Abstract
Hematopoietic stem cell transplant (HSCT) plays a central role in the treatment of hematologic cancers. With the increasing survival of patients after HSCT, survivorship issues experienced by this population have become an important outcome. Cognitive impairment is an established sequela of HSCT, with studies to date establishing its presence, associated risk factors, and clinical phenotype. There are multiple potential contributors to cognitive impairment after HSCT. Efforts are ongoing to further characterize its clinical phenotype, associated biomarkers, and biologic underpinnings. A fundamental knowledge of post‐HSCT cognitive impairment is of value for all clinicians who interface with this population, and further academic efforts are needed to more fully understand the impact of this cancer treatment on brain health.
Implications for Practice
As survival outcomes after hematopoietic stem cell transplant (HSCT) improve, an awareness of the post‐treatment challenges faced by this population has become central to its care. HSCT can have a sustained and broad impact on brain health, causing cognitive dysfunction, fatigue, disturbed mood, and sleep. In affected patients, autonomy, return to work, relationships, and quality of life may all be affected. A fundamental fluency in this area is important for clinicians interfacing with HSCT survivors, facilitating the identification and management of cognitive dysfunction and concurrent symptom clusters, and stimulating interest in these sequelae as areas for future clinical research.
Keywords: Cancer, Cognitive dysfunction, Hematologic cancer, Hematopoietic stem cell transplantation, Oncology
Short abstract
This review summarizes the current literature surrounding the nature of cognitive dysfunction after hematopoietic stem cell transplant (HSCT), potential contributing factors and associated biomarkers, and avenues for further intervention and research.
Introduction
Hematopoietic stem cell transplant (HSCT) has revolutionized the treatment of hematologic cancers and certain nonmalignant conditions. Through manipulation of the immune system, HSCT can afford potentially curative treatments to patients whose conditions were once deemed terminal. However, the potency of this intervention is not without consequence. Many of the post‐transplant sequelae have been well characterized, including infectious complications, autoimmune phenomena, chemotherapy toxicities, and end‐organ dysfunction [1]. An area that has received less attention to date is the impact of HSCT on brain and cognitive functioning.
Over one third of patients with autologous and allogeneic stem cell transplants disagree with the statement “life has returned to normal” at 2 years after transplant [2], a time when most of these patients are out of hospital and off active treatment. Of those alive at 5 years, only 60% had returned to full‐time work [3]. These data suggest pervasive functional changes that are sustained beyond the acute post‐transplant period. Changes in neurocognitive function have been identified as a significant issue among patients and caregivers after HSCT [4]. Neurocognitive impairments may also affect survivors' independence and sense of personal agency. Improved patient survival outcomes have increased the importance of long‐term survivorship issues, such as cognition, within this population.
Research on cognitive impairment in post‐HSCT is in its infancy, but it is estimated that nearly half of all patients show some change from baseline cognitive function after transplant [5]. For this reason, clinical awareness of cognitive impairment among HSCT recipients has been advocated, with recommendation for annual clinical monitoring of cognitive deficits [6]. It is essential that clinicians caring for HSCT recipients are aware of potential cognitive changes associated with treatment, given the potential significance of their impact. The biologic processes incited by HSCT are complex, making it challenging to study the underlying neurobiological mechanisms of cognitive changes. This narrative review summarizes the current literature surrounding the nature of cognitive dysfunction after HSCT, potential contributing factors and associated biomarkers, and avenues for further intervention and research. We first provide an overview of the HSCT procedure, describe the cognitive changes and associated risk factors reported in this population, explain potential underlying mechanisms of cognitive changes in HSCT recipients, and discuss clinical implications including considerations for returning to work and managing symptoms.
Materials and Methods
A narrative review of relevant academic literature was performed by the authors. Levels of evidence were not assessed prior to inclusion. The review is limited to published data; no unpublished data were included.
The HSCT Procedure
The standard process for HSCT involves a conditioning regimen followed by the transplantation. Regimens are of variable intensity and toxicity, with regimens dependent on the underlying malignancy, patient comorbidities, performance status, and risk of graft rejection. In autologous transplant the recipient's endogenous stem cells are collected and then reinfused after high‐dose chemotherapeutic conditioning. In allogeneic transplant the patient's stem cells are replaced with closely matched donor cells. With the goal of eradicating marrow cells, myeloablative regimens are generally characterized as having the greatest potential for toxicity (both acute and chronic), whereas nonmyeloablative regimens are generally chosen for their greater graft‐versus‐tumor immune effect. The type of conditioning regimen and transplant differentially affect the underlying malignant condition, as well as the patient's other organ systems.
The transplant procedure and associated hospitalization renders HSCT recipients vulnerable to a host of medical complications. An illustration of the transplant period is provided in Figure 1. Bone marrow transplant–related complications can be broadly classified into early/acute (<3 months after HSCT) and late/chronic (>3 months after HSCT). In the early post‐transplant period, hematologic complications, such as profound cytopenias, can render patients vulnerable to vascular and infectious complications, including central nervous system (CNS) infections. Mucositis and diarrheal illness from infection or acute graft‐versus‐host disease (GVHD) can lead to nutritional compromise, and patients may require total parenteral nutrition to supplement deficiencies from oral intake. Pulmonary, renal, and hepatic dysfunction also occur at increased frequency, with many organ toxicities being linked to chemotherapies used as part of the HSCT regimen. Along with polypharmacy, these multiorgan toxicities contribute to the high incidence of delirium in this population, which is estimated at 50% in the month after transplant [7]. GVHD is a common and significant complication of allogeneic HSCT where the alloreactive donor T lymphocytes recognize histocompatibility antigens on host cells and initiate secondary inflammatory response.
Figure 1.

Transplant timeline.
Chronic issues after HSCT are also well characterized in the literature. Chronic GVHD and its treatments can be profoundly debilitating for patients and contribute to organ failure, infection, immune dysregulation, and functional disability. Acute GVHD is the most important risk factor for development of chronic GVHD, which is the leading cause of nonrelapse late mortality in allogeneic HSCT survivors. A breadth of end organs may be implicated in GVHD, including, rarely, the central nervous system [8]. Risk of GVHD often requires long‐term immunosuppression to prevent graft rejection. End‐organ toxicities, including cardiovascular risks, renal failure, CNS changes, and hepatic toxicity (to name a few), can thus be chronic. Aside from chronic GVHD, which is attributed to the cell graft itself, chronic organ toxicities have also been attributed to chemotherapy and radiation during HSCT conditioning.
Identification and Characterization of Cognitive Impairment
Trajectory and Nature of Deficits
Prior to HSCT, it is estimated that up to half of individuals experience impairment in one or more cognitive domains [5, 9, 10, 11, 12, 13, 14, 15], with deficits in attention, executive function, verbal fluency, verbal learning and memory, graphomotor speed, and motor functioning identified [11, 15, 16]. Dynamic multidomain impairment has been identified in the acute post‐transplant period. One month after HSCT, deficits in learning, memory, and motor functioning have been identified, with nearly half of HSCT recipients experiencing a decline from their pre‐HSCT baseline [5].
These acute deficits often evolve into chronic neurocognitive change. Between 2 and 6 months, declines in verbal memory, complex attention, graphomotor processing speed, verbal fluency, and motor functioning have been found when analyzing populations of both allogeneic and autologous HSCT recipients [10, 15]. At 3 months after HSCT, Jones and colleagues (2013) found that almost half of patients in their 53‐patient cohort had a clinically significant decline in learning, memory, and/or executive function compared with performance at 1 month [5]. In another cohort of HSCT recipients evaluated at 6 months after transplant, half had a deficit in at least one cognitive domain, with greatest impairment found in psychomotor speed followed by complex executive functioning [17]. Of note, although many of these studies analyze autologous and allogeneic HSCT recipients as a collective, the incidence of cognitive impairment does appear to be distinct between the groups, with allogeneic recipients having nearly double the incidence of cognitive impairment in comparison with autologous recipients [18].
Longitudinal studies examining the trajectory of cognitive functioning following HSCT have identified domain‐specific deficits. Harder and colleagues (2007) found that at 20 months, those who underwent HSCT exhibited poorer performance across measures of attention, complex attention, and verbal memory compared with a disease‐specific reference group [11].
At 36 months, poorer performance in executive function, verbal speed, processing speed, auditory memory, and fine motor dexterity for those who underwent full‐intensity allogeneic HSCT compared with healthy controls has been identified [18].
Conversely, other studies have identified improvements in function at 12 to 18 months across similar cognitive domains, including attention, working memory, executive functioning, graphomotor processing speed, verbal fluency, and verbal learning and memory, with these improvements extending out 5 years in one study [11, 15, 16, 19, 20]. Despite these improvements, however, compared with healthy controls, 35%–40% of allogeneic and approximately 19% of autologous HSCT recipients showed evidence of global cognitive impairment 3 to 5 years after transplant [18, 20]. The trend toward improvement over time may be related in part to an interaction effect between time at evaluation and cancer diagnosis. For example, one prospective study found longitudinal improvement in verbal fluency among patients with chronic myeloid leukemia when comparing baseline performance with performance at 12 and 18 months, but this change was not seen in patients transplanted for myelodysplastic syndrome [21]. This may suggest that the disease pathology itself plays a role in cognitive dysfunction.
Neuroimaging Biomarkers
Neuroimaging evaluations have been limited in adult‐onset cancers and patients undergoing HSCT thus far. Several studies have noted white matter abnormalities on magnetic resonance imaging (MRI) in HSCT recipients [22, 23, 24, 25, 26, 27, 28]. In their study on neurologic and cognitive statuses and brain MRI in long‐term survivors (average 34 months ±26 months) of allogeneic HSCT, Padovan and colleagues found that two thirds of their subjects (33 in 55, total) had pathological MRI findings [25]. The MRI scans demonstrated white matter lesions in 54%, mainly in the frontal, parietal, or temporal lobes. Among their tested predictors (older age [>40 years], intrathecal methotrexate, total body irradiation [TBI], lengthier period since transplant [>25 months], blood pressure, long‐term cyclosporine medication, microangiopathy early after bone marrow transplant, chronic GVHD evolved from acute GVHD, corticosteroid use, and marrow donor status), only older age (odds ratio [OR], 4.0; confidence interval [CI], 1.1–15.3), intrathecal methotrexate (OR, 1.8; CI, 0.3–10.1), long‐term cyclosporine (OR, OR, 2.6; CI, 0.8–8.1), microangiopathy (OR, 1.7; CI, 0.4–6.4), chronic GVHD evolved from acute GVHD (OR, 5.3; CI, 1.3–21.4), and corticosteroid use (OR, 3.1; CI, 0.9–10.6) were associated with pathological MRI. However, Correa and colleagues conducted two studies of patients receiving chemotherapy and HSCT with or without TBI. In the first [9], patients who received high‐dose chemotherapy with HSCT demonstrated a significant decrease in bilateral middle frontal gyrus volume over time compared with controls. There were no significant correlations between brain volumes and cognitive test performances, making these results difficult to interpret in terms of their clinical meaning.
The follow‐up study by Correa et al. involved a subset of the prior cohort and focused on quantification of white matter integrity using diffusion tensor imaging [29]. Patients who received HSCT demonstrated significant decreases in mean and axial diffusivity over time compared with controls, which was correlated with improved cognitive test scores. However, patients who received allogeneic HSCT demonstrated regions of higher mean and axial diffusivity and lower fractional anisotropy 1 year after transplant compared with those who received autologous HSCT. These findings suggest that compromise to white matter tract integrity may contribute to cognitive impairment after transplant.
The literature on structural changes in the brain after HSCT has collectively included patients with varying underlying cancers and disorders, including hematologic malignancy (most common) both with and without CNS involvement, breast cancers, and nonmalignant hematologic disorders. Despite the modest number of studies, the breadth of significant findings on neuroimaging across diagnoses suggest that these structural changes (or, perhaps, a greater proportion of them) may be due to risk factors other than the diagnoses themselves. In fact, some studies found no association between finding on neuroimaging and underlying disease [25]. Below, we synthesize the literature on the risk factors already supported by research.
White matter abnormalities seem to be a consistent finding thus far in an albeit small body of literature [22, 23, 24, 25, 26, 27, 28]. White matter pathways are critical for supporting integrated communication between different specialized information processing centers in the brain. White matter damage has been observed in other cancers as well and tends to be attributed to chemotherapy and/or radiation treatments [30, 31]. Lower white matter integrity among allogeneic HSCT recipients noted in the study by Correa and colleagues were attributed to differences in chemotherapy regimens prior to transplant and/or factors associated with disease biology. Decreased diffusivity in certain brain regions, which, in general, tends to reflect higher white matter integrity, could indicate improvement over time in HSCT recipients, a finding associated with better cognitive performance. Other brain regions of decreased diffusivity were correlated with lower cognitive scores. These findings illustrate an important caveat in neuroimaging research: as with most biologic processes, more is not always better with respect to brain structure and function, as has been illustrated in studies of other cancer types [32, 33].
Additionally, associations between structural brain changes and neurological status or cognitive impairment are inconsistent across studies. Pathological findings on neuroimaging did not always correlate with clinical findings. Padovan and colleagues found that, although intrathecal methotrexate was associated with pathological MRI results, it had no clear effect on clinical status, and although time since transplant indicated increased cognitive and neurological deficits, time had little association with MRI outcomes [25]. Correa and colleagues, in contrast, found modest correlations among mean diffusivity and cognitive performance [29].
Neuroimaging studies are costly, limited by contraindications that many patients with cancer possess (e.g., surgical implants, anxiety, pain), and can be biased toward patients who are healthier. The clinical interpretation of neuroimaging findings is often difficult because of, in no small part, limitations of the behavioral assessments that are used as clinical correlates. Despite the limitations, neuroimaging assessments offer unique insights regarding the neurobiologic mechanisms underlying cognitive impairment. Neuroimaging assessments also tend to be more sensitive measures of outcome compared with many behavioral tests [34]. Accordingly, neuroimaging metrics tend to show greater effects of breast cancer and its treatments on brain function compared with neuropsychological tests, for example [35, 36, 37].
Neuroimaging may be useful for predicting cognitive outcomes after cancer [38, 39] and could be implemented to identify high‐risk patients in order to inform treatment decision‐making or prioritize patients for early interventions/prehabilitation. Specific brain networks corresponding to gene expression networks [40, 41] show neurotransmitter receptor fingerprints [42] and unique metabolic patterns [43, 44] that could guide investigations of molecular mechanisms of cognitive impairment and associated treatment development. Many analogous neuroimaging measures can be obtained in animal models, which may provide a point of preclinical translation [45, 46]. Further neuroimaging research, especially with longitudinal designs, is needed in HSCT. Guidelines have been published to help improve standardization of neuroimaging studies of cancer‐related cognitive impairment [47].
Risk Factors for Neurocognitive Impairment After HSCT
Patients with hematologic malignancies, comprising the majority of patients receiving HSCT, have an increased incidence of baseline cognitive impairment, with 15%–32% of patients demonstrating pretreatment cognitive impairment prior to HSCT [48]. Pretreatment impairment is also an established risk factor for post‐HSCT cognitive impairment [49]. Certain common therapeutics used in many standard regimens, including methotrexate [29], cytarabine [29], cyclosporine [28], total body/cranial irradiation [28, 44], and corticosteroids and immunosuppressants [28, 44], have been implicated in cognitive changes that occur even before patients reach HSCT. Various baseline demographic factors have also been found to increase the likelihood of developing cognitive changes after HSCT, including age, minority status, and educational background [5, 49]. These variables contribute to cognitive reserve [50, 51], affecting the brain's resiliency to aging and neurotoxic insults. Of note, however, many of these studies have heterogeneous patient populations and small sample sizes, rendering more detailed understanding of these factors in the population of HSCT recipients a challenge.
HSCT treatment‐related factors may also influence cognitive outcomes. Given the complexity of HSCT regimens, their evolution in recent decades, and the small sample sizes of many studies, a granular approach to each therapeutic element and its impact on patient outcomes is challenging. Multiple variables, including the number of induction cycles, history of prophylactic cranial irradiation, TBI, intrathecal chemotherapy, prior chemotherapy regimens, transplant type, donor relationship, length of hospital stay, and days until engraftment, have all been associated with poorer cognitive outcomes [5, 18, 49]. In prospective study of 477 HSCT recipients, those treated with myeloablative allogeneic HSCT showed greater impairment in verbal fluency and processing at 6 months after HSCT than reduced‐intensity autologous HSCT recipients [18]. After 3 years, however, reduced‐intensity autologous HSCT recipients displayed poorer performance across measures of executive functioning, verbal fluency, and working memory, whereas only fine motor dexterity was shown to be reduced for those who underwent myeloablative allogeneic HSCT, suggesting a delayed decline in those undergoing autologous transplant. At 3 years after HSCT, global cognitive impairment rates were 18.7% in those receiving autologous transplant versus 35.7% in those with allogeneic transplant [18]. Notably, the outcomes varied by intensity of the applied therapy in the latter group, with those having myeloablative treatment having marked early cognitive impairment, and those having reduced‐intensity allogeneic transplant having less severe and more delayed cognitive changes. These findings support intensity of cytotoxic chemotherapy as having a direct contribution to the more pronounced cognitive impact of allogeneic transplant, in comparison with autologous HSCT. Additional contributors to their distinct outcomes may include the increased incidence of autoimmune phenomena in patients with allogeneic transplant [52], with potential for chronic proinflammatory state affecting brain health, even in the absence of frank GVHD. With GVHD, these patients are also more likely to require chronic calcineurin inhibitors, which may themselves be neurotoxic, causing posterior reversible encephalopathy syndrome, tremor, and thrombotic microangiopathy [53]. This population is also more vulnerable to post‐transplant infections; both CNS and systemic infections have the potential to affect cognitive performance. These multiple variables likely contribute to the poorer cognitive outcomes of allogeneic transplant recipients.
Discrepancies in the cognitive outcomes for different transplant types may be secondary to different biologic contributors to these processes or to the conditioning regimen that is used prior to transplant. Although some studies have demonstrated no significant association between cognitive decline and specific therapeutic exposures [13, 14, 18], the complexity and variability of the regimens, as well as small sample sizes, involved in the aforementioned studies make drawing conclusions about treatment effects difficult, and more rigorous studies are needed.
Post‐transplant complications can have a significant impact on neurocognitive function. In a prospective study that evaluated patients at baseline, 6 months, and 12 months, length of hospital stay was a significant risk for neurocognitive decline [49]. The length of hospital course may be seen as a proxy for post‐transplant recovery. As noted earlier in this review, post‐transplant delirium is estimated to occur in half of all transplant recipients [7]. Delirium is a robust predictor of long‐term cognitive impairment, with duration of delirium being an independent predictor of impairment on objective cognitive testing in medical intensive care unit patients [54], as well as increased incidence of age‐related cognitive decline [55]. The magnitude and nature of the impact of delirium during hospitalization for HSCT on cognitive outcomes requires further definition, but the literature in other populations suggests it is likely contributory. CNS infections and nutritional deficiencies can also be significant contributors during this period. Additionally, presence of relapse, systemic steroid treatment, and presence of GVHD have been identified as long‐term contributors to cognitive impairment after HSCT [49].
Ascribing the contributions of specific risk factors for neurocognitive impairment after HSCT is thus difficult. Studies are limited by the diverse background stories that accompany each participant when they arrive to HSCT, including their psychosocial status, medical history, previous treatment with chemotherapy and radiation, immunosuppression, and likely (although severely unstudied) their genetic and epigenetic factors. In addition, their peritransplant regimens (such as pre‐HSCT conditioning, infections, length of stay, metabolic complications, GVHD, and acute toxicities) coupled with nuances of measurement across studies (time since transplant, number of transplant, time since diagnosis, operationalization of neurologic and cognitive changes) make the concept inherently difficult to describe using any one theory or framework. Likely (as suggested by authors of previous work) the best explanation of risk factors for neurocognitive impairment after HSCT is that many factors represent a variety of insults and contribute (albeit perhaps unequally) a cumulative effect on neurocognitive outcomes [25, 28, 29].
Mechanisms of Cognitive Impairment After HSCT
Post‐HSCT cognitive impairment is likely multifactorial, with biologic, psychological, and social factors contributing to its development, as outlined in Figure 2.
Figure 2.
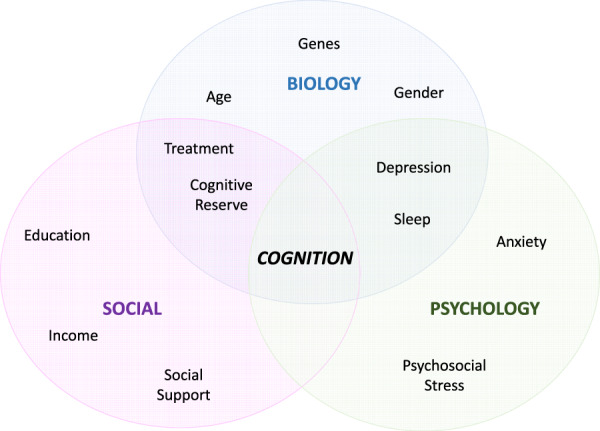
Contributors to the development of cognitive impairment after hematopoietic stem cell transplant. Adapted from Engel [169].
Inflammation
Proinflammatory cytokine activity in patients with non‐CNS cancers has been linked to cognitive dysfunction [56, 57, 58, 59, 60]. Preclinical models indicate that some chemotherapies may upregulate expression of certain inflammatory markers (tumor necrosis factor [TNF]‐α, interleukin [IL]‐1) [4, 61], resulting in neuroinflammation and neurocognitive dysfunction. A recent study by Hoogland and colleagues [62] demonstrated that elevated circulating proinflammatory cytokines (IL‐6, soluble TNF receptors) were significantly associated with poorer cognitive function after allogeneic HSCT. It is well known that many chemotherapies used in HSCT pose a risk for inflammation or neurotoxicity; however, evidence supporting these underlying mechanisms of HSCT cognitive impairment is lacking [4, 61].
An interrelationship between CNS immune biomarkers and cognitive function has been identified [63, 64], but this relationship in the population of HSCT recipients is not fully developed. One study found that higher levels of circulating IL‐6 were associated with poorer executive function in patients with acute myeloid leukemia or myelodysplastic syndrome [65]. These levels, as well as TNF‐α and IL‐1 receptor antagonist levels, were also associated with quality of life and fatigue. Certain transplant sequelae such as GVHD, or allogeneic/unrelated donors, may also have the potential to lead to chronic subclinical CNS inflammation. This theory is supported by the finding of more complications in patients with alternative donor allogeneic HSCT, rather than those who had human leukocyte antigen–identical siblings or autologous transplants [66], and by a meta‐analysis showing autologous transplant recipients demonstrating greater improvement in cognitive function over time [67]. Importantly, metabolic encephalopathy and CNS infections are the first and second (respectively) most prominent sources of post‐HSCT neurological complications [55]. Inflammation as a result of either metabolic complications or primary infectious pathology is considered a major mechanism for neurocognitive changes after transplant.
Proposed contributions of inflammation to post‐transplant cognitive impairment are illustrated in Figure 3.
Figure 3.
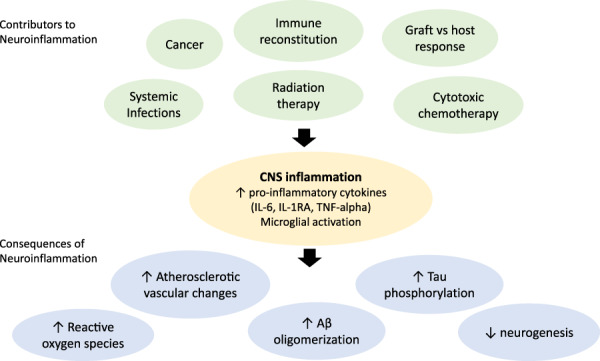
Proposed contributions of inflammation to post‐transplant cognitive impairment.
Abbreviations: Aβ, amyloid beta; IL‐1RA, interleukin‐1 receptor antagonist; IL‐6, interleukin‐6; TNF‐alpha, tumor necrosis factor alpha.
CNS GVHD
GVHD is a donor T‐cell–mediated response that can affect allogeneic HSCT recipients months to years after transplant [68]. GVHD may involve profound immune dysregulation, multiorgan dysfunction, and poorer quality of life in survivors of allogeneic HSCT [69]. GVHD most often presents in the liver, gastrointestinal tract, and skin and more rarely may affect the CNS [8, 68]. There is ongoing debate regarding CNS manifestations in the post‐HSCT period and its precise phenotype, although diagnostic criteria have been proposed [8]. In the last decade, three primary mechanisms of CNS GVHD have been postulated: vasculitis/angiitis, encephalitis, and demyelination [8, 70, 71]. However, patients with mild cognitive impairment after HSCT may not routinely receive spinal fluid testing or other paraclinical tests to look for CNS inflammation, opening the possibility of smoldering CNS inflammation in some patients that is thus far underidentified.
Microbiome Alterations
The microbiome is the collection of bacteria and microorganisms that naturally inhabit a host's body [72]. The impact of the microbiome in human health and disease has been increasingly recognized in recent years [73]. Chemotherapy and cancer itself may have a direct impact on the microbiome [74]. Disruption and de‐diversification of the microbiota (dysbiosis) may lead to systemic inflammation, ultimately resulting in neuroinflammation through the neurophysiologic cascade and cognitive sequalae in patients with who receive chemotherapy [74, 75, 76]. A landmark study by Taur et al. [77] demonstrated that, after receiving allogeneic HSCT, recipients' gut diversification markedly decreased. The gut‐brain axis can also be threatened by way of epithelial injury, also termed barrier breach. Chemotherapies, radiation, and infection are risk factors for epithelial injury. Transient bacteremias upregulate the systemic inflammatory cascade [74]. These studies highlight the established relationship between gut and brain biology, and the potential for HSCT to influence the biology of both. Although literature regarding the relationship between the gut microbiome and cancer‐related cognitive impairment is lacking, research to date does support a link with cognitive performance. In murine models, alterations in gut microbiota are associated with changes in learning and memory [78, 79] and neurobiologic alterations in brain‐derived neurotropic factor levels [80] and hippocampal serotonin levels [81]. Human studies in this area are few but do support a relationship [82, 83] that warrants further exploration.
Neurovascular Changes
Certain drugs used in some HSCTs are known to cause injury to the neurovascular system and neural progenitor cells. Calcineurin inhibitors such as cyclosporin and tacrolimus can damage the vascular endothelium [58]. Subdural hematoma is a cerebrovascular event that may occur in either autologous or allogeneic HSCT, with a documented incidence of at least 2.6% [58]. Subdural hematoma risk factors in this specific population include prolonged thrombocytopenia with refractoriness to platelet transfusions, arterial hypertension, acute GVHD, and fibrinogen serum levels. Thrombotic events are also described, with links to chronic GVHD and steroid treatment, infections, and in the case of hemorrhagic infarcts, multiple organ failure [55]. Cerebral infarcts after HSCT have also been associated with nonbacterial thrombotic endocarditis, although not all cases co‐occur [55]. The aforementioned first mechanism underlying CNS GVHD, cerebrovascular disease, is a function of vasculitis in the brain marked by inflammatory markers and (in some cases) white matter changes [58]. Insults to the small and medium arterial vessels of the meninges and cerebral parenchyma may be caused by neurotoxic chemotherapy or radiation, although definitive diagnosis requires brain biopsy. Transplantation‐associated thrombotic microangiopathy is another pathology of endothelial origin and arterial thrombosis, which may be linked to increased C5b‐9 serum concentration (during complement activation) or to genetic expression of various proteins involved in the coagulation pathway [58].
Oxidative Stress
Oxidative stress has been described as a mechanism underlying neurocognitive changes in recipients of chemotherapy and HSCT [64, 65]. Reactive oxygen species (ROS), which are produced as a normal part of respiration and energy metabolism, may accumulate intracellularly and disrupt cell apoptosis and regulation while increasing inflammation [65]. Oxidative stress from ROS has been implicated in certain behavioral toxicities like mild cognitive impairment, and there is evidence suggesting that it may be a mediator in chemotherapy‐induced cognitive impairment [65].
Accelerated Brain Aging
A leading conceptual framework for the neurobiologic changes that occur in patients with cancer is accelerated brain aging. Many of the processes already discussed, including oxidative stress, microtubule instability, and impaired functional connectivity, are found in normal brain aging and appear to contribute to cancer‐related neurotoxicity [84, 85, 86]. This process is likely at play in patients who have received HSCT. Although studies to date are limited, one study of patients with multiple myeloma who had undergone autologous stem cell transplant found an association with changes in p16INK4a, a cellular marker of physiologic aging, consistent with 33.7 years of chronologic age.
Genetic Biomarkers
Molecular mechanisms of cognitive impairment have been suggested to explain individual susceptibility to cognitive impairment in patients with cancer [87, 88] and in older healthy individuals [89]. The established association of variants along the APOE gene in Alzheimer's disease [90] have shown a mixed association pattern in populations of patients with cancer [91]. It has been linked to cognitive decline in patients with breast cancer [92] but not in patients with colorectal cancer [93]. The most recent systematic review of genetic risk factors for cognitive impairment in populations of patients with cancer maintains the inconsistent nature of evidence on the role of APOE variants [94]. Genetic risk variants on COMT [95, 96] and DNA repair and oxidative stress genes [97, 98] have been reported in breast cancer survivors and GNB3 in prostate cancer survivors [99]. Although variants on BDNF gene have been repeatedly studied, no clear significant association with cancer‐related cognitive impairment has been identified to date [94]. In HSCT recipients, the evidence on genetic determinants of cognitive impairment is similarly scarce. In one study, variants across DNA repair, blood‐brain barrier, and telomere homeostasis genes were associated with global cognitive deficits after transplant [100]. It is important to note that all studies reported to date rely on a candidate gene approach with its inherent limitations and possibility of missing novel associations. Nonetheless, candidate genetic variants were shown to enhance risk prediction of cognitive impairment following HSCT beyond that offered by clinical and demographic characteristics [100]. Utility of genetics in identifying high‐risk individuals will only be realized through full characterization of genetic risk of cognitive impairment in HSCT recipients and warrants further investigation.
Practical Implications
Psychoneurological Symptom Clusters
Cognitive impairment frequently co‐occurs with other symptoms, including sleep disruption, fatigue, anxiety, and depression, in patients with and survivors of cancer [101, 102, 103]. Co‐occurrence of two or more of these symptoms is referred to as a psychoneurological symptom cluster [101, 104, 105, 106]. Symptom clusters have a larger impact on quality of life and functional status than individual symptoms [107, 108, 109], and increased symptom burden has been associated with decreased survival [110, 111]. Therefore, it is important for providers caring for HSCT recipients to consider their cognitive symptoms within the context of psychoneurological symptom clusters. Given that the component symptoms of psychoneurological symptom clusters are each multidimensional and are likely to be interrelated, it can be difficult to tease out their relative contribution to cognition dysfunction, particularly within the context of post‐transplant neurobiologic alterations. In addition, although symptoms such as depression, fatigue, and sleep changes have all been associated with self‐reported cognitive changes in populations of patients with breast cancer [112], the literature examining the relationship of these factors to performance on objective cognitive measures has been mixed [113], and exploration in the population of HSCT recipients specifically is lacking. A better understanding of symptom clusters and their mechanisms may guide development of clinical management strategies that can address multiple symptoms simultaneously [114].
Fatigue
Sustained and intensive cancer treatment is an established risk factor for cancer‐related fatigue [115]. Persistent moderate to severe fatigue has been estimated to occur in more than 40% of patients following HSCT [116] and has been found to be an independent predictor of quality of life in this population up to 5 years after transplant [117]. The biologic underpinnings of cancer‐related fatigue are broad, but potential etiologies in the population of HSCT recipients can be postulated. Neuroinflammation as well as systemic inflammatory response are some of the mechanisms that putatively underlie both fatigue and cognitive dysfunction in patients with cancer, as well as in other populations of patients who undergo HSCT [118, 119]. Furthermore, a breadth of comorbid conditions, including metabolic disorders, chronic anemia, chronic infections, polypharmacy, and deconditioning, also have the capacity to affect fatigue. The relationship of fatigue to cognitive performance in patients with cancer is not fully elucidated, however, and is unlikely to be linear. One study in patients with breast cancer that evaluated between‐persons and within‐persons performance on cognitive tasks as they relate to fatigue ratings found that objective cognitive performance on tasks of processing speed were not affected by average level fatigue; however, on within‐persons comparison, longer response latencies were noted when a patient with breast cancer had more fatigue [120]. The nature of the relationship between fatigue and cognition has been mixed. Although there appears to be some interdependence of fatigue on cognition, a prospective study of 75 patients with breast cancer undergoing chemotherapy found their trajectories to be distinct, suggesting there may be distinct biologic underpinnings and contributors to both [121]. Other work in the population of patients with breast cancer has found a direct relationship between cognitive symptoms, but not cognitive performance, and fatigue [122]. Another study in fatigued cancer survivors supports this, with direct intervention aimed at improving fatigue leading to improved perceived cognition but not objective cognitive testing [123]. At this time, further study in the population of HSCT recipients is needed to better understand their profile.
Depression
Identification and management of depression and depressive symptoms in HSCT recipients are imperative. Pretransplant depression has been found to be a risk factor for post‐transplant complications and is associated with early death, lower overall survival, and higher risk of acute GVHD after transplant [124, 125]. In a study of risk factors for depression and fatigue among survivors of HSCT, moderate to severe depression was reported by 43% of survivors, and moderate to severe fatigue was reported by 42% [49]. However, the risk of depression is high following HSCT even in patients who were not depressed prior to transplant [126]. Cognitive changes and depression or depressive symptoms are prevalent in the population of HSCT recipients [126, 127], even in those with no premorbid depressive history [126]. Depression has been found to have a significant negative effect on performance on objective cognitive tests across various patient populations [128], but not reliably so, with one study in patients with breast cancer finding no impact of depressed mood on cognitive performance [120]. Depression has also been found to be predictive of self‐reported neurocognitive complaints [129, 130] and thus may affect an individual's experience of their cognitive function and warrants following.
Sleep Disruption
Sleep disruption, including difficulty falling and staying asleep, early awakening, and/or nonrestorative sleep is both common and distressing in patients who have undergone HSCT [131]. A review by Jim et al. (2014) indicated that more than 50% of patients experience sleep disruption prior to HSCT, up to 82% during transplant, and up to 43% after transplant [132]. Sleep disruption prior to HSCT may be attributed to preparatory chemotherapy regimens, and there are mixed data regarding whether sleep disruption is more common among patients undergoing autologous versus allogenic transplants [132]. Sleep disruption after HSCT may have overlapping mechanisms with medication effects and/or complications including GVHD‐related symptoms and other psychoneurological symptoms [132].
Depression, anxiety, sleep disruption, and fatigue can worsen cognitive dysfunction in patients with cancer [133, 134, 135, 136, 137]. Conversely, cognitive decline can elevate depression and anxiety and also impede benefit from treatments for these symptoms [138, 139, 140]. Depression and anxiety are known to increase sleep dysfunction and vice versa [141, 142, 143, 144, 145]. Biologic pathways subserving psychoneurological symptom clusters remain unclear but may involve neurotoxic effects of cancer and its therapies, including inflammation/cytokine‐induced sickness behavior, hypothalamic‐pituitary‐adrenal axis dysfunction, DNA damage, and oxidative stress [101]. The incidence and phenotypes of psychoneurological symptom clusters in HSCT are currently unknown.
Return to Work
Cognitive functioning and the other psychoneurological symptoms discussed above are all important factors that affect rates at which patients with cancer successfully return to work [146]. Although difficulty returning to work has been identified as a potential long‐term complication following HSCT [3, 146], there is a paucity of data examining this issue. An early study found that only 60% of patients who had been previously working and were alive and relapse‐free 5 years after transplant had returned to full‐time work, with female patients being less likely to return to work [3]. A retrospective study in Switzerland found that of 203 HSCT recipients surviving at least 5 years after transplant, 76 (37%) were receiving disability pensions compared with 3.17% of the Swiss working population [147]. In a U.S. study, only 62.4% of 690 HSCT recipients had returned to work 1 year after transplant. Those who had not returned to work reported higher rates of fatigue and lower quality of life and were most likely to report the perception of having poor health [148]. Allogeneic transplant recipients and patients with extensive GVHD have shown a trend of delayed return to work, although these associations were not significant [15]. Three years after transplant, 19.5% of autologous, 31.2% of reduced‐intensity allogeneic, and 46.3% of myeloablative allogeneic HSCT recipients had not returned to work [18]. Allogeneic HSCT recipients with evidence of global cognitive deficits had approximately 10‐fold increased odds of not returning to work [18]. Furthermore, HSCT recipients with higher self‐reported cognitive problems had fourfold higher odds of not returning to work 3 years after transplant [149]. As such, issues of return to work seem to be prevalent among this population. It is logical to infer cognitive impairment may influence patients' return to work; however, more research is needed to better understand occupational outcomes in HSCT recipients.
Therapeutic Interventions
At this time, there are no established interventions for post‐HSCT cognitive impairment. Data from studies of cognitive impairment in other patients with cancer could be evaluated in HSCT survivors experiencing cognitive dysfunction. This may involve some combination of pharmacotherapy, cognitive training and rehabilitation, exercise, and cognitive behavioral therapy (Table 1). Further understanding of the biologic underpinnings of cognitive impairment in HSCT recipients will inform more targeted interventions. Studies evaluating therapeutic interventions for cognitive impairment in this specific population are warranted.
Table 1.
Potential pharmacologic and nonpharmacologic interventions for post–hematopoietic stem cell transplant cognitive impairment
| Pharmacologic interventions | Nonpharmacologic interventions |
|---|---|
|
Stimulant medications [152, 153, 154] Pharmacologic therapy of comorbid depression, fatigue [157] |
Computerized cognitive training [91, 158, 159, 160] Cognitive behavioral therapy [160] Transcranial direct current stimulation experiments [161] Physical activity (aerobic and/or resistance exercise) [164, 165, 166] Integrative therapies (e.g., yoga, healthy diet maintenance, mindfulness meditation) [167, 168] |
Conclusion
In this narrative review, we outline the incidence and nature of post‐HSCT cognitive impairment, associated risk factors, proposed biologic mechanisms, and therapeutic considerations. Research in this area is in its early stages, and our review can facilitate research question development and generation of hypotheses to move this body of research forward. Given the rising number of HSCTs performed per year [150], it is of increasing importance to understand the impact of this treatment on neurocognitive outcomes. At this time, clinical studies have highlighted cognitive impairment and its prevalence in the population of HSCT recipients. Cognitive phenotypes and associated biomarkers have also been evaluated; however, much remains to be elucidated regarding the nature, severity, risk factors, and biology of cognitive changes in this population.
Studies examining cognitive changes from HSCT remain limited. Small sample size [5, 9, 11, 14, 15, 16, 20, 151], lack of controls [5, 10, 11, 12, 13, 14, 15, 16, 17, 19, 20, 151], paucity of longitudinal follow‐up, and variability in cognitive testing methods limit the quality of available data. Of note, prospective selection of study populations is of value, given the interrelationship between cognitive performance and patient survival and, thus, the potential for sample bias to occur and be influenced by early clinical events. Additionally, samples evaluated have been very heterogeneous with respect to type of HSCT and various cancer types [5, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 49, 151]. Importantly, future studies should address the contributions of co‐occurring symptoms, including fatigue, anxiety, depression, and sleep disruption, as these tend to form symptom clusters with cognitive impairment in patients with cancer.
More robust, longitudinal assessments are warranted to evaluate cognitive function in this population. Many of the studies cease enrollment in the weeks or months after transplant, although it is clear the occupational and functional impacts are years later. Furthermore, rigorous mechanistic studies in this population, including biomarker and neuroimaging evaluation, could help to better understand the neurobiological processes that influence these patients and the impact on the nervous system, aiding in the development of appropriate targeted interventions. In addition to objective clinical testing for neurocognitive function, evaluation of patient‐reported outcomes, psychiatric well‐being, and understanding of other comorbidities related to cognitive function will also contribute to a broader understanding of the impact of transplant on brain health.
Author Contributions
Conception/design: Rebecca A Harrison, Shelli R. Kesler
Collection and/or assembly of data: Rebecca A Harrison, Noha Sharafeldin, Jennie L. Rexer, Brennan Melissa Petersen, Ashley M Henneghan, Shelli R. Kesler
Data analysis and interpretation: Rebecca A Harrison, Noha Sharafeldin, Jennie L. Rexer, Brennan Streck, Melissa Petersen, Ashley M Henneghan, Shelli R. Kesler
Manuscript writing: Rebecca A Harrison, Noha Sharafeldin, Jennie L. Rexer, Brennan Streck, Melissa Petersen, Ashley M Henneghan, Shelli R. Kesler
Final approval of manuscript: Rebecca A Harrison, Noha Sharafeldin, Jennie L. Rexer, Brennan Streck, Melissa Petersen, Ashley M Henneghan, Shelli R. Kesler
Disclosures
Rebecca A. Harrison: Orbus Therapeutics, Oncoceutics (RF: Institutional). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We would like to acknowledge and thank Lorie Kmetz for her assistance in preparation of this manuscript. Noha Sharafeldin is supported by the Leukemia and Lymphoma Society Career Development Award (LLS 3386‐19). Rebecca A. Harrison and Shelli Kesler are supported by the National Institutes of Health Research Projects Grant Program (R01CA226080, R01CA172145).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Socié G, Mary JY, Esperou H et al. Health and functional status of adult recipients 1 year after allogeneic haematopoietic stem cell transplantation. Br J Haematol 2001;113:194–201. [DOI] [PubMed] [Google Scholar]
- 2. Lee SJ, Fairclough D, Parsons SK et al. Recovery after stem‐cell transplantation for hematologic diseases. J Clin Oncol 2001;19:242–252. [DOI] [PubMed] [Google Scholar]
- 3. Kirchhoff AC, Leisenring W, Syrjala KL. Prospective predictors of return to work in the 5 years after hematopoietic cell transplantation. J Cancer Surviv 2010;4:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchbinder D, Kelly DL, Duarte RF et al. Neurocognitive dysfunction in hematopoietic cell transplant recipients: Expert review from the late effects and Quality of Life Working Committee of the CIBMTR and Complications and Quality of Life Working Party of the EBMT. Bone Marrow Transplant 2018;53:535–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones D, Vichaya EG, Wang XS et al. Acute cognitive impairment in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplant. Cancer 2013;119:4188–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Majhail NS, Rizzo JD, Lee SJ et al. Recommended screening and preventive practices for long‐term survivors after hematopoietic cell transplantation. Bone Marrow Transplant 2012;47:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fann JR, Roth‐Roemer S, Burington BE et al. Delirium in patients undergoing hematopoietic stem cell transplantation. Cancer 2002;95:1971–1981. [DOI] [PubMed] [Google Scholar]
- 8. Grauer O, Wolff D, Bertz H et al. Neurological manifestations of chronic graft‐versus‐host disease after allogeneic haematopoietic stem cell transplantation: Report from the Consensus Conference on Clinical Practice in chronic graft‐versus‐host disease. Brain 2010;133:2852–2865. [DOI] [PubMed] [Google Scholar]
- 9. Correa DD, Root JC, Baser R et al. A prospective evaluation of changes in brain structure and cognitive functions in adult stem cell transplant recipients. Brain Imaging Behav 2013;7:478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedman MA, Fernandez M, Wefel JS et al. Course of cognitive decline in hematopoietic stem cell transplantation: A within‐subjects design. Arch Clin Neuropsychol 2009;24:689–698. [DOI] [PubMed] [Google Scholar]
- 11. Harder H, Van Gool AR, Duivenvoorden HJ et al. Case‐referent comparison of cognitive functions in patients receiving haematopoietic stem‐cell transplantation for haematological malignancies: Two‐year follow‐up results. Eur J Cancer 2007;43:2052–2059. [DOI] [PubMed] [Google Scholar]
- 12. Meyers CA, Weitzner M, Byrne K et al. Evaluation of the neurobehavioral functioning of patients before, during, and after bone marrow transplantation. J Clin Oncol 1994;12:820–826. [DOI] [PubMed] [Google Scholar]
- 13. Scherwath A, Schirmer L, Kruse M et al. Cognitive functioning in allogeneic hematopoietic stem cell transplantation recipients and its medical correlates: A prospective multicenter study. Psychooncology 2013;22:1509–1516. [DOI] [PubMed] [Google Scholar]
- 14. Schulz‐Kindermann F, Mehnert A, Scherwath A et al. Cognitive function in the acute course of allogeneic hematopoietic stem cell transplantation for hematological malignancies. Bone Marrow Transplant 2007;39:789–799. [DOI] [PubMed] [Google Scholar]
- 15. Syrjala KL, Langer SL, Abrams JR et al. Recovery and long‐term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA 2004;291:2335–2343. [DOI] [PubMed] [Google Scholar]
- 16. Beglinger LJ, Duff K, Van Der Heiden S et al. Neuropsychological and psychiatric functioning pre‐ and posthematopoietic stem cell transplantation in adult cancer patients: A preliminary study. J Int Neuropsychol Soc 2007;13:172–177. [DOI] [PubMed] [Google Scholar]
- 17. Booth‐Jones M, Jacobsen PB, Ransom S et al. Characteristics and correlates of cognitive functioning following bone marrow transplantation. Bone Marrow Transplant 2005;36:695–702. [DOI] [PubMed] [Google Scholar]
- 18. Sharafeldin N, Bosworth A, Patel SK et al. Cognitive functioning after hematopoietic cell transplantation for hematologic malignancy: Results from a prospective longitudinal study. J Clin Oncol 2018;36:463–475. [DOI] [PubMed] [Google Scholar]
- 19. Chang G, Meadows ME, Orav EJ et al. Mental status changes after hematopoietic stem cell transplantation. Cancer 2009;115:4625–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Syrjala KL, Artherholt SB, Kurland BF et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol 2011;29:2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meadows ME, Chang G, Jones JA et al. Predictors of neuropsychological change in patients with chronic myelogenous leukemia and myelodysplastic syndrome. Arch Clin Neuropsychol 2013;28:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown MS, Stemmer SM, Simon JH et al. White matter disease induced by high‐dose chemotherapy: Longitudinal study with MR imaging and proton spectroscopy. AJNR Am J Neuroradiol 1998;19:217–221. [PMC free article] [PubMed] [Google Scholar]
- 23. Brown MS, Simon JH, Stemmer SM et al. MR and proton spectroscopy of white matter disease induced by high‐dose chemotherapy with bone marrow transplant in advanced breast carcinoma. AJNR Am J Neuroradiol 1995;16:2013–2020. [PMC free article] [PubMed] [Google Scholar]
- 24. Stemmer SM, Stears JC, Burton BS et al. White matter changes in patients with breast cancer treated with high‐dose chemotherapy and autologous bone marrow support. AJNR Am J Neuroradiol 1994;15:1267–1273. [PMC free article] [PubMed] [Google Scholar]
- 25. Padovan CS, Yousry TA, Schleuning M et al. Neurological and neuroradiological findings in long‐term survivors of allogeneic bone marrow transplantation. Ann Neurol 1998;43:627–633. [DOI] [PubMed] [Google Scholar]
- 26. Doolittle ND, Korfel A, Lubow MA et al. Long‐term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology 2013;81:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peper M, Steinvorth S, Schraube P et al. Neurobehavioral toxicity of total body irradiation: A follow‐up in long‐term survivors. Int J Radiat Oncol Biol Phys 2000;46:303–311. [DOI] [PubMed] [Google Scholar]
- 28. Sostak P, Padovan CS, Yousry TA et al. Prospective evaluation of neurological complications after allogeneic bone marrow transplantation. Neurology 2003;60:842–848. [DOI] [PubMed] [Google Scholar]
- 29. Correa DD, Wang Y, West JD et al. Prospective assessment of white matter integrity in adult stem cell transplant recipients. Brain Imaging Behav 2016;10:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deprez S, Vandenbulcke M, Peeters R et al. Longitudinal assessment of chemotherapy‐induced alterations in brain activation during multitasking and its relation with cognitive complaints. J Clin Oncol 2014;32:2031–2038. [DOI] [PubMed] [Google Scholar]
- 31. Schuitema I, Deprez S, Van Hecke W et al. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J Clin Oncol 2013;31:3378–3388. [DOI] [PubMed] [Google Scholar]
- 32. Apple AC, Schroeder MP, Ryals AJ et al. Hippocampal functional connectivity is related to self‐reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. Neuroimage Clin 2018;20:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krull KR, Hardy KK, Kahalley LS et al. Neurocognitive outcomes and interventions in long‐term survivors of childhood cancer. J Clin Oncol 2018;36:2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gabrieli JD, Ghosh SS, Whitfield‐Gabrieli S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron 2015;85:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng Y, Zhang XD, Zheng G et al. Chemotherapy‐induced brain changes in breast cancer survivors: Evaluation with multimodality magnetic resonance imaging. Brain Imaging Behav 2019;13:1799–1814. [DOI] [PubMed] [Google Scholar]
- 36. Kesler SR. Default mode network as a potential biomarker of chemotherapy‐related brain injury. Neurobiol Aging 2014;35(suppl 2):S11–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li M, Caeyenberghs K. Longitudinal assessment of chemotherapy‐induced changes in brain and cognitive functioning: A systematic review. Neurosci Biobehav Rev 2018;92:304–317. [DOI] [PubMed] [Google Scholar]
- 38. Kesler S, Rao A, Blayney DW et al. Predicting long‐term cognitive outcome following breast cancer with pre‐treatment resting state fMRI and random forest machine learning. Front Human Neurosci 2017;11:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen VC, Lin TY, Yeh DC et al. Predicting chemo‐brain in breast cancer survivors using multiple MRI features and machine‐learning. Magn Reson Med 2019;81:3304–3313. [DOI] [PubMed] [Google Scholar]
- 40. Wang GZ, Belgard TG, Mao D et al. Correspondence between resting‐state activity and brain gene expression. Neuron 2015;88:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richiardi J, Altmann A, Milazzo AC et al. Brain networks. Correlated gene expression supports synchronous activity in brain networks. Science 2015;348:1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zilles K, Bacha‐Trams M, Palomero‐Gallagher N et al. Common molecular basis of the sentence comprehension network revealed by neurotransmitter receptor fingerprints. Cortex 2015;63:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shah NJ, Arrubla J, Rajkumar R et al. Multimodal fingerprints of resting state networks as assessed by simultaneous trimodal MR‐PET‐EEG imaging. Sci Rep 2017;7:6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tomasi D, Wang GJ, Volkow ND. Energetic cost of brain functional connectivity. Proc Natl Acad Sci USA 2013;110:13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chiu GS, Boukelmoune N, Chiang ACA et al. Nasal administration of mesenchymal stem cells restores cisplatin‐induced cognitive impairment and brain damage in mice. Oncotarget 2018;9:35581–33597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shah D, Blockx I, Keliris GA et al. Cholinergic and serotonergic modulations differentially affect large‐scale functional networks in the mouse brain. Brain Struct Funct 2016;221:3067–3079. [DOI] [PubMed] [Google Scholar]
- 47. Deprez S, Kesler SR, Saykin AJ et al. International Cognition and Cancer Task Force recommendations for neuroimaging methods in the study of cognitive impairment in non‐CNS cancer patients. J Natl Cancer Inst 2018;110:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smedler AC, Winiarski J. Neuropsychological outcome in very young hematopoietic SCT recipients in relation to pretransplant conditioning. Bone Marrow Transplant 2008;42:515–522. [DOI] [PubMed] [Google Scholar]
- 49. Jim HS, Small B, Hartman S et al. Clinical predictors of cognitive function in adults treated with hematopoietic cell transplantation. Cancer 2012;118:3407–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weuve J, Barnes LL, Mendes de Leon CF et al. Cognitive aging in Black and White Americans: Cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology 2018;29:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilson RS, Yu L, Lamar M et al. Education and cognitive reserve in old age. Neurology 2019;92:e1041–e1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Buxbaum NP, Pavletic SZ. Autoimmunity following allogeneic hematopoietic stem cell transplantation. Front Immunol 2020;11:2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bechstein WO. Neurotoxicity of calcineurin inhibitors: Impact and clinical management. Transpl Int 2000;13:313–326. [DOI] [PubMed] [Google Scholar]
- 54. Girard TD, Jackson JC, Pandharipande PP et al. Delirium as a predictor of long‐term cognitive impairment in survivors of critical illness. Crit Care Med 2010;38:1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Davis DH, Muniz‐Terrera G, Keage HA et al. Association of delirium with cognitive decline in late life: A neuropathologic study of 3 population‐based cohort studies. JAMA Psychiatry 2017;74:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cleeland CS, Bennett GJ, Dantzer R et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine‐immunologic model of cancer symptoms. Cancer 2003;97:2919–2925. [DOI] [PubMed] [Google Scholar]
- 57. Kesler S, Janelsins M, Koovakkattu D et al. Reduced hippocampal volume and verbal memory performance associated with interleukin‐6 and tumor necrosis factor‐alpha levels in chemotherapy‐treated breast cancer survivors. Brain Behav Immun 2013;30(suppl):S109–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel SK, Wong AL, Wong FL et al. Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J Natl Cancer Inst 2015;107:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Henneghan A, Haley AP, Kesler S. Exploring relationships among peripheral amyloid beta, tau, cytokines, cognitive function, and psychosomatic symptoms in breast cancer survivors. Biol Res Nurs 2020;22:126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Henneghan AM, Palesh O, Harrison M et al. Identifying cytokine predictors of cognitive functioning in breast cancer survivors up to 10 years post chemotherapy using machine learning. J Neuroimmunol 2018;320:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kelly DL, Buchbinder D, Duarte RF et al. Neurocognitive dysfunction in hematopoietic cell transplant recipients: Expert review from the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and Complications and Quality of Life Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2018;24:228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoogland AI, Nelson AM, Gonzalez BD et al. Worsening cognitive performance is associated with increases in systemic inflammation following hematopoietic cell transplantation. Brain Behav Immun 2019;80:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shi E, Shi K, Qiu S et al. Chronic inflammation, cognitive impairment, and distal brain region alteration following intracerebral hemorrhage. FASEB J 2019;33:9616–9626. [DOI] [PubMed] [Google Scholar]
- 64. Sartori AC, Vance DE, Slater LZ et al. The impact of inflammation on cognitive function in older adults: Implications for healthcare practice and research. J Neurosci Nurs 2012;44:206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer 2005;104:788–793. [DOI] [PubMed] [Google Scholar]
- 66. de Brabander C, Cornelissen J, Smitt PA et al. Increased incidence of neurological complications in patients receiving an allogenic bone marrow transplantation from alternative donors. J Neurol Neurosurg Psychiatry 2000;68:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Phillips KM, McGinty HL, Cessna J et al. A systematic review and meta‐analysis of changes in cognitive functioning in adults undergoing hematopoietic cell transplantation. Bone Marrow Transplant 2013;48:1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ruggiu M, Cuccuini W, Mokhtari K et al. Case report: Central nervous system involvement of human graft versus host disease: Report of 7 cases and a review of literature. Medicine 2017;96:e8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kurosawa S, Oshima K, Yamaguchi T et al. Quality of life after allogeneic hematopoietic cell transplantation according to affected organ and severity of chronic graft‐versus‐host disease. Biol Blood Marrow Transplant 2017;23:1749–1758. [DOI] [PubMed] [Google Scholar]
- 70. Maffini E, Festuccia M, Brunello L et al. Neurologic complications after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2017;23:388–397. [DOI] [PubMed] [Google Scholar]
- 71. Matsuo Y, Kamezaki K, Takeishi S et al. Encephalomyelitis mimicking multiple sclerosis associated with chronic graft‐versus‐host disease after allogeneic bone marrow transplantation. Intern Med 2009;48:1453–1456. [DOI] [PubMed] [Google Scholar]
- 72. Roy S, Trinchieri G. Microbiota: A key orchestrator of cancer therapy. Nat Rev Cancer 2017;17:271–285. [DOI] [PubMed] [Google Scholar]
- 73. Althani AA, Marei HE, Hamdi WS et al. Human microbiome and its association with health and diseases. J Cell Physiol 2016;231:1688–1694. [DOI] [PubMed] [Google Scholar]
- 74. Jordan KR, Loman BR, Bailey MT et al. Gut microbiota‐immune‐brain interactions in chemotherapy‐associated behavioral comorbidities. Cancer 2018;124:3990–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Johnston IN. Chemotherapy‐induced cognitive deficits, white matter pathologies and cytokines. Brain Behav Immun 2014;35:21–22. [DOI] [PubMed] [Google Scholar]
- 76. Vichaya EG, Chiu GS, Krukowski K et al. Mechanisms of chemotherapy‐induced behavioral toxicities. Front Neurosci 2015;9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Taur Y, Xavier JB, Lipuma L et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012;55:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang T, Hu X, Liang S et al. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef Microbes 2015;6:707–717. [DOI] [PubMed] [Google Scholar]
- 79. Savignac HM, Tramullas M, Kiely B et al. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res 2015;287:59–72. [DOI] [PubMed] [Google Scholar]
- 80. Bercik P, Denou E, Collins J et al. The intestinal microbiota affect central levels of brain‐derived neurotropic factor and behavior in mice. Gastroenterology 2011;141:599–609, .e1–3. [DOI] [PubMed] [Google Scholar]
- 81. Clarke G, Grenham S, Scully P et al. The microbiome‐gut‐brain axis during early life regulates the hippocampal serotonergic system in a sex‐dependent manner. Mol Psychiatry 2013;18:666–673. [DOI] [PubMed] [Google Scholar]
- 82. Chung YC, Jin HM, Cui Y et al. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J Funct Foods 2014;10:465–474. [Google Scholar]
- 83. Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr 2007;61:355–361. [DOI] [PubMed] [Google Scholar]
- 84. Chiang ACA, Huo X, Kavelaars A et al. Chemotherapy accelerates age‐related development of tauopathy and results in loss of synaptic integrity and cognitive impairment. Brain Behav Immun 2019;79:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Moruno‐Manchon JF, Uzor NE, Kesler SR et al. Peroxisomes contribute to oxidative stress in neurons during doxorubicin‐based chemotherapy. Mol Cell Neurosci 2018;86:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Henneghan A, Rao V, Harrison RA et al. Cortical brain age from pre‐treatment to post‐chemotherapy in patients with breast cancer. Neurotox Res 2020;37:788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ahles TA, Root JC, Ryan EL. Cancer‐ and cancer treatment‐associated cognitive change: An update on the state of the science. J Clin Oncol 2012;30:3675–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Janelsins MC, Kohli S, Mohile SG et al. An update on cancer‐ and chemotherapy‐related cognitive dysfunction: Current status. Semin Oncol 2011;38:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Harris SE, Deary IJ. The genetics of cognitive ability and cognitive ageing in healthy older people. Trends Cogn Sci 2011;15:388–394. [DOI] [PubMed] [Google Scholar]
- 90. Belloy ME, Napolioni V, Greicius MD. A quarter century of APOE and Alzheimer's disease: Progress to date and the path forward. Neuron 2019;101:820–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chung NC, Walker AK, Dhillon HM et al. Mechanisms and treatment for cancer‐ and chemotherapy‐related cognitive impairment in survivors of non‐CNS malignancies. Oncology (Williston Park) 2018;32:591–598. [PubMed] [Google Scholar]
- 92. Ahles TA, Saykin AJ, Noll WW et al. The relationship of APOE genotype to neuropsychological performance in long‐term cancer survivors treated with standard dose chemotherapy. Psychooncology 2003;12:612–619. [DOI] [PubMed] [Google Scholar]
- 93. Vardy JL, Dhillon HM, Pond GR et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. J Clin Oncol 2015;33:4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Buskbjerg CDR, Amidi A, Demontis D et al. Genetic risk factors for cancer‐related cognitive impairment: A systematic review. Acta Oncol 2019;58:537–547. [DOI] [PubMed] [Google Scholar]
- 95. Cheng H, Li W, Gan C et al. The COMT (rs165599) gene polymorphism contributes to chemotherapy‐induced cognitive impairment in breast cancer patients. Am J Transl Res 2016;8:5087–5097. [PMC free article] [PubMed] [Google Scholar]
- 96. Li W, Zhao J, Ding K et al. Catechol‐O‐methyltransferase gene polymorphisms and the risk of chemotherapy‐induced prospective memory impairment in breast cancer patients with varying tumor hormonal receptor expression. Med Sci Monit 2020;26:e923567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bender CM, Merriman JD, Sereika SM et al. Trajectories of cognitive function and associated phenotypic and genotypic factors in breast cancer. Oncol Nurs Forum 2018;45:308–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Koleck TA, Bender CM, Sereika SM et al. Polymorphisms in DNA repair and oxidative stress genes associated with pre‐treatment cognitive function in breast cancer survivors: An exploratory study. Springerplus 2016;5:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gonzalez BD, Jim HS, Booth‐Jones M et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen‐deprivation therapy: A controlled comparison. J Clin Oncol 2015;33:2021–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sharafeldin N, Richman J, Bosworth A et al. Clinical and genetic risk prediction of cognitive impairment after blood or marrow transplantation for hematologic malignancy. J Clin Oncol 2020;38:1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kim HJ, Barsevick AM, Fang CY et al. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs 2012;35:E1–E20. [DOI] [PubMed] [Google Scholar]
- 102. Tometich DB, Small BJ, Carroll JE et al. Pretreatment psychoneurological symptoms and their association with longitudinal cognitive function and quality of life in older breast cancer survivors. J Pain Symptom Manage 2019;57:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mandelblatt JS, Zhai W, Ahn J et al. Symptom burden among older breast cancer survivors: The Thinking and Living with Cancer (TLC) study. Cancer 2020;126:1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim HJ, McGuire DB, Tulman L et al. Symptom clusters: Concept analysis and clinical implications for cancer nursing. Cancer Nurs 2005;28:270–282; quiz 83–84. [DOI] [PubMed] [Google Scholar]
- 105. Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum 2001;28:465–470. [PubMed] [Google Scholar]
- 106. Angela RS, Debra EL, Elswick RK et al. A conceptual model of psychoneurological symptom cluster variation in women with breast cancer: Bringing nursing research to personalized medicine. Curr Pharmacogenomics Person Med 2013;11:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dodd MJ, Cho MH, Cooper BA et al. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs 2010;14:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cleeland CS, Mayer M, Dreyer NA et al. Impact of symptom burden on work‐related abilities in patients with locally recurrent or metastatic breast cancer: Results from a substudy of the VIRGO observational cohort study. Breast 2014;23:763–769. [DOI] [PubMed] [Google Scholar]
- 109. Kim HJ, Barsevick AM, Beck SL et al. Clinical subgroups of a psychoneurologic symptom cluster in women receiving treatment for breast cancer: A secondary analysis. Oncol Nurs Forum 2012;39:E20–E30. [DOI] [PubMed] [Google Scholar]
- 110. Jimenez A, Madero R, Alonso A et al. Symptom clusters in advanced cancer. J Pain Symptom Manage 2011;42:24–31. [DOI] [PubMed] [Google Scholar]
- 111. Aktas A, Walsh D, Rybicki L. Symptom clusters and prognosis in advanced cancer. Support Care Cancer 2012;20:2837–2843. [DOI] [PubMed] [Google Scholar]
- 112. Pullens MJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: A systematic review. Psychooncology 2010;19:1127–1138. [DOI] [PubMed] [Google Scholar]
- 113. Von Ah D, Tallman EF. Perceived cognitive function in breast cancer survivors: Evaluating relationships with objective cognitive performance and other symptoms using the functional assessment of cancer therapy‐cognitive function instrument. J Pain Symptom Manage 2015;49:697–706. [DOI] [PubMed] [Google Scholar]
- 114. Aktas A. Cancer symptom clusters: Current concepts and controversies. Curr Opin Support Palliat Care 2013;7:38–44. [DOI] [PubMed] [Google Scholar]
- 115. Gielissen MF, Schattenberg AV, Verhagen CA et al. Experience of severe fatigue in long‐term survivors of stem cell transplantation. Bone Marrow Transplant 2007;39:595–603. [DOI] [PubMed] [Google Scholar]
- 116. Hacker ED, Kim I, Park C et al. Real‐time fatigue and free‐living physical activity in hematopoietic stem cell transplantation cancer survivors and healthy controls: A preliminary examination of the temporal, dynamic relationship. Cancer Nurs 2017;40:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Esser P, Kuba K, Scherwath A et al. Stability and priority of symptoms and symptom clusters among allogeneic HSCT patients within a 5‐year longitudinal study. J Pain Symptom Manage 2017;54:493–500. [DOI] [PubMed] [Google Scholar]
- 118. Lacourt TE, Heijnen CJ. Mechanisms of neurotoxic symptoms as a result of breast cancer and its treatment: Considerations on the contribution of stress, inflammation, and cellular bioenergetics. Curr Breast Cancer Rep 2017;9:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang XS, Shi Q, Shah ND et al. Inflammatory markers and development of symptom burden in patients with multiple myeloma during autologous stem cell transplantation. Clin Cancer Res 2014;20:1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Small BJ, Jim HSL, Eisel SL et al. Cognitive performance of breast cancer survivors in daily life: Role of fatigue and depressed mood. Psychooncology 2019;28:2174–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gullett JM, Cohen RA, Yang GS et al. Relationship of fatigue with cognitive performance in women with early‐stage breast cancer over 2 years. Psychooncology 2019;28:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Klemp JR, Myers JS, Fabian CJ et al. Cognitive functioning and quality of life following chemotherapy in pre‐ and peri‐menopausal women with breast cancer. Support Care Cancer 2018;26:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Goedendorp MM, Knoop H, Gielissen MF et al. The effects of cognitive behavioral therapy for postcancer fatigue on perceived cognitive disabilities and neuropsychological test performance. J Pain Symptom Manage 2014;47:35–44. [DOI] [PubMed] [Google Scholar]
- 124. El‐Jawahri A, Chen YB, Brazauskas R et al. Impact of pre‐transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer 2017;123:1828–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Loberiza FR Jr, Rizzo JD, Bredeson CN et al. Association of depressive syndrome and early deaths among patients after stem‐cell transplantation for malignant diseases. J Clin Oncol 2002;20:2118–2126. [DOI] [PubMed] [Google Scholar]
- 126. Artherholt SB, Hong F, Berry DL et al. Risk factors for depression in patients undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant 2014;20:946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ahern E, Semkovska M. Cognitive functioning in the first‐episode of major depressive disorder: A systematic review and meta‐analysis. Neuropsychology 2017;31:52–72. [DOI] [PubMed] [Google Scholar]
- 128. Rock PL, Roiser JP, Riedel WJ et al. Cognitive impairment in depression: A systematic review and meta‐analysis. Psychol Med 2014;44:2029–2040. [DOI] [PubMed] [Google Scholar]
- 129. LE Kinsinger SW, Mohr DC. Relationship between depression, fatigue, subjective cognitive impairment, and objective neuropsychological functioning in patients with multiple sclerosis. Neuropsychology 2010;24:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bender CM, Pacella ML, Sereika SM et al. What do perceived cognitive problems reflect? J Support Oncol 2008;6:238–242. [PMC free article] [PubMed] [Google Scholar]
- 131. Bevans MF, Mitchell SA, Marden S. The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation (HSCT). Support Care Cancer 2008;16:1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jim HS, Evans B, Jeong JM et al. Sleep disruption in hematopoietic cell transplantation recipients: Prevalence, severity, and clinical management. Biol Blood Marrow Transplant 2014;20:1465–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yang Y, Hendrix CC. Cancer‐related cognitive impairment in breast cancer patients: Influences of psychological variables. Asia Pac J Oncol Nurs 2018;5:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bedillion MF, Ansell EB, Thomas GA. Cancer treatment effects on cognition and depression: The moderating role of physical activity. Breast 2019;44:73–80. [DOI] [PubMed] [Google Scholar]
- 135. Henneghan AM, Carter P, Stuifbergan A et al. Relationships between self‐reported sleep quality components and cognitive functioning in breast cancer survivors up to 10 years following chemotherapy. Psychooncology 2018;27:1937–1943. [DOI] [PubMed] [Google Scholar]
- 136. Henneghan A, Stuifbergen A, Becker H et al. Modifiable correlates of perceived cognitive function in breast cancer survivors up to 10 years after chemotherapy completion. J Cancer Surviv 2018;12:224–233. [DOI] [PubMed] [Google Scholar]
- 137. Tager FA, McKinley PS, Schnabel FR et al. The cognitive effects of chemotherapy in post‐menopausal breast cancer patients: A controlled longitudinal study. Breast Cancer Res Treat 2010;123:25–34. [DOI] [PubMed] [Google Scholar]
- 138. Caudle DD, Senior AC, Wetherell JL et al. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry 2007;15:680–689. [DOI] [PubMed] [Google Scholar]
- 139. Thompson DG, Kesler SR, Sudheimer K et al. FMRI activation during executive function predicts response to cognitive behavioral therapy in older, depressed adults. Am J Geriatr Psychiatry 2015;23:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Vinkers DJ, Gussekloo J, Stek ML et al. Temporal relation between depression and cognitive impairment in old age: Prospective population based study. BMJ 2004;329:881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Spoormaker VI, van den Bout J. Depression and anxiety complaints; relations with sleep disturbances. Eur Psychiatry 2005;20:243–245. [DOI] [PubMed] [Google Scholar]
- 142. Taylor DJ, Lichstein KL, Durrence HH et al. Epidemiology of insomnia, depression, and anxiety. Sleep 2005;28:1457–1464. [DOI] [PubMed] [Google Scholar]
- 143. Sivertsen B, Salo P, Mykletun A et al. The bidirectional association between depression and insomnia: The HUNT study. Psychosom Med 2012;74:758–765. [DOI] [PubMed] [Google Scholar]
- 144. Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep 2007;30:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Redeker NS, Lev EL, Ruggiero J. Insomnia, fatigue, anxiety, depression, and quality of life of cancer patients undergoing chemotherapy. Sch Inq Nurs Pract 2000;14:275–290; discussion 91–98. [PubMed] [Google Scholar]
- 146. Dorland HF, Abma FI, Van Zon SKR et al. Fatigue and depressive symptoms improve but remain negatively related to work functioning over 18 months after return to work in cancer patients. J Cancer Surviv 2018;12:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tichelli A, Gerull S, Holbro A et al. Inability to work and need for disability pension among long‐term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant 2017;52:1436–1442. [DOI] [PubMed] [Google Scholar]
- 148. Morrison EJ, Ehlers SL, Bronars CA et al. Employment status as an indicator of recovery and function one year after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2016;22:1690–1695. [DOI] [PubMed] [Google Scholar]
- 149. Murdaugh DL, Bosworth A, Patel SK et al. Self‐endorsed cognitive problems versus objectively assessed cognitive impairment in blood or bone marrow transplantation recipients: A longitudinal study. Cancer 2020;126:2174–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Pasquini M, Wang Z, Horowitz MM et al. 2013. report from the Center for International Blood and Marrow Transplant Research (CIBMTR): Current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl 2013:187–197. [PubMed] [Google Scholar]
- 151. Andrykowski MA, Schmitt FA, Gregg ME et al. Neuropsychologic impairment in adult bone marrow transplant candidates. Cancer 1992;70:2288–2297. [DOI] [PubMed] [Google Scholar]
- 152. Mar Fan HG, Clemons M, Xu W et al. A randomised, placebo‐controlled, double‐blind trial of the effects of d‐methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 2008;16:577–583. [DOI] [PubMed] [Google Scholar]
- 153. Kohli S, Fisher SG, Tra Y et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer 2009;115:2605–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Karschnia P, Parsons MW, Dietrich J. Pharmacologic management of cognitive impairment induced by cancer therapy. Lancet Oncol 2019;20:e92–e102. [DOI] [PubMed] [Google Scholar]
- 155. King S, Green HJ. Psychological intervention for improving cognitive function in cancer survivors: A literature review and randomized controlled trial. Front Oncol 2015;5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gehring K, Roukema JA, Sitskoorn MM. Review of recent studies on interventions for cognitive deficits in patients with cancer. Expert Rev Anticancer Ther 2012;12:255–269. [DOI] [PubMed] [Google Scholar]
- 157. Ollat H, Laurent B, Bakchine S et al. Effects of the association of sulbutiamine with an acetylcholinesterase inhibitor in early stage and moderate Alzheimer disease [in French]. Encephale 2007;33:211–215. [DOI] [PubMed] [Google Scholar]
- 158. Kesler S, Hadi Hosseini SM, Heckler C et al. Cognitive training for improving executive function in chemotherapy‐treated breast cancer survivors. Clin Breast Cancer 2013;13:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Poppelreuter M, Weis J, Mumm A et al. Rehabilitation of therapy‐related cognitive deficits in patients after hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;41:79–90. [DOI] [PubMed] [Google Scholar]
- 160. Fernandes HA, Richard NM, Edelstein K. Cognitive rehabilitation for cancer‐related cognitive dysfunction: A systematic review. Support Care Cancer 2019;27:3253–3279. [DOI] [PubMed] [Google Scholar]
- 161. Liu CS, Rau A, Gallagher D et al. Using transcranial direct current stimulation to treat symptoms in mild cognitive impairment and Alzheimer's disease. Neurodegener Dis Manag 2017;7:317–329. [DOI] [PubMed] [Google Scholar]
- 162. Luctkar‐Flude M, Groll D, Tyerman J. Using neurofeedback to manage long‐term symptoms in cancer survivors: Results of a survey of neurofeedback providers. Eur J Integr Med 2017;12:172–176. [Google Scholar]
- 163. Alvarez J, Meyer FL, Granoff DL et al. The effect of EEG biofeedback on reducing postcancer cognitive impairment. Integr Cancer Ther 2013;12:475–487. [DOI] [PubMed] [Google Scholar]
- 164. Strohle A, Schmidt DK, Schultz F et al. Drug and exercise treatment of Alzheimer disease and mild cognitive impairment: A systematic review and meta‐analysis of effects on cognition in randomized controlled trials. Am J Geriatr Psychiatry 2015;23:1234–1249. [DOI] [PubMed] [Google Scholar]
- 165. Myers JS, Erickson KI, Sereika SM et al. Exercise as an intervention to mitigate decreased cognitive function from cancer and cancer treatment: An integrative review. Cancer Nurs 2018;41:327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Campbell KL, Zadravec K, Bland KA et al. The effect of exercise on cancer‐related cognitive impairment and applications for physical therapy: Systematic review of randomized controlled trials. Phys Ther 2020;100:523–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Jean‐Pierre P, McDonald BC. Neuroepidemiology of cancer and treatment‐related neurocognitive dysfunction in adult‐onset cancer patients and survivors. Handb Clin Neurol 2016;138:297–309. [DOI] [PubMed] [Google Scholar]
- 168. Zhang Q, Gao X, Liu S et al. Therapies for cognitive impairment in breast cancer survivors treated with chemotherapy: A protocol for systematic review. Medicine (Baltimore) 2020;99:e20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Engel GL. The need for a new medical model: A challenge for biomedicine. Science 1977;196:129–136. [DOI] [PubMed] [Google Scholar]


