male | Asian | — | — | Kidney | BARD1, CEBPA, FANCA, NF1, RECQL4 |
| 38 | FANCM | c.5791C > T (p.Arg1931*) | 65 | Male | Black/African American | — | — | — | |
| 39 | MUTYH | c.933 + 3A > C (intronic) | 61 | Female | NHW | — | — | Lymphoma (60) | AKT1 |
| 40 | PALB2 | c.2642_2645dupGTTG (p.Cys882Trpfs*3) | 66 | Male | Unknown | — | PSU (70) | Breast, breast, breast | BAP1, PTCH2, TSC2 |
| 41 | PALB2 | c.3116delA (p.Asn1039Ilefs*2) | 69 | Female | NHW | — | Breast | Breast | RAD50, SMARCA4 |
| 42 | PMS2 | c.137G > T (p.Ser46Ile) | 59 | Male | NHW | — | Esophageal | — | MLH3, RET |
| 43 | SDHB | c.656_707dup (p.Pro237_Phe238insAspTer) (possibly mosaic) | 48 | Male | Asian | — | — | Lymphoma (69) | GALNT12, SUFU |
| 44 | SPINK1 | c.194 + 2T > C (splice donor) | 63 | Male | Asian | — | — | Stomach (70) |
Abbreviations: —, none; NHW, non‐Hispanic White; PSU, primary site unknown to the patient; VUS, variant of unknown significance.
Discussion
Universal genetic counseling and germline testing are essential tools to reduce PDAC incidence and mortality. In this study, we demonstrate that the implementation of a systematic patient engagement workflow featuring an embedded in‐clinic GTS successfully improves germline testing rates, decreases patient attrition in the referral/testing process, and increases detection of pathogenic mutations. To our knowledge, our real‐world rate of confirmed genetic counseling/testing (71%) is the highest reported for patients with PDAC.
This study highlights the benefits of an automated workflow to maximize PDAC germline testing, as well as the utility of condensed pretest genetic counseling to expedite the testing process and minimize patient attrition. Our innovation builds upon previously reported programs similarly intended to increase testing rates. For example, Chittenden and colleagues from the Dana Farber Cancer Institute recently created a workflow in which all patients with PDAC are automatically offered a genetic counseling referral when scheduling an initial oncology clinic visit [21]. Compared with a prior system relying on clinician‐directed genetics referrals, the automated process successfully increased germline testing rates from 16.5% to 38.0% (p < .001). However, this report also demonstrated the challenges of integrating testing into routine clinical practice: the workflow did not ascertain whether patients underwent testing at another institution, and the majority of patients still did not complete genetic counseling/testing. Furthermore, a genetic counseling appointment was scheduled prior to germline testing, necessitating the capacity for increased GC availability which may not be replicable at other institutions. This latter hurdle has been previously addressed by Symecko and colleagues, who created a system of video‐based pretest counseling and point‐of‐care PDAC genetic testing at the University of Pennsylvania in 2018 [22]. Although this system relied on provider referrals, streamlining of pretest procedures nevertheless increased the volume of germline testing by greater than sixfold. Similar approaches of video‐assisted or in‐clinic genetic counseling to expedite testing have been successfully employed in other settings for which germline testing is recommended, such as ovarian cancer [23, 24, 25]. Finally, providers at the Cleveland Clinic Taussig Cancer Center recently improved PDAC GC referrals from 9% to 58% by implementing reminders in the electronic medical record as well as engaging in direct physician and patient education [26].
Our systematic workflow and in‐clinic GTS combine best practices from these prior studies to achieve our six primary objectives. (a) The proportion of patients referred to Clinical Genetics/GTS more than doubled after GTS implementation, suggesting that the prior low rate of testing reflected a lack of provider‐driven referrals rather than patient preference. These data will be used to further optimize the GTS workflow, as even with the systematic approach, germline testing was not discussed with 6% of patients, and another 4% did not have referrals placed. (b) The intake workflow included documentation of testing at other institutions, which allowed identification of 10% of patients for whom repeat testing was unnecessary. (c) The GTS kiosk featured standardized educational resources and a GCA to provide additional information or refer to a GC prior to testing. (d) Because nurse navigators introduced the concept of germline testing in advance of the initial clinic appointment, same‐day scheduling of sample collection could be coordinated with the initial oncology visit. This minimized attrition between referral and sample collection due to disease severity/death, which was previously observed in the traditional testing model. Among those who received a Clinical Genetics/GTS referral, the proportion of referred patients who attended their appointment and completed germline testing increased from 64% to 97% after implementation of the GTS system. (e) By employing GCAs and using standardized pretest video‐based educational materials, increased testing volume could be accommodated without undue strain on clinic resources or personnel, and the cost associated with a pretest GC visit could be avoided. (f) Finally, the comprehensive nature of the GTS system allowed for tracking of demographic data to guide improvements in testing practices.
GTS implementation was associated with a marked increase in the rate of confirmed genetic counseling/testing. Although this represents progress toward achieving guideline‐recommended universal testing, our data reveal that additional work is required to ameliorate barriers to equitable health care. We identified demographic characteristics associated with higher risk for attrition: identifying as non‐White race/ethnicity, speaking a non‐English primary language, and older age. These findings corroborate well‐established racial/ethnic disparities in PDAC treatment and outcomes [27, 28] and may reflect issues with culturally competent communication of the rationale or risks/benefits of germline testing, either in person, by telephone interpreter, or via standardized educational materials. In addition, although the use of video‐based educational materials helped increase overall testing rates, these may be more effective for younger patients more familiar with the requisite technology. Our findings will be used to guide future improvements in testing practices and educational materials to ensure equitable care for all patients with PDAC.
Regarding test results, implementation of the GTS and systematic testing workflow increased the detection rate of pathogenic variants from 20% to 33%. The relevance of some of these alterations to PDAC oncogenesis is uncertain, but all have implications for familial testing. The increased detection rate among a more inclusive population is counterintuitive, as genetic testing in the traditional workflow was guided by referrals informed by personal or family medical history and thus would be expected to result in a higher frequency of pathogenic variants due to selection bias. However, germline testing through the GTS is standardized with a broad testing panel of 133 genes, and therefore the likelihood of identifying a pathogenic variant is greater than with smaller, more targeted germline panels used prior to GTS implementation, despite the less enriched patient population. These data underscore the importance of broad, unbiased germline testing to inform treatment options and guide familial cascade testing.
Strengths of this study include the comprehensive nature of data collection, the nearly universal engagement of eligible patients to minimize selection bias, and the comparison of GTS data with data from the traditional model at the same cancer center to minimize confounding. Limitations include the single‐center nature of this study at an academic comprehensive cancer center possessing the infrastructure to create and staff an in‐clinic GTS kiosk, which may affect generalizability to other care settings. Similarly, our access to philanthropic funds defrayed the cost of counseling/testing and may have increased testing rates. However, germline testing has become more accessible since the completion of this study (e.g., Medicare now covers BRCA1/BRCA2 testing for patients with PDAC, and the Invitae Detect Hereditary Pancreatic Cancer Program [29] now sponsors no‐charge germline testing and posttest genetic counseling), and so our results remain generalizable to centers without similar funds. Our comparison of clinical efficacy between the traditional and GTS models may be confounded by 2018 guideline updates recommending universal testing, which independently may have increased referral rates. However, reports of persistently lower rates of germline testing at other centers [21], as well as our demonstrated reduction in attrition rates among patients referred to Clinical Genetics/GTS, suggest that the improvement in testing can be largely attributed to the GTS. Lastly, the traditional testing workflow did not ascertain which patients completed testing at outside medical centers, and so the overall proportion of patients receiving testing prior to GTS implementation may have been higher than reported. However, based on our GTS data, this subset of patients is likely <10% and would not be expected to materially alter the study findings.
Conclusion
Germline testing is now integral to PDAC treatment, and innovations are required to provide this testing for all patients. This study reports the most clinically efficacious real‐world PDAC genetic counseling/testing workflow to date. We markedly improved referral and testing rates by enlisting a multidisciplinary team to engage in a systematic counseling and testing workflow and by creating an in‐clinic embedded GTS. We also discovered opportunities to improve equitable PDAC care by identifying demographic groups for whom better recruitment and communication is needed. Our study provides a model for similar innovation at other medical centers in order to provide guideline‐concordant care and improve outcomes for all patients with PDAC.
Author Contributions
Conception/design: Evan J. Walker, Dena Goldberg, Kelly M. Gordon, Christina Pedley, Amie M. Blanco, Mallika Dhawan
Provision of study material or patients: Christina Pedley, Julia Carnevale, Pelin Cinar, Eric A. Collisson, Margaret A. Tempero, Andrew H. Ko, Amie M. Blanco, Mallika Dhawan
Collection and/or assembly of data: Evan J. Walker, Dena Goldberg, Kelly M. Gordon, Christina Pedley, Amie M. Blanco, Mallika Dhawan
Data analysis and interpretation: Evan J. Walker, Dena Goldberg, Kelly M. Gordon, Christina Pedley, Julia Carnevale, Pelin Cinar, Eric A. Collisson, Margaret A. Tempero, Andrew H. Ko, Amie M. Blanco, Mallika Dhawan
Manuscript writing: Evan J. Walker, Dena Goldberg, Kelly M. Gordon, Christina Pedley, Julia Carnevale, Pelin Cinar, Eric A. Collisson, Margaret A. Tempero, Andrew H. Ko, Amie M. Blanco, Mallika Dhawan
Final approval of manuscript: Evan J. Walker, Dena Goldberg, Kelly M. Gordon, Christina Pedley, Julia Carnevale, Pelin Cinar, Eric A. Collisson, Margaret A. Tempero, Andrew H. Ko, Amie M. Blanco, Mallika Dhawan
Disclosures
Eric A. Collisson: Takeda, Merck, Loxo, Pear Diagnostics (C/A), AstraZeneca, Ferro Therapeutics, Senti Biosciences, Merck KgA, Bayer/Loxo (RF—institution), Tatara Therapeutics, Clara Health, BloodQ, Guardant Health (OI), Nant (other—expert witness); Margaret A. Tempero: Abbvie, AstraZeneca, Biotech Research, Boehringer Ingelheim, Bristol‐Myers Squibb, Corcept Therapeutics, Geistlich Pharma, Merck & Co., Steba Biotech (SAB), GlaxoSmithKline, Seagen, Inc., ISPEN (C/A); Andrew H. Ko: Tyme, FivePrime, Turning Point Therapeutics, Signatera (C/A), Merck, Celgene, Bristol‐Myers Squibb, Roche/Genentech, Apexigen, Abgenomics, LEAP Therapeutics (RF—institution), Ipsen, Roche/Genentech, Imugene, Erytech, SynCore (other—Data Safety Monitoring Board); Amie M. Blanco: BioMarin Pharmaceuticals, Inc. (E—spouse); Mallika Dhawan: Merck (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Appendix S2. Supporting Information.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Siegel RL, Miller KD, Fuchs HE et al. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 3. Rahib L, Wehner MR, Matrisian LM et al. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open 2021;4:e214708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Lakoma A, Zogopoulos G. Building towards precision oncology for pancreatic cancer: Real‐world challenges and opportunities. Genes (Basel) 2020;11:1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Travaline C, Elliott LA, Ramdin NN et al. Compliance with germline testing in pancreatic cancer in a rural tertiary care hospital. J Clin Oncol 2021;39(suppl 15):10599a. [Google Scholar]
- 6. Mizrahi JD, Surana R, Valle JW et al. Pancreatic cancer. Lancet 2020;395:2008–2020. [DOI] [PubMed] [Google Scholar]
- 7. Golan T, Hammel P, Reni M et al. Maintenance olaparib for germline BRCA‐mutated metastatic pancreatic cancer. N Engl J Med 2019;381:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marcus L, Lemery SJ, Keegan P et al. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability‐high solid tumors. Clin Cancer Res 2019;25:3753–3758. [DOI] [PubMed] [Google Scholar]
- 9. O'Reilly EM, Lee JW, Zalupski M et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol 2020;38:1378–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wattenberg MM, Asch D, Yu S et al. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer 2020;122:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vasen H, Ibrahim I, Ponce CG et al. Benefit of surveillance for pancreatic cancer in high‐risk individuals: Outcome of long‐term prospective follow‐up studies from three European expert centers. J Clin Oncol 2016;34:2010–2019. [DOI] [PubMed] [Google Scholar]
- 12. Canto MI, Almario JA, Schulick RD et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long‐term surveillance. Gastroenterology 2018;155:740‐751.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shindo K, Yu J, Suenaga M et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol 2017;35:3382–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lowery MA, Wong W, Jordan EJ et al. Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. J Natl Cancer Inst 2018;110:1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu C, Hart SN, Polley EC et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 2018;319:2401–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yurgelun MB, Chittenden AB, Morales‐Oyarvide V et al. Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet Med 2019;21:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tempero MA, Malafa MP, Chiorean EG et al. Pancreatic adenocarcinoma, version 1.2019. J Natl Compr Canc Netw 2019;17:202–210. [DOI] [PubMed] [Google Scholar]
- 18. Stoffel EM, McKernin SE, Brand R et al. Evaluating susceptibility to pancreatic cancer: ASCO provisional clinical opinion. J Clin Oncol 2019;37:153–164. [DOI] [PubMed] [Google Scholar]
- 19. Hicks JK, Howard R, Reisman P et al. Integrating somatic and germline next‐generation sequencing into routine clinical oncology practice. JCO Precis Oncol 2021;5:PO.20.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walker EJ, Carnevale J, Pedley C et al. Referral frequency, attrition rate, and outcomes of germline testing in patients with pancreatic adenocarcinoma. Fam Cancer 2019;18:241–251. [DOI] [PubMed] [Google Scholar]
- 21. Chittenden A, Haraldsdottir S, Ukaegbu C et al. Implementing systematic genetic counseling and multigene germline testing for individuals with pancreatic cancer. JCO Oncol Pract 2021;17:e236–e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Symecko H, Mueller R, Spielman K et al. Ten‐fold increase in genetic testing in pancreatic and metastatic prostate cancer with implementation of point of care (POC) testing. J Clin Oncol 2019;37(suppl 15):1506a. [Google Scholar]
- 23. Kentwell M, Dow E, Antill Y et al. Mainstreaming cancer genetics: A model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol Oncol 2017;145:130–136. [DOI] [PubMed] [Google Scholar]
- 24. Senter L, O'Malley DM, Backes FJ et al. Genetic consultation embedded in a gynecologic oncology clinic improves compliance with guideline‐based care. Gynecol Oncol 2017;147:110–114. [DOI] [PubMed] [Google Scholar]
- 25. Blackburn P, Watson CH, Gordon J et al. Video‐assisted genetic counseling in patients with ovarian, fallopian, and peritoneal carcinoma: A prospective, randomized controlled trial. Gynecol Oncol 2020;159(suppl 1):9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nair KG, Leach B, Sledge S et al. Improving the referral rate of universal genetic counseling for pancreatic ductal adenocarcinoma (PDAC) at the Cleveland Clinic Taussig Cancer Center: A quality improvement project. J Clin Oncol 2020;38(suppl 29):196a. [Google Scholar]
- 27. Noel M, Fiscella K. Disparities in pancreatic cancer treatment and outcomes. Health Equity 2019;3:532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zavala VA, Bracci PM, Carethers JM et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer 2021;124:315–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Invitae . Detect Hereditary Pancreatic Cancer. Available at https://www.invitae.com/en/detect-hereditary-pancreatic-cancer/. Accessed August 23, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Appendix S2. Supporting Information.


