Abstract
Isatuximab is a monoclonal antibody that binds to the human CD38 antigen. On May 30, 2020, a marketing authorization valid through the European Union (EU) was issued for isatuximab in combination with pomalidomide and dexamethasone (IsaPd) for the treatment of adult patients with relapsed and refractory (RR) multiple myeloma (MM). The recommended dose of isatuximab was 10 mg/kg, administered intravenously weekly at cycle 1 and then biweekly in subsequent 28‐day cycles. Isatuximab was evaluated in a phase III, open‐label, multicenter, randomized trial that randomly allocated IsaPd versus pomalidomide plus dexamethasone (Pd) to adult patients with RR MM. The primary endpoint of the trial was progression‐free survival, as assessed by an independent review committee, which was superior for the IsaPd arm (hazard ratio, 0.596; 95% confidence interval, 0.436–0.814; p = .001) compared with the Pd arm. Treatment with IsaPd led to higher incidences of treatment‐related adverse events (AEs), grade ≥ 3 AEs, and serious AEs compared with Pd treatment. Most frequently observed AEs that occurred more often in the IsaPd arm were infusion‐related reactions, infections, respiratory AEs, neutropenia (including neutropenic complications), and thrombocytopenia. The aim of this article is to summarize the scientific review of the application leading to regulatory approval in the EU.
Implications for Practice
Isatuximab was approved in the European Union, in combination with pomalidomide and dexamethasone, for the treatment of patients with multiple myeloma who have already received therapy but whose disease did not respond or relapsed afterward. The addition of isatuximab resulted in a clinically meaningful and significant prolongation of the time from treatment initiation to further disease relapse or patient's death. The safety profile was considered acceptable, and the benefit‐risk ratio was determined to be positive.
Keywords: Multiple myeloma, Isatuximab, Pomalidomide, Dexamethasone, EMA • Anti‐CD38 antibody
Short abstract
This article summarizes the scientific review of the application leading to EMA regulatory approval of isatuximab combination therapy for multiple myeloma.
Introduction
Multiple myeloma (MM) is a hematological malignancy resulting from the uncontrolled proliferation of monoclonal plasma cells, leading to the production of a monoclonal immunoglobulin, immune suppression, and end‐organ damage. The incidence of MM in Europe is 6.0 cases per 100,000 inhabitants per year, with a median age at diagnosis between 65 and 70 years and age‐standardized 5‐year relative survival around 40% [1]. Patients with MM may experience a variety of disease‐related events and symptoms, including renal failure, fatigue, bone pain and fractures, hypercalcemia, and recurrent infections. A deterioration in quality of life is particularly marked in elderly/frail patients, who represent approximately 30% of patients with MM [2]. Prognostic factors are serum β2‐microglobulin, albumin, C‐reactive protein, lactate dehydrogenase, and cytogenetic alterations such as t(4;14), deletion(17p), t(14;16), and chromosome 1 abnormalities [3].
Treatment is recommended in patients with symptomatic disease and typically contains one proteasome inhibitor (PI) and/or immunomodulatory drug (IMiD) plus dexamethasone, followed by autologous stem cell transplantation in younger eligible patients [3]. Patients with relapsed and/or refractory (RR) disease typically receive salvage therapy, generally followed by consolidation or maintenance therapy until progression or intolerance, and then proceed to the next option. In this setting, PI‐ and IMiD‐based regimens are commonly used in combination with corticosteroids, but the landscape is changing since the approval of novel classes of agents, specifically the histone deacetylase inhibitor panobinostat [4] and two monoclonal antibodies (mAbs) against the cell surface antigens CD38 (daratumumab) and SLAMF7 (elotuzumab) [5, 6, 7]. Furthermore, the addition of the second‐generation IMiDs, lenalidomide [8] and pomalidomide [9], and the second‐generation PIs carfilzomib [10] and ixazomib [11] provides additional within‐class treatment options for patients with RR MM. With the advent of newer therapies, the median overall survival (OS) of patients with MM has improved and is currently around 45–60 months from diagnosis [12].
On April 30, 2019, the Sanofi‐Aventis Groupe (Paris, France) applied for a marketing authorization via the European Medicines Agency (EMA) centralized procedure for isatuximab (trade name Sarclisa). Isatuximab had been designated an orphan medicine by the European Commission in April 2014. To qualify for orphan designation, a medicine must be intended for the treatment, prevention, or diagnosis of a life‐threatening or chronically debilitating disease; the prevalence of the condition in the European Union (EU) must not be more than 5 in 10,000; and the medicine must be of significant benefit to those affected by the condition. On April 23, 2020, the marketing authorization holder requested the withdrawal of the orphan designation.
The review of the benefit‐risk balance was conducted by the Committee for Medicinal Products for Human Use (CHMP), and the positive opinion was issued on March 26, 2020. The indication approved in the EU is as follows: “Sarclisa in combination with pomalidomide and dexamethasone is indicated for the treatment of adult patients with relapsed and refractory multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor and have demonstrated disease progression on the last therapy.” The aim of this article is to summarize the scientific review of the application leading to the regulatory approval of isatuximab in the EU.
Nonclinical Aspects and Clinical Pharmacology
Isatuximab is an mAb that binds to the human cell surface antigen molecule classified as cluster of differentiation 38 (CD38), which is strongly expressed by MM tumor cells. Isatuximab acts through antibody‐dependent cell‐mediated cytotoxicity (ADCC), antibody‐dependent cellular phagocytosis, and complement‐dependent cytotoxicity. Isatuximab can also trigger tumor cell death by induction of apoptosis via an Fc‐independent mechanism, and it exhibits multiple tumor targeting and immunomodulatory effects that may contribute to tumor growth inhibition [13, 14]. The combination of isatuximab and pomalidomide was supported by several nonclinical experiments. In vitro, the combination enhanced the cell lysis of CD38‐expressing MM cells by ADCC and direct tumor cell killing compared with isatuximab alone. In vivo experiments using an MM xenograft model demonstrated that the combination of isatuximab and pomalidomide resulted in enhanced antitumor activity compared with isatuximab or pomalidomide alone [15].
From sequence comparisons of the mAbs and the CD38 sequence, it was concluded that isatuximab binds to a different CD38 epitope compared with daratumumab, the other anti‐CD38 mAb approved in the EU [13].
Biweekly intravenous administration of isatuximab to mice or once weekly intravenous administration to nonhuman primates resulted in dose‐dependent accumulation of isatuximab in line with its half‐life. Using the population pharmacokinetic modeling approach, a plasma concentration threshold of 128.8 μg/mL was needed for tumor eradication in mice, which corresponded to 10–20 mg/kg in humans.
Trial Design
The submission was based on the phase III trial EFC14335/ICARIA, a randomized, open‐label, multicenter study comparing isatuximab plus pomalidomide and dexamethasone (IsaPd) with pomalidomide and dexamethasone alone (Pd) in adult patients with RR MM [16]. The appropriate isatuximab dose was set at 10 mg/kg in two dose‐finding studies as a single agent [17, 18] and a phase Ib study of isatuximab in combination with pomalidomide and dexamethasone [19].
In trial EFC14335/ICARIA, patients had to have received at least two prior lines of therapy, including lenalidomide and a PI (bortezomib, carfilzomib, or ixazomib), and had to have progressed to the last therapy. Patients could have received other anti‐CD38 mAbs, but they could not be refractory to them. In contrast, prior therapy with pomalidomide therapy was not allowed.
Treatment was administered in 28‐day cycles. In the IsaPd arm, dexamethasone 40 mg (or 20 mg in patients older than 75 years) was administered orally or intravenously on days 1, 8, 15, and 22; isatuximab 10 mg/kg was administered intravenously on the same days at cycle 1 and then on days 1 and 15 for subsequent cycles; and pomalidomide 4 mg was administered orally on days 1 to 21. In the Pd arm, patients received dexamethasone and pomalidomide in the same manner as patients allocated to the IsaPd arm. If a patient were clinically stable, he or she could remain on treatment until progressive disease or unacceptable toxicity.
The primary endpoint was progression‐free survival (PFS) as assessed by an independent review committee (IRC). Key secondary endpoints were objective response rate (ORR) and OS. Other secondary endpoints were time to progression (TTP), time to next treatment (TTNT), PFS in high‐risk cytogenetic population, duration of response, safety, pharmacokinetics, immunogenicity, and health‐related quality of life. A sample size of 300 patients was planned, assuming a median PFS of 4.0 months for the control arm and a 40% risk reduction in hazard ratio (HR) in patients allocated to the IsaPd arm. The study was also powered to detect a statistically significant difference in OS.
Clinical Efficacy
The study randomized 307 subjects: 154 to the IsaPd arm and 153 to the Pd arm (intention‐to‐treat population). Six of these patients did not receive study drug: four in the Pd arm and two in the IsaPd arm. Reasons for not receiving therapy were adverse events (AEs) in three patients, progressive disease in one patient, consent withdrawal in one patient, and one woman unwilling to be tested for pregnancy. The 301 patients who received the study drug comprised the safety population: 152 in the IsaPd arm and 149 in the Pd arm. Patients’ baseline characteristics were reasonably well balanced across both arms except for high‐risk cytogenetics, which were more frequent in the Pd arm (15.6% vs. 23.5%, respectively).
With a median follow‐up of 11.6 months (cutoff date: October 11, 2018), the primary endpoint (IRC‐assessed PFS) showed an HR of 0.596 (95% confidence interval [CI], 0.436–0.814; p = .001) in favor of the IsaPd arm. Moreover, the median IRC‐confirmed PFS was 11.53 months (95% CI, 8.94–13.9) for the IsaPd arm and 6.47 months (95% CI, 4.47–8.28) for the Pd arm (Fig. 1; Table 1). Sensitivity analyses consistently favored the IsaPd arm, showing HRs ranging from 0.568 to 0.602, including the CHMP‐recommended PFS analysis (not censoring for subsequent therapy) and investigator‐assessed PFS analysis.
Figure 1.
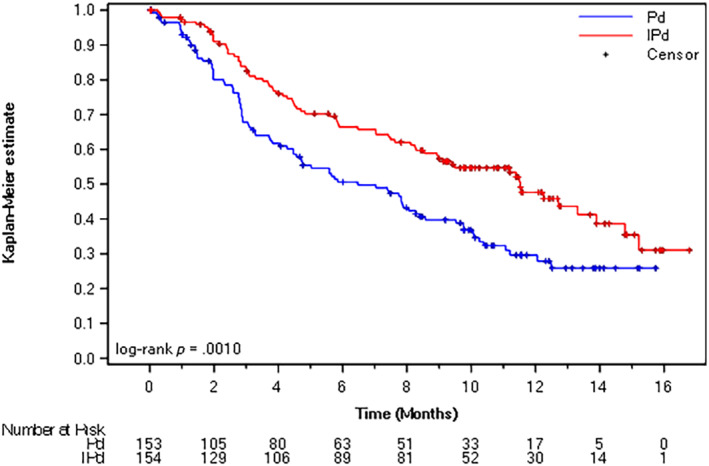
Kaplan‐Meier plots for progression‐free survival by independent review committee assessment (primary endpoint, cutoff date October 11, 2018).
Abbreviations: IPd, isatuximab, pomalidomide, and dexamethasone; Pd, pomalidomide and dexamethasone.
Table 1.
Key favorable and unfavorable results of isatuximab therapy in combination with pomalidomide and dexamethasone for adult patients with relapsed/refractory multiple myeloma (ICARIA study, cutoff date: October 11, 2018, for efficacy analysis and November 22, 2018, for safety analysis)
| Effect | Experimental (IsaPd, n = 154) | Control (Pd, n = 153) | Uncertainties, strength of evidence |
|---|---|---|---|
| Favorable effects | |||
| PFS by IRC, months | 11.53 | 6.47 |
Supported by sensitivity analyses Not yet supported by mature OS data |
| HR, 0.596; 95% CI, 0.44–0.81; p = .001 | |||
| ORR by IRC, % | 60.4 | 35.3 | |
| OS, % at 12 months | 72 | 63.3 |
Not mature yet Mature data probably available in 2021 |
| HR, 0.687; 95% CI, 0.46–1.02; p = .0631 | |||
| Unfavorable effects, % | |||
| AEs, overall (treatment‐related) | 99.3 (90.8) | 98.0 (79.9) | |
| Grade ≥3 AEs, overall (treatment‐related) | 86.8 (71.7) | 70.5 (47.7) | |
| SAEs, overall (treatment‐related) | 61.8 (35.5) | 53.7 (16.1) | |
| IRRs, overall (grade ≥3) | 38.2 (2.6) | 0 (0) | |
| Infections and infestations, overall (grade ≥3) | 80.9 (42.8) | 64.4 (30.2) | |
| SPMs, overall | 3.9 | 0.7 | |
| Neutropenia, grade ≥3 (neutropenic complications) | 84.9 (30.3) | 70.1 (20.1) | |
| Thrombocytopenia, grade ≥3 (bleeding) | 30.9 (8.6) | 24.5 (11.4) | |
Abbreviations: AE, adverse event; CI, confidence interval; HR, hazard ratio; IRC, independent review committee; IRR, infusion‐related reaction; IsaPd, isatuximab, pomalidomide, and dexamethasone; ORR, objective response rate; OS, overall survival; Pd, pomalidomide and dexamethasone; PFS, progression‐free survival; SAE, severe adverse event; SPM, secondary primary malignancy.
Regarding key secondary endpoints, the ORR was 60.4% (95% CI, 52.2%–68.2%) versus 35.3% (95% CI, 27.7%–43.4%) for the IsaPd and Pd arms, respectively. At the time of the primary analysis for PFS, the OS data were still immature, but there was a trend favoring the IsaPd arm with an HR of 0.687 (95% CI, 0.46–1.02; p = .0631).
The other secondary endpoints TTP (median 12.71 months [95% CI, 11.20–15.21] for the IsaPd arm vs. 7.75 months [95% CI, 5.03–9.76] for the Pd arm) and TTNT (median not reached [95% CI, 12.12–not reached] for the IsaPd arm vs. 9.10 months [95% CI, 6.37–12.26] for the Pd arm) supported the primary endpoint.
Clinical Safety
The safety population included 152 patients and 149 patients in the IsaPd and Pd groups, respectively. Overall treatment exposure was greater (median 41 vs. 24 weeks) for patients allocated to the IsaPd versus Pd arms, respectively. The median relative dose intensity was 92.28%, 85.14%, and 87.76% for isatuximab, pomalidomide, and dexamethasone, respectively, in the IsaPd group and 93.33% and 96.32% for pomalidomide and dexamethasone, respectively, in the Pd group. The incidence of treatment‐emergent AEs (TEAEs) was higher in the IsaPd group when considering treatment‐related TEAEs (90.8% vs. 79.9%, respectively), grade ≥ 3 TEAEs (86.8% vs. 70.5%), and serious TEAEs (61.8% vs. 53.7%) (Table 1). The incidence of TEAEs with a fatal outcome during the treatment period was 7.2% versus 8.7%, respectively, and of TEAEs leading to definitive treatment discontinuation was 7.2% versus 12.8%.
The incidences of treatment‐related TEAEs that were ≥ 5% greater in the IsaPd arm included neutropenia (42.8% vs. 32.2%), infusion‐related reactions (IRRs, 36.2% vs. 0.0%), upper respiratory tract infection (9.9% vs. 4.0%), febrile neutropenia (10.5% vs. 2.0%), and bronchitis (8.6% vs. 2.0%). The treatment‐related grade ≥ 3 TEAEs with an incidence ≥5% greater in the IsaPd arm were neutropenia (42.1% vs. 30.9%) and febrile neutropenia (10.5% vs. 2.0%). There were no treatment‐related TEAEs with an incidence ≥5% greater in the Pd group compared with the IsaPd group.
Regarding AEs of special interest (AESIs), IRRs occurred in 38.2% of patients from the IsaPd arm. These were mostly grade 2 and led to discontinuation of isatuximab in 2.6% of patients. Second primary malignancies were reported in one patient in the Pd arm (skin squamous cell carcinoma [SCC]) and in six patients in the IsaPd arm: four cases of skin SCC, one of angiosarcoma, and one of myelodysplastic syndrome. The incidence of lower respiratory AEs was 36.8% versus 25.5% in the IsaPd versus Pd arms (grade ≥ 3 7.9% vs. 3.4%). AEs contributing the most to this imbalance were dyspnea and productive cough. The incidence of all grades (grade ≥ 3) respiratory infections was 74.3% versus 53.0% (36.2% vs. 24.2%) in the IsaPd versus Pd arms, respectively. Table 1 also displays AESI rates related to the total population of patients exposed to isatuximab at different dose levels, either as a single agent or in combination (n = 576).
Benefit‐Risk Assessment
The proposed target population of isatuximab has a very advanced stage of disease (third‐line setting and beyond), a setting in which pomalidomide, daratumumab, and panobinostat are approved. Since definitive cure is not available, a significant delay in disease progression associated with acceptable toxicity would represent a benefit for these patients.
The primary endpoint (PFS as per IRC) was reached, with a significant and clinically relevant improvement for IsaPd compared with Pd. The PFS subgroup (including the high‐risk cytogenetic population) and sensitivity analyses generally supported the primary analysis. Some secondary endpoints (ORR and TTP) also supported the primary endpoint. Immature results in OS revealed a trend in favor of the IsaPd arm, which should be interpreted in the context of subsequent therapy. The final OS analysis, including subgroup analyses by refractoriness to lenalidomide, PI inhibitors, or both, will be submitted in line with CHMP recommendations.
Since daratumumab is already approved for patients with previously untreated and RR MM, the applicant was asked about the potential use of isatuximab in patients who are refractory to daratumumab. The applicant provided preliminary results from studies TCD14079 and TED14154 suggesting that isatuximab had some activity in this patient population, but the interpretation was difficult because of low patient numbers, patient heterogeneity, and single‐arm design. The applicant was also asked to add the following sentence to the Summary of Product Characteristics: “Insufficient data is available to conclude on the efficacy of isatuximab in patients previously treated with daratumumab.”
Compared with Pd, drug exposure to IsaPd was higher, but the relative dose intensity for each separate drug lower, because more dose adjustments were needed. Treatment with IsaPd led to more treatment‐related AEs, grade ≥ 3 AEs, and severe AEs compared with Pd treatment. The type of AEs was not unexpected for a Pd backbone combined with an anti‐CD38 antibody. The toxicity profile of isatuximab was manageable and mostly reversible and will be monitored in routine pharmacovigilance activities. Since treatment duration is until progressive disease or unacceptable toxicity, long‐term exposure might occur. However, safety data for long‐term exposure (>12 months) are limited. To gain more information about long‐term exposure, the applicant was asked to evaluate the safety data at the time of the final OS analysis of study EFC14335 and provide an addendum.
In summary, the clinical benefit of adding isatuximab to pomalidomide and dexamethasone was demonstrated in patients with RR MM who have received at least two prior therapies including lenalidomide and a PI. Toxicity was higher for IsaPd compared with isatuximab monotherapy, but this did not result in increased treatment discontinuation or death. The type of AEs was generally as expected based on the mechanism of action of the IsaPd components. The added toxicity of combining isatuximab with Pd was justified by the demonstrated clinical benefit.
Conclusion
Based on the review of data on quality, safety, and efficacy, the EMA CHMP concluded by consensus that the risk‐benefit balance of isatuximab in combination with pomalidomide and dexamethasone was favorable for the treatment of adult patients with relapsed and refractory multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor and have demonstrated disease progression on the last therapy.
Author Contributions
Data analysis and interpretation: Malgorzata Zienowicz, Paula Boudewina van Hennik, Alexandre Moreau, Christian Gisselbrecht, Harald Enzmann, Francesco Pignatti
Manuscript writing: Julio Delgado, Malgorzata Zienowicz, Francesco Pignatti
Final approval of manuscript: Julio Delgado, Malgorzata Zienowicz, Paula Boudewina van Hennik, Alexandre Moreau, Christian Gisselbrecht, Harald Enzmann, Francesco Pignatti
Disclosures
The authors indicated no financial relationships.
Acknowledgments
This publication is a summary of the European Public Assessment Report, the Summary of Product Characteristics, and other product data as published on the EMA Web site (https://www.ema.europa.eu/en/medicines). For the most current information on this marketing authorization, please refer to the EMA Web site. The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the regulatory agency/agencies or organizations with which the authors are employed/affiliated.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Sant M, Allemani C, Tereanu C et al. Incidence of hematologic malignancies in Europe by morphologic subtype: Results of the HAEMACARE project. Blood 2010;116:3724–3734. [DOI] [PubMed] [Google Scholar]
- 2. Rosko A, Giralt S, Mateos MV et al. Myeloma in elderly patients: When less is more and more is more. Am Soc Clin Oncol Educ Book 2017;37:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moreau P, San Miguel J, Sonneveld P et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28(suppl 4):iv52–iv61. [DOI] [PubMed] [Google Scholar]
- 4. San‐Miguel JF, Hungria VT, Yoon SS et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double‐blind phase 3 trial. Lancet Oncol 2014;15:1195–1206. [DOI] [PubMed] [Google Scholar]
- 5. Lokhorst HM, Plesner T, Laubach JP et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015;373:1207–1219. [DOI] [PubMed] [Google Scholar]
- 6. Dimopoulos MA, Dytfeld D, Grosicki S et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med 2018;379:1811–1822. [DOI] [PubMed] [Google Scholar]
- 7. Lonial S, Dimopoulos M, Palumbo A et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015;373:621–631. [DOI] [PubMed] [Google Scholar]
- 8. Dimopoulos M, Spencer A, Attal M et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 2007;357:2123–2132. [DOI] [PubMed] [Google Scholar]
- 9. Miguel JS, Weisel K, Moreau P et al. Pomalidomide plus low‐dose dexamethasone versus high‐dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM‐003): A randomised, open‐label, phase 3 trial. Lancet Oncol 2013;14:1055–1066. [DOI] [PubMed] [Google Scholar]
- 10. Stewart AK, Rajkumar SV, Dimopoulos MA et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015;372:142–152. [DOI] [PubMed] [Google Scholar]
- 11. Moreau P, Masszi T, Grzasko N et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016;374:1621–1634. [DOI] [PubMed] [Google Scholar]
- 12. Turesson I, Bjorkholm M, Blimark CH et al. Rapidly changing myeloma epidemiology in the general population: Increased incidence, older patients, and longer survival. Eur J Haematol 2018;101:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deckert J, Wetzel MC, Bartle LM et al. SAR650984, a novel humanized CD38‐targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res 2014;20:4574–4583. [DOI] [PubMed] [Google Scholar]
- 14. Moreno L, Perez C, Zabaleta A et al. The mechanism of action of the anti‐cd38 monoclonal antibody isatuximab in multiple myeloma. Clin Cancer Res 2019;25:3176–3187. [DOI] [PubMed] [Google Scholar]
- 15. Jiang H, Acharya C, An G et al. SAR650984 directly induces multiple myeloma cell death via lysosomal‐associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia 2016;30:399–408. [DOI] [PubMed] [Google Scholar]
- 16. Attal M, Richardson PG, Rajkumar SV et al. Isatuximab plus pomalidomide and low‐dose dexamethasone versus pomalidomide and low‐dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA‐MM): A randomised, multicentre, open‐label, phase 3 study. Lancet 2019;394:2096–2107. [DOI] [PubMed] [Google Scholar]
- 17. Martin T, Strickland S, Glenn M et al. Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma. Blood Cancer J 2019;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mikhael J, Richter J, Vij R et al. A dose‐finding phase 2 study of single agent isatuximab (anti‐CD38 mAb) in relapsed/refractory multiple myeloma. Leukemia 2020;34:3298–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mikhael J, Richardson P, Usmani SZ et al. A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood 2019;134:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]


