Abstract
Background
With increased use of immune checkpoint inhibitors (ICIs) among patients with cancer, there is substantial interest in understanding clinical and economic outcomes and management of immune‐related adverse events (irAEs).
Patients, Materials, and Methods
A retrospective study was conducted using Premier Healthcare Database, a U.S. national hospital discharge database, from March 1, 2015, through December 31, 2017. The database comprises more than 880 million inpatient and hospital‐based outpatient encounters, with more than 200 million unique patients reported by 966 hospitals. Patients with four solid tumors known to benefit from ICI therapy were included. The list of irAEs assessed was defined a priori per American Society of Clinical Oncology clinical guidelines for irAE management. Baseline irAE‐related inpatient and outpatient visits were defined as the first inpatient or hospital‐based outpatient visit with discharge diagnosis of any irAE of interest following confirmed ICI usage within 90 days prior to the baseline visit. Patients were followed for 90 days after baseline irAE‐related inpatient discharge date or outpatient visit date to assess irAE‐related inpatient admissions, all‐cause in‐hospital mortality, ICI reinitiation, and to determine costs and health care resource utilization.
Results
Records from 673,957 patients with four tumor types were reviewed for ICI therapy. Of 13,030 patients receiving ICIs, approximately 40% experienced at least one irAE, with a total of 10,121 irAEs occurring within 90 days of the ICI visit. The most frequent (>1,000 events) irAEs were anemia, impaired ventricular function with heart failure and vasculitis, thrombocytopenia, thyroid conditions, and peripheral edema. As might be expected, compared with those with baseline irAE‐related outpatient visits, patients with baseline irAE‐related inpatient visits had a significantly higher percentage of irAE‐related inpatient admissions (23% vs. 14%) and all‐cause in‐hospital mortality (22% vs. 6%) and lower reinitiation of ICI therapy (31% vs. 71%). Baseline irAE‐related inpatient visits had significantly higher mean costs ($29,477 vs. $5,718) with longer hospital stays (12.6 vs. 7.8 days).
Conclusion
Findings from a U.S. national hospital discharge database suggest that irAEs in patients treated with ICIs are common, occur in multiples and with greater frequency in those with pre‐existing comorbidities. Those with inpatient admissions have poorer outcomes.
Implications for Practice
The present work addressed the knowledge gap in understanding real‐world outcomes of immune‐related adverse events (irAEs) associated with immune checkpoint inhibitors (ICIs). Patients who experienced irAEs had significantly higher baseline comorbidities and were more likely to have immune‐related or immune‐compromised comorbid conditions. Patients with baseline irAE‐related hospitalizations were more likely to be rehospitalized and to experience in‐hospital mortality and less likely to reinitiate ICI treatment. Real‐world patients are more diverse than clinical trials, and clinicians should consider both the efficacy and safety profile of ICI treatments, especially for patients with comorbidity conditions. Close monitoring is needed after patients have experienced an irAE.
Keywords: Immune checkpoint inhibitors, Immune‐related adverse events, Real‐world study, Clinical outcomes, Economic outcomes
Short abstract
This large real‐world evidence study assessed the prevalence of immune‐related adverse events among patients who received immune checkpoint inhibitors and associated clinical and economic outcomes
Introduction
Cancer immunotherapy with monoclonal antibodies has proven to be an efficacious alternative to chemotherapy and targeted therapies for late‐stage tumors [1]. In particular, immune checkpoint inhibitor (ICI) treatment for advanced malignancies has brought significant improvements in treating some solid tumors, by increasing response rates, demonstrating durability, and extending survival over existing treatment options [2]. In recent years the use of ICI therapies, specifically programmed death‐1 (PD‐1) inhibitors and programmed death‐ligand 1 (PD‐L1) inhibitors, has grown considerably among patients with highly immunogenic tumors such as Merkel cell carcinoma (MCC), non‐small cell lung cancer (NSCLC), renal cell carcinoma (RCC) and urothelial carcinoma (UC) [3].
Data from both clinical trials and real‐world studies have shown that ICI monotherapy had lower adverse event (AE) incidence than chemotherapies and as a result was associated with fewer AE‐related visits in the real world [4, 5]. Although generally well‐tolerated [2, 6, 7, 8, 9, 10], ICIs are associated with adverse immunologic reactions related to their mechanism of action [11]. The spectrum of immune‐related adverse events (irAEs) is quite different from the side effects seen with other systemic therapies such as cytotoxic chemotherapy [2, 6, 7, 8, 9, 10, 12]. These irAEs occur in multiple organ systems with dermatologic, gastrointestinal, endocrine, musculoskeletal, renal, neurological, hematologic, cardiovascular, and ocular being the most commonly affected [13]. For mild irAEs, ICI treatment is typically continued with close monitoring. Moderate to severe irAEs can be associated with a decline in organ function, quality of life, and death [13, 14]. Depending on the severity of the toxicities, ICI treatment may be discontinued, and may or may not be reinitiated. Management of irAEs is associated with significant costs [15]. Thus, early clinical management of patients with irAEs is critical to optimize clinical and economic outcomes.
Despite increased use of ICI treatments, there is a paucity of published literature on interventions used to manage irAEs and resulting outcomes. Much of the available information about ICI‐associated irAEs was reported from randomized clinical trials, case studies, and case reports. Moreover, management guidelines primarily rely on informal consensus provided by the American Society of Clinical Oncology (ASCO) and underscore the need for evidence‐based data [11]. To help address this knowledge gap and better understand clinical and economic outcomes associated with inpatient and outpatient management of irAEs, this real‐world evidence study assessed current use of PD‐1 and PD‐L1 ICIs for treatment of selected solid tumors using a U.S. national hospital discharge database.
Patients, Materials, and Methods
The Premier Healthcare Database
The Premier Healthcare Database (PHD) was used to conduct this retrospective observational study of patients with select solid tumors (MCC, NSCLC, RCC, or UC) who received ICI treatment (PD‐1 or PD‐L1 antibodies). The PHD is a large hospital‐based, service‐level, all‐payer database containing discharge information from inpatient and hospital‐based outpatient visits. It represents approximately 20%–25% of all U.S. inpatient admissions from geographically diverse nongovernmental community and teaching hospitals and health systems from rural and urban settings. Outpatient visits to emergency departments, ambulatory surgery centers, and hospital‐based outpatient clinics are included. The PHD contains data from standard hospital discharge files, including patient demographics and disease states, insurance type, admission and discharge diagnoses, admission source and type, and discharge status and disposition. Information on billed services include overall, departmental, and service‐level costs for inpatient and outpatient encounters. Hospital pharmacy medication use is also included.
Unique masked identifiers allow patients to be tracked in the same hospital across inpatient and hospital‐based outpatient settings. More than 880 million inpatient and hospital‐based outpatient encounters with more than 200 million unique patients were reported by 966 hospitals contributing data to the PHD at the time of this study. All data in the PHD are deidentified and compliant with the Health Insurance Portability and Accountability Act. Institutional review board approval for this study was not required, based on U.S. Title 45 Code of Federal Regulations, Part 46, because the study used existing deidentified hospital discharge data, and recorded information could not be identified directly or through identifiers linked to individuals.
Study Population
Patients were included in the study if all of the following criteria were met: (a) had an inpatient or hospital‐based outpatient visit, (b) were ≥ 12 years of age with MCC, or ≥ 18 years of age with RCC, UC, or NSCLC, and (c) received ICI treatment during the main study period of March 1, 2015, through December 31, 2017. MCC, RCC, UC, and NSCLC were defined using primary or secondary International Classification of Diseases‐9th/10th Revision, Clinical Modification (ICD‐9/10) Discharge Diagnosis Codes (Fig. 1). In addition, a multiple cancers group was formed for patients who had no primary but two or more secondary ICD codes for the solid tumors of interest. Patients who were pregnant were excluded from the study. ICI treatments included PD‐L1 antibodies (avelumab, atezolizumab, or durvalumab), and PD‐1 antibodies (nivolumab or pembrolizumab), and ICIs were identified using text searches of both generic and brand names in the hospital chargemaster data.
Figure 1.
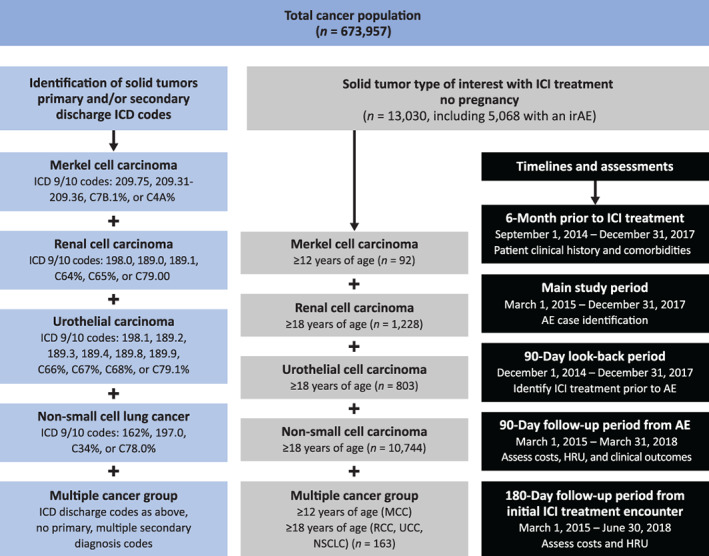
Sample attrition and case definition.Abbreviations: AE, adverse event; HRU, health care resource utilization; ICD 9/10; International Classification of Diseases‐9th/10th Revision; ICI, immune checkpoint inhibitors; irAE, immune‐related adverse event; MCC, Merkel cell carcinoma; NSCLC, non‐small lung cancer; RCC, renal cell carcinoma; UCC, urothelial cell carcinoma.
Study Timeline
The main study period for AE case identification was from March 1, 2015, through December 31, 2017, with follow‐up through March 31, 2018, for clinical and economic outcomes for patients who experienced ICI‐associated irAEs (Figs. 1, 2). To determine whether an adverse event was indeed an irAE, there was a 90‐day look‐back period for assessment of ICI treatment (Figs. 1, 2). If ICI treatment was identified, the AE was deemed to be related to ICI use and was an irAE; otherwise, it was not deemed an irAE. From the time of the first ICI treatment in the main study period, there was a 6‐month look‐back period to assess patient history and comorbidities, as well as another 180 days of follow‐up to assess treatment costs and health care resource utilization (HRU) for all patients, regardless of whether they experienced an irAE (Figs. 1, 2).
Figure 2.
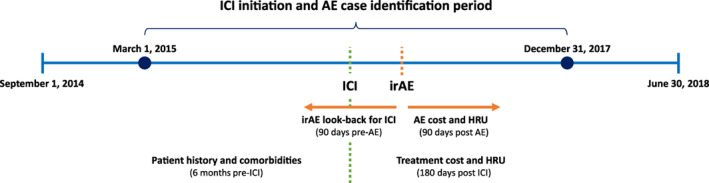
Study timeline. Reinitiation within 90 days of the initial encounter discharge; note that there were no initial outpatient encounter irAEs for myocarditis, transverse myelitis, and toxic epidermal necrolysis and no initial inpatient encounter irAEs for acquired hemophilia.Abbreviations: AE, adverse event; HRU, health care resource utilization; ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event.
Immune‐Related Adverse Event Identification and Definitions
irAEs were selected using ASCO clinical practice guidelines on irAE management [11] and defined using ICD‐9/10 discharge diagnosis codes. A total of 45 irAEs were assessed at the beginning of the study but only 28 were captured in the data and included in this study. Baseline irAE‐related inpatient and hospital‐based outpatient visits were defined as the first hospital visit during the study period with a certain irAE diagnosis within 90 days of a confirmed ICI usage. All corresponding irAEs of the same type during the 90 days post the irAEs‐related baseline visit were counted as part of follow‐up care of the irAEs. If no ICI treatment was identified during the 90‐day look‐back period, then the visit was not designated as an irAE‐related visit. If a patient had multiple visits with diagnosis of different irAEs, the earliest visit is considered as the baseline‐irAE‐related visit. Multiple irAEs per patient were counted and assessed in accordance with the definitions described earlier, and baseline irAE‐related inpatient and outpatient visits were reported separately.
To account for pre‐existing conditions when determining whether an event was indeed an irAE, patients were not counted as experiencing a post‐ICI treatment irAE for the same condition if they had a history of anemia, thyroid condition, venous thromboembolism, colitis, Guillain‐Barré syndrome, pneumonitis, adrenal insufficiency, encephalitis, hepatitis, impaired ventricular function with heart failure and vasculitis, myocarditis, nephritis, or pituitary conditions during the 6 months prior to the first ICI treatment.
Patient, Visit, and Hospital Characteristics
Patient, visit, and hospital characteristics were examined at the time of first ICI treatment in the main study period. Patient demographics included age, sex, race/ethnicity, and primary payor. Admission status was also assessed (inpatient vs. outpatient), as well as the hospital setting (urban vs. rural), and teaching status. The Deyo‐modified Charlson Comorbidity Index (CCI) [16, 17] was also assessed at time of first ICI treatment in the main study period. Various immune‐related and immuno‐compromised comorbidities were identified in the 6‐month look‐back period prior to the first ICI treatment using ICD‐9/10 discharge diagnosis codes.
Patient, visit, and hospital characteristics as well as comorbid conditions were compared between patients who went on to experience an ICI‐associated selected irAE during the study period and those who did not.
Clinical Outcomes, Cost, and HRU
Patients were followed for 90 days post‐irAE (through March 31, 2018) to determine clinical outcomes, cost, and HRU related to irAE. Clinical outcomes assessed included irAE‐related inpatient admissions, all‐cause in‐hospital mortality, and ICI treatment reinitiation. To be considered as a subsequent irAE‐related inpatient admission, patients must have had an ICD code indicating the same irAE. Total costs were calculated by irAE type and whether the baseline irAE‐related visit was an inpatient or outpatient visit. ICI treatment costs were excluded. Mean costs were calculated inclusive of the baseline irAE‐related visit cost, whereas mean readmission length of stay (LOS) for follow‐up inpatient hospitalizations was calculated exclusive of the baseline irAE‐related visit. The follow‐up costs and LOS included all discharges with ICD‐9/10 discharge diagnosis codes for the irAEs of interest at the baseline irAE‐related visit.
Additionally, to compare the all‐cause costs and HRU differences for patients with versus without irAEs, these outcomes were also assessed through 180 days of follow‐up (through June 30, 2018) from the time of the first ICI treatment administration in the main study period.
Statistical Analysis
Descriptive statistics were calculated for all study variables including irAEs and clinical and economic outcomes. Data measured on a continuous scale were expressed as means and SDs. Categorical data were expressed as counts and percentages.
Bivariate analysis was performed, and p values were calculated, using χ2 test, t‐test, analysis of variance, Wilcoxon and Kruskal‐Wallis as appropriate to compare outcomes (e.g., costs, LOS, ICI reinitiation, mortality, inpatient admission during the follow‐up) between patients who experienced an irAE versus those who did not experience an irAE. These tests were also used to compare inpatient versus outpatient baseline irAE‐related visits among patients experiencing irAEs on all outcomes.
Results
A total of 673,957 patients were initially identified with MCC (3,316 [0.5%]), RCC (98,400 [14.6%]), UC (110,281 [16.4%]), and NSCLC (454,623 [67.5%]). Of these, 13,030 patients received ICI treatment. Among which, 92 had MCC (0.7%), 1,228 had RCC (9.4%), 803 had UC (6.2%), 10,744 had NSCLC (82.4%), and 163 (1.2%) had multiple cancers (Fig. 1).
Patient, Visit, and Hospital Characteristics
Among the 13,030 patients with ICI treatment, 5,068 (39%) experienced at least one irAE during the main study period (March 1, 2015, to December 31, 2017). At the time of first ICI treatment during the main study period, patients who did not experience an irAE and those who went on to experience at least one irAE were similar in age, gender, race, insurance status, and hospital characteristics, all p > .05. (Table 1). The majority of patients were insured by Medicare followed by commercial carriers, then Medicaid and other payer types. Hospitals served mostly urban populations, and less than half were teaching hospitals (Table 1). The mean CCI was significantly higher among patients who experienced at least one irAE during the study period (5.0 ± 3.2 for irAE vs. 4.5 ± 3.1 for no irAE; p < .001).
Table 1.
Patient, visit, and hospital characteristics at the time of first immune checkpoint inhibitor treatment by irAE status and baseline comorbidities
| Baseline characteristics | No irAE (n = 7,962) | Any irAE (n = 5,068) | p value a |
|---|---|---|---|
| Age, mean ± SD | 66.9 ± 10.6 | 67.1 ± 10.6 | .29 |
| Gender, n (%) | |||
| Male | 4,597 (57.7) | 2,913 (57.5) | .76 |
| Female | 3,365 (42.3) | 2,155 (42.5) | |
| Race/Ethnicity, n (%) | |||
| White | 6,246 (78.5) | 3,931 (77.6) | .68 |
| Black | 711 (8.9) | 478 (9.4) | |
| Hispanic/Latino | 343 (4.3) | 223 (4.4) | |
| Unknown/Other | 662 (8.3) | 436 (8.6) | |
| Admission Status, n (%) | |||
| Inpatient | 540 (6.8) | 360 (7.1) | .48 |
| Outpatient | 7,422 (93.2) | 4,708 (92.9) | |
| Primary payer, n (%) | |||
| Commercial | 1,964 (24.7) | 1,222 (24.1) | .68 |
| Medicare | 4,916 (61.7) | 3,168 (62.5) | |
| Medicaid | 802 (10.1) | 515 (10.2) | |
| Other payer | 280 (3.5) | 163 (3.2) | |
| Hospital setting, n (%) | |||
| Urban | 6,622 (83.2) | 4,201 (82.9) | .68 |
| Rural | 1,304 (16.8) | 867 (17.1) | |
| Teaching status, n (%) | |||
| Teaching | 3,739 (47.0) | 2,354 (46.5) | .57 |
| Nonteaching | 4,223 (53.0) | 2,714 (53.6) | |
| Baseline comorbidities, n (%) | |||
| Lung, hematologic, cardiovascular | |||
| Anemia | 2,156 (27.1) | 1,541 (30.4) | <.001 |
| Hypergammaglobulinemia | 13 (0.2) | 12 (0.2) | .35 |
| Idiopathic thrombocytopenic purpura | 10 (0.1) | 30 (0.6) | <.001 |
| Venous thromboembolism | 824 (10.4) | 708 (14.0) | <.001 |
| Sarcoidosis | 6 (0.1) | 5 (0.1) | .66 |
| Musculoskeletal/neurological | |||
| Guillain‐Barré syndrome | 2 (0.03) | 2 (0.04) | .65 |
| Lupus | 12 (0.2) | 16 (0.3) | .05 |
| Multiple sclerosis | 8 (0.1) | 10 (0.2) | .15 |
| Ankylosing spondylitis | 7 (0.1) | 7 (0.1) | .39 |
| Spondyloarthropathy | 126 (1.6) | 119 (2.4) | .002 |
| Psoriatic arthritis | 6 (0.1) | 2 (0.04) | .5 |
| Rheumatoid arthritis | 92 (1.2) | 72 (1.4) | .19 |
| Myasthenia gravis | 4 (0.1) | 1 (0.02) | .66 |
| Dermatologic | |||
| Psoriasis | 185 (2.3) | 186 (3.7) | <.001 |
| Vitiligo | 3 (0.04) | 5 (0.1) | .28 |
| Gastrointestinal | |||
| Celiac | 5 (0.1) | 4 (0.1) | .74 |
| Crohn's disease | 15 (0.2) | 13 (0.3) | .41 |
| Colitis | 169 (2.1) | 148 (2.9) | .004 |
| Endocrine | |||
| Diabetes | 994 (12.5) | 805 (15.9) | <.001 |
| Thyroid | 952 (12.0) | 709 (14.0) | .001 |
| Other | |||
| Multiple myeloma | 21 (0.3) | 11 (0.3) | .6 |
| Nephropathy | 2 (0.03) | 4 (0.1) | .22 |
| Organ or allogeneic stem cell transplant | 3 (0.04) | 6 (0.1) | .09 |
| Peripheral edema | 316 (4.0) | 505 (10.0) | <.001 |
| Solid organ transplant | 28 (0.4) | 15 (0.3) | .64 |
At the time of first ICI treatment during the study period (March 1, 2015, through December 31, 2017); any irAE is at least one irAE during the main study period.
p values are by χ2 or Fisher's exact test and are for comparisons between irAE vs. no irAE.
Abbreviation: irAE, immune‐related adverse event.
Patients experiencing any irAE had a higher average total number of immune‐related or immune‐compromised comorbid conditions in the previous 6 months compared with those without an irAE (0.27 ± 0.53 vs. 0.21 ± 0.47; p < .001; Table 1). Overall, the most frequent comorbid conditions were history of anemia, diabetes, thyroid disorders, and venous thromboembolism (all >10% in the overall sample). These comorbidities were also significantly more frequent among patients experiencing an irAE compared with those patients not experiencing an irAE during the study period, all p < .001 (Table 1). Additionally, previously documented idiopathic thrombocytopenic purpura (p < .001), spondyloarthropathy (p = .002), psoriasis (p < .001), colitis (p = .004), or peripheral edema (p < .001) were significantly more frequent among patients with irAEs (Table 1).
Rates of Selected irAEs
Among patients with irAEs, 57% had one irAE of any type, 26% had two, 11% had three, and 6% had more than four irAEs. The total number of irAEs ranged from one to eight and the average number of irAEs per patient was 1.67 ± 0.97. The percentage of patients experiencing irAEs was similar across the selected solid tumor types: 47% for MCC, 42% for multiple cancers, 39% for NSCLC, 40% for RCC, and 40% for UC, p = 0.23. A total of 10,121 irAEs at baseline were identified among patients who experienced at least one irAE (Table 2), of which 4,244 (42%) were identified in baseline irAE‐related inpatient visits and 5,877 (58%) were identified in baseline irAE‐related outpatient visits. The proportion of inpatient versus outpatient visits varied considerably across specific types of irAEs (Table 2).
Table 2.
Number of inpatient and outpatient immune checkpoint inhibitor treatment–associated irAEs by irAE type
| irAE type | Total events, n | Inpatient n (%) | Outpatient n (%) |
|---|---|---|---|
| Lung/hematologic/cardiovascular | |||
| Acquired hemophilia | 1 | 0 (0) | 1 (100) |
| Arrhythmia | 192 | 102 (53) | 90 (47) |
| Anemia | 1,717 | 936 (55) | 781 (45) |
| Hemolytic anemia | 13 | 4 (31) | 9 (69) |
| Impaired ventricular function with heart failure and vasculitis | 1,285 | 830 (65) | 455 (35) |
| Myocarditis | 4 | 4 (100) | 0 (0) |
| Pericarditis | 18 | 11 (61) | 7 (39) |
| Pneumonitis | 10 | 5 (50) | 5 (50) |
| Thrombocytopenia | 1,069 | 555 (52) | 514 (48) |
| Venous thromboembolism | 678 | 387 (57) | 291 (43) |
| Endocrine | |||
| Adrenal insufficiency | 325 | 42 (13) | 283 (87) |
| Diabetes | 87 | 27 (31) | 60 (69) |
| Pituitary | 180 | 128 (71) | 52 (29) |
| Thyroid | 1,568 | 366 (23) | 1,202 (77) |
| Musculoskeletal/neurological | |||
| Encephalitis | 10 | 8 (80) | 2 (20) |
| Guillain‐Barré syndrome | 2 | 1 (50) | 1 (50) |
| Inflammatory arthritis | 343 | 76 (22) | 267 (78) |
| Myositis | 13 | 8 (62) | 5 (38) |
| Peripheral or autonomic neuropathy | 890 | 245 (28) | 645 (72) |
| Polymyalgia‐like syndrome | 21 | 2 (10) | 19 (90) |
| Transverse myelitis | 5 | 5 (100) | 0 (0) |
| Gastrointestinal/kidney | |||
| Colitis | 384 | 223 (58) | 161 (42) |
| Hepatitis | 81 | 45 (56) | 36 (44) |
| Nephritis | 97 | 64 (66) | 33 (34) |
| Dermatologic | |||
| Stevens‐Johnson syndrome | 8 | 1 (13) | 7 (88) |
| Toxic epidermal necrolysis | 1 | 1 (100) | 0 (0) |
| Other | |||
| Blepharitis | 7 | 3 (43) | 4 (57) |
| Peripheral edema | 1,112 | 165 (15) | 947 (85) |
| Totals | 10,121 | 4,244 (42) | 5,877 (58) |
Abbreviation: irAE, immune‐related adverse event.
Twenty‐eight distinct irAEs from multiple organ systems were identified in the current study (Table 2). The selected ICI treatment–associated irAEs occurred with varying frequency. Across both baseline irAE‐related inpatient and outpatient visits, the most frequent (>1,000 events) irAEs were anemia, impaired ventricular function with heart failure and vasculitis, thrombocytopenia, thyroid conditions, and peripheral edema. The least frequent (<20 events) were acquired hemophilia, hemolytic anemia, myocarditis, pericarditis, pneumonitis, encephalitis, Guillain‐Barré syndrome, myositis, transverse myelitis, Stevens‐Johnson syndrome, toxic epidermal necrolysis, and blepharitis (Table 2).
Clinical Outcomes
Overall, subsequent irAE‐related inpatient admissions (23% vs. 14%), all‐cause in‐hospital mortality (22% vs. 6%), and lack of reinitiation of ICI treatment (31% vs. 71%) were significantly different (p < .001, all) for those with a baseline irAE‐related inpatient visit versus those with a baseline irAE‐related hospital‐based outpatient visit. Clinical outcomes associated with specific individual irAEs were summarized in Figs. 3, 4, and 5, showing that ICI reinitiation, in‐hospital mortality, and subsequent inpatient admission rate varied by irAE type and baseline irAE‐related visit (inpatient vs. outpatient). Patients experiencing myocarditis and toxic epidermal necrolysis were least likely to reinitiate ICI treatment (0%) and patients experiencing blepharitis and thyroid disorder were most likely to reinitiate ICI treatment (86% and 66%, respectively).
Figure 3.
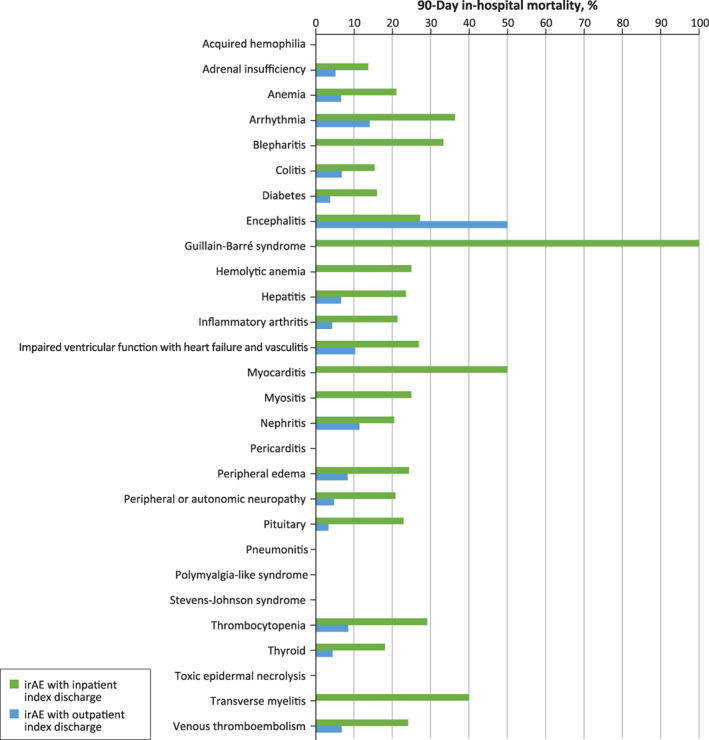
In‐hospital mortality during 90‐day follow‐up by type of baseline irAE‐related visit (inpatient vs. outpatient) and by irAE type. There was no mortality identified for patients with acquired hemophilia, pericarditis, polymylagia‐like syndrome, Stevens‐Johnson syndrome, or toxic epidermal necrolysis.Abbreviation: irAE, immune‐related adverse event.
Figure 4.
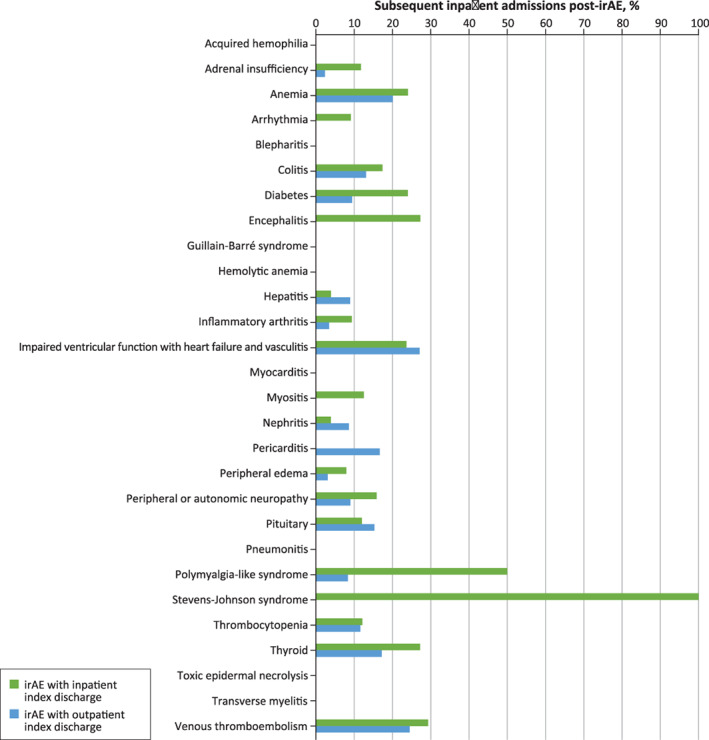
Percentage of subsequent inpatient admissions post–irAE‐related baseline inpatient and outpatient visits by irAE type. Subsequent inpatient admissions within 90 days post the baseline irAE‐related visit; note that there were no subsequent inpatient admissions for acquired hemophilia, blepharitis, Guillain‐Barré syndrome, hemolytic anemia, myocarditis, pneumonitis, toxic epidermal necrolysis, or transverse mylelitis.Abbreviation: irAE, immune‐related adverse event.
Figure 5.
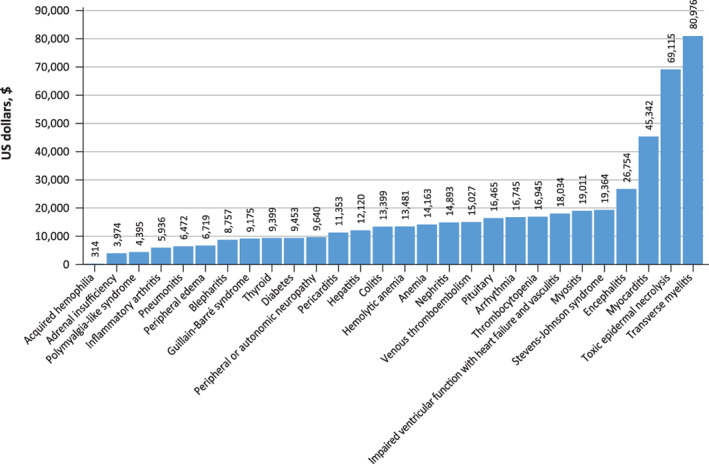
Average total costs by immune‐related adverse event type.
Cost and Health Care Resource Utilization Outcomes
During the 90‐day follow‐up period post‐irAE and inclusive of the baseline irAE‐related visit, the overall mean ± SD cost was $29,477 ± $48,087 for patients with an inpatient baseline irAE‐related visit and $5,718 ± $13,720 for patients with a baseline irAE‐related outpatient visit, p < .001. The mean ± SD overall readmission LOS, exclusive of baseline irAE‐related visit, during the follow‐up period was 12.6 ± 17.1 days for patients with baseline irAE‐related inpatient visit and 7.8 ± 7.0 days for those with baseline irAE‐related outpatient visit, p < .001. When mean costs and LOS were calculated by individual type of irAEs (supplemental online Fig. 1), the highest mean ± SD costs were for transverse myelitis ($80,976 ± $95,282), toxic epidermal necrolysis ($69,115 ± $0), and myocarditis ($45,341 ± $25,895), whereas the lowest were for acquired hemophilia ($314 ± $0), adrenal insufficiency ($3,319 ± $9,012), and polymyalgia‐like syndrome ($4,395 ± $6,025; Fig. 5).
During the 180‐day follow‐up from the time of the first ICI treatment during the main study period, the average ± SD total cost for patients who experienced an irAE during the study period was $57,366 ± $60,064 versus $46,921 ± $83,136 for those with no irAE, p < .001. For LOS, the mean ± SD was 9.7 ± 9.2 days among patients who experienced an irAE during the study period compared with 7.2 ± 7.4 days for those with no irAE, p < .001.
Discussion
This retrospective observational real‐world evidence study demonstrated that across the solid tumor types of interest, approximately 40% of patients treated with ICIs experienced 1 or more of the 28 irAEs selected pre‐hoc based on ASCO guidelines. Patients who developed irAEs had higher comorbidity burdens during the 6 months preceding ICI treatment. Among patients experiencing an irAE, the majority of irAEs (58%) were treated in the hospital‐based outpatient setting. Although overall fewer patients had baseline irAE‐related inpatient visits, these patients had significantly higher clinical and economic burdens during the 90‐day follow‐up period compared with those with baseline irAE‐related outpatient visits. Greater subsequent inpatient admissions and higher in‐hospital mortality during the follow‐up period resulted in significantly higher costs and longer hospital stays for patients with baseline irAE‐related inpatient visits. A lower percentage of patients with baseline irAE‐related inpatient visits were able to resume ICI treatment than those with baseline irAE‐related outpatient visits.
This study used real‐world data to reflect current clinical practice and used ICD codes to identify 28 ASCO‐defined irAEs associated with use of PD‐1 and PD‐L1 inhibitors for treatment of MCC, RCC, UC, and NSCLC. irAE frequency across the selected solid tumor types was similar; the most common irAEs were anemia, impaired ventricular function with heart failure and vasculitis, thrombocytopenia, thyroid conditions, and peripheral edema. In a 2019 systematic review and meta‐analysis of 125 clinical trials involving 20,128 patients that assessed 75 treatment‐related irAEs, 66% of patients experienced at least one irAE [18]. In that meta‐analysis, fatigue, pruritus, and diarrhea were the most frequently reported overall, with the most common endocrine irAEs being hypothyroidism and hyperthyroidism [18]. Other studies have reported percentages of patients experiencing ICI‐associated irAEs ranging from 30% [19, 20] to upward of 80% [3]. The wide‐ranging percentages of patients experiencing an irAE in these studies most likely reflects the methods for determining irAEs (ICD codes vs. clinical criteria), study designs (retrospective observational vs. clinical trials), and data sources (hospital‐based discharge data vs. trial data). To put this finding in context of existing treatment options, a systematic review and meta‐analysis of 12,727 patients with advanced solid organ malignancies from randomized clinical trials found that patients receiving ICIs are less likely to develop severe AEs than those receiving chemotherapy and have fewer discontinuations and deaths due to AEs [4].
The role of pre‐existing comorbidities on irAEs was assessed by determining the CCI at the time of the first ICI treatment in the main study period. Patients who developed irAEs compared with those who did not have similar demographics but higher CCI scores. The current study also considered immune‐related and immunocompromised comorbidities and other comorbid conditions in the 6 months preceding the first ICI treatment. Notably, in this real‐world evidence study, patients with autoimmune or immunocompromised conditions received ICI treatments. This differs from clinical trials, from which these patients have largely been excluded [14], although several studies suggest that patients with underlying autoimmune disease can be successfully treated with ICIs if patients are closely monitored and managed [21, 22, 23, 24]. Evidence on the successful use of ICI treatment in patients with immunocompromised conditions is lacking, with one study suggesting possibly increased graft risk among patients who had solid organ transplants [25]. Patients in the current study who experienced an irAE had a somewhat higher average number of immune‐related or immune‐compromised comorbid conditions in the previous 6 months compared with those without an irAE. Although some of these differences were statistically significant, it is unknown whether the differences are of clinical importance.
This study also assessed several clinical outcomes during a 90‐day follow‐up period post–irAE‐related baseline visit. With the 90‐day follow‐up [26], patients with baseline irAE‐related inpatient visits are presumed to have more severe irAEs that could not be treated in the outpatient setting. The study did find that these patients had a higher proportion of in‐hospital mortality and subsequent inpatient admissions during follow‐up. Available evidence on subsequent inpatient admission or in‐hospital mortality among patients who have experienced irAEs is lacking, and this study provides some metrics by both irAE type and type of baseline irAE‐related visits (inpatient vs. outpatient).
A significantly lower percentage of patients with an inpatient baseline irAE‐related visit reinitiated on ICI therapy. Among 49 patients with NSCLC who experienced a serious irAE in a small observational study, approximately 57% did not reinitiate ICI treatment [27]. In a small cohort study conducted in France of 93 patients with various solid tumor types, who were treated with ICIs and experienced an irAE, also 57% of patients did not reinitiate ICI treatment [28]. In another study of 499 patients with renal cell carcinoma receiving ICIs, 80 patients experienced an irAE, and of these, 55% permanently discontinued ICI treatment [29]. Our findings are consistent with these published studies. In the current study, among n = 5,068 patients experiencing an irAE, 46% did not reinitiate ICI treatment within 90 days post‐irAE; among patients with a baseline irAE‐related inpatient visit, 71% did not reinitiate ICIs, whereas among those with a baseline irAE‐related outpatient visit, 31% did not reinitiate ICI therapy. Not surprisingly, if the initial irAE didn't result in hospitalization, which was indicative of a milder irAE, patients were more likely to reinitiate ICI treatment.
Costs and HRU related to the management of irAEs were also a focus of this study. In the current study, patients treated with ICIs who did not develop irAEs had significantly lower costs and HRU than those developing irAEs that required treatment. Studies previously published examining the costs related to the management of irAEs have been conducted in France [30] and Italy [31], and thus are not comparable to the current study conducted in the U.S. owing to differing health care systems. Compared with chemotherapy, a U.S. real‐world study found that ICI monotherapy resulted in fewer AE‐related visits among patients with metastatic NSCLC receiving first‐line treatment [5].
Considering the clinical and cost sequences associated with irAEs, it is important to closely monitor patients in the real‐world clinical setting for irAEs to achieve better clinical outcomes. Previous meta‐analyses reported that the risk of irAEs appears lower with anti–PD‐L1 versus anti–PD‐1 antibodies [18, 32]. A more recent meta‐analysis reported a lower risk of overall any‐grade irAEs with atezolizumab and avelumab versus pembrolizumab, and a higher risk of any‐grade irAEs with durvalumab versus other agents [33]. To assess ICI value, clinical efficacy measures, safety outcomes, and reported patient outcomes all require consideration [15, 34, 35].
Although strengths and limitations of this study are mostly related to study design and use of real‐world data, there is a recognized need for real‐world evidence to assess cancer treatment efficacy, tolerability, and adverse events [36]. Although the PHD is not a random sample and may not be generalizable to the U.S. patient population, availability of patient, discharge, and hospital‐level data allowed for the retrospective design and longitudinal collection of irAEs, costs, and health care resource utilization in a real‐world setting among patients receiving ICI treatments across several tumor types. The PHD also encompasses approximately 25% of all hospital discharges in the U.S. One limitation of our study is the potential underreporting of patients requiring hospital admissions and patients reinitiating ICI treatment in the follow‐up period if they did not return to the same health system for subsequent treatment. For infrequently occurring irAEs, costs and health care resource utilization may not be reflective or generalizable to a larger population of patients with these particular irAEs.
Conclusion
The current study used the largest hospital discharge database in the U.S. to address the gap of knowledge identified by ASCO for the management and outcomes of selected irAEs. Findings suggest that patients with baseline irAE‐related inpatient visits have poorer outcomes and higher economic burden during a subsequent 90‐day follow‐up period and often do not resume ICI treatment. This study also adds important information regarding real‐world treatment of patients with autoimmune diseases or immune‐compromised conditions with ICIs, as well as information on ICI treatment reinitiation among patients who have experienced an irAE. Future studies should evaluate differences in irAEs between different ICIs to enable patient‐centered treatment choices as well as the impact of ICIs on irAEs in additional tumors. Future work will also include a more thorough accounting for pre‐existing comorbid conditions and medication use when determining the occurrence of irAEs. Successful use of ICIs in the future will depend on the early recognition and effective management of irAEs.
Author Contributions
Conception/design: Ying Zheng, Ruth Kim, Ting Yu, Jill Dreyfus, Julie A. Gayle, Christina L. Wassel, Hemant Phatak, Saby George
Provision of study material or patients: Ying Zheng, Ruth Kim, Ting Yu, Jill Dreyfus, Julie A. Gayle, Christina L. Wassel, Hemant Phatak, Saby George
Collection and/or assembly of data: Julie A. Gayle, Christina L. Wassel
Data analysis and interpretation: Ying Zheng, Ruth Kim, Ting Yu, Jill Dreyfus, Julie A. Gayle, Christina L. Wassel, Hemant Phatak, Saby George
Manuscript writing: Ying Zheng, Ruth Kim, Ting Yu, Jill Dreyfus, Julie A. Gayle, Christina L. Wassel, Hemant Phatak, Saby George
Final approval of manuscript: Ying Zheng, Ruth Kim, Ting Yu, Jill Dreyfus, Julie A. Gayle, Christina L. Wassel, Hemant Phatak, Saby George
Disclosures
Ying Zheng: EMD Serono (E); Ruth Kim: Pfizer (E), Exelixis (H), Bristol‐Myers Squibb (OI); Jill Dreyfus: Premier, Avalere (E); Hemant Phatak: EMD Serono (E); Saby George: Bristol‐Myers Squibb, Bayer, Corvus, Genentech, Exelixis, Sanofi, EMD Serono, Seattle Genetics, Pfizer, Eisai, Merck & Co. (C/A), Bristol‐Myers Squibb, Bayer, Eisai, Pfizer, Seattle Genetics/Astellas, Agensys, Immunomedics, Novartis, Merck, Calithera, Corvus (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1 Mean Length of Stay for Follow‐up Hospitalizations by Type of Baseline irAE‐Related Visit (inpatient vs. outpatient) and and by irAE Type.
Acknowledgments
The authors thank Frank Liu and Stan Krulewicz for their feedback to the study and the manuscript. The authors would also like to acknowledge Dr. Ning Rosenthal at Premier Inc. for providing expert input on revising the manuscript. This study was funded by EMD Serono (CrossRef Funder ID: 10.13039/100004755), as part of an alliance between the healthcare business of Merck KGaA, Darmstadt, Germany, and Pfizer.
The current affiliations of Ying Zheng, Hemant Phatak, and Ting Yu are Sanofi, Cambridge, MA, USA, Acceleron Pharma, Cambridge, MA, USA, and AstraZeneca Hematology, South San Francisco, CA, USA, respectively.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Ventola CL. Cancer immunotherapy, part 2: Efficacy, safety, and other clinical considerations. P T 2017;42:452–463. [PMC free article] [PubMed] [Google Scholar]
- 2. Patnaik A, Kang SP, Rasco D et al. Phase I study of pembrolizumab (MK‐3475; Anti‐PD‐1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015;21:4286–4293. [DOI] [PubMed] [Google Scholar]
- 3. Eun Y, Kim IY, Sun JM et al. Risk factors for immune‐related adverse events associated with anti‐PD‐1 pembrolizumab. Sci Rep 2019;9:14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magee DE, Hird AE, Klaassen Z et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: A systematic review and meta‐analysis of randomized clinical trials. Ann Oncol 2020;31:50–60. [DOI] [PubMed] [Google Scholar]
- 5. Engel‐Nitz N, Ryan K, Johnson MP et al. Health care utilization associated with adverse events (AEs) among metastatic non‐small cell lung cancer (mNSCLC) patients treated with immunotherapy or chemotherapy. J Clin Oncol 2019;37(suppl 15):e20655. [Google Scholar]
- 6. D'Angelo SP, Russell J, Lebbe C et al. Efficacy and safety of first‐line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: A preplanned interim analysis of a clinical trial. JAMA Oncol 2018;4:e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaufman HL, Russell J, Hamid O et al. Avelumab in patients with chemotherapy‐refractory metastatic Merkel cell carcinoma: A multicentre, single‐group, open‐label, phase 2 trial. Lancet Oncol 2016;17:1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aguiar PN, Jr , De Mello RA, Barreto CMN et al. Immune checkpoint inhibitors for advanced non‐small cell lung cancer: Emerging sequencing for new treatment targets. ESMO Open 2017;2:e000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atkins MB, Clark JI, Quinn DI. Immune checkpoint inhibitors in advanced renal cell carcinoma: Experience to date and future directions. Ann Oncol 2017;28:1484–1494. [DOI] [PubMed] [Google Scholar]
- 10. Massari F, Di Nunno V, Cubelli M et al. Immune checkpoint inhibitors for metastatic bladder cancer. Cancer Treat Rev 2018;64:11–20. [DOI] [PubMed] [Google Scholar]
- 11. Akamatsu H, Murakami E, Oyanagi J et al. Immune‐related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non‐small cell lung cancer. The Oncologist 2020;25:e679–e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nghiem PT, Bhatia S, Lipson EJ et al. PD‐1 blockade with pembrolizumab in advanced merkel‐cell carcinoma. N Engl J Med 2016;374:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kartolo A, Sattar J, Sahai V et al. Predictors of immunotherapy‐induced immune‐related adverse events. Curr Oncol 2018;25:e403–e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelly ZR, Davar D. The financial and physical toxicity of immune checkpoint inhibitors in cancer. ASCO Daily News September 11, 2019. Available at https://dailynews.ascopubs.org/do/10.1200/ADN.19.190405/full/. Accessed August 09, 2021. [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Zhou S, Yang F et al. Treatment‐related adverse events of PD‐1 and PD‐L1 inhibitors in clinical trials: A systematic review and meta‐analysis. JAMA Oncol 2019;5:1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Das S, Johnson DB. Immune‐related adverse events and anti‐tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Connolly C, Bambhania K, Naidoo J. Immune‐related adverse events: A case‐based approach. Front Oncol 2019;9:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdel‐Wahab N, Shah M, Lopez‐Olivo MA et al. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease. Ann Intern Med 2018;169:133–134. [DOI] [PubMed] [Google Scholar]
- 22. La‐Beck NM, Jean GW, Huynh C et al. Immune checkpoint inhibitors: New insights and current place in cancer therapy. Pharmacotherapy 2015;35:963–976. [DOI] [PubMed] [Google Scholar]
- 23. Haanen J, Ernstoff MS, Wang Y et al. Autoimmune diseases and immune‐checkpoint inhibitors for cancer therapy: Review of the literature and personalized risk‐based prevention strategy. Ann Oncol 2020;31:724–744. [DOI] [PubMed] [Google Scholar]
- 24. Martins F, Sofiya L, Sykiotis GP et al. Adverse effects of immune‐checkpoint inhibitors: Epidemiology, management and surveillance. Nat Rev Clin Oncol 2019;16:563–580. [DOI] [PubMed] [Google Scholar]
- 25. Babey H, Quere G, Descourt R et al. Immune‐checkpoint inhibitors to treat cancers in specific immunocompromised populations: A critical review. Expert Rev Anticancer Ther 2018;18:981–989. [DOI] [PubMed] [Google Scholar]
- 26. Owen DH, Wei L, Bertino EM et al. Incidence, risk factors, and effect on survival of immune‐related adverse events in patients with non‐small‐cell lung cancer. Clin Lung Cancer 2018;19:e893–e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mouri A, Kaira K, Yamaguchi O et al. Clinical difference between discontinuation and retreatment with nivolumab after immune‐related adverse events in patients with lung cancer. Cancer Chemother Pharmacol 2019;84:873–880. [DOI] [PubMed] [Google Scholar]
- 28. Simonaggio A, Michot JM, Voisin AL et al. Evaluation of readministration of immune checkpoint inhibitors after immune‐related adverse events in patients with cancer. JAMA Oncol 2019;5:1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abou Alaiwi S, Xie W, Nassar AH et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune‐related adverse events in metastatic renal cell carcinoma. J Immunother Cancer 2020;8:e000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chouaid C, Loirat D, Clay E et al. Cost analysis of adverse events associated with non‐small cell lung cancer management in France. Clinicoecon Outcomes Res 2017;9:443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mennini FS, Bini C, Marcellusi A et al. Cost estimate of immune‐related adverse reactions associated with innovative treatments of metastatic melanoma. Clin Drug Investig 2018;38:967–976. [DOI] [PubMed] [Google Scholar]
- 32. Pillai RN, Behera M, Owonikoko TK et al. Comparison of the toxicity profile of PD‐1 versus PD‐L1 inhibitors in non‐small cell lung cancer: A systematic analysis of the literature. Cancer 2018;124:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sonpavde GP, Grivas P, Lin Y et al. Immune‐related adverse events (irAEs) with single agent PD‐1 vs PD‐L1 inhibitors: A meta‐analysis of 8,730 patients from clinical trials. Ann Oncol 2019;30(suppl 5):Abstract 475. [Google Scholar]
- 34. Kaufman HL, Atkins MB, Dicker AP et al. The value of cancer immunotherapy summit at the 2016 Society for Immunotherapy of Cancer 31st Anniversary annual meeting. J Immunother Cancer 2017;5:Abstract 38. [Google Scholar]
- 35. Verma V, Sprave T, Haque W et al. A systematic review of the cost and cost‐effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer 2018;6:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Maio M, Perrone F, Conte P. Real‐world evidence in oncology: Opportunities and limitations. The Oncologist 2020;25:e746–e752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1 Mean Length of Stay for Follow‐up Hospitalizations by Type of Baseline irAE‐Related Visit (inpatient vs. outpatient) and and by irAE Type.


